Published online Jun 15, 2012. doi: 10.4239/wjd.v3.i6.118
Revised: May 11, 2012
Accepted: June 10, 2012
Published online: June 15, 2012
AIM: To assess the effect of sitagliptin therapy on seasonal fluctuation of glycemic control in Japanese type 2 diabetic patients.
METHODS: Participating patients (age: 29-80 years) had been treated with conventional oral antidiabetic agents and/or diet and exercise therapy for over 6 mo. From December 2009, 35 patients were additionally prescribed oral sitagliptin starting from 50 mg once daily, while 19 patients taking α-glucosidase inhibitors were switched to sitagliptin. Twenty-four patients who refused sitagliptin formed the control group. Changes of mean monthly hemoglobin A1c (HbA1c) during the “winter holiday season” were compared between groups using Student’s t-test (2008-2009 vs 2009-2010). Statistical significance was accepted at P < 0.05. Multivariate analysis was performed to assess whether sitagliptin use was associated with deterioration or improvement of glycemic control.
RESULTS: Both add-on sitagliptin and switching from α-glucosidase inhibitors to sitagliptin prevented the seasonal deterioration of glycemic control and tended to improve HbA1c. Multivariate analysis revealed that both adding and switching to sitagliptin were negatively correlated with deterioration of glycemic control. In 44 patients who continued sitagliptin therapy for another year, elevation of HbA1c was suppressed without adverse effects.
CONCLUSION: Sitagliptin is a suitable oral agent for preventing deterioration of glycemic control during the winter holiday season.
- Citation: Matsuhashi T, Sano M, Fukuda K, Kohsaka S, Suzuki Y. Sitagliptin counteracts seasonal fluctuation of glycemic control. World J Diabetes 2012; 3(6): 118-122
- URL: https://www.wjgnet.com/1948-9358/full/v3/i6/118.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i6.118
In Japan, glycemic control typically deteriorates during the New Year winter holiday season[1-3], since diabetic patients (like other Japanese) celebrate with a high calorie diet and alcohol. In 2009, the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin was approved as the first incretin enhancer for use in Japan[4-11]. Although it has been suggested that seasonal fluctuations of hemoglobin A1c (HbA1c) are noted in patients with type 2 diabetes, no reports have been published concerning the efficacy of antidiabetic agents for such fluctuations. Because the hypoglycemic effect of sitagliptin (a DPP-4 inhibitor) becomes stronger with an increase of the blood glucose level, it has the potential to inhibit seasonal HbA1c fluctuations[12-14]. To evaluate the effect of sitagliptin on seasonal fluctuation of glycemic control, we studied patients with relatively good blood glycemic control over 2 years while on treatment with conventional oral antidiabetic agents and/or diet and exercise.
Patients with type 2 diabetes aged 29-80 years were enrolled. Type 2 diabetes was diagnosed from clinical criteria according to the Japan Diabetes Society guidelines. They were all patients periodically attending our hospital. They were prescribed adequate diet/exercise therapy by specialists and nutritionists and received other appropriate treatment depending on their condition. There were no differences of baseline treatment between the sitagliptin and control groups. Exclusion criteria were type 1 diabetes, treatment with insulin or steroids, and poor glycemic control (HbA1c≥ 10%). Each patient provided informed consent for monthly blood tests and the study was approved by the ethics committee of our institution. Patients receiving DPP-4 inhibitors or glucagon-like peptide-1 receptor agonists were also excluded.
Sitagliptin was released in December 2009 as the first DPP-4 inhibitor to be approved in Japan. Because this clinical study was started simultaneously with its release, patients who had already received DPP-4 inhibitor therapy were not enrolled. There is a rule in Japan that patients receiving a new drug should be examined every 2 wk for 1 year after release of the drug, so subjects were assigned to the sitagliptin and control groups solely based on whether they could attend hospital at fortnightly intervals or not. Because basal treatment was identical and there were no differences of other baseline characteristics between the two groups, the subjects were considered to be comparable. Laboratory data from 2008-2009 before the start of this study were used for baseline values. From December 2009, 35 patients were additionally prescribed oral sitagliptin starting from 50 mg once daily (add-on group), while 19 patients taking α-glucosidase inhibitors were switched to sitagliptin (switching group). Twenty-four patients who refused sitagliptin formed the control group. Throughout the 2 year observation period, the doses of oral diabetic agents other than sitagliptin were not changed. To test baseline characteristics, analysis of variance was employed for age, disease duration and body mass index, while the χ2 test was performed for sex and use of sulfonylureas. Changes of mean monthly HbA1c during the “winter holiday season” were compared between groups using Student’s t-test (2008-2009 vs 2009-2010) and statistical significance was accepted at P < 0.05. Multivariate analysis was performed to assess whether sitagliptin use was associated with deterioration or improvement of glycemic control.
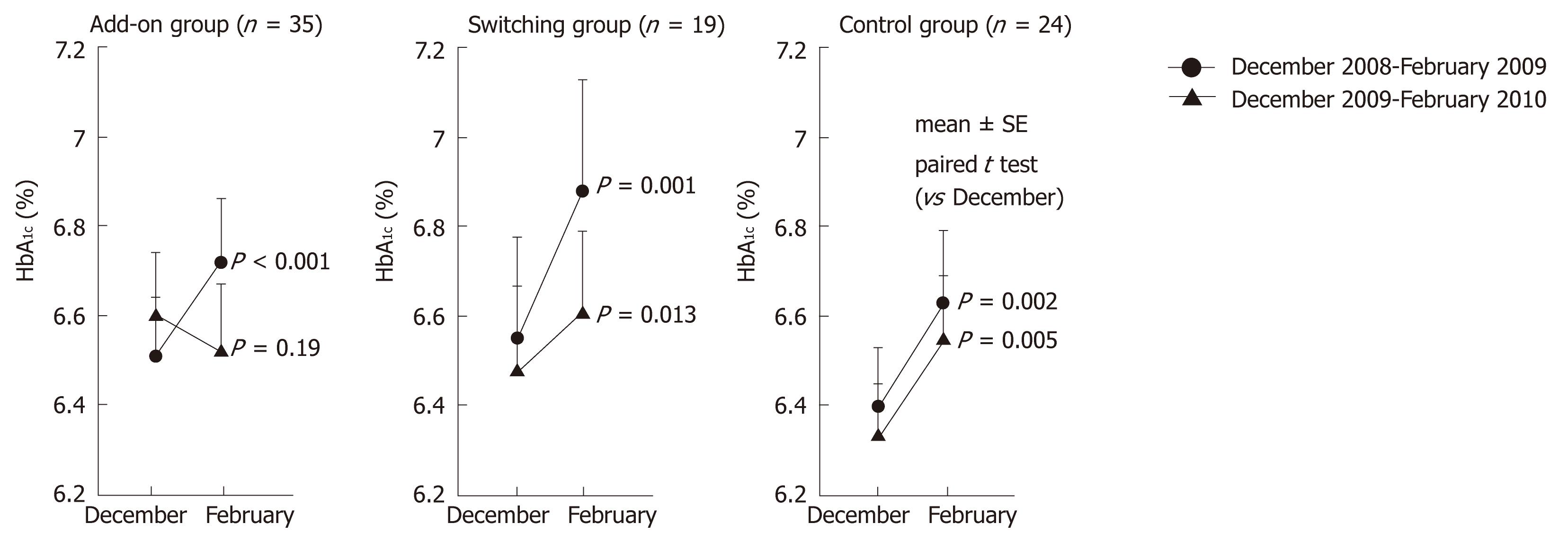
There were no significant differences of baseline characteristics among the three groups (Table 1). When this study was started, the 54 subjects had already been treated for at least 1 year at our hospital and had a good relationship with their physicians. There were no differences of patient education between the sitagliptin group attending hospital every 2 wk and the control group attending every 4 wk because compliance with diet/exercise therapy was adequate in both groups. Since the subjects were assigned to the treated and control groups solely based on their ability to attend the hospital, there was no bias of baseline characteristics between the two groups, making it appropriate to compare the two groups in this study. From December 2008 to February 2009, the mean change of HbA1c was + 0.19% (6.51% ± 0.13% vs 6.72% ± 0.14%, P < 0.001) in the add-on group and + 0.23% (6.40% ± 0.13% vs 6.63% ± 0.16%, P = 0.002) in the control group (Figure 1). Thus, both groups showed an increase while on conventional antidiabetic therapy. From December 2009 to February 2010, the mean change of HbA1c was -0.08% (6.60% ± 0.14% vs 6.52% ± 0.15%, P = 0.19) in the add-on group and 0.22% (6.33% ± 0.12% vs 6.55% ± 0.14%, P = 0.005) in the control group. Seasonal deterioration of HbA1c was prevented in the add-on group (0.19% vs -0.08%). In the switching group, the mean change of HbA1c from December 2008 to February 2009 was 0.33% (6.55% ± 0.23% to 6.88% ± 0.25%, P < 0.001), while the mean change from December 2009 to February 2010 was 0.13% (6.48% ± 0.19% to 6.61% ± 0.18%, P = 0.013). Thus, deterioration of HbA1c was less marked (0.33% vs 0.13%). There were no changes of body weight in any group.
| Add-on group (n = 35) | Switching group (n = 19) | Control group (n = 24) | P value | |
| Age (yr) | 64.66 ± 10.63 | 55.84 ± 12.96 | 63.04 ± 8.85 | 0.171 |
| Gender | ||||
| Male | 28 | 15 | 18 | 0.897 |
| Female | 7 | 4 | 6 | |
| Disease duration (yr) | 11.98 ± 9.66 | 10.00 ± 11.22 | 8.31 ± 8.25 | 0.797 |
| Body mass index (kg/m2) | 24.28 ± 3.49 | 23.94 ± 3.69 | 25.20 ± 3.27 | 0.536 |
| Using sulfonylureas | 16 | 12 | 13 | 0.463 |
Multivariate analysis showed that both adding sitagliptin and switching to sitagliptin were negatively correlated with deterioration of glycemic control (defined as an increase of HbA1c by > 0.1%) after adjustment for age, gender, duration of antidiabetic therapy and body mass index [odds ratio (OR): 0.07, 95% confidence interval (CI): 0.02-0.31, and P = 0.007 for adding sitagliptin; OR: 0.20, 95% CI: 0.04-0.94, and P = 0.041 for switching to sitagliptin]. Sitagliptin treatment was also significantly correlated with a decrease of HbA1c by > 0.1% (OR: 9.85, 95% CI: 2.75-35.1, and P < 0.001 for adding; OR: 4.71, 95% CI: 1.11-19.8, and P = 0.034 for switching).
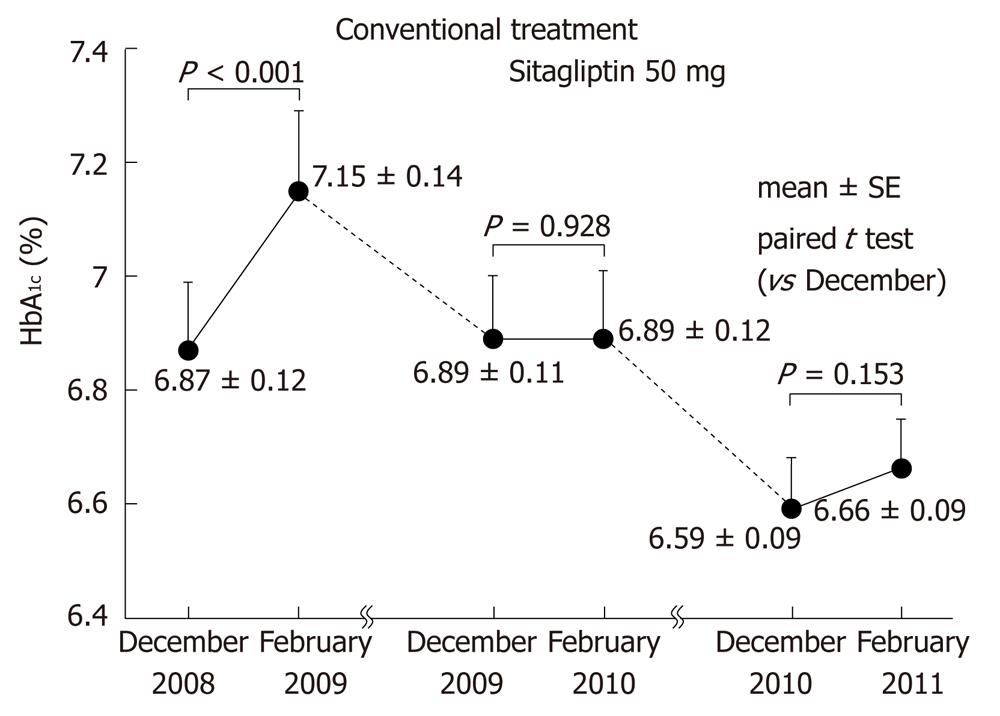
We also followed 44 patients who continued to receive sitagliptin for another year without any changes in dosages of concomitant drugs for another year (Figure 2). As occurred during the first year of sitagliptin treatment, elevation of HbA1c in February was suppressed in the second year. No adverse events or changes of weight were observed.
In Japanese patients, the effect of overeating around New Year is usually reflected by elevation of monthly HbA1c values between December and February. Although we focused on type 2 diabetic patients with good glycemic control for 2 years, HbA1c levels still increased significantly during the winter holiday season, suggesting that conventional oral antidiabetic therapy cannot prevent seasonal deterioration of glycemic control. However, the present study showed that add-on therapy with sitagliptin prevented seasonal deterioration of glycemic control and tended to improve HbA1c despite the increased calorie intake and decrease of physical activity during the New Year holiday period.
In 44 patients who continued sitagliptin therapy for an additional year, elevation of HbA1c was also suppressed in the second year, demonstrating the characteristics of incretin therapy, which exerts a stronger hypoglycemic effect when blood glucose levels are high. Our results suggest that sitagliptin, which has been reported to suppress diurnal variation of blood glucose levels, may also suppress seasonal variation and is a suitable oral agent for preventing deterioration of glycemic control during the winter holiday season in Japanese patients with type 2 diabetes.
Although this was a relatively small study, the results are considered to be reliable because: (1) all of the patients who visited our hospital during a one month period (December) were enrolled, except for those who met the exclusion criteria; and (2) patients assigned to the control group were selected solely on the basis that they could not attend the hospital fortnightly and all participating patients received similar basal treatment (including diet).
According to Takao et al[15] who investigated glycemic control over 10 years in Japanese type 2 diabetic patients, there was a correlation between the change of blood glucose and progression of diabetic retinopathy. In addition, Wadén et al[16] reported that HbA1c variability could not only predict incident microalbuminuria and progression of renal disease, but also cardiovascular events in type 1 diabetes patients. Bouchi et al[17] recently reported that there is a relationship between blood glucose changes and cardiovascular events in Japanese patients with type 2 diabetes. Thus, the importance of good glycemic control has continued to attract attention. There is a possibility that cardiovascular events can be prevented by regulating blood glucose excursion. Because previous reports concerning cardiovascular events in Japanese type 2 diabetic patients have not clarified this issue, whether blood glucose excursion is related to cardiovascular events remains to be determined[18]. HbA1c elevation during the winter holiday season was also attenuated by switching from α-glucosidase inhibitors to sitagliptin (HbA1c increased by 0.33% before switching vs 0.13% after switching). This 0.2% difference of HbA1c over 2 mo between α-glucosidase inhibitor therapy and sitagliptin is clinically important.
It is too early to draw definite conclusions from our study without placebo control. Further investigations are needed to confirm whether better glycemic control by using sitagliptin with or without other oral hypoglycemic agents can improve pre-existing atheroma and thus prevent major cardiovascular events[19-26].
Epidemiological studies have suggested that seasonal fluctuations of hemoglobin A1c (HbA1c) are noted in patients with type 2 diabetes, but no reports have been published concerning the efficacy of antidiabetic agents for such HbA1c fluctuations.
Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, was the first incretin enhancer approved in Japan (in 2009). This drug causes few side effects when regulating blood glucose levels but the effect of sitagliptin therapy on seasonal fluctuation of glycemic control in Japanese type 2 diabetic patients is unknown.
This is the first study to demonstrate that add-on therapy with sitagliptin can prevent seasonal deterioration of glycemic control and even improve HbA1c levels despite the increased calorie intake and decrease of physical activity during the New Year holiday period.
In Japan, glycemic control typically deteriorates during the New Year winter holiday season, since Japanese people (including diabetic patients) celebrate with a high calorie diet and alcohol. By understanding and utilizing the response to sitagliptin demonstrated in the present study, treatment can be tailored to better manage the seasonal deterioration of glycemic control, which is unfavorable for patients with type 2 diabetes.
DPP-4 inhibitors, of which sitagliptin was the first to be released in Japan, inhibit the enzyme DPP-4 and are used to treat type 2 diabetes. Inhibition of DPP-4 enhances the activity of incretins that play an important role in regulating insulin secretion and blood glucose.
This is an interesting study that suggests the beneficial effects of sitagliptin during the winter holiday period in Japanese diabetic patients. The authors report that HbA1c was significantly reduced during the holiday period in diabetic patients switching to sitagliptin or using it as add-on therapy compared with control patients.
Peer reviewers: Dr. Arulmozhi D Kandasamy, Department of Pharmacology, University of Alberta, Edmonton T6G 2S2, Canada; Dr. Joshua J Neumiller, Washington State University, Spokane, WA 99210-1495, United States
S- Editor Wu X L- Editor Roemmele A E- Editor Zhang DN
| 1. | Tseng CL, Brimacombe M, Xie M, Rajan M, Wang H, Kolassa J, Crystal S, Chen TC, Pogach L, Safford M. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol. 2005;161:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Sohmiya M, Kanazawa I, Kato Y. Seasonal changes in body composition and blood HbA1c levels without weight change in male patients with type 2 diabetes treated with insulin. Diabetes Care. 2004;27:1238-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Sakura H, Tanaka Y, Iwamoto Y. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res Clin Pract. 2010;88:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, Suzuki H, Hirayama Y, Ahmed T, Davies MJ. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Nonaka K, Tsubouchi H, Okuyama K, Fukao Y, Johnson-Levonas AO, Amatruda JM. Effects of once-daily sitagliptin on 24-h glucose control following 4 weeks of treatment in Japanese patients with type 2 diabetes mellitus. Horm Metab Res. 2009;41:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Iwamoto Y, Taniguchi T, Nonaka K, Okamoto T, Okuyama K, Arjona Ferreira JC, Amatruda J. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J. 2010;57:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Iwamoto Y, Tajima N, Kadowaki T, Nonaka K, Taniguchi T, Nishii M, Arjona Ferreira JC, Amatruda JM. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes Obes Metab. 2010;12:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Mori Y, Taniguchi Y, Matsuura K, Sezaki K, Yokoyama J, Utsunomiya K. Effects of sitagliptin on 24-h glycemic changes in Japanese patients with type 2 diabetes assessed using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Maeda H, Kubota A, Tanaka Y, Terauchi Y, Matsuba I. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e20-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Nomiyama T, Akehi Y, Takenoshita H, Nagaishi R, Terawaki Y, Nagasako H, Kudo T, Kodera T, Kobayashi K, Urata H. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e27-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Yanai H, Masui Y, Yoshikawa R, Kunimatsu J, Kaneko H. Dipeptidyl peptidase-4 inhibitor for steroid-induced diabetes. World J Diabetes. 2010;1:99-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 794] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Xu L, Man CD, Charbonnel B, Meninger G, Davies MJ, Williams-Herman D, Cobelli C, Stein PP. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab. 2008;10:1212-1220. [PubMed] |
| 14. | Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 631] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 15. | Takao T, Ide T, Yanagisawa H, Kikuchi M, Kawazu S, Matsuyama Y. The effect of fasting plasma glucose variability on the risk of retinopathy in type 2 diabetic patients: retrospective long-term follow-up. Diabetes Res Clin Pract. 2010;89:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649-2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A. Fluctuations in HbA1c are associated with a higher incidence of cardiovascular disease in Japanese patients with type 2 diabetes. J Diabetes Invest. 2012;3:148-155. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ehara H, Yamamoto-Honda R, Kitazato H, Takahashi Y, Kawazu S, Akanuma Y, Noda M. ApoE isoforms, treatment of diabetes and the risk of coronary heart disease. World J Diabetes. 2012;3:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Sauvé M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 439] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 21. | Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, Akiyama E, Kurokawa H, Nozaki T, Ohba K, Konishi M. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. [PubMed] |
| 24. | Frederich R, Alexander JH, Fiedorek FT, Donovan M, Berglind N, Harris S, Chen R, Wolf R, Mahaffey KW. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 25. | Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27 Suppl 3:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |