Published online Oct 15, 2013. doi: 10.4239/wjd.v4.i5.210
Revised: March 24, 2013
Accepted: August 12, 2013
Published online: October 15, 2013
Processing time: 272 Days and 16.8 Hours
AIM: To investigate whether impaired fasting glucose (IFG) confers cardiovascular risk.
METHODS: A non-diabetic population-based sample representative of middle-aged and elderly Turks was studied at 8.5 years’ follow-up for incident diabetes and coronary heart disease (CHD). Metabolic syndrome (MetS) was defined by ATP-III criteria modified for male abdominal obesity, and IFG and type 2 diabetes were identified by criteria of the American Diabetes Association. Stratification by presence of MetS was used. Outcomes were predicted providing estimates for hazard ratio (HR) obtained by use of Cox proportional hazards regression analysis in models that controlled for potential confounders.
RESULTS: In 3181 adults (aged 52 ± 11.5 years at baseline), analysis stratified by MetS, gender and IFG status distinguished normoglycemic subjects by a “hypertriglyceridemic waist” phenotype consisting of significantly higher waist circumference, fasting triglyceride and lower high-density lipoprotein-cholesterol, regardless of gender and MetS. Additionally, lipoprotein (Lp) (a) tended to be lower in (especially female) participants with MetS. Multivariable linear regression in a subset of the sample demonstrated decreased Lp (a) levels to be associated with increased fasting glucose and insulin concentrations, again particularly in women. In Cox regression analysis, compared with normoglycemia, baseline IFG adjusted for major confounders significantly predicted incident diabetes at a 3-fold HR in men and only women with MetS. Cox models for developing CHD in 339 individuals, adjusted for conventional risk factors, revealed that IFG status protected against CHD risk [HR = 0.37 (95%CI: 0.14-0.998)] in subjects free of MetS, a protection that attenuated partly in male and fully in female participants with MetS.
CONCLUSION: IFG status in non-diabetic people without MetS displays reduced future CHD risk, yet is modulated by MetS, likely due to autoimmune activation linked to serum Lp (a).
Core tip: The study investigated whether and to what extent impaired fasting glucose (IFG) conferred risk of type 2 diabetes or coronary heart disease in 3181 middle-aged adults, by separately stratifying to gender and metabolic syndrome. Follow-up over 8.5 years revealed both factors to modulate future risk. In women without metabolic syndrome, IFG was not associated with either cardiometabolic disorder, while in men, IFG imparted risk of diabetes alone. Coronary heart disease risk appeared to depend on the core components of the metabolic syndrome, especially in the female. The results implicate autoimmune activation involving lipoprotein(a) to be of high relevance in the pathogenesis of new-onset diabetes.
- Citation: Onat A, Aydın M, Can G, Çakmak HA, Köroğlu B, Kaya A, Ademoğlu E. Impaired fasting glucose: Pro-diabetic, “atheroprotective” and modified by metabolic syndrome. World J Diabetes 2013; 4(5): 210-218
- URL: https://www.wjgnet.com/1948-9358/full/v4/i5/210.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i5.210
Fasting blood glucose is one of the five components of metabolic syndrome (MetS), the prevalence and the impact on cardiometabolic risk of which is on the rise worldwide. Fasting glucose has been reported to be log-linearly associated with risk of vascular disease in non-diabetic people although related data are inconclusive[1,2]. Impaired fasting glucose (IFG), one of the two pre-diabetic states based on an underlying insulin resistance and impaired β-cell function, is recognized to carry excess risk for diabetes but clearly less risk than impaired glucose tolerance (IGT) for cardiovascular risk that is no more than modest[3,4]. The meta-analysis by the Emerging Risk Factors Collaboration on nearly 280000 non-diabetic individuals showed that fasting glucose concentration was modestly and non-linearly associated with risk of vascular disease[1]. Compared with fasting glucose concentrations of 70-100 mg/dL, HR for coronary heart disease (CHD) were 1.07 for lower than 70 mg/dL; 1.11 for 100-110 mg/dL; and 1.17 for 110-126 mg/dL. IFG status did not significantly improve metrics of vascular disease prediction when added to information about several conventional risk factors[1]. Heterogeneity in screened populations is suggested by individual studies that have reported no excess cardiovascular risk[5-8]. A meta-analysis of clinical trials on interventions in subjects with pre-diabetes did not show reductions in all causes or cardiovascular mortality, contrasted to preventing progression to overt diabetes[9].
We have confirmed, among nearly 2900 Turkish adults, increased multi-adjusted (J-shaped) risk of incident CHD for fasting glucose category < 90 mg/dL, compared with the category 90-110 mg/dL[10], an observation that was at variance with one meta-analysis[11] but in agreement with another[1]. There was evidence that higher CHD risk observed in low compared to higher normal glucose concentrations was likely related to an associated pro-inflammatory state that required further investigation.
However, these analyses did not discriminate the role of the presence of MetS regarding the impact of IFG on CHD risk. Our previous observations indicated that age-adjusted MetS prevailed more in people with the lowest than the intermediate lipoprotein (Lp) (a) tertile[12], and an 11% mean decline in sex- and age-adjusted Lp (a) levels was reported in individuals with diabetes[13], which is usually associated with activation of complement pathways.
We, therefore, aimed to examine the impact of IFG simultaneously on incident type 2 diabetes and CHD in non-diabetic middle-aged Turkish adults of the general population by stratifying to the presence of MetS lending care to potential sex differences that had been shown in Turkish adults with respect to cardiometabolic risk[14,15]. The association of serum Lp (a) and apolipoprotein apoA-I with fasting glucose was also investigated in a subset of the study sample. Such an approach yielded the novel information that IFG, although conferring excess diabetes risk, represented an atheroprotective status compared with normoglycemia in non-diabetic people without MetS, and involvement of Lp (a) in autoimmune activation might be an underlying factor.
The Turkish Adult Risk Factor study is a longitudinal population-based cohort study on cardiac disease and its risk factors in adults in Turkey, carried out biennially in 59 communities in all geographical regions[16]. It involves a random sample of the Turkish adult population, representatively stratified for sex, age, geographical regions and for rural-urban distribution[16]. Combined measurements of waist circumference and high-density lipoprotein (HDL) cholesterol being first available at the follow-up visit in 1997-1998, the latter examination formed the baseline. Participants, 28 years of age or older at baseline, were examined periodically up to the survey 2010-2011. When individuals with prevalent diabetes and missing values for fasting (or 2-h postprandial) glucose at baseline were excluded, the remaining 3181 non-diabetic participants formed the cohort of the current study. The survey conformed to the principles embodied in the Declaration of Helsinki and was approved by the Istanbul University Ethics Committee. Individuals of the cohort gave written consent for participation. Data were obtained by history of the past years via a questionnaire, physical examination of the cardiovascular system, sampling of blood and recording of a resting 12-lead electrocardiogram.
Blood pressure (BP) was measured using a sphygmomanometer (Erka, Bad Tölz, Germany) after at least 5 min of rest while seated, and the mean of two recordings was recorded. Waist circumference was measured with a tape (Roche LI95 63B 00), the subject standing, at the level midway between the lower rib margin and the iliac crest. Body mass index (BMI) was computed as weight divided by height squared (kg/m2). Self-reported cigarette smoking was categorized into never smokers, former smokers (discontinuance of 3 mo or more) and current smokers (regularly 1 or more cigarettes daily).
Plasma concentrations of total and high-density lipoprotein (HDL) cholesterol, (> 11 h) fasting triglycerides and glucose were determined at baseline examination by the enzymatic dry chemistry method using a Reflotron apparatus. Low-density lipoprotein (LDL) cholesterol values were computed according to the Friedewald formula. In the final five surveys, the stated parameters, as well as insulin and C-reactive protein (CRP) values, were assayed in a single central laboratory. Blood samples were shipped to Istanbul and stored in deep-freeze at -75 °C until analyzed. Concentrations of insulin were determined by the electrochemiluminescence immunoassay ECLIA on Roche Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). Serum concentrations of apoA-I, apoB, apoE, and Lp (a) were measured by the Behring nephelometry (Behring Diagnostics, Marburg, Germany). Serum γ-glutamyltransferase activity was assayed by the kinetic method using Glucana as substrate (Thermo Trace, Noble Park, Victoria, Australia). External quality control was performed with a reference laboratory in a random selection of 5%-6% of participants. Data on baseline fasting insulin, Lp (a) and apoA-I were available in 71%, 50% and 34% of participants, respectively.
Subjects in whom plasma glucose was measured after breakfast outside a 1.5-2.5 h period were not included. As recommended by the American Diabetes Association, individuals with IFG were identified with fasting glucose concentrations of 5.56-6.98 mmol/L, or (in one-fifth of the sample) with levels of 5.60-7.78 mmol/L 1.5-2.5 h after breakfast; type 2 diabetes with ≥ 7.0 mmol/L (or 2 h postprandial glucose ≥ 11.1 mmol/L) and/or the current use of diabetes medication[17]. MetS was identified when 3 out of the 5 criteria of the National Cholesterol Education Program ATP-III were met, modified for pre-diabetes (fasting glucose 5.56-6.95 mmol/L)[18] and further for male abdominal obesity using as a cut point ≥ 95 cm[19], as assessed in the Turkish Adult Risk Factor study. Biological evidence of functional defectiveness of HDL and apoA-I particles as discerned by outcomes of diabetes and/or CHD has been designated as HDL dysfunction[15,20,21].
Diagnosis of nonfatal CHD was based on the presence of angina pectoris, of a history of myocardial infarction with or without accompanying Minnesota codes of the electrocardiogram (ECG)[22], or on a history of myocardial revascularization. Typical angina and age > 45 years in women were prerequisite for a diagnosis when angina was isolated. ECG changes of “ischemic type” of greater than minor degree (codes 1.1-2, 4.1-2, 5.1-2, 7.1) were considered as myocardial infarct sequelae or myocardial ischemia, respectively. Cause of death was assigned in accordance with the information on the mode of death obtained from first degree relatives and/or local health personnel, also considering pre-existing clinical and laboratory findings elicited during biennial surveys.
Descriptive parameters were shown as mean ± SD or in percentages. Due to skewed distribution, values derived from log-transformed (geometric) means were used for serum triglycerides, insulin, apoE and Lp (a). Multiple linear regression analysis was performed with continuous parameters using Lp (a) as a dependent variable and apoA-I/HDL-C ratio as an independent variable. This ratio was considered to potentially reflect greater proportional increase in serum apoA-I than HDL-C, in line with the notion of preβ -1 HDL[23]. In predicting outcomes at baseline examination in multivariate analyses, estimates (and 95%CI) for hazard ratio (HR) were obtained by use of Cox proportional hazards regression analysis in models that controlled for potential confounders. HRs were expressed in terms of 1 SD increment. A value of P < 0.05 on the two-sided test was considered statistically significant. Statistical analyses were performed using SPSS-10 for Windows (SPSS Inc., Chicago, IL, United States, Nr. 9026510).
Mean age of the study sample at baseline (1564 men and 1617 women) was 52.0 ± 11.5 years. Mean follow-up with respect to diabetes amounted to 8.5 ± 3.5 years (range 2-13 years) and to CHD 8.4 ± 3.7 years (range 1-13 years). Diabetes developed at a mean rate of 11.3 per 1000 person-years, CHD at 15.0 per 1000 person-years.
Normoglycemic men without MetS were younger, had a wider waist, higher triglycerides, LDL and total cholesterol, and lower HDL-C than men with IFG but without MetS (Table 1). They tended also to have higher apoB levels. Normoglycemic women without MetS had a wider waist, higher triglycerides and lower HDL-C, and tended to lower Lp (a) than women with IFG yet free of MetS. This suggests that aggregation between Lp (a) and a protective protein was in operation, enhancing the pro-inflammatory state.
| Without metabolic syndrome | ||||||||||||||
| Men | Women | Men | Women | |||||||||||
| N’glyc. 91.4 | IFG, 110.5 mg/dL | ANOVA | N’glyc. 92 | IFG, 109 mg/dL | ANOVA | N’glyc. 93.3 | ANOVA | N’glyc. 93.3 | IFG, 111 mg/dL | ANOVA | ||||
| n | n= 857 | n= 79 | n= 813 | n= 77 | n | n= 460 | n= 168 | n= 471 | n= 256 | |||||
| Age (yr) | 1826 | 50.8 ± 12.7 | 54.3 ± 14.2 | 0.03 | 49.7 ± 12.2 | 49.7 ± 11.9 | 0.99 | 1355 | 54.1 ± 11.4 | 57 ± 12 | 0.008 | 55.3 ± 11.3 | 56.2 ± 12.2 | 0.31 |
| Waist circumference (cm) | 1826 | 89.3 ± 9.6 | 85.4 ± 8.5 | 0.001 | 84.7 ± 11 | 81.7 ± 9.8 | 0.023 | 1289 | 101.1 ± 8.4 | 98.9 ± 9.3 | 0.004 | 98.3 ± 10.3 | 95 ± 12 | < 0.001 |
| F. triglycerides (mg/dL) | 1336 | 111.6 ±1.60 | 93 ± 1.33 | < 0.001 | 95.7 ± 1.48 | 83.9 ± 1.36 | 0.001 | 1043 | 195 ± 1.62 | 155.2 ± 1.67 | < 0.001 | 164.7±1.61 | 143 ± 1.65 | 0.001 |
| HDL cholesterol (mg/dL) | 1826 | 40.5 ± 12.5 | 43.9 ± 12.3 | 0.021 | 48.5 ± 13 | 52.3 ± 13.8 | 0.017 | 1291 | 32.3 ± 7.7 | 35 ± 10.1 | 0.002 | 39.4 ± 9 | 42.1 ± 11.7 | < 0.001 |
| LDL cholesterol (mg/dL) | 1826 | 113 ± 30.9 | 102.8 ± 25.9 | 0.003 | 116 ± 33.8 | 109.1 ± 35.1 | 0.11 | 1355 | 116 ± 32.5 | 115.5 ± 33 | 0.90 | 127.4 ± 34.3 | 120.2 ± 34 | 0.015 |
| Total cholest. (mg/dL) | 1826 | 180 ± 36.5 | 167.4 ± 30.2 | 0.003 | 185 ± 38 | 179 ± 36.5 | 0.18 | 1355 | 189.2 ± 37.7 | 186.1 ± 35.8 | 0.35 | 199 ± 38.2 | 195 ± 39.7 | 0.18 |
| Apo B (mg/dL) | 618 | 111.8 ± 35 | 100.7 ± 22.7 | 0.07 | 108.9 ± 32.7 | 115 ± 41.6 | 0.34 | 505 | 128.6 ± 38.4 | 120.8 ± 43.5 | 0.18 | 131.6 ± 50.2 | 122.5 ± 41.7 | 0.13 |
| Apo A-I (mg/dL) | 583 | 127.7 ± 29.7 | 127.7 ± 35.8 | 1.00 | 145.9 ± 32 | 150.7 ± 35.2 | 0.44 | 486 | 123 ± 28.3 | 124.8 ± 30.8 | 0.67 | 137 ± 30 | 145 ± 36.4 | 0.067 |
| Apo E (mg/dL) | 574 | 4.26 ± 3.73 | 3.36 ± 0.98 | 0.24 | 4.03 ± 1.25 | 4.01 ± 1.11 | 0.94 | 455 | 4.50 ± 2.11 | 4.67 ± 2.99 | 0.64 | 4.67 ± 1.97 | 4.21 ± 1.39 | 0.063 |
| Fast insulin (mIU/L) | 1310 | 7.14 ± 2.06 | 5.97 ± 2.23 | 0.063 | 7.52 ± 2.01 | 7.41 ± 1.87 | 0.88 | 952 | 10.4 ± 1.80 | 9.7 ± 1.92 | 0.28 | 10.3 ± 1.75 | 9.5 ± 1.93 | 0.13 |
| Lipoprotein(a) (mg/dL) | 884 | 9.14 ± 2.92 | 8.48 ± 2.67 | 0.70 | 10.98 ± 2.93 | 15.96 ± 3.25 | 0.073 | 687 | 7.75 ± 3.01 | 8.75 ± 2.67 | 0.39 | 10.23 ± 2.96 | 11.32 ± 3.1 | 0.39 |
| Diastolic BP (mmHg) | 1826 | 77.1 ± 11 | 76.1 ± 7.8 | 0.43 | 78.6 ± 12.6 | 77 ± 12.3 | 0.18 | 1355 | 88 ± 13 | 87 ± 14.6 | 0.44 | 90.3 ± 13.5 | 87.7 ± 14.3 | 0.013 |
| γ-glutamyltransf (U/L) | 984 | 24.7 ± 1.78 | 22.5 ± 1.94 | 0.32 | 16.5 ± 1.80 | 19.2 ± 1.80 | 0.13 | 750 | 29.7 ± 1.85 | 27.6 ± 1.84 | 0.33 | 19.9 ± 1.88 | 21.1 ± 1.93 | 0.40 |
| Creatinine (mg/dL) | 1060 | 0.977 ± 0.18 | 0.963 ± 0.17 | 0.63 | 0.783 ± 0.31 | 0.75 ± 0.12 | 0.51 | 745 | 1.04 ± 0.25 | 0.96 ± 0.18 | 0.64 | 0.808 ± .19 | 0.77 ± 0.14 | 0.023 |
| Smoking; curr., fast (%) | 1800 | 58.9 ± 18.9 | 55.3 ± 21.1 | 0.82 | 21.9 ± 4 | 26.3 ± 6.6 | 0.33 | 1331 | 46 ± 25.6 | 37.7 ± 27.5 | 0.82 | 11.5 ± 3.8 | 15.7 ± 4.3 | 0.24 |
Normoglycemic men with MetS were younger, had a wider waist, higher triglycerides and creatinine, and lower HDL-C than men with IFG and MetS. Normoglycemic women with MetS had a wider waist, higher triglycerides, LDL and total cholesterol, diastolic BP, lower HDL-C levels and tended to higher apoE, apoB and lower apoA-I than women with IFG and MetS.
In men, while no material difference in circulating Lp (a) existed in individuals with MetS and IFG compared to men free of MetS, concentrations were lower in normoglycemic MetS by 15% (P = 0.069) (Table 1). In women, whereas serum Lp (a) was lower in normoglycemia by 7% (P = 0.41), concentrations were lower by 29% (P = 0.14) in IFG. The data tend to indicate a reduction of Lp (a) concentrations in established MetS.
Multiple linear regression analysis in separate sexes with Lp (a) as a dependent and age, glucose, insulin and apoA-I/HDL cholesterol ratio as independent variables showed inverse associations with age and fasting glucose (P < 0.02) in women and a tendency to inverse associations in both sexes with insulinemia (Table 2).
| Men (n = 165) | Women (n = 173) | |||||
| β | SE | P value | β | SE | P value | |
| Age (11 yr) | 1.05 | 1.11 | 0.59 | 0.78 | 1.01 | 0.007 |
| Fasting glucose (24 mg/dL) | 0.82 | 1.12 | 0.14 | 0.66 | 1.18 | 0.018 |
| Fasting insulin (mIU/L) | 0.82 | 1.28 | 0.43 | 0.75 | 1.32 | 0.31 |
| Apo A-I/HDL-chol ratio (1 U) | 1.06 | 1.09 | 0.48 | 1.08 | 1.09 | 0.40 |
| R2 = 0.01; P = 0.45 | R2 = 0.06; P = 0.014 | |||||
Compared with normoglycemia, IFG predicted incident type 2 diabetes with an HR of 2.83 (95%CI: 2.17-3.71), after adjustment for sex, age, BMI, smoking status, non-HDL and HDL cholesterol, and usage of antihypertensive and statin medication (Table 3). BMI alone among the latter confounders contributed significantly, albeit at a lesser magnitude than IFG, towards predicting diabetes. In individuals free of MetS (57% of the sample), women differed from the whole group in as much as IFG did not confer diabetes risk against which current smoking tended to protect, whereas in men the difference concerned statin usage which was associated with future diabetes risk (corresponding to an absolute risk of 1 excess diabetes case for every 145 male users free of MetS).
| Whole sample | With MetS | |||||||||||
| Total, n = 238/23821 | Men, n = 130/1140 | Women, n = 108/1242 | Total, n= 75/13601 | Men, n = 49/6871 | Women, n = 26/6731 | |||||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | HR | 95%CI | HR | 95%CI | HR | 95%CI | |
| Sex (female) | 0.53 | 0.38; 0.75 | 0.33 | 0.18-0.61 | ||||||||
| Age (11 yr) | 1.10 | 0.97-1.26 | 1.19 | 1.00-1.41 | 1.00 | 0.82-1.23 | 1.13 | 0.90-1.40 | 1.14 | 0.87-1.48 | 1.09 | 0.73-1.62 |
| IFG vs normoglycemia | 2.83 | 2.17-3.71 | 2.65 | 1.82-3.86 | 3.10 | 2.10-4.58 | 2.06 | 1.04-4.06 | 2.59 | 1.19-5.64 | 1.38 | 0.32-5.97 |
| Body m. index (5 kg/m2) | 1.74 | 1.53-1.98 | 1.91 | 1.55-2.34 | 1.64 | 1.39-1.94 | 1.77 | 1.34-2.33 | 1.86 | 1.24-2.78 | 1.70 | 1.18-2.46 |
| HDL-cholest (12 mg/dL) | 0.95 | 0.82-1.10 | 0.99 | 0.79-1.22 | 0.92 | 0.75-1.13 | 1.05 | 0.83-1.31 | 1.10 | 0.85-1.41 | 0.96 | 0.66-1.43 |
| Statin usage | 1.25 | 0.59-2.68 | 2.62 | 0.96-7.18 | 0.76 | 0.24-2.43 | 2.13 | 0.49-9.26 | 7.63 | 1.66-35.2 | 0 | few users |
| Antihypertensive drugs | 1.18 | 0.85-1.64 | 1.32 | 0.81-2.14 | 1.12 | 0.71-1.76 | 1.70 | 0.84-3.42 | 2.19 | 0.88-5.44 | 1.32 | 0.46-3.82 |
| Current vs never smoking | 1.04 | 0.73-1.47 | 1.11 | 0.72-1.71 | 0.86 | 0.47-1.59 | 0.67 | 0.37-1.23 | 0.85 | 0.42-1.73 | 0.20 | 0.03-1.48 |
| Former vs never smoking | 1.38 | 0.93-2.05 | 1.24 | 0.79-1.97 | 1.98 | 0.86-4.54 | 1.24 | 0.63-2.44 | 1.25 | 0.58-2.70 | 1.47 | 0.20-11.0 |
Table 4 displays Cox proportional hazard models for incident CHD risk stratified to gender and presence of MetS, and comprised of sex, age, systolic BP, smoking status, non-HDL, HDL cholesterol and lipid-lowering drug usage as well. Adults free of MetS but with IFG displayed a clearly reduced HR 0.37 (95%CI: 0.14-0.998) compared with normoglycemic subjects. In participants with MetS, this reduced risk was attenuated in men to an HR of 0.75 and was abolished in women. Aside from age, non-HDL cholesterol (especially in people free of MetS) and systolic BP (especially in men) were major determinants of incident CHD.
| Whole sample | Without MetS | With MetS | ||||||||||||||||
| Total,n = 359/271812 | Men,n = 175/13242 | Women,n = 184/13942 | Total,n = 133/15691 | Men,n = 74/795 | Women,n = 59/7741 | Total,n = 226/11381 | Men,n = 101/524 | Women,n = 125/614 | ||||||||||
| HR | 95%CI | RR | 95%CI | RR | 95%CI | HR | 95%CI | RR | 95%CI | RR | 95%CI | HR | 95%CI | RR | 95%CI | RR | 95%CI | |
| Female sex | 0.94 | 0.72-1.22 | 0.82 | 0.54-1.27 | 0.98 | 0.70-1.38 | ||||||||||||
| Age (11 yr) | 1.49 | 1.34-1.66 | 1.52 | 1.31-1.76 | 1.46 | 1.26-1.71 | 1.71 | 1.46-2.02 | 1.71 | 1.37-2.13 | 1.75 | 1.37-2.24 | 1.33 | 1.15-1.52 | 1.40 | 1.14-1.73 | 1.24 | 1.02-1.51 |
| IFG (FG > 100 mg/dL) | 0.93 | 0.71-1.21 | 0.71 | 0.46-1.10 | 1.10 | 0.78-1.55 | 0.37 | 0.14-0.998 | 0.47 | 0.15-1.53 | 0.22 | 0.03-1.64 | 0.96 | 0.72-1.29 | 0.75 | 0.46-1.23 | 1.10 | 0.76-1.61 |
| Non-HDL chol. (35 mg/dL) | 1.37 | 1.23-1.47 | 1.42 | 1.23-1.63 | 1.32 | 1.15-1.52 | 1.47 | 1.23-1.68 | 1.57 | 1.28-1.93 | 1.37 | 1.07-1.68 | 1.28 | 1.11-1.42 | 1.32 | 1.07-1.57 | 1.28 | 1.11-1.52 |
| HDL cholest. (12 mg/dL) | 0.84 | 0.76-0.95 | 0.87 | 0.73-1.04 | 0.83 | 0.71-0.98 | 0.90 | 0.76-1.07 | 0.93 | 0.73-1.20 | 0.85 | 0.66-1.09 | 0.90 | 0.74-1.09 | 0.83 | 0.62-1.13 | 0.95 | 0.75-1.22 |
| Systolic BP (25 mmHg) | 1.42 | 1.28-1.56 | 1.72 | 1.49-2.04 | 1.31 | 1.16-1.49 | 1.35 | 1.13-1.60 | 1.60 | 1.22-2.15 | 1.22 | 0.95-1.52 | 1.35 | 1.19-1.56 | 1.72 | 1.38-2.15 | 1.22 | 1.03-1.42 |
| Current vs never smoking | 1.13 | 0.86-1.49 | 1.52 | 1.04-2.21 | 0.78 | 0.49-1.25 | 1.19 | 0.78-1.84 | 1.34 | 0.76-2.37 | 1.09 | 0.52-2.26 | 1.13 | 0.78-1.64 | 1.60 | 0.96-2.67 | 0.70 | 0.37-1.31 |
| Former vs never smoking | 1.13 | 0.79-1.60 | 1.23 | 0.81-1.88 | 1.42 | 0.66-3.05 | 1.05 | 0.58-1.90 | 0.93 | 0.45-1.92 | 2.22 | 0.90-6.22 | 1.15 | 0.74-1.79 | 1.38 | 0.81-2.33 | 0.95 | 0.30-3.03 |
In this sample representative of middle-aged non-diabetic Turkish adults, yet prone to MetS, salient findings were normoglycemic participants (without or with) MetS exhibited a more adverse risk profile than those with ADA-defined IFG (specifically regarding waist circumference, triglyceride and HDL cholesterol); fasting glucose levels (especially in women) were associated with serum Lp (a) inversely and independently of fasting insulin concentrations; IFG status conferred a risk of diabetes, as anticipated, 3-fold as high compared with normoglycemic participants, but did not impart future risk for CHD in subjects with MetS and even protected significantly in those free of MetS. Novel findings elicited via such stratified analyses may be attributed in normoglycemic individuals without MetS to the intriguing operation of a tendency to a decline in serum Lp (a), presumably associated with elevation of fasting glucose, while lower levels (particularly in women with IFG) reflect autoimmune activation and, in turn, induce increased risk for MetS and CHD.
Stratification by gender and presence of MetS clearly and consistently showed significant differences in each of the four groups across normoglycemic and pre-diabetic people, specifically in regard to waist circumference, fasting triglyceride and HDL cholesterol levels. This indicates that the hypertriglyceridemic waist (HtgW) phenotype, together with low HDL-C is the main feature distinguishing normoglycemic from pre-diabetic adults, a remarkable and a priori unexpected finding. This finding may, however, be explained by presuming changes in circulating Lp (a) to be the primary determinant in individuals without MetS, which may have mediated changes in fasting plasma glucose. Indeed, Lp (a) was independently linearly associated inversely with fasting insulin concentrations[12] (approximately 2% lower Lp (a) per 2-fold increment of insulin). Irrespective of MetS in men, higher insulin levels (by 15%) existed in normoglycemia than in IFG, a constellation noted in women to a lesser magnitude (by 5%).
Our multiple regression analysis model for serum Lp (a), albeit in a relatively small subgroup of 338 subjects due to missing values of apo A-I, Lp (a) and fasting insulin, yielded valuable information with respect to independent inverse associations in women of lower Lp (a) with increasing age and higher glycemia. This finding not only supports a notion of autoimmune activation whereby part of Lp (a) protein becomes non-assayable, but also may account for the findings of diminished levels of Lp (a) in type 2 diabetes[13] and MetS[12]. Lower Lp (a) in non-diabetic females with increasing age further suggests consumption of Lp (a) protein during the slow process of autoimmune complex formation.
The present study demonstrated nearly a 3-fold diabetes risk for IFG in male participants and females with MetS, after adjustment for variables often used in major diabetes algorithms. This is essentially in line with reports on other populations[24-26], although several validated risk scores do not include IFG as a parameter[26-28].
Yet, IFG was associated with substantially lower future CHD risk than normoglycemia in all men, and women free of MetS. IFG tended to confer slight CHD risk only in women with MetS (i.e., abdominal obesity, atherogenic dyslipidemia and associated pro-inflammatory state). An analogous situation is related to risk of chronic kidney disease (CKD): IFG was the only component of MetS not significantly predicting it in a meta-analysis of 11 studies[29].
At variance from women, normoglycemic men persist to carry a more adverse risk profile than male IFG, presumably in the process of the development of MetS, along with a slow decline in serum Lp (a), a process congruent with normoglycemia still with a non-significantly higher CHD risk than IFG. A further difference is the lack of change in Lp (a) concentration in males with IFG which is consistent with MetS, rather than diabetes, mediating CHD risk in men, in contrast to Turkish women in whom impaired glucose tolerance[8] and diabetes lead to the development of CHD[14].
In the transition to MetS in men, beyond the development of atherogenic dyslipidemia and apoB, LDL and total cholesterol levels and the global risk profile are only marginally pronounced, regardless of the fasting glucose status. The transition to MetS in women, however, seems to be associated with increase in LDL-chol, apoB, apoE, diastolic BP and little elevation in serum creatinine despite aging by 6 years. This may be attributed to the effect of apoB concentration substantially released from Lp (a) consequent to its aggregation to apoE/apoA-I in women with IFG, in whom a substantial decline of circulating Lp (a) (by 29%) seems to occur during a transition to MetS.
Beyond the augmentation of the three core components of MetS, two fundamental aspects in MetS that differ from non-MetS are compared with participants free of MetS: circulating Lp (a) is assayed to be lower by 7.5% (10.06 mg/dL vs 9.3 mg/dL); as a surrogate of immature pre-β HDL being more predominant in MetS in both sexes, apoA-I/HDL-C ratio was higher (3.48 vs 3.01) in female MetS vs non-MetS, and (3.51 vs 3.15, respectively) in males. Each of these differences may partly account for the component of pro-inflammatory state in MetS.
We have pointed out that Turkish women are more prone to IGT than men due to a greater participation of chronic low-grade inflammation and HDL dysfunction[8]. The current study supports this notion and further provides evidence that autoimmune activation induced by Lp (a) may be the major determinant of IGT (and diabetes).
Current findings indicate further that relatively low levels of HDL cholesterol already occur in people free of MetS associated with a relatively wide waist and high triglycerides and that this trend is more pronounced in normoglycemic men and women. This observation is largely at variance with the proposition by Mahley et al[30] that low HDL cholesterol in Turkish adults has an isolated genetic origin irrespective of serum triglycerides.
The presence of IFG in non-diabetic adults without MetS (8%-9%) indicates an atheroprotective risk factor profile since these people carry the “rudimentary phenotype” of abdominal obesity and atherogenic dyslipidemia (the crux of MetS) less. Males with IFG have, moreover, lower serum Lp (a) than normoglycemic men. Women generally possess substantially higher circulating Lp (a) than men and are subject to immune complex formation with loss of measurable Lp (a) (along with apoB) and concomitant increase in glycemia (yielding IFG) without elevated apoB-containing lipoproteins.
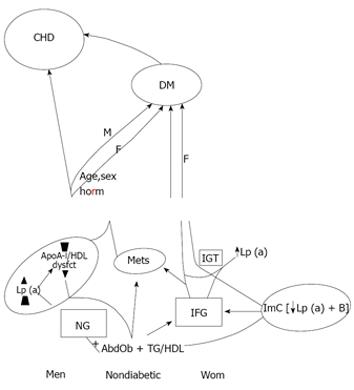
The slow process of established MetS develops as abdominal obesity and atherogenic dyslipidemia become pronounced and apoB-containing lipoproteins rise, during which modest loss of Lp (a) protein alone takes place in normoglycemic men and women, and major loss of both Lp (a) protein and apoB emerges in women with IFG. IFG in women represents commonly IGT as well rendering, as is known, diabetes more readily (Figure 1).
IFG confers a 3-fold risk of diabetes in each sex and the risk profile in IFG associated with MetS reduces the atheroprotective milieu in both sexes, especially in women due to the contributing effect of autoimmune activation. The limited immune complex formation in normoglycemic men requires merely the disappearance of atheroprotective properties of apoA-I (attained by effect of HtgW) to induce diabetes, contrasted to normoglycemic women who tend to hyperinsulinemia and need additional factors (changes in serum SHBG and testosterone) related to aging by several years. HDL dysfunction independent of IFG is an important contributor to the risk of CHD in MetS (as notable by HRs near unity).
Present findings support a concept that MetS is preceded over a long period by persistent minimal autoimmune activation manifesting as partly lower circulating assayable Lp (a) along with apoA-I dysfunctionality. This process represents increased insulin resistance despite normoglycemic levels prevailing.
A clinical implication of our IFG-related finding is to determine in a given individual whether IFG is or is not part of MetS (Table 5). If not, no further action may be needed other than assessing global cardiometabolic risk and undertaking preventive measures against diabetes in men. In subjects, especially women, with IFG associated with MetS (or hypertriglyceridemic waist phenotype), an oral GTT is mandatory to ascertain whether the clinician is faced with IGT which should be regarded as nearly a DM-equivalent accompanied by autoimmune activation and HDL dysfunction. Standard efforts to reduce the pro-inflammatory state need to then be instituted, including insulin-sensitizing agents.
The lack of performance of a standard oral glucose tolerance test in this study evidently limits the important differentiation between the two types of pre-diabetic status, IFG and IGT, the absence of which, however, does not enhance and would rather tend to attenuate, the main finding of IFG representing a cardioprotective element in subjects free of MetS.
IFG status in non-diabetic people without MetS is wrought with excess diabetes risk, mainly due to a commonly underlying IGT, but is otherwise associated with a less adverse cardiovascular risk profile and reduced future CHD risk. “Protection” against CHD risk disappears as currently-defined MetS becomes established. Underlying the slowly progressive pro-inflammatory state is likely to be autoimmune activation linked to serum Lp (a).
Impaired fasting glucose (IFG), a common pre-diabetic state in middle-aged adults, often leads to diabetes but whether and to what extent it confers risk of coronary heart disease is controversial. A modest and non-linear association with risk of vascular disease was found in a meta-analysis.
Gender and presence of metabolic syndrome modulate cardiometabolic risk and might modulate IFG status as well. The authors stratified baseline characteristics distinguishing IFG from normoglycemia and future cardiometabolic risk by stratifying to gender and metabolic syndrome in this study. They showed that, surprisingly, normoglycemic subjects were distinct by a phenotype consisting of a significantly higher waist circumference, fasting triglyceride and lower high-density lipoprotein-cholesterol (“hypertriglyceridemic waist”), regardless of gender and metabolic syndrome (MetS). And notably, lipoprotein(a) tended to be lower in (particularly female) participants with MetS.
This is the first study to report that IFG status in non-diabetic people without metabolic syndrome is independently associated not only with a less adverse cardiovascular risk profile than in normoglycemia, but also with a reduced future coronary heart disease risk. Only in men but not women without metabolic syndrome does IFG independently predict diabetes and the relative “protection” IFG affords against coronary heart disease disappears fully with the development of metabolic syndrome (“hypertriglyceridemic waist”) only in women., The authors hypothesize autoimmune activation linked to serum lipoprotein(a) as responsible for the modulating phenomenon, which they found to be independently and apparently “inversely” related to components of insulin resistance in women.
This knowledge may be utilized in population screening and more precise undertaking of preventive measures against diabetes and coronary heart disease, as well as in assessment of individual cardiometabolic risk. The hypothesis put forward also opens new avenues of research in the area of pathogenesis of new-onset diabetes and coronary heart disease.
The authors examined whether and to what extent IFG confers risk of diabetes or coronary heart disease by separately stratifying to gender and the presence of metabolic syndrome. The study disclosed that both factors modulated future risk. In women without metabolic syndrome, IFG was not associated with any cardiometabolic risk and in men it imparted only risk of diabetes. Coronary heart disease risk appeared to be dependent on the “hypertriglyceridemic waist” phenotype of the metabolic syndrome, especially in the female. The results suggest autoimmune activation involving lipoprotein(a) may be highly relevant in the pathogenesis of new-onset type 2 diabetes, with implications in both risk assessment and prevention of cardiometabolic risk, and open new avenues for research.
P- Reviewers González-Ortiz M, Raghow R, Tarantino G S- Editor Gou SX L- Editor Roemmele A E- Editor Liu XM
| 1. | Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3857] [Cited by in RCA: 3461] [Article Influence: 230.7] [Reference Citation Analysis (0)] |
| 2. | Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Qiao Q, Pyörälä K, Pyörälä M, Nissinen A, Lindström J, Tilvis R, Tuomilehto J. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108:3B-24B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 820] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 6. | Hanefeld M, Temelkova-Kurktschiev T, Schaper F, Henkel E, Siegert G, Koehler C. Impaired fasting glucose is not a risk factor for atherosclerosis. Diabet Med. 1999;16:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore Longitudinal Study on Aging. Diabetes. 2004;53:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Onat A, Can G, Ciçek G, Ayhan E, Doğan Y, Kaya H. Fasting, non-fasting glucose and HDL dysfunction in risk of pre-diabetes, diabetes, and coronary disease in non-diabetic adults. Acta Diabetol. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil. 2011;18:813-823. [PubMed] |
| 10. | Onat A, Can G, Ciçek G, Doğan Y, Yüksel H. Coronary disease risk and fasting glucose levels in a non-diabetic population. Diabetes Res Clin Pract. 2011;91:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1160] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 12. | Onat A, Hergenç G, Ozhan H, Kaya Z, Bulur S, Ayhan E, Can G. Lipoprotein(a) is associated with coronary heart disease independent of metabolic syndrome. Coron Artery Dis. 2008;19:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1333] [Cited by in RCA: 1260] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 14. | Onat A, Hergenç G, Keleş I, Doğan Y, Türkmen S, Sansoy V. Sex difference in development of diabetes and cardiovascular disease on the way from obesity and metabolic syndrome. Metabolism. 2005;54:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Onat A, Hergenç G. Low-grade inflammation, and dysfunction of high-density lipoprotein and its apolipoproteins as a major driver of cardiometabolic risk. Metabolism. 2011;60:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis. 2001;156:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167. [PubMed] |
| 18. | Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3603] [Cited by in RCA: 3668] [Article Influence: 174.7] [Reference Citation Analysis (0)] |
| 19. | Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Can G. Determinants and definition of abdominal obesity as related to risk of diabetes, metabolic syndrome and coronary disease in Turkish men: a prospective cohort study. Atherosclerosis. 2007;191:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Onat A, Can G, Ayhan E, Kaya Z, Hergenç G. Impaired protection against diabetes and coronary heart disease by high-density lipoproteins in Turks. Metabolism. 2009;58:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Onat A, Hergenç G, Bulur S, Uğur M, Küçükdurmaz Z, Can G. The paradox of high apolipoprotein A-I levels independently predicting incident type-2 diabetes among Turks. Int J Cardiol. 2010;142:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods, 2nd ed. Geneva: World Health Organization 1982; 124-127. |
| 23. | Guey LT, Pullinger CR, Ishida BY, O’Connor PM, Zellner C, Francone OL, Laramie JM, Naya-Vigne JM, Siradze KA, Deedwania P. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol. 2011;108:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Abdul-Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care. 2006;29:1613-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068-1074. [PubMed] |
| 26. | Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1235] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 27. | Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev. 2011;33:46-62. [PubMed] |
| 29. | Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 30. | Mahley RW, Palaoğlu KE, Atak Z, Dawson-Pepin J, Langlois AM, Cheung V, Onat H, Fulks P, Mahley LL, Vakar F. Turkish Heart Study: lipids, lipoproteins, and apolipoproteins. J Lipid Res. 1995;36:839-859. [PubMed] |