Published online Jan 15, 2012. doi: 10.4239/wjd.v3.i1.19
Revised: November 18, 2011
Accepted: January 9, 2012
Published online: January 15, 2012
Redox balance is fundamentally important for physiological homeostasis. Pathological factors that disturb this dedicated balance may result in oxidative stress, leading to the development or aggravation of a variety of diseases, including diabetes mellitus, cardiovascular diseases, metabolic syndrome as well as inflammation, aging and cancer. Thus, the capacity of endogenous free radical clearance can be of patho-physiological importance; in this regard, the major reactive oxygen species defense machinery, the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) system needs to be precisely modulated in response to pathological alterations. While oxidative stress is among the early events that lead to the development of insulin resistance, the activation of Nrf2 scavenging capacity leads to insulin sensitization. Furthermore, Nrf2 is evidently involved in regulating lipid metabolism. Here we summarize recent findings that link the Nrf2 system to metabolic homeostasis and insulin action and present our view that Nrf2 may serve as a novel drug target for diabetes and its complications.
- Citation: Yu ZW, Li D, Ling WH, Jin TR. Role of nuclear factor (erythroid-derived 2)-like 2 in metabolic homeostasis and insulin action: A novel opportunity for diabetes treatment? World J Diabetes 2012; 3(1): 19-28
- URL: https://www.wjgnet.com/1948-9358/full/v3/i1/19.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i1.19
The association between oxidative stress and insulin resistance has been recognized for more than a decade[1,2]. While the initial interpretation of this phenomenon was that oxidative stress was among the consequences of impaired insulin action during hyperglycemia[1,2], further studies have revealed the causative role of oxidative stress in insulin resistance[3,4]. More recently, the initiation of oxidative stress in inducing insulin resistance has been more specifically linked to the elevated mitochondrial reactive oxidative species (ROS) production[5]. The progress in the research on the etiological role of oxidative stress in insulin resistance has deepened our knowledge of the patho-physiological alterations in metabolic disorders, including type 2 diabetes mellitus (T2D).
The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) system was originally discovered as one of the important antioxidant machineries[6,7]. In the middle of the 1990s, two teams independently isolated the cDNA that encodes Nrf2[6,8]. The function of Nrf2 in regulating redox balance was identified following the discovery of Nrf2 in up-regulating genes encoding antioxidant enzymes[9]. In addition, another major function of the Nrf2 system in detoxification was revealed[10,11]. The function of this system as a master regulator of redox balance in cellular cytoprotective response is now widely accepted[12].
From practical point of view, Nrf2 has drawn our attention as a promising drug target. Several Nrf2 activators have already been developed for treating diseases, including tumors and inflammatory diseases[13,14]. Interestingly, Nrf2 activators have been shown to modulate insulin action[15]. In addition, certain natural chemicals, including those in the category of Chinese herbal medicine, were shown to both up-regulate Nrf2 action and sensitize insulin action[16]. Further exploration of mechanisms underlying the function of Nrf2 will not only help the proper utilization of traditional medicines in treating metabolic diseases, but also lead to the discovery of novel therapeutic targets for various diseases.
In the past few years, a number of excellent reviews have updated our knowledge about the molecular basis of the Nrf2 system, the crosstalk between Nrf2 and other cell signaling pathways, as well as its capability in repressing inflammation, tumorigenesis and promoting longevity[17-20]. Here we review recent findings which link the Nrf2 system to metabolic homeostasis and insulin action. In addition, we present our view that Nrf2 may serve as a novel drug target for diabetes and its complications.
Insulin resistance, i.e., impaired insulin action in its sensitive tissues (muscles, liver and adipose tissue), was recognized as a common feature of obesity and diabetes more than half a century ago[21]. This abnormality is also associated with other prevalent metabolic diseases, including hypertension, dyslipidemia and cardiovascular disorders[22]. The spectrum of insulin resistant syndrome causes a broad health hazard and enormous financial burden, which make the pharmacological combat of insulin resistance an urgent task.
For effective drug intervention of insulin resistance and related diseases, the first important task is to identify a proper drug target. This is based on our understanding the molecular mechanisms underlying insulin insensitivity. Great efforts have been made in the exploration of the cellular aberrant related to insulin resistance. Early observations suggested that the defect in insulin signaling, including insulin receptor substrate-1 (IRS-1), is apparently involved[23]. We have then gradually recognized that the impairment in IRS-1 signaling is not primary but secondary to other alterations[24], including the inflammatory responses (kinase complex IKK-β activation)[25], endoplasmic reticulum (ER) stress[26] and mitochondrial dysfunction[27]. These abnormalities can blunt IRS-1 tyrosine phosphorylation and subsequent insulin signaling transduction[24].
Currently, it is still not known which pathological factor initiates insulin resistance. Several pioneer studies indicated that an apparent impairment of insulin signaling is not prerequisite for the occurrence of insulin resistance in the early stage. A study by Dr. Hoehn et al[28] found that treatment of insulin sensitive cells with a variety of insulin resistance inducers, such as tumor necrosis factor-α (TNF-α), oxidative stressor and dexamethasone, did not always impair the insulin signaling transduction, but still produced the impairment in insulin action. Moreover, in mice fed with a high fat diet, leading eventually to initial insulin resistance, there was no insulin signaling alteration involved[28]. This is reinforced by a recent study showing that mitochondrial derived oxidative stress is tightly linked to impaired insulin action, while the traditionally defined insulin signaling transduction appears to be intact[29]. Moreover, in high fat-induced insulin resistance, oxidative stress is evident in adipose tissue at the initial stage of insulin resistance[4,30]. Thus, oxidative stress derived from mitochondrial ROS overproduction after excessive nutrient uptake is likely to be the early aberrance that causes insulin resistance[5]. Furthermore, antioxidants were shown to ameliorate insulin resistance[3]. These findings collectively support the notion that oxidative stress plays an initial role in the development of insulin resistance. This theory also makes sense if considering that insulin resistance is actually an adaptive response to block energy over supply and the mitochondria is a major energy producer responding to energy overwhelming by producing ROS, causing a negative feedback to block insulin action.
Although at this stage we do not fully understand mechanistically how oxidative stress causes insulin resistance, existing scientific evidence has indicated a few potential pathways by which oxidative stress interferes with insulin action. Experimental data indicate that oxidative stress may lead to a direct impairment of insulin signaling molecules via modifying their oxidative status[31]. More importantly, oxidative stress can interact with inflammation, ER stress and mitochondrial dysfunction, which are among the causative factors of insulin resistance[25-27]. While over-nutrition may promote mitochondrial oxidant production and oxidative stress[5], inflammatory signals can be activated by oxidative stress[32]. ER is an initial stress sensing organelle. Responding to oxidative stress, it can also promote the oxidant production by an unfold protein reaction which produces ROS, leading to activation of redox sensitive kinases, such as NF-κB, to initial inflammatory responses[33]. NF-κB-regulated cytokine production in turn negatively affects the function of ER via various routes including the increase of TNF-α[34]. One of the fundamental consequences of ER stress response is the inhibition of protein synthesis, which will ultimately affect mitochondrial biogenesis and function[35]. Furthermore, oxidative stress may damage mitochondrial DNA directly and further impair its function[36]. Therefore, oxidative stress is linked to a variety of pathological factors that are important for impairing insulin action.
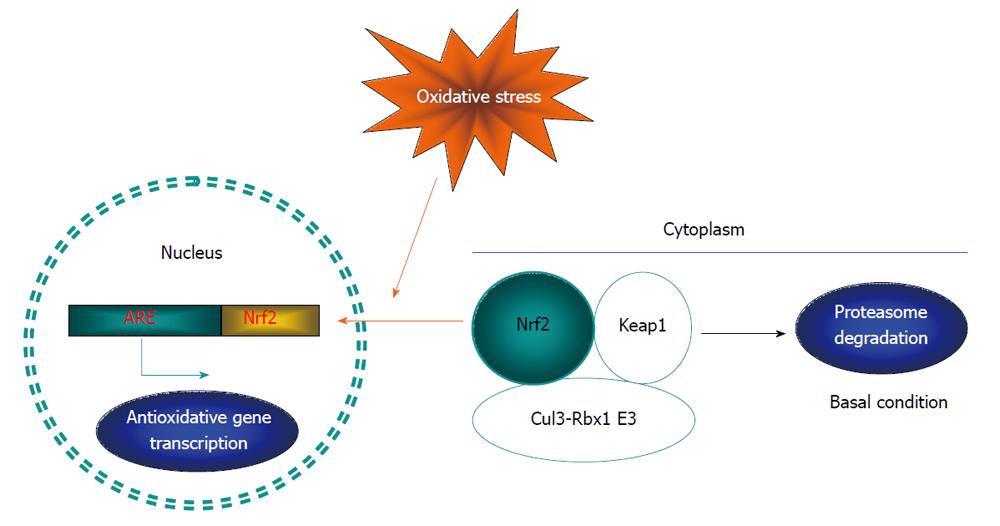
One of the most important antioxidant machineries is the Nrf2 system, with the transcription factor Nrf2 as the central component[37]. Nrf2 binds to the nucleotide sequence, namely antioxidant response element (ARE), in the promoter region of a battery of genes that encode antioxidant enzymes. The major Nrf2 regulated antioxidant enzymes include heme oxygenase-1, Mn-superoxide dismutase, sequestosome 1, NAD(P)H quinone oxidoreductase 1, glutathione peroxidase, glutathione S-transferase A1 and glutamate-cysteine ligase[37]. Without stimulation, Nrf2 molecules mainly reside in the cytoplasm, anchored with Kelch-like ECH-associated protein 1 (Keap1). The association between Nrf2 and Keap1 may trigger Nrf2 ubiquitination and subsequent proteasome degradation. In response to oxidative stress, certain lysine residues in Keap1 are modified, resulting in the disruption of the complex and the increase of free Nrf2 molecules. Nrf2 free molecules will then be translocated into the nucleus to stimulate gene transcription (Figure 1). Nrf2 nuclear translocation can also be triggered by other signaling kinases. For example, an early study found that ARE-directed transcription was activated by the protein kinase C (PKC) activator, phorbol 12-myristate 13-acetate, while the PKC catalytic subunit was also able to phosphorylate Nrf2 directly in vitro, indicating a direct regulation of PKC on Nrf2 translocation and activation[38]. Several other protein kinases, including MARK, PERK and Akt, may also be able to phosphorylate Nrf2 and stimulate its nuclear translocation and action[38-41].
The Nrf2 system is evolutionally conserved and ubiquitously expressed in a variety of cell lineages and systems. This, along with the large spectrum of Nrf2 regulated enzymes, renders it with a great capacity to prevent oxidative stress-induced damage. In addition to its role in regulating redox balance[17,42], recent evidence suggests that the Nrf2 system is involved in certain other important functions, including regulating lipid metabolism and insulin action, which will be detailed in the following sections.
The interaction of the Nrf2 system with insulin action is an emerging research theme. In one way, insulin and its effector Akt/PKB were shown to modulate the function of Nrf2. In Caenorhabditis elegans (C. elegans), it was shown that Nrf2 (SKN-1, a synonyms in C. elegans) can be directly phosphorylated by Akt, leading to the repression of its nuclear translocation[43]. Since oxidative stress is associated with aging and Akt/insulin signaling is a critical signaling that causes aging[44], this repression may be among the mechanisms for insulin signaling in accelerating aging. However, in mammals, a number of studies have actually shown that insulin signaling is required for Nrf2 activation[45,46]. Interestingly, Nrf2 function was found to be defective in aged mice[47] and aging is usually accompanied by insulin resistance. Whether impaired insulin signaling blunts Nrf2 function or the reverse in mammals remains to be examined.
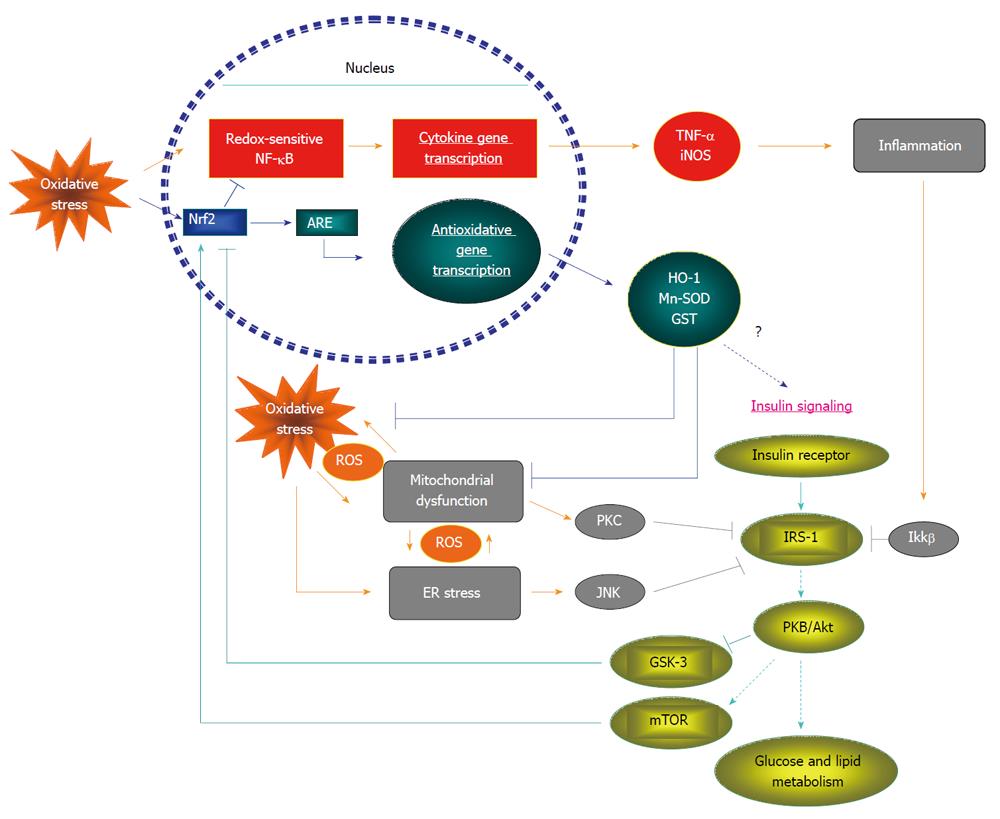
On the other hand, the modulation of the Nrf2 system to insulin signaling in mammals has just been recognized recently, particularly in conditions of insulin resistance. In fact, oxidants are not always detrimental and a certain amount of ROS is important to maintain normal insulin signaling transduction as redox balance is dedicatedly regulated in physiological conditions[48]. However, ROS overproduction will destroy this balance, resulting in oxidative stress and impaired insulin action. To combat oxidative stress, the Nrf2 system may directly or indirectly interact with insulin signaling via several potential pathways to sensitize insulin action (Figure 2). It has been demonstrated that in high fat diet (HFD) fed mouse models, Nrf2 activation was shown to repress oxidative stress and ameliorate blunted insulin signaling[15]. In addition, Nrf2 activation may enhance insulin signaling via inhibiting the inflammation signaling pathway and ER stress in vivo[15]. Our group reported recently that a direct depletion of Nrf2 by siRNA in the hepatic HepG2 cell line resulted in impaired insulin-stimulated Akt phosphorylation[15]. Furthermore, in injured liver, Nrf2 was shown to be required to promote liver regeneration in response to Akt activation[53].
In addition to the regulation of redox balance, Nrf2 activation may negatively regulate lipid synthesis and exert an antiobesity function. Several reports have revealed the role of Nrf2 on lipid metabolism in adipocytes. Nrf2 stimulation by carnosic acid and carnosol was shown to inhibit preadipocyte differentiation and adipogenesis[54]. Since adipocyte differentiation is affected by both redox balance and transcription factors[55], we do not know now whether Nrf2 affects adipocyte differentiation through redox modulation or by regulating key transcription factors, such as peroxisome proliferator-activated receptor (PPARγ)[56]. The role of Nrf2 in regulating whole body weight and obesity has been investigated recently by our group and others. Oltipraz and oleanolic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole (CDDO-Im), two known activators of Nrf2, were shown to prevent HFD-induced increase of body weight, adipose mass and hepatic lipid accumulation[15,57]. Importantly, the effects of CDDO-Im were dependent on the Nrf2 system, while no such responses were observed in Nrf2-disrupted mice[57].
In the liver, a recent study using proteomic analysis of Nrf2 deficient transgenic mice suggests that the Nrf2 system is responsible for inhibition of expression of lipid synthetic and metabolic enzymes, such as ATP-citrate lyase[58]. When the methionine- and choline-deficient (MCD) diet was utilized to induce fatty liver, a more profound hepatosteatosis was observed in the Nrf2-null mice, compared with that of the wild type littermates[59]. In contrast, Keap1-null mice showed a delay of onset in hepatosteatosis and the degree of hepatosteatosis was milder than the control wild type littermates[59]. These observations collectively suggest that the Nrf2 system plays a role in repression of hepatic lipid accumulation.
Interestingly, a seemly opposite role of Nrf2 in lipid metabolism and lipogenesis was observed in the analysis of Nrf2-/- mice. These mice show reduced liver weight, hepatic fatty acid content as well as serum lipids[60]. Furthermore, in hepatocytes of the Nrf2-/- mice, the expression level of PPARγ gamma, fatty-acid synthase, stearoyl-CoA desaturase and regulatory-element binding protein that are involved in de novo lipogenesis were found to be reduced[60]. We suggest that the role of the Nrf2 system in lipid homeostasis is certain but complex. The different role of Nrf2 in lipid homeostasis may be related to the status of Nrf2 activation. Permanent inactivation of Nrf2 by genetic modulation versus temporary Nrf2 activation by its chemical activators may give rise different adaptive mechanisms that affect lipogenic gene expression and lipid metabolism.
As Nrf2 affects insulin signaling and lipid metabolism, it is anticipated that Nrf2 would also modulate glucose metabolism. Following STZ treatment, compared with the wild type mice, Nrf2-null mice had a higher blood glucose level, accompanied by enhanced hepatic gluconeogenesis[61]. In the high fat diet-fed C57BL/6J mouse model, the administration of Nrf2 activator oltipraz significantly attenuated glucose intolerance, accompanied by the blockage of the development of obesity and dyslipidemia[15]. Table 1 summarizes the recent findings of the Nrf2 system on metabolic regulation.
| Effect | Model | Ref. |
| Preadipocyte differentiation2 | Carnosic acid and carnosol stimulated Nrf2 activation in 3T3-L1 adipocytes | [54] |
| Preadipocyte differentiation1 | Nrf2 deficient 3T3-L1 adipocytes | [56] |
| Adipocyte differentiation1 | The Nrf2 activator CDDO-Im-treated mouse embryonic fibroblasts from C57BL/6J Nrf2-/- mice | [62] |
| Obesity2 | HFD-induced obesity in C57BL/6J mice fed with the Nrf2 activator oltipraz | [15] |
| Obesity2 | HFD-induced obesity in C57BL/6J mice fed with the Nrf2 activator CDDO-Im | [57] |
| Hepatic lipogenesis1 | Nrf2 mice deficient mice | [58] |
| Hepatic steatosis1 | MCD diet-induced hepatic steatosis in Nrf2 null mice | [59] |
| Hepatic steatosis2 | Nrf2-/- mice | [60] |
| Hepatic gluconeogenesis1 | STZ-induced diabetes in Nrf2 null mice | [61] |
| Blood glucose1, serum lipid1 | STZ-induced diabetes in Nrf2 null mice | [61] |
| Blood glucose2, serum lipid2 | HFD-induced obesity in C57BL/6J mice fed with the Nrf2 activator oltipraz | [15] |
| Blood glucose2 | STZ-induced diabetes in mice treated with oltipraz | [61] |
| Insulin signaling1 | HFD-induced obesity in C57BL/6J mice fed with the Nrf2 activator oltipraz | [15] |
| Insulin signaling1 | Oltipraz treated- mice with partial hepatectomy | [63] |
| Insulin signaling2 | Hepatectomy in Nrf2-/- mice | [53] |
| Insulin signaling2 | Nrf2 knockdown in human liver cell line HepG2 cells | [15] |
| AMPK signaling1 | Oltipraz treated HepG2 cells | [64] |
| Pancreatic β-cell damage2 | Cytokine or STZ-induced RIN β-cell damage with the Nrf2 activator, sulforaphane | [65] |
| Mitochondria damage2 | ROS-induced mitochondrial damage in HepG2 cell with oltipraz | [64] |
With the causal link of oxidative stress with insulin resistance[15,66], it is reasonable to expect that Nrf2 activation can be the potential drug target for diabetes treatment[67-71]. Based on existing studies, several aspects of Nrf2 activation can benefit diabetic patients: (1) Nrf2 activation protects pancreatic β-cells from damage[65] and subsequently prevents the onset of diabetes; (2) The sensitizing action of Nrf2 on insulin may bring benefit for diabetic patients with better glucose control; (3) In addition, hyperglycemia-induced endothelial dysfunction, vascular complications and cardiomyocyte damage[72,73] may be prevented by Nrf2 activation by reducing oxidative stress[74-77]; and (4) A protective role of the Nrf2 system in diabetic nephropathy and neuropathy is another potential function for a Nrf2 modulating drug[78-81]. Certainly, to further explore its in vivo efficacy, clinical trials are required to prove its usefulness on glucose control and prevention of diabetic complications.
One should note that applying an anti-oxidative stress strategy to treat diabetes was raised a long time ago. However, this approach is questionable because of the experimental observation that exogenous supplement antioxidants, such as vitamin C, do not generate effective and consistent results for the control of glucose level and diabetic complications[81-83]. A potential problem of long-term vitamin C administration with its suppressing effect on the endogenous Nrf2 system[84], therefore, is unable to produce sufficient antioxidant function. The utilization of Nrf2 activator may provide additional advantages compared with external antioxidant intake to treat oxidative stress and prove the effectiveness of this strategy[81].
Natural compounds may provide a rich resource for the pharmacist to explore Nrf2 activators with sufficient safety. Substantial evidence indicates that many natural compounds or nutraceuticals can activate Nrf2[19,85,86]. Notably, resveratrol, curcumin and epigallocatechin-3-gallate are all reported to act as insulin sensitizing agents, reverse hyperglycemia, hyperlipidemia and other symptoms linked to obesity[87-90]. These natural compounds may initially cause a depolarization of mitochondrial membrane potential and ROS production, then activate the Nrf2 system to exert subsequent protective responses[19,91-93]. Furthermore, these plant-derived polyphenols are electrophilic and can modulate the reactive cysteine residues in Keap1 molecules, leading to a dissociation of Nrf2 from the Nrf2-Keap complex and increasing the free Nrf2 level[94,95]. Therefore, released Nrf2 together with the inhibition of Keap1-mediated Nrf2 degradation increases the free Nrf2 level, resulting in its translocation to the nucleus and action (Figure 1). It can be expected that further exploring more potent natural compounds or synthetic derivatives that activate Nrf2 to sensitize insulin action[96] could lead to a new drug generation for diabetes treatment.
As the major cellular defense machinery against oxidative stress, the Nrf2 system has drawn extensive attention. However, its functional alteration in metabolic diseases has been realized recently and needs to be explored further. Impaired Nrf2 function is evident in several pathological conditions, such as aging, neurodegeneration diseases and insulin resistance, that are mechanistically linked to oxidative stress, while Nrf2 activation reverses the functional abnormality of these diseases[15,51]. Therefore, the malfunction of the Nrf2 system is anticipated to contribute to the pathological development of these diseases. The characterization of this system in the regulation of glucose and lipid metabolism would evoke more studies to determine if it is a promising drug target. The capability of Nrf2 activation in preventing obesity, protecting pancreatic β cells and enhancing insulin action makes Nrf2 activators a novel category in diabetic therapeutics. Particularly, natural compounds have been proven to be effective in insulin sensitization and preventing diabetic complications in animal and pre-clinical human studies[97]. Further clinical trials are needed to confirm their benefits for diabetics and possible usefulness in clinical treatment. In order to develop Nrf2 activators as therapeutic agents in T2D, we suggest that several tasks need to be carried out for further exploration of the beneficial effects of Nrf2 activation on insulin signaling, as well as glucose and lipid homeostasis: (1) Further investigations are needed to clarify whether and how Nrf2 activation leads to insulin sensitization. Obviously, these investigations may lead to the recognition of novel targets of the Nrf2 system. Nrf2-null mice as well as the in vitro Nrf2 knockdown approach are essential tools for this purpose; (2) The Nrf2 system has been shown to be activated by mitochondrial ROS production[98] and when activated can protect mitochondrial function by eliminating ROS[99,100]. Whether this effect is related to its beneficial action in insulin resistance requires further studies; (3) Many natural compounds, such as resveratrol, curcumin and epigallocatechin-3-gallate, have been shown to activate Nrf2, along with improved insulin sensitization. It is essential to determine whether their stimulatory effect on insulin signaling is dependent on Nrf2 activity. Again, the Nrf2 null mice will be the asset for these studies; (4) The AMP-dependent kinase activator metformin, PPARγ agonists and α-folic acid were shown to improve glucose control and also to attenuate oxidative stress[101-104]. Whether these existing drugs exert these effects via Nrf2 activation requires further investigations; and (5) Nrf2 can upregulate CD36 expression involved in lipid uptake. However, this effect promotes lipid accumulation in blood vessels and accelerates atherosclerosis[105,106]. While Nrf2 activation can be beneficial to insulin action, these potential side effects must be carefully evaluated.
Peer reviewers: Min Du, Dr., Department of Animal Science, University of Wyoming, 1000 E University Avenue, Laramie, WY 82070 United States; Christina Piperi, Assistant Professor, Department of Biological Chemistry, University of Athens Medical School, Mikras Asias 75, Goudi, Athens 11527, Greece; Serap Yalın, Dr., Pharmacy Faculty, Mersin University, Mersin 33169, Turkey
S- Editor Wu X L- Editor Roemmele A E- Editor Zhang DN
| 1. | Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 363] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 480] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 3. | Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1895] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 4. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 5. | Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573-581. [PubMed] |
| 6. | Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926-9930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1274] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 7. | Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184-4193. [PubMed] |
| 9. | Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960-14965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 874] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 10. | Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 471] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Numazawa S, Yoshida T. Nrf2-dependent gene expressions: a molecular toxicological aspect. J Toxicol Sci. 2004;29:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 596] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther. 2008;7:3470-3479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, Fantus IG, Jin T. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54:922-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 19. | Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Reaven GM. Resistance to insulin-stimulated glucose uptake and hyperinsulinemia: role in non-insulin-dependent diabetes, high blood pressure, dyslipidemia and coronary heart disease. Diabete Metab. 1991;17:78-86. [PubMed] |
| 22. | Bruce KD, Byrne CD. The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J. 2009;85:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1158] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 24. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1943] [Cited by in RCA: 2024] [Article Influence: 106.5] [Reference Citation Analysis (3)] |
| 25. | Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1434] [Cited by in RCA: 1424] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 26. | Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2756] [Cited by in RCA: 2870] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 27. | Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9-S15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 614] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 28. | Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 29. | Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 30. | Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071-1077. [PubMed] |
| 31. | Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511-7518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4137] [Cited by in RCA: 3708] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 33. | Kitamura M. Control of NF-κB and inflammation by the unfolded protein response. Int Rev Immunol. 2011;30:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917-33925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 332] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 35. | Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 2011;18:769-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 36. | Kang D, Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin Chem Lab Med. 2003;41:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291-13295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2104] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 38. | Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci USA. 2000;97:12475-12480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 39. | Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Brunt KR, Fenrich KK, Kiani G, Tse MY, Pang SC, Ward CA, Melo LG. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol. 2006;26:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198-7209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1008] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 42. | Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 43. | Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 791] [Cited by in RCA: 718] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 44. | Parr T. Insulin exposure and aging theory. Gerontology. 1997;43:182-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Chang CL, Au LC, Huang SW, Fai Kwok C, Ho LT, Juan CC. Insulin up-regulates heme oxygenase-1 expression in 3T3-L1 adipocytes via PI3-kinase- and PKC-dependent pathways and heme oxygenase-1-associated microRNA downregulation. Endocrinology. 2011;152:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Geraldes P, Yagi K, Ohshiro Y, He Z, Maeno Y, Yamamoto-Hiraoka J, Rask-Madsen C, Chung SW, Perrella MA, King GL. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem. 2008;283:34327-34336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 48. | Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281:14841-14851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 435] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 50. | Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095-9105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Ndisang JF, Lane N, Jadhav A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2009;296:E1029-E1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106:17787-17792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 394] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 53. | Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 54. | Takahashi T, Tabuchi T, Tamaki Y, Kosaka K, Takikawa Y, Satoh T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase2 enzymes and activation of glutathione metabolism. Biochem Biophys Res Commun. 2009;382:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Ducluzeau PH, Priou M, Weitheimer M, Flamment M, Duluc L, Iacobazi F, Soleti R, Simard G, Durand A, Rieusset J. Dynamic regulation of mitochondrial network and oxidative functions during 3T3-L1 fat cell differentiation. J Physiol Biochem. 2011;67:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 2010;285:9292-9300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 57. | Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, Goldring CE, Powell H, Sanderson C, Williams S. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics. 2010;73:1612-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Zhang YK, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 60. | Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211-G1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ, Klaassen CD. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J Pharmacol Exp Ther. 2010;333:140-151. [PubMed] |
| 62. | Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27:7188-7197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 273] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 63. | Cho IJ, Sung DK, Kang KW, Kim SG. Oltipraz promotion of liver regeneration after partial hepatectomy: The role of PI3-kinase-dependent C/EBPbeta and cyclin E regulation. Arch Pharm Res. 2009;32:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Kwon YN, Shin SM, Cho IJ, Kim SG. Oxidized metabolites of oltipraz exert cytoprotective effects against arachidonic acid through AMP-activated protein kinase-dependent cellular antioxidant effect and mitochondrial protection. Drug Metab Dispos. 2009;37:1187-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol. 2009;235:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 66. | Li M, Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 559] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 68. | Maher J, Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4-15. [PubMed] |
| 69. | de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 358] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 70. | Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424-6431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 295] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 71. | Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S-3001S. [PubMed] |
| 72. | He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809-2817. [PubMed] |
| 74. | He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis. 2011;21:277-285. [PubMed] |
| 75. | Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 76. | Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 77. | Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133-H1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 708] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 79. | Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812:719-731. [PubMed] |
| 80. | Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13:1159-1170. [PubMed] |
| 81. | Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 82. | Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. 2011;7:106-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 83. | McGrowder D, Ragoobirsingh D, Dasgupta T. The enhancement of the hyperglycemic effect of S-nitrosoglutathione and S-nitroso-N-acetylpenicillamine by vitamin C in an animal model. BMC Pharmacol. 2002;2:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 84. | Wagner AE, Boesch-Saadatmandi C, Breckwoldt D, Schrader C, Schmelzer C, Döring F, Hashida K, Hori O, Matsugo S, Rimbach G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes - role of the redox-regulated transcription factor Nrf2. BMC Complement Altern Med. 2011;11:1. [PubMed] |
| 85. | Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 86. | Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 87. | Kim JA. Mechanisms underlying beneficial health effects of tea catechins to improve insulin resistance and endothelial dysfunction. Endocr Metab Immune Disord Drug Targets. 2008;8:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002;50:7182-7186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3166] [Cited by in RCA: 3245] [Article Influence: 170.8] [Reference Citation Analysis (0)] |
| 90. | Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS, Kim HJ, Chang KC, Lee JH, Lee HT, Kim JH. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radic Biol Med. 2007;43:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Noda C, He J, Takano T, Tanaka C, Kondo T, Tohyama K, Yamamura H, Tohyama Y. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochem Biophys Res Commun. 2007;362:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Wang CT, Chang HH, Hsiao CH, Lee MJ, Ku HC, Hu YJ, Kao YH. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol Nutr Food Res. 2009;53:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56:4813-4818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 94. | Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 812] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 95. | McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165-172. [PubMed] |
| 96. | Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18-H24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 97. | Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 98. | Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925-22936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 100. | Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 825] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 101. | Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 510] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 102. | Habeos IG, Ziros PG, Chartoumpekis D, Psyrogiannis A, Kyriazopoulou V, Papavassiliou AG. Simvastatin activates Keap1/Nrf2 signaling in rat liver. J Mol Med (Berl). 2008;86:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Ogborne RM, Rushworth SA, O'Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol. 2005;25:2100-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Wang X, Wang Z, Liu JZ, Hu JX, Chen HL, Li WL, Hai CX. Double antioxidant activities of rosiglitazone against high glucose-induced oxidative stress in hepatocyte. Toxicol In Vitro. 2011;25:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 105. | Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One. 2008;3:e3791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 106. | Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 537] [Article Influence: 25.6] [Reference Citation Analysis (0)] |