INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) appears to be an integral part of the metabolic syndrome that comprises a cluster of abnormalities (dysglycemia, dyslipidemia, hypertension, procoagulant tendency, etc.) with insulin resistance as a central pathogenic factor[1,2]. NAFLD is significantly associated with insulin resistance[3,4]. Subjects with NAFLD had significantly higher values of body mass index (BMI), waist circumference, hip circumference, fasting blood glucose, fasting insulin, total cholesterol and serum triglycerides[5,6]. A reduction in hepatic insulin sensitivity due to triglyceride accumulation in liver has been documented[5]. Triglyceride storage in the liver could be a protective mechanism and does not necessarily impair insulin sensitivity nor contribute to liver damage. Current understanding suggests that inappropriate triglyceride storage and formation of harmful lipid derivatives or increased free fatty acids may be harmful. Despite much research, the exact pathophysiological mechanisms of NAFLD are not clear.
NAFLD AS A LOW-GRADE SYSTEMIC INFLAMMATORY CONDITION
NAFLD could be a low-grade systemic inflammatory condition since liver and adipose tissue tumor necrosis factor-α (TNF-α) and TNF receptor 1 transcripts[7] as well as serum TNF-α levels[8] are increased in patients with NAFLD and IL-6-deficient mice are less prone to NAFLD[9]. Yet, deficiency of TNF receptors does not prevent elevation of serum ALT in ob/ob mice[10] or after intragastric overfeeding of a high-fat diet[11]. TNF-α can induce insulin resistance. This implies that TNF-α and other pro-inflammatory cytokines may have a role in NAFLD but are not the sole cause of the same.
Hepatic fat deposition with hepatocellular damage, a feature of NAFLD, may also be mediated by pro-inflammatory prostaglandins (PGs). Among the more than twenty isozymes of mammalian PLA2, group IVA PLA2 (IVAPLA2) is a key enzyme responsible for the release of arachidonic acid (AA), a precursor of PGs. IVA-PLA2-knockout mice fed normal chow diets showed a decrease in hepatic triacylglycerol content and the size of epididymal adipocytes was smaller with a lower serum level of pro-inflammatory prostaglandin E2 (PGE2) compared with wild-type mice, suggesting that the circulating level of PGE2 is related to the levels of intracellular triglyceride (TG) in the liver and adipose tissues[12]. Stimulation of rat hepatocytes with PGE2 and the administration of PGE2 to rats induced increases in TG level in the cells and the liver, respectively[13,14], suggesting that IVA-PLA2 mediates fat deposition in the liver and adipose tissues through the generation of PGs that are pro-inflammatory in nature and thus, could predispose to the development of NAFLD. A deficiency of IVA-PLA2 alleviated fatty liver damage caused by high-fat diets[15] as a result of the lower generation of IVA-PLA2 metabolites, such as PGE2 that has pro-inflammatory action. Thus, NAFLD is a low-grade systemic inflammatory condition.
COULD NAFLD BE A RESULT OF DEFICIENCY OF ANTI-INFLAMMATORY CYTOKINES AND BIOACTIVE LIPIDS?
Fatty livers of obese fa/fa rats are vulnerable to injury when challenged by insults such as endotoxin, ischemia-reperfusion or acute ethanol treatment that could lead to NAFLD. When obese fa/fa rats and their lean littermates were fed a diet low in fat (12% of total calories) or a diet with 60% calories as lard for 8 wk, hyperglycemia and steatohepatitis occurred in the fa/fa rats fed the high-fat diet. This was accompanied by liver injury as evidence by enhanced levels of hepatic enzymes (such as alanine aminotransferase) that was found to be associated with increased TNF-α and TGF-β, collagen deposition, up-regulation of α-smooth muscle actin, increased TIMP1 (a component of family of tissue inhibitors of metalloproteinases), and elevated oxidative stress, lipid peroxides, protein carbonyls and reduced glutathione and antioxidant enzymes in the fa/fa rats fed with the high-fat diet[16]. Despite the fact that inflammatory events play a significant role in NAFLD, relatively little attention has been paid to anti-inflammatory events.
It is possible that enhanced IL-6, TNF-α, PGE2 levels and insulin resistance seen in NAFLD could be due to a deficiency of anti-inflammatory molecules. For instance, AA released by IVA-PLA2 can form a precursor to anti-inflammatory bioactive lipids such as lipoxins (LXs), resolvins and protectins that suppress IL-6, TNF-α and PGE2 production and ameliorate insulin resistance[17-19]. This is supported by the observation that enteral and intravenous supplementation of omega-3 fatty acids can ameliorate hepatic steatosis in a murine model of NAFLD[20]. In addition, a relative depletion in polyunsaturated fatty acids (PUFAs), particularly of the n-6 and n-3 series in hepatic triacylglycerols and of the n-3 series in liver phospholipids, with decreased 20:4, n-6/18:2, n-6 and (20:5, n-3 + 22:6, n-3)/18:3, n-3 ratios with simultaneously higher n-6/n-3 ratios in liver and adipose tissue, 18:1, n-9 trans contents in adipose tissue, and hepatic lipid peroxidation and protein oxidation indexes was reported in NAFLD patients[21]. These results suggest that an alteration in the metabolism of both n-6 and n-3 fatty acids occur in NAFLD.
Thus, it is likely that an imbalance between the pro- and anti-inflammatory molecules that is tilted more in favor of the former could trigger the development of NAFLD. Hence, I propose that failure to produce adequate amounts of anti-inflammatory molecules such as LXs, resolvins and protectins and cytokines IL-4 and IL-10 play a role in the pathobiology of NAFLD.
ESSENTIAL FATTY ACID METABOLISM
In view of the proposal that altered metabolism of EFAs in the form of enhanced formation of pro-inflammatory eicosanoids and decreased formation of anti-inflammatory bioactive lipids, especially those from ω-3 fatty acids, play a significant role in NAFLD, a close look at the metabolism of EFAs is necessary.
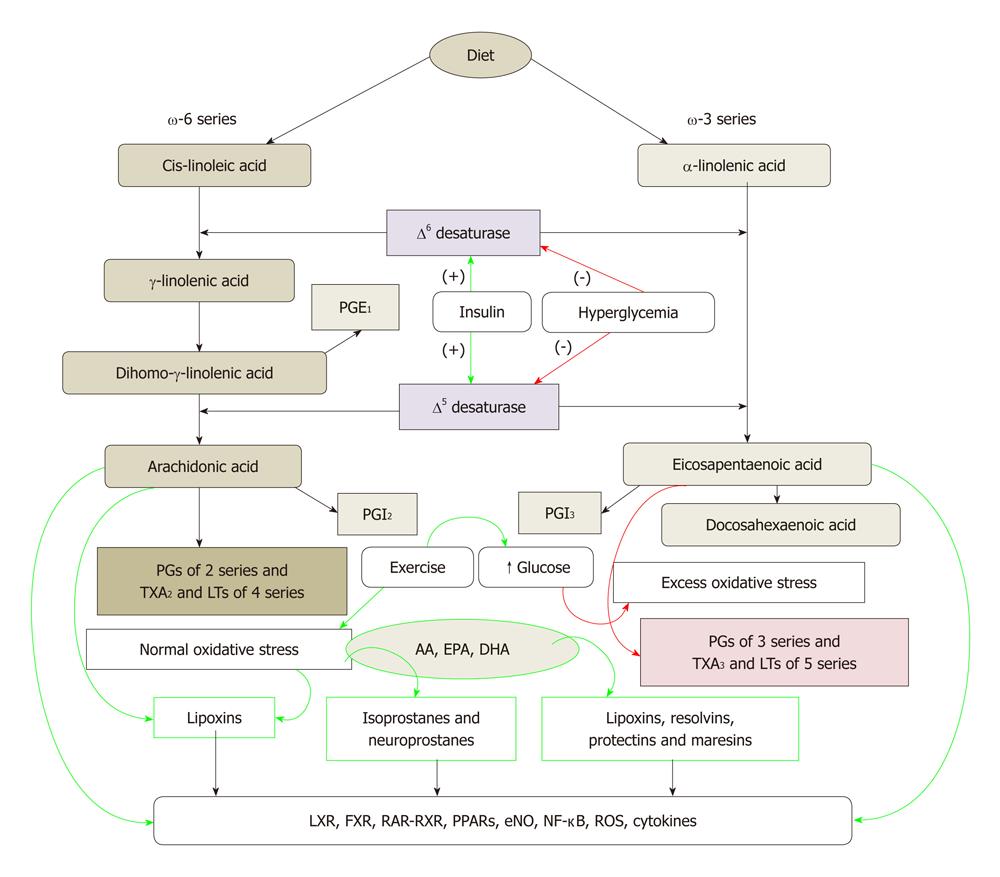
Cis-linoleic acid (LA, 18:2 ω-6) and α-linolenic acid (ALA, 18:3 ω-3) are EFAs. LA is converted to γ-linolenic acid (GLA, 18:3, ω-6) by the action of the enzyme ∆6 desaturase and GLA is elongated to form di-homo-GLA (DGLA, 20:3, ω-6), the precursor of the 1 series of prostaglandins. DGLA can be converted to AA (20:4, ω-6) by the action of the enzyme ∆5 desaturase. AA forms the precursor of 2 series of prostaglandins, thromboxanes and the 4 series LTs. ALA is converted to eicosapentaenoic acid (EPA, 20:5, ω-3) by ∆6 and ∆5 desaturases. EPA forms the precursor of the 3 series of prostaglandins and the 5 series of Leukotrienes (LTs). EPA can be elongated to form docosahexaenoic acid (DHA, 22:6, ω-3). AA, EPA and DHA also form precursors to a group of novel compounds: LXs, resolvins, protectins and maresins[22-32] that have anti-inflammatory action (Figure 1). Eicosanoids bind to G protein-coupled receptors on many cell types and mediate virtually every step of inflammation, are found in inflammatory exudates and their synthesis is increased at sites of inflammation. Non-steroidal anti-inflammatory drugs such as aspirin inhibit cyclo-oxygenase (COX) activity and thus, are believed to bring about their anti-inflammatory action.
Figure 1 Metabolism of essential fatty acids and their modulation by insulin, glucose, exercise and oxidative stress.
Calorie restriction increases the activity of Δ6 and Δ5 desaturases. High-fat diet, trans-fats and cholesterol block the activities of Δ6 and Δ5 desaturases. (-): Inhibition of synthesis or action; (+): Enhancement of synthesis or action. Green arrows indicate beneficial action and/or anti-inflammation whereas red arrows indicate harmful action and/or pro-inflammation. PG: Pro-inflammatory prostaglandin; AA: Arachidonic acid; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; LTs: Leukotrienes.
LXS, RESOLVINS, PROTECTINS AND MARESINS
There are two COX enzymes, the constitutively expressed COX-1 and the inducible enzyme COX-2. Different types of PGs are formed by the action of COX enzymes depending on the substrate fatty acid from which they are derived.
There are 3 types of lipoxygenases and are present in only a few types of cells. 5-lipoxygenase (5-LO), present in neutrophils, produces 5-hydroxyeicosatetraenoic acid (5-HETE), which is chemotactic for neutrophils and is converted into LTs. LTB4, a potent chemotactic and activator of neutrophils, induces aggregation and adhesion of leukocytes to vascular endothelium, generation of reactive oxygen species and release of lysosomal enzymes. The cysteinyl-containing leukotrienes C4, D4, and E4 (LTC4, LTD4 and LTE4) induce vasoconstriction, bronchospasm and vascular permeability in venules. LTs are more potent than histamine in increasing vascular permeability and causing bronchospasm. LTs mediate their actions by binding to cysteiny leukotreine 1 (CysLT1) and CysLT2 receptors. In general, PGs, LTs and thromboxanes (TXs) formed from DGLA and AA are pro-inflammatory in nature. PGs, TXs and LTs formed from EPA also have pro-inflammatory action but are generally less pro-inflammatory compared to those formed from AA.
AA, EPA and DHA also form precursors to potent anti-inflammatory compounds: LXs, resolvins, protectins and maresins. LXs are generated from AA, EPA and DHA (LXA4 is formed from AA; LXA5 is formed from EPA; resolvins are formed from EPA and DHA and protectins from DHA; and all these products have potent anti-inflammatory actions) by transcellular biosynthetic mechanisms involving two cell populations. Neutrophils produce intermediates in LX synthesis and these are converted to LXs by platelets interacting with leukocytes. LXA4 and LXB4 are generated by the action of platelet 12-LO on neutrophil-derived LTA4. LXs inhibit leukocyte recruitment, neutrophil chemotaxis and adhesion to endothelium[28]. LXs have a negative regulation on LT synthesis and action and help in the resolution of inflammation. An inverse relationship generally exists between LXs and LTs and the balance between these two molecules appears to be crucial in the determination of degree of inflammation and its final resolution[22,25,27,30-32].
ASPIRIN-TRIGGERED 15 EPIMER LXS, RESOLVINS AND PROTECTINS
The formation of aspirin-triggered 15 epimer LXs are potent counter regulators of polymorphonuclear neutrophils (PMNs)-mediated injury and acute inflammation. Acetylated COX-2 enzyme of endothelial cells generates 15R-HETE from AA that is converted by activated PMNs to the 15-epimeric LXs that have potent anti-inflammatory properties[23-32]. This cross-talk between endothelial cells and PMNs leading to the formation of 15R-HETE and its subsequent conversion to 15-epimeric LXs by aspirin-acetylated COX-2 is a protective mechanism to prevent local inflammation on the vessel wall by regulating the motility of PMNs, eosinophils and monocytes[30-32]. Endothelial cells also oxidize AA, EPA and DHA via P450 enzyme system to form various hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids such as 11,12-epoxy-eicosatetraenoic acid(s) that have many biological actions that include blocking endothelial cell activation, while non-enzymatic oxidation products of EPA inhibit phagocyte-endothelium interaction and suppress the expression of adhesion molecules[33-38].
Akin to the formation of 15R-HETE and 15-epimeric LXs from AA, similar compounds are also formed from EPA and DHA. In the presence of aspirin, activated COX-2 of human endothelial cells converts EPA to 18R-HEPE, 18-HEPE and 15R-HEPE. Activated human PMNs, in turn, converts 18R-HEPE to 5,12,18R-triHEPE and 15R-HEPE to 15-epi-LXA5 by their 5-LO. Both 18R-HEPE and 5,12,18R-triHEPE inhibited LTB4-stimulated PMN transendothelial migration. 5,12,18R-triHEPE effectively competed with LTB4 for its receptors and inhibited PMN infiltration suggesting that it suppresses LT-mediated responses at the sites of inflammation[22,25,27,31,32,39,40].
The conversion of EPA by human endothelial cells with upregulated COX-2 treated with ASA of EPA to 15-epi-LX, also termed aspirin-triggered LX (ATL), and to 18R-HEPE and 15R-HEPE is interesting. These compounds in turn, are used by polymorphonuclear leukocytes to generate separate classes of novel trihydroxy-containing mediators, including 5-series 15R-LX(5) and 5,12,18R-triHEPE, which are potent inhibitors of human polymorphonuclear leukocyte transendothelial migration and infiltration in vivo (ATL analogue > 5,12,18R-triHEPE > 18R-HEPE). Acetaminophen and indomethacin also permitted 18R-HEPE and 15R-HEPE generation with recombinant COX-2. The formation of these bioactive lipid mediators via COX-2-nonsteroidal anti inflammatory drug-dependent oxygenations and cell-cell interactions may have significant therapeutic benefits in inflammation[17,27,31,32,39,40].
Leukocytes, brain and glial cells transform enzymatically DHA to 17R series of hydroxy DHAs that, in turn, is converted enzymatically to di- and tri-hydroxy containing docosanoids[31,32,40-42]. The conversion of DHA to 17S-hydroxy-containing docosanoids denoted as docosatrienes (the main bioactive member of the series was 10,17S-docosatriene) and 17S series resolvins serve as regulators of both leukocytes reducing infiltration in vivo and glial cells blocking their cytokine production. Thus, DHA is the precursor to novel docosatrienes and 17S series resolvins that have anti-inflammatory action and resolve inflammation.
Similar small molecular weight compounds are also generated from AA, EPA and DHA: 15R-hydroxy containing compounds from AA, 18R series from EPA and 17R-hydroxy series from DHA. All these compounds have potent anti-inflammatory actions, resolve inflammation and hence are called “resolvins”. Resolvins inhibit cytokine generation, leukocyte recruitment, leukocyte diapedesis and exudate formation. The formation of resolvins from AA, EPA and DHA from acetylated COX-2 are generated via transcellular biosynthesis (e.g. due to cell-cell communication between endothelial cells and PMNs) and their main purpose appears to be to suppress inflammation. Resolvins inhibit brain ischemia-reperfusion injury[31,32,40-42]. It is likely that LXs, resolvins and protectins (docosanoids are also called as protectins since they have neuroprotective actions) serve as endogenous anti-inflammatory and cytoprotective molecules[17,18,28]. The general cytoprotective properties that have been attributed to AA, EPA and DHA can be related to their conversion to LXs, resolvins and docosanoids (protectins) (Figures 2-5). Hence, defects in the formation and action of LXs, resolvins and protectins could lead to perpetuation of inflammation[31,32].
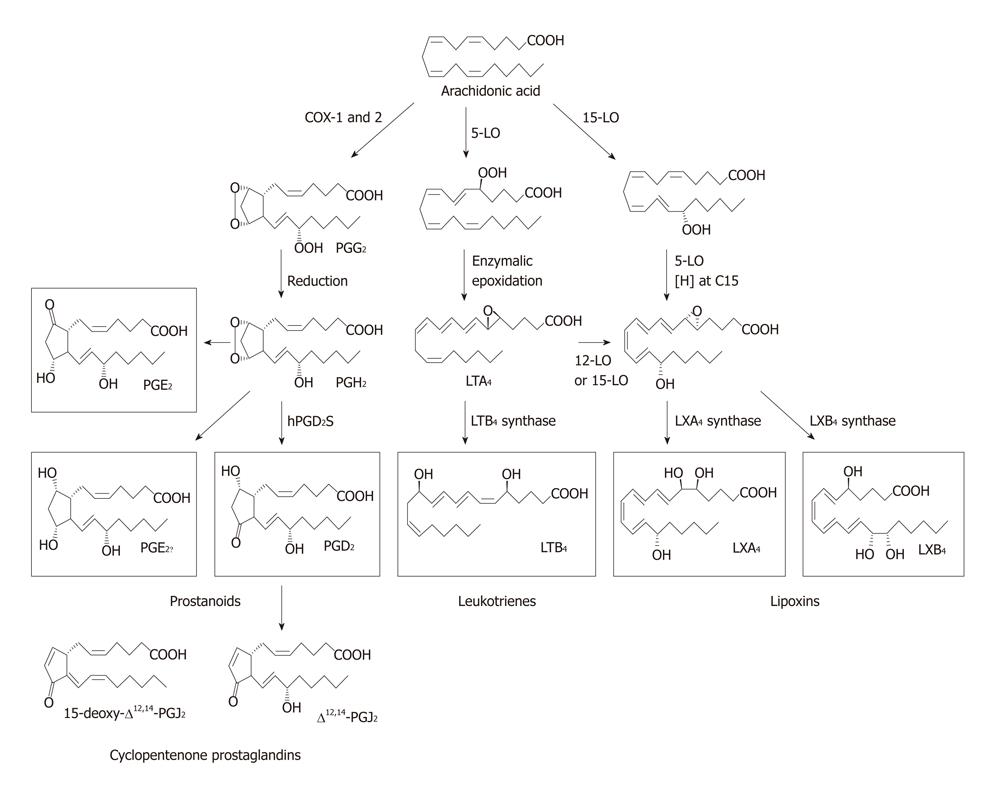
Figure 2 Metabolism of arachidonic acid showing different metabolites formed from it.
LO: Lipoxygenase; LXs: Lipoxins; LTs: Leukotrienes; PG: Pro-inflammatory prostaglandin.
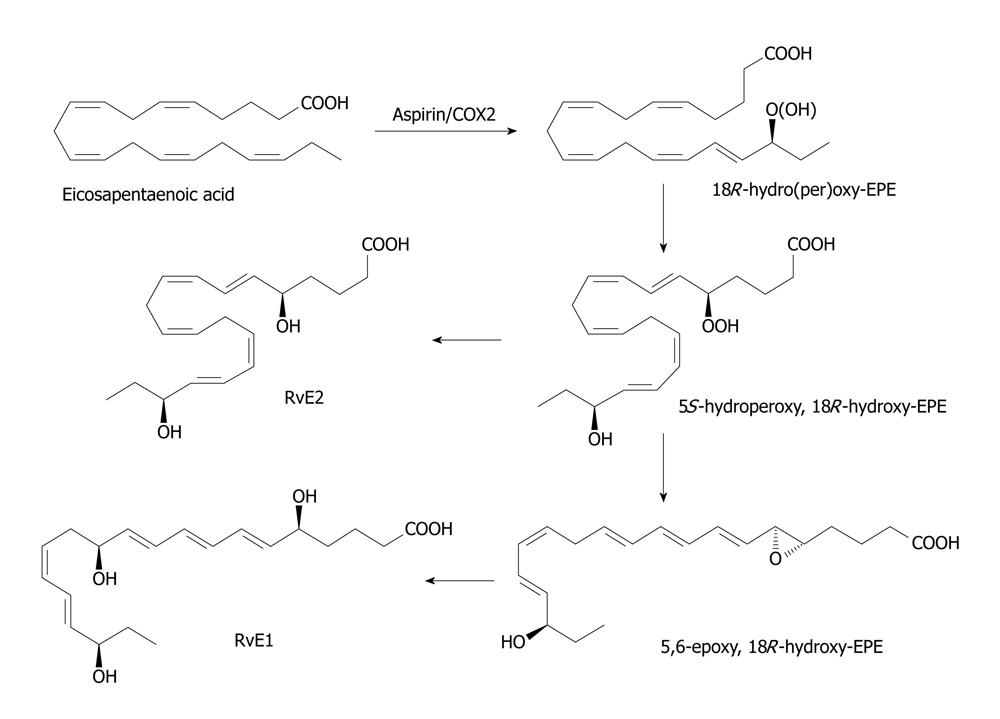
Figure 3 Scheme showing the formation of resolvin E derived from eicosapentaenoic acid.
In the endothelial cells, the cyclo-oxygenase (COX)-2 enzyme that has been acetylated introduces an 18R hydroperoxy-group into the eicosapentaenoic acid molecule (c.f. the role of aspirin in the biosynthesis of the epi-lipoxins). This is reduced to the corresponding hydroxy compound before a 5S-hydroperoxy group is introduced into the molecule by the action of 5-lipoxygenase as in the biosynthesis of leukotrienes. A further reduction step produces 15S,18R-dihydroxy-EPE or resolvin E2. Alternatively, the 5S-hydrpperoxy, 18R-hydroxy-EPE intermediates is converted to a 5,6-epoxy fatty acids in polymorphonuclear leukocytes I humans and eventually to 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eiocsapentaenoic acid or resolvin E1 by process similar to the formation of leukotrienes in leukocytes.
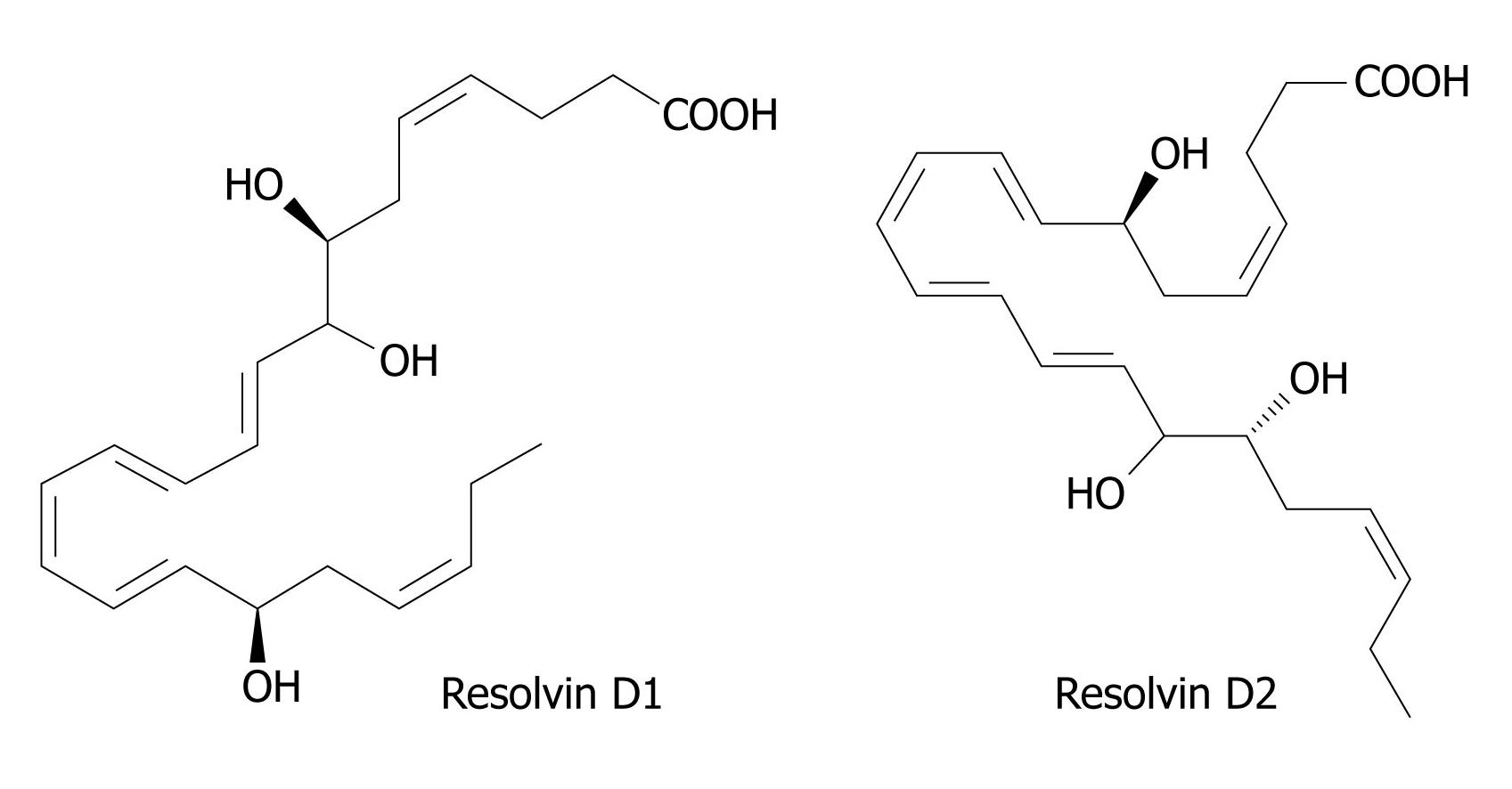
Figure 4 Structures of Resolvin D1 and D2.
DHA is converted to 17R-resolvins by a similar aspirin-triggered mechanism similar to the scheme shown in Figure 2. In the absence of aspirin, COX-2 of endothelial cells converts DHA to 13S-hydroxy-DHA. In the presence of aspirin, the initial product is 17R-hydroxy-DHA, which is converted to 7S-hydroperoxy, 17R-hydroxy-DHA by the action of a lipoxygenase, and thence via an epoxy intermediate to epimeric resolvins D1 and D2. An alternative lipoxygenase-generated intermediate, 4S-hydroperoxy, 17R-hydroxy-DHA, is transformed via an epoxide to epimeric resolvins D3 and D4. 17S Resolvins of the D series are produced in cells in the absence of aspirin by a reaction catalyzed in the first step by a lipoxygenase. COX: Cyclo-oxygenase; DHA: Docosahexaenoic acid.
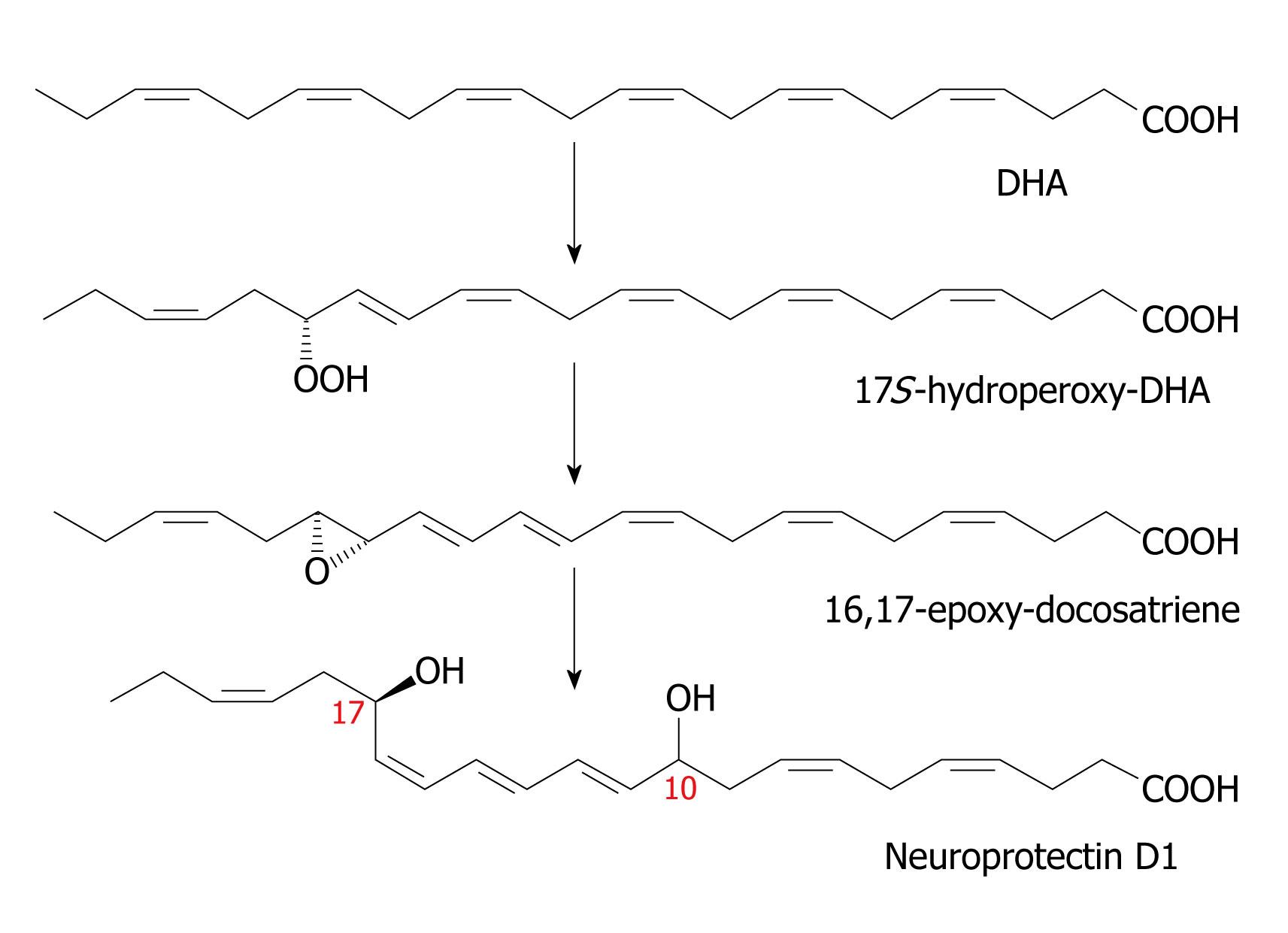
Figure 5 Scheme showing the synthesis of neuroprotectin D1.
Resolvins are generated in brain tissue in response to aspirin treatment and in addition, docosatrienes termed neuroprotectins are also produced. The lipoxygenase product 17S-hydroperoxy-DHA is converted first to a 16(17)-epoxide and then to the 10, 17-dihydroxy docosatriene denoted as 10, 17 S-DT or NPD1. As with the leukotrienes, there are three double bonds in conjugation, hence the term “triene”, although there are six double bonds in total. Figures 2-5 are from[27,31,32,64,119]. DHA: Docosahexaenoic acid.
ANTI-INFLAMMATORY MOLECULE LXA4 IS DETECTABLE IN URINE
LXA4, generated by LO transformation of AA possess potent anti-inflammatory activity in vivo and temporal biosynthesis of LXs, concurrent with spontaneous resolution, has been observed during exudate formation[29-31,42-44]. LXs, resolvins, protectins and maresins are detectable in the plasma[17,31,32]. Recently, it was reported that urine from healthy subjects contains LXA4[45] and strenuous exercise significantly increased its urinary excretion in healthy volunteers[46], suggesting that alterations in the urinary excretion of LXA4 can used as a reflection of changes in its (LXA4) formation to monitor changes in the inflammatory events/diseases. It is possible that other anti-inflammatory bioactive lipids such as resolvins, protectins and maresins may also be detectable in the urine. Since urinary levels of LXA4 was decreased (similar decrease may occur of other bioactive lipids such as resolvins, protectins and maresins) while that of cysteinyl leukotrienes (cysLTs) increased in volunteers aged from 26 to over 100 years, leading to a profound unbalance of the LXA(4)/cysLTs ratio, that may serve as an index of the endogenous anti-inflammatory potential[47]. Hence, measurement of urinary and plasma levels of LXs, resolvins, protectins, maresins and leukotrienes could be used to monitor the inflammatory process that occurs in various diseases including NAFLD.
ALTERED EFA METABOLISM IN NAFLD IN THE FORM OF A DECREASE IN ANTI-INFLAMMATORY AND AN INCREASE IN PRO-INFLAMMATORY BIOACTIVE LIPIDS
NAFLD consists of a variety of pathological states ranging from the simple buildup of fat in the liver (hepatic steatosis) to nonalcoholic steatohepatitis, cirrhosis and ultimately liver failure[48-51]. Current statistics suggest that NAFLD is a major cause of liver-related morbidity and mortality and is believed to account for approximately 80% of individuals with elevated serum liver enzymes[52] and further to that, up to 30% of the Western population may have NAFLD[53]. NAFLD is associated with metabolic disorders such as obesity[54] and diabetes[55], as well as with prolonged chemotherapy[56] and total parenteral nutrition[57-59]. In view of such wide spread incidence and prevalence of NAFLD, it is important to understand its etiopathogenesis to develop suitable remedial measures.
Intravenous administration of fish oil that is rich in ω-3 fatty acids EPA and DHA reduced parenteral nutrition-induced cholestasis in newborn piglets[60] and rats[61,62] and dietary ω-3 and ω-6 PUFAs have the ability to regulate hepatic lipogenesis by reducing sterol regulatory element-binding protein-1 in the liver[63,64]. In a clinical study, wherein analysis of liver and abdominal adipose tissue fatty acids was carried out in normal controls and those with NAFLD, it was noted that NAFLD patients had a depletion in PUFAs of the ω-6 and ω-3 series in liver triacylglycerols, with decreased AA/LA and EPA + DHA/ALA ratios, whereas liver phospholipids contained higher ω-6 and lower ω-3 PUFAs[21]. These findings were accompanied by an enhancement of (1) ω-6/ω-3 ratio in liver and adipose tissue; (2) 18:1, ω-9 trans levels in adipose tissue; and (3) hepatic lipid peroxidation and protein oxidation indexes. These results suggest that a marked enhancement in PUFA ω-6/ω-3 ratio occurs in the liver of NAFLD patients. Based on these results, it was suggested that depletion of hepatic PUFA content may result from both defective desaturation of EFAs (both LA and ALA) that could be due to both inadequate intake of EFAs (both LA and ALA) and higher intake of the 18:1, ω-9 trans isomer leading to desaturase inhibition and, possibly, from an increased peroxidation of PUFAs due to oxidative stress[21].
Prolonged use of total parenteral nutrition can lead to nonalcoholic fatty liver disease, ranging from hepatic steatosis to cirrhosis and liver failure. Mice that receive fat-free, high-carbohydrate diet develop severe liver damage as determined by histology and magnetic resonance spectroscopy as well as elevation of serum liver function tests. In such a murine model of NAFLD in which all animals develop steatosis and liver enzyme disturbances, intravenous administration of ω-3 fatty acid emulsion attenuated NAFLD and prevented hepatic pathology and normalized liver function tests[20], suggesting that ω-3 fatty acids protect liver against injury including NAFLD. In addition, NAFLD is associated with low levels of adiponectin and relatively high levels of TNF-α[65]. This lends support to the proposal that NAFLD could be an inflammatory condition and methods designed to suppress inflammation could be of significant benefit. In addition, adiponectin antagonizes both the production and activity of TNF-α, whereas TNF-α inhibits adiponectin. Adiponectin acts directly on hepatocytes to inhibit fatty acid synthesis and uptake while stimulating fatty acid oxidation. Thus, it is likely that a combination of low adiponectin and high TNF-α levels in the context of increased hepatic exposure to free fatty acids results in hepatic steatosis, severe hepatic insulin resistance and ultimately NAFLD[65].
It is interesting to note that PUFAs, especially ω-3 EPA and DHA and ω-6 AA, DGLA and GLA and their products such as PGE1, LXs, resolvins, protectins and maresins suppress the production of IL-6, TNF-α and MIF that are pro-inflammatory cytokines, free radical generation and lipid peroxidation process[22-32,39-44,64,66-73]. Furthermore, insulin resistance itself may perpetuate NAFLD since insulin has anti-inflammatory actions[74-78], whereas exercise is beneficial since it (exercise) is anti-inflammatory in nature[79,80]. In the initial stages of exercise, there will be an increase in the production of IL-6 that triggers elevation in the production of endogenous anti-oxidants such as superoxide dismutase[81] and LXA4. In addition, exercise enhances the production of LXA4 that may explain its anti-inflammatory action[81]. Thus, there is a close relationship that exists between high fat diet, EFA/PUFA metabolism, pro-inflammatory cytokines, insulin resistance, insulin and exercise (Figure 6). NAFLD is a low-grade systemic inflammatory condition. Increased formation of pro-inflammatory cytokines and eicosanoids and/or reduced formation of anti-inflammatory cytokines and inflammation resolving bioactive lipids may participate in the pathobiology of NAFLD. Thus, release and timely formation of anti-inflammatory bioactive lipids is necessary to prevent NAFLD and/or resolution of inflammation seen in NAFLD. The release and formation of anti-inflammatory bioactive lipids depends on the activity of phospholipase A2. This scheme is applicable to both acute and chronic inflammation. NAFLD that starts may initially have an acute inflammatory component and will become chronic due to the changes in the activities of various sub-classes of phospholipase A2 and continued exposure to pro-inflammatory stimuli such as high-fat diet.
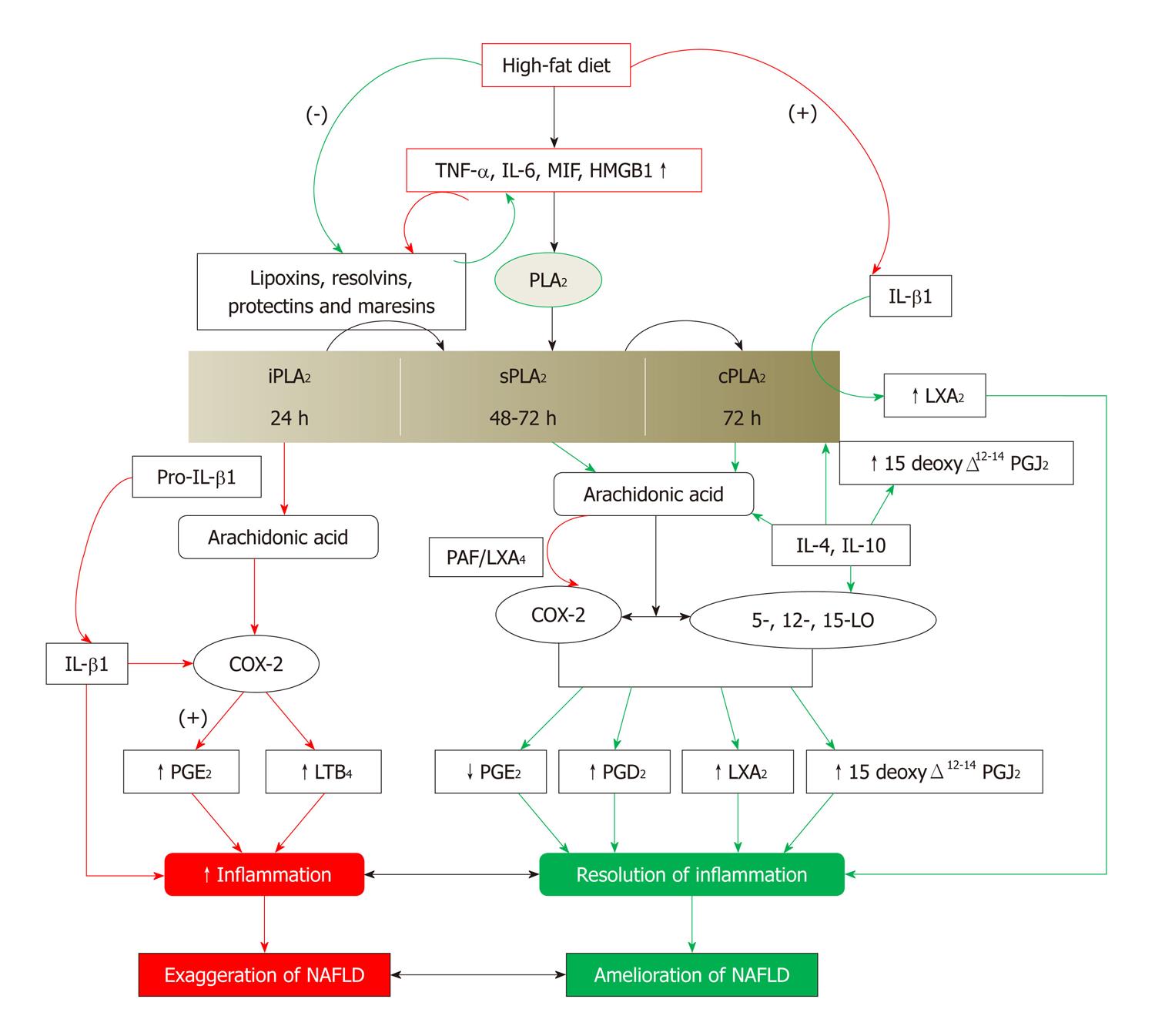
Figure 6 Scheme showing the role of eicosanoids, lipoxins, resolvins, protectins and maresins in resolution of non-alcoholic fatty liver disease.
Green arrows indicate events that will lead to resolution of non-alcoholic fatty liver disease (NAFLD). Red arrows indicate events that will lead to initiation and/or progression of NAFLD. (-): Inhibition or suppression of action; (+): Activation or enhancement of action; TNF-α: Tumor necrosis factor-α; LXs: Lipoxins; COX: Cyclo-oxygenase; LO: Lipoxygenase; PG: Pro-inflammatory prostaglandin. For further details see[82-90,119].
There are three classes of phospholipases that control the release of AA and other PUFAs: calcium-independent PLA2 (iPLA2), secretory PLA2 (sPLA2) and cytosolic PLA2 (cPLA2)[82]. Each class of PLA2 is further divided into isoenzymes for which there are 10 for mammalian sPLA2, at least 3 for cPLA2 and 2 for iPLA2. During the early phase of inflammation, COX-2 derived PGs and TXs and lipoxygenase-derived LTs initiate exudate formation and inflammatory cell influx[83]. TNF-α causes an immediate influx of neutrophils concomitant with PGE2 and LTB4 production, whereas during the phase of resolution of inflammation, an increase in LXA4 (LX A4), PGD2 and its product 15deoxyΔ12-14PGJ2 formation occurs that induces resolution of inflammation with a simultaneous decrease in PGE2 synthesis that stops neutrophil influx and enhances phagocytosis of debris[84,85]. Thus, there appears to be two waves of release of AA and other PUFAs: one at the onset of inflammation that causes the synthesis and release of PGE2 and a second at resolution for the synthesis of anti-inflammatory PGD2, 15deoxyΔ12-14PGJ2, and LXs that are necessary for the suppression of inflammation. Thus, COX-2 enzyme has both harmful and useful actions by virtue of its ability to give rise to pro-inflammatory and anti-inflammatory PGs and LXs.
Increased type VI iPLA2 protein expression was found to be the principal isoform expressed from the onset of inflammation up to 24 h, whereas type IIa and V sPLA2 was expressed from the beginning of 48 h till 72 h while type IV cPLA2 was not detectable during the early phase of acute inflammation but increased progressively during resolution, peaking at 72 h. This increase in type IV cPLA2 was mirrored by a parallel increase in COX-2 expression[86]. The increase in cPLA2 and COX-2 occurred in parallel, suggesting a close enzymatic coupling between these two. Thus, there is a clear-cut role for different types of PLA2 in distinct and different phases of inflammation. Selective inhibition of cPLA2 resulted in the reduction of pro-inflammatory molecules PGE2, LTB4, IL-1β and platelet-activating factor (PAF). Furthermore, inhibition of types IIa and V sPLA2 not only decreased PAF and LXA4 (LX A4) but also resulted in a reduction in cPLA2 and COX-2 activities. These results suggest that sPLA2-derived PAF and LXA4 induce COX-2 and type IV cPLA2. IL-1β induced cPLA2 expression. This suggests that one of the functions of IL-1 is not only to induce inflammation but also to induce cPLA2 expression to initiate resolution of inflammation[87,88].
Synthetic glucocorticoid dexamethasone inhibited both cPLA2 and sPLA2 expression, whereas type IV iPLA2 expression is refractory to its suppressive actions[89-91]. Activated iPLA2 contributes to the conversion of inactive proIL-1β to active IL-1β, which in turn induces cPLA2 expression that is necessary for resolution of inflammation.
LXs, especially LXA4 inhibit TNF-α-induced production of ILs, promote TNF-α mRNA decay, TNF-α secretion and leukocyte trafficking and thus attenuated inflammation. Based on these evidences, in NAFLD there could occur increased formation of PGE2 and other pro-inflammatory eicosanoids and decreased production of PGD2, 15 deoxyΔ12-14 PGJ2, LXs, resolvins, protectins and maresins that have anti-inflammatory action. Defective function of sPLA2 and cPLA2, as a result of which decreased release of AA, EPA and DHA could occur that, in turn, leads to reduced formation of pro-resolving and anti-inflammatory PGD2, 15 deoxyΔ12-14 PGJ2, LXs, resolvins, protectins and maresins leads to the initiation and perpetuation of NAFLD.
It is noteworthy that high-fat diet, trans-fats and cholesterol interfere with EFA metabolism by blocking the actions of Δ6 and Δ5 desaturases and thus, decrease the levels of GLA, DGLA, AA, EPA and DHA[25,27,32,64]. As a result, the formation of anti-inflammatory bioactive lipids, PGE1, 15 deoxyΔ12-14 PGJ2, LXs, resolvins, protectins and maresins, will also be decreased due to substrate deficiency that leads to initiation and perpetuation of the inflammatory process[31,32,64]. On the other hand, insulin and diet restriction enhance the action of Δ6 and Δ5 desaturases[25,27,92], augment tissue levels of GLA, DGLA, AA, EPA and DHA that leads to increased formation of PGE1, 15 deoxyΔ12-14 PGJ2, LXs, resolvins, protectins and maresins that could ameliorate inflammation and NAFLD.
DEFECT IN THE ACTIVITY OF Δ6 AND Δ5 DESATURASES AND ANTI-INFLAMMATORY BIOACTIVE LIPIDS IN NAFLD
Based on the preceding discussion, it is proposed that patients with NAFLD have low plasma and hepatic levels of AA, EPA and DHA due to decreased Δ6 and Δ5 desaturases, reduced formation of PGD2, 15 deoxyΔ12-14 PGJ2, LXs, resolvins, protectins and maresins as a result of substrate deficiency, and a defect in the production of adequate amounts of anti-inflammatory cytokines IL-4, IL-10 and significantly higher levels of pro-inflammatory molecules PGE2, TXs, LTs and cytokines IL-6, and TNF-α compared to normal healthy controls. It is also possible that there may be a direct correlation between the concentrations of pro-inflammatory and anti-inflammatory molecules and the degree of NAFLD.
In a study, patients with dyslipidemia (Fredrickson type IIb), who had asymptomatic persistent transaminasemia lasting 24 wk and evidence of hepatic fat infiltration on ultrasonography and liver biopsy, were studied with regard to the efficacy and safety of ω-3 fatty acids (rich in EPA and DHA), atorvastatin, an HMG-CoA reductase inhibitor, and orlistat, which inhibits both gastric and pancreatic lipases in the enteric lumen, and it was found that all three drugs were effective[93]. It may be mentioned here that both ω-3 fatty acids EPA and DHA and HMG-CoA reductase inhibitors are known to have anti-inflammatory actions (reviewed in 25, 27, 32) that may explain their beneficial action in NAFLD. The role of PUFAs in NAFLD is further supported by the observation that reductions in AA in free fatty acids, triacylglycerol and phosphatidylcholine and a decrease in EPA and DHA in diacylglycerol fractions in the liver biopsy specimens occurred[94]. These results reiterate the proposal that deficiency of AA, EPA and DHA occurs even in the hepatic tissue in NAFLD.
Further support to the role of PUFAs and their pro- and anti-inflammatory products and pro- and anti-inflammatory cytokines in the pathobiology of NAFLD is derived from studies performed using mice in which 12/15-LO gene (Alox 15) has been disrupted. 12/15-LO is a member of the lipoxygenase family that converts AA into lipid mediators such as 12-HETE and 15-HETE[95]. 12/15-LO products have pro-inflammatory actions and activate nuclear factor κB and c-Jun amino-terminal kinase and stimulate the expression of proinflammatory cytokines[96,97]. It is known that 12/15-LO plays an important role in the metabolic syndrome, which is a low-grade systemic inflammatory condition[98-104]. Disruption of gene encoding for 12/15-LO (Alox15) in mice delayed the onset of atherosclerosis[105,106], were resistant to the development of streptozotocin-induced and autoimmune diabetes[107,108] and protected from high-fat diet-induced obesity and metabolic consequences, including adipose tissue inflammation and insulin resistance[109,110]. Conversely, transgenic mice overexpressing 12/15-LO in cardiomyocytes displayed exacerbated cardiac inflammation and fibrosis and more advanced heart failure[111].
In the ob/ob mice, an obesity model of insulin resistance and fatty liver disease, supplementation of EPA and DHA improved insulin-sensitivity in adipose tissue and liver, upregulated hepatic PPAR-γ, glucose transport (GLUT-2/GLUT-4) and insulin receptor signaling (IRS-1/IRS-2) genes, increased adiponectin levels and induced AMPK phosphorylation, a fuel-sensing enzyme and a gatekeeper of the energy balance. Hepatic steatosis was alleviated by ω-3-PUFAs in this animal model as a result of increased formation of resolvin E1 and protectin D1. Both resolvin E1 and protectin D1 mimicked the insulin-sensitizing and antisteatotic effects of ω-3-PUFAs and induced adiponectin expression[112]. These results lend direct support to the proposal that ω-3 PUFAs and their anti-inflammatory products LXs and resolvins prevent obesity-induced insulin resistance and NAFLD. Furthermore, obese subjects who are more prone to develop NAFLD are known to have decreased hepatic Δ6 and Δ5 desaturase activity[113] that may, in turn, lead to decrease in the hepatocyte content of AA, EPA and DHA that form precursors to anti-inflammatory LXs, resolvins and protectins. Park et al[114] showed that long-term use of ezetimibe (for 24 mo), a lipid-lowering drug, significantly improved metabolic parameters including visceral fat area, fasting insulin, homeostasis model assessment of insulin resistance, triglycerides, total cholesterol, low-density lipoprotein cholesterol, oxidative-LDL, the net electronegative charge modified-LDL, profiles of lipoprotein particle size and fatty acids component, estimated desaturase activity and lowered serum alanine aminotransferase and high-sensitivity C-reactive protein levels in patients with NAFLD. It is noteworthy that these patients also showed an increase Δ5 desaturase activity indicating that plasma and hepatic levels of AA, EPA and DHA, the precursors of LXs, resolvins and protectins, have increased. On the other hand, Fujita et al[115] showed that antiplatelet drugs, aspirin, ticlopidine and cilostazol, significantly attenuated liver steatosis, inflammation and fibrosis in the Fisher 344 male rats that were given a choline-deficient, l-amino acid-defined (CDAA) diet with high-fat high-calorie diet that induced NAFLD. It may be noted here that aspirin enhances the formation of LXA4 as discussed above, suggesting that perhaps, enhanced formation of LXA4 is responsible for the beneficial action observed.
It was reported[116] that increased PGE2 produced in Kupffer cells attenuated insulin-dependent glucose utilization by interrupting the intracellular signal chain downstream of the insulin receptor in hepatocytes. In addition, PGE2 stimulated oncostatin M (OSM) production by Kupffer cells that, in turn, attenuated insulin-dependent Akt activation and inhibited the expression of key enzymes of hepatic lipid metabolism. Since both COX-2 and OSM mRNA are induced early in the course of the development of NAFLD and NASH, it indicates that induction of OSM production in Kupffer cells by an autocrine PGE2-dependent feed-forward loop may be an additional mechanism that contributes to hepatic insulin resistance and the development of NAFLD and NASH. The importance of activation of Kupffer cells in NASH and NAFLD lies in the fact that the metabolic abnormalities seen in these conditions in the form of insulin resistance and low-grade systemic inflammation could lead to enhanced release of free fatty acid flux and changes in adipocytokines production such as leptin, adiponectin and IL-6 as discussed above. As a result, the nuclear transcription factor peroxisome proliferator-activated receptor γ and the endocannabinoid system (that are also formed from AA) are activated that may predispose to the development of liver fibrosis[117,118]. Hence, early identification and management of NASH and NAFLD is important.
CONCLUSION
NAFLD is associated with decreased levels of AA, EPA and DHA and their anti-inflammatory products PGE1, PGD2, LXs, resolvins and protectins with a concomitant increase in pro-inflammatory cytokines IL-6 and TNF-α and bioactive lipids PGE2, LTs and TXs. The low levels of AA, EPA and DHA could be a result of decreased activity of Δ6 and Δ5 desaturases. In view of this, administration of AA/EPA/DHA and/or more stable synthetic analogues of LXs, resolvins and protectins may prove to be useful in the prevention management and assessing prognosis of NAFLD and possibly other inflammatory diseases[91]. This proposal can be verified by estimating plasma, liver and adipose tissue content of AA, EPA, DHA, LXs, resolvins and protectins and the activity of Δ6 and Δ5 desaturases to the stage and activity of NASH and NAFLD. Periodic estimation of plasma, adipose and hepatic content of various PUFAs, LXs, resolvins and protectins and the activity of Δ6 and Δ5 desaturases and correlating them to the response to treatment is recommended. It is predicted that those in whom the plasma, adipose and hepatic content of various PUFAs, LXs, resolvins and protectins and the activity of Δ6 and Δ5 desaturases show an increase can be regarded as responding favorably to treatment while those in whom there is no change or decrease in the levels of these bioactive lipids and activity of Δ6 and Δ5 desaturases are likely to have progressive disease or unresponsive treatment that is being offered. Such patients need more aggressive therapy. Thus, plasma, adipose and hepatic content of various PUFAs, LXs, resolvins and protectins and the activity of Δ6 and Δ5 desaturases can be used to predict prognosis of NASH and NAFLD.