INTRODUCTION
Hyperglycemia and insulin resistance has been described in the critical care literature[1-5]. Evidence has suggested tight blood sugar control in the critically ill patients, specifically surgical intensive care unit (ICU) patients, may improve outcomes in these patients[6]. Hyperglycemia has been associated with increase in cytokines and counter-regulatory hormones which, in turn, lead to insulin resistance[2]. An increase in insulin resistance has also been described in patients with sepsis, renal failure, and a variety of critical illnesses[4,7,8].
Chromium is a trace element which plays an important role in carbohydrate, protein and lipid metabolism[9,10]. There have been several studies which have shown improvement in blood sugar control with the addition of extrinsic chromium[11-18]. In addition, chromium has shown to improve the insulin sensitivity. This agent’s resulting decrease in insulin resistance, increases low density lipoprotein cholesterol, decreases high density lipoprotein cholesterol, increases body fat mass, and decrease in lean body mass. We present a case of a patient with septic shock and severe insulin resistance, in whom intravenous chromium was utilized with good clinical results.
CASE REPORT
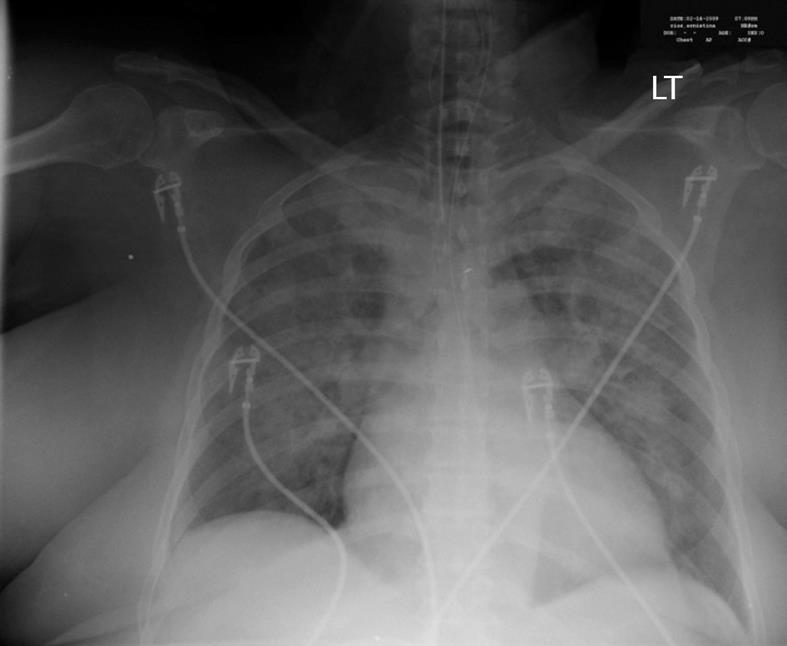
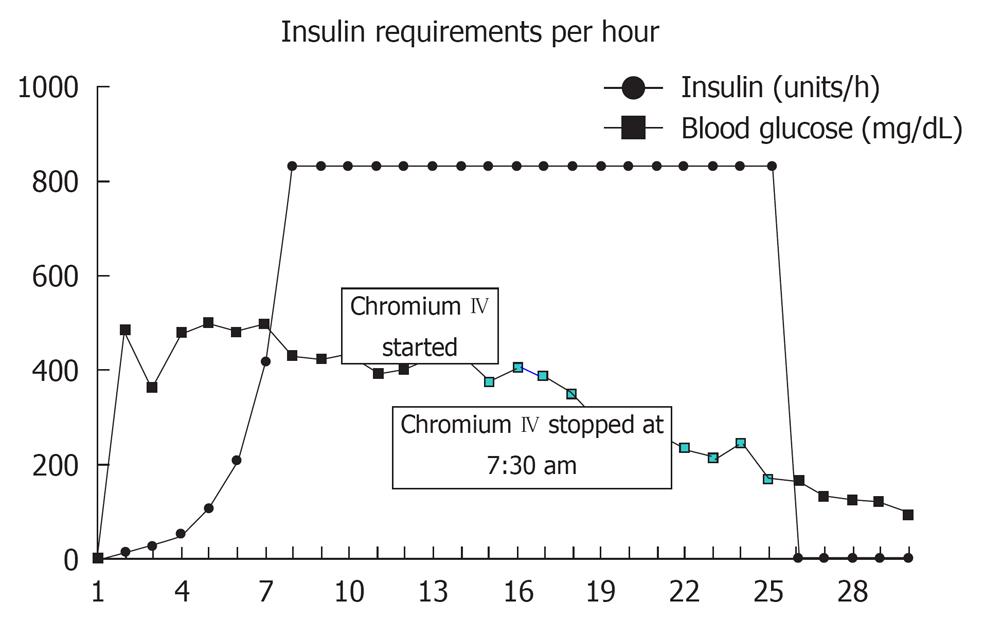
A 62-year-old Caucasian woman with a past medical history of diabetes mellitus, coronary artery disease and hypercholesterolemia was brought to the emergency department after suffering a witnessed cardiac arrest. Initial bystander cardiopulmonary resuscitation was initiated. The patient was found to be in ventricular fibrillation and underwent electroshock into normal sinus rhythm and was then intubated. She was awake and able to follow simple commands in the ICU while on assisted mechanical ventilation. Chest X-ray revealed bilateral pulmonary infiltrates and increased vascular markings suggesting a combination of pulmonary edema and possible aspiration pneumonia (Figure 1). The patient was observed over the the next several hours where, despite adequate volume resuscitation, the patient’s blood pressure would decline, requiring norepinephrine to keep the systolic blood pressure above 90 mmHg. A pulmonary artery catheter was placed revealing a cardiac output of 8.2 L/min, a calculated systemic vascular resistance (SVR) of 424 dynes/cm2, and a pulmonary capillary wedge pressure of 16 torr. The findings of high cardiac output and low SVR were consistent with septic shock. Patient white blood cell count was recorded as 21.4 × 103 cells/mm3. Approximately 24 h later, the patient’s blood sugar began to increase above 1500 mg/L, which required initiation of intravenous insulin therapy. After 4 h of insulin administration, the patient was found to still require 832 units of insulin per hour with blood sugar levels above 4000 mg/L. After 7 h on an insulin drip administered at 832 units/h, the patient’s blood sugar was still above 4000 mg/L. The patient was then started on chromium chloride at 3 μg/h intravenous infusion for 5 h. By the fifth hour of chromium infusion, the patient’s blood sugar was less than 2000 mg/L, and by the sixth hour of the patient was off insulin, the blood sugar having been determined to be within normal values (Figure 2).
Figure 1 Chest X-ray showing bilateral lung infiltrates.
Figure 2 Insulin requirements and corresponding blood sugar levels.
DISCUSSION
Resistance to insulin has been well documented in the literature[1-5]. The gold standard for measuring insulin resistance is by the insulin clamp test[1]. Saberi and coworkers studied the prevalence, incidence and clinical resolution of insulin resistance in critically-ill patients[5]. They found that on admission to an ICU, 67% of patients demonstrated overt insulin resistance, 9.4% of patients had non-overt insulin resistance, and 24% of patients were insulin sensitive. During the course of ICU stay, an additional 16% developed overt insulin resistance, leaving only 10% of critically-ill patients insulin sensitive. These observations in of themselves highlight the importance of insulin resistance in critically-ill patients. The ICU stay is complicated by renal insufficiency in almost 3%-16% of ICU patients, depending on the population studied[19]. Basi and co-investigators found that glucose concentrations among survivors were significantly lower than among non-survivors throughout the 5-wk study period (P = 0.013)[7].
The mechanism by which chromium is believed to affect changes in glucose uptake has been posited by various sources. Chromium (III), the trivalent form of the element, is the elemental form in question, as chromium demonstrates six (I-VI) naturally occurring valences, the number of which determines subsequent molecular forms found in nature. Chromium (III) circulates in the blood bound to transferrin, a transport metal binding protein usually associated with iron. Four steps characterize the autoamplification that characterizes chromium (III) effects on insulin bound insulin receptors. Insulin, binding to insulin sensitive cells, stimulates autophosphorylation of intracellular β-subunit tyrosine residues, an autocatalytic pathway constituting the first step in the classical cell signaling pathway of insulin. Increased insulin plasma concentrations are postulated to simultaneously activate a transient permeability of the cell membrane to chromium (III) which dissociates from transferrin to enter the cell. The flux of chromium (III) into the cell is captured by pre-synthesized apocalmodulin proteins present intracellularly. Calmodulin, also referred to in the literature as low molecular weight chromium binding protein, strongly binds the free form of chromium (III), in a ratio of 4:1. The calmodulin-chromium complex binds to the β-subunit intracellular portion of the insulin receptor and further enhances autophosphorylation. The end-result of the traditionally understood insulin-mediated uptake of glucose in insulin sensitive cells follows. Following increase in intracellular glucose concentration, insulin concentration drops. The affinity for the calmodulin-chromium complex for the insulin receptor is dependent directly to the concentration of insulin, and calmodulin dissociates from the insulin receptor. It is then released from the cell; however, this loss is of the calmodulin-chromium complex, rather than of chromium or calmodulin individually. This may be understood by the magnitude of the association constant Kf of approximately 1021 M-1[20].
Chromium is an essential component of the human diet. The Food and Drug Administration proposes a reference dietary intake of 120 μg/d, whereas the National Academy of Science has proposed a daily intake of 20 μg/d for females and 30 μg/d for males. This, in contrast to the calculated reference dose of 70 mg/d, represents an almost 350 fold difference between the recommended and the safety dose[21,22]. There have been several studies suggesting a beneficial effect of chromium[11-18]. For example, supplementation with chromium picolinate, a stable and highly bio-available form of chromium, has been shown to reduce the risk of cardiovascular disease and type 2 diabetes[23]. Existing data suggests using chromium picolinate supplementation of at least 200 μg/d. Other studies have suggested higher doses. Anderson et al[14], suggests that a dose of chromium of 1000 μg/d has a more pronounced effect during a 4 mo trial. Administering a 1000 μg/d of chromium also lead to a significant decrease in the HbA1c (glycosylated hemoglobin) after the second month of treatment. A follow-up study demonstrated that the effect of chromium supplementation was long lasting[24]. The long lasting beneficial effects were also seen with a 500 μg/d dose of chromium picolinate.
In addition to oral supplementation, chromium supplementation has been a part of multi-trace element formulation in total parental nutrition (TPN), which provides on average only 10 μg/d. Some authors have suggested that the high glucose content in TPN also increases the renal excretion of chromium[25]. Wongseelashote et al[26] did an observational study on patients with parental chromium supplementation. They found a mixed response to parental chromium supplementation.
A case of extreme insulin resistance in a cardiothoracic ICU patient treated with chromium infusion has previously been described[27]. That particular patient developed severe insulin resistance following surgical repair of the thoracic aorta. Post-operatively, the patient required 2110 units of insulin over 40 h while receiving vasopressors and glucocorticoids. After administration of intravenous chromium at a rate of 3 μg/h (100 μg elemental chromium chloride in 1 L of normal saline infused at 30 mL/h) the patient had a significant response. The insulin infusion rate was decreased and 12 h later insulin infusion was stopped.
In the case of our patient, an even more significant response to chromium intravenous infusion was noted. Our patient demonstrated an extreme insulin resistance which responded well to intravenous chromium. This case also served as a learning model and led us to reassess our protocols for insulin as well as several other protocols, and emphasizes the need of placing a ceiling on amount of insulin which a patient can receive. Chromium has been used orally to improve insulin sensitivity. Its role in parental infusion has not been evaluated. Our case represents the second successful case of improving insulin sensitivity in a patient with extreme insulin resistance. In view of an excellent response to blood sugar control with the addition of chromium, we suggest that future studies be conducted to study the effect of chromium on insulin resistance in critically-ill patients.