Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108789
Revised: May 19, 2025
Accepted: June 23, 2025
Published online: July 15, 2025
Processing time: 83 Days and 16 Hours
Recent studies have potentiated the essential role of androgens in normal folliculogenesis and, therefore, female fertility. Contrastingly, excess androgen levels, i.e., hyperandrogenism (HA), a hallmark characteristic of polycystic ovary syndrome, overrides the delicate balance of folliculogenesis, leading to follicular arrest and ovulatory issues. Insulin resistance (IR) has a profound effect on elevating androgen secretion and is considered one of the primary factors driving both ovarian androgen production and metabolic dysfunction in polycystic ovary syndrome. Together with IR, disruptions in key intraovarian and systemic factors, including activin, inhibin, follistatin, anti-Mullerian hormone, bone morphogenetic proteins, growth differentiation factor-9 and Kit ligand, as well as dysregulation in both the insulin and the transforming growth factor-β superfamily signaling pathway, contribute to follicular arrest, elevated androgen levels and metabolic dysfunction, exacerbating HA. Additionally, suppression of sex hormone-binding globulin, disrupted adipose-neuroendocrine signaling and altered microRNA expression heighten HA, with IR serving as the fundamental contributor. Emerging evidence implicates impaired atresia together with non-apoptotic cell death, such as ferroptosis and pyroptosis, which have also been associated with ovarian dysfunction. A comprehensive understanding of the most significant factors, particularly IR, which amplifies androgen production through hyperinsulinemia-mediated stimulation of theca cells, is essential for identifying targeted therapeutic strategies.
Core Tip: Polycystic ovary syndrome is a multifactorial disorder characterized by excess ovarian androgen production, frequently amplified by insulin resistance. This review explores how insulin resistance intersects with systemic and intraovarian factors such as activin, inhibin, follistatin, anti-Mullerian hormone, bone morphogenetic proteins, growth differentiation factor-9 and Kit ligand, as well as the dysregulation in both the insulin and the transforming growth factor-β superfamily signaling pathway, to create a vicious cycle of reproductive and metabolic dysfunction. A deeper understanding of these factors and pathways emphasizes the need for individualized, multi-targeted therapeutic approaches beyond conventional treatments.
- Citation: Rambaran N, Islam MS. Decoding androgen excess in polycystic ovary syndrome: Roles of insulin resistance and other key intraovarian and systemic factors. World J Diabetes 2025; 16(7): 108789
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108789.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108789
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that affects women of reproductive age[1]. The key pathophysiological features of PCOS include hyperandrogenism (HA), chronic anovulation (oligomenorrhea or amenorrhea), and polycystic ovarian morphology (PCOM). While traditionally viewed as a multifactorial condition, insulin resistance (IR) plays a central role as one of the primary factors in the development of PCOS[2]. Elevated insulin levels due to IR drive increased ovarian androgen production, which contributes to the hallmark symptoms of PCOS, including hirsutism, acne, and irregular menstrual cycles[3]. Additionally, PCOS is commonly accompanied by subclinical inflammation, metabolic abnormalities, oxidative stress, and obesity[2,4].
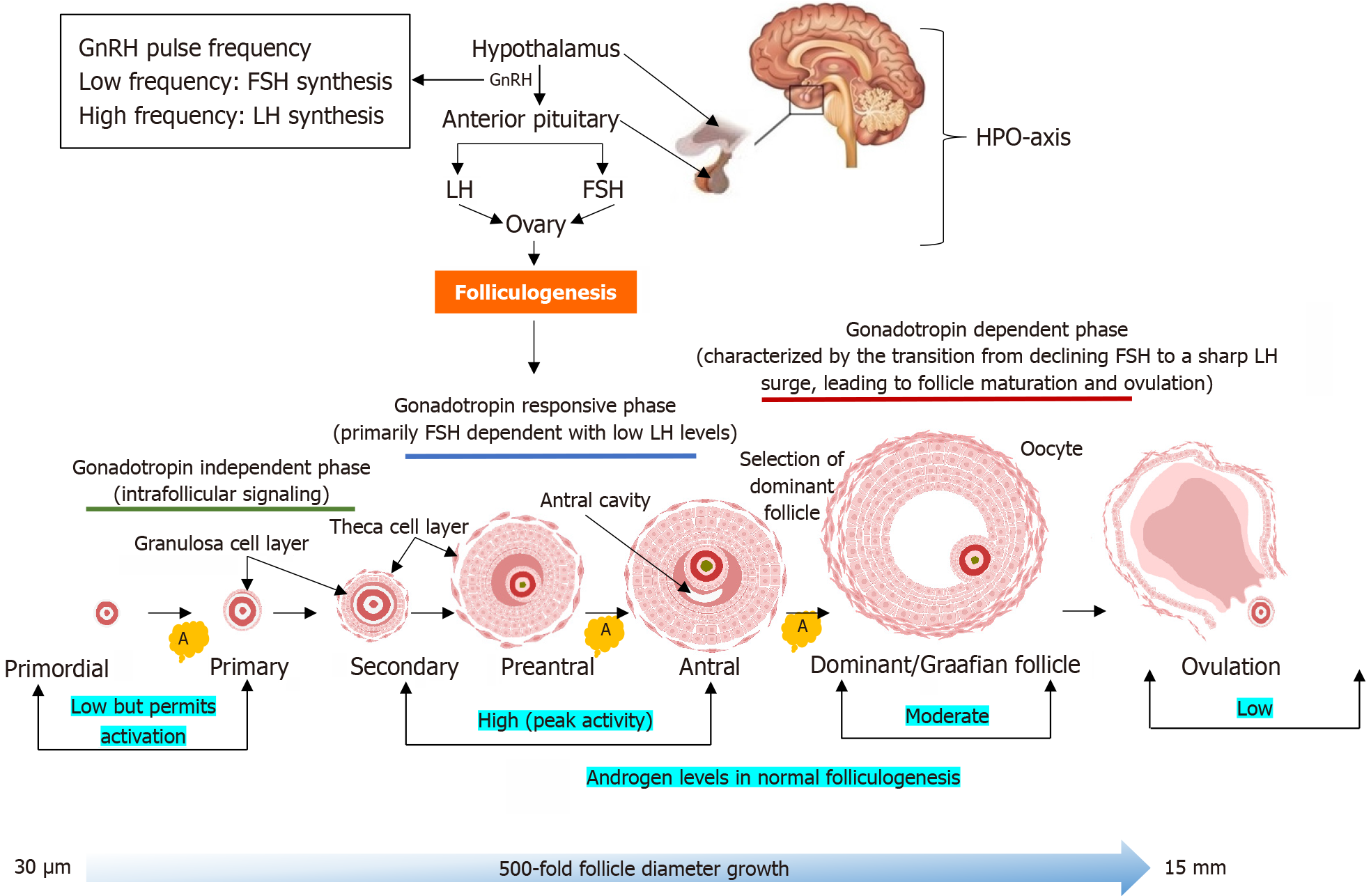
The role of androgen in the process of ovulation is perplexing at best. On the one hand, androgen plays an imperative role in the process of normal folliculogenesis, and on the other hand, excess androgen is central to the pathophysiology of PCOS. Under normal conditions, folliculogenesis is tightly regulated by the hypothalamic-pituitary-ovarian (HPO) axis, where gonadotropin-releasing hormone (GnRH) pulsatility controls the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland[5]. Rapid GnRH pulse frequency favors LH secretion, while slower pulses enhance FSH secretion[5]. A balanced LH/FSH ratio is critical for coordinated follicular development, i.e., FSH promotes granulosa cell proliferation, estradiol synthesis, and follicle maturation, while LH stimulates theca cells to produce androgens, which serve as substrates for aromatization[6]. At normal physiological levels, androgens enhance FSH receptor (FSHR) expression in granulosa cells, supporting follicle growth and selection[7].
In PCOS, this balance is dysregulated. Driven by HA, increased GnRH pulse frequency elevates LH secretion over FSH, contributing to the characteristic elevated LH/FSH ratio[8]. The excess LH promotes overproduction of androgens by the theca cells, while the decreased FSH levels impair aromatization of these androgens to estrogens in granulosa cells[9]. This hormonal imbalance disrupts normal folliculogenesis, leading to arrested follicle development and anovulation. The premature and heightened expression of androgen receptors (ARs) in the ovarian follicles maybe a significant factor that enables androgen excess to alter GnRH pulsatility, which disrupts the normal regulation of LH and FSH secretion, further promoting the androgenic states implicated in PCOS[10].
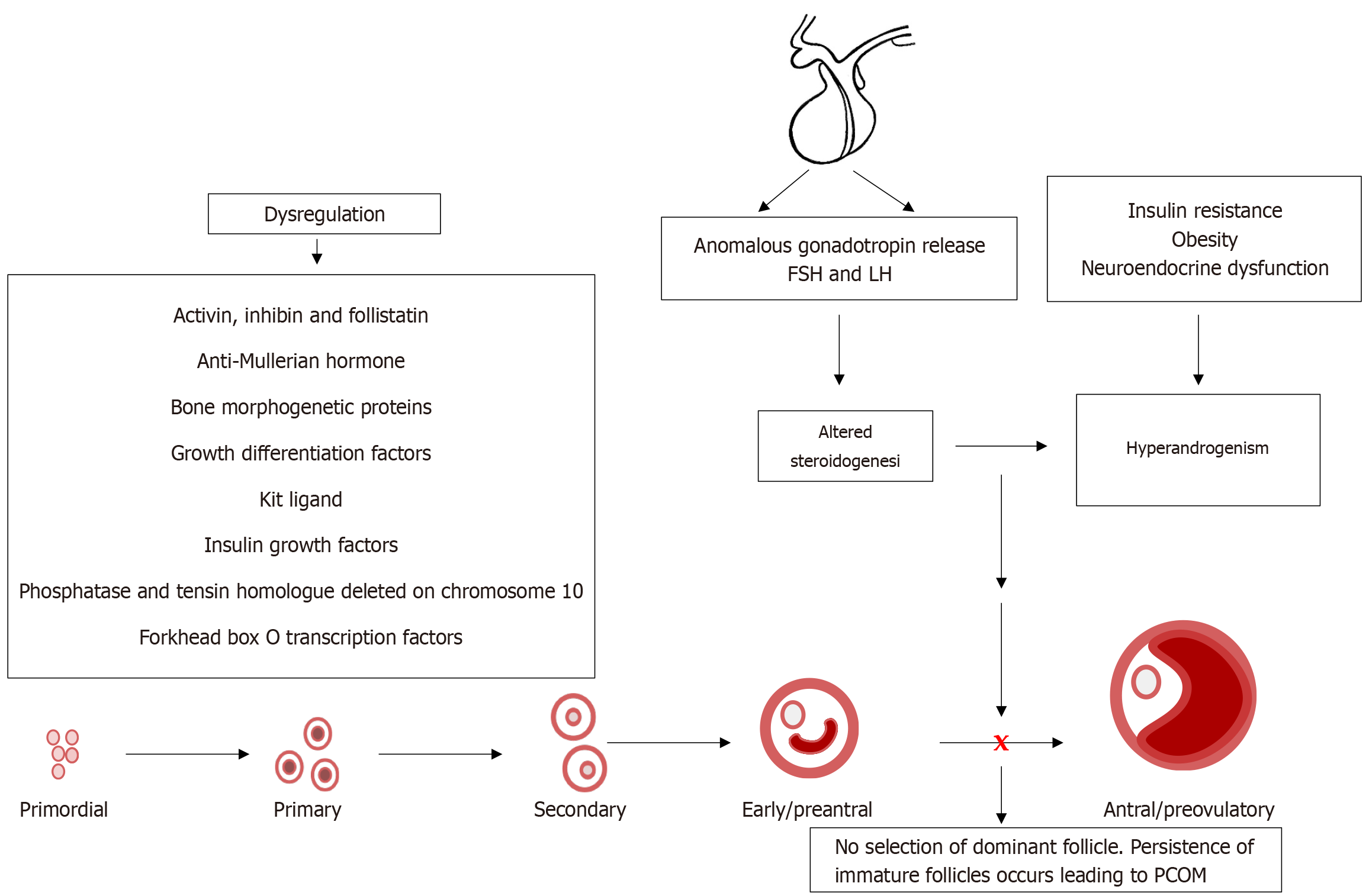
Concurrently, IR also affects the neuroendocrine axis[8]. Elevated insulin enhances the release of kisspeptin, a neuropeptide that stimulates GnRH-LH hypersecretion, which in turn, amplifies androgen synthesis, worsening HA[11]. Additionally, IR decreases the synthesis of hepatic sex hormone-binding globulin (SHBG), increasing free androgen levels[9,12]. Thus, this heralds a vicious cycle where both metabolic and reproductive issues persist, progressively worsening. Furthermore, members of the transforming growth factor-β (TGF-β) superfamily, including activin, inhibin, follistatin, anti-Mullerian hormone (AMH), bone morphogenetic proteins (BMPs), growth differentiation factor-9 (GDF-9) and Kit ligand (KL), play crucial roles in granulosa cell differentiation, oocyte maturation, and follicular selection[6]. Their dysregulation, together with the impairment of the insulin and the TGF-β superfamily signaling pathways, primarily influenced by excessive insulin, may impair these intra-ovarian regulatory mechanisms, perpetuating follicular arrest and contributing to the hyperandrogenic state of PCOS. Accordingly, this review aimed to shed light on these mechanisms to improve our understanding of the underlying pathophysiology and steer the advancement of targeted diagnostic and therapeutic strategies.
Recent epidemiological studies show the growing global burden in the prevalence of PCOS[13]. A 2025 systematic review involving over 561000 women reported global PCOS prevalence estimates ranging from approximately 6.6% to 10.9%, depending on the diagnostic criteria used[14]. Notably, there was a significant lack of data from the African region, highlighting the need for more research in these areas[14]. Complementing this, a comparative study showed that the global age-standardized point prevalence and annual incidence rates for PCOS have increased by 30.4% and 29.5% since 1990, respectively, with sub-Saharan Africa having lower-than-expected burdens across the measurement period. However, it has been noted that 68%-75% of patients with PCOS remain undiagnosed even after visiting many medical institutions, indicating that the incidence of this condition is probably underestimated[15,16]. Additionally, the lower detection rates may result from poor healthcare infrastructure. For example, the limited availability of ultrasound imaging equipment in Africa[17]. Furthermore, the diverse clinical manifestations, unknown etiology, and complicated pathophysiology have amplified the poor diagnosis of PCOS[18].
Furthermore, an analysis of Global Burden of Disease data from 1990 to 2021 showed that the number of women with PCOS nearly doubled globally, from 34.81 million to 65.77 million, with corresponding increases in incidence and disability-adjusted life years[19]. Of concern is the significant gap in public awareness and understanding of PCOS. This was reflected in a 2023 cross-sectional study in the United Arab Emirates, which found that 84.3% of women were familiar with the term PCOS, but only 21.7% demonstrated sufficient awareness of its causes, symptoms and complications[20]. Similarly, in Malaysia, a study conducted with women in the Klang Valley region indicated that nearly half of the respondents had poor knowledge and health-related practices concerning PCOS[21]. These findings suggest the need for targeted educational initiatives to enhance PCOS awareness and promote early diagnosis and management.
Additionally, as the symptoms of the syndrome worsen and intensify, it sets in motion a significant deterioration in quality of life, an increase in stress, negatively affecting the psychological and emotional well-being of women with PCOS[22,23]. The implications of this have been exemplified by Yadav et al[24] by indicating that the healthcare-related economic burden of PCOS exceeds 15 billion dollars yearly in the United States, considering the costs of PCOS diagnosis, costs related to PCOS-associated mental health, reproductive, vascular, and metabolic disorders. Given that PCOS shows a global prevalence, the economic burden it imposes is substantial, highlighting the need for greater attention from both scientific and policy-making sectors[24].
The heterogenous nature of the syndrome has produced considerable scientific debate and as yet, no conclusive clinical definition of PCOS has been reached[25]. Most commonly used are the Rotterdam diagnostic criteria[26], which defines PCOS when at least two of the following three features are present: Oligo or anovulation, clinical and/or biochemical signs of HA, and polycystic ovaries[27,28]. To have a consensus, the International Guidelines for the Assessment and Management of PCOS[29] integrates the criteria from the different scientific bodies, i.e., National Institute of Health 1990, Rotterdam 2003, and Androgen Excess and PCOS Society[30]. Thus, the consolidated definition has established four clinical phenotypes of PCOS, with different metabolic repercussions for each criterion (Table 1)[31]. Patients diagnosed with fullblown PCOS (phenotype A) are at higher risk of adverse metabolic and cardiovascular outcomes, while phenotype D is the least severe phenotype[32]. The correct diagnosis and phenotyping facilitates a more effective treatment strategy for the cardiometabolic risk of patients[31].
| Phenotype | Synonym | Diagnostic criteria |
| Phenotype A | Classic phenotype (full-blown PCOS) | Hyperandrogenism + oligo-ovulation + polycystic ovaries |
| Phenotype B | Classic phenotype (non-polycystic ovaries) | Hyperandrogenism + oligo-ovulation |
| Phenotype C | Ovulatory phenotype | Hyperandrogenism + polycystic ovaries |
| Phenotype D | Non-hyperandrogenic phenotype | Oligo-ovulation + polycystic ovaries |
Emerging advancements in molecular and omics-based technologies have identified several novel biomarkers that may enhance diagnosis. For instance, circular RNAs (circRNAs), a class of non-coding RNAs, have been found to be differentially expressed in PCOS patients and may influence processes like cell proliferation, apoptosis, and steroidogenesis[33]. Growing evidence indicates that circRNAs are involved in insulin secretion and pancreatic β-cell function and aberrations in circRNAs may reflect disruptions in insulin-related signaling pathways[34]. The strong association between circRNAs and IR may provide therapeutic targets for managing IR in PCOS[33]. Additionally, circRNAs are present in the follicular fluid of women with PCOS, indicating their potential as diagnostic biomarkers[33]. Similarly, lipidomic and proteomic studies of follicular fluid have identified novel metabolic signatures in women with PCOS, for example, Wnt1-inducible signaling pathway protein 1 as a downstream effector of Wnt/β-catenin signaling pathway[35]. Wnt1-inducible signaling pathway protein 1 may function in the interaction between IR, inflammation, and obesity and can be a therapeutic target for PCOS patients[35].
Lifestyle and dietary modifications that include a balanced diet and exercise are the cornerstone of the initial treatment of PCOS before pharmacological intervention[36]. This holistic approach plays a key role in alleviating symptoms associated with the metabolic profile of the patient[37]. Unfortunately, lifestyle interventions are mostly ineffective due to the lack of compliance. Furthermore, the heterogeneous nature of the pathophysiology of PCOS makes it difficult to manage. Thus, various pharmacological treatment modalities are available to address the intricacies associated with PCOS. Given the different phenotypes of PCOS, the management of PCOS centers around these areas: HA, metabolic aberrations, and anovulation[37].
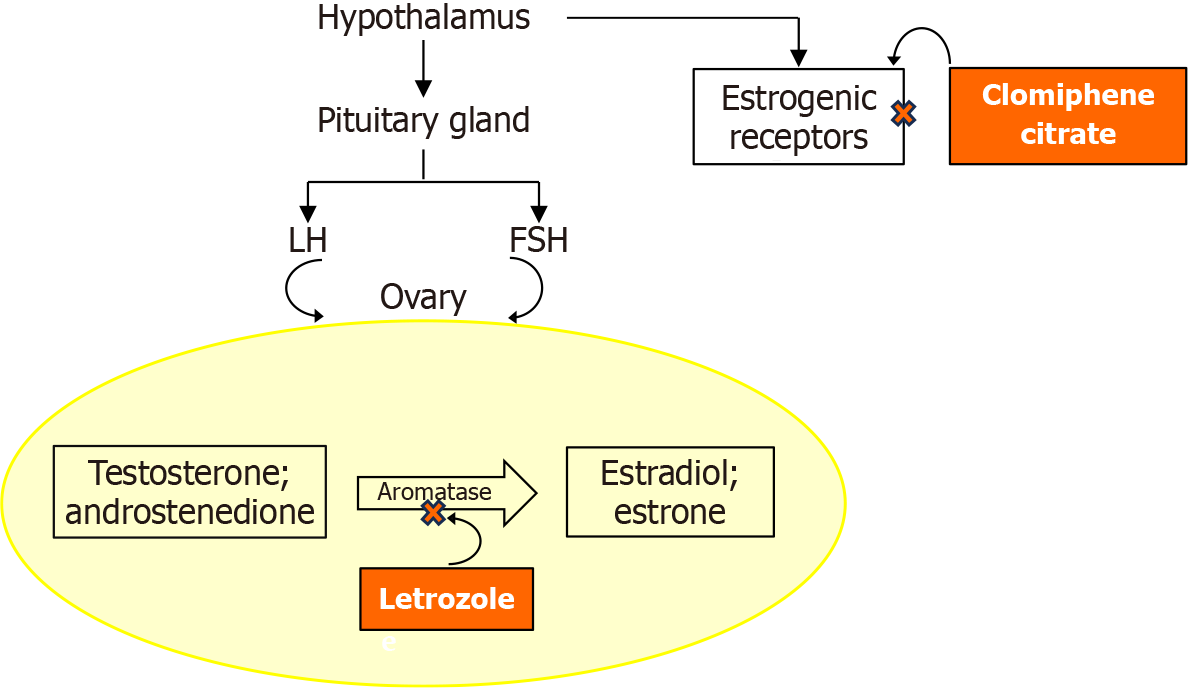
Pharmacological remedies currently used to manage PCOS are presented in Table 2[2,12,37-48]. The table compares these pharmacological drugs based on clinical benefits, mechanisms, adverse effects, and safety considerations. Additionally, comparative information, such as between letrozole and clomiphene citrate, is summarized in the table and accompanying Figure 1. The table provides a practical summary of treatment options and may serve as a patient-specific guide.
| Drug class and treatment | Clinical benefits | Mechanism of action | Adverse effects | Safety considerations | Ref. |
| Hormonal regulators (combined oral contraceptives): Ethinylestradiol + drospirenone | Menstrual regulation, androgen suppression, reduces hirsutism and acne | Inhibits ovarian androgen production by suppressing gonadotropin, primarily, LH | Nausea, bloating, and mood changes | Weight gain, alterations in cardiometabolic parameters and venous thromboembolism risk | [38,39] |
| Insulin sensitizers (biguanides): Metformin | Improves insulin sensitivity, menstrual regularity, and ovulation | Inhibits hepatic gluconeogenesis and improves peripheral glucose uptake | GI upset | Caution with persistent use, increases homocysteine levels and increase risk of CVD | [12,37,40] |
| Insulin sensitizers (thiazolidinediones): Pioglitazone | Improves IR, ovulation, and excess androgen regulation | Decreases hepatic and peripheral IR through the activation of the nuclear hormone receptor (PPARα) | Weight gain and peripheral edema | Liver toxicity, not a first-line treatment option due to cardiovascular concerns | [37,41] |
| GLP-1 agonists: Liraglutide | Improves metabolic profile, including weight loss | Binds to insulin receptors on beta cells, stimulates insulin secretion, reduces glucagon secretion, inhibits hunger centers and delays gastric emptying | Nausea and emesis | Avoid during pregnancy; effective contraception is required and a washout period prior to pregnancy | [2,42,43] |
| SGLT2 inhibitors: Empagliflozin | Weight loss, improves insulin sensitivity, and reduces androgen levels | Renal glucose reabsorption is inhibited, promoting glycosuria | Increased urination, urinary tract infections, and dehydration | Monitor hydration and renal function; limited long-term data in PCOS | [44,45] |
| Anti-androgens: Spironolactone | Reduces hirsutism and acne | Reduces androgen production by blocking AR | May cause menstrual irregularity | Teratogenic; requires effective contraception; potassium monitoring advised due to hyperkalemia risk | [46] |
| Ovulation inducers: Clomiphene citrate | Ovulation induction | Selective estrogen receptor modulator | Mood swings, hot flashes | Risk of multiple pregnancies, endometrial thinning and thickening of cervical mucus | [47] |
| Ovulation inducers: Letrozole | Enhances ovulation and pregnancy rates | Aromatase inhibitor, reduces estrogen feedback to increase FSH | Fatigue, dizziness, hot flashes | Possibly teratogenic effects | [48] |
Emerging clinical evidence continues to enhance the therapeutic framework of PCOS. A meta-analysis by Zeng et al[49] demonstrated that combining metformin with spironolactone resulted in greater improvements in body mass index (BMI), fasting glucose and androgen levels than metformin alone. A meta-analysis of randomized controlled trials showed the efficacy and safety of glucagon-like peptide-1 agonists in PCOS women living with obesity by promoting weight loss and hormonal regulation by improving BMI, waist circumference, and androgen levels[50]. Interestingly, a recent network meta-analysis of non-pharmacological strategies, including electroacupuncture, diet, and exercise, yielded significant improvements in androgen-related symptoms[51]. Vitamin D supplementation was also associated with improved endometrial thickness, inflammatory markers and androgen profiles[52]. These findings endorse the growing trend of integrative and metabolic-targeted interventions in PCOS management.
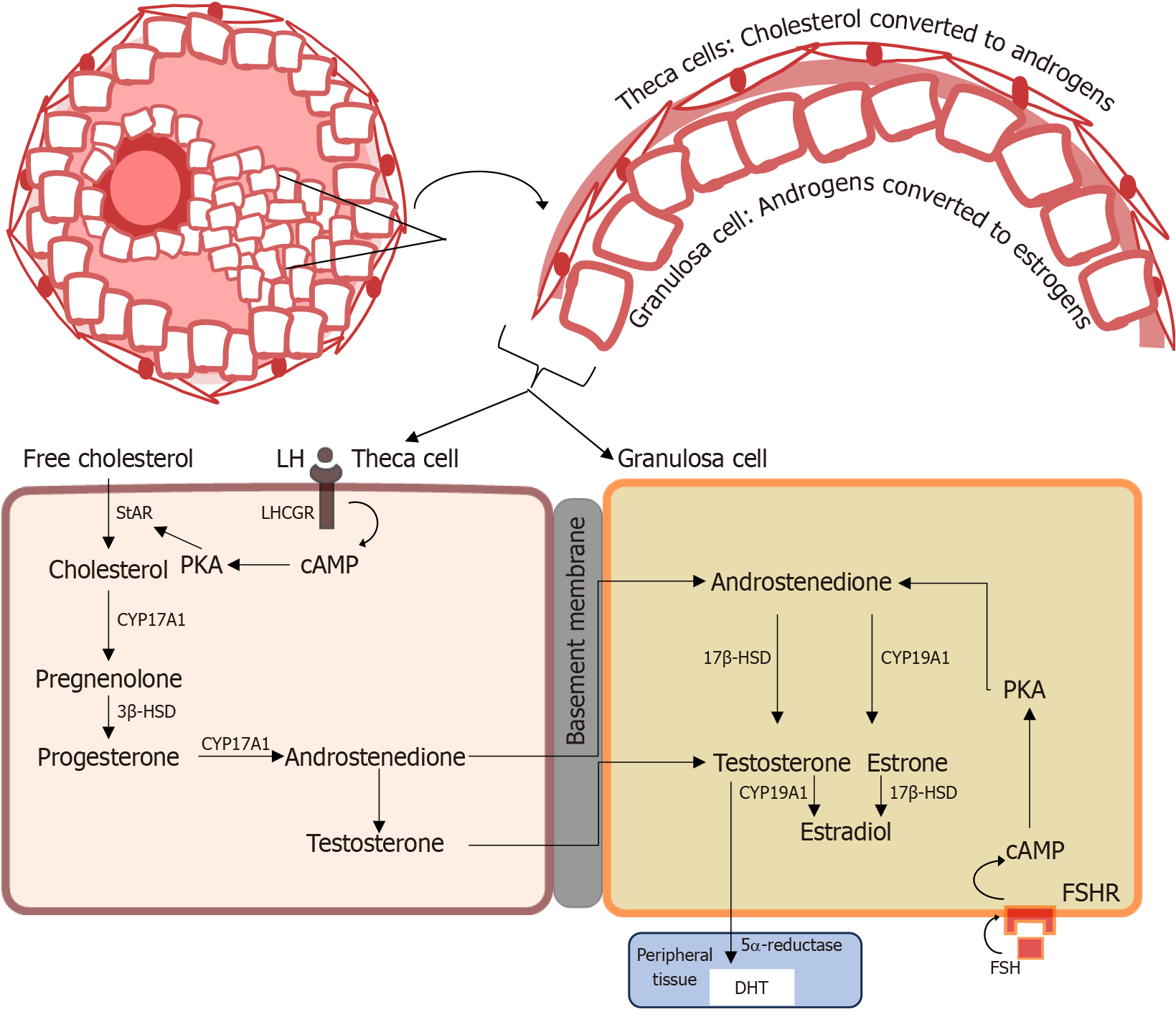
The binding of LH to respective LH receptors on theca interna cells leads to an elevation in intracellular 3’,5’-cyclic adenosine monophosphate levels and subsequent activation of protein kinase A[53]. This elevation, in turn, promotes the activity of steroidogenesis by increasing the expression of steroidogenic acute regulatory protein (StAR) (Figure 2). Thus, the two-cell, two-gonadotropin model compartmentalizes steroidogenesis, where two types of cells (granulosa cells and theca cells) together with two gonadotropins (LH and FSH) function together in estrogen synthesis[6]. In this process, LH-influenced theca cells produce androgens that are aromatized to estrogen in the granulosa cells by the cytochrome P450 19A1 (CYP19A1) aromatase enzyme via the FSH signaling pathway (Figure 2)[54].
Androgens play critical roles in female reproductive physiology, especially in the regulation of normal folliculogenesis. Their actions are mediated primarily through ARs expressed in ovarian theca and granulosa cells.
During the early phase of folliculogenesis, androgens appear to support the activation of dormant primordial follicles[6]. Animal studies suggest that, despite the absence of AR expression in the primordial follicles, androgens may enhance the initial recruitment of follicles by upregulating intraovarian growth factors such as insulin-like growth factor 1 (IGF-1) and KL, promoting the transition from primordial to primary follicles (Figure 3)[55,56]. Notably, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway is one of the intracellular signal transduction pathways involved in primordial follicle activation in the ovary. Phosphatase and tensin homolog (PTEN), a key negative regulator of the PI3K-Akt pathway, acts to preserve the dormancy of primordial follicles by inhibiting premature activation[57,58]. Conversely, androgens may enhance PI3K-Akt signaling indirectly via stimulation of local growth factors, thereby promoting follicle activation[6]. This suggests a functional opposition: While PTEN maintains dormancy, androgens will encourage the balance toward activation. Disruption in this regulatory balance, especially excessive androgens, may contribute to early follicle recruitment with implications for disorders such as PCOS[59].
Androgens exert their most prominent effects during the preantral and early antral stages. Here, they act synergistically with FSH to promote granulosa cell mitosis, induction of FSHRs and stimulate estradiol production via increased aromatase activity[7]. This androgen-mediated sensitization to FSH is critical for promoting healthy follicle growth and selection as well as making the follicle more resistant to atresia[7,60].
In later stages, appropriate androgen signaling has been implicated in regulating the final stages of follicle maturation and ovulation[60]. However, androgen levels must be tightly controlled, i.e., while physiological concentrations are beneficial, excessive androgens, as seen in PCOS, disrupt the follicular environment, inhibit aromatase expression and impair granulosa cell function[10]. This imbalance contributes to follicular arrest, anovulation, and the development of PCOM[61].
Although androgens are less dominant during ovulation, their earlier effects set the stage for successful follicular rupture and corpus luteum formation[62]. Indirectly, by supporting FSH-driven estradiol production and granulosa cell differentiation, androgens facilitate the LH surge necessary for ovulation. Proper androgen balance may also contribute to luteal phase adequacy, although further research is needed in this area[10].
Follicular atresia, the degenerative process that eliminates non-dominant follicles, is indirectly influenced by androgen signaling[63]. Physiological androgen levels can exert anti-apoptotic effects by increasing FSHRs in preantral and early antral follicles, enhancing follicular survival during early development[7]. However, excessive androgen exposure activates pro-apoptotic signaling in granulosa cells of larger follicles, thereby accelerating atresia[7]. In PCOM ovaries contain an abnormally large population of small antral follicles, but it is not static, as the follicles show high rates of atresia, with constant replacement from newer developing follicle cohorts[64,65]. Thus, in normal folliculogenesis, androgens are essential in early follicle activation, granulosa cell proliferation, and FSH responsiveness[7]. Androgens either support survival at critical periods of folliculogenesis or, in excess, induce degeneration[60]. This duality extends to the role of androgen in follicular survival vs atresia and demonstrates the importance of androgen balance for reproductive health. Disruption of this homeostasis, as in PCOS, leads to aberrant follicle development and anovulation.
The biological activity of androgens occurs predominantly through the binding and activation of their AR, a member of the nuclear receptor superfamily that functions as a ligand-activated transcription factor[66]. Within ovarian follicles, AR is localized in the granulosa cells, stromal cells and oocytes, with expression highest in the granulosa cells[60]. It has been shown that the inactivation of AR results in premature ovarian failure, alluding to the crucial role of AR-mediated androgen action in normal folliculogenesis[67]. In studies using granulosa cell-specific AR knockout mice, it was revealed that selective loss of granulosa cell AR actions during preantral and antral developmental stages resulted in subfertility, defective follicle dynamics, altered ovulation rates, follicle depletion, increased follicle atresia, and reduced embryo viability[68]. Thus, the granulosa cells of preantral and antral follicles are essential sites for AR-mediated actions involved in maintaining follicle and embryo survival and, ultimately, optimal female fertility[68]. Contrary to previous assumptions, which have implicated androgens to inhibit antral follicle development, recent findings reveal that androgens have a growth-promoting effect on the early stages of follicle development[6]. This highlights the complex and intricate nature of androgens and their impact on folliculogenesis.
Several studies have shown that expression of AR decreases as the follicle develops[60], functioning predominantly as a substrate of estrogen synthesis. Thus, the decrease in AR expression coincides with rising granulosa cells CYP aromatase expression, allowing androgens produced by LH-stimulated theca cells to undergo FSH-stimulated aromatization to estrogen (Figure 2)[69]. Additionally, it has been postulated that androgens indirectly modulate P450 aromatase activity by increasing FSHR expression and FSH activity[70]. Likewise, Fujibe et al[71] demonstrated in their investigations that androgens could support follicle development during the FSH-dependent preantral stage by enhancing FSH action via increased expression of FSHR mRNA levels in prenatal follicles of mice. These findings indicate the indispensable role of androgen in early to preantral follicle development.
HA is often exacerbated by IR, a common but variable metabolic feature linked to PCOS[12]. The co-occurrence of HA and IR creates a pathological loop that worsens ovarian dysfunction, metabolic imbalance, and systemic inflammation[2]. These disturbances are mediated by the dysregulation of several intraovarian factors, molecular pathways, and hormonal systems, many of which are intricately involved in follicular growth, steroidogenesis, apoptosis, and cellular signaling. The sections below explore IR, key intraovarian and systemic factors, and their role in hyperandrogenic PCOS states by highlighting their regular physiological roles and altered states in PCOS.
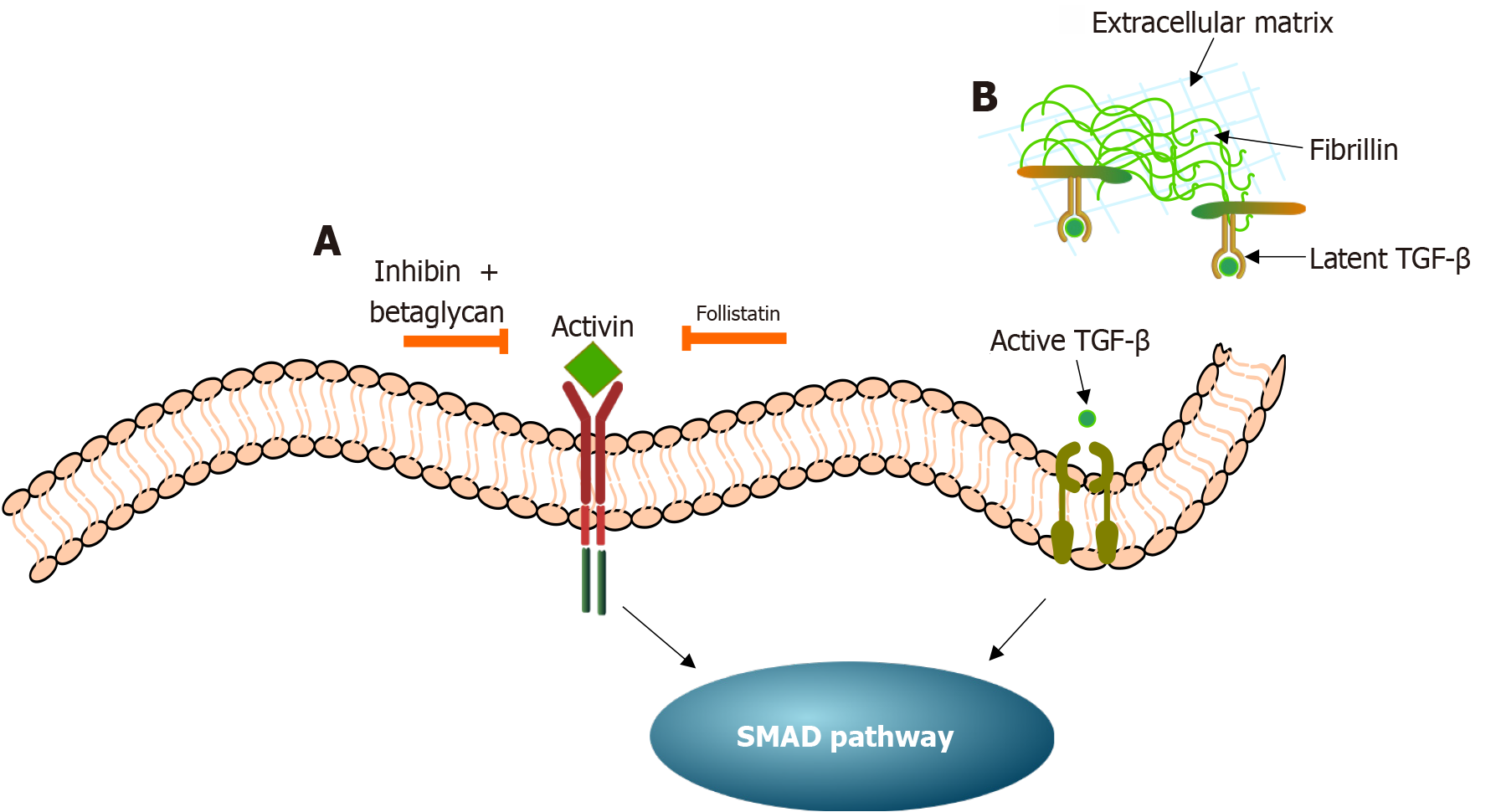
Interplay between IR, activin, and inhibin in PCOS: Under normal physiological conditions, two glycoproteins, activin and inhibin, members of the TGF-β superfamily, modulate theca cell steroidogenesis in the ovary[72]. Several studies have confirmed that the biological effects of activin on theca cell androgen production are inhibitory. In contrast, inhibin acts in an opposing manner by causing an increase in the production of androgens[73]. More specifically, in the granulosa cells, activin appears to elevate the levels of CYP aromatase, the FSHR, and estradiol. Additionally, it has been shown that deleting the activin receptors leads to FSH deficiency, with female mice becoming acyclic and sterile[74]. Thus, favoring the hypothesis that activin is an FSH-stimulating agent[75]. Contrastingly, in the theca cells, activin seems to decrease the levels of StAR, which is responsible for transporting cholesterol esters into the mitochondria to initiate steroidogenesis, thereby downregulating androgen production[72,73].
Inversely, inhibin, which is produced by the granulosa cells, modulates activin bioactivity[72]. Inhibin complexed with betaglycan (an inhibin co-receptor) functions to antagonize activin activity by competitively binding to activin receptors, thereby blocking the effects of activin (Figure 4). Another glycoprotein, follistatin, although not classified as a member of the TGF-β superfamily, interacts and regulates the activity of activin (Figure 4). Ultimately, follistatin and inhibin downregulate activin activity[75]. The activin-follistatin-inhibin system is considered a key regulator of the HPO axis[76].
In PCOS, this balance is disrupted, particularly due to the effects of IR[77-79]. Chronic hyperinsulinemia, central to IR, not only stimulates ovarian theca cells directly to increase androgen production but also upregulates follistatin levels, thereby suppressing activin’s beneficial role in FSH support and androgen suppression. Interestingly, Sylow et al[80] showed in their study that elevated circulating follistatin, but not activin A, was strongly associated with IR in patients with type 2 diabetes (T2D). In the mouse model, follistatin was found to mediate diabetes by fostering IR specifically in white adipose tissue[81].
Similarly, an impaired activin A and inhibin B ratio is associated with PCOS, which may further dampen FSH signaling and contribute to arrested follicular development[72]. Furthermore, Walton et al[82] generated a mouse model with inhibin loss of function and their analyses revealed that complete inactivation of inhibin resulted in an elevation in circulating FSH, which triggered ovarian overstimulation and pregnancy loss. However, a partial reduction in inhibin levels enhanced female fecundity. Their conclusions were further supported by Brûlé et al[83]. These studies garnered confirmatory evidence regarding the delicate balance of the activin, inhibin, and follistatin necessary for the physiology of the female reproductive system, and an imbalance in their signaling can trigger pathologies such as PCOS[72].
Crosstalk between IR and AMH in PCOS: In typical physiological conditions, the glycoprotein AMH is secreted by the granulosa cells from the primary follicle stage and peaks in the pre-antral stage and early antral stage in human follicles[84]. The secretion starts to decrease as the follicle becomes FSH-dependent. In folliculogenesis, AMH has two windows of action. Firstly, AMH exerts an inhibitory effect on the initial recruitment of resting primordial follicles[85], possibly as an adaptive measure to prevent excessive recruitment, thereby maintaining homeostasis in the ovary[86]. Secondly, AMH negatively affects preantral and small antral follicle growth by attenuating their responsiveness to FSH[87]. In human studies, AMH was shown to reduce FSH-stimulated aromatase expression in granulosa cells and also decrease FSHR mRNA expression[88].
As an inhibitor of aromatase activity, AMH may drive an elevated androgenized state, leading to HA, oligo/anovulation, and infertility that are symptomatic of patients with PCOS[87]. Elevated serum AMH expression may play a central role in the follicular arrest characteristically observed in anovulatory PCOS (Figure 5)[89]. Recent studies have indicated a complex and, to some extent, inconsistent relationship between AMH levels and IR in women with PCOS. Some studies report a positive correlation between AMH and IR markers. For instance, a 2023 retrospective cohort study in Chinese women with PCOS found that serum AMH levels were significantly higher in those with IR, with positive correlations noted between AMH, homeostasis model assessment of IR, fasting insulin, androgens, and LH/FSH ratio, suggesting that elevated AMH may be linked to increased IR in this population[90]. Similarly, a study in adolescents with PCOS identified AMH as an independent determinant of IR, indicating that AMH may play a role in the early development of metabolic dysfunction in this group[91].
However, a systematic review and meta-analysis of 22 studies concluded that the overall association between AMH and IR in PCOS is weak and statistically insignificant (pooled correlation coefficient = 0.089), highlighting considerable heterogeneity across studies[92]. This variability may be attributed to several confounding factors, such as different PCOS phenotypes, different BMI classifications, variation in environmental factors and genetics across regions, and various age groups[92]. Together, these factors demonstrate the complexity of the AMH-IR relationship and suggest the need for well-controlled, phenotype-specific investigations to elucidate underlying mechanisms.
Interactions between IR, GDFs, and BMPs in PCOS: Under conditions of physiological equilibrium, BMPs and GDFs have been identified to play essential roles in the regulation of folliculogenesis by paracrine/autocrine mechanisms[93]. In GDF-deficient mouse models, there was anomalous follicular growth beyond the primary stage[93,94]. It was confirmed in initial studies by Orisaka et al[6] that GDF-9 plays an essential role in promoting follicle growth by up-regulating follicular androgen biosynthesis. Conversely, BMPs, specifically BMP4, have been shown to have potent suppressive action on androgen production via the theca cells[95]. The knockdown models of BMP4 showed increased androgen levels and decreased estrogen levels[96]. When ligands of BMP4 bind to their respective receptors on the cell surface, they initiate signal transduction that leads to the activation of suppressor of mothers against decapentaplegic proteins[97]. Mechanistically, the impaired suppressor of mothers against decapentaplegic proteins expression negatively regulates BMP4 signaling, in turn impacting steroid hormone formation[98]. Liu et al[98] showed that ovarian BMP4 levels significantly decreased in HA. Conversely, BMP4 treatment downregulated the androgen production by modulating the expression of steroidogenic enzymes (Figure 5).
Sproul et al[99] showed in their study that BMP15 polymorphisms were linked to anovulation and infertility in PCOS. Additionally, variants in GDF-9 have been associated with impaired reproductive outcomes, such as reduced parity, possibly due to altered oocyte development or early pregnancy loss[99]. These genetic disruptions may not directly cause PCOS but could contribute to fertility issues in affected individuals. Furthermore, in the context of IR, the dysregulation of these oocyte-derived factors could exacerbate follicular arrest and anovulation, emphasizing the multifactorial nature of PCOS-related infertility.
In an estradiol valerate-induced polycystic ovary mouse model, folliculogenesis was disrupted, with follicles arrested at the preantral-to-antral transition[100]. This coincided with the reduced expression of GDF-9 and BMP receptor II, a granulosa cell-expressed receptor crucial for BMP15 and GDF-9[100]. Since GDF-9 and BMP15 are oocyte-derived growth factors essential for granulosa cell proliferation and follicle maturation, their dysregulation may underlie ovulatory dysfunction. This is supported by prior studies showing that GDF-9-null mice fail to develop healthy follicles beyond the primary stage[93], and GDF-9 variants in women are associated with subfertility, including reduced parity and potential early pregnancy loss[99]. Thus, continued research is needed to clarify the consequences of GDF-9 and BMP15 polymorphisms, particularly in metabolically compromised PCOS phenotypes.
TGF-β superfamily signaling pathway and IR: The TGF-β superfamily signaling pathway is intricately involved in the regulation of follicular growth and development[101,102]. In addition to the above-mentioned ligands (i.e., inhibin, activin, AMH, GDFs, and BMPs), the TGF-β family consists of three primary isoforms: TGF-β1, TGF-β2, and TGF-β3[102]. The activity of the TGF-β superfamily is regulated by an extracellular matrix (ECM) protein known as fibrillin (FBN) (Figure 4). The ligands of the TGF-β superfamily are sequestered in an inactive latent form bound to latent TGF-β binding proteins[103]. These then bind to FBN and are stored in their latent form in the ECM until required[104].
Thus, FBN regulates TGF-β signaling by managing its activation and availability in the ECM and its dysfunction may contribute to PCOS disease mechanisms[102]. Genetic studies have identified an association between the FBN3 gene variant D19S884 A8 and metabolic disturbances in women with PCOS[102]. Notably, women with PCOS who have one or two copies of the D19S884 A8 allele exhibit significantly elevated fasting insulin levels, and increased homeostasis model assessment-IR scores compared to those with other genotypes, indicating the presence of a more pronounced IR[105]. This polymorphism has also been linked with β-cell dysfunction and dysregulation in basal glucose homeostasis, further linking FBN3 to the metabolic phenotype of PCOS[102]. These findings suggest that genetic variation in FBN3 may impair the delicate balance of TGF-β signaling, possibly contributing to both androgen excess and IR in PCOS. Considering that multiple ECM-binding proteins, including follistatin, influence the TGF-β pathway, it is likely that FBN3 and follistatin variants function together to disrupt TGF-β-dependent regulation of metabolic and reproductive function in PCOS[102]. The regulation of the FBN-TGF-β interactions offers a novel and promising strategy to manage HA, IR, and anovulation in PCOS[104]. Integrating such approaches with existing therapies could significantly improve outcomes for individuals with PCOS.
The KL system, also called stem cell factor or steel factor, is a granulosa cell-derived factor that regulates theca cell function[106]. The membrane receptor of KL, c-Kit, is a receptor tyrosine kinase protein. C-Kit is expressed on the secondary and antral theca cells and may be involved in androgen production in the theca cells[107]. Thus, KL impacts the crosstalk between granulosa cells and theca cells, which is crucial for androgen and estrogen production. Tuck et al[108] observed a markedly increased KL in follicles at all stages of development within the examined PCOS ovaries. The authors postulated that the increased levels of KL observed in PCOS ovaries may underlie several abnormalities of PCOS, such as increased ovarian reserve due to the diverse roles KL has in animal models (Figure 5)[108]. Thus, the overexpression of KL or c-Kit signaling in PCOS may amplify HA by increasing theca cell activity. This impairs granulosa cell function, including a decrease in aromatase activity, leading to the reduced conversion of androgens to estrogens.
In the context of PCOS, where both IR and hyperinsulinemia are prominent features, the interplay between c-Kit signaling and the insulin receptor pathway may have important implications. C-Kit is not only active in pancreatic β-cells but also in ovarian cells[109]. In β-cells, c-Kit activation promotes insulin secretion by upregulating insulin receptor and insulin receptor substrate 1 (IRS-1), supporting PI3K/Akt pathway[108,109]. However, prolonged c-Kit activation results in negative feedback through serine phosphorylation of IRS-1, inhibiting insulin receptor signaling and reducing insulin output[109]. This may indicate that dysregulated c-Kit expression could contribute to the metabolic infertility phenotype in PCOS. Chronic c-Kit-mediated hyperinsulinemia may exacerbate ovarian androgen production, while long-term impairment of insulin signaling could worsen metabolic dysfunction. Thus, tight regulation of c-Kit-IR interactions may be critical in managing the metabolic aspects of PCOS. Hence, targeting c-Kit activation may offer potential therapeutic targets to address abnormal follicular development and IR[109].
Essential for physiological androgen steroidogenesis are IGF-1 that function to enhance FSH function in granulosa cells by stimulating the differentiation and proliferation of theca cells and granulosa cells[110]. The synergistic action of IGF-1 and LH stimulates androgen production from the theca cells[111]. IGF-1 contributes to steroidogenesis by mimicking and amplifying LH actions[112]. Furthermore, the stimulatory effects of FSH on aromatase expression depend on IGF-1 action and the expression and activation of the IGF-1 receptor (IGF-1R)[113-115]. The inactivation of IGF-1R resulted in completely sterile mice, with only 10% to 60% surviving to adulthood[116]. More adversely, IGF-1R knockout mice die at birth, indicating that several other critical pathways may become dysfunctional in the absence of IGF-1R[116].
Interestingly, the action of insulin on the production of ovarian androgens is believed to be through IGF-1R on theca cells[117]. High levels of insulin and IGF-1 amplify the effect of LH on granulosa cells, which disrupts follicular development and ovulation, leading to anovulation[118]. A recent meta-analysis showed that women with PCOS had significantly higher insulin levels, which reduces the hepatic production of IGF-binding protein 1[119]. Decreased levels of IGF-binding protein 1 result in increased bioavailability of free IGF-1, further triggering androgen production and contributing to the cycle of HA and IR[120]. It has been shown that IGF-1R is necessary to induce the PI3K/Akt pathway[121]. The IGF-1/PI3K pathway functions as a crucial downstream signaling mechanism activated by the binding of IGF-1 to IGF-1R.
Under normal conditions, when IGF-1 binds to its receptor, IGF-1R, it activates the intracellular signaling cascade, particularly the PI3K/Akt pathway[112]. There is evidence emerging that the IGF-1R/PI3K pathway may be central in the pathogenesis of PCOS. Thus, the increase in androgen levels characteristic of PCOS patients is more related to dysfunction in the IGF-1R/PI3K pathway[122]. Two other role players in the pathway are the IRS-1 and IRS-2 proteins that contribute to the binding and subsequent activation of the PI3K pathway[122,123]. He et al[122] concluded from their studies that the IGF-1R/PI3K pathway is differently expressed in PCOS granulosa cells compared with controls, with IGF-1R, IRS-1 and IRS-2 significantly increased while PTEN decreased. PTEN plays an important regulatory role. The selective deletion of PTEN in mice resulted in the overactivation of PI3K signaling in the ovaries[59]. The mice exhibited elevated androgen levels, ovary enlargement, antral follicle accumulation, and early fertility loss, hallmark features of human PCOS[59]. Thus, it can be concluded that dysfunctional PTEN may be involved in PCOS pathogenesis[122]. Another earlier study by Nahum et al[124] showed the “progonadotrophic” roles for insulin and IGF-1 in regulating normal ovarian androgen production. Their study also revealed the effects of insulin in driving the etiology of HA, interestingly, both with and without hyperinsulinemia in PCOS[124]. In line with this, recent studies have found comparable evidence[125,126]. Targeting the IGF-1/PI3K pathway could provide therapeutic advantages by addressing ovarian and metabolic anomalies in PCOS.
Under normal conditions, a critical downstream effector of PI3K is the serine/threonine kinase Akt[58]. The activation of the PI3K/Akt pathway promotes insulin-mediated glucose uptake in target tissues by facilitating the transport of glucose transporter 4 to the plasmalemma[127]. It has been shown that the PI3K/Akt signaling pathway plays a crucial role in the ovary. The PI3K/Akt pathway exerts an anti-apoptotic effect, partly by phosphorylating the forkhead box O (FOXO1/3/4/6) transcription factors[128]. Phosphorylation is a regulatory mechanism that controls the activity of FOXO proteins. Thus, in the presence of insulin, FOXO transcription factors are phosphorylated in response to the activation of the PI3K/Akt pathway[129]. Their transcriptional functions are turned off following nuclear exclusion and sequestered into the cytoplasm[129]. In the cytoplasm, FOXO is degraded by proteasomes, which accompanies transcriptional activity loss, thereby preventing it from re-entering the nucleus and activating its target genes[130]. FOXO signaling can induce apoptosis in oocytes and granulosa cells and inhibit the activation of primordial follicles[130]. In particular, FSH is a protective factor that promotes the survival of antral follicles because it antagonizes apoptosis in granulosa cells. Thus, an inverse relationship exists between FOXO and FSH, where FOXO activity is negatively influenced by FSH[131].
Dysregulation of the PI3K/Akt and FOXO signaling pathways in PCOS is pivotal in linking IR with HA and follicular dysfunction[132]. Concurrently, the impairment of the PI3K/Akt pathway, characteristic in most PCOS patients, may lead to the activation and overexpression of FOXO3 in granulosa cells, resulting in higher apoptosis levels in these cells[128]. When hormones and/or growth factors are depleted, FOXO3 dephosphorylates and translocates into the nucleus, resulting in transcription of pro-apoptotic factors and loss of mitochondrial membrane potential[128]. Furthermore, it has been evidenced that FOXO1, which has been extensively studied, mediates gluconeogenesis by upregulating several key genes, including phosphoenolpyruvate carboxykinase and glucose 6-phosphatase[133,134]. Interestingly, during IR, FOXO1 becomes less phosphorylated and therefore more active to promote expression of gluconeogenic genes, which may promote a hyperglycemic state[133]. Some studies have identified aberrant FOXO1-mediated signaling to contribute to the development of low-grade chronic inflammation in the body, leading to HA in PCOS patients[135,136]. Thus, the aberrant activation of the PI3K/Akt and FOXO pathways can contribute to the pathophysiology of PCOS. Hence, modulation of these pathways may provide therapeutic interventions that can address the hormonal and metabolic dysfunctions associated with PCOS.
Under normal physiological conditions, insulin and growth factors such as IGF-1 activate both the PI3K/Akt and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways in a synchronized way, maintaining proper regulation of metabolism, cell growth, and ovarian function. Both these pathways are associated with LH-induced changes in steroid biosynthesis in ovarian theca cells[137]. Although the PI3K/Akt and MAPK/ERK pathways operate in a balanced and complementary manner, disruption of their coordination can impair PI3K/Akt signaling while relatively amplifying the MAPK/ERK activity, contributing to the development of IR[138].
Recent evidence has implicated the dysfunctional activation of the MAPK/ERK cascade in PCOS, leading to excess androgen biosynthesis and suppressed activity of IRS, thus promoting IR development[139]. The link between IR and activation of the MAPK pathway was demonstrated by Zhang et al[140], where the authors assessed the effects of berberine on MAPK-related protein expression and observed a significant berberine-mediated suppression of p38, ERK, and c-Jun N-terminal kinases. These molecules can provoke the abnormal activation of serine/threonine kinase signaling pathways[141]. Thus, their downregulation was accompanied by reduced PCOS symptoms and IR values in the berberine-treated rat model[140]. These findings were further supported by another study, which demonstrated that ovaries from PCOS rats exhibited decreased PI3K/Akt signaling alongside increased MAPK/ERK pathway activation, suggesting a significant disruption in ovarian glucose metabolism[138].
Furthermore, studies have indicated that impairment in the MAPK/ERK signaling pathway can influence steroidogenic enzyme expression and androgen production[142]. For instance, research suggests that in PCOS theca cells, reduced phosphorylation of ERK1/2 correlated with increased CYP17A1 mRNA expression and increased androgen biosynthesis, implying that decreased ERK1/2 activity may contribute to HA in PCOS[142]. Interestingly, Fukuda et al[143] concluded that LH stimulates CYP17A1 mRNA expression and androgen production in theca cells via activation of the PI3K/Akt pathway, with MAPK/ERK signaling facilitating this process. Insulin appears to act synergistically with LH to trigger StAR protein, stimulating steroidogenic action, with IR, possibly fuelling the impaired MAPK/ERK signaling pathway[144]. IR may play a prominent role in causing gonadotropin dysfunction and steroid hormone imbalance among individuals with PCOS[144]. Tailored strategies to address IR may constitute the cornerstone of remedying both the symptoms and root causes of PCOS.
In normal homeostatic conditions, the SHBG, a glycoprotein secreted by the liver, plays a critical role as a gatekeeper by binding androgen with high affinity and specificity[145]. Frequently, PCOS patients have abnormally low serum SHBG levels, contributing to a hyperandrogenic state[146]. Total testosterone and SHBG concentrations are inversely related, where a suppressed SHBG results in an increase of bioavailable androgen, thereby leading to a rise in peripheral an
Recent studies have supported the inverse correlation between SHBG levels and markers of IR. A study involving European women with PCOS found that SHBG levels below 40 nmol/L indicated metabolic dysregulation, with waist circumference significantly predicting SHBG levels[148]. Waist circumference is an essential predictor of SHBG levels, particularly in IR states[148]. A larger waist circumference may indicate more abdominal fat, which is metabolically active and contributes to IR by promoting the release of inflammatory molecules and free fatty acids into the blood
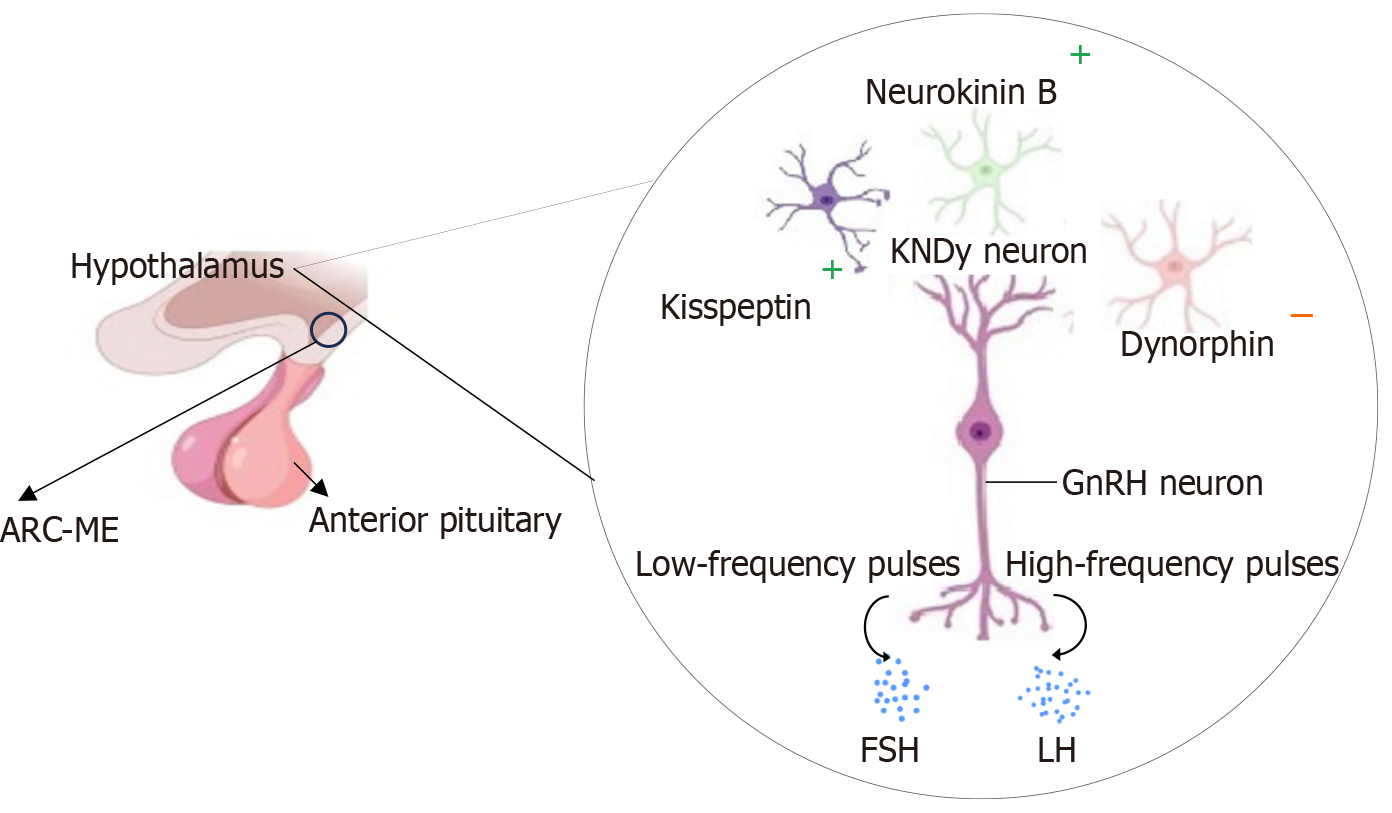
Aberrant neuroendocrine signaling has been proposed to play a fundamental role in the development of PCOS. In the neuroendocrine system, the hierarchical arrangement of the hypothalamus, pituitary, and ovary forms the HPO axis that produces hormones in response to signals from the nervous system[149]. In PCOS, dysregulation often occurs in the HPO axis, resulting in a change in the GnRH pulsatile pattern (Figure 6), favoring the release of LH. The elevated LH concentrations can aggravate the hyperandrogenic PCOS state[150]. The exact mechanism driving the disruptions in GnRH pulsatility in PCOS is not fully understood. However, studies have implicated imbalances in the neurotransmitters and neurohormones that regulate GnRH secretions[151].
Of particular interest are the kisspeptin/neurokinin B/dynorphin (KNDy) neurons that are recognized for their co-expression of KNDy, which are involved in orchestrating the rhythmic release of GnRH, which in turn triggers the secretion of LH and FSH[8] (Figure 6). Recent studies have indicated the potential link between KNDy, PCOS and IR[11]. IR in PCOS can disrupt the finely tuned balance of KNDy neurons, causing hormonal dysregulation and reproductive disturbances[152]. Due to the complexity of these interactions, further research is required to understand the specific mechanisms underlying insulin’s effects on KNDy neurons in the context of PCOS.
Additionally, the disruptions in the neural circuits of the neuron-glia network in the arcuate nucleus and median eminence may advance the neuroendocrine disturbances characteristic of PCOS[153]. Glial cells, including astrocytes and tanycytes, play a pivotal role in modulating the secretions of the GnRH by regulating synaptic inputs to GnRH neurons and structural changes in response to the fluctuations in the steroid hormone feedback (such as estrogen)[153]. Thus, the dysregulation in the interaction between neurons and glial cells may contribute to the amplified androgen production observed in PCOS[153].
Recent research has increasingly centered on the pathophysiology of neuroinflammation, or neurodegeneration linked to gut dysbiosis[153]. Numerous studies have linked alterations in gut microbiomes in preclinical PCOS models and clinical PCOS patients[154-157]. Thus, the dysfunction of the gut-inflammation-brain axis can be a crucial link between the metabolic, hormonal, and neuroendocrine disturbances characteristic of PCOS. Targeting this axis can offer promising targets for discovering novel strategies to remedy PCOS[153].
Adipose tissue, mainly white adipose tissue, plays a pivotal role in the pathophysiology of PCOS because of its capacity to produce and metabolize steroid hormones[158]. In individuals with PCOS, alterations in insulin sensitivity and adi
To further elucidate the neuroendocrine impacts and adipose irregularities, Cox et al[161] developed an adipocyte and brain-specific AR knockout model with a dihydrotestosterone-induced PCOS mouse model. The wildtype mice de
Recent studies have shown that miRNAs, i.e., small RNA molecules, can bind to mRNA of target genes, resulting in their degradation or inhibiting mRNA translation[164]. Furthermore, miRNAs have been implicated in altered steroidogenesis and may play an essential role in the pathogenesis of HA in PCOS[165,166]. Additionally, the variance in miRNA ex
Conversely, PCOS patients often have a decreased expression of miRNA-592, which triggers the increased expression of mRNA for LH/chorionic gonadotropin receptor (LHCGR). The binding of LH or chorionic gonadotropin to the LHCGR, found on the cell surface of the ovaries, prompts various cellular responses, including the production of androgens, exacerbating HA in PCOS[165]. Overall, miRNAs act as crucial gene expression mediators in both the neuroendocrine and adipose tissue, and their dysregulation may contribute to the hormonal imbalances observed in PCOS[164].
It has been recognized that follicular atresia is an essential regulatory process in women to maintain a healthy reproductive system[169]. Ultimately, only about 400 follicles (< 1%) ovulate throughout the reproductive lifespan of females, and the majority (> 99%) undergo atresia[170]. The apoptosis of granulosa cells is considered the principal mechanism of atresia[169]. Initial studies in the pathophysiology of PCOS have touted androgen excess for retarding the follicular atresia process. However, the downregulation of FSH action by androgen is consistent with a role for androgen in follicular atresia. During cyclic recruitment, a cohort of antral follicles escapes apoptosis due to the protective action of FSH[171]. One of these follicles, the dominant follicle, grows faster than the rest of the cohort and secretes higher levels of estrogen and inhibin[172,173]. A negative feed loop ensues that suppresses pituitary FSH release, depriving subordinate follicles of adequate FSH required for survival, thus causing them to regress and eventually disintegrate[172].
Plenty of evidence shows that androgens modulate FSH responsiveness at intermediate stages of follicular de
In PCOS patients with PCOM, the follicles are arrested in development and do not show apparent signs of atresia. The expression of AR and FSHR may be amplified, as observed in granulosa cells from PCOS patients compared to controls[84]. Aberrant granulosa cell function has been observed, particularly in anovulatory women with PCOS, exhibiting premature responsiveness to LH, implying an early acquisition of LH receptors[179]. Consequently, this may culminate in the failure to select a dominant follicle and a lack of ovulation[64]. Furthermore, the elevated LH stimulation induces androgen production in ovarian theca cells by upregulating the key androgen-producing enzyme (CYP17A1). Thus, accelerating the hyperandrogenic state that may result in the premature stagnation of follicular growth or follicular “arrest”[64,180]. Consequently, the hyperandrogenic state may protect subordinate follicles from atresia, resulting in follicular persistence as seen in patients with PCOM[64]. However, prevailing conditions such as obesity need to be taken into consideration. Recent findings have shown suppressed antral follicle development in women with obesity, resulting in follicles being selected at a small diameter[181]. The smaller size of follicles at selection may reflect premature responsiveness to LH, which correlates with the anovulatory issues associated with PCOS[181].
Insulin and the IGF system may profoundly affect the hyperandrogenic state observed in PCOS[182-184]. Under normal physiological conditions, insulin enhances LH-induced androgen synthesis in the ovary. Thus, in PCOS ano
Recently identified forms of regulated cell death, notably pyroptosis and ferroptosis, have been recognized as essential contributors to the metabolic and reproductive irregularities noted in PCOS[186,187]. These processes, which are driven by oxidative stress, inflammation, and iron dysregulation, interconnect closely with insulin signaling pathways and may exacerbate IR[186,187]. The functional and regulatory roles of pyroptosis and ferroptosis in the context of PCOS are explored in the following sections.
Ferroptosis in follicular atresia: Ferroptosis is a form of cell death characterized by the accumulation of lipid peroxides and iron-dependent reactive oxygen species, which causes the disruption of cellular membranes and eventually cell death[188,189]. Recently, ferroptosis has increasingly been recognized for its role in ovarian granulosa cell dysfunction in PCOS[186]. Glutathione is a critical cofactor for glutathione peroxidase 4 (GPX4), an enzyme that prevents ferroptosis by reducing lipid peroxides[186,190]. Under normal conditions, GPX4 prevents cell damage by converting fatty acid hydroperoxides into non-toxic fatty acid alcohols[191]. Thus, inhibiting GPX4 can interfere with intracellular iron homeostasis and increase lipid peroxidation[192].
In PCOS, the dysregulation of GPX4 function may worsen the oxidative stress-induced inflammation, a key feature of PCOS, further advancing the androgen synthesis and secretion. Iron overload and oxidative stress can also impair insulin signaling pathways, contributing to IR[193]. Therefore, controlling ferroptosis may play an essential role in PCOS pathophysiology. The targeted regulation of ferroptosis, including restoring GPX4 function, regulating iron metabolism and managing oxidative stress, may help improve metabolic and reproductive outcomes in women with PCOS[194]. Interestingly, natural compounds like leonurine have been found to significantly improve ovarian function, hormone disorders, and IR, while reducing granulosa cell ferroptosis[186].
Pyroptosis in follicular atresia: Pyroptosis, an inflammatory type of programmed cell death, may contribute to the chronic inflammation characteristic of PCOS by the excessive release of proinflammatory cytokines, such as interleukin-1β, interleukin-18 and the formation of pyroptotic bodies, which may exacerbate ovarian dysregulation and hormonal imbalance[195]. Additionally, nucleotide-binding oligomerization domain, leucine-rich repeat, and NLR family pyrin domain-containing protein 3 (NLRP3) is a cytosolic sensor involved in activating the inflammasome complex[187]. Studies have implicated NLRP3 as a significant factor leading to inflammation and IR induced by obesity[196]. Yang et al[187] found that the expression levels of NLRP3 were positively correlated with those of IGF-1. This may indicate that the upregulation of NLRP3 in PCOS may reveal a complex interplay between the increased inflammatory and pyroptosis-related factors and IR[187].
Additionally, studies have indicated that persistent excessive inflammation can cause cell dysfunction and activate pyroptosis[195]. The amplified inflammatory response impairs ovarian function, exacerbating the development of PCOS, resulting in the abnormal overactivation of pyroptosis[195]. Targeted therapies investigating mechanisms that help suppress pyroptosis or regulate the relevant inflammatory pathways could help alleviate inflammation and improve both ovarian function and metabolic health in women with PCOS. For instance, mogroside V, an active ingredient found in the monk fruit, has been shown to inhibit NLRP3-mediated pyroptosis in granulosa cells, thereby improving insulin sensitivity and ovarian function in PCOS models[187].
Despite accumulating findings linking HA and IR to disruptions in key signaling pathways in PCOS, several limitations must be acknowledged. First, many mechanistic insights are based on animal models and in vitro systems. While these models provide important findings, they may not accurately denote the intricate hormonal and metabolic environment of human PCOS. Interspecies differences in ovarian physiology and insulin signaling pathways hinder the direct translation of these results to clinical practice. Second, the inherent heterogeneity of PCOS, including variability in androgen excess, metabolic dysfunction, and ovulatory status, complicates the generalization of pathway-specific findings. Individual signaling pathways may vary across different PCOS phenotypes, making it difficult to identify universal therapeutic targets. Third, although pathways such as PI3K/Akt, MAPK/ERK, and miRNA networks have been studied individually, their interactions within the ovarian microenvironment remain poorly understood. A more integrative approach is needed to understand how these pathways influence each other in PCOS. Fourth, there is a lack of long-term effects and intervention studies specifically targeting the molecular pathways involved in PCOS. Current treatments, such as insulin sensitizers or antiandrogens, often have a widespread impact on the body, making it challenging to determine their exact influence on specific signaling mechanisms. Fifth, a significant limitation is the difficulty in accounting for the diverse environmental and lifestyle exposures, which can substantially influence disease pathogenesis. Emerging pollutants, including pharmaceuticals, personal care products, microplastics, and endocrine-disrupting chemicals, have been implicated in the etiology of PCOS[197]. However, the complexity and variability of these environmental factors, coupled with challenges in determining exposure levels and timing, complicate the establishment of defined cause-and-effect relationships. Additionally, most studies rely on animal models or in vitro systems that may not fully replicate human exposure, limiting the translation of the findings. Furthermore, the cumulative and synergistic effects of various environmental toxins, often encountered simultaneously, are poorly understood, necessitating more research to elucidate their role in PCOS pathophysiology[197].
In continuation of the above discussion, maternal health during pregnancy is crucially important in fetal programming and the future risk of PCOS in offspring. Animal models suggest that maternal HA at a critical period of fetal deve
Finally, developing areas of investigation, such as ferroptosis and pyroptosis, including the epigenetic regulation, are gaining attention. However, these mechanisms require further validation through studies involving human ovarian tissue and larger, well-defined patient cohorts to determine causality and therapeutic significance.
Emerging insights suggest that the complex interplay between genetic expression and epigenetic regulation is a promising avenue in understanding and managing IR and HA in PCOS[199]. Epigenetic modifications include DNA methylation, histone modifications and non-coding RNA activity that can modulate genes, either by exacerbating or mitigating their expression[200]. For instance, aberrant methylation has been implicated in the downregulation of insulin-signaling genes, such as INSR and IRS1, aggravating IR in PCOS patients[201]. Likewise, hypomethylation of the LHCGR gene promoter has been linked with increased receptor expression, which may increase LH signaling and promote excessive steroidogenesis, impairing normal folliculogenesis in PCOS[202].
Plant-derived compounds such as curcumin have exhibited the ability to reverse these epigenetic abnormalities by modulating gene expression related to oxidative stress and metabolism, by focusing on two key genes: Sirtuin 1 and peroxisome proliferator-activated receptor gamma coactivator-1α[203]. The peroxisome proliferator-activated receptor gamma coactivator-1α acts to amplify the body’s antioxidant defense mechanisms by elevating detoxifying enzymes like superoxide dismutase and GPX[204]. Sirtuin 1 regulates insulin secretion, antioxidant action, apoptosis and protects DNA[205].
The gut microbiome has also emerged as a dynamic epigenetic regulator by producing metabolites like short-chain fatty acids, which can influence gene expression[206,207]. Dysbiosis commonly seen in PCOS may contribute to systemic IR and HA through epigenetic mechanisms. Additionally, dysbiosis can interfere with how the body processes estrogen, affecting the gut-HPO axis and triggering inflammation by allowing harmful substances like endotoxins to leak into the bloodstream[208,209]. These effects can disrupt the HPO axis and worsen IR and high androgen levels. These findings highlight the importance of integrated therapeutic approaches that combine phytochemicals, microbiome modulation, and epigenetics, aiming to target the multifaceted nature of PCOS.
The dysregulation caused by excess androgen production and action on follicle maturation and the development of PCOS is well established. However, recent research suggests that several ovarian and antecedent factors may contribute to HA. The most critical may be the dysfunction in the insulin signaling pathway and pathogenicity of IR in PCOS patients. The physiological abnormalities associated with IR appear to be a forerunner in the upsurge of the metabolic aberrations related to HA. Metabolic disturbances such as obesity, dyslipidemia, and T2D exacerbate IR, leading to a vicious cycle that may cause long-term health issues such as cardiovascular disease. Furthermore, IR negatively affects the HPO axis, resulting in anomalous gonadotropin release and impaired folliculogenesis. Further studies investigating the mechanisms that target the impact of IR on the HPO axis and GnRH secretions may help control the hormonal imbalances and PCOS.
A deep understanding of the molecular disruptions in PCOS, especially the interaction between IR, HA, and signaling pathways like PI3K/Akt and MAPK/ERK, offers potential for more targeted therapies. Enhancing PI3K/Akt activity or modulating MAPK/ERK signaling might improve insulin sensitivity and reduce androgen excess. Similarly, targeting the activin-inhibin-follistatin axis and restoring GDF-9 and Kit signaling suggests beneficial prospects of improving folliculogenesis and fertility. Heightening SHBG levels to reduce free androgen may contribute to lowering androgenic symptoms. Additionally, emerging research on miRNAs, ferroptosis, and pyroptosis holds promise for novel, molecular-guided treatments. These strategies highlight the shift toward personalized medicine in PCOS, where treatments are aligned with individual molecular profiles to enhance outcomes.
Furthermore, advancements in the PCOS landscape continues to unveil novel biomarkers and therapeutic targets for clinical application. Biomarkers associated with IR and HA offer potential for early and more precise diagnosis and guide more individualized treatment approaches. Molecules such as AMH, SHBG, adiponectin and emerging epigenetic regulators like certain circRNAs may help bridge the gap between metabolic and reproductive phenotypes. As research evolves, integrating these biomarkers into clinical practice could enhance metabolic outcomes and reproductive health in women with PCOS. Leveraging emerging treatment options may offer hope to women who suffer from PCOS, especially in terms of mental, reproductive, and metabolic health.
| 1. | Zirak Sharkesh E, Keshavarz SA, Nazari L, Abbasi B. The dietary inflammatory index is directly associated with polycystic ovary syndrome: A case-control study. Clin Endocrinol (Oxf). 2022;96:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 3. | Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12:616-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 4. | Kruszewska J, Laudy-Wiaderny H, Kunicki M. Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients-The Debate Is Still Open. Int J Environ Res Public Health. 2022;19:2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Chen Y, Wang G, Chen J, Wang C, Dong X, Chang HM, Yuan S, Zhao Y, Mu L. Genetic and Epigenetic Landscape for Drug Development in Polycystic Ovary Syndrome. Endocr Rev. 2024;45:437-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Orisaka M, Miyazaki Y, Shirafuji A, Tamamura C, Tsuyoshi H, Tsang BK, Yoshida Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod Med Biol. 2021;20:169-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Rose BI, Brown SE. A review of the physiology behind letrozole applications in infertility: are current protocols optimal? J Assist Reprod Genet. 2020;37:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 8. | Panda SP, Kesharwani A, Singh GD, Prasanth D, Vatchavai BR, Kumari PVK, Panda SK, Mallick SP. Impose of KNDy/GnRH neural circuit in PCOS, ageing, cancer and Alzheimer's disease: StAR actions in prevention of neuroendocrine dysfunction. Ageing Res Rev. 2023;92:102086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Su P, Chen C, Sun Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J Ovarian Res. 2025;18:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Walters KA, Rodriguez Paris V, Aflatounian A, Handelsman DJ. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242:R23-R50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Dong J, Rees DA. Polycystic ovary syndrome: pathophysiology and therapeutic opportunities. BMJ Med. 2023;2:e000548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Armanini D, Boscaro M, Bordin L, Sabbadin C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int J Mol Sci. 2022;23:4110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 13. | Safiri S, Noori M, Nejadghaderi SA, Karamzad N, Carson-Chahhoud K, Sullman MJM, Collins GS, Kolahi AA, Avery J. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990-2019. Hum Reprod. 2022;37:1919-1931. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Amiri M, Hatoum S, Buyalos RP, Sheidaei A, Azziz R. The Influence of Study Quality, age, and Geographic Factors on Pcos Prevalence - A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2025;110:2082-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8:96351-96358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Liu J, Wu Q, Hao Y, Jiao M, Wang X, Jiang S, Han L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum Reprod. 2021;36:1108-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 17. | Chima SC, Mamdoo F. Ethical and legal dilemmas around termination of pregnancy for severe fetal anomalies: A review of two African neonates presenting with ventriculomegaly and holoprosencephaly. Niger J Clin Pract. 2015;18 Suppl:S31-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Deswal R, Narwal V, Dang A, Pundir CS. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J Hum Reprod Sci. 2020;13:261-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 19. | Meng Y, Zhao T, Zhang R, Zhu X, Ma C, Shi Q. Global burden of polycystic ovary syndrome among women of childbearing age, 1990-2021: a systematic analysis using the global burden of disease study 2021. Front Public Health. 2025;13:1514250. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Zaitoun B, Al Kubaisi A, AlQattan N, Alassouli Y, Mohammad A, Alameeri H, Mohammed G. Polycystic ovarian syndrome awareness among females in the UAE: a cross-sectional study. BMC Womens Health. 2023;23:181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Goh JE, Farrukh MJ, Keshavarzi F, Yap CS, Saleem Z, Salman M, Ramatillah DL, Goh KW, Ming LC. Assessment of prevalence, knowledge of polycystic ovary syndrome and health-related practices among women in klang valley: A cross-sectional survey. Front Endocrinol (Lausanne). 2022;13:985588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Cesta CE, Kuja-Halkola R, Lehto K, Iliadou AN, Landén M. Polycystic ovary syndrome, personality, and depression: A twin study. Psychoneuroendocrinology. 2017;85:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Podfigurna A, Meczekalski B, Petraglia F, Luisi S. Clinical, hormonal and metabolic parameters in women with PCOS with different combined oral contraceptives (containing chlormadinone acetate versus drospirenone). J Endocrinol Invest. 2020;43:483-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Yadav S, Delau O, Bonner AJ, Markovic D, Patterson W, Ottey S, Buyalos RP, Azziz R. Direct economic burden of mental health disorders associated with polycystic ovary syndrome: Systematic review and meta-analysis. Elife. 2023;12:e85338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 25. | Ruddenklau A, Campbell RE. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology. 2019;160:2230-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3363] [Cited by in RCA: 4016] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 27. | Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 958] [Article Influence: 159.7] [Reference Citation Analysis (0)] |
| 28. | Polak AM, Adamska A, Krentowska A, Łebkowska A, Hryniewicka J, Adamski M, Kowalska I. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J Clin Med. 2020;9:732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, Abbott DH. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr Rev. 2020;41:bnaa010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 30. | Azziz R. Defining what is normal: the key to the diagnosis of polycystic ovary syndrome (and any other disorder for that matter…). Fertil Steril. 2019;111:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Ortiz-Flores AE, Luque-Ramírez M, Escobar-Morreale HF. Polycystic ovary syndrome in adult women. Med Clin (Barc). 2019;152:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Sachdeva G, Gainder S, Suri V, Sachdeva N, Chopra S. Comparison of the Different PCOS Phenotypes Based on Clinical Metabolic, and Hormonal Profile, and their Response to Clomiphene. Indian J Endocrinol Metab. 2019;23:326-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Jing T, Wu Y, Wan A, Ge C, Chen ZJ, Du Y. Circular RNA as a Novel Regulator and Promising Biomarker in Polycystic Ovary Syndrome. Biomolecules. 2023;13:1101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Zeng Y, Zheng Z, Liu F, Yi G. Circular RNAs in metabolism and metabolic disorders. Obes Rev. 2021;22:e13220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Qian Y, Tong Y, Zeng Y, Huang J, Liu K, Xie Y, Chen J, Gao M, Liu L, Zhao J, Hong Y, Nie X. Integrated lipid metabolomics and proteomics analysis reveal the pathogenesis of polycystic ovary syndrome. J Transl Med. 2024;22:364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Kim CH, Lee SH. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life (Basel). 2022;12:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 37. | Papadakis G, Kandaraki EA, Garidou A, Koutsaki M, Papalou O, Diamanti-Kandarakis E, Peppa M. Tailoring treatment for PCOS phenotypes. Expert Rev Endocrinol Metab. 2021;16:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Pkhaladze L, Russo M, Unfer V, Nordio M, Basciani S, Khomasuridze A. Treatment of lean PCOS teenagers: a follow-up comparison between Myo-Inositol and oral contraceptives. Eur Rev Med Pharmacol Sci. 2021;25:7476-7485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 39. | Forslund M, Melin J, Alesi S, Piltonen T, Romualdi D, Tay CT, Witchel S, Pena A, Mousa A, Teede H. Different kinds of oral contraceptive pills in polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2023;189:S1-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 40. | Song Y, Wang H, Huang H, Zhu Z. Comparison of the efficacy between NAC and metformin in treating PCOS patients: a meta-analysis. Gynecol Endocrinol. 2020;36:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Zhao H, Xing C, Zhang J, He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 42. | Jensterle M, Herman R, Janež A. Therapeutic Potential of Glucagon-like Peptide-1 Agonists in Polycystic Ovary Syndrome: From Current Clinical Evidence to Future Perspectives. Biomedicines. 2022;10:1989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Borner T, Tinsley IC, Doyle RP, Hayes MR, De Jonghe BC. Glucagon-like peptide-1 in diabetes care: Can glycaemic control be achieved without nausea and vomiting? Br J Pharmacol. 2022;179:542-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Zhang L, Wang Z, Kong L, Liu H, Ma Z, Xu M, Yushanjiang S, Yuan D, Yu L. Effect of SGLT2 Inhibitors on Improving Glucolipid Metabolism and Reproductive Hormone Status in Overweight/Obese Women with PCOS: A Systematic Review and Meta-Analysis. Reprod Sci. 2024;31:1190-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Roy M, Parveen R, Khan P, Majid H, Pathak M, Saxena R, Nidhi. A systematic review on effect of sodium-glucose cotransporter-2 inhibitors on the metabolic and endocrinological profile of patients with polycystic ovarian syndrome. Expert Opin Pharmacother. 2024;25:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | deOliveira TA, Marchesan LB, Spritzer PM. Potassium levels in women with polycystic ovary syndrome using spironolactone for long-term. Clin Endocrinol (Oxf). 2024;100:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Chera-Aree P, Tanpong S, Thanaboonyawat I, Laokirkkiat P. Clomiphene citrate plus letrozole versus clomiphene citrate alone for ovulation induction in infertile women with ovulatory dysfunction: a randomized controlled trial. BMC Womens Health. 2023;23:602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Franik S, Le QK, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for ovulation induction in infertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2022;9:CD010287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Zeng H, Zhang Y, Huang S, Wu J, Ren W, Zhou L, Huang L, Ye Y. Metformin combined with spironolactone vs. metformin alone in polycystic ovary syndrome: a meta-analysis. Front Endocrinol (Lausanne). 2023;14:1223768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Austregésilo de Athayde De Hollanda Morais B, Martins Prizão V, de Moura de Souza M, Ximenes Mendes B, Rodrigues Defante ML, Cosendey Martins O, Rodrigues AM. The efficacy and safety of GLP-1 agonists in PCOS women living with obesity in promoting weight loss and hormonal regulation: A meta-analysis of randomized controlled trials. J Diabetes Complications. 2024;38:108834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 51. | Jin Q, Xu G, Ying Y, Liu L, Zheng H, Xu S, Yin P, Chen Y. Effects of non-pharmacological interventions on biochemical hyperandrogenism in women with polycystic ovary syndrome: a systematic review and network meta-analysis. J Ovarian Res. 2025;18:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 52. | Zhang B, Yao X, Zhong X, Hu Y, Xu J. Vitamin D supplementation in the treatment of polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Heliyon. 2023;9:e14291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 53. | Abedel-Majed MA, Romereim SM, Davis JS, Cupp AS. Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Front Endocrinol (Lausanne). 2019;10:832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab. 2022;36:101665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 55. | Knapczyk-Stwora K, Grzesiak M, Duda M, Koziorowski M, Slomczynska M. Effect of flutamide on folliculogenesis in the fetal porcine ovary--regulation by Kit ligand/c-Kit and IGF1/IGF1R systems. Anim Reprod Sci. 2013;142:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149:R193-R218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 464] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 58. | West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 454] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 59. | Lan ZJ, Krause MS, Redding SD, Li X, Wu GZ, Zhou HX, Bohler HC, Ko C, Cooney AJ, Zhou J, Lei ZM. Selective deletion of Pten in theca-interstitial cells leads to androgen excess and ovarian dysfunction in mice. Mol Cell Endocrinol. 2017;444:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Walters KA, Handelsman DJ. Role of androgens in the ovary. Mol Cell Endocrinol. 2018;465:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36:487-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 622] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 62. | Bharati J, Kumar S, Kumar S, Mohan NH, Islam R, Pegu SR, Banik S, Das BC, Borah S, Sarkar M. Androgen receptor gene deficiency results in the reduction of steroidogenic potential in porcine luteal cells. Anim Biotechnol. 2023;34:2183-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 63. | Casarini L, Paradiso E, Lazzaretti C, D'Alessandro S, Roy N, Mascolo E, Zaręba K, García-Gasca A, Simoni M. Regulation of antral follicular growth by an interplay between gonadotropins and their receptors. J Assist Reprod Genet. 2022;39:893-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 64. | Jarrett BY, Vanden Brink H, Oldfield AL, Lujan ME. Ultrasound Characterization of Disordered Antral Follicle Development in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2020;105:e3847-e3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Walters KA, Moreno-Asso A, Stepto NK, Pankhurst MW, Rodriguez Paris V, Rodgers RJ. Key signalling pathways underlying the aetiology of polycystic ovary syndrome. J Endocrinol. 2022;255:R1-R26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 371] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 67. | Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101:11209-11214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 68. | Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87:151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Guedikian AA, Lee AY, Grogan TR, Abbott DH, Largaespada K, Chazenbalk GD, Dumesic DA. Reproductive and metabolic determinants of granulosa cell dysfunction in normal-weight women with polycystic ovary syndrome. Fertil Steril. 2018;109:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Prizant H, Gleicher N, Sen A. Androgen actions in the ovary: balance is key. J Endocrinol. 2014;222:R141-R151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Fujibe Y, Baba T, Nagao S, Adachi S, Ikeda K, Morishita M, Kuno Y, Suzuki M, Mizuuchi M, Honnma H, Endo T, Saito T. Androgen potentiates the expression of FSH receptor and supports preantral follicle development in mice. J Ovarian Res. 2019;12:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Appiah Adu-Gyamfi E, Tanam Djankpa F, Nelson W, Czika A, Kumar Sah S, Lamptey J, Ding YB, Wang YX. Activin and inhibin signaling: From regulation of physiology to involvement in the pathology of the female reproductive system. Cytokine. 2020;133:155105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | Young JM, McNeilly AS. Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells. J Mol Endocrinol. 2012;48:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Schang G, Ongaro L, Schultz H, Wang Y, Zhou X, Brûlé E, Boehm U, Lee SJ, Bernard DJ. Murine FSH Production Depends on the Activin Type II Receptors ACVR2A and ACVR2B. Endocrinology. 2020;161:bqaa056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Wijayarathna R, de Kretser DM. Activins in reproductive biology and beyond. Hum Reprod Update. 2016;22:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 76. | Perakakis N, Upadhyay J, Ghaly W, Chen J, Chrysafi P, Anastasilakis AD, Mantzoros CS. Regulation of the activins-follistatins-inhibins axis by energy status: Impact on reproductive function. Metabolism. 2018;85:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Teede H, Ng S, Hedger M, Moran L. Follistatin and activins in polycystic ovary syndrome: relationship to metabolic and hormonal markers. Metabolism. 2013;62:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Sylow L, Vind BF, Kruse R, Møller PM, Wojtaszewski JFP, Richter EA, Højlund K. Circulating Follistatin and Activin A and Their Regulation by Insulin in Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2020;105:dgaa090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Tao R, Wang C, Stöhr O, Qiu W, Hu Y, Miao J, Dong XC, Leng S, Stefater M, Stylopoulos N, Lin L, Copps KD, White MF. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med. 2018;24:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 82. | Walton KL, Goney MP, Peppas Z, Stringer JM, Winship A, Hutt K, Goodchild G, Maskey S, Chan KL, Brûlé E, Bernard DJ, Stocker WA, Harrison CA. Inhibin Inactivation in Female Mice Leads to Elevated FSH Levels, Ovarian Overstimulation, and Pregnancy Loss. Endocrinology. 2022;163:bqac025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Brûlé E, Wang Y, Li Y, Lin YF, Zhou X, Ongaro L, Alonso CAI, Buddle ERS, Schneyer AL, Byeon CH, Hinck CS, Mendelev N, Russell JP, Cowan M, Boehm U, Ruf-Zamojski F, Zamojski M, Andoniadou CL, Sealfon SC, Harrison CA, Walton KL, Hinck AP, Bernard DJ. TGFBR3L is an inhibin B co-receptor that regulates female fertility. Sci Adv. 2021;7:eabl4391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 85. | Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 488] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 86. | Sahmay S, Aydogan Mathyk B, Sofiyeva N, Atakul N, Azemi A, Erel T. Serum AMH levels and insulin resistance in women with PCOS. Eur J Obstet Gynecol Reprod Biol. 2018;224:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Chang HM, Klausen C, Leung PC. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100:585-92.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 88. | Sacchi S, D'Ippolito G, Sena P, Marsella T, Tagliasacchi D, Maggi E, Argento C, Tirelli A, Giulini S, La Marca A. The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet. 2016;33:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of Anti-Müllerian Hormone in pathophysiology, diagnosis and treatment of Polycystic Ovary Syndrome: a review. Reprod Biol Endocrinol. 2015;13:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 90. | Zhao H, Zhou D, Liu C, Zhang L. The Relationship Between Insulin Resistance and Obesity and Serum Anti-Mullerian Hormone Level in Chinese Women with Polycystic Ovary Syndrome: A Retrospective, Single-Center Cohort Study. Int J Womens Health. 2023;15:151-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 91. | Guo G, Zheng H, Wu X. Association of anti-Müllerian hormone and insulin resistance in adolescent girls with polycystic ovary syndrome. Endokrynol Pol. 2024;75:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 92. | Md Muslim MZ, Mohammed Jelani A, Shafii N, Yaacob NM, Che Soh NAA, Ibrahim HA. Correlation between anti-mullerian hormone with insulin resistance in polycystic ovarian syndrome: a systematic review and meta-analysis. J Ovarian Res. 2024;17:106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 94. | Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1115] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 95. | Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R, Rodgers RJ, Knight PG. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci U S A. 2013;110:E1426-E1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Gu R, Dai F, Xiang C, Chen J, Yang D, Tan W, Wang Z, Liu H, Cheng Y. BMP4 participates in the pathogenesis of PCOS by regulating glucose metabolism and autophagy in granulosa cells under hyperandrogenic environment. J Steroid Biochem Mol Biol. 2023;235:106410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 97. | Wang K, Li Y. Signaling pathways and targeted therapeutic strategies for polycystic ovary syndrome. Front Endocrinol (Lausanne). 2023;14:1191759. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 98. | Liu Y, Du SY, Ding M, Dou X, Zhang FF, Wu ZY, Qian SW, Zhang W, Tang QQ, Xu CJ. The BMP4-Smad signaling pathway regulates hyperandrogenism development in a female mouse model. J Biol Chem. 2017;292:11740-11750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG. 2010;117:756-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Asghari R, Shokri-Asl V, Rezaei H, Tavallaie M, Khafaei M, Abdolmaleki A, Majdi Seghinsara A. Alteration of TGFB1, GDF9, and BMPR2 gene expression in preantral follicles of an estradiol valerate-induced polycystic ovary mouse model can lead to anovulation, polycystic morphology, obesity, and absence of hyperandrogenism. Clin Exp Reprod Med. 2021;48:245-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Yang H, Cao C, Wu C, Yuan C, Gu Q, Shi Q, Zou J. TGF-βl Suppresses Inflammation in Cell Therapy for Intervertebral Disc Degeneration. Sci Rep. 2015;5:13254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The role of TGF-β in polycystic ovary syndrome. Reprod Sci. 2014;21:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 103. | Shen H, Wang Y. Activation of TGF-β1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J Cell Physiol. 2019;234:11976-11985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 104. | D Prabhu Y, Valsala Gopalakrishnan A. Can polyunsaturated fatty acids regulate Polycystic Ovary Syndrome via TGF-β signalling? Life Sci. 2021;276:119416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 105. | Yalamanchi SK, Sam S, Cardenas MO, Holaday LW, Urbanek M, Dunaif A. Association of fibrillin-3 and transcription factor-7-like 2 gene variants with metabolic phenotypes in PCOS. Obesity (Silver Spring). 2012;20:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Liu T, Qin QY, Qu JX, Wang HY, Yan J. Where are the theca cells from: the mechanism of theca cells derivation and differentiation. Chin Med J (Engl). 2020;133:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 107. | Mori T. Regulatory Principles of Follicular Development. In: Allahbadia GN, Morimoto Y. Ovarian Stimulation Protocols. New Delhi: Springer, 2016: 1-16. [DOI] [Full Text] |
| 108. | Tuck AR, Robker RL, Norman RJ, Tilley WD, Hickey TE. Expression and localisation of c-kit and KITL in the adult human ovary. J Ovarian Res. 2015;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Oakie A, Wang R. β-Cell Receptor Tyrosine Kinases in Controlling Insulin Secretion and Exocytotic Machinery: c-Kit and Insulin Receptor. Endocrinology. 2018;159:3813-3821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Gao L, Gao H, Wang W. Androgens improve ovarian follicle function impaired by glucocorticoids through an androgen-IGF1-FSH synergistic effect. Front Endocrinol (Lausanne). 2022;13:951928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 111. | Dou L, Zheng Y, Li L, Gui X, Chen Y, Yu M, Guo Y. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reprod Biol Endocrinol. 2018;16:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 112. | Khan MZ, Zugaza JL, Torres Aleman I. The signaling landscape of insulin-like growth factor 1. J Biol Chem. 2025;301:108047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 113. | Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, Stocco C. FSH Regulates IGF-2 Expression in Human Granulosa Cells in an AKT-Dependent Manner. J Clin Endocrinol Metab. 2015;100:E1046-E1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 114. | Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 115. | Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 116. | Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59-72. [PubMed] |
| 117. | Nagamani M, Stuart CA. Specific binding sites for insulin-like growth factor I in the ovarian stroma of women with polycystic ovarian disease and stromal hyperthecosis. Am J Obstet Gynecol. 1990;163:1992-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Li H, Zhang Y, Liu C, Zhang Y, Yang H, Fu S, Lv H. Association of Insulin-Like Growth Factor-1 With Polycystic Ovarian Syndrome: A Systematic Review and Meta-analysis. Endocr Pract. 2023;29:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 119. | Jin Y, Sun F, Yang A, Yu X, Li Y, Liang S, Jing X, Wang K, Zhang L, Xiao S, Zhang W, Wang X, Zhao G, Gao B. Insulin-like growth factor binding protein-1 and insulin in polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1279717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 120. | Seibel SA, Chou KH, Capp E, Spritzer PM, von Eye Corleta H. Effect of metformin on IGF-1 and IGFBP-1 levels in obese patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2008;138:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 121. | Chen C, Jiang X, Ding C, Sun X, Wan L, Wang C. Downregulated lncRNA HOTAIR ameliorates polycystic ovaries syndrome via IGF-1 mediated PI3K/Akt pathway. Gynecol Endocrinol. 2023;39:2227280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 122. | He T, Liu Y, Zhao S, Liu H, Wang Z, Shi Y. Comprehensive assessment the expression of core elements related to IGFIR/PI3K pathway in granulosa cells of women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2019;233:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Valverde AM, Lorenzo M, Pons S, White MF, Benito M. Insulin receptor substrate (IRS) proteins IRS-1 and IRS-2 differential signaling in the insulin/insulin-like growth factor-I pathways in fetal brown adipocytes. Mol Endocrinol. 1998;12:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod. 1995;10:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Feng M, Liu F, Xing J, Zhong Y, Zhou X. Anemarrhena saponins attenuate insulin resistance in rats with high-fat diet-induced obesity via the IRS-1/PI3K/AKT pathway. J Ethnopharmacol. 2021;277:114251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 126. | Liang A, Zhang W, Wang Q, Huang L, Zhang J, Ma D, Liu K, Li S, Chen X, Li S, Lei X. Resveratrol regulates insulin resistance to improve the glycolytic pathway by activating SIRT2 in PCOS granulosa cells. Front Nutr. 2022;9:1019562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 127. | King GL, Park K, Li Q. Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes. 2016;65:1462-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 128. | Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, Abbasi M. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet. 2016;294:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Li L, Shi X, Shi Y, Wang Z. The Signaling Pathways Involved in Ovarian Follicle Development. Front Physiol. 2021;12:730196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 130. | Zhang H, Lin F, Zhao J, Wang Z. Expression Regulation and Physiological Role of Transcription Factor FOXO3a During Ovarian Follicular Development. Front Physiol. 2020;11:595086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 131. | Shen M, Liu Z, Li B, Teng Y, Zhang J, Tang Y, Sun SC, Liu H. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 2014;5:e1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 132. | Guo R, Zheng H, Li Q, Qiu X, Zhang J, Cheng Z. Melatonin alleviates insulin resistance through the PI3K/AKT signaling pathway in ovary granulosa cells of polycystic ovary syndrome. Reprod Biol. 2022;22:100594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 133. | Xiong X, Tao R, DePinho RA, Dong XC. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One. 2013;8:e74340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 134. | Xu R, Wang Z. Involvement of Transcription Factor FoxO1 in the Pathogenesis of Polycystic Ovary Syndrome. Front Physiol. 2021;12:649295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 135. | Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril. 2008;89:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Barthelmess EK, Naz RK. Polycystic ovary syndrome: current status and future perspective. Front Biosci (Elite Ed). 2014;6:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 137. | Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Li CG, Zhou L, Zhang YJ, Li Y, Zhao LY. Effect of irisin on ovarian phosphatidylinositol-3-kinase/protein kinase B signaling pathway and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways of rats with polycystic ovary syndrome. J Obstet Gynaecol Res. 2024;50:1945-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 139. | Guney G, Taşkın MI, Sener N, Tolu E, Dodurga Y, Elmas L, Cetin O, Sarigul C. The role of ERK-1 and ERK-2 gene polymorphisms in PCOS pathogenesis. Reprod Biol Endocrinol. 2022;20:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 140. | Zhang N, Liu X, Zhuang L, Liu X, Zhao H, Shan Y, Liu Z, Li F, Wang Y, Fang J. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol. 2020;110:104544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 141. | Zeber-Lubecka N, Ciebiera M, Hennig EE. Polycystic Ovary Syndrome and Oxidative Stress-From Bench to Bedside. Int J Mol Sci. 2023;24:14126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 142. | Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF 3rd, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 143. | Fukuda S, Orisaka M, Tajima K, Hattori K, Kotsuji F. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP Kinase in bovine theca cells. J Ovarian Res. 2009;2:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 144. | Malini NA, Roy GK. Influence of Insulin on LH, Testosterone and SHBG in various PCOS Categories based on the Mode of Secretion of LH in relation to FSH Levels. Acta Endocrinol (Buchar). 2021;17:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Qu X, Donnelly R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int J Mol Sci. 2020;21:8191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 146. | Deswal R, Yadav A, Dang AS. Sex hormone binding globulin - an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst Biol Reprod Med. 2018;64:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 147. | Winters SJ, Scoggins CR, Appiah D, Ghooray DT. The hepatic lipidome and HNF4α and SHBG expression in human liver. Endocr Connect. 2020;9:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 148. | van Bree BE, van der Heijden DMB, van Golde RJT, Brouwers MCGJ, Spaanderman MEA, Valkenburg O. Sex hormone-binding globulin as a biomarker for metabolic risk in European women with polycystic ovary syndrome. Gynecol Endocrinol. 2025;41:2500462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 149. | Zheng CY, Yu YX, Cao SY, Bai X. Epigenetics of inflammation in hypothalamus pituitary gonadal and neuroendocrine disorders. Semin Cell Dev Biol. 2024;154:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 150. | Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 151. | Szeliga A, Rudnicka E, Maciejewska-Jeske M, Kucharski M, Kostrzak A, Hajbos M, Niwczyk O, Smolarczyk R, Meczekalski B. Neuroendocrine Determinants of Polycystic Ovary Syndrome. Int J Environ Res Public Health. 2022;19:3089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 152. | Pérez-López FR, Ornat L, López-Baena MT, Santabárbara J, Savirón-Cornudella R, Pérez-Roncero GR. Circulating kisspeptin and anti-müllerian hormone levels, and insulin resistance in women with polycystic ovary syndrome: A systematic review, meta-analysis, and meta-regression. Eur J Obstet Gynecol Reprod Biol. 2021;260:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 153. | Dai R, Sun Y. Altered GnRH neuron-glia networks close to interface of polycystic ovary syndrome: Molecular mechanism and clinical perspectives. Life Sci. 2025;361:123318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 154. | Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, Thackray VG. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103:1502-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 155. | Feng X, Wang D, Hu L, Lu H, Ling B, Huang Y, Jiang Q. Dendrobium officinale polysaccharide ameliorates polycystic ovary syndrome via regulating butyrate dependent gut-brain-ovary axis mechanism. Front Endocrinol (Lausanne). 2022;13:962775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 156. | Yu Z, Qin E, Cheng S, Yang H, Liu R, Xu T, Liu Y, Yuan J, Yu S, Yang J, Liang F. Gut microbiome in PCOS associates to serum metabolomics: a cross-sectional study. Sci Rep. 2022;12:22184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 157. | Rodriguez Paris V, Wong XYD, Solon-Biet SM, Edwards MC, Aflatounian A, Gilchrist RB, Simpson SJ, Handelsman DJ, Kaakoush NO, Walters KA. The interplay between PCOS pathology and diet on gut microbiota in a mouse model. Gut Microbes. 2022;14:2085961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 158. | Lemaitre M, Christin-Maitre S, Kerlan V. Polycystic ovary syndrome and adipose tissue. Ann Endocrinol (Paris). 2023;84:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 159. | O'Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102:3327-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 160. | Kempegowda P, Melson E, Manolopoulos KN, Arlt W, O'Reilly MW. Implicating androgen excess in propagating metabolic disease in polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2020;11:2042018820934319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 161. | Cox MJ, Edwards MC, Rodriguez Paris V, Aflatounian A, Ledger WL, Gilchrist RB, Padmanabhan V, Handelsman DJ, Walters KA. Androgen Action in Adipose Tissue and the Brain are Key Mediators in the Development of PCOS Traits in a Mouse Model. Endocrinology. 2020;161:bqaa061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 162. | Bril F, Ezeh U, Amiri M, Hatoum S, Pace L, Chen YH, Bertrand F, Gower B, Azziz R. Adipose Tissue Dysfunction in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2023;109:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 163. | Lionett S, Kiel IA, Camera DM, Vanky E, Parr EB, Lydersen S, Hawley JA, Moholdt T. Circulating and Adipose Tissue miRNAs in Women With Polycystic Ovary Syndrome and Responses to High-Intensity Interval Training. Front Physiol. 2020;11:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 164. | Udesen PB, Sørensen AE, Svendsen R, Frisk NLS, Hess AL, Aziz M, Wissing MLM, Englund ALM, Dalgaard LT. Circulating miRNAs in Women with Polycystic Ovary Syndrome: A Longitudinal Cohort Study. Cells. 2023;12:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 165. | Mu L, Sun X, Tu M, Zhang D. Non-coding RNAs in polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2021;19:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 166. | Guan G, Zhang D, Zheng Y, Wen L, Yu D, Lu Y, Zhao Y. microRNA-423-3p promotes tumor progression via modulation of AdipoR2 in laryngeal carcinoma. Int J Clin Exp Pathol. 2014;7:5683-5691. [PubMed] |
| 167. | Sebastiani G, Guay C, Latreille M. Circulating Noncoding RNAs as Candidate Biomarkers of Endocrine and Metabolic Diseases. Int J Endocrinol. 2018;2018:9514927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Singh A, Bora P, Krishna A. Systemic adiponectin treatment reverses polycystic ovary syndrome-like features in an animal model. Reprod Fertil Dev. 2018;30:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 169. | Zhou J, Peng X, Mei S. Autophagy in Ovarian Follicular Development and Atresia. Int J Biol Sci. 2019;15:726-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 170. | Jin LY, Yu JE, Xu HY, Chen B, Yang Q, Liu Y, Guo MX, Zhou CL, Cheng Y, Pang HY, Wu HY, Sheng JZ, Huang HF. Overexpression of Pde4d in rat granulosa cells inhibits maturation and atresia of antral follicles to induce polycystic ovary. Biochim Biophys Acta Mol Basis Dis. 2024;1870:166869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 171. | McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 446] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 172. | Findlay JK, Dunning KR, Gilchrist RB, Hutt KJ, Russell DL, Walters KA. Follicle Selection in Mammalian Ovaries. In: Leung PCK, Adashi EY. The Ovary (Third Edition). United States: Academic Press, 2019: 3-21. [DOI] [Full Text] |
| 173. | Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, Ernst E, Macklon KT, Andersen CY. Hallmarks of Human Small Antral Follicle Development: Implications for Regulation of Ovarian Steroidogenesis and Selection of the Dominant Follicle. Front Endocrinol (Lausanne). 2017;8:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 174. | Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111:3008-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 175. | Słomczyńska M, Szołtys M, Duda M, Sikora K, Tabarowski Z. Androgens and FSH affect androgen receptor and aromatase distribution in the porcine ovary. Folia Biol (Krakow). 2003;51:63-68. [PubMed] |
| 176. | Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Møllgård K, Wreford Andersen E, Byskov AG, Yding Andersen C. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 177. | Casarini L, Simoni M. Membrane estrogen receptor and follicle-stimulating hormone receptor. Vitam Horm. 2023;123:555-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 178. | Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524-E1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 179. | Deswal R, Nanda S, Dang AS. Association of Luteinizing hormone and LH receptor gene polymorphism with susceptibility of Polycystic ovary syndrome. Syst Biol Reprod Med. 2019;65:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 180. | Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 181. | Oldfield AL, Vanden Brink H, Carter FE, Jarrett BY, Lujan ME. Obesity is associated with alterations in antral follicle dynamics in eumenorrheic women. Hum Reprod. 2023;38:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 182. | Genazzani AD, Lanzoni C, Ricchieri F, Baraldi E, Casarosa E, Jasonni VM. Metformin administration is more effective when non-obese patients with polycystic ovary syndrome show both hyperandrogenism and hyperinsulinemia. Gynecol Endocrinol. 2007;23:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 183. | Wang F, Zhang Z, Wang Z, Xiao K, Wang Q, Su J, Wang Z. Expression and clinical significance of the HIF-1a/ET-2 signaling pathway during the development and treatment of polycystic ovary syndrome. J Mol Histol. 2015;46:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 184. | Wang F, Wang S, Zhang Z, Lin Q, Liu Y, Xiao Y, Xiao K, Wang Z. Defective insulin signaling and the protective effects of dimethyldiguanide during follicular development in the ovaries of polycystic ovary syndrome. Mol Med Rep. 2017;16:8164-8170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 185. | Shen YH, Peng S, Zhu T, Shen MJ. Mechanisms of Granulosa Cell Programmed Cell Death and Follicular Atresia in Polycystic Ovary Syndrome. Physiol Res. 2025;74:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Huang X, Geng H, Liang C, Xiong X, Du X, Zhuan Q, Liu Z, Meng L, Zhou D, Zhang L, Fu X, Qi X, Hou Y. Leonurine restrains granulosa cell ferroptosis through SLC7A11/GPX4 axis to promote the treatment of polycystic ovary syndrome. Free Radic Biol Med. 2025;226:330-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 187. | Yang W, Ma Y, Wu Y, Lei X, Zhang J, Li M. Study on the effects of Mogroside V in inhibiting NLRP3-mediated granulosa cell pyroptosis and insulin resistance to improve PCOS. J Ovarian Res. 2025;18:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 188. | Ding H, Xiang Y, Zhu Q, Wu H, Xu T, Huang Z, Ge H. Endoplasmic reticulum stress-mediated ferroptosis in granulosa cells contributes to follicular dysfunction of polycystic ovary syndrome driven by hyperandrogenism. Reprod Biomed Online. 2024;49:104078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 189. | Xi H, Chen X, Wang X, Jiang F, Niu D. Role of programmed cell death in mammalian ovarian follicular atresia. J Steroid Biochem Mol Biol. 2025;247:106667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 190. | Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 1461] [Article Influence: 292.2] [Reference Citation Analysis (0)] |
| 191. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 1011] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 192. | Wang M, Zhang BQ, Ma S, Xu Y, Zhao DH, Zhang JS, Li CJ, Zhou X, Zheng LW. Broadening horizons: the role of ferroptosis in polycystic ovary syndrome. Front Endocrinol (Lausanne). 2024;15:1390013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 193. | Mo M, Pan L, Deng L, Liang M, Xia N, Liang Y. Iron Overload Induces Hepatic Ferroptosis and Insulin Resistance by Inhibiting the Jak2/stat3/slc7a11 Signaling Pathway. Cell Biochem Biophys. 2024;82:2079-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 194. | Ji R, Wang S, Chen X, Yang Z, Zhang Z, Bao S, Xiao Z, Zhang Y, Yin T, Yang J. Platycodin D ameliorates polycystic ovary syndrome-induced ovarian damage by upregulating CD44 to attenuate ferroptosis. Free Radic Biol Med. 2024;224:707-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 195. | Xiang Y, Wang H, Ding H, Xu T, Liu X, Huang Z, Wu H, Ge H. Hyperandrogenism drives ovarian inflammation and pyroptosis: A possible pathogenesis of PCOS follicular dysplasia. Int Immunopharmacol. 2023;125:111141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 196. | Barnett KC, Li S, Liang K, Ting JP. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell. 2023;186:2288-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 197. | Gautam R, Prambil AM, Patel AK, Arora T. Emerging pollutants in etiology and pathophysiology of polycystic ovary syndrome. Reprod Toxicol. 2024;123:108515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 198. | Gur EB, Karadeniz M, Turan GA. Fetal programming of polycystic ovary syndrome. World J Diabetes. 2015;6:936-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 199. | Rawat K, Sandhu A, Gautam V, Saha PK, Saha L. Role of genomic DNA methylation in PCOS pathogenesis: a systematic review and meta-analysis involving case-controlled clinical studies. Mol Hum Reprod. 2022;28:gaac024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 200. | Korolenko A, Skinner MK. Generational stability of epigenetic transgenerational inheritance facilitates adaptation and evolution. Epigenetics. 2024;19:2380929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 201. | Zhong X, Jin F, Huang C, Du M, Gao M, Wei X. DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of Polycystic Ovary Syndrome (PCOS). Technol Health Care. 2021;29:11-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 202. | Liu YN, Qin Y, Wu B, Peng H, Li M, Luo H, Liu LL. DNA methylation in polycystic ovary syndrome: Emerging evidence and challenges. Reprod Toxicol. 2022;111:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 203. | Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, Tanbakooei S, Shidfar F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 204. | Qian L, Zhu Y, Deng C, Liang Z, Chen J, Chen Y, Wang X, Liu Y, Tian Y, Yang Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct Target Ther. 2024;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 205. | Wu YX, Yang XY, Han BS, Hu YY, An T, Lv BH, Lian J, Wang TY, Bao XL, Gao L, Jiang GJ. Naringenin regulates gut microbiota and SIRT1/ PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed Pharmacother. 2022;153:113286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 206. | Pepke ML, Hansen SB, Limborg MT. Unraveling host regulation of gut microbiota through the epigenome-microbiome axis. Trends Microbiol. 2024;32:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Reference Citation Analysis (0)] |
| 207. | Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, Yao S, Maynard CL, Singh N, Dann SM, Liu Z, Cong Y. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 648] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 208. | Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y, Jiang C, Qiao J. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 209. | Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 560] [Article Influence: 70.0] [Reference Citation Analysis (0)] |