Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108946
Revised: June 2, 2025
Accepted: July 14, 2025
Published online: August 15, 2025
Processing time: 109 Days and 3.2 Hours
Wolfram syndrome is a rare autosomal recessive genetic disorder characterized by early-onset diabetes and progressive neurodegeneration, most notably sen
A 2-year-old Han Chinese girl was admitted with a 1-month history of polydipsia, polyuria, and polyphagia, and was diagnosed with diabetic ketoacidosis and im
This case identifies WFS1 c.986T>C (p.Phe329Ser) as a novel pathogenic variant causing Wolfram syndrome. It highlights the importance of early genetic testing in pediatric patients with atypical diabetes presentations to enable timely diagnosis, individualized therapy, and comprehensive family support.
Core Tip: A 2yearold girl with diabetic ketoacidosis and congenital deafness was found to carry a novel heterozygous WFS1 mutation (c.986T>C, p.Phe329Ser). Rapid wholeexome sequencing confirmed Wolfram syndrome, guiding timely me
- Citation: Gao AM, Deng WL, Yang XP, Wu WY, Ma CY, Liu Y. WFS1 gene mutation associated with pediatric diabetes mellitus and congenital deafness: A case report. World J Diabetes 2025; 16(8): 108946
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108946.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108946
Diabetes mellitus is a significant global public health issue and a major endocrine disorder impacting child health[1]. In recent years, the incidence of pediatric diabetes mellitus has progressively increased. Globally, there is a rising incidence of type 1 diabetes mellitus among children, especially those younger than 5 years old[2]. The global prevalence of Wolfram syndrome is estimated to be approximately 1 in 770000 in the United Kingdom, and 1 in 710000 in Japan[3-5]. In China, although large-scale epidemiological data are lacking, case reports have been increasingly published in recent years, suggesting potential underdiagnosis or misclassification as type 1 diabetes[6]. This geographic variability may be attributed to differences in genetic backgrounds, diagnostic awareness, and access to molecular testing. With rapid advancements in genetic sequencing technologies and precision medicine, an increasing number of specific types of diabetes, predominantly monogenic diabetes, have been distinguished from traditional classifications such as type 1 diabetes, type 2 diabetes, and gestational diabetes[7,8]. Wolfram syndrome has been formally recognized in the International Classification of Diseases 11th Revision as a distinct, rare subtype of diabetes mellitus (code 5A16.1), reflecting its unique genetic and clinical profile and underscoring the need for precise diagnostic classification.
Monogenic diabetes results from mutations in one or more genes that impair pancreatic β-cell function or insulin action, leading to dysregulated blood glucose levels[7,8]. This form of diabetes typically manifests early in life and includes neonatal diabetes, maturity-onset diabetes of the young (MODY), mitochondrial diabetes, and diabetes-related genetic syndromes. Despite representing only 1%-6% of all diabetes cases, monogenic diabetes has a low diagnosis rate due to its substantial clinical heterogeneity. More than 40 subtypes of monogenic diabetes have been identified, and mutations in these genes impair insulin secretion and/or insulin sensitivity, resulting in hyperglycemia and diabetes[9]. Research into monogenic diabetes provides novel insights into the pathophysiology, etiology, and potential treatments of diabetes mellitus. Several other genetic syndromes share overlapping clinical features with Wolfram syndrome, such as mitochondrial diabetes, which may also present with diabetes mellitus combined with sensorineural hearing loss[10]. Additionally, syndromes like Alström syndrome and certain mitochondrial DNA mutations present phenotypes that include early-onset diabetes and progressive neurodegeneration[11]. However, these conditions differ in their genetic etiology, clinical course, and associated systemic manifestations. This phenotypic overlap can complicate early diagnosis, highlighting the critical role of genetic testing to differentiate Wolfram syndrome from other rare monogenic or syndromic diabetes forms, thus enabling timely and precise patient management. In this report, we present the clinical diagnosis and management of a pediatric patient diagnosed with diabetes mellitus and congenital deafness associated with a heterozygous WFS1 gene mutation, aiming to provide reference for clinicians and reduce misdiagnosis and inappropriate treatment in similar cases.
A 2-year-old Han Chinese girl was admitted with a 1-month history of polydipsia, polyuria, and polyphagia, and recent-onset fever and cough. Her parents brought her to our hospital for further treatment on February 20, 2023.
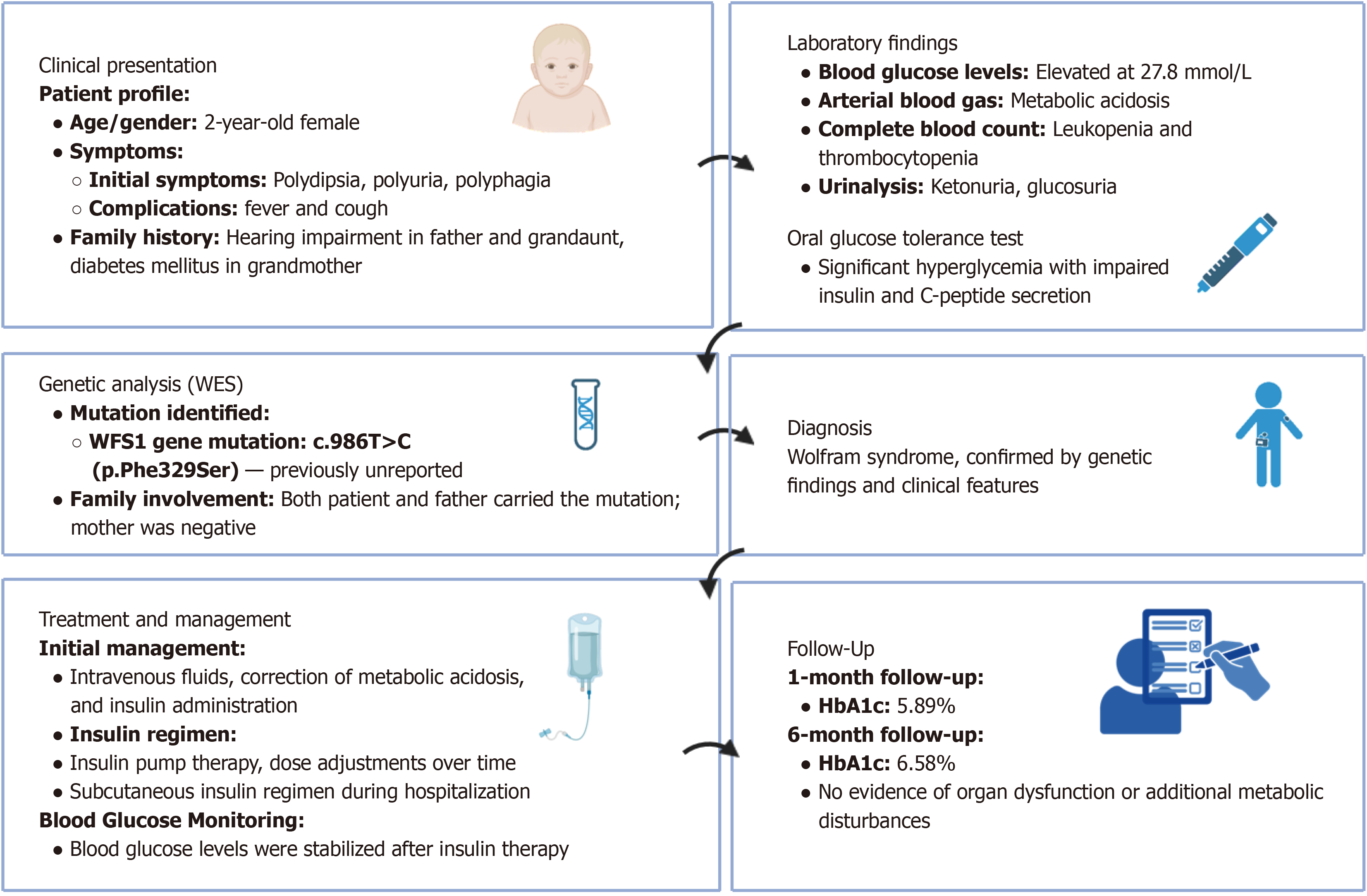
Figure 1 provides a graphical overview of the case report. The patient’s symptoms initially manifested without any identifiable triggers. Her daily milk consumption was approximately 800-900 mL, along with three meals daily predominantly composed of rice and sweet foods. She experienced increased nocturia (4-5 episodes nightly) but did not present with nocturnal enuresis, fatigue, altered consciousness, seizures, excessive sweating, or motor abnormalities. Two days before admission, lethargy, drowsiness, and fever (maximum temperature: 38.5 °C) developed, accompanied by pa
Medical history revealed sensorineural hearing loss diagnosed at 9 months of age, with subsequent cochlear implantation at 18 months. There was no history of mumps, pancreatitis, medication allergies, trauma, or prior surgeries.
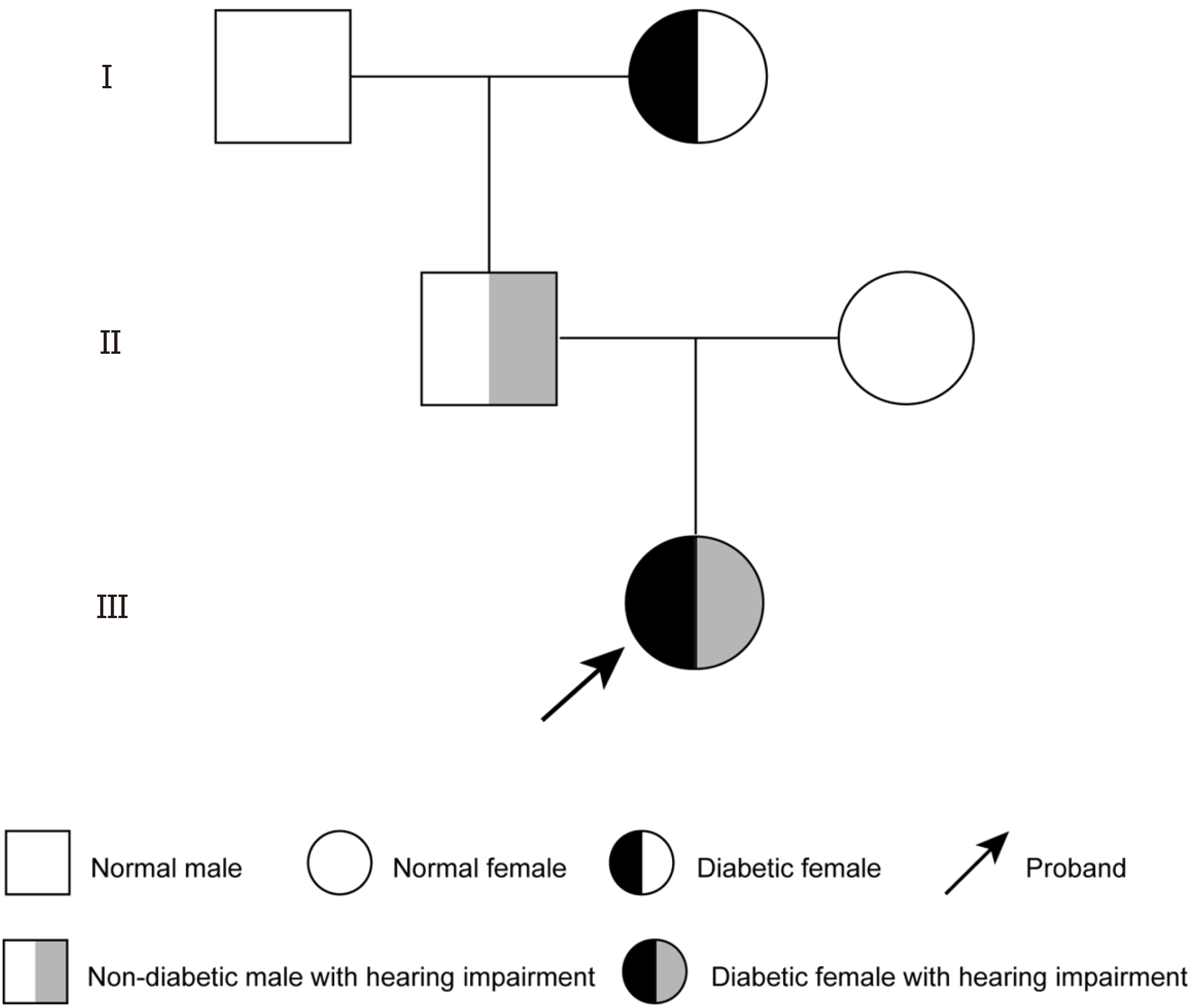
A detailed clinical history was obtained from the patient's parents, including symptom onset, progression, dietary habits, urination frequency, and family medical history. Family history indicated hearing impairment in her father and grandaunt (age at onset unknown), while her grandmother had diabetes mellitus (Figure 2). The patient was born at term by cesarean section, with a birth weight of 3.85 kg. She was breastfed after birth, and her parents were not consanguineous. No family history of diabetes was reported. The parents have no smoking or drinking habits. Apart from the father's history of abnormal hearing, the parents of the child patient have no other family medical history.
At admission, vital signs were as follows: Temperature 36.3 °C, pulse rate 121 bpm, respiratory rate 24 breaths per minute, weight 9 kg, oxygen saturation 96%, and blood pressure 82/42 mmHg. Clinical examination revealed lethargy, mild pallor, moderate dehydration evidenced by mildly sunken eyes and reduced tear secretion, grade I tonsillar enlargement, coarse breath sounds, and mild intercostal retractions. Abdominal and neurological examinations were unremarkable. A comprehensive assessment included evaluation of hydration status, respiratory and cardiovascular systems, abdominal examination, and neurological evaluation.
Initial laboratory results showed marked hyperglycemia (27.8 mmol/L), ketonuria, and metabolic acidosis (pH 7.26, bicarbonate 13.9 mmol/L). C-peptide and insulin levels were significantly reduced, and diabetes-related autoantibodies [zinc transporter 8 (ZnT-8), insulin autoantibodies (IAA), islet cell antibodies (ICA), glutamic acid decarboxylase (GAD)] were negative. Complete blood count revealed mild leukopenia and thrombocytopenia. Thyroid function and adrenal hormone levels were within normal ranges. An oral glucose tolerance test (OGTT), performed after stabilization of the patient’s acute metabolic condition, demonstrated persistent hyperglycemia and impaired insulin and C-peptide secretion, consistent with insulinopenic diabetes (Table 1). The OGTT was conducted not for initial diagnosis, which had already been established based on marked hyperglycemia and ketoacidosis, but to better characterize the patient’s glycemic profile and residual β-cell function during follow-up.
| Test parameter | 0 minute | 30 minutes | 60 minutes | 120 minutes | 180 minutes |
| Blood glucose (mmol/L) | 9.3 | 16.7 | 21.1 | 21.62 | 13.45 |
| Plasma insulin (μIU/mL) | 1.41 | 2.85 | 2.39 | 1.52 | 1.19 |
| C-peptide (nmol/L) | 0.176 | 0.23 | 0.271 | 0.23 | 0.27 |
Diagnostic imaging included abdominal ultrasonography and echocardiography to assess internal organs, and the results were unremarkable except for mild valvular regurgitation, while chest radiography demonstrated mildly increased pulmonary markings.
Whole-exome sequencing (WES) was performed using peripheral blood samples collected from the patient and both parents after obtaining informed consent. DNA was extracted from peripheral leukocytes using standard extraction kits following the manufacturer’s protocol. Whole-exome capture and sequencing were conducted on an Illumina NovaSeq platform (Illumina, San Diego, CA, United States). Data processing included alignment to the human reference genome (GRCh37/hg19) using the Burrows-Wheeler Aligner, variant calling using GATK software, and annotation of identified variants using databases such as dbSNP, 1000 Genomes Project, ExAC, and ClinVar. Candidate variants were evaluated for pathogenicity using in silico predictive tools including REVEL, SIFT, PolyPhen-2, MutationTaster, and GERP+. Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines. Confirmation of the identified variants in the patient and parents was performed by Sanger sequencing.
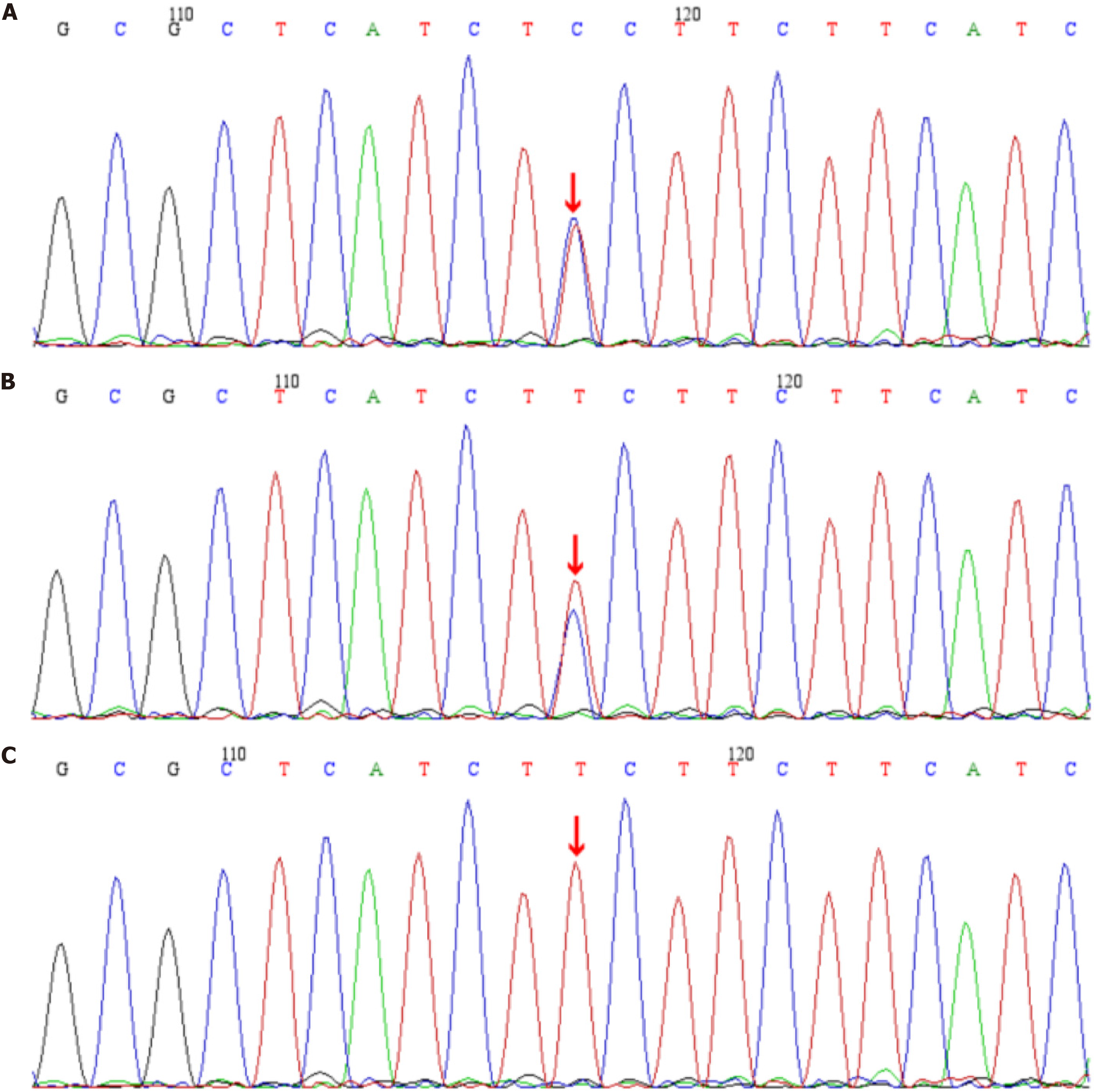
Based on clinical features, family history, and laboratory findings, the patient was diagnosed with: (1) Diabetes mellitus complicated by ketoacidosis; and (2) Congenital deafness. Given the early onset of diabetes, negative autoimmune antibodies, impaired insulin secretion, congenital hearing loss, and significant family history, WES was performed. Genetic analysis identified a previously unreported heterozygous WFS1 mutation (c.986T>C, p.Phe329Ser) in the patient and her father, while her mother had no mutation at this locus (Figure 3)[12]. Bioinformatic analyses (REVEL, SIFT, PolyPhen_2, MutationTaster, GERP+) classified this variant as likely pathogenic (PM2_Supporting+PP3_Strong+PM5) according to the ACMG guidelines, confirming the diagnosis of Wolfram syndrome[8]. To date, this mutation has not been reported in major public databases or the literature, expanding the known mutation spectrum.
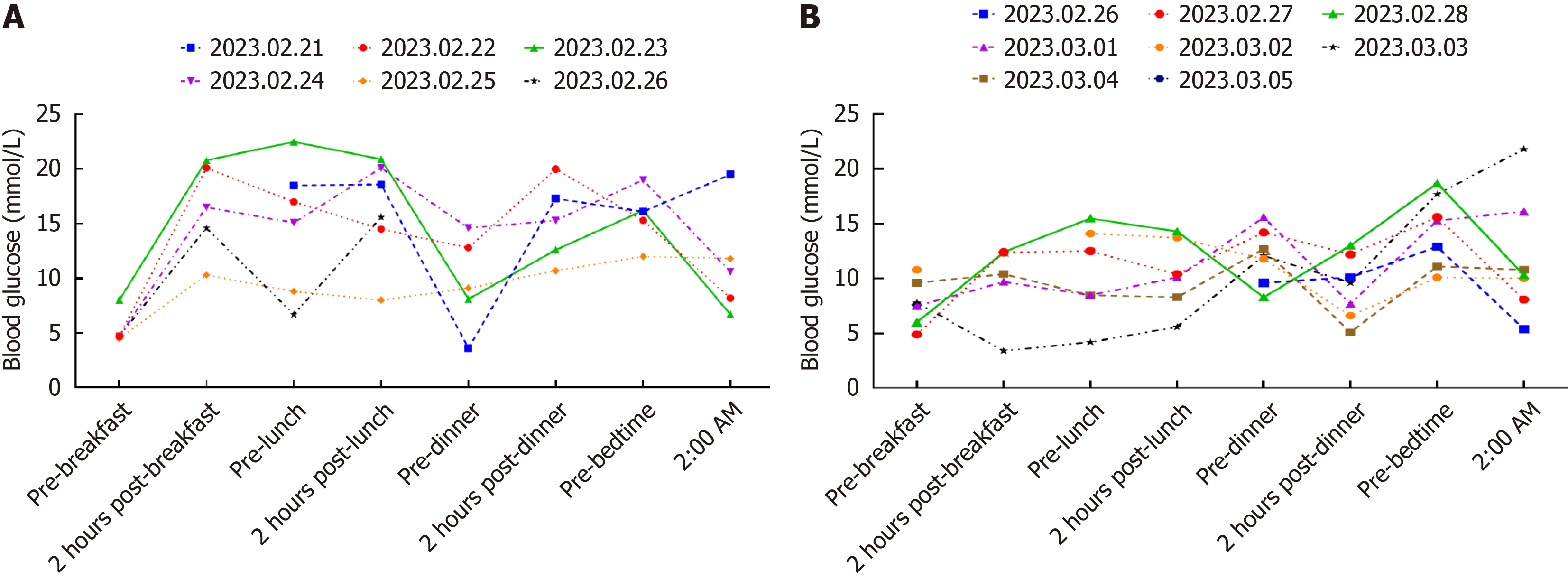
Initial treatment included intravenous fluid therapy, correction of metabolic acidosis, and insulin administration. Subsequently, glycemic control was maintained using continuous subcutaneous insulin infusion (Supplementary Table 1) and intermittent subcutaneous insulin injections (Supplementary Table 2), resulting in stabilized blood glucose levels (Figure 4).
Post-discharge, the patient underwent regular follow-up at 1- and 6-month intervals. Follow-up assessments included measurements of glycosylated hemoglobin (HbA1c), fasting plasma glucose, liver and renal function tests, blood lipids, electrolytes, thyroid function, and uric acid levels. Clinical evaluations were conducted to monitor growth, development, and potential complications. At the 1- and 6-month follow-ups, glycemic control was satisfactory (HbA1c: 5.89% and 6.58%, respectively), without evidence of organ dysfunction or additional metabolic disturbances.
Wolfram syndrome is a rare autosomal recessive monogenic disorder characterized by juvenile-onset diabetes mellitus, diabetes insipidus, optic nerve atrophy, and sensorineural hearing loss, with diabetes typically manifesting as the initial symptom[13,14]. The global incidence ranges from 1 in 100000 to 1 in 805000. This syndrome predominantly results from mutations in the WFS1 gene, with over 100 pathogenic missense mutations identified to date[15]. The WFS1 gene encodes an 890-amino acid transmembrane glycoprotein localized to the endoplasmic reticulum and highly expressed in pancreatic β-cells, neurons, and cardiomyocytes. Although the precise mechanisms are not fully elucidated, current evidence suggests that mutations in WFS1 disrupt endoplasmic reticulum calcium homeostasis, inducing endoplasmic reticulum stress, β-cell dysfunction, and subsequent apoptosis, thereby contributing to diabetes pathogenesis[16,17].
In this case report, a novel heterozygous WFS1 mutation (c.986T>C, p.Phe329Ser) was identified through WES in a 2-year-old patient presenting with diabetes mellitus complicated by ketoacidosis and congenital deafness. In addition to targeted analysis of the WFS1 variant found in the proband, the mother underwent extended sequencing to check for other potentially pathogenic variants in WFS1, and in additional genes associated with monogenic diabetes and syndromic forms of congenital deafness. No pathogenic or likely pathogenic variants were identified. As for the father, who is a heterozygous carrier of the p.Phe329Ser variant, we have recommended genetic counseling and long-term clinical surveillance, given the theoretical possibility of milder or subclinical manifestations. Importantly, he has been informed of the potential reproductive implications of his carrier status. In future pregnancies, options such as prenatal genetic testing or preimplantation genetic diagnosis will be discussed to assist the family in making informed reproductive choices.
The diagnosis of Wolfram syndrome can be challenging due to its heterogeneous presentation, potentially resulting in misdiagnosis as type 1 or type 2 diabetes[18]. Early clinical features such as juvenile-onset diabetes mellitus combined with sensorineural hearing loss should prompt clinicians to consider Wolfram syndrome. Importantly, negative diabetes-related autoantibodies (ZnT-8, IAA, ICA, GAD), as observed in this case, further support a monogenic rather than autoimmune etiology. Comprehensive genetic analysis is crucial for confirming the diagnosis and guiding appropriate genetic counseling, especially in asymptomatic family members who may carry the mutation.
A previously reported mutation at the same amino acid position (p.Phe329Ile) was identified through genetic screening in patients with longstanding type 1 diabetes, underscoring the complexity of monogenic diabetes diagnosis and the critical role of molecular genetic testing[12]. Bioinformatics analyses conducted in this report, including REVEL, SIFT, PolyPhen-2, MutationTaster, and GERP+, consistently classified the novel p.Phe329Ser mutation as likely pathogenic, further supporting its clinical significance. Nonetheless, we acknowledge that the p.Phe329Ser variant may exhibit variable phenotypic expressivity, including incomplete penetrance or a potential association with other diabetes subtypes, depending on the broader genetic or environmental context. To date, this specific variant has not been reported in published patient cohorts or public databases. While our findings expand the mutational spectrum of WFS1-related disease, we also recognize that experimental functional validation is crucial for definitive pathogenic classification, and such studies are planned in future work. This case highlights the utility of genetic testing in early diagnosis and family counseling for Wolfram syndrome. Additionally, as illustrated by prior examples-such as KLF11 variants initially thought to cause MODY but later reclassified as non-pathogenic[19]-we emphasize the need for cautious interpretation of novel variants. Bioinformatic predictions, although valuable, must be complemented by clinical correlation, functional assays, and independent cohort data. Therefore, we advocate a prudent and evidence-based approach to the classification of novel genetic findings.
Differentiating monogenic diabetes from other forms, such as type 1 or type 2 diabetes, requires careful consideration of clinical presentations and familial aggregation. Monogenic diabetes typically manifests earlier in life and often exhibits a clear family history, though de novo mutations or incomplete family history assessment can obscure this pattern[9,20]. Syndromic features like optic atrophy, hearing loss, and diabetes insipidus are important clinical indicators for monogenic syndromes such as Wolfram syndrome. Additionally, assessments of insulin sensitivity and the presence of concurrent endocrine disorders can facilitate accurate diagnosis and appropriate management strategies[21].
Early and definitive diagnosis of Wolfram syndrome is crucial not only for implementing appropriate multidisciplinary clinical management, but also for improving long-term patient outcomes and quality of life. Timely identification facilitates proactive interventions such as tailored insulin regimens, auditory rehabilitation, visual monitoring, and psychological support, which may slow disease progression and alleviate symptom burden. Furthermore, from a public health and policy perspective, accurate genetic diagnosis reinforces the need for strategic allocation of healthcare resources to expand access to molecular testing technologies. In resource-limited settings, underrecognition and underdiagnosis of monogenic diabetes may lead to treatment delays and mismanagement. Therefore, the integration of genetic screening into pediatric diabetes evaluation protocols, particularly for patients presenting with atypical features such as sensorineural hearing loss, is essential to improving diagnostic precision, optimizing treatment, and promoting health equity globally.
Monogenic diabetes, including Wolfram syndrome, represents a critical but often underrecognized subset of diabetes characterized by early onset and distinctive clinical manifestations. Early genetic testing is indispensable for children presenting with atypical features of diabetes, particularly those with sensorineural hearing loss, as it facilitates timely and accurate diagnosis. Genetic testing enables personalized treatment strategies and proactive management, which can significantly improve long-term patient outcomes and quality of life. This case highlights the need for increased clinical vigilance and the integration of genetic testing into routine evaluation of pediatric diabetes patients with unusual presentations. Continued research and comprehensive case reporting will further advance our understanding and optimize care for these rare disorders.
| 1. | Subspecialty Group of Endocrinologic; Hereditary and Metabolic Diseases, the Society of Pediatrics; Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. [Expert consensus on the standardized diagnosis and management of type 1 diabetes mellitus in Chinese children (2020)]. Zhonghua Er Ke Za Zhi. 2020;58:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61:12-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 3. | Urano F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr Diab Rep. 2016;16:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Zmysłowska A, Borowiec M, Fendler W, Jarosz-Chobot P, Myśliwiec M, Szadkowska A, Młynarski W. The prevalence of Wolfram syndrome in a paediatric population with diabetes. Endokrynol Pol. 2014;65:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Lopes CP, Gonçalves GF, Paulino MFVM, Esquiaveto-Aun AM, de Mello MP, Pavin EJ, Breder ISS, Pu MZMH, de Lemos-Marini SHV, Guerra G. Insights from a Wolfram syndrome cohort: clinical and molecular findings from a specialized diabetes reference center. Arch Endocrinol Metab. 2024;68:e240091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Xie Y, Xu K, Chang H, Zhang X, Li Y. Comprehensive Genetic Analysis Unraveled the Missing Heritability in a Chinese Cohort With Wolfram Syndrome 1: Clinical and Genetic Findings. Invest Ophthalmol Vis Sci. 2022;63:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 7. | Tsoi STF, Lim C, Ma RCW, Lau ESH, Fan B, Chow E, Kong APS, Chan JCN, Luk AOY. Monogenic diabetes in a Chinese population with young-onset diabetes: A 17-year prospective follow-up study in Hong Kong. Diabetes Metab Res Rev. 2024;40:e3823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Greeley SAW, Polak M, Njølstad PR, Barbetti F, Williams R, Castano L, Raile K, Chi DV, Habeb A, Hattersley AT, Codner E. ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1188-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Bonnefond A, Unnikrishnan R, Doria A, Vaxillaire M, Kulkarni RN, Mohan V, Trischitta V, Froguel P. Monogenic diabetes. Nat Rev Dis Primers. 2023;9:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (1)] |
| 10. | Karaa A, Goldstein A. The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes. Pediatr Diabetes. 2015;16:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Ferch M, Peitsch I, Kautzky-Willer A, Greber-Platzer S, Stättermayer AF, Krebs M, Scherer T. Effectiveness of the dual GIP/GLP1-agonist Tirzepatide in two cases of Alström syndrome, a rare obesity syndrome. J Clin Endocrinol Metab. 2025;dgaf258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Yu MG, Keenan HA, Shah HS, Frodsham SG, Pober D, He Z, Wolfson EA, D'Eon S, Tinsley LJ, Bonner-Weir S, Pezzolesi MG, King GL. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest. 2019;129:3252-3263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Salzano G, Rigoli L, Valenzise M, Chimenz R, Passanisi S, Lombardo F. Clinical Peculiarities in a Cohort of Patients with Wolfram Syndrome 1. Int J Environ Res Public Health. 2022;19:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Lee EM, Verma M, Palaniappan N, Pope EM, Lee S, Blacher L, Neerumalla P, An W, Campbell T, Brown C, Hurst S, Marshall B, Hershey T, Nunes V, López de Heredia M, Urano F. Genotype and clinical characteristics of patients with Wolfram syndrome and WFS1-related disorders. Front Genet. 2023;14:1198171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | De Franco E, Flanagan SE, Yagi T, Abreu D, Mahadevan J, Johnson MB, Jones G, Acosta F, Mulaudzi M, Lek N, Oh V, Petz O, Caswell R, Ellard S, Urano F, Hattersley AT. Dominant ER Stress-Inducing WFS1 Mutations Underlie a Genetic Syndrome of Neonatal/Infancy-Onset Diabetes, Congenital Sensorineural Deafness, and Congenital Cataracts. Diabetes. 2017;66:2044-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Wang L, Liu H, Zhang X, Song E, Wang Y, Xu T, Li Z. WFS1 functions in ER export of vesicular cargo proteins in pancreatic β-cells. Nat Commun. 2021;12:6996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Barrabi C, Zhang K, Liu M, Chen X. Pancreatic beta cell ER export in health and diabetes. Front Endocrinol (Lausanne). 2023;14:1155779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Zmyslowska A, Borowiec M, Fichna P, Iwaniszewska B, Majkowska L, Pietrzak I, Szalecki M, Szypowska A, Mlynarski W. Delayed recognition of Wolfram syndrome frequently misdiagnosed as type 1 diabetes with early chronic complications. Exp Clin Endocrinol Diabetes. 2014;122:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Mancera-Rincón P, Luna-España MC, Rincon O, Guzmán I, Alvarez M. Maturity-onset Diabetes of the Young Type 7 (MODY7) and the Krüppellike Factor 11 Mutation (KLF11). A Review. Curr Diabetes Rev. 2024;20:e210323214817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Zhang H, Colclough K, Gloyn AL, Pollin TI. Monogenic diabetes: a gateway to precision medicine in diabetes. J Clin Invest. 2021;131:e142244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 21. | Rigoli L, Caruso V, Salzano G, Lombardo F. Wolfram Syndrome 1: From Genetics to Therapy. Int J Environ Res Public Health. 2022;19:3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |