INTRODUCTION
Before describing and discussing the involvement of the mitochondria in the fetal programming of adult diseases, a brief introduction on the biogenesis and function of mitochondria will be presented.
Mitochondria, their biogenesis and function
It is only recently that the mitochondrial proteome has been considered as a dynamic system generated by the nuclear DNA (nDNA) and the mitochondrial DNA (mtDNA). In most human cells, mitochondria contain 103-104 copies of a circular genome of 16 569 base-pairs without introns. It contains 37 genes encoding 2 ribosomal RNAs, 22 tRNAs required for mitochondrial protein synthesis and 13 polypeptides[1]. These include 7 of the 46 polypeptides of the complex I (NADH dehydrogenase; ND 1, 2, 3, 4L, 4, 5, 6), one of the 11 proteins of complex III (cytochrome b), 3 of the 13 polypeptides of complex IV (cytochrome c oxidase; COX-1, -2, -3) and 2 of the 16 proteins of complex V (ATP synthase; ATPase-6, -8)[2].
Mitochondrial biogenesis requires a tight coordination between the nDNA and mtDNA to transcribe the genes in the nucleus, as well as in mitochondria. The nDNA-encoded mitochondrial proteins are translated by using cytosolic ribosomes and selectively imported into the mitochondrion through various import systems[3,4]. These proteins include the four units of the complex II, the mtDNA polymerase γ, mitochondrial RNA polymerase, the mitochondrial transcription factor (Tfam), the mitochondrial ribosomal proteins and elongation factors, and the mitochondrial metabolic enzymes[5].
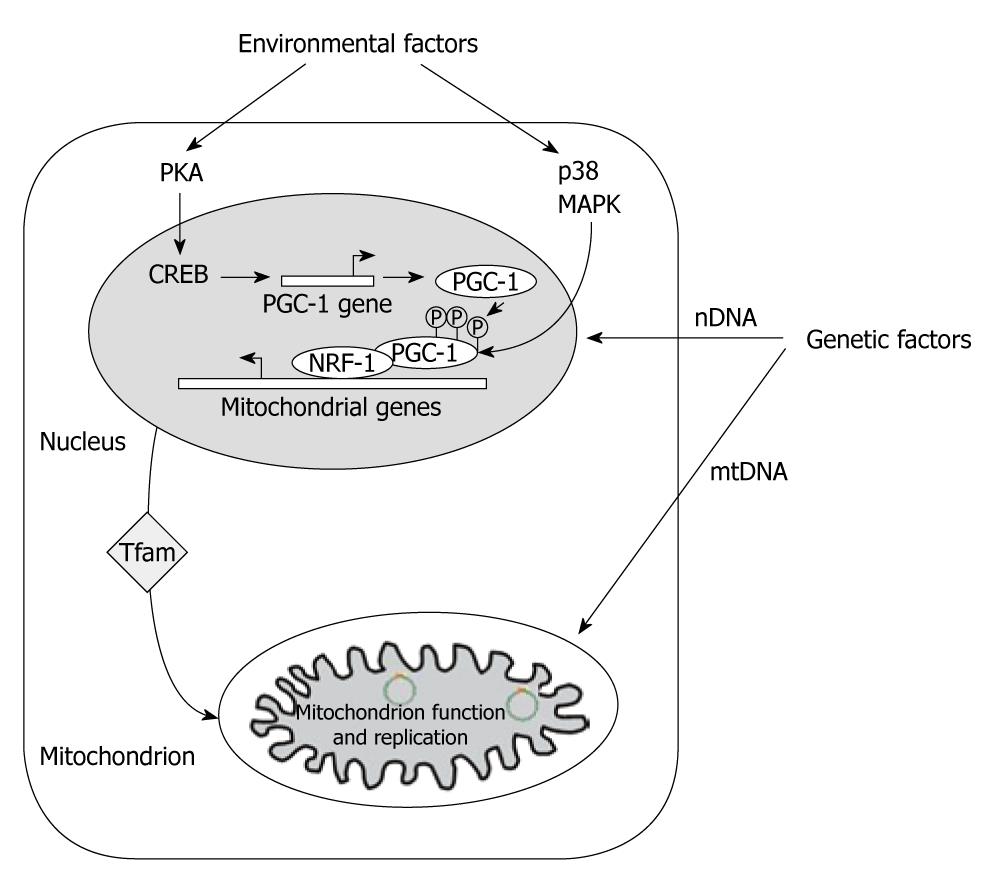
Three factors, i.e. peroxisome proliferator activated receptor γ (PPARγ) coactivator-1α (PGC-1α), nuclear respiratory factor 1 (NRF-1) and Tfam provide a molecular basis for the connection between environmental stimuli and mitochondrial biogenesis. PGC-1α is part of the PGC-1 coactivator family which, in addition to its role in the mitochondrial biogenesis and through its interaction with the PPARγ[6], regulates several functions, including adaptive thermogenesis, glucidic metabolism, fatty acid oxidation and mitochondrial anabolic and catabolic function. NRF-1 and -2 bind to the promoter region of a broad range of mitochondrial genes encoded in the nucleus, including Tfam. NRF-1 turns on Tfam, a key transcriptional factor that translocates into the mitochondria and activates mitochondrial biogenesis and function through mtDNA replication and transcription (Figure 1)[7]. NRF-1 may also affect expression of mitochondrial and metabolic genes[8].
Figure 1 Mitochondrial gene expression and biogenesis.
Environmental factors induce PKA and p38 MAPK pathways. PKA phosphorylates CREB transcription factor, which is involved in the induction of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α gene expression. Activation of p38 MAPK phosphorylates PGC-1α protein, resulting in its stabilization and activation. PGC-1α activates the expression of the subunits of mitochondrial electron transport chain and Tfam, one of the major regulatory factors for mitochondrial transcription and replication, through the co-activation of nuclear respiratory factor 1-mediated transcription. Tfam subsequently translocates in the mitochondrion and directly increases the transcription and replication of mitochondrial DNA (Adapted from Remacle et al[45], 2007).
In addition, PGC-1α may promote the mitochondrial biogenesis in a cell type-specific manner with the co-activation of PPARγ. It seems that PPARγ affects mitochondrial biogenesis indirectly by enhancing the expression of PGC-1α since the agonist of PPARγ rosiglitazone, induced endogenous expression of PGC-1α in adipose tissue[9,10]. Through this way, PGC-1α may drive PPARγ[6] and ameliorate symptoms of metabolic disease. In a cell-selective manner, the efficiency of the oxidative phosphorylation process may also be regulated by PGC-1α through the transcriptional control of uncoupling proteins (UCP)[11].
There is a great variation in the mtDNA across different cell types. Whereas somatic cells contain up to 4 000 copies, maternal oocytes may contain as many as 200 000 copies and sperm as few as 100[12]. This is the reason why it is usually accepted that mtDNA is exclusively maternally inherited.
Mitochondria are responsible for the production of energy by oxidizing pyruvate through the tricarboxylic acid (TCA) cycle and lipids through -oxydation. These processes produce reducing equivalents that then drive the electron transport chain (ETC) enclosed within the inner membrane to produce ATP. Inevitably, by the products of oxidative phosphorylation, mitochondria are also the major source of reactive oxygen/nitrogen species (ROS/RNS). Electrons leaking into the mitochondrial matrix can react with molecular oxygen. ROS can occur when electrons are in excess in case of inhibition of oxidative phosphorylation and they can damage macromolecules[13]. ROS can also inhibit the activity of the ETC, specifically the iron-sulfur center-containing enzymes of the complex I and III, and mitochondrial aconitase of the TCA cycle[5]. Mitochondria also possesses a major role in the regulation of apoptosis. Indeed, several proapoptotic proteins reside in the intermembrane space, including cytochrome c and apoptosis inducing factor[14]. Due to the absence of protective histone proteins, to the close vicinity and the limited DNA repair mechanism, mtDNA is a sensitive target for oxidative DNA damage by ROS[15]. The mutation rate of mtDNA is at least 10 times higher than that of nuclear DNA[5,16].
Equally important, the TCA cycle is critical for several metabolic functions, where its intermediates are used as substrates for de novo synthesis of biomolecules[17]. Beside this anabolic process, the TCA cycle also plays a critical role in the catabolism where non-essential as well as essential amino acids are broken down to TCA cycle intermediates and fatty acids are oxidized to acetyl-CoA. So, the different anabolic and catabolic functions of the mitochondria are tightly regulated in response to nutrients such as glucose, amino acids and fatty acids. As shown in case of caloric restriction, adipose tissue features a strong down-regulation of genes involved in energy-generating process such as the TCA cycle and oxidative phosphorylation[18,19]. In the liver, which participates to maintain an adequate level of sugar in the blood, an up-regulation of genes involved in glucogenesis and β-oxidation was noted, whereas genes involved in the TCA cycle and oxidative phosphorylation were down regulated[19]. In the muscle, caloric restriction increased mitochondrial activity, at least in human[20].
Impaired mitochondrial function in metabolic diseases
Given the crucial role of mitochondria for multiple metabolic pathways, tight control of mitochondrial abundance and function is imperative for cellular homeostasis. Therefore, it is not surprising that a link exists between mitochondrial alteration and various diseases including diabetes, cancer and precocious aging[5]. Polymorphic variation in mtDNA has been associated with metabolic diseases. It should be noted that several studies indicate that genomic variation in the 37 mitochondrial genes plays a critical role in apoptotic and metabolic pathways in many tissues including the brain. It is only recently that the mitochondrial proteome has been seen as a dynamic cross talking system generated to adapt the mitochondrial functional capacity to meet the specific needs of the tissue or the disease state[21]. According to the tissue and depending of the functional requirements, the nuclear transcriptional programming of the mitochondrial proteome may vary. This is also true for disease state. For instance, in type 1 diabetes, an adaptation of the liver mitochondrial proteome to support ATP production and fatty acid oxidation was observed[21]. The posttranscriptional modifications are also tissue and disease specific and may modify the localization and function of the mitochondrial proteins and enzymes.
During the process of reduction of oxygen to water by the ETC, ROS/RNS, such as superoxide, hydrogen peroxide, the hydroxyl radical and nitric oxide are generated and cause oxidative damage to target structures. An imbalance between the production of ROS/RNS and antioxidant defenses plays a major role in inducing alterations in insulin signaling pathways[22].
ROS and RNS are formed during both pro-inflammatory cytokines-mediated β-cell aggression in type 1 diabetes and glucolipotoxicity-mediated β-cell dysfunction in type 2 diabetes[23-26].
At least 1.5% of diabetic patients exhibit mutations in mtDNA[27]. Many studies suggest that mitochondrial dysfunction is critical in insulin-linked pathologies. Fewer mitochondria, lower expression of mitochondrial genes, abnormal mitochondrial morphology and disturbed oxidative phosphorylation are commonly described in insulin target tissues such as the liver, muscle and adipose tissue in the case of type 2 diabetes[28] or obesity[29]. Decrease in the number of mitochondria causes mitochondrial dysfunction[30] and mtDNA density is closely associated with oxidative function which itself is linked to insulin sensitivity. Indeed, it has been shown that a decrease in mtDNA density in peripheral blood cells preceded the development of type 2 diabetes[31]. Moreover, mtDNA density was also associated with abnormal obesity before the onset of type 2 diabetes[32,33]. Mitochondrial dysfunction results in an accumulation of fatty acid metabolites, diacylglycerol and long chain fatty acid CoA which will induce insulin resistance via the activation of the phosphokinase C. These changes are accompanied by a decrease in both mitochondrial oxidative activity and ATP biosynthesis.
As already mentioned, several studies with type 2 diabetic patients and non-diabetic subjects with a family history of diabetes featured down regulation of nDNA-encoded mitochondrial genes. For some authors, this may lead to alteration at the level of the mitochondrial biogenesis like the control by PGC-1α and NRF-1[34-36]. However, Morino et al[30] did not observe any difference in such factors and suspected a confounding influence such as being overweight. Disruption of the nuclear gene Tfam in cells reproduced pathophysiological features of diabetes[37]. Moreover, maternally inherited alterations in mtDNA that disrupt mitochondrial function are known to cause an insulin-deficient form of diabetes resembling type 1 diabetes[38].
FETAL MITOCHONDRIAL PROGRAMMING
Intrauterine environment is a major contributor to the future of individuals and disturbance at a critical period of development may compromise their health. After the observation made by Hales et al[39] in 1991 that men with low birth weight had increased susceptibility to develop type 2 diabetes and cardio-vascular disease, the same association was found throughout the world. Therefore, the concept of “the thrifty phenotype hypothesis” suggested 19 years ago by Hales et al[40] is now accepted by the scientific community as being involved in several pathologies such as obesity, insulin resistance, diabetes, hypertension, cardiovascular disease and even cancer and precocious aging. The term “thrifty phenotype” suggests that in case of poor fetal nutrition, resulting from either poor maternal nutrition or poor delivery of nutrients to the fetus due to other causes such as placental dysfunction, an adaptive response is set up by the fetus to optimize the growth of key organs like the brain at the expense of other tissues such as muscles, kidneys and endocrine pancreas. It is also accompanied by programmed changes in metabolism, enabling the organisms to efficiently use and store nutrients. Such adaptations are beneficial for the survival of the fetus but may be detrimental later in life, namely when a mismatch occurs between the environment predicted and that one encountered after birth. Then, the concept evolved, introducing the notion of “developmental plasticity” and “predictive adaptive response”[41]. If the insufficient metabolic and nutritional environment is the same during fetal life and early after birth, the adaptation set up by the fetus will be efficient to cope with it but if not, the adaptations are not appropriate and further enhance the risk of developing metabolic diseases later in life.
It is only recently that attention was paid to the involvement of the mitochondria as putative targets for the fetal programming of adult disease. Indeed, it has been proposed that a key adaptation enabling a fetus to survive in a limited energy environment may be a programming of mitochondrial function[42].
The Simmons’ group was the first to show in rat that utero-placental insufficiency provoked by uterine artery ligation targeted the mitochondria because it induced a lower pyruvate oxidation in the muscle[43] and liver[44] of young adult offspring. In muscle, this defect leads to a chronic reduction of ATP available from oxidative phosphorylation, which compromises glucose transporter 4 (GLUT4) recruitment, glucose transport and glycogen synthesis, contributing to insulin resistance and hyperglycaemia of type 2 diabetes[43]. The concept of mitochondrial programming could be especially true for cells that have a high energy requirement, such as the β-cells. Indeed, uteroplacental insufficiency also induces mitochondrial dysfunction in fetal β-cells leading to increased production of ROS, reduced ATP production and decline in mitochondrial ETC complex I and III. In turn, this drives damage to mtDNA that may progressively deteriorate the mitochondrial and β-cell function and diabetes may ensue[42].
Although the model of placental insufficiency induced severe fetal growth restriction due to reduction of transfer of nutrients as well as of oxygen to the fetus, more subtle nutritional disturbances in the intrauterine environment have also been shown to program key organ during development. In a general population, nutritional imbalance in the presence of an adequate quantity of calories and oxygen is obviously less drastic but is probably more frequent and may have substantial consequence for the progeny.
For many years we have investigated several models of early malnutrition in rats to understand by which mechanism developmental programming could occur. Most of our research focused on the β-cell development in the fetus and newborn and we have evaluated long-term consequences in offspring of mother fed a low protein diet (LP). We pointed to an alteration at the level of the mitochondria but because insulin resistance, diabetes and obesity are burning throughout the world, we also investigated if a mitochondrial programming could be a common mechanism for several types of nutrient imbalance, including calorie restriction or HF.
If the quantity of calories is adequate during development but the proteins are low, the development of many organs is altered and the islet cell is specifically targeted as reported in several reviews[45] and in some articles of this book. Briefly, although the fetal growth of the offspring from dams fed a LP diet was only reduced by 5%-10%, the fetal β-cell mass was smaller. Such a reduction was demonstrated to be due to a low β-cell proliferation, a reduced islet vascularisation[46-49] and an increased susceptibility of the insulin secreting cell to be destroyed by apoptosis in response to aggressive molecules[50,51]. In addition, these fetal islets secreted less insulin in response to glucose and amino acids[52]. The lower insulin secretion was maintained in young adulthood[51,53]. Later in life, the LP offspring featured also an increased vulnerability to cytokines, ROS[51] and poor capacity to regenerate after streptozotocin destruction (unpublished data). On the basis of such pathological characteristics, we investigated by proteome and microarray analysis if a common pathway could be found and we demonstrated that the mitochondrion through its TCA cycle was the main target. Indeed, 11% of the altered genes founded in the LP fetal islets coded for mitochondrial protein and the expression of almost every gene involved in the TCA cycle was changed by the maternal LP diet[54,55].
Antioxidants defenses
We knew from the literature that the normal adult β-cells possess particularly weak antioxidant defenses activity compared to other organs such as the liver[56,57], but no data were available for fetal and neonatal pancreatic islets. With their first breath, newborns are directly exposed to an increase in oxygen concentration. A few hours later when lactation starts, they are also exposed to another type of nutrition, switching from a diet rich in glucose and amino acids in utero to a fatty diet during lactation. A microarray analysis performed on mtRNA from cord whole blood collected after human cesarean section revealed a higher expression of genes involved oxidative stress pathways such as superoxide dismutase (SOD), catalase, peroxiredoxins and UCP[58]. Thus, we investigated the islet antioxidant activity at birth and after weaning in normal rats. While SOD and catalase activity were much lower in islets than in the liver, we found an as efficient glutathione peroxidase activity (GPX) but that, however, decreased thereafter when compared to the liver, weakening the general antioxidant capacity in normal rats postnatally[59]. GPX removes H2O2 produced through the dismutation by SOD of the superoxide anion to O2. When the mother was fed the LP diet we found that the GPX activity was decreased in fetal islets[59]. Then, a temporary efficient GPX activity counterbalancing SOD activity that occurs in normal islets was not possible in LP fetal islets. This alteration may be one explanation for the increased susceptibility of these fetal islets to cytotoxic aggression. If a switch to a normal diet is given to the mother after birth, a reduction of islet antioxidant capacity was observed in the newborn. If the LP diet was maintained until weaning such lowering was not reported. This observation supports the concept of the detrimental effect of a mismatch between a suboptimal environment and a richer environment after birth[60].
We were the first to measure the oxidative stress (OS) and the antioxidant capacity in the islets of 3-month old adult offspring from LP mother. Nitrotyrosine levels were significantly higher in the plasma of offspring when the LP diet was present during fetal life or during fetal life and lactation[59]. Adult islets expressed higher iNOS levels and consequently secreted large amounts of NO[61]. The best way to verify the antioxidant potential of a cell is to measure the activity of the antioxidant enzymes. Maternal LP diet provoked an increased SOD activity in adult islets which should increase the level of H2O2, but no concomitant activation of catalase and GPX was observed. This imbalance could lead to higher hydrogen peroxide production that may concur to increased oxidative stress contributing to the alteration of the insulin secretion and the increased vulnerability of the β-cell later in life[51,59].When total SOD activity was measured, the analysis did not allow making a difference between the manganese superoxide dismutase (MnSOD) and the Cu/ZnSOD. An increased expression of Cu/ZnSOD gene but not of MnSOD was observed in the offspring that received a LP diet during gestation or during gestation and lactation. When the LP offspring was analyzed at 15 mo, the expression of both Cu/Zn and MnSOD genes was decreased in the islets[62].
Mitochondrial biogenesis and function
As mentioned above, the participation of mitochondria in the programming of β-cell dysfunction observed in offspring submitted to environmental disorders during early life was proposed recently[27]. Simmons et al[42] found that uteroplacental insufficiency during late gestation, which implies nutrient as well as oxygen depletion, induced OS and marked mitochondrial dysfunction in pancreatic islets of the intrauterine growth retardation (IUGR) progeny. Showing that mitochondrial dysfunction was not limited to pancreatic islets[43,44], they proposed that a key factor enabling a fetus to survive in a limited energy environment is a reprogramming of mitochondrial function, which can lead to deleterious effects.
In order to assess whether maternal malnutrition, without restriction of the oxygen supply, should lead to mitochondrial programming in islets, we analyzed parameters of mitochondrial biogenesis and function in adult offspring of dams fed either a protein restriction (LP), a high fat diet (HF) or exposed to a global food restriction (GFR) during gestation.
We found that, independently of the type of prenatal malnutrition, mitochondrial function was affected in pancreatic islets of the adult offspring[53,63]. Thus, maternal malnutrition itself caused mitochondrial dysfunction in pancreatic islets from 3-month old progeny that may predispose to glucose intolerance later in life, namely by affecting insulin secretion[64,65]. In vitro, male and female islets from control offspring increased their insulin secretion in response to glucose. This enhancement was less marked in LP offspring and absent in GFR and HF 3-month old animals. This could be associated with dysfunctions in energy metabolism, located for a large part in mitochondria because ATP production was blunted after glucose challenge in islets of male and female progeny from malnourished dams.
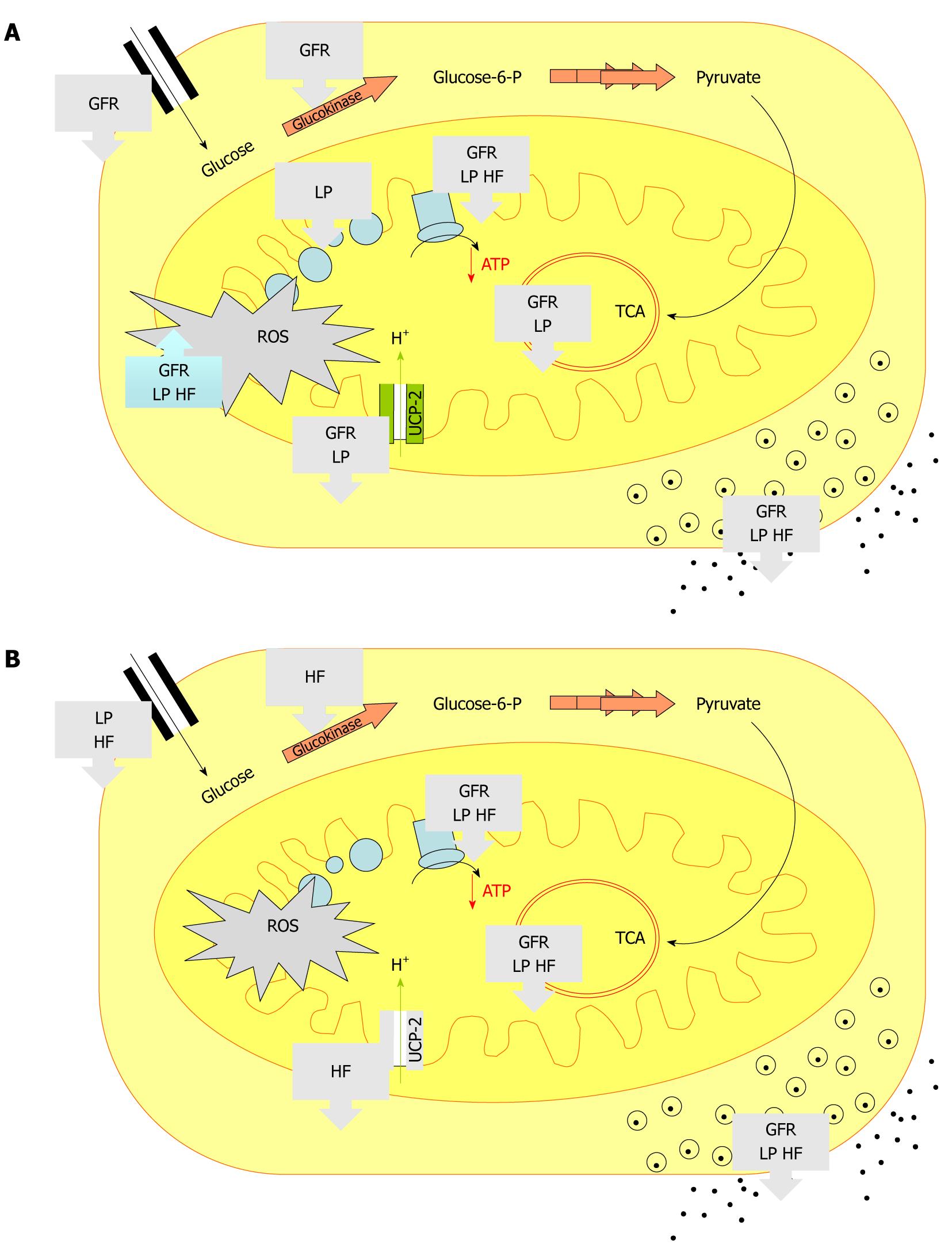
It is becoming obvious that the programming is a sex-specific phenomenon[53,63]. Although the common alteration cited above exists, some changes were specific to the maternal diet as well as to the sex of the progeny (Figure 2). For instance, in male progeny, the restriction of nutrients seemed to have more consequences since β-cell mass, as well as the expression of genes coding for proteins involved in energy metabolism and TCA cycle, were found altered to a greater extent in LP and GFR rats than in HF male animals (Figure 2A). Conversely, a maternal diet enriched with animal fat was more pernicious for females because HF females presented much more damage than LP and GFR females (Figure 2B). Also, independently of the type of early malnutrition, the pathway leading to blunted ATP production in malnourished offspring appeared differently in males and females. Indeed, increased basal production of ROS was found only in males of the 3 groups (Figure 2A). This latter long-term consequence of prenatal malnutrition could be a determinant for inducing sex-specific cellular and molecular effects since ROS are known to be able to inactivate the iron-sulfur centers of the ETC complexes and TCA cycle enzymes, resulting in shutdown of mitochondrial energy production[66]. It should be noted that higher ROS production in male islets from LP offspring was congruent with our previous observation showing the influence of early malnutrition on adult antioxidant potential[59]. Manifestation of progression of OS was also reported by others for IUGR male offspring of rats exposed to uteroplacental insufficiency[42]. In these rats, OS was linked to accumulation of mtDNA mutations in islets and blunted ATP production. Indeed, IUGR males presented a reduction by 50% of the activities of both complexes I and III at 7-week of age that dropped at 15-week to less than 25% of those of controls[42]. In female offspring that were exposed to low protein, low calorie or HF during prenatal life, we reported that the poor capacity of ATP biosynthesis directly involved a down regulation of crucial factors. Indeed, independently of the type of early malnutrition, each female group showed a reduction in the expression of both malate dehydrogenase and ATP6 which could decrease the mitochondrial energy production through the TCA cycle and the ETC (Figure 2B).
Figure 2 Summary of the main consequences of prenatal malnutrition on metabolic pathways in islets from low protein diet, global food restriction and high fat diet.
As shown in the text and extensively described in the related publications[53,63], the entrance of glucose was analyzed through GLUT-2 expression, the glycolysis through glucokinase expression, the TCA through citrate synthase and malate dehydrogenase expression. Reactive oxygen species production, ATP content and insulin secretion in response to glucose were also determined. The arrows indicated an increased or decreased level compared to control offspring. A: Males; B: Females; GLUT: Glucose transporter; TCA: Tricarboxylic acid cycle; GFR: Global food restriction; LP: Low protein diet; HF: High fat diet; ROS: Reactive oxygen; UCP: Uncoupling proteins species.
The effect of altered nutrient availability to the fetus on β-cell mitochondrial DNA is puzzling. While reduction of placental blood flow first provoked an increase in the number of mtDNA copies at fetal stage, this number decreased with age under the normal value[42]. We did not find any modification in LP progeny mtDNA but an increase in offspring of mothers 50% underfed or fed a HF during gestation.
Another consequence of early malnutrition which is sex specific was the over expression of PPARγ in islets from LP, GFR and HF males. This strong PPARγ expression might increase ROS production, via an enhanced lipid uptake in cells that are not metabolically adjusted to handle this challenge[67]. Moderate amounts of PPARγ are known to be expressed in normal pancreatic β-cell but its fundamental role in these cells is not fully understood[68]. PPARγ appears to be important for glucose homeostasis since PPARγ ligands reduced insulin levels by targeting the insulin gene transcription[69]. Improvement of mitochondrial biogenesis was also associated with enhanced PPARγ function in adipose tissue[9]. Emerging evidence suggests that PPARγ ligands, named thiazolidinediones, offer benefits for preventing or delaying the decline in β-cell function[70,71] through effects on lipid transport and metabolism, by modulating the expression of genes involved in glucose sensing[68,72] and by reducing ER stress[69]. Although activation of PPARγ results from ligand-dependent heterodimerization of PPARγ with RXR, over expression of PPARγ may induce by itself an increase in GSIS in the absence of exogenous PPARγ ligand[68]. These data could also help to explain that LP males maintained insulin release despite a blunted ATP biosynthesis after glucose challenge. However, we did not show the same correlation for GFR and HF, suggesting that the excessive level of over expression of PPARγ in LP rats could be determinant to GSIS. PPARγ has been reported to induce expression of UCP-2 in β-cells[73], as observed in LP male islets. Thus, as postulated above, the particularly high level of PPARγ expression could be a key factor inducing UCP-2 transcription in LP male islets.
Mitochondrial programming in other organs
Mitochondrial dysfunction is not limited to the pancreatic islets. In the liver, a marked resistance to insulin was observed in the young IUGR progeny prior to the occurrence of diabetes. Oxidation rates of pyruvate, glutamate and succinate were blunted in isolated hepatic mitochondria of very young IUGR offspring. Increased MnSOD protein expression as well as high levels of 4-hydroxynonenal was found already at fetal stage and maintained later in life[44]. We also reported a programming that was sex-specific, at the level of mitochondria in the liver of offspring of malnourished mother[53,63]. After a maternal LP diet, although mtDNA content was reduced in male liver, no expression of genes involved in mitochondrial biogenesis, function and metabolism was found altered while the female offspring presented a lower expression of citrate synthesis and malate dehydrogenase, suggesting that the ATP production could be affected[53]. The liver of GFR and HF males featured a higher expression of ND4L and COX-1, respectively subunits of complexes I and IV of the ETC encoded by the mtDNA and a reduced level of citrate synthase and malate dehydrogenase mRNA[63]. In the LP offspring, key enzymes that regulate glucose homeostasis were found altered in the young and adult progeny[74]. An increase in hepatic carbonyl concentration and an up-regulation of GPX were also observed in the LP adult progeny which may be indicative of higher oxidative stress[75].
In muscle, the reduced pyruvate oxidation provoked by uteroplacental insufficiency results in a chronic reduction in the supply of ATP available from oxidative phosphorylations, which compromises GLUT4 recruitment, glucose transport and glycogen synthesis, contributing to insulin resistance and hyperglycemia of type 2 diabetes[43].
Park et al[76] found that the offspring of dams fed a LP diet during pregnancy and weaning have a lower mtDNA content as well as mtDNA-encoded gene expression in the liver and skeletal muscle. They also reported lower mtDNA levels in the total pancreas[76] which was, however, not corroborated by us when only endocrine pancreas was analyzed[53].
Several reports documented that vascular structure and function can be programmed in early life. It was shown that maternal low protein diet impaired vascularization in the islets[46-49] as well as in the brain[77] and muscle[78]. The vascular change may be associated or not with hypertension later in life. A clear mitochondrial programming at the level of endothelial cell is not yet demonstrated. What is known is that growth restricted neonates exhibited endothelial dysfunction very early in life, predisposing them to atherosclerosis. Higher mitochondrial ROS generation and function are associated with cardio-vascular disease. In neonates with IUGR, increased lipid peroxidation was observed in association with low levels of antioxidants and antioxidant enzyme activity[79]. It is possible that excessive ROS production by placental mitochondria may be released in the fetal circulation and may alter vascular mtDNA[80]. Taylor et al[81] searched for mitochondrial abnormalities in the aorta of adult offspring from a mother fed a HF during gestation and lactation and revealed a lower expression of the mitochondrial genome. Four genes of the mitochondrial encoded mRNA were down regulated among which was ATPase-6 and six genes of the nuclear mRNA encoding mitochondrial proteins were under expressed, among which was MnSOD.