INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep related breathing disorder characterized by collapse of the upper airway leading to cessation of airflow in the setting of continued respiratory effort[1]. The resultant hypoxia leads to frequent arousals causing sleep fragmentation and symptoms of excessive daytime sleepiness. Also, sleep fragmentation increases sympathetic activity, which can increase blood sugar levels by decreasing insulin sensitivity and glucose effectiveness[2].
According to the American Diabetes Association (ADA), diabetes results from a defect in insulin secretion, insulin action or a combination of both[3]. The current global prevalence of diabetes is 135 million and is expected to be 300 million by 2025[4]. Obesity, male gender and older age are well known risk factors for development of OSA and these risk factors are also associated with the increased likelihood of developing type 2 diabetes mellitus (T2DM)[5]. There is a growing body of evidence describing the association between OSA, insulin resistance and the subsequent development of T2DM[6-11]. Also, the importance of effective treatment of OSA in individuals with T2DM has been well studied[12,13]. In spite of this, OSA remains an underdiagnosed and under-treated condition in individuals with T2DM[11-13]. This article provides a concise review of current literature on the relationship between OSA and T2DM, the effect of OSA on the secondary complications of T2DM, and the effect of OSA treatment on outcomes related to T2DM.
OBSTRUCTIVE SLEEP APNEA AND ITS RELATIONSHIP TO GLUCOSE METABOLISM
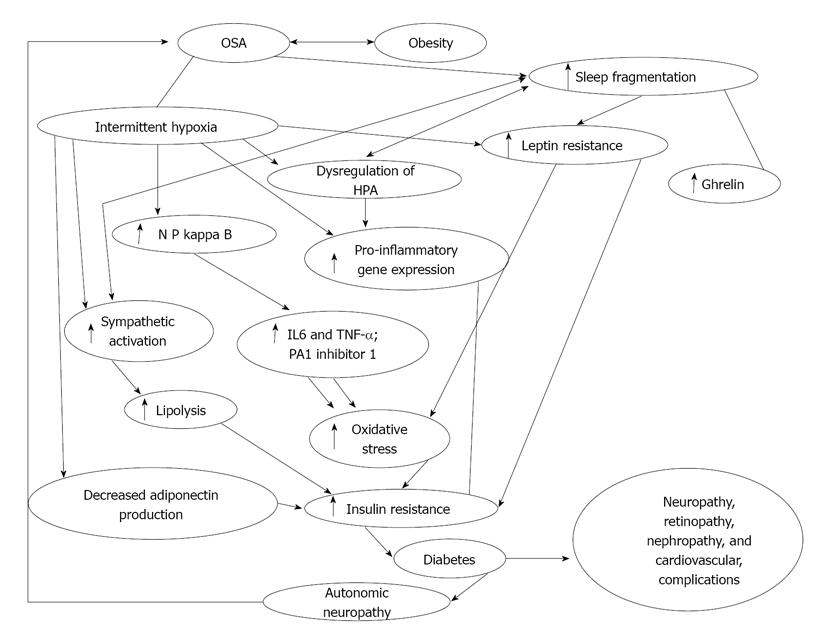
OSA is known to induce a severe state of insulin resistance (which is a risk factor for cardiovascular disease even in the absence of T2DM), resulting in marked compensatory hyperinsulinemia and thereby increasing the requirement for higher doses of exogenous insulin[5,14]. Two recent studies suggested that sleep disordered breathing is independently associated with glucose intolerance and insulin resistance[12,15]. In this large population based cohort, the investigators found that individuals with OSA were more likely to have lower levels of insulin sensitivity (34% vs 54%, P≤ 0.0001) and higher levels of fasting insulin production compared to those without OSA. The pathophysiological basis of hyperglycemia in OSA appears to be twofold; hypoxia and sleep fragmentation (Figure 1). The mechanisms involved in the development of hypoxia induced hyperglycemia and insulin resistance have been extensively studied[8-13]. Sympathomimetic hormone (epinephrine, nor-epinephrine and cortisol) levels were noted to be elevated in healthy volunteers subjected to transient hypoxia[16-19]. These studies demonstrate that hypoxia causes a significant elevation in epinephrine levels leading to an increase in hepatic gluconeogenesis and decrease in skeletal muscle reuptake of glucose resulting in hyperglycemia. Additionally, the genesis of metabolic dysfunction in sleep disordered breathing likely involves several distinct but synergistic processes, including activation of the sympathetic nervous system, increase in oxidative stress, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and low-grade systemic inflammation[9]. Elevation of systemic inflammatory markers such as tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), fibrinogen and uric acid, all of which could be secondary to the combined interactions of obesity, hyperglycemia and nocturnal hypoxia, are also possible contributing factors[20]. Elevation of these inflammatory markers leads to insulin resistance and impaired glucose utilization[21]. Furthermore, sleep fragmentation for as little as two nights has been shown to decrease insulin sensitivity and impair glucose metabolism[2]. Sleep fragmentation induced hyperglycemia and decreased insulin sensitivity appears to be mediated through alterations in sympathovagal balance, with a shift toward increased sympathetic nervous system activity during sleep and wakefulness[2]. These findings were also specifically studied in women with sleep apnea[15]. Women were noted to have a decrease in insulin sensitivity and beta-cell function, and higher loss of the Homeostasis Model Assessment (HOMA) product[15]. These two abnormalities were associated with poorer glycemic control and a 10% higher hemoglobin A1c (HbA1c) even after adjusting for intensive treatment for T2DM. In fact in this study, women with sleep apnea received more insulin than women without sleep apnea. Therefore, the relationship between OSA as a significant contributing factor to impaired glucose metabolism appears well proven.
Figure 1 Flow diagram demonstrating interplay between obstructive sleep apnea, intermittent hypoxia, sleep fragmentation and diabetes.
OSA: Obstructive sleep apnea; NF-κB: Nuclear factor kappa B; PA1: Plasminogen activator inhibitor 1; HPA: Hypothalamic-pituitary axis; IL: Interleukin; TNF-α: Tumor necrosis factor-α. Modified from Ref [58].
OBSTRUCTIVE SLEEP APNEA AND DIABETES MELLITUS TYPE 2
A vast body of literature has been published establishing the relationship between OSA and T2DM. Cross-sectional estimates from clinic populations and population studies suggest that up to 40% of patients with OSA have diabetes[7,22,23], but the incidence of new diabetes in patients with OSA is not known. Likewise, in patients who are known to have diabetes, the prevalence of OSA may be up to 23%[11], and the prevalence of some form of sleep disordered breathing (SDB) may be as high as 58%[24]. In patients with established T2DM there is a significant relationship between SDB and fasting insulin, glucose, and HbA1c levels, that is independent of obesity as determined by the waist-hip ratio[16]. Although there is now convincing evidence of the association between OSA and decreased insulin sensitivity, the exact pathophysiological mechanism linking OSA as a causative factor of T2DM remains elusive. The converse (T2DM causing OSA) is postulated to involve the dysregulation of the autonomic nervous system leading to SDB. Diabetic autonomic neuropathy likely leads to ventilatory dysfunction through decreased heart rate variability and impaired central control of breathing leading to SDB[25-29]. One study showed that 25% of diabetic individuals with autonomic neuropathy have sleep apnea, a proportion greater than in diabetic subjects without autonomic neuropathy[25]. Based up on the current body of literature it is therefore possible to postulate that there is a strong relationship between T2DM and OSA.
RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA AND MICROVASCULAR COMPLICATIONS OF DIABETES MELLITUS
Long standing poorly controlled T2DM is associated with the development of microvascular complications such as retinopathy, nephropathy, neuropathy and macrovascular vascular disease such as coronary artery and cerebrovascular disease. In this section, we review the literature on the association between OSA and the risk of developing T2DM its related microvascular complications. The macrovascular complications of T2DM have been well recognized. OSA leads to increase in insulin resistance and T2DM, which in turn can lead to increase in the inflammatory markers leading to cardiovascular complications. Studies have shown that treatment with continuous positive airway pressure (CPAP) can decrease the levels of IL-6 and CRP, which in turn can lead to decrease in inflammation and reduce vascular complications[6,30]. Several studies have demonstrated an association between OSA and cardiovascular abnormalities[15,31], and improvement with CPAP therapy. Discussing this is beyond the scope of this review. Here we discuss briefly the role of OSA in diabetic retinopathy, neuropathy, nephropathy and insulin resistance and its treatment. The complex interplay of sleep and diabetes demonstrating interplay between OSA, T2DM, intermittent hypoxia, sleep fragmentation and insulin resistance is shown in Figure 1.
DIABETIC RETINOPATHY
The relationship between diabetes mellitus and the development of proliferative retinopathy is well established. Although one of the first studies[32] to assess the potential relationship between OSA and diabetic retinopathy did not find a strong association, other recent studies have found[33-36] that OSA remained an independent predictor of proliferative retinopathy even after adjusting for conventional risk factors and novel biomarkers for diabetic retinopathy. Also, in individuals with OSA, where hypertension and obesity are co-morbid to T2DM, there appears to be an increased risk of proliferative retinopathy[34]. Diabetic retinopathy is mediated by high levels of serum vascular endothelial growth factors and other biomarkers[33,37,38]. Similarly, the increased risk of diabetic retinopathy in OSA appears to be mediated by elevated levels of inflammatory markers, reduced endothelial regulatory function and increased insulin resistance[39,40]. A recent study by Fujita et al[41] noted an increase in the levels of inflammatory markers such as acylation stimulating protein, high sensitive CRP and components of the membrane attack complex (which leads to activation of alternative complement pathway) in obese T2DM patients with retinopathy. As a result, complement mediated activation of inflammation likely leads to acceleration of diabetic microangiopathy leading to development and worsening of diabetic retinopathy. Previous studies[42,43] looking in to complement activation in individuals with OSA have noted increased levels of C3 and a decrease in the levels of IgM and NK cell percentage. All this data strongly points towards a strong relationship between OSA and increased risk of proliferative retinopathy in individuals with OSA and T2DM.
DIABETIC NEPHROPATHY
Diabetes Mellitus is the leading cause of end stage renal disease[44]. Nephropathy in T2DM is postulated to be mediated through an increase in activity of angiotensin II, platelet derived growth factor, and thromboxane. These agents upregulate protein kinase c activity and this leads to activation of transforming growth factor-beta (TGF-β). TGF-β leads to proliferation of extracellular matrix and glomerulosclerosis leading to diabetic nephropathy[45]. Progression of renal failure in the presence of OSA is likely secondary to hypoxia mediated increase in sympathetic activation and inflammatory cytokines[46]. Additionally, a recent study has also shown increased cystatin C levels in individuals with severe OSA[47]. Cystatin C has been recognized as an early biomarker for renal failure development and cardiovascular events[47]. Other studies have shown that the severity of OSA appears to be directly correlated to the degree of loss of renal function[48]. The literature on OSA leading to the progression of diabetic nephropathy is limited. A recent small study evaluated OSA as an independent risk factor for microalbuminuria and did not find any significant relationship[49]. This study consisted of a small sample of patients with moderate to severe OSA (Apnea hypopnea index > 15), and was underpowered to detect a difference. However, given the overall similarity in the pathophysiological mechanisms, it is likely that OSA may contribute to the development of diabetic nephropathy. Future larger population based studies will be necessary to better understand this relationship.
PERIPHERAL NEUROPATHY
Peripheral neuropathy secondary to T2DM is very common, affecting 60%-70% of individuals with diabetes[44]. Severe forms of diabetic neuropathy are a major contributing cause of lower-extremity amputations[44]. The pathophysiologic mechanism of diabetic peripheral neuropathy appears to be complex; involving metabolic and ischemic pathways[50]. Metabolic factors mediated by hyperglycemia lead to abnormal nerve energy transport, impaired axonal transport, increased activity of the sorbitol pathway, non-enzymatic nerve protein glucosylation and abnormal myo-inositol metabolism. The ischemic pathway is mediated through thickening and hyalinization of the microvasculature wall leading to neuronal ischemia[50]. Previous studies have noted the relationship between OSA and peripheral neuropathy and the improvement in peripheral neuropathy with treatment of OSA using CPAP[51-54]. A recent study specifically looking into the relationship between OSA and diabetic peripheral neuropathy found that there was a fourfold increase in the odds of peripheral neuropathy in diabetic patients with OSA compared those without[36]. Also, noted was a significant trend in the prevalence of diabetic peripheral neuropathy with lower levels of oxygen saturations. In the same study, individuals with OSA and diabetic peripheral neuropathy were found to have higher levels of nitrotyrosine and lipid peroxide levels compared to those without OSA. Nitrotyrosine and lipid peroxide leads to nitrosative and oxidative stress that reduces nerve perfusion, resulting in impairment of vascular reactivity of the epineurial arterioles[55]. Overall, there is stronger data now supporting the hypothesis that OSA is an independent risk factor for the development of diabetic peripheral neuropathy. A recent study-linking OSA to diabetic peripheral neuropathy has been postulated suggesting protein kinase, advanced glycation end products, hexosamine and polyol pathway each of those leading to microvascular complications including diabetic peripheral neuropathy[36].
TREATMENT OF OBSTRUCTIVE SLEEP APNEA AND OUTCOMES WITH IMPAIRED GLUCOSE TOLERANCE AND DIABETES MELLITUS
The strong association between insulin resistance and OSA would imply that treatment with CPAP should lead to improved glucose control in patients with T2DM. The effect of CPAP on glucose control has been variable[56]. Review of the literature found that most studies exploring the effect of CPAP on insulin sensitivity show a positive effect[57-65], although other studies have not shown a significant improvement in hyperglycemia or level of insulin resistance[66-69]. The variability in results is likely due to methodological weaknesses such as small sample sizes, lack of adjustment for confounders and absence of polysomnographic evidence to support sleep duration. Other studies have shown that racial or ethnic differences among the study populations could contribute to variability in response to CPAP[70,71]. In one study the response to CPAP was significantly greater if the body mass index (BMI) was less than 30[60]. The likely explanation appears to be that obesity is a more important determinant of insulin resistance than OSA. The less obese the patients are, the greater is the improvement in insulin sensitivity brought about by CPAP treatment[60]. Another study observed a significant decrease in glycated hemoglobin values with CPAP, although there was no significant difference in fasting blood sugars and insulin resistance on a day-to-day basis[68,72]. The likely explanation for this finding is that glycated hemoglobin is a better marker of long term glucose control unlike blood glucose and insulin resistance, which can fluctuate on a day-to-day basis[68]. This suggests that monitoring HbA1c levels as a marker of efficacy of CPAP therapy is likely a better indicator than monitoring indices of insulin resistance or glucose utilization (i.e., HOMA product). A recent randomized control study[64] noted a dose-response effect for both the severity of disease and adherence to CPAP treatment. Insulin sensitivity was significantly better after treatment with CPAP in patients with severe OSA (Apnea hypopnea index ≥ 30) compared to patients with less severe OSA. Also, the same study revealed that each additional hour of active CPAP usage was associated with a significant improvement in insulin sensitivity. The reason for a lack of effect of CPAP on the less severe OSA patients is unknown. In spite of the variability in results of the studies, treatment with CPAP remains an important modality for patients with OSA to improve glucose metabolism. Treatment appears particularly important for patients with severe OSA and subjective symptoms of sleepiness. In light of the current results, clinicians should ensure patients with OSA are effectively treated, particularly with the epidemic of obesity and diabetes.
CONCLUSION
There appears to be an association between OSA and the development of insulin resistance leading to impaired fasting glucose tests and the development of T2DM. Furthermore, data supports the development and progression of complications from long standing T2DM in the setting of inadequately treated OSA. There is an abundance of evidence demonstrating the link between OSA and impaired glucose metabolism. Irrespective of the direction of causality, the association between OSA and T2DM remains irrefutable. Therefore, in spite of the controversial nature of the data regarding the improvement in glycemic control with CPAP therapy, judgment should be reserved until long term rigorously conducted prospective studies can expand knowledge in this area. In the interim, although a strong recommendation for treatment of OSA with CPAP to control diabetes remains controversial, physicians should individualize their decisions based on the particular needs of their patients.
P- Reviewers: Cui WP, Sanchez-MargaletV, Schaffer S S- Editor: Wen LL L- Editor: A E- Editor: Liu XM