Published online Jul 15, 2011. doi: 10.4239/wjd.v2.i7.108
Revised: June 21, 2011
Accepted: June 28, 2011
Published online: July 15, 2011
The association between diabetes and hyperglycemia and the associated increased risk of several solid and hematologic malignancies has been the subject of investigation for many years. Although the association is not fully understood, current knowledge clearly indicates that diabetes may influence malignant cell transformation by several mechanisms, including hyperinsulinemia, hyperglycemia and chronic inflammation. In this context, the receptor for advanced glycation end-products (RAGE) has emerged as a focal point in its contribution to malignant transformation and tumor growth. We highlight how RAGE, once activated, as it manifests itself in conditions such as diabetes or hyperglycemia, is able to continuously bring about an inflammatory milieu, thus supporting the contribution of chronic inflammation to the development of malignancies.
- Citation: Rojas A, González I, Morales E, Pérez-Castro R, Romero J, Figueroa H. Diabetes and cancer: Looking at the multiligand/RAGE axis. World J Diabetes 2011; 2(7): 108-113
- URL: https://www.wjgnet.com/1948-9358/full/v2/i7/108.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i7.108
The association between diabetes and hyperglycemia and cancer, has been investigated extensively. Most studies, but not all, have found that both conditions are associated with an increased risk of several solid and hematologic malignancies. Currently, more than 250 million people live with diabetes; hence any impact derived even in smaller increases in the risk of cancer may have important consequences at world population level, and on associated costs to healthcare systems worldwide[1]. Although this association has been consistently reported for the most common cancer, more research efforts are needed, particularly in connection with the less common cancers, where data are limited or absent[2].
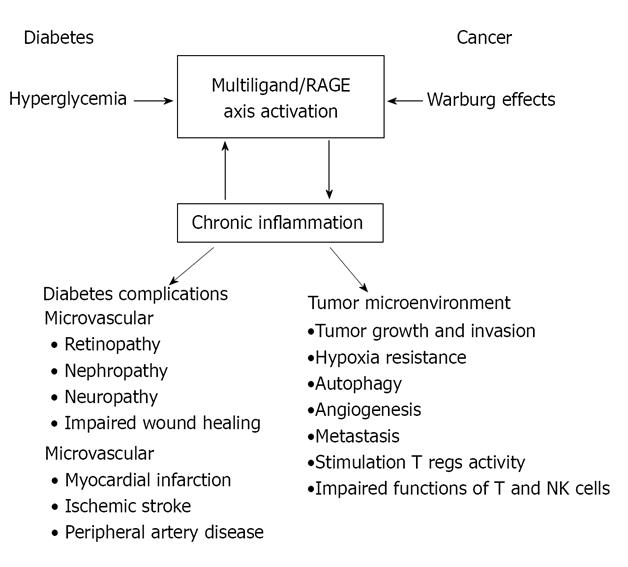
From the biological point of view, an essential question is raised when the association is analyzed: What are the mechanistic links between diabetes and cancer risk? Obviously, the answer to this question is not easy to find. However, and based on current knowledge, diabetes may influence malignant cell transformation by several mechanisms, including hyperinsulinemia, hyperglycemia and chronic inflammation. These three mechanisms are closely related to the receptor for advanced glycation end-products (RAGE), which may represent a focal point in their respective contributions to malignant transformation.
In 1927, Otto Warburg and co-workers reported the increased uptake of glucose and production of lactate by tumors. At present, resurgent research interest in the Warburg effect, as it is now known, have brought about a growing body of evidence supporting the dependence of many tumors on glycolysis for energy production. One consequence of the rise of glycolysis is the non-enzymatic glycation of proteins, leading to the formation of advanced glycation end-products (AGEs)[3,4]. AGEs were the first identified RAGE ligands, particularly N-carboxymethyllysine [CML]-modified proteins[5] .
The formation of AGEs is based on the non-enzymatic reaction of the reactive aldehyde moiety of glucose with the amino groups of proteins, forming slowly reversible Amadori products. Rearrangement reactions then occur to produce a chemically related group of moieties, termed AGEs, which remain irreversibly bound to proteins[6].
The major AGEs in vivo appear to be formed from highly reactive intermediate carbonyl groups, known as α-dicarbonyls or oxoaldehydes, including 3-deoxyglucosone, glyoxal, and methylglyoxal[7,8].
There is considerable evidence linking hyperglycemia with the accelerated formation of irreversible AGEs, which subsequently accumulate in different tissue locations[9,10,11]. Of note, the presence of AGEs has been detected in human cancer tissues, and their expression is markedly varied between different types of tumors[12].
It has been demonstrated by different authors that the circulating level of AGEs is associated with insulin resistance even in non-obese, non-diabetic subjects, independent of adiponectin levels[13,14,15].
How AGEs can impact insulin actions has been recently reviewed by Schalkwijk and co-workers[16]. Experimental data, obtained from both animal and isolated muscle and adipose tissue, suggest that glycation of insulin significantly impairs its biological activity[17].
It is also known that the increase of endogenous methylglyoxal accumulation impairs the insulin-signaling pathway and decreases insulin-stimulated glucose uptake in adipose tissue, which, in turn, may contribute to the development of insulin resistance[18,19].
Reduced intake of dietary AGEs has been shown to decrease the incidence of type 1 diabetes in non-obese diabetic mice[20], as well as the formation of atherosclerotic lesions in diabetic apolipoprotein E-deficient mice[21]. Vlassara and co-workers[22] have also shown that reduced AGE intake leads to lower levels of circulating AGEs and to improved insulin sensitivity in db/db mice. Furthermore, AGEs are reported to impair insulin action in muscle tissue by the formation of a multi-molecular complex, including RAGE/IRS-1/Src and PKCα[23].
The receptor for advanced glycation end-products (RAGE) is a member of the immunoglobulin protein family of cell surface molecules[24] and shares structural homology with other immunoglobulin-like receptors. Firstly described in 1992, RAGE has attracted increasing attention, due to its diverse ligand repertoire and its involvement in several pathophysiological processes associated with inflammation, such as diabetes, cancer, renal and heart failure, as well as neurodegenerative diseases[25,26].
The RAGE gene is localized on chromosome 6 in the vicinity of the MHC class III complex region in humans and mice, and in close proximity to the homeobox gene HOX12 and the human counterpart of the mouse mammary tumor gene int-3[27,28].
RAGE is highly expressed during development, especially in the brain, but its expression level decreases in adult tissues. However, RAGE expression is also markedly augmented by increased levels of ligands, as observed in some pathologic states[29]. The mature 382 amino-acid long RAGE is composed of an extracellular domain (85 aa), a single transmembrane spanning helix (27 aa) and a short cytosolic region (41 aa)[30]. The extracellular domain of RAGE contains one variable, like V-domain, and two constants, like C type domains, which are frequently referred to as C1 and C2 domains. Recent studies suggest that RAGE forms oligomers at the cell surface[31]. RAGE possesses two N-glycosylation sites, one adjacent to the V-domain and the second one located within the V-domain[32].
Recently, RAGE splice variants have been classified and renamed according to the Human Gene Nomenclature Committee[33], and many of them appear to be more abundant under various pathological conditions. At DNA level, the RAGE gene consists of 11 introns/exons that can alternatively be spliced into different variants. In terms of prevalence, the three major isoforms appear to be the full-length RAGE, a secreted form RAGE_v1 (previously named as sRAGE, secretory C-truncated RAGE, esRAGE, hRAGEsec or sRAGE1/2/3) and a N-terminally truncated isoform RAGE_v2 (previously named Nt-RAGE, N-RAGE or N-truncated RAGE). It is important to point out that RAGE_v1 is released into the extracellular compartment, where it can interact with free RAGE ligands, then working as a “decoy receptor”, thereby preventing ligands from interacting with cell surface RAGE[34].
In addition to AGEs, other molecules have been identified as RAGE ligands, as has been demonstrated for S100/calgranulins; high-mobility group box 1 (HMGB1) have also been identified as ligands of this promiscuous receptor. The S100/calgranulin protein family comprises several members of non-ubiquitous Ca-binding proteins of the EF-hand type that have both intracellular and extracellular functions. At intracellular level, S100 proteins are responsible for different roles in the cell cycle, cell differentiation and cell motility. However, some members of the family have additional relevant extracellular roles, particularly at sites of chronic inflammation, where they are able to activate, via RAGE, endothelial cells, macrophages and peripheral blood mononuclear cells, including T lymphocytes[35].
The DNA binding protein HMGB1 stabilizes nucleosome function, and acts as a transcription factor that regulates the expression of several genes[36]. HMGB1 belongs to the so-called “damage associated molecular pattern molecules” or alarmins, which are released in response to infection or inflammatory stimuli, especially during tissue damage[37].
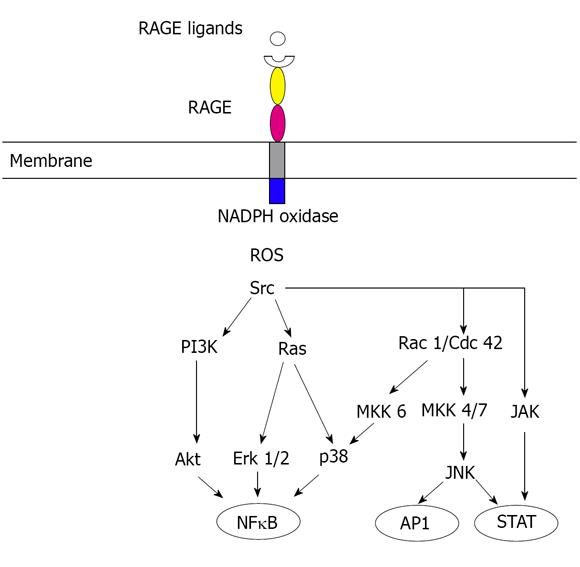
Although glucose may be the triggering stimulus to draw RAGE into diabetes pathology, consequent cellular stress results in the release of pro-inflammatory RAGE ligands S100/calgranulins and HMGB1. Thus, RAGE engagement in diabetic tissue produces a vicious cycle of ligand-RAGE perturbation, leading not only to chronic tissue injury, but also suppression of repair mechanisms[38]. RAGE engagement activates multiple signaling pathways (Figure 1), including reactive oxygen species, p21ras, erk1/2 (p44/p42) mitogen-activated protein kinases, p38 and SAPK/JNK mitogen-activated protein kinases, rhoGTPases, phosphoinositol-3 kinase and JAK/STAT pathway, with important downstream inflammatory consequences, such as the activation of nuclear factor-kappaB (NFκB), AP-1 and STATs, which are involved in the inflammatory process seen in both diabetes and cancer.
In the nineteenth century, Rudolph Virchow first launched the idea about a putative connection between inflammation and cancer. At present, resurgent research interest in this topic has raised a growing body of evidence supporting the contribution of chronic inflammation to the development of malignancies, as well as an association between the usage of non-steroidal anti-inflammatory agents, and protection against various tumor types[39,40,41,42].
For many years, the relationship between the expression of the receptor of advanced glycation end-products (RAGE) and cancer has been well-documented, as reported in gastric, prostate, lung, pancreas, and liver malignancies. However, the contribution of RAGE to cancer biology seems to be much more functional than initially thought, because it has now emerged as a relevant element that can continuously fuel an inflammatory milieu at the tumor microenvironment[43].
Most of the cancer-promoting effects of RAGE ligands are the result of their interaction with RAGE. Signals downstream of RAGE, drive the strength and maintenance of an inflammatory reaction during tumor promotion in a mouse model of skin cancer, as well as a marked reduction in the number of infiltrating immune cells and the levels of proinflammatory mediators in RAGE-/- animals[44]. In addition, the interaction of the ligands S100A8/A9 with RAGE involve carboxylated glycans; the transition from acute to chronic inflammatory conditions in the study cited did not occur in RAGE-/ - mice, which in turn, produced fewer tumors in a colitis-associated cancer model[45].
The consequences of RAGE activation to tumor biology also reach key processes, such as the acquisition of an hypoxia–resistant phenotype in carcinoma cells[46]. Recently, it has been reported that S100A8/A9 proteins contribute to the recruitment and retention of myeloid suppressor cells through a mechanism mediated, at least in part, by the binding to carboxylated N-glycans expressed on the receptor for advanced glycation end-products, and the subsequent activation of the NFκB signaling pathway[47]. AGEs can also down-regulate in vitro the ability of dendritic cells (DCs) to express co-stimulatory signals and to activate T cells[48]. Similar results have been described after a blockade of the autocrine secretion of HMGB1, and of RAGE activation[49,50].
In recent years, a growing body of evidence supports the role of ligands/RAGE axis in angiogenesis. Upon RAGE engagement, profound effects are reported in endothelial cells, including up-regulation of VEGF and metalloproteinase-2, as well as the disruption of the VE-cadherine/catenins complex, thus favoring capillary tube formation[51,52]. Additionally, RAGE activation also increases endothelial permeability to macromolecules, a condition very common in tumor microvasculature[53].
Although many aspects of differentiation, mobilization and recruitment of endothelial progenitor cells (EPCs) remain controversial, it has been reported that the levels of peripheral blood EPCs have been shown to be increased in certain malignant states[54].
HMGB1 increased EPCs adhesion to the immobilized integrin ligands intercellular adhesion molecule-1 and fibronectin in a RAGE-dependent manner, thus stimulating EPC homing to ischemic tissues[55].
In 2000, a seminal report on the contribution of multiligand/RAGE axis on invasion and metastasis demonstrated that a blockade of RAGE-HGMB1-derived signaling decreased growth and metastases of both implanted tumors, and tumors developing spontaneously in susceptible mice[56].
During the last decade, relevant advances in our understanding of the pathophysiologic role of the multiligand/RAGE axis have lead to a substantial knowledge of how this promiscuous receptor, once activated, is able to continuously bring about an inflammatory milieu (Figure 2). The current relevance of Virchow´s postulate about the role of chronic inflammation in cancer development highlights the facts associated with the presence of an activated RAGE axis, smoldering inflammation such as that occurring in diabetes, and thus its contribution towards the understanding of the mechanistic scenario supporting the link between diabetes and cancer.
Peer reviewers: Chifeng Liu, Associate Professor, National Taipei University of Nursing and Sciences, NO.365, Ming Te Road, Peitou, Taipei 11211, Taiwan, China; Hong Ding, Assistant Professor, Weill Cornell Medical College in Qatar, PO Box 24144, Education city, Doha 24144, Qatar
S- Editor Zhang HN L- Editor Herholdt A E- Editor Zhang L
| 1. | van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103-1123. [PubMed] [DOI] [Full Text] |
| 3. | Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11785] [Article Influence: 736.6] [Reference Citation Analysis (0)] |
| 5. | Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740-31749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 694] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6145] [Cited by in RCA: 6194] [Article Influence: 258.1] [Reference Citation Analysis (0)] |
| 8. | Kim W, Hudson BI, Moser B, Guo J, Rong LL, Lu Y, Qu W, Lalla E, Lerner S, Chen Y. Receptor for advanced glycation end products and its ligands: a journey from the complications of diabetes to its pathogenesis. Ann N Y Acad Sci. 2005;1043:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Méndez JD, Xie J, Aguilar-Hernández M, Méndez-Valenzuela V. Trends in advanced glycation end products research in diabetes mellitus and its complications. Mol Cell Biochem. 2010;341:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1761] [Cited by in RCA: 1790] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 11. | Yamagishi S, Matsui T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | van Heijst JW, Niessen HW, Hoekman K, Schalkwijk CG. Advanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann N Y Acad Sci. 2005;1043:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Tan KC, Shiu SW, Wong Y, Tam X. Serum advanced glycation end products is associated with insulin resistance. Diabetes Metab Res Rev. 2011;[Epub ahead of print]. [PubMed] |
| 14. | Sarkar P, Kar K, Mondal MC, Chakraborty I, Kar M. Elevated level of carbonyl compounds correlates with insulin resistance in type 2 diabetes. Ann Acad Med Singapore. 2010;39:909-904. [PubMed] |
| 15. | Tahara N, Yamagishi SI, Matsui T, Takeuchi M, Nitta Y, Kodama N, Mizoguchi M, Imaizumi T. Serum Levels of Advanced Glycation End Products (AGEs) are Independent Correlates of Insulin Resistance in Nondiabetic Subjects. Cardiovasc Ther. 2010;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Schalkwijk CG, Brouwers O, Stehouwer CD. Modulation of insulin action by advanced glycation endproducts: a new player in the field. Horm Metab Res. 2008;40:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Hunter SJ, Boyd AC, O’Harte FP, McKillop AM, Wiggam MI, Mooney MH, McCluskey JT, Lindsay JR, Ennis CN, Gamble R. Demonstration of glycated insulin in human diabetic plasma and decreased biological activity assessed by euglycemic-hyperinsulinemic clamp technique in humans. Diabetes. 2003;52:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Jia X, Wu L. Accumulation of endogenous methylglyoxal impaired insulin signaling in adipose tissue of fructose-fed rats. Mol Cell Biochem. 2007;306:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | He C, Li J, Sabol J, Hattori M, Chang M, Mitsuhashi T, Vlassara H. AGE-restricted diet decreases incidence of diabetes and prolongs survival in NOD mice (Abstract). Diabetes. 1999;48:A144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Lin RY, Choudhury RP, Cai W, Lu M, Fallon JT, Fisher EA, Vlassara H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Cassese A, Esposito I, Fiory F, Barbagallo AP, Paturzo F, Mirra P, Ulianich L, Giacco F, Iadicicco C, Lombardi A. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem. 2008;283:36088-36099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 325] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Rojas A, Mercadal E, Figueroa H, Morales MA. Advanced Glycation and ROS: a link between diabetes and heart failure. Curr Vasc Pharmacol. 2008;6:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151-D1160. [PubMed] |
| 28. | Sugaya K, Fukagawa T, Matsumoto K, Mita K, Takahashi E, Ando A, Inoko H, Ikemura T. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics. 1994;23:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998-15004. [PubMed] |
| 31. | Xie J, Reverdatto S, Frolov A, Hoffmann R, Burz DS, Shekhtman A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J Biol Chem. 2008;283:27255-27269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH. N -Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem. 2002;80:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004;322:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 644] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 36. | Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1947] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 37. | Coffelt SB, Scandurro AB. Tumors sound the alarmin(s). Cancer Res. 2008;68:6482-6485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl). 2009;87:235-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 40. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8317] [Article Influence: 489.2] [Reference Citation Analysis (0)] |
| 41. | Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 706] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 42. | Harald zur Hausen; Infections Causing Human Cancer. Weinheim: Wiley-VCH; 2007. p. 532. . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 706] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 43. | Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Gebhardt C, Riehl A, Durchdewald M, Németh J, Fürstenberger G, Müller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 319] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 45. | Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 46. | Hiwatashi K, Ueno S, Abeyama K, Kubo F, Sakoda M, Maruyama I, Hamanoue M, Natsugoe S, Aikou T. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol. 2008;15:923-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666-4675. [PubMed] |
| 48. | Price CL, Sharp PS, North ME, Rainbow SJ, Knight SC. Advanced glycation end products modulate the maturation and function of peripheral blood dendritic cells. Diabetes. 2004;53:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2184-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506-7515. [PubMed] |
| 51. | Hoffmann S, Friedrichs U, Eichler W, Rosenthal A, Wiedemann P. Advanced glycation end products induce choroidal endothelial cell proliferation, matrix metalloproteinase-2 and VEGF upregulation in vitro. Graefes Arch Clin Exp Ophthalmol. 2002;240:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Yamagishi S, Yonekura H, Yamamoto Y, Katsuno K, Sato F, Mita I, Ooka H, Satozawa N, Kawakami T, Nomura M. Advanced glycation end products-driven angiogenesis in vitro. Induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. J Biol Chem. 1997;272:8723-8730. [PubMed] |
| 53. | Otero K, Martínez F, Beltrán A, González D, Herrera B, Quintero G, Delgado R, Rojas A. Albumin-derived advanced glycation end-products trigger the disruption of the vascular endothelial cadherin complex in cultured human and murine endothelial cells. Biochem J. 2001;359:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Ding YT, Kumar S, Yu DC. The role of endothelial progenitor cells in tumour vasculogenesis. Pathobiology. 2008;75:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 56. | Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 968] [Article Influence: 38.7] [Reference Citation Analysis (0)] |