INTRODUCTION
Diabetic retinopathy (DR) is a leading cause of vision loss in working-age patients around the world. DR is related to 1% of all cases of blindness worldwide, and it may be related to 5% of blindness in some countries[1,2] (Figure 1). The main cause of vision impairment in diabetic patients is diabetic macular edema (DME)[3-5]. DME may occur at any stage of non-proliferative or proliferative DR[6,7]. Macular edema is divided into two types: focal and diffuse. Focal macular edema is caused by focal leakage from microaneurysms and dilated retinal capillaries with abnormal permeability. Complete or partial rings, as a circinate pattern of hard exudates, often demarcate the macular edema[8] (Figure 2A). In diffuse macular edema, generalized leakage from dilated capillaries is observed throughout the posterior pole (Figure 2B). Occlusion of a portion of the capillary bed causes dilation of the patent capillaries, which tend to leak, leading to edema[9]. The risk factors associated with diffuse macular edema are systemic hypertension, adult-onset diabetes mellitus and poor blood glucose control, cardiovascular disease, impaired renal function, increased number of retinal microaneurysms, advanced retinopathy and vitreomacular traction[9,10]. It is estimated that DME occurs in 3% to 6% of all patients with diabetes aged 18 or older[11]. A large epidemiological study indicated that macular edema was present in 26% of the study patients with DR[12].
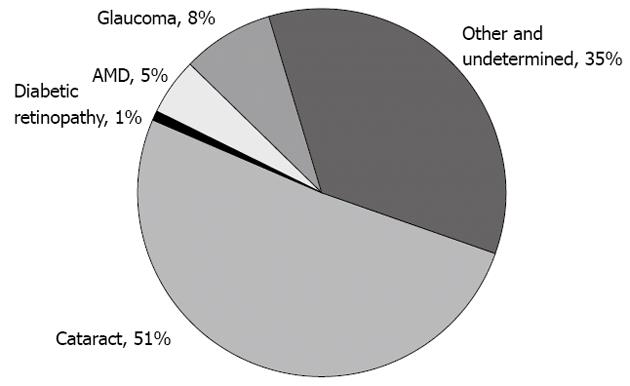
Figure 1 Pie chart displaying the distribution of global causes of blindness.
Although cataracts are responsible for more than half of the cases, they are potentially reversible. When considering the causes of permanent vision impairment, diabetic retinopathy contributes significantly to 1%-5% of cases of blindness. In addition, diabetic retinopathy is the major cause of irreversible blindness in the working-age patients worldwide. AMD: Age-related macular disease.
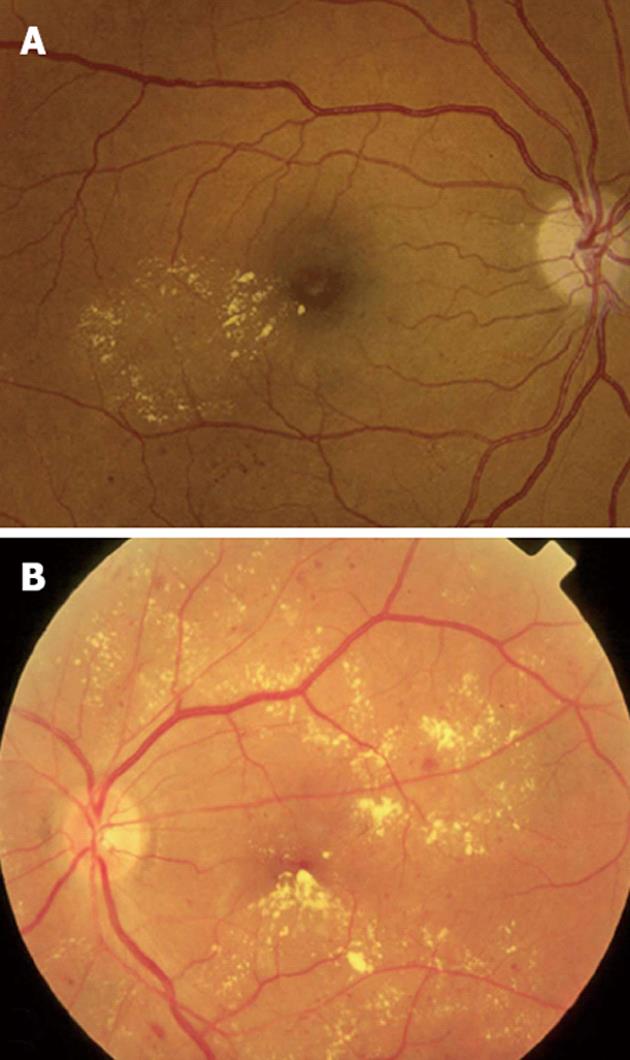
Figure 2 Clinical patterns of diabetic macular edema.
A: Focal macular edema marked by focal leakage from microaneurysms and dilated retinal capillaries with abnormal permeability, making a complete ring as a localized circinate pattern of hard exudates; B: Diffuse macular edema, characterized by hard exudates with generalized leakage from dilated capillaries throughout the posterior pole.
The most efficient tool for preventing vision loss from DR is screening and identification of at-risk patients, along with regular office visits to educate patients on the importance of tight blood sugar and blood pressure control in both type 1 and type 2 diabetes[3].
Once a patient develops DME, the gold standard treatment in recent decades has been macular photocoagulation (MPC) using the laser technique, which reduces the risk of moderate visual loss by approximately 50% (Figure 3)[13]. A review of the data from the Early Treatment DR Study (ETDRS) demonstrated that approximately 40% of the patients who demonstrated improvement with focal laser treatment and a baseline best-corrected visual acuity (BCVA) worse than 20/40 had gained 6 or more letters at 3-year post follow-up[13,14]. Recently, the Diabetic Retinopathy Clinical Research Network (DRCR.net) has demonstrated BCVA improvement of more than 5 letters of vision in 51%, 47% and 62% of eyes treated with MPC after 1, 2 and 3 years of follow-up, respectively[5,15-17].
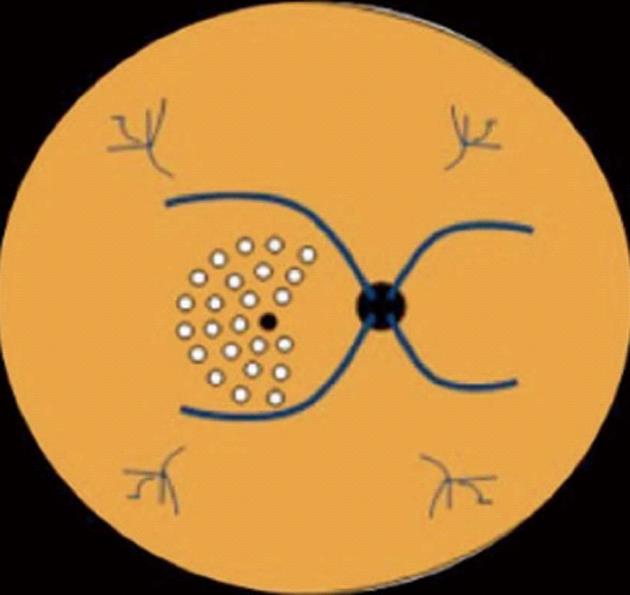
Figure 3 Macular area treated by laser photocoagulation using the scheme proposed by the Early Treatment Diabetic Retinopathy Study.
VASCULAR ENDOTHELIAL GROWTH FACTOR INHIBITORS AND DME
In recent years, alternative or adjunct treatments for DME have been studied, and various pharmacological compounds are under investigation, such as intravitreal triamcinolone acetonide (IVTA) and therapies using inhibitors of vascular endothelial growth factor (VEGF)[4]. Studies performed by DRCR.net demonstrated that despite the early benefits of intravitreal injection of 4 mg of triamcinolone acetonide (TA), the BCVA and retinal thickening at 4 mo compared with a 1-mg TA dose or with focal/grid photocoagulation, the final mean BCVA at 2 and 3 years was better in the MPC group[15,16].
VEGF expression and signaling are deregulated in DR, and VEGF is an important mediator of blood retinal barrier breakdown, which leads to fluid leakage below the macula and the development of macular edema. Therefore, at present, treatment with anti-VEGF agents is one of the most promising approaches for the treatment of vision loss due to DME[18,19]. Several studies have been conducted that have addressed the efficacy and safety of anti-VEGF agents, including ranibizumab (Lucentis, Genentech, Inc., United States), pegaptanib (Macugen, OSI/Eyetech, United States), and aflibercept (EYLEA; Regeneron, United States) and bevacizumab (Avastin, Genentech, Inc., United States), in the treatment of DME.
It has been shown that pegaptanib inhibits VEGF permeability effects[20,21]. The VEGF Inhibition Study in Ocular Neovascularization trial established the safety and efficacy in neovascular age-related macular disease (AMD)[22]. For DME, the efficacy and safety of 0.3 mg of pegaptanib sodium vs sham injections was studied in a phase-2/3, multicenter, randomized, double-blinded trial[23]. After 102 wk, the pegaptanib group presented significantly better results than the sham injection group in BCVA change, letters gained and reduced need for focal/grid laser photocoagulation.
Recently, 2 mg/0.05 mL aflibercept (EYLEA; Regeneron, United States) received regulatory approval from the Food and Drug Administration (FDA) for the treatment of neovascular AMD. For management of DME, a multicenter, randomized, double-masked, phase-2 clinical trial, the DA VINCI Study, tested different dosing regimens of aflibercept (VEGF Trap-Eye) and compared them with laser photocoagulation: 0.5 mg every 4 wk, 2 mg every 4 wk, 2 mg for the 3 initial doses then every 8 wk, 2 mg for the 3 initial doses then as needed. Subjects in the VEGF Trap-Eye groups experienced mean reductions in central retina thickness and, at their 6-mo follow-up, had better results for BCVA than those who were treated with laser photocoagulation. However, it is important to note that a considerable number of re-injections were necessary.
The drug was well tolerated. The phase-3 trials on aflibercept in patients with visual loss due to DME are ongoing[7,24].
Ranibizumab is approved for the treatment of neovascular AMD and just received FDA approval (August 2012) for the treatment of visual impairment due to DME, based on the RIDE and RISE clinical trials. Several clinical trials have been performed examining the use of ranibizumab for the treatment of visual impairment due to DME. The RESTORE study demonstrated superiority after 12 mo of ranibizumab monotherapy (0.5 mg) administered as needed or as an adjunct to laser photocoagulation vs laser monotherapy[25,26]. The READ-2 study found that ranibizumab (0.5 mg) alone or in combination with laser photocoagulation improved BCVA over 2 years in DME patients[27]. RIDE and RISE, two identicallydesigned, parallel, double-blinded, 3-year clinical trials that were sham-treatment controlled for 24 mo had preliminary results that demonstrated that patients who received 0.3 mg of ranibizumab experienced significant, early and sustained improvements in vision. The DRCR.net conducted a study to investigate the role of ranibizumab and also steroid treatment combined with laser photocoagulation. The 2-year results of this study indicated that 0.5 mg of ranibizumab administered as needed and combined with laser therapy produced a rapid and sustained improvement in the BCVA of patients with DME compared with laser treatment[28].
RESULTS AND DISCUSSION
The DRCR.net conducted a randomized study of 121 eyes over a 12-wk period[33]. It consisted of five treatment arms: (1) focal photocoagulation; (2) two intravitreal injections of 1.25 mg of bevacizumab at 0 and 6 wk; (3) two intravitreal injections of 2.5 mg of bevacizumab at 0 and 6 wk; (4) 1.25 mg of bevacizumab at week 0 followed by a sham injection at 6 wk; and (5) 1.25 mg of bevacizumab at 0 and 6 wk combined with focal photocoagulation at 3 wk. The majority of eyes, 69%, were refractory to previous treatment for DME. The eyes of two groups that received two bevacizumab injections without laser, 2 and 3, had a significant BCVA improvement over the laser-only group 1, and this difference persisted through the 12 wk. These two groups also had a greater improvement in central subfield thickness at the 3-wk visit. No differences were observed between groups 2 and 3 (1.25-mg and 2.5-mg doses, respectively). The single injection group had no advantage over the photocoagulation group in this study. Group 5, which combined bevacizumab with photocoagulation, had results comparable with laser-only treatment. This study suggested that bevacizumab was an effective drug for the management of DME as a primary treatment and also for refractory eyes. Safety data were reported for 24 wk, and no safety concerns were detected. Two trends were identified: (1) the eyes that received primary treatment had greater improvement (P = 0.04) than the refractories; and (2) the presence of subretinal fluid at the initial therapy [measured by optical coherence tomography (OCT)] may be associated with a greater improvement in BCVA (P = 0.06).
The DRCR.net study identified no difference between 1.25 mg and 2.5 mg of bevacizumab, and similar outcomes have been previously reported by other colleagues in retrospectively designed studies[35,36]. One of these studies involved three initial injections monthly and a follow-up period of 6 mo[33]; another study followed the same design but with a 12-mo follow-up[34]. Both studies demonstrated significant reductions in central foveal thickness (CFT) by OCT evaluation and also significant improvements in BCVA[33,34]. There were statistically similar outcomes for the two study groups throughout the 6 initial months and a trend toward recurrence of edema at the 1-year follow-up, suggesting a trend of reducing the CFT during the 2-3 mo following the intravitreal bevacizumab injection (IVBI)[33].
Another study focused on IVB for DME investigated a remarkably diverse group of eyes, with no exclusions based on previous treatment, ischemia, or poor initial BCVA[37]. The study consisted of a noncomparative trial of 1.25 mg of bevacizumab at baseline, with subsequent re-treatment based on improvement in OCT or BCVA response to the initial injection. At 6 mo, there was no significant improvement in mean BCVA, but there were significant decreases in the mean CFT according to OCT evaluation. Although some characteristics of this study led to difficulty in analyzing its results, such as the diverse baseline data and a variable number of treatments, the results corroborated the idea that bevacizumab should be the object of further studies for eyes with DME refractory to previous treatments, as this therapeutic approach was able to decrease the CFT as measured by OCT.
When investigating the long-term effects of intravitreal bevacizumab in patients with chronic diffuse DME, Kook et al[38] observed a decrease in central macular thickness (CMT) and again in BCVA following repeated intravitreal injections of bevacizumab, even in cases with chronicdiffuse ischemic DME.
Bonini-Filho et al[39] performed a pilot study of IVBtreatment for macular edema in ten eyes with severecapillary loss. The treatment used 1.5-mg dosing, and all ten eyes underwent an injection at baseline. Re-treatment at follow-up visits was based on the presence of intraretinal or subretinal fluid on OCT. After 54 wk, the CMT and BCVA improved significantly. No progression of capillary loss was observed in fluorescein angiogram at the end of the study.
The BOLT Study, a prospective, randomized, blinded, single-center study, compared IVB and macular laser photocoagulation in patients with persistent CSME after at least one macular laser treatment[40]. Eighty eyes were randomized into a bevacizumab treatment group (with injections every 6 wk), with a minimum of 3 and a maximum of 9 injections, or a photocoagulation group, with sessions every 4 mo and a minimum of 1 and a maximum of 4 treatments. After 1 year, the mean BCVA measured by ETDRS evaluation increased in the bevacizumab group and deteriorated in the laser group. The CMT results were also favorable for the bevacizumab group. The median number of injections in this first year was 9 in the bevacizumab group, and the median number of laser treatments was 3.
The 2-year outcome report of the BOLT Study was published recently and presented similar results to the first year report[41]. The mean ETDRS equivalent Snellen was 20/50 in the bevacizumab group and 20/80 in the laser group (P = 0.005). The bevacizumab group gained a median of 9 ETDRS letters vs 2.5 letters for the laser treatment group (P = 0.005), with a mean gain of 8.6 letters for bevacizumab vs a mean gain of 0.5 letters for the laser group. Among the eyes treated with bevacizumab, 32% gained at least 15 letters vs 4% for the laser-treated eyes (P = 0.004). The percentage of patient eyes that lost fewer than 15 letters in the macular laser treatment group was 86% vs 100% for the bevacizumab group (P = 0.03). At 2 years, the CMT decreased significantly in both groups. At the 2-year follow-up, the median number of injections was 13, and the median number of laser treatments was 4.
In addition to MPC,some of the largest trials published examining bevacizumab use for DME have compared intravitreal bevacizumab and intravitreal triamcinolone (IVT).
Ahmadieh et al[30] conducted a 24-wk trial randomizing 115 eyes to one of three study arms: a bevacizumab-only arm, an IVTA/bevacizumab combination arm, and a placebo arm. The two treatment arms received three 1.25-mg bevacizumab injections every 6 wk, and the IVTA/bevacizumab group received an additional injection of 2 mg of triamcinolone at the baseline visit only. No difference in BCVA or CMT was detected between the bevacizumab and IVTA/bevacizumab groups.
In a study performed by Faghihi et al[42], IVB-only was compared with bevacizumab associated to triancinolone and with MPC in eyes with no history of treatment. Dosings of 1.25 mg of bevacizumab and 2 mg of triamcinolone were used, and injections were performed at the baseline visit only. The three groups had significant improvements in CMT at both the 6- and 16-wk visits vs baseline. A similar trend was observed for BCVA; the bevacizumab group outperformed the laser group in CMT and BCVA at 6 wk but not at 16 wk. The bevacizumab/IVTA group outperformed the laser group in CMT andBCVA at both 6 and 16 wk.
A randomized clinical trial comparing IVB injection alone or in combination with IVTA vs macular laser photocoagulation as a primary treatment for DME was conducted by Soheilian et al[5,43], and the 2-year outcomes results were recently published. In total, 150 eyes were randomly assigned to 1 of the 3 study arms: the 1.25-mg IVB group; the IVB/IVT group, with 1.25 mg of IVB and 2 mg of IVT; and the macular laser group. There was significant superiority of visual acuity improvement in the IVB group after 6 mo, but this was not sustained after 24 mo. The mean BCVA improvement was greater in the IVB group than in the other groups and also in the IVB/IVT group compared with the laser group. The same was noted for the reduction of CMT, which was more evident in the IVB group compared to the other groups. However, the difference among the groups was not significant, which may be related to some methodological aspects, such as the 3-mo re-treatment intervals, when indicated, or the missing data in 24.6% of the cases at the final follow-up.
In a retrospective study, Wu et al[44] aimed to identify OCT patterns in diabetic DME that were predictive of visual outcomes after IVBIs. Thirty-one eyes with clinically significant DME[13] and without previous treatment underwent complete ophthalmic examination and OCT. The eyes were classified into 4 groups, based on the cross-sectional retinal morphologies, by using OCT features: diffuse retinal thickening, cystoid macular edema (CME), serous retinal detachment and vitreomacular interface abnormalities. The minimum required follow-up was 3 mo. Changes in CMT and total macular volume after IVB injections were evaluated as well as the BCVA. Patients with CME exhibited greater improvement in all evaluated parameters compared with other groups. The study concluded that OCT patterns in DME may be helpful in deciding the best treatment and predicting the outcome after IVBI. In addition, the study indicates that IVBI could be a primary therapeutic modality for CME[44]. Similar results were found in a retrospective study conducted by Roh et al[32].
The Pan-American Collaborative Retina Study Group has published the 24-mo results of a study examining intravitreal bevacizumab as the primary treatment for diffuse DME (DDME). For these retrospective, multicenter, interventional, comparative case series, the clinical data of 139 eyes with DDME at 11 centers from 8 countries were reviewed. All of the eyes were treated with off-label IVB with at least 1 intravitreal injection of 1.25 or 2.5 mg of bevacizumab. The dose received at baseline was the same dose delivered throughout the study. The exclusion criteria were as follows: patients with DDME that were treated with laser photocoagulation or intravitreal triamcinolone previously, macular ischemia, intraocular inflammation, a prior history of vitreoretinal surgery or cataract surgery within the past 6 mo, uncontrolled intraocular pressure, and the presence of an epiretinal membrane or vitreomacular traction syndrome. Each patient underwent BCVA measurement with ETDRS charts, ophthalmic examination and OCT at baseline and 1, 3, 6, 12 and 24 mo after the initial injection. Fluorescein angiography was performed at the discretion of the examiner (usually every 6 mo). Patients received re-injections whenever there was a recurrence of DDME.
One month after the initial bevacizumab injection, improvements in the BCVA and CMT measurements were observed, and these significant changes continued during the 24-mo follow-up period. The improvement of the BCVA and OCT from one study after 6 injections during the 2-year period is shown (Figure 5). BCVA analysis demonstrated that after 24 mo, 72 (51.8%) eyes improved 2 or more ETDRS lines, 62 (44.6%) eyes remained stable, and 5 (3.6%) eyes decreased 2 or more ETDRS lines of BCVA. A twenty-four-month OCT analysis indicated that CMT measurements decreased from 446.4 ± 154.4 μm to 279.7 ± 80 μm. The mean number of IVB injections per eye was 5.8 (range, 1-15 injections) at a mean interval of 12.2 ± 10.4 wk. The data analysis of BCVA and CMT found no significant differences between the 1.25- and 2.5-mg dose groups[45].
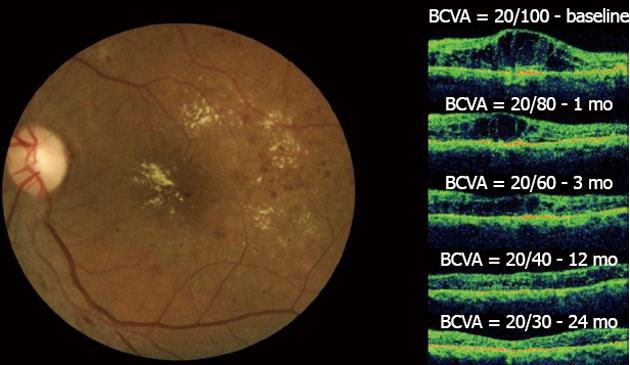
Figure 5 Diffuse diabetic macular edema treated with bevacizumab.
In the left figure, the clinical fundus photograph shows the macular edema and hard exudates at the foveal center.In the right figure, a series of optical coherence tomographys (OCTs) taken at a 24-mo follow-up can be observed. The OCT image at baseline shows the intraretinal fluid with increased central macular thickness (CMT) and best-corrected visual acuity (BCVA) = 20/100. One month after the first injection, improvement in both BCVA and CMT was observed. This result was maintained throughout the 24-mo follow-up period after six injections and with final central macular thickness within normal limits without intraretinal fluid and the improvement of BCVA to 20/30. No laser photocoagulation was performed in this case.
A systematic review of IVBI for the treatment of primary DME was conducted by Yilmaz et al[34] and published in 2011. The review compared IVB injection vs MPC vs a combination IVB/IVTA injection in improving the BCVA of patients without previous treatment for DME[34]. The review included four randomized clinical trials comparing IVB injection with macular laser and three of them also comparing IVB injection with IVB/IVTA. The outcomes indicated that IVB injection is effective in improving BCVA in primary DME for 6 wk, but the benefits are no longer present at 12 wk after injection. IVTA had no detectable adjunctive effect.
Throughout the discussion of this systematic review, various limitations may be responsible for these observed outcomes, which somewhat contradict the trends shown in previous studies. First, this review was limited to four randomized controlled trials, and all of them had varied baseline characteristics. The DRCR.net study provided BCVA and CMT values that were not estimable in our analysis because there was a mixture of patients with and without prior treatment for DME. However, that study was included in the systematic review to emphasize that patients from IVB groups did improve in their BCVA and CMT values compared to the laser group. Another relevant aspect is that a decrease in efficiency may be related to the cessation of treatment in those studies in which just one injection was performed. The DRCR.net demonstrated that the improvement results were sustained for 12 wk with two IVB injections.
Therefore, the limitations of this analysis may corroborate the idea that IVB is effective in treating primary DME; however, IVB should not be considered the first line of treatment.
The safety of the intravitreal use of bevacizumab has also been studied. A retrospective study involving 1173 patients who received intravitreal bevacizumab and were followed for 12 mo is likely the largest series regarding the use of bevacizumab in DME. In this study, the following adverse effects were observed: seven cases of acute elevation of blood pressure, six strokes, five myocardial infarctions, five deaths, seven cases of bacterial endophthalmitis, seven cases of tractional retinal detachment (TRD), and four cases of uveitis[46]. These numbers were similar to those found in the prospective, controlled studies of the other anti-VEGF agents[3].
TRD in proliferative diabetic retinopathy following intravitreal bevacizumab may happen because of natural history or rapid neovascular involution with accelerated fibrosis and posterior hyaloidal contraction as a response to decreased levels of VEGF. Arevalo et al[47], in a retrospective review, identified a 5.2% incidence of development or progression of TRD after treatment with intravitreal bevacizumab. Therefore, treatment with bevacizumab for patients with proliferative DR and DME must be cautiously applied, especially in cases with elevated glycosylated hemoglobin, patients with type 1 diabetes with poor glycemic control, patients without previous PRP or refractory to this treatment and the presence of areas of isolated TRD.
Although delivered intravitreously, anti-VEGF drugs can potentially circulate systemically[19]. Systemic side-effects such as arterial thromboembolism, gastrointestinal perforation, hemorrhage, hypertensive crisis, and nephrotic syndrome are the main safety concerns surrounding the use of intravenous bevacizumab in patients with a diagnosis of colorectal cancer and other important systemic comorbidities.