Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108724
Revised: May 25, 2025
Accepted: July 9, 2025
Published online: August 15, 2025
Processing time: 113 Days and 2.8 Hours
Type 1 diabetes mellitus (T1DM) is an autoimmune disease with a multifactorial pathogenesis. Viral infections have been proposed as contributing triggers, sup
To investigate the incidence and seasonality of T1DM before and during COVID-19 pandemic in relation to global viral infection rates.
This population-based retrospective study utilized a nationwide computerized database. Extracted data included the number of new T1DM cases over the 8 years preceding and during the COVID-19 pandemic, demographic characteristics of affected individuals, and nationwide respiratory virus polymerase chain reaction data from weekly nasal wash sample collections.
A total of 2176 patients were diagnosed with new-onset T1DM during the pre-pandemic period, compared to 348 cases during the pandemic. In the same periods, 33727 respiratory virus-positive polymerase chain reaction results from nasal wash samples were recorded pre-pandemic, compared to 2603 during the pandemic. Additionally, 363399 positive COVID-19 cases were reported during the pandemic period. Seasonality analysis revealed a higher rate of new-onset T1DM cases and a weaker seasonal pattern during the pandemic. Trend analysis showed a consistent increase in T1DM incidence prior to COVID-19, with a more variable trend observed during the pandemic. Correlation analysis between T1DM incidence and respiratory viruses demonstrated a weak correlation between T1DM incidence and a few respiratory viruses.
The observed increase in new-onset T1DM cases and the disruption of its typical seasonal pattern during the COVID-19 pandemic suggest a potential association between respiratory virus exposure and the development of T1DM.
Core Tip: This population-based study assessed incidence and seasonality of type 1 diabetes mellitus (T1DM) before and during the coronavirus disease 2019 pandemic. A higher rate of new-onset T1DM and diminished seasonal pattern were observed during the pandemic, coinciding with reduced circulation of common respiratory viruses due to lockdown measures. These findings suggest respiratory viruses, including severe acute respiratory distress syndrome corona virus-2, may serve as environmental triggers for T1DM. Weak positive correlations were identified between T1DM incidence and respiratory viruses (e.g., respiratory syncytial virus, influenza). The results support a potential link between viral exposure and T1DM pathogenesis, highlighting the need for further research into underlying mechanisms and preventive strategies.
- Citation: Carmon L, Bachar Y, Babiev AS, Hazn I, Hershkovitz E, Shaki D, Loewenthal N, Haim A, Hazan G. Impact of COVID-19 outbreak on the seasonality and incidence of type 1 diabetes mellitus: A nationwide cohort study. World J Diabetes 2025; 16(8): 108724
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108724.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108724
Type 1 diabetes mellitus (T1DM) is a T cell-mediated autoimmune disorder that targets pancreatic beta cells[1,2], leading to insulin deficiency, hyperglycemia, and, in some cases, diabetic ketoacidosis, a life-threatening complication[3]. The global incidence of T1DM has been rising over the past several decades, reaching approximately 15 cases per 100000 individuals per year[4,5]. Both genetic predisposition and environmental exposures contribute to T1DM pathogenesis[1,2].
Several viruses, including enterovirus, rubella, mumps, rotavirus, parvovirus, and cytomegalovirus, have been proposed as potential environmental triggers for T1DM[1,2]. Suggested mechanisms include molecular mimicry, direct beta cell cytotoxicity, and regulatory T cell dysfunction[2]. Although respiratory viruses are less frequently implicated, some studies suggest they may also play a contributing role[6,7].
Seasonality refers to regular, periodic fluctuations that occur within a 1-year period. In time series analysis, Fourier terms are mathematical components used to model and quantify seasonal variation. Epidemiological studies have shown a seasonal pattern in T1DM incidence, with increased case rates during autumn and winter[8], potentially reflecting seasonal viral exposures[1,2,8-11]. While seasonality may be influenced by geographical position[8] and patient age[9], it has been consistently observed across populations.
The coronavirus disease 2019 (COVID-19) pandemic, which began in March 2020, resulted in widespread public health measures, including lockdowns, movement restrictions, and mandatory face mask use. These interventions led to a marked decline in seasonal respiratory virus infections during the first year of the pandemic[12,13]. This epidemiological shift provided a unique opportunity to examine the relationship between respiratory virus circulation, particularly COVID-19, and the incidence of T1DM, as well as the effect of lockdowns on T1DM seasonality as an indirect indicator of other respiratory viruses as potential triggers. Several studies have reported an increase in T1DM incidence during the pandemic[14-17], while others found no significant change[18-20], and one systematic review yielded inconclusive results[21]. Some studies also noted a reduction in the typical seasonal variation of T1DM incidence during COVID-19 period[14,15,18-20]. One possible explanation is that decreased exposure to respiratory viruses during lockdown may have attenuated their role as environmental triggers for T1DM. This study aimed to investigate the correlation between respiratory virus exposure and the incidence of T1DM before and during the COVID-19 Lockdown, and to assess the impact of viral exposure on the seasonal pattern of T1DM incidence.
This population-based retrospective study utilized a nationwide computerized database from Clalit Healthcare Services (CHS) electronic medical records (the MDCLONE system). The CHS database includes extensive demographic data, anthropometric measurements, diagnoses from community clinics and hospitals, medication dispensing records, and comprehensive laboratory results. CHS is the largest health maintenance organization in Israel, serving over 5 million patients. All data were de-identified prior to analysis, and the study involved secondary use of existing clinical records.
The study period was divided into two intervals: The pre-COVID-19 period (“pre-pandemic”, January 2012 to February 2020) and the COVID-19 period (“pandemic”, March 2020 to February 2021), consistent with a previous study[12]. Extracted data included: (1) The number of participants aged 0-18 years with at least two insulin prescriptions, used as a proxy for T1DM diagnosis, following prior methodology[19]. The date of the first insulin prescription was considered the diagnosis date. Demographic variables recorded were age at diagnosis, sex, and ethnicity; and (2) Nationwide respiratory virus polymerase chain reaction (PCR) data from nasal wash samples collected weekly across all Clalit institutions, without age restrictions. Samples were categorized by virus type, and different test methods (e.g., antigen and PCR) were combined. New T1DM diagnoses were defined as those occurring more than 90 days after a previous diagnosis. IgG and IgM antibody test results were excluded. The study protocol was approved by the Medical Ethics Committee of Review Board of Soroka University Medical Center.
Descriptive analyses were performed to compare T1DM incidence between the pre-pandemic and pandemic periods. Population-based incidence rates were calculated by dividing the number of new T1DM diagnoses by the total number of CHS members. Statistical analysis began with an assessment of data distribution and measures of central tendency. Temporal trends in T1DM incidence were evaluated using Poisson regression models adjusted for time periods. Seasonal patterns were analyzed using an additive generalized linear regression model incorporating Fourier terms. The time series model was defined as: “Yt = α0 + α2t + α2t2 + α3t3 + b1cos(ωt) + c1sin(ωt) + ϵt” where Yt is the number of diabetes cases at time t, α terms represent polynomial trend components, ω denotes yearly periodicity (ω = 2π/365), and sine and cosine terms (Fourier terms) capture seasonal variation. The term ϵt represents random variation. Poisson regression was used to account for count-based data.
For the pre-pandemic period, associations between respiratory viruses and T1DM incidence were analyzed using weekly aggregated data. Spearman correlation tests were conducted between the number of T1DM diagnoses and each type of viral infection This was followed by individual Poisson regression analyses that controlled for seasonal effects. The analysis was performed at the population level, not at the level of individual T1DM patients. All statistical analyses were conducted using R software (version 4.2.3; R Core Team, 2023).
A total of 2524 patients with new-onset T1DM were included, comprising 2176 cases during the pre-pandemic period and 348 during the pandemic period. Approximately half of the participants were female, 39% were of Bedouin ethnicity, and the median age at diagnosis was 11 years (interquartile range: 7-14). No significant demographic differences were observed between the two groups (Table 1).
| Characteristic | Pre-COVID-19 period (n = 2176) | COVID-19 period (n = 348) | P value1 | Overall (n = 2524) |
| Gender, male, n (%) | 1132 (52) | 186 (53) | 0.6 | 1318 (52) |
| Ethnicity, Arab, n (%) | 858 (39) | 122 (35) | 0.12 | 980 (39) |
| Age at diagnosis (years), median (IQR) | 11.0 (7.0-14.0) | 11.0 (8.0-13.0) | 0.7 | 11.0 (7.0-14.0) |
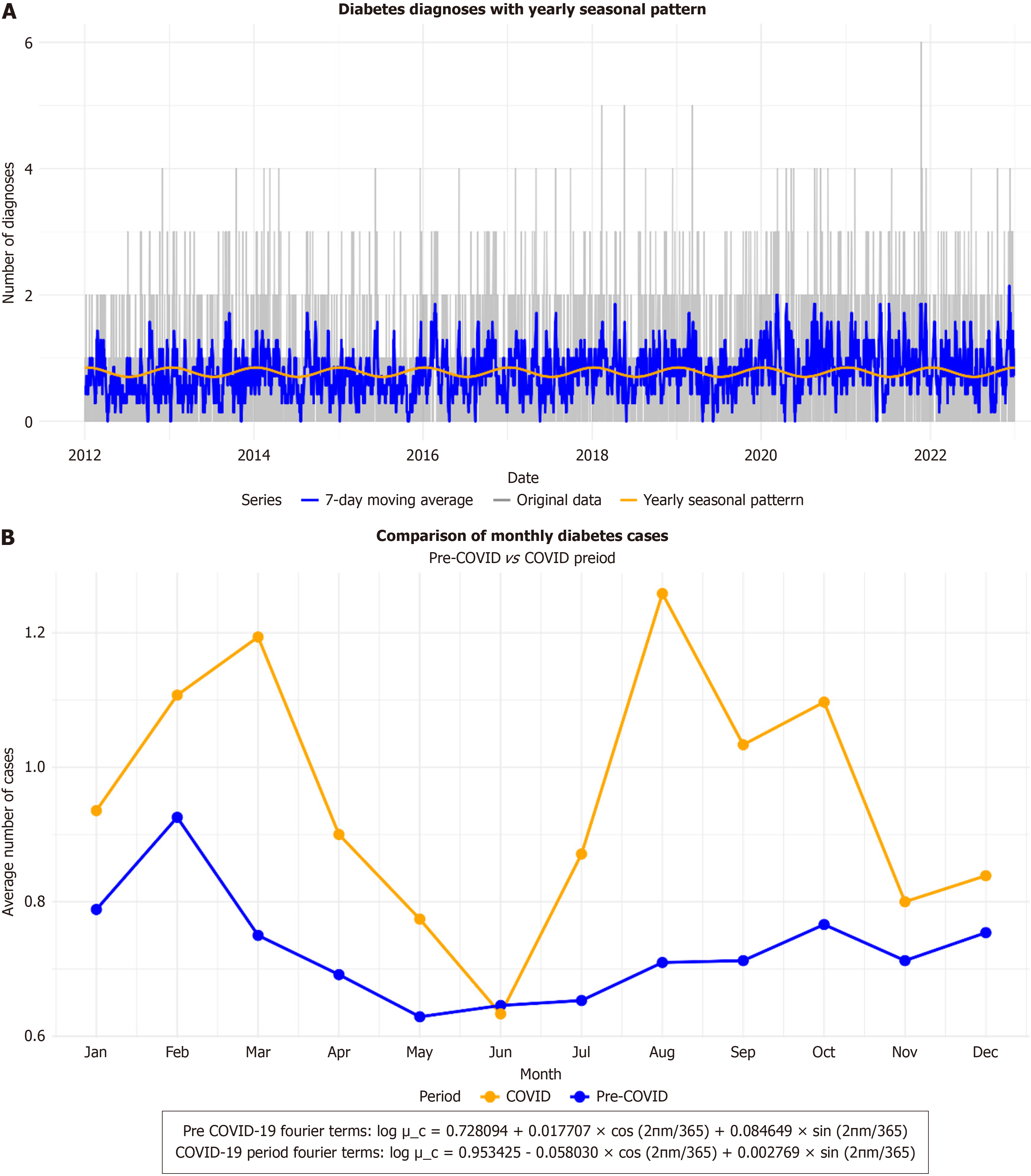
Seasonality analysis of T1DM was conducted separately for the pre-pandemic and pandemic periods. During the pre-pandemic period, 33727 viral-positive PCR results from nasal wash samples were recorded, compared to 2603 during the pandemic. Additionally, 363399 positive COVID-19 cases were reported throughout the pandemic period. A clear seasonal pattern was observed pre-pandemic, with higher T1DM incidence during autumn and winter (Figure 1).
During the pre-pandemic period, the seasonality analysis yielded a log value of 0.728094, with a small positive cosine term and a larger sine term, indicating a moderate seasonal pattern characterized by rhythmic sinusoidal waves (Figure 1A). In contrast, the pandemic period showed an increased average log value of 0.953425, reflecting a higher rate of new-onset T1DM cases. However, the cosine term was negative and the sine term was minimal, indicating a weakened seasonal pattern during this time (yellow line in Figure 1B).
Trend analysis revealed a steady increase in T1DM incidence throughout the pre-pandemic period, whereas the trend during the pandemic period was more variable (Supplementary Figure 1). Correlation analysis using Spearman’s method showed weak positive correlations between T1DM incidence and several respiratory viruses, including respiratory syncytial virus (RSV) (commonly referred to as RSV), parainfluenza, influenza, and adenovirus (Table 2, Supplementary Figure 2).
| Value | Total viruses | Adeno | Entero | Influenza A | Influenza B | Parainfluenza | HMPV | RSV | Rhino |
| Rho | 0.193 | 0.196 | 0.045 | 0.196 | 0.165 | 0.151 | 0.032 | 0.130 | 0.077 |
| P value | < 0.001 | < 0.001 | 0.343 | < 0.001 | < 0.001 | 0.001 | 0.49 | 0.006 | 0.107 |
This study identified a moderate seasonal pattern of new-onset T1DM cases during the pre-pandemic period, characterized by higher incidence in autumn and winter. In contrast, during the pandemic period, there was an almost complete loss of this seasonal pattern. The question of triggers and causality in T1DM pathogenesis remains unresolved[22,23]. However, the potential role of respiratory viral infections as contributing factors has been widely explored[6,7], with supporting evidence from the observed seasonality of T1DM incidence, which peaks in autumn and winter[8,9].
During the pandemic period, the incidence of new-onset T1DM was higher compared to the pre-pandemic period, consistent with findings from previous studies[14-17]. This increase may suggest COVID-19 as a potential trigger for T1DM[24]. Proposed mechanisms for COVID-19’s impact on pancreatic beta cells include direct cellular damage as well as enhanced autoimmunity through epitope spreading, molecular mimicry, and bystander activation[24].
However, alternative explanations for the shifts in T1DM incidence during the COVID-19 pandemic have been proposed, including limited access to medical care, changes in healthcare-seeking behavior, and increased stress[16,18]. The seasonal pattern observed in the pre-pandemic period was diminished during the pandemic, consistent with findings from other studies[18-20]. Given the marked reduction in respiratory virus transmission during COVID-19 Lockdowns[12], our results suggest that exposure to respiratory viruses may influence T1DM seasonality and contribute to its development. Moreover, the increased incidence of T1DM during the pandemic and the potential role of COVID-19 as a trigger support this hypothesis. Initial post-pandemic trend data indicating a decline in T1DM incidence may further reinforce this theory[25].
Influenza, parainfluenza, adenovirus, and RSV were found to have a weak positive correlation with T1DM incidence in the pre-pandemic period. These viruses are known to follow distinct seasonal patterns[26], and our findings suggest they may contribute to the observed seasonality of T1DM incidence. This study’s strengths include a large, population-based cohort and an extended pre-pandemic observation period, enabling robust trend and seasonality analyses. However, several limitations should be noted. The relatively short duration of the pandemic period (1 year) restricts the depth of seasonal trend evaluation during this time frame. Additionally, the use of insulin prescription as a proxy for new-onset T1DM, while consistent in previous studies[19], may lead to misclassification, as insulin is occasionally prescribed for type 2 diabetes. Nonetheless, limiting the cohort to individuals aged 0-18 years likely mitigated this concern. Further research is needed to clarify the role of respiratory viruses in T1DM pathogenesis, elucidate underlying causal me
This study suggests that the diminished seasonal variation in T1DM incidence observed during the COVID-19 Lockdown, alongside an overall increase in new cases, may reflect the potential role of respiratory viruses, including severe acute respiratory distress syndrome corona virus-2, as environmental triggers for T1DM. These findings underscore the need for further research to confirm causal relationships and to inform preventive and public health strategies.
| 1. | Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863-2871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | van der Werf N, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev. 2007;23:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 4. | Gomez-Lopera N, Pineda-Trujillo N, Diaz-Valencia PA. Correlating the global increase in type 1 diabetes incidence across age groups with national economic prosperity: A systematic review. World J Diabetes. 2019;10:560-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 5. | Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10:98-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 6. | Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, Burkhardt BR, Briese T, Hagopian WA, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Hyöty H; TEDDY Study Group. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med. 2009;26:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Samuelsson U, Carstensen J, Löfman O, Nordfeldt S. Seasonal variation in the diagnosis of type 1 diabetes in south-east Sweden. Diabetes Res Clin Pract. 2007;76:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Afoke A, Ludvigsson J, Hed J, Lindblom B. Raised IgG and IgM in "epidemic" IDDM suggest that infections are responsible for the seasonality of type I diabetes. Diabetes Res. 1991;16:11-17. [PubMed] |
| 11. | Principi N, Berioli MG, Bianchini S, Esposito S. Type 1 diabetes and viral infections: What is the relationship? J Clin Virol. 2017;96:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Hazan G, Fox C, Mok H, Haspel J. Age-dependent rebound in asthma exacerbations after COVID-19 lockdown. J Allergy Clin Immunol Glob. 2022;1:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Amram T, Duek OA, Golan-Tripto I, Goldbart A, Greenberg D, Hazan G. The Interplay Between Respiratory Syncytial Virus and Asthma Inception: Insights Gained From the COVID-19 Pandemic. Pediatr Pulmonol. 2025;60:e27474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | McKeigue PM, McGurnaghan S, Blackbourn L, Bath LE, McAllister DA, Caparrotta TM, Wild SH, Wood SN, Stockton D, Colhoun HM. Relation of Incident Type 1 Diabetes to Recent COVID-19 Infection: Cohort Study Using e-Health Record Linkage in Scotland. Diabetes Care. 2023;46:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Schiaffini R, Deodati A, Rapini N, Pampanini V, Cianfarani S. Increased incidence of childhood type 1 diabetes during the COVID-19 pandemic. Figures from an Italian tertiary care center. J Diabetes. 2022;14:562-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Rahmati M, Keshvari M, Mirnasuri S, Yon DK, Lee SW, Il Shin J, Smith L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J Med Virol. 2022;94:5112-5127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 17. | Vlad A, Serban V, Timar R, Sima A, Botea V, Albai O, Timar B, Vlad M. Increased Incidence of Type 1 Diabetes during the COVID-19 Pandemic in Romanian Children. Medicina (Kaunas). 2021;57:973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Kostopoulou E, Eliopoulou MI, Rojas Gil AP, Chrysis D. Impact of COVID-19 on new-onset type 1 diabetes mellitus - A one-year prospective study. Eur Rev Med Pharmacol Sci. 2021;25:5928-5935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | van den Boom L, Kostev K, Kuss O, Rathmann W, Rosenbauer J. Type 1 diabetes incidence in children and adolescents during the COVID-19 pandemic in Germany. Diabetes Res Clin Pract. 2022;193:110146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Reschke F, Lanzinger S, Herczeg V, Prahalad P, Schiaffini R, Mul D, Clapin H, Zabeen B, Pelicand J, Phillip M, Limbert C, Danne T; SWEET Study Group. The COVID-19 Pandemic Affects Seasonality, With Increasing Cases of New-Onset Type 1 Diabetes in Children, From the Worldwide SWEET Registry. Diabetes Care. 2022;45:2594-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Nassar M, Nso N, Baraka B, Alfishawy M, Mohamed M, Nyabera A, Sachmechi I. The association between COVID-19 and type 1 diabetes mellitus: A systematic review. Diabetes Metab Syndr. 2021;15:447-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Duncan LJP, Clarke BF. Changing Concepts of the Cause of Diabetes Mellitus. Res Medica. 2013;5. [DOI] [Full Text] |
| 23. | Buschard K. What causes type 1 diabetes? Lessons from animal models. APMIS Suppl. 2011;1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Guo H, Wang G, Zhai J, Du B. COVID-19 as a Trigger for Type 1 Diabetes. J Clin Endocrinol Metab. 2023;108:2176-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Kamrath C, Eckert AJ, Lignitz S, Hillenbrand N, Dost A, Warncke K, Klose D, Grohmann-Held K, Holl RW, Rosenbauer J. Wave in Pediatric Type 1 Diabetes Incidence After the Emergence of COVID-19: Peak and Trough Patterns in German Youth-A Population-Based Study From the Prospective Multicenter DPV Registry. Diabetes Care. 2025;48:e47-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. 2020;7:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 655] [Article Influence: 131.0] [Reference Citation Analysis (0)] |