Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108310
Revised: May 19, 2025
Accepted: July 14, 2025
Published online: August 15, 2025
Processing time: 125 Days and 16.5 Hours
This editorial comments on the study by Yang et al, emphasizing the Ras homolog enriched in brain 1 (Rheb1) core function in restoring functional β-cell mass in diabetes, as crucial for β-cell proliferation and survival. It has been revealed that Rheb1 promotes β-cell regeneration through a dual pathway, activating mamma
Core Tip: This editorial emphasizes the critical role of β-cells in diabetes risk, particularly regarding brain dysfunction and ulceration in young individuals, and underscores the need for further investigation. Recent studies, including that of Yang et al, demonstrate the key influence of Ras homolog enriched in brain 1 on β-cell function and proliferation, which is linked to insulin secretion and glucose levels. This suggests that Ras homolog enriched in brain 1 influences the mammalian target of rapamycin complex 1 and AMP-activated protein kinase pathways, confirming the existence of the islet reflex instead of relying solely on one pathway. This metabolic role opens up therapeutic avenues for advanced treatment targets of diabetes-related brain dysfunction.
- Citation: Gouda MM. Rheb1 as a novel β-cell regulator connecting mTORC1, AMPK, and NOTCH1 pathways for efficient diabetes therapy. World J Diabetes 2025; 16(8): 108310
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108310.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108310
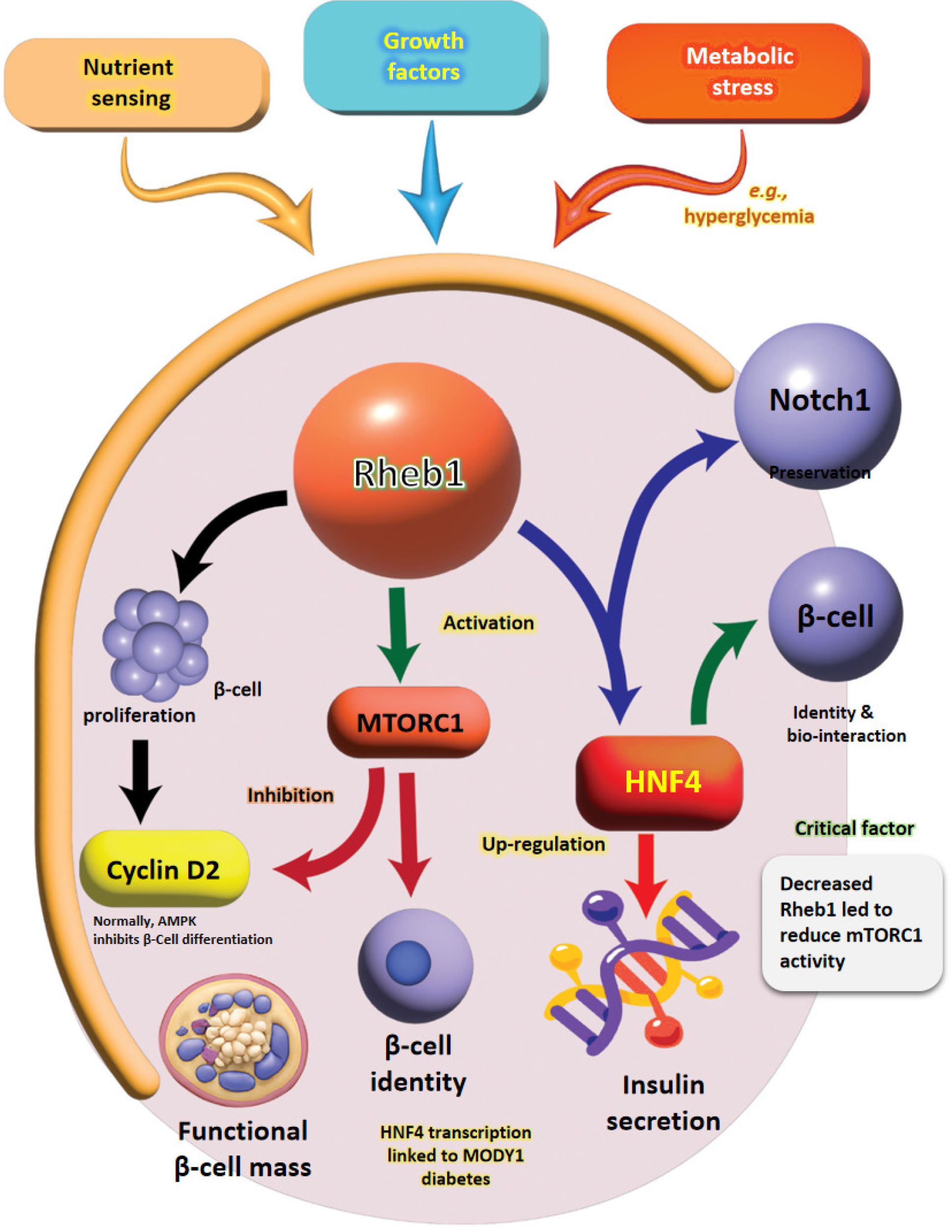
Diabetes mellitus poses a significant global health crisis characterized by β-cell dysfunction and insufficient insulin secretion[1]. While current therapies predominantly emphasize glycemic control, restoring functional β-cell mass remains a critical challenge[2]. In this context, Yang et al[3] shed light on the essential role of Ras homolog enriched in brain (Rheb) in regulating β-cell proliferation and identity through the mammalian target of rapamycin complex 1 (mTORC1)/AMP-activated protein kinase (AMPK)/neurogenic locus notch homolog protein 1 (NOTCH1) signaling pathways. Specifically, Rheb, a GTPase, activates mTORC1 signaling, vital for both β-cell proliferation and maintenance[4]. It was established that Rheb1 promotes β-cell proliferation through mTORC1 activation while simultaneously inhibiting AMPK, thereby preserving β-cell identity via the mTORC1 and NOTCH1 pathways[3,5].
This interplay suggests that manipulating Rheb1 might serve as a promising therapeutic strategy for diabetes management. The regulation of β-cell mass is complex, necessitating a balance between proliferation, dedifferentiation, and neogenesis. As a pivotal mTORC1 activator, Rheb1 effectively integrates nutrient and energy-sensing pathways, highlighting its multifaceted role in β-cell biology[6]. Previous research by Ardestani et al[7] has demonstrated that mTORC1 activation leads to increased β-cell proliferation, although the underlying mechanisms have yet to elucidate fully. In addition, Yang et al[3] findings reveal that Rheb1 not only stimulates mTORC1 but also inhibits AMPK signaling, which plays a crucial role in enhancing β-cell expansion. AMPK functions as a cellular energy sensor, typically inhibiting mTORC1 activation under low-energy conditions, which are often associated with diabetes[8]. By suppressing AMPK, Rheb1 effectively prevents growth arrest, fostering sustained β-cell proliferation and suggesting its role as a molecular switch. This switch helps align proliferative and metabolic signals to maintain β-cell homeostasis.
Furthermore, Rheb1 integrates Wnt and energy signals through phosphorylation by AMPK and glycogen synthase kinase-3, indicating its involvement in multiple pathways. Beyond its role in promoting proliferation, Rheb1 is also essential for preserving β-cell identity by activating NOTCH1 signaling, which is closely linked to cell fate determination. Recent evidence indicates that β-cell dedifferentiation plays a significant role in the pathogenesis of diabetes, reinforcing the importance of maintaining β-cell function. In addition, Cai et al[9] mentioned that the upstream inhibitor Grb10, known to inhibit mTORC1, promotes β-cell dedifferentiation, further emphasizing the critical role of mTORC1 in maintaining β-cell function. Therefore, Rheb1 emerges as a central regulator that increases β-cell mass and protects against functional decline. This editorial highlights Rheb1’s novel contribution to diabetes research. The authors in the current study convincingly demonstrate that Rheb1 enhances β-cell proliferation through the dual modulation of mTORC1 and AMPK while concurrently preserving identity through NOTCH1 signaling and interaction with hepatocyte nuclear factor 4 alpha (HNF4α). They position Rheb1 as a potential therapeutic target for diabetes, advancing a multi-pathway approach to β-cell regeneration.
The key mechanistic findings include dual regulation of β-Cell proliferation via mTORC1 and AMPK like Rheb1 promotes β-cell proliferation by activating mTORC1 (increasing cyclin D2) while inhibiting AMPK (Table 1). Altogether, the link between lower Rheb1 Levels and reduced mTORC1 activity in older tissues demonstrates how metabolic dysfunction contributes to inadequate insulin secretion and β-cell mass loss, key features of diabetes. Moreover, the evidence gathered from β-cell-specific Rheb1 knockout mice illustrates Rheb1’s essential role in maintaining β-cell mass and proliferation. Additionally, the discovery that Rheb1 suppresses AMPK in β-cells contrasts with its role in other metabolic tissues, where AMPK activation is generally beneficial for glucose homeostasis (Figure 1). This divergence underscores the complexity of targeting Rheb1 in diabetes and highlights the importance of further mechanistic studies. Besides, the reduction in the anti-apoptotic genes, such as those from the B-cell lymphoma-2 family, suggests that impaired mTORC1 signaling due to the absence of Rheb1 contributes significantly to β-cell failure[10]. Notably, the decreased phos
| Aspect | Yang et al’s study[3] | Prior studies | Implications | Ref. |
| Rheb1 & β-cell proliferation | Dual regulation via mTORC1 and AMPK (Figure 4 in the study of Yang et al[3]) | mTORC1 alone drives β-cell growth | Reveals AMPK as a critical co-regulator | [16] |
| β-cell identity | NOTCH1 activation prevents dedifferentiation (Figure 5 in the study of Yang et al[3]) | mTORC1 maintains identity | Suggests NOTCH1 as a new therapeutic axis | [5] |
| HNF4α interaction | Rheb1 binds and upregulates HNF4α (Figure 6 in the study of Yang et al[3]) | HNF4α mutations cause MODY1 | Links Rheb1 to genetic diabetes mechanisms | [13] |
| Age-dependent Rheb1 | Higher in young human islets (Figure 1A in the study of Yang et al[3]) | β-cell replication declines with age | Supports rejuvenation strategies targeting Rheb1 | [8] |
The study’s findings open new avenues for diabetes through restoring β-cell mass, where Rheb1 overexpression could enhance β-cell regeneration in type 1 diabetes and late-stage type 2 diabetes[3,5,11]. Combined mTORC1 activation and AMPK inhibition may be more effective than single-pathway targeting[8]. Moreover, preventing β-cell NOTCH1 agonists could complement Rheb1-based therapies to maintain functional β-cells[3,12]. Personalized medicine by regulating HNF4α through Rheb1 benefits patients with HNF4α mutations (MODY1) from Rheb1 modulation[13]. Given its pivotal role in β-cell proliferation and identity maintenance, Rheb1 represents a promising therapeutic target for diabetes. Current strategies to restore β-cell mass, such as exogenous insulin administration or islet transplantation, are limited by their inability to address the underlying loss of functional β-cells.
The pharmacological modulation of Rheb1 could offer a novel approach that promotes proliferation and prevents dedifferentiation. Besides, mTORC1 inhibitors like rapamycin have been explored for their anti-diabetic effects but often exhibit paradoxical outcomes due to their broad suppression of anabolic processes. The data in Yang et al’s study[3] suggest that selectively enhancing Rheb1 activity, rather than globally inhibiting mTORC1, may provide a more precise strategy. Small-molecule activators of Rheb1 or inhibitors of its negative regulators (e.g., tuberous sclerosis complex 1/2) could be investigated for their ability to boost β-cell mass without disrupting metabolic homeostasis. Moreover, the interplay between opens new avenues for combination therapies. Since AMPK activation alone does not fully reverse Rheb1-induced proliferation, co-targeting both pathways might optimize therapeutic efficacy. For instance, metformin, an AMPK activator, could be combined with Rheb1 modulators to fine-tune β-cell growth while maintaining energy balance. Finally, the role of Rheb1 in NOTCH1 signaling suggests potential applications in preventing β-cell dedifferentiation. Notch pathway modulators, currently under investigation in cancer and regenerative medicine, could be repurposed to enhance β-cell stability in diabetic patients.
The study bridges pancreatic stress responses and β-cell adaptation, highlighting the metabolic stress (e.g., hyper
Yang et al[3] reported that Rheb1 overexpression enhances β-cell proliferation and upregulates cyclin D1, cyclin D2, and HNF4α without inducing stress. Their study included islets and cells collected from mice and young human donors (below the age of 18), as well as a murine pancreatic beta cell line (Min6)[3]. However, their study examined only short-term effects, and sustained overexpression might cause stress or dysregulation over time[8-10]. The model simulated diabetic conditions by exposing cells and islets to high glucose and insulin, thereby mimicking metabolic stress. Interestingly, Rheb1’s involvement in β-cell proliferation extends beyond mTORC1 activation; the fact that mTORC1 blockade with rapamycin only partially inhibited the proliferative response from Rheb1 indicates that additional pa
Importantly, Yang et al[3] mentioned that Rheb1 overexpression elevates HNF4α levels while its knockout reduces these levels, pointing to a potentially pivotal mechanism through which Rheb1 regulates β-cell function. This interaction warrants deeper investigation and suggests a fascinating angle on the genetic forms of diabetes, particularly about how Rheb1 modulation could reveal novel mechanisms behind β-cell dysfunction. In β-cells, Rheb1 promotes proliferation without leading to malignant transformation, likely due to differing regulatory pathways at play. In contrast, the studied models were relevant to the type 2 diabetes glucotoxicity environment. Since Rheb1’s effects were more focused on characterizing β-cell dysfunction and reduced proliferation related to type 2 diabetes. Additionally, Rheb1 regulated β-cell function by modulating mTORC1, AMPK, and NOTCH1 signaling, which are crucial for growth, energy sensing, and differentiation of type 2 diabetes. In type 1 diabetes, Rheb1 activation offers limited benefits without immunomodulatory strategies, as it cannot prevent immune-mediated β-cell destruction on its own.
The current study positions Rheb1 as a central regulator of β-cells, opening new therapeutic possibilities for diabetes by targeting its proliferation and preventing dedifferentiation. Future research should explore tissue-specific modulation of Rheb1 and its interactions with other signaling pathways to optimize diabetes treatment strategies. Preclinical studies have tested the Rheb1 overexpression in diabetic animal models (e.g., non-obese diabetic mice, db/db mice). Where, develop β-cell-specific Rheb1 activators to avoid systemic mTORC1 side effects. Additionally, clinical biomarkers that validate Rheb1 Levels in human diabetic compared to the non-diabetic islets explore the blood-based Rheb1 activity markers for patient stratification. Furthermore, the drug development approach should focus on screening the small-molecule Rheb1 modulators with dual mTORC1/AMPK effects. That should be aligned with investigating the NOTCH1-HNF4α crosslinks in β-cell differentiation protocols. Furthermore, the impact of β-cell-specific Rheb1 knockout on food intake response in mice should be further investigated to explore the potential systemic metabolic consequences of Rheb1 deletion, which are yet to be explored. As food intake influences overall energy homeostasis and insulin demand, future studies incorporating metabolic phenotyping would be valuable to determine whether Rheb1 deficiency in β-cells has broader physiological effects. This combination will allow us to dissect molecular mechanisms under controlled conditions and validate the physiologically relevant system. Thus, the inclusion of more in vivo islet studies will help to bridge the gap between cellular mechanisms and organismal physiology.
Yang et al’s study[3] redefines Rheb1 as a master regulator of β-cell mass and function, integrating mTORC1, AMPK, NOTCH1, and HNF4α pathways. Their work underscores the potential of multi-pathway targeting for diabetes therapy, moving beyond conventional insulin-centric approaches. Future research should focus on translational validation, ensuring Rheb1-based strategies are safe and effective for clinical use. For the World Journal of Diabetes readership, this study advances mechanistic understanding and paves the way for next-generation diabetes therapeutics for β-cell re
| 1. | Elsharkawy ER, Alqahtani A, Uddin MN, Khan F, He Y, Li X, Gouda MM. The antidiabetic, haematological, and antioxidant implications of Schimpera arabica natural plant on Streptozotocin-diabetic rats. J Agric Food Res. 2025;21:101891. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Mallik S, Paria B, Firdous SM, Ghazzawy HS, Alqahtani NK, He Y, Li X, Gouda MM. The positive implication of natural antioxidants on oxidative stress-mediated diabetes mellitus complications. J Genet Eng Biotechnol. 2024;22:100424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Song WJ, Zhang JJ. Ras homolog enriched in brain 1 regulates β cell mass and β cell function via mTORC1/AMPK/Notch1 pathways. World J Diabetes. 2025;16:104973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (4)] |
| 4. | Li M, Lazorchak AS, Ouyang X, Zhang H, Liu H, Arojo OA, Yan L, Jin J, Han Y, Qu G, Fu Y, Xu X, Liu X, Zhang W, Yang Z, Ruan C, Wang Q, Liu D, Huang C, Lu L, Jiang S, Li F, Su B. Sin1/mTORC2 regulate B cell growth and metabolism by activating mTORC1 and Myc. Cell Mol Immunol. 2019;16:757-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Yang L, Zhang Z, Wang D, Jiang Y, Liu Y. Targeting mTOR Signaling in Type 2 Diabetes Mellitus and Diabetes Complications. Curr Drug Targets. 2022;23:692-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Mahoney SJ, Narayan S, Molz L, Berstler LA, Kang SA, Vlasuk GP, Saiah E. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nat Commun. 2018;9:548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Ardestani A, Lupse B, Kido Y, Leibowitz G, Maedler K. mTORC1 Signaling: A Double-Edged Sword in Diabetic β Cells. Cell Metab. 2018;27:314-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Jaafar R, Tran S, Shah AN, Sun G, Valdearcos M, Marchetti P, Masini M, Swisa A, Giacometti S, Bernal-Mizrachi E, Matveyenko A, Hebrok M, Dor Y, Rutter GA, Koliwad SK, Bhushan A. mTORC1 to AMPK switching underlies β-cell metabolic plasticity during maturation and diabetes. J Clin Invest. 2019;129:4124-4137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Cai Z, Liu F, Yang Y, Li D, Hu S, Song L, Yu S, Li T, Liu B, Luo H, Zhang W, Zhou Z, Zhang J. GRB10 regulates β-cell mass by inhibiting β-cell proliferation and stimulating β-cell dedifferentiation. J Genet Genomics. 2022;49:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Perez-Serna AA, Guzman-Llorens D, Dos Santos RS, Marroqui L. Bcl-2 and Bcl-xL in Diabetes: Contributions to Endocrine Pancreas Viability and Function. Biomedicines. 2025;13:223. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Böhm R, Imseng S, Jakob RP, Hall MN, Maier T, Hiller S. The dynamic mechanism of 4E-BP1 recognition and phosphorylation by mTORC1. Mol Cell. 2021;81:2403-2416.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Eom YS, Gwon AR, Kwak KM, Youn JY, Park H, Kim KW, Kim BJ. Notch1 Has an Important Role in β-Cell Mass Determination and Development of Diabetes. Diabetes Metab J. 2021;45:86-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94:13209-13214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 294] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Nishad R, Meshram P, Singh AK, Reddy GB, Pasupulati AK. Activation of Notch1 signaling in podocytes by glucose-derived AGEs contributes to proteinuria. BMJ Open Diabetes Res Care. 2020;8:e001203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Cai Z, Pan Z, Liu F, Li D, Ji Y, Zhong J, Luo H, Hu S, Song L, Yu S, Li T, Li J, Ma X, Zhang W, Zhou Z, Liu F, Zhang J. Rheb1 promotes glucose-stimulated insulin secretion in human and mouse β-cells by upregulating GLUT expression. Metabolism. 2021;123:154863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem. 2009;284:7832-7842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |