Published online Aug 15, 2011. doi: 10.4239/wjd.v2.i8.127
Revised: August 11, 2011
Accepted: August 14, 2011
Published online: August 15, 2011
Obesity is increasing around the globe. While adult lifestyle factors undoubtedly contribute to the incidence of obesity and its attendant disorders, mounting evidence suggests that programming of obesity may occur following under- and over-nutrition during development. As hypothalamic control of appetite and energy expenditure is set early in life and can be perturbed by certain exposures such as undernutrition and altered metabolic and hormonal signals, in utero exposure to altered maternal nutrition and inadequate nutrition during early postnatal life may contribute to programming of obesity in offspring. Data from animal studies indicate both intrauterine and postnatal environments are critical determinants of the development of pathways regulating energy homeostasis. This review summarizes recent evidence of the impact of maternal nutrition as well as postnatal nutrition of the offspring on subsequent obesity and disease risk of the offspring. While much of the experimental work reviewed here was conducted in the rodent, these observations provide useful insights into avenues for future research into developing preventive measures to curb the obesity epidemic.
- Citation: Velkoska E, Morris MJ. Mechanisms behind early life nutrition and adult disease outcome. World J Diabetes 2011; 2(8): 127-132
- URL: https://www.wjgnet.com/1948-9358/full/v2/i8/127.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i8.127
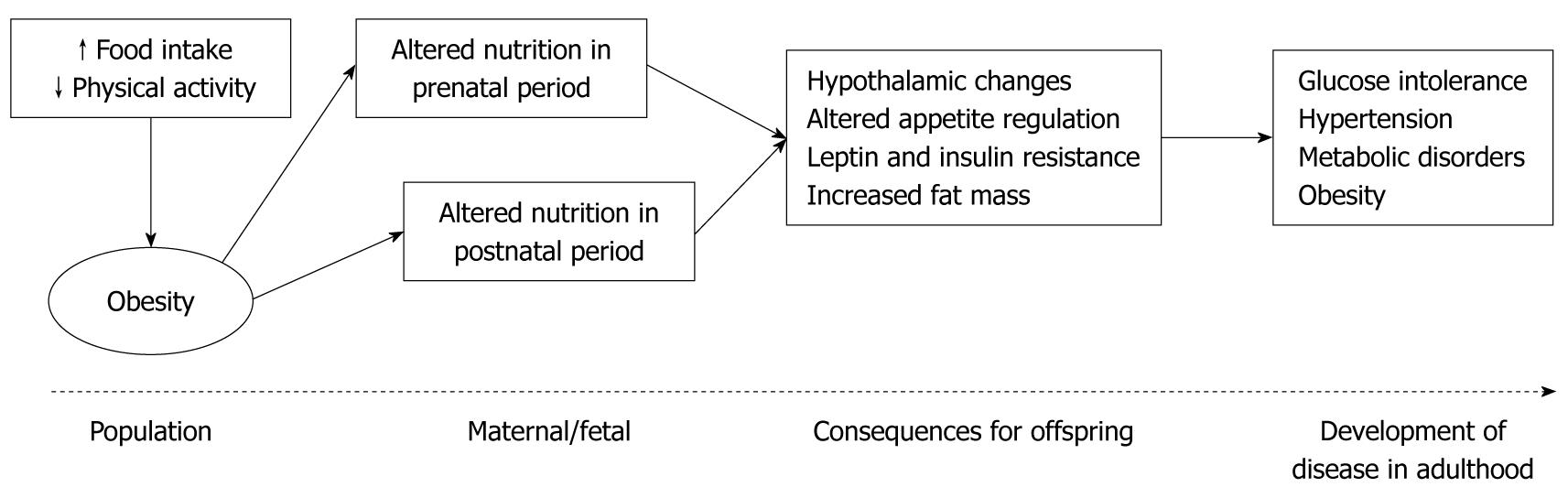
Obesity is a common disorder and an important risk factor for many chronic diseases. As the second biggest cause of mortality after smoking, obesity-associated complications account for 10% of health-care costs in most countries[1]. The prevalence of obesity, particularly childhood obesity, is rising worldwide. The reasons behind this epidemic are not clearly understood, however this metabolic disease can result from a complex interaction between many factors including genetic, physiological, behavioural and environmental influences. The rate at which this disease has increased suggests that environmental and behavioural factors such as increased consumption of high-fat and high-energy foods, coupled with reduced physical activity play a greater role than genetic causes[2-4]. It is therefore particularly relevant that recent epidemiological and animal studies have suggested that long-term health can be influenced by events in fetal and early infant phases of life. Nutritional status during “critical windows” in early development is thought to influence or “program”, the onset of major diseases in adulthood[5] (Figure 1). The mechanisms underlying this development of obesity and associated disease in adulthood are not yet completely understood. This review will discuss the effects of nutritional imbalances in utero and in early postnatal life as well as the mechanisms that may contribute to the development of adult disease.
One of the first studies to highlight the fetal origins of disease was a population study in Hertfordshire demonstrating a link between low birth weight and weight at 1 year of age and increased death rate from ischemic heart disease, impaired glucose tolerance and type 2 diabetes[6,7]. This led to the “Thrifty Phenotype” hypothesis, where poor nutrition in utero led to fetal adaptations that produced permanent changes in insulin and glucose metabolism, increasing the risk of developing the metabolic syndrome in adulthood[8]. A more recent hypothesis is the “Predictive Adaptive Response” hypothesis[9], which proposes that the fetus makes adaptations in utero or during the early postnatal developmental period based on the predicted postnatal environment. When the predictive adaptive response is appropriate the phenotype is normal, however when the predicted and actual environments do not match, disease manifests[9]. Epidemiological data indicate that maternal obesity is linked to offspring obesity, and a child’s body mass index (BMI) correlates with that of the mother[10]. Thus both undernutrition, and maternal obesity have been shown to increase the risk of obesity in offspring. In support of these hypotheses, a large number of studies have been carried out, where maternal nutrition has been altered during gestation and early postnatal life.
In determining the mechanisms involved in metabolic programming, the use of animal models has been paramount. A benefit of using non-human species is the capacity to rigorously control diet and other relevant environmental factors that impact on obesity. In altricial species such as rat the lactation period correlates with the third trimester of human gestation. Initial animal experiments examining early life programming influences on subsequent obesity risk dealt with the impact of undernutrition during gestatsaion, utilizing restricted feeding, uterine ligation or protein deprivation of the mother. The effects of maternal undernutrition, low protein diets and nutritional excess have diverse effects on the offspring, as recently reviewed[11-13].
Maternal protein restriction during gestation has previously been shown to result in low birth weight of the off spring and impaired development of organs such as the pancreas and kidney[14] leading to impaired glucose tolerance and insulin resistance in peripheral tissues. There is also strong evidence that these animals will develop obesity later on in life[15]. In a rat model of total caloric restriction during pregnancy, offspring are hyperphagic, hyperinsulinemic and develop obesity and hypertension[16]. Other models of early growth restriction have produced similar findings, together with an amplification of the metabolic disturbances when a highly-palatable or high-fat diet is introduced postnatally[16-18].
Rodent models of maternal overnutrition usually involve the feeding of a high fat diet to pregnant dams, resulting in the development of a phenotype comparable to that of the human metabolic syndrome[11]. Offspring have altered neuron development[19,20], increased adiposity and blood pressure, impaired cardiovascular function[21], and become hyperinsulinemic and hyperglycemic in adulthood[22]. More recent studies have shown that offspring from obesity-prone rats developed adiposity and impaired glucose and lipid metabolism as early as postnatal day 20[23,24] and this was maintained until adulthood[25]. Furthermore, maternal high fat diet during the preconceptional period and/or throughout pregnancy and lactation has also been shown to result in a similar obesity phenotype in the offspring independent of postnatal nutrition[26]. These recent studies highlight the profound impact that dietary interventions during pregnancy could have on the long-term health of the offspring.
Maternal diet during the suckling period is also important as several regulatory mechanisms not fully developed at birth undergo significant maturation in the early postnatal period. This is more marked in rodents, as they undergo rapid maturation of most organ systems after birth. The important influence of the suckling period is supported by rodent studies where reducing rat litter sizes to 3-4 pups from 10-12 pups per dam, increases milk availability resulting in offspring with dyslipidemia, hyperinsulinemia, hyperleptinemia, increased body weight and fat pad mass[27-34]. This model was designed by McCance who demonstrated that the adjustment of rat pup litter size during lactation changes the milk intake of the pups and this resulted in a lifetime of programming of the growth trajectory[35]. A subsequent study added to these initial observations suggesting that the amount of food consumed in early life plays an important role in determining the pattern of food intake in later life[36]. To further support the importance of the postnatal period, a recent epidemiological study demonstrated that rapid weight gain in neonatal life is associated with increased risk of obesity in later life, independent of birth weight and weight at 1 year of age[37]. Thus, rapid weight gain mainly results from neonatal overfeeding, highlighting the importance of this period of development in programming of adult disease. Although a recent study demonstrated that maternal obesity exerted a stronger detrimental impact on the offspring phenotype compared to overnutrition during the early postnatal period, pre- and postnatal nutritional excess were shown to interact with each other to exert additive detrimental effects on programming of central appetite regulators and glucose and lipid metabolism[24,38].
There are a growing number of signals and pathways that have been shown to be involved in energy homeostasis, some of which are listed in Table 1. Alteration of one or more relevant pathways during early development plays a major role in the programming of obesity and associated adulthood diseases. These mechanistic pathways can be located both centrally and peripherally.
| Orexigenic | Anorexigenic |
| Peripheral | |
| Adipose tissue: | Adipose tissue: |
| Adipsin | Leptin |
| Glucocortiocoids | Adiponectin |
| Angiotensin II | Resistin |
| Tumour necrosis factor α | |
| Stomach: | Gut: |
| Ghrelin | Cholecystokinin |
| Peptide YY | |
| Obestatin | |
| Pancreas: | |
| Insulin | |
| Amylin | |
| Pancreatic polypeptide | |
| Central | |
| Neuropeptide Y | α-melanocyte stimulating hormone |
| Agouti related peptide | Cocaine and amphetamine regulated transcript |
| Melanin concentrating hormone | Corticotrophin releasing hormone |
| Orexin A and B | Urocortin |
| Galanin | Serotonin |
| Noradrenaline | Dopamine |
| Cannabinoid |
Alteration in the environment during a “critical period” of development may alter the normal development of the neuronal circulatory regulating food intake. Recent evidence shows that there are physiological differences in the regulation of energy balance between adults and neonates. Although much is known about the neurocircuitry in adults, the development of important appetite regulating systems such as the neuropeptide Y (NPY) and melanocortin systems remains unclear.
The ontogeny of the NPY system has been extensively studied by Grove and associates. Initial studies demonstrated that NPY was not only abundantly expressed in the arcuate nucleus (ARC) but transient expression of NPY was also observed in the other hypothalamic regions, including the dorsomedial hypothalamus, paraventricular nucleus, lateral hypothalamus and the perifornical region which is not evident in adulthood[39]. NPY levels in all areas were low at postnatal day 2 (P2), increased rapidly to peak at P15-16 and returned to levels observed in adulthood in the ARC, while in the other areas NPY was no longer apparent after P30[39].
The development of important neuronal circuits regulating appetite occurs late in gestation and continues postnatally in rodents, suggesting that the normal development of this system may be susceptible to environmental and nutritional changes after birth. A series of early studies demonstrated that the amount of food consumed during suckling in the rat plays an important role in determining food intake later in life[36]. This may contribute to long-term development of Syndrome X-like alterations, such as insulin resistance, obesity and increased blood pressure[30]. Recent data extends these observations, demonstrating that maternal consumption of “junk” food during gestation and lactation led to increased preference for “junk” food in offspring as they matured[40].
Leptin and insulin signaling appears to be important for the development of the appetite regulating system. In the rodent during the first 3 wk of life, leptin is unable to alter feeding or energy expenditure[41]. During the neonatal period a surge of leptin is evident, which does not correlate with body fat[42]. In rodents, fetal adipocytes and placenta produce very low levels of leptin late in gestation[43], so the main source for this surge of leptin may be the transplacental transfer of maternal leptin to the fetus[44]. This neonatal hyperleptinemia however, is not able to affect growth, food intake or energy expenditure in mice and rats as the neuronal circuits are still not developed[32,45]. Recently it has been suggested that this leptin surge is actually an important signal for the initiation of the development of ARC projections in the rodent[46]. The main evidence for this is the incomplete development of ARC projections in ob/ob and db/db mice that do not have a functioning leptin system[47]. On the other hand, exogenous leptin treatment during the early postnatal period in rodents can also cause abnormal expression of NPY, agouti-related peptide and pro-opiomelanocortin in the ARC[45], however the effect on the projections is unknown. Hyperleptinemia caused by overfeeding during this period can also cause abnormalities in hypothalamic circuits[48,49]. Collectively these findings suggest that a certain level of leptin is required during the “critical period” of development and both deficiency and excess can have long-term detrimental effects on the hypothalamic circuitry that regulates energy homeostasis.
Insulin receptors are also highly expressed in the fetal brain of rodents and humans, with expression declining during the postnatal period[50]. Insulin treatment during the postnatal period results in increased body weight, chronic hyperinsulinemia and increased blood pressure that persists into adulthood[51], suggesting abnormal insulin levels during a “critical period” of development may cause long-term defects in the regulation of energy homeostasis[49]. Insulin may also be an important trophic factor, however more studies are needed to determine its role in the development of the feeding circuits.
Adipose tissue development can also be affected during the fetal and postnatal periods. Development of adipose tissue commences in utero, where adipocytes have the ability to develop into either brown or white adipose tissue (WAT)[52]. The main function of brown adipose tissue (BAT) is to convert energy into heat[53], whereas the WAT represents an endogenous energy store that is capable of secreting a number of mediators involved in the regulation of energy metabolism, neuroendocrine function and immune function[54].
BAT is present in rodents throughout life but until recently it was thought that BAT in humans was only present in early life and did not have any important function in adults[53]. The presence of BAT in rodents has been shown to be important in body weight and energy regulation as well as glucose metabolism[53,55] while active BAT in adult humans has been demonstrated following cold exposure[56]. A recent study was able to demonstrate a functioning BAT in adult humans, particularly females, using combined positron-emission tomography and computed tomography scanning[57]. Furthermore, the same study demonstrated an inverse correlation between the amount of BAT and BMI[57]. The ability to measure and locate the mass and activity of BAT will help to better understand the physiological role of BAT in adult humans and its potential as a therapeutic target in the management of obesity[57].
Over the last decade WAT has become recognized as an important endocrine organ able to secrete a vast number of hormones as well as expressing numerous receptors that allow it to respond to traditional hormone systems as well as signals from the central nervous system[54,58]. The wide range of protein signals and factors that have been identified in WAT highlights the complexity of this system which is highly integrated into the general homeostatic mechanisms of mammals[59]. WAT development is characterized by a rapid increase in fat cell number until 4 wk of age, followed by slower rate of growth until puberty, whereas increase in adipose tissue mass during maturity is mainly due to increased adipocyte size[60]. The increase in fat mass during early life appears to be dependent on an increase in local glucocorticoid action during the postnatal period[61]. The ability of glucocorticoids to promote lipogenesis and decrease lipolysis[62], highlights their role as important mediators in the development of central obesity, which can contribute to hypertension and glucose intolerance[63]. Corticosterone production driven by the enzyme 11β-HSD1 could play a pivotal role in the growth and development of the adipose tissue[64]. Further evidence of programming of the adipose tissue was recently provided by a study, which showed that increased maternal nutrition in the sheep led to upregulation of peroxisome proliferator activated receptor γ, lipoprotein lipase and leptin in fetal tissue, thereby predisposing the offspring to enhanced adipose accumulation[65].
Evidence from both epidemiological and animal studies suggests that the programming of obesity and adulthood disease arises from multifactorial influences occurring early in life. Adipocyte development, leptin, insulin and glucocorticoid signaling, as well as the plasticity of the hypothalamus all play a major role in the programming of appetite and metabolism, possibly leading to development of associated diseases. While the intrauterine environment and early postnatal window are critical determinants, recent data from our laboratory highlighting the detrimental impact of paternal high fat diet-induced obesity on offspring glucose tolerance and pancreatic β cell function, highlights the possibility that unhealthy paternal diets can reprogram gene expression in offspring, implicating epigenetics in these trans-generational effects[66].
Peer reviewer: Siamak Bidel, MD, PhD, Department of Public Health, University of Helsinki, PO Box 41 (Mannerheimintie 172, 6 krs.), Helsinki FIN-00014, Finland
| 1. | Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16:2323-2330. [PubMed] |
| 2. | Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55-92. [PubMed] |
| 3. | Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1-5. [PubMed] |
| 4. | Jeffery RW, Harnack LJ. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes. 2007;56:2673-2676. [PubMed] |
| 5. | McMillen IC, Adam CL, Mühlhäusler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9-17. [PubMed] |
| 6. | Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577-580. [PubMed] |
| 7. | Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019-1022. [PubMed] |
| 8. | Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20. [PubMed] |
| 9. | Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183-187. [PubMed] |
| 10. | Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323:1331-1335. [PubMed] |
| 11. | Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3-8. [PubMed] |
| 12. | Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103-121. [PubMed] |
| 13. | Buckley AJ, Jaquiery AL, Harding JE. Nutritional programming of adult disease. Cell Tissue Res. 2005;322:73-79. [PubMed] |
| 14. | Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591-603. [PubMed] |
| 15. | Ozanne SE, Lewis R, Jennings BJ, Hales CN. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci (Lond). 2004;106:141-145. [PubMed] |
| 16. | Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83-E87. [PubMed] |
| 17. | Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411-412. [PubMed] |
| 18. | Morris MJ. Early life influences on obesity risk: maternal overnutrition and programming of obesity. Expert Rev Endocrinol Metab. 2009;4:625-637. |
| 19. | Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond). 2009;33:115-122. [PubMed] |
| 20. | Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One. 2009;4:e5870. [PubMed] |
| 21. | Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127-R133. [PubMed] |
| 22. | Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134-R139. [PubMed] |
| 23. | Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a 'junk food' diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586:3219-3230. [PubMed] |
| 24. | Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348-5356. [PubMed] |
| 25. | White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464-R1472. [PubMed] |
| 26. | Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905-915. [PubMed] |
| 27. | Bassett DR, Craig BW. Influence of early nutrition on growth and adipose tissue characteristics in male and female rats. J Appl Physiol. 1988;64:1249-1256. [PubMed] |
| 28. | Faust IM, Johnson PR, Hirsch J. Long-term effects of early nutritional experience on the development of obesity in the rat. J Nutr. 1980;110:2027-2034. [PubMed] |
| 29. | Hahn P. Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents. J Nutr. 1984;114:1231-1234. [PubMed] |
| 30. | Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dörner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146-155. [PubMed] |
| 31. | Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dörner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541-546. [PubMed] |
| 32. | Schmidt I, Fritz A, Schölch C, Schneider D, Simon E, Plagemann A. The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disord. 2001;25:1168-1174. [PubMed] |
| 33. | Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ. Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. J Nutr. 2008;138:1622-1627. [PubMed] |
| 34. | Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab. 2005;288:E1236-E1243. [PubMed] |
| 36. | Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol. 1978;235:R141-R144. [PubMed] |
| 37. | Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194-199. [PubMed] |
| 38. | Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One. 2009;4:e6259. [PubMed] |
| 39. | Singer LK, Kuper J, Brogan RS, Smith MS, Grove KL. Novel expression of hypothalamic neuropeptide Y during postnatal development in the rat. Neuroreport. 2000;11:1075-1080. [PubMed] |
| 40. | Bayol SA, Farrington SJ, Stickland NC. A maternal 'junk food' diet in pregnancy and lactation promotes an exacerbated taste for 'junk food' and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843-851. [PubMed] |
| 41. | Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277:R742-R747. [PubMed] |
| 42. | Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020-1027. [PubMed] |
| 43. | Kawai M, Yamaguchi M, Murakami T, Shima K, Murata Y, Kishi K. The placenta is not the main source of leptin production in pregnant rat: gestational profile of leptin in plasma and adipose tissues. Biochem Biophys Res Commun. 1997;240:798-802. [PubMed] |
| 44. | Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024-3030. [PubMed] |
| 45. | Proulx K, Richard D, Walker CD. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology. 2002;143:4683-4692. [PubMed] |
| 46. | Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621-2626. [PubMed] |
| 47. | Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108-110. [PubMed] |
| 48. | Davidowa H, Plagemann A. Different responses of ventromedial hypothalamic neurons to leptin in normal and early postnatally overfed rats. Neurosci Lett. 2000;293:21-24. [PubMed] |
| 49. | Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47-63. [PubMed] |
| 50. | Marks JL, Eastman CJ. Ontogeny of insulin binding in different regions of the rat brain. Dev Neurosci. 1990;12:349-358. [PubMed] |
| 51. | Harder T, Plagemann A, Rohde W, Dörner G. Syndrome X-like alterations in adult female rats due to neonatal insulin treatment. Metabolism. 1998;47:855-862. [PubMed] |
| 53. | Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277-359. [PubMed] |
| 54. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [PubMed] |
| 55. | Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med. 1997;48:307-316. [PubMed] |
| 56. | Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444-E452. [PubMed] |
| 57. | Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509-1517. [PubMed] |
| 58. | Frühbeck G, Gómez-Ambrosi J. Control of body weight: a physiologic and transgenic perspective. Diabetologia. 2003;46:143-172. [PubMed] |
| 59. | Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827-E847. [PubMed] |
| 60. | Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res. 1974;15:474-483. [PubMed] |
| 61. | Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T. Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1407-R1415. [PubMed] |
| 63. | Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab (Lond). 2005;2:3. [PubMed] |
| 64. | Wake DJ, Walker BR. 11 beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol Cell Endocrinol. 2004;215:45-54. [PubMed] |
| 65. | Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-gamma, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007;148:878-885. [PubMed] |