Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108344
Revised: May 19, 2025
Accepted: June 23, 2025
Published online: July 15, 2025
Processing time: 95 Days and 5.3 Hours
Exercise plays a key role in managing chronic conditions such as diabetes mellitus (DM), a major contributor to end-stage renal disease (ESRD), a serious public health issue.
To investigate the relationship between exercise intensity, DM duration, and ESRD incidence.
This retrospective cohort study analyzed data from 2495031 individuals with DM who underwent the Korean National Health Screening between 2015 and 2016, with follow-up through 2022. The Cox proportional hazards model was adjusted for confounders, including age, sex, income, smoking, and baseline comorbidities.
Longer DM duration was associated with a significantly higher risk of ESRD, with durations ≥ 10 years showing the highest risk [hazard ratio (HR): 2.624, 95% confidence interval (CI): 2.486-2.770]. Increased exercise intensity reduced the risk of developing ESRD across all diabetes duration groups, with the highest exercise category (≥ 1500 metabolic equivalents of task-min/week) demonstrating a protective effect compared to that of no exercise (HR: 0.837, 95%CI: 0.791-0.886). Exercise benefits were more pronounced in patients without hypertension, non-smokers, and those with lower alcohol consumption. Additionally, ESRD risk reduction was significant among patients with a body mass index ≥ 25 and those without proteinuria or chronic kidney disease.
Longer diabetes duration is associated with increased ESRD risk, while high-intensity exercise may mitigate this risk. These findings suggest promoting exercise is important for managing diabetes to reduce renal complications.
Core Tip: This large-scale nationwide cohort study explored the association between exercise intensity and the risk of end-stage renal disease (ESRD) in patients with diabetes. The findings indicate that high-intensity physical activity (≥ 1500 metabolic equivalents of task-min/week) significantly reduced ESRD risk, particularly in those with diabetes for over 10 years. Subgroup analyses revealed the greatest benefit in patients without hypertension, non-smokers, and those with higher body mass index. These findings highlight the potential of targeted exercise interventions to delay renal complications in diabetes management.
- Citation: Bae EH, Lim SY, Kim BS, Han KD, Suh SH, Choi HS, Kim CS, Ma SK, Kim SW. Exercise intensity and the risk of end-stage renal disease in diabetes: A nationwide population-based study. World J Diabetes 2025; 16(7): 108344
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108344.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108344
Exercise is increasingly becoming a key strategy in preventing and managing various medical conditions, including arthritis and diabetes (termed “exercise as medicine”)[1]. It reduces cardiovascular risk, inflammation, cachexia, and hypertension while enhancing physical function, strength, and cardiorespiratory fitness. Combined with dietary changes, exercise also helps regulate glucose levels[2].
Chronic kidney disease (CKD) is a global public health concern, exacerbated by reduced muscle strength and activity levels in patients with CKD compared to those of healthy individuals. This decrease in physical activity further decreases their quality of life and increases mortality[3]. Although exercise is known to improve CKD-related outcomes, such as blood pressure, renal perfusion, and proteinuria, evidence remains inconsistent, possibly owing to variations in study designs, patient populations, and exercise adherence[4,5]. Nonetheless, exercise has shown benefits for lipid metabolism and renal function in patients with cardiovascular and renal comorbidities[6].
Despite these benefits, the specific impact of exercise intensity on the progression of diabetic complications such as end-stage renal disease (ESRD) remains underexplored. This gap persists largely due to difficulties in accurately measuring exercise intensity and adherence in routine clinical practice.
Therefore, this study aimed to clarify how varying exercise intensities affect the risk of developing ESRD among patients with diabetes, stratified by diabetes duration, using a large, population-based dataset.
We used the Korean National Health Insurance Service (NHIS) claims database, which includes data from the NHIS and Medical Aid programs. The NHIS, a mandatory social insurance scheme, covers approximately 97% of the Korean population, while the remaining 3% is covered by the Medical Aid program[7,8]. Therefore, the NHIS database represents nearly the entire South Korean population (approximately 50 million). Under this system, all insured individuals aged > 40 years undergo a biannual health checkup, and employees aged > 20 years must undergo annual health checkups. The Korean National Health Screening (KNHS) database, obtained through these checkups, provides various information, including anthropometric measurement data, health questionnaire data, and laboratory results. We combined this with nationwide medical records to form the study cohort following NHIS approval. The study was approved by Chonnam National University Hospital (approval number: CNUH-EXP-2023-183) and conducted in accordance with the principles of the Declaration of Helsinki. The review board waived the requirement for written informed consent.
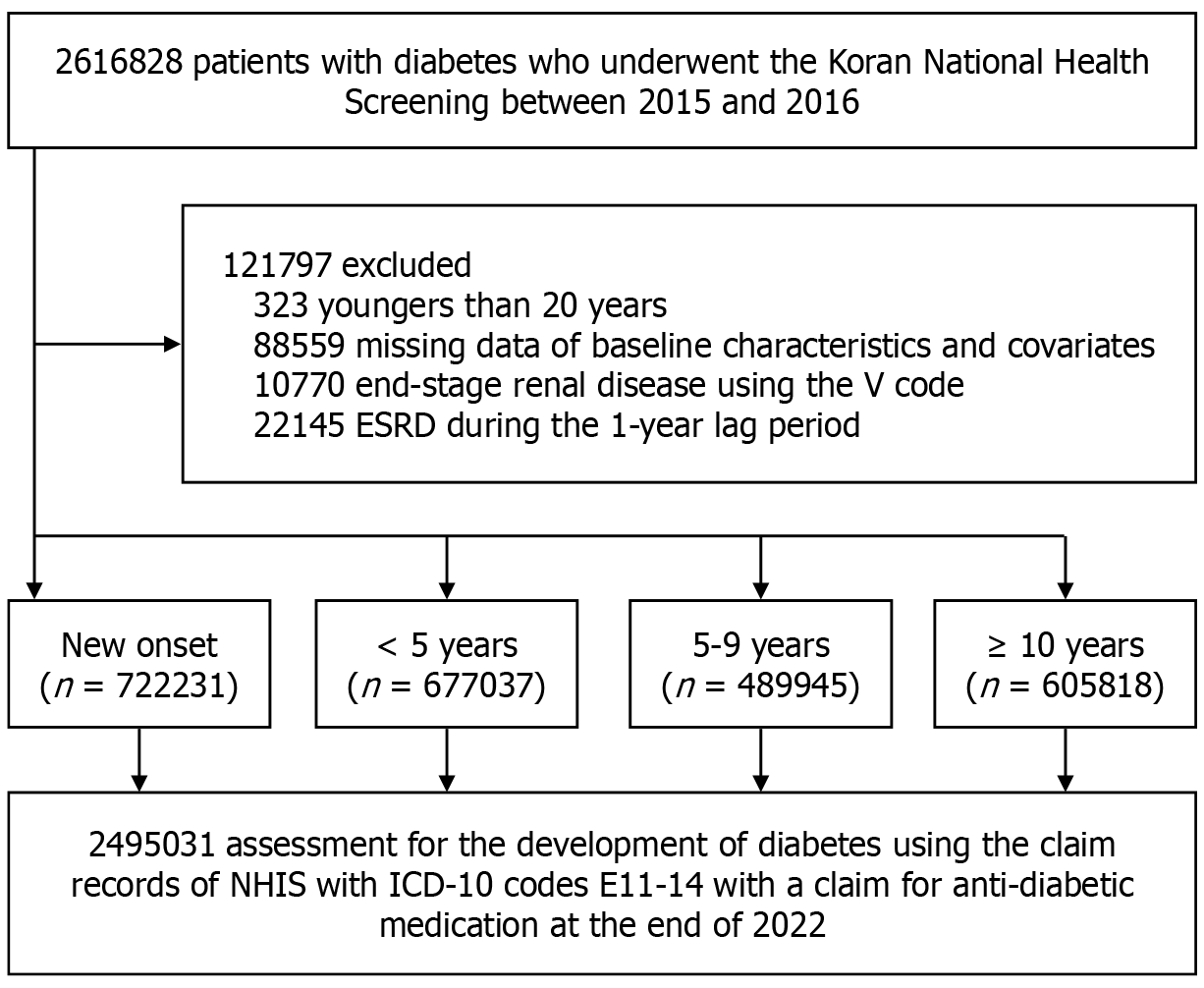
All 2616828 participants who underwent the KNHS from 2015 to 2016 were initially included in the study and followed up until the end of 2022. We excluded those aged < 20 years (n = 323), with missing baseline characteristics and covariates data (n = 88559), and those with pre-existing ESRD at baseline (n = 10770) or during a 1-year lag period (n = 22145). The final sample size, comprising 2495031 participants, was evaluated based on their diabetes mellitus (DM) duration and exercise intensity (Figure 1).
Diabetes at baseline was diagnosed based on fasting blood glucose (FBG) ≥ 126 mg/dL or ICD-10 codes E11–14 with anti-diabetic medication claims[9]. Hypertension or hyperlipidemia was confirmed using laboratory data (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg; total cholesterol ≥ 240 mg/dL) or ICD codes (I10-I15 or E78) with relevant medication claims for each disease. Ischemic heart disease was defined by ICD-10 codes I21-25. Cancer was identified by NHIS registration with ICD-10 code C, and CKD was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula[10]. Participants were categorized by smoking status as non-smokers, current smokers, or former smokers, and by alcohol drinking as non-drinkers, moderate, or heavy drinkers (> 30 g of alcohol per day). Regular exercise was defined as vigorous activity over 20 minutes, at least 3 days in the past week, causing near breathlessness. Income was divided into quartiles (Q): Q1 (the lowest), Q2, Q3, and Q4 (the highest), with low income defined as the lowest 25%. Urban residence was also assessed. Laboratory test quality is ensured by the Korean Association for Laboratory Medicine, and the NHIS certifies hospitals participating in health check-up programs. Proteinuria was assessed using the dipstick method and categorized as negative, trace, or graded from 1+ to 4+. Energy expenditure was calculated from a self-reported survey on exercise frequency and minimum duration, assigning 2.9, 4.0, and 7.0 metabolic equivalents of tasks (METs) to light-, moderate-, and vigorous-intensity exercise, respectively[11]. Total energy expenditure, computed by summing MET values multiplied by exercise frequency and minimum duration, was stratified into < 500, 500-999, 1000-1499, and ≥ 1500 MET-min/week for exploratory analysis.
The primary endpoint was incident ESRD, defined by ICD-10 codes (N18-19, Z49, Z94.0, and Z99.2) combined with special V codes assigned for renal replacement therapy initiation: Hemodialysis (HD) V001, peritoneal dialysis (PD) V003, or kidney transplantation (KT, V005) during hospitalization. Dialysis costs are fully reimbursed through the Korean Health Insurance Review and Assessment Service. The patients were also registered as special medical aid beneficiaries. Therefore, we identified all patients with ESRD nationwide and analyzed the data for patients starting dialysis. Treatment and claim codes included V005 (KT), V001 (HD), and V003 (PD). We excluded individuals without previous CKD who had transplant or dialysis codes on the same day as an acute renal failure code, as well as those on continuous renal replacement therapy or acute PD. Follow-up began 365 days after the health checkup to minimize reverse causation (1-year lag period). Time to ESRD was measured from this point to the first ESRD diagnosis, as determined by ICD-10 (N18-19, Z49, Z94.0, and Z99.2) and V (V001, V003, and V005) codes. Participants without ESRD were censored at death or December 31, 2022, whichever came first.
Data are presented as means ± SD for normally distributed continuous variables, medians (25th, 75th percentiles) for non-normal variables, and proportions for categorical variables. Student’s t-test was used to compare continuous variables, while the χ2 test was used for comparing binary and categorical variables between the cohorts. Mortality rates were calculated per 1000 person-years. Multivariable Cox proportional hazard models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between DM duration, exercise intensity, and ESRD incidence. Model 1 was unadjusted. Model 2 was adjusted for age and sex. Model 3 included model 2 adjustments plus income, smoking, drinking, regular exercise, hypertension, dyslipidemia, FBG, use of > 3 oral hyperglycemic agents, and eGFR. Furthermore, model 4 included model 3 adjustments plus proteinuria. Variance inflation factors (VIF) were calculated to assess multicollinearity in the multivariate analysis. All VIF values were below the threshold of 10, indicating no significant multicollinearity among the variables (Supplementary Table 1). No formal multiple-testing correction (e.g., Bonferroni) was applied due to the exploratory nature of the analyses. All statistical tests were two-tailed, with P < 0.05 considered statistically significant. All data analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC, United States).
Table 1 presents the baseline characteristics of participants with and without ESRD. Patients with ESRD were older (63.43 vs 59.56 years), had longer diabetes duration (≥ 10 years in 63.65% vs 11.12%), lower body mass index (BMI) but higher waist circumference, and higher systolic and diastolic blood pressure, FBG, and worse lipid profiles. Table 2 summarizes the demographic characteristics based on DM duration. Those with DM > 10 years had lower eGFR compared to those other groups. Table 3 presents the baseline characteristics by exercise intensity. Participants with MET ≥ 1500/week had the lowest BMI, triglycerides, low-density lipoprotein, and a longer DM duration compared to those of other groups.
| Variable | None ESRD (n = 2471672) | ESRD (n = 23359) | P value | Variable | None ESRD (n = 2471672) | ESRD (n = 23359) | P value |
| Age | 59.56 ± 12 | 63.43 ± 11.4 | 0.0001 | DM duration | 0.0001 | ||
| Age group | 0.0001 | New onset | 720322 (29.14) | 1909 (8.17) | |||
| < 40 | 120544 (4.88) | 542 (2.32) | < 5 years | 674433 (27.29) | 2604 (11.15) | ||
| 40-64 | 1502637 (60.79) | 11435 (48.95) | 5-9 years | 485967 (19.66) | 3978 (17.03) | ||
| ≥ 65 | 848491 (34.33) | 11382 (48.73) | ≥ 10 years | 590950 (23.91) | 14868 (63.65) | ||
| Sex male (%) | 1486398 (60.14%) | 15637 (66.94%) | 0.0001 | BMI (kg/m2) | 25.34 ± 3.54 | 25 ± 3.67 | 0.0001 |
| Low income1 | 530489 (21.46) | 5878 (25.16) | 0.0001 | BMI_5 level | 0.0001 | ||
| Smoking | 0.0001 | < 18.5 | 33269 (1.35) | 477 (2.04) | |||
| Never | 1360211 (55.03) | 12376 (52.98) | 18.5-23 | 575343 (23.28) | 6379 (27.31) | ||
| Ex- | 553739 (22.4) | 5820 (24.92) | 23-25 | 597529 (24.18) | 5557 (23.79) | ||
| Current | 557722 (22.56) | 5163 (22.1) | 25-30 | 1032471 (41.77) | 8857 (37.92) | ||
| Drinking | 0.0001 | ≥ 30 | 233060 (9.43) | 2089 (8.94) | |||
| None | 1423230 (57.58) | 16532 (70.77) | WC cm | 86.15 ± 8.98 | 87.08 ± 9.54 | 0.0001 | |
| Moderate | 822332 (33.27) | 5590 (23.93) | WC level (M/F) | 0.0001 | |||
| Heavy2 | 226110 (9.15) | 1237 (5.3) | < 80/75 | 361141 (14.61) | 3633 (15.55) | ||
| Regular exercise | 537065 (21.73) | 4437 (18.99) | 0.0001 | < 85/80 | 489684 (19.81) | 4167 (17.84) | |
| Hypertension | 1424600 (57.64) | 20241 (86.65) | 0.0001 | < 90/85 | 604127 (24.44) | 5045 (21.6) | |
| Dyslipidemia | 1411286 (57.1) | 16770 (71.79) | 0.0001 | < 95/90 | 485423 (19.64) | 4660 (19.95) | |
| CKD3 | 223756 (9.05) | 16054 (68.73) | 0.0001 | < 100/95 | 291335 (11.79) | 2911 (12.46) | |
| Proteinuria | 156756 (6.34) | 13927 (59.62) | 0.0001 | ≥ 100/95 | 239962 (9.71) | 2943 (12.6) | |
| SBP (mmHg) | 128.4 ± 14.99 | 134.66 ± 18.01 | 0.0001 | FBS mg/dL | 144.46 ± 45.34 | 156.02 ± 74.42 | 0.0001 |
| DBP (mmHg) | 78.08 ± 9.94 | 78.17 ± 11.13 | 0.0001 | TC mg/dL | 185.3 ± 43.63 | 181.57 ± 51.26 | 0.0001 |
| DM medication | 1751350 (70.86) | 21450 (91.83) | 0.0001 | HDL-C mg/dL | 51 ± 14.71 | 46.97 ± 14.97 | 0.0001 |
| OHA ≥ 3 | 578752 (23.42) | 8376 (35.86) | 0.0001 | LDL-C mg/dL | 103.17 ± 38.36 | 99.19 ± 42.32 | 0.0001 |
| Metformin | 1598169 (64.66) | 14570 (62.37) | 0.0001 | eGFR | 89.81 ± 52.98 | 52.22 ± 40.69 | 0.0001 |
| SGLT-2 inhibitor | 65835 (2.66) | 567 (2.43) | 0.0256 | TG (mg/dL)4 | 137.57 (137.47-137.66) | 155.88 (154.77-157.01) | 0.0001 |
| Variables | New onset (n = 722231) | < 5 years (n = 677037) | 5-9 years (n = 489945) | ≥ 10 years (n = 605818) | P value |
| Age | 53.72 ± 12.36 | 58.75 ± 11.32 | 62.09 ± 10.54 | 65.53 ± 9.77 | < 0.0001 |
| Age group | < 0.0001 | ||||
| < 40 | 85092 (11.78) | 26721 (3.95) | 6488 (1.32) | 2785 (0.46) | |
| 40-64 | 504540 (69.86) | 446861 (66) | 286658 (58.51) | 276013 (45.56) | |
| ≥ 65 | 132599 (18.36) | 203455 (30.05) | 196799 (40.17) | 327020 (53.98) | |
| Sex male (%) | 501523 (69.44) | 388124 (57.33) | 277851 (56.71) | 334537 (55.22) | < 0.0001 |
| Low income1 | 149558 (20.71) | 148265 (21.9) | 111167 (22.69) | 127377 (21.03) | < 0.0001 |
| Smoking | < 0.0001 | ||||
| Never | 337007 (46.66) | 377005 (55.68) | 283606 (57.89) | 374969 (61.89) | |
| Ex- | 163967 (22.7) | 150191 (22.18) | 110089 (22.47) | 135312 (22.34) | |
| Current | 221257 (30.64) | 149841 (22.13) | 96250 (19.65) | 95537 (15.77) | |
| Drinking | < 0.0001 | ||||
| None | 306986 (42.51) | 404179 (59.7) | 309644 (63.2) | 418953 (69.15) | |
| Moderate | 318767 (44.14) | 214381 (31.66) | 143223 (29.23) | 151551 (25.02) | |
| Heavy2 | 96478 (13.36) | 58477 (8.64) | 37078 (7.57) | 35314 (5.83) | |
| Regular exercise | 147260 (20.39) | 141581 (20.91) | 109065 (22.26) | 143596 (23.7) | < 0.0001 |
| Hypertension | 318886 (44.15) | 396558 (58.57) | 316466 (64.59) | 412931 (68.16) | < 0.0001 |
| Dyslipidemia | 258643 (35.81) | 447804 (66.14) | 322958 (65.92) | 398651 (65.8) | < 0.0001 |
| CKD3 | 33398 (4.62) | 45686 (6.75) | 51080 (10.43) | 109646 (18.1) | < 0.0001 |
| Proteinuria | 38161 (5.28) | 37292 (5.51) | 31993 (6.53) | 63237 (10.44) | < 0.0001 |
| SBP (mmHg) | 129.44 ± 15.27 | 127.7 ± 14.66 | 127.98 ± 14.77 | 128.5 ± 15.3 | < 0.0001 |
| DBP (mmHg) | 80.31 ± 10.31 | 78.36 ± 9.68 | 77.3 ± 9.48 | 75.73 ± 9.57 | < 0.0001 |
| DM medication | None | 677037 (100) | 489945 (100) | 605818 (100) | < 0.0001 |
| OHA ≥ 3 | None | 122456 (18.09) | 166263 (33.94) | 296615 (48.96) | < 0.0001 |
| Metformin | None | 621307 (91.77) | 443228 (90.46) | 540323 (89.19) | < 0.0001 |
| SGLT-2 inhibitor | None | 26509 (3.92) | 16904 (3.45) | 22732 (3.75) | < 0.0001 |
| BMI (kg/m2) | 25.59 ± 3.71 | 25.77 ± 3.61 | 25.29 ± 3.4 | 24.57 ± 3.23 | < 0.0001 |
| BMI-level | < 0.0001 | ||||
| < 18.5 | 10909 (1.51) | 6977 (1.03) | 5778 (1.18) | 10082 (1.66) | |
| 18.5-23 | 154876 (21.44) | 132903 (19.63) | 113133 (23.09) | 180810 (29.85) | |
| 23-25 | 163960 (22.7) | 156199 (23.07) | 122022 (24.91) | 160905 (26.56) | |
| 25-30 | 311871 (43.18) | 302572 (44.69) | 206796 (42.21) | 220089 (36.33) | |
| ≥ 30 | 80615 (11.16) | 78386 (11.58) | 42216 (8.62) | 33932 (5.6) | |
| WC (cm) | 22.87 ± 3.19 | 24.27 ± 3.24 | 25.18 ± 3.34 | 25.71 ± 4.75 | < 0.0001 |
| WC level (M/F) | < 0.0001 | ||||
| < 80/75 | 119073 (16.49) | 84582 (12.49) | 65915 (13.45) | 95204 (15.71) | |
| < 85/80 | 147428 (20.41) | 125227 (18.5) | 94982 (19.39) | 126214 (20.83) | |
| < 90/85 | 173565 (24.03) | 164474 (24.29) | 120917 (24.68) | 150216 (24.8) | |
| < 95/90 | 136216 (18.86) | 138283 (20.42) | 99184 (20.24) | 116400 (19.21) | |
| < 100/95 | 79117 (10.95) | 87794 (12.97) | 60251 (12.3) | 67084 (11.07) | |
| ≥ 100/95 | 66832 (9.25) | 76677 (11.33) | 48696 (9.94) | 50700 (8.37) | |
| FBS (mg/dL) | 151.55 ± 39.14 | 137.74 ± 46.25 | 140.25 ± 45.05 | 147.38 ± 51.13 | < 0.0001 |
| TC (mg/dL) | 207.12 ± 41.98 | 184.24 ± 43.26 | 174.27 ± 39.29 | 169.26 ± 38.74 | < 0.0001 |
| HDL-C (mg/dL) | 52.62 ± 16.28 | 50.62 ± 14.18 | 50.34 ± 13.73 | 49.88 ± 13.92 | < 0.0001 |
| LDL-C (mg/dL) | 119.45 ± 38.36 | 102.28 ± 38.38 | 94.54 ± 35.01 | 91.57 ± 34.23 | < 0.0001 |
| eGFR | 93.6 ± 59.94 | 91.59 ± 51.48 | 88.39 ± 49.71 | 83 ± 47.49 | < 0.0001 |
| TG (mg/dL)4 | 156.25 (156.03-156.46) | 139.11 (138.93-139.29) | 130.23 (130.03-130.42) | 122.61 (122.44-122.77) | < 0.0001 |
| Variables/MET (min/week) | 0 (n = 572290) | 1-499 (n = 705215) | 500-999 (n = 731798) | 1000-1499 (n = 310973) | ≥ 1500 (n = 174755) | P value |
| Age | 61.55 ± 12.18 | 58.61 ± 12.17 | 59.06 ± 12.02 | 58.74 ± 11.3 | 60.93 ± 10.86 | < 0.0001 |
| Age group | < 0.0001 | |||||
| < 40 | 20308 (3.55) | 39493 (5.6) | 40420 (5.52) | 15084 (4.85) | 5781 (3.31) | |
| 40-64 | 324181 (56.65) | 443248 (62.85) | 446531 (61.02) | 198691 (63.89) | 101421 (58.04) | |
| ≥ 65 | 227801 (39.81) | 222474 (31.55) | 244847 (33.46) | 97198 (31.26) | 67553 (38.66) | |
| Sex male (%) | 302373 (52.84) | 411840 (58.4) | 459889 (62.84) | 207056 (66.58) | 120877 (69.17) | < 0.0001 |
| Low income1 | 135748 (23.72) | 149801 (21.24) | 152920 (20.9) | 62674 (20.15) | 35224 (20.16) | < 0.0001 |
| Smoking | < 0.0001 | |||||
| Never | 354678 (61.98) | 388019 (55.02) | 382636 (52.29) | 156832 (50.43) | 90422 (51.74) | |
| Ex- | 87965 (15.37) | 150711 (21.37) | 180327 (24.64) | 88686 (28.52) | 51870 (29.68) | |
| Current | 129647 (22.65) | 166485 (23.61) | 168835 (23.07) | 65455 (21.05) | 32463 (18.58) | |
| Drinking | < 0.0001 | |||||
| None | 381736 (66.7) | 402174 (57.03) | 398383 (54.44) | 161354 (51.89) | 96115 (55) | |
| Moderate | 136879 (23.92) | 240927 (34.16) | 266239 (36.38) | 121719 (39.14) | 62158 (35.57) | |
| Heavy2 | 53675 (9.38) | 62114 (8.81) | 67176 (9.18) | 27900 (8.97) | 16482 (9.43) | |
| Regular exercise | 0 (0) | 9754 (1.38) | 89956 (12.29) | 267037 (85.87) | 174755 (100) | < 0.0001 |
| Hypertension | 351028 (61.34) | 399757 (56.69) | 417175 (57.01) | 174445 (56.1) | 102436 (58.62) | < 0.0001 |
| Dyslipidemia | 331813 (57.98) | 406736 (57.68) | 415042 (56.72) | 175765 (56.52) | 98700 (56.48) | < 0.0001 |
| CKD3 | 68870 (12.03) | 65189 (9.24) | 64770 (8.85) | 24924 (8.01) | 16057 (9.19) | < 0.0001 |
| Proteinuria | 42377 (7.4) | 49028 (6.95) | 48986 (6.69) | 19388 (6.23) | 10904 (6.24) | < 0.0001 |
| SBP (mmHg) | 128.75 ± 15.51 | 128.22 ± 14.97 | 128.48 ± 14.9 | 128.27 ± 14.65 | 128.65 ± 14.84 | < 0.0001 |
| DBP (mmHg) | 77.96 ± 10.04 | 78.26 ± 9.98 | 78.14 ± 9.95 | 78 ± 9.79 | 77.59 ± 9.79 | < 0.0001 |
| DM medication | 416149 (72.72) | 492161 (69.79) | 515255 (70.41) | 219636 (70.63) | 129599 (74.16) | < 0.0001 |
| OHA ≥ 3 | 141666 (24.75) | 166098 (23.55) | 168821 (23.07) | 69974 (22.5) | 40569 (23.21) | < 0.0001 |
| Metformin | 376811 (65.84) | 450165 (63.83) | 469403 (64.14) | 199852 (64.27) | 116508 (66.67) | < 0.0001 |
| SGLT-2 inhibitor | 15152 (2.65) | 19962 (2.83) | 19079 (2.61) | 8073 (2.6) | 4136 (2.37) | < 0.0001 |
| BMI (kg/m2) | 25.38 ± 3.69 | 25.48 ± 3.6 | 25.3 ± 3.5 | 25.2 ± 3.37 | 24.95 ± 3.22 | < 0.0001 |
| WC (cm) | 86.45 ± 9.27 | 86.44 ± 9.1 | 86.1 ± 8.88 | 85.66 ± 8.67 | 85.27 ± 8.47 | < 0.0001 |
| DM duration | < 0.0001 | |||||
| New onset | 156141 (27.28) | 213054 (30.21) | 216543 (29.59) | 91337 (29.37) | 45156 (25.84) | |
| < 5 years | 158290 (27.66) | 195372 (27.7) | 196962 (26.91) | 82296 (26.46) | 44117 (25.25) | |
| 5-9 years | 114441 (20) | 136083 (19.3) | 142641 (19.49) | 61138 (19.66) | 35642 (20.4) | |
| ≥ 10 years | 143418 (25.06) | 160706 (22.79) | 175652 (24) | 76202 (24.5) | 49840 (28.52) | |
| FBS (mg/dL) | 145.15 ± 48.36 | 146.16 ± 46.64 | 144.32 ± 44.93 | 142.82 ± 42.52 | 140.41 ± 41.09 | < 0.0001 |
| TC (mg/dL) | 185.61 ± 44.33 | 186.75 ± 44.17 | 185.21 ± 43.47 | 183.88 ± 42.77 | 180.93 ± 41.95 | < 0.0001 |
| HDL-C (mg/dL) | 50.5 ± 14.73 | 50.51 ± 14.5 | 51.18 ± 14.7 | 51.68 ± 14.82 | 52.15 ± 15.26 | < 0.0001 |
| LDL-C (mg/dL) | 103.35 ± 39.06 | 104.16 ± 38.79 | 102.98 ± 38.17 | 102.25 ± 37.6 | 100.49 ± 36.83 | < 0.0001 |
| eGFR | 87.82 ± 49.04 | 89.88 ± 54.14 | 90.05 ± 53.23 | 90.35 ± 55.92 | 89.03 ± 54.37 | < 0.0001 |
| TG (mg/dL)4 | 140.72 (140.52-140.93) | 142.5 (142.31-142.69) | 137.04 (136.86-137.22) | 131.75 (131.49-132.02) | 123.62 (123.29-123.95) | < 0.0001 |
Tables 4 and 5 show the event rates and adjusted HRs for renal outcomes according to DM duration and exercise intensity, respectively. ESRD risk increased with longer diabetes duration. Patients with diabetes > 10 years had a 2.6-fold higher risk than those with new-onset diabetes after adjusting for confounding factors (HR: 2.624, 95%CI: 2.486-2.770; Table 4). Conversely, greater physical activity was associated with lower ESRD risk in patients with diabetes. Individuals engaging in physical activity ≥ 1500 MET-min/week had a reduced risk of developing ESRD (HR: 0.837, 95%CI: 0.791-0.886), even after adjusting for confounders (Table 5). When evaluating ESRD risk according to physical activity and diabetes duration, no consistent risk reduction was observed in new-onset DM cases. However, individuals with DM > 5 years showed a consistent reduction in ESRD risk with ≥ 1500 MET-min/week of physical activity (Table 6). Supplementary Table 2 presents the HRs and CIs for all covariates included in the final adjusted model, highlighting the influence of age, BMI, income, proteinuria, and baseline eGFR on ESRD risk.
| DM duration | Total (n) | ESRD (n) | Duration PY | IR 1000 PY | Adjusted HR (95% confidence interval) | |||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| New onset | 722231 | 1909 | 4155941 | 0.46 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| < 5 years | 677037 | 2604 | 3946270 | 0.66 | 1.430 (1.3481.517) | 1.442 (1.3591.530) | 1.179 (1.1101.252) | 1.162 (1.0941.234) |
| 5-9 years | 489945 | 3978 | 2829657 | 1.41 | 3.050 (2.8883.221) | 2.989 (2.8273.159) | 1.941 (1.8322.056) | 1.790 (1.6901.896) |
| ≥ 10 years | 605818 | 14868 | 3389455 | 4.39 | 9.558 (9.11410.025) | 9.135 (8.6929.600) | 3.334 (3.1583.520) | 2.624 (2.4862.770) |
| METs-min/week | Total (n) | ESRD (n) | Duration, PY | IR, 1000 PY | Adjusted HR (95% confidence interval) | |||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| 0 | 572290 | 6431 | 3234371 | 1.99 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 1-499 | 705215 | 6497 | 4056207 | 1.60 | 0.805 (0.7780.833) | 0.856 (0.8270.886) | 0.938 (0.9060.971) | 0.946 (0.9130.979) |
| 500-999 | 731798 | 6501 | 4215771 | 1.54 | 0.775 (0.749 0.802) | 0.791 (0.7640.819) | 0.917 (0.886 0.950) | 0.919 (0.8880.952) |
| 1000-1499 | 310973 | 2422 | 1804581 | 1.34 | 0.674 (0.643 0.706) | 0.683 (0.6520.716) | 0.831 (0.7930.871) | 0.845 (0.806, 0.886) |
| ≥ 1500 | 174755 | 1508 | 1010392 | 1.49 | 0.749 (0.7090.793) | 0.697 (0.6580.737) | 0.806 (0.7620.853) | 0.837 (0.7910.886) |
| DM duration | METs-min/week | Total (n) | ESRD (n) | Duration, PY | IR, 1000 PY | Adjusted HR (95% Confidence interval) | |||
| Composite | Subgroup | ||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | ||||||
| New onset | 0 | 156141 | 473 | 888675 | 0.53 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 1-499 | 213054 | 613 | 1227517 | 0.50 | 0.938 (0.8321.058) | 1.031 (0.9141.162) | 0.938 (0.8321.058) | 1.031 (0.9141.162) | |
| 500-999 | 216543 | 509 | 1249361 | 0.41 | 0.765 (0.6750.867) | 0.864 (0.7620.980) | 0.765 (0.6750.867) | 0.864 (0.7620.980) | |
| 1000-1499 | 91337 | 195 | 529164 | 0.37 | 0.692 (0.5850.817) | 0.753 (0.6370.890) | 0.692 (0.5850.817) | 0.753 (0.6370.890) | |
| ≥ 1500 | 45156 | 119 | 261225 | 0.46 | 0.855 (0.6991.045) | 0.890 (0.7281.088) | 0.855 (0.6991.045) | 0.890 (0.7271.088) | |
| < 5 years | 0 | 158290 | 731 | 914709 | 0.80 | 1.495 (1.3321.678) | 1.203 (1.0711.351) | 1 (Reference) | 1 (Reference) |
| 1-499 | 195372 | 720 | 1139960 | 0.63 | 1.181 (1.0521.327) | 1.063 (0.9461.195) | 0.790 (0.7130.876) | 0.884 (0.7980.980) | |
| 500-999 | 196962 | 750 | 1149962 | 0.65 | 1.220 (1.0871.368) | 1.082 (0.9641.214) | 0.816 (0.7370.903) | 0.899 (0.8120.996) | |
| 1000-1499 | 82296 | 258 | 483431 | 0.53 | 0.997 (0.8571.161) | 0.949 (0.8151.104) | 0.667 (0.5790.769) | 0.789 (0.6840.909) | |
| ≥ 1500 | 44117 | 145 | 258209 | 0.56 | 1.050 (0.8711.264) | 0.967 (0.8031.165) | 0.702 (0.5880.839) | 0.804 (0.6730.961) | |
| 5-9 years | 0 | 114441 | 1090 | 651145 | 1.67 | 3.137 (2.8163.494) | 1.771 (1.5881.976) | 1 (Reference) | 1 (Reference) |
| 1-499 | 136083 | 1110 | 787142 | 1.41 | 2.640 (2.3712.940) | 1.690 (1.5161.884) | 0.842 (0.7740.915) | 0.954 (0.8781.038) | |
| 500-999 | 142641 | 1139 | 826498 | 1.38 | 2.579 (2.3172.871) | 1.697 (1.5231.891) | 0.822 (0.7570.894) | 0.958 (0.8821.041) | |
| 1000-1499 | 61138 | 402 | 357285 | 1.13 | 2.104 (1.8422.403) | 1.508 (1.3191.724) | 0.671 (0.5980.752) | 0.852 (0.7590.955) | |
| ≥ 1500 | 35642 | 237 | 207588 | 1.14 | 2.135 (1.8272.496) | 1.461 (1.2491.709) | 0.681 (0.5920.783) | 0.825 (0.7170.949) | |
| ≥ 10 years | 0 | 143418 | 4137 | 779842 | 5.30 | 9.998 (9.09110.996) | 2.635 (2.3882.908) | 1 (Reference) | 1 (Reference) |
| 1-499 | 160706 | 4054 | 901589 | 4.50 | 8.454 (7.6869.298) | 2.484 (2.2522.741) | 0.846 (0.8100.883) | 0.943 (0.9030.985) | |
| 500-999 | 175652 | 4103 | 989951 | 4.14 | 7.788 (7.0818.565) | 2.423 (2.1972.672) | 0.779 (0.7460.813) | 0.919 (0.8800.960) | |
| 1000-1499 | 76202 | 1567 | 434702 | 3.60 | 6.764 (6.1037.497) | 2.284 (2.0562.538) | 0.677 (0.6380.717) | 0.867 (0.8180.919) | |
| ≥ 1500 | 49840 | 1007 | 283371 | 3.55 | 6.671 (5.9807.441) | 2.213 (1.9792.475) | 0.667 (0.6230.715) | 0.840 (0.7840.900) | |
Subgroup analysis by age: In new-onset DM, physical activity had no impact on the risk of ESRD regardless of age group (> 65 or < 65 years). In those with DM > 10 years, physical activity consistently reduced ESRD risk across both age groups. For DM < 10 years, exercise intensity influenced ESRD risk only in individuals aged < 65 years, with no effect in those aged > 65 years (Figure 2A).
Subgroup analysis by sex: Among participants with diabetes > 10 years, higher exercise intensity was associated with lower ESRD risk in both men and women (Figure 2B).
Subgroup analysis by BMI: In individuals with diabetes > 10 years, increased physical activity was linked to reduced ESRD risk regardless of BMI. Specifically, in those with BMI ≥ 25 kg/m², all levels of physical activity were statistically significant. However, in the group with BMI < 25 kg/m², only those with ≥ 1500 MET-min/week of physical activity showed a significant effect (Figure 2C).
Subgroup analysis for hypertension: The impact of exercise on ESRD based on diabetes duration was more pronounced in individuals without hypertension. Among those with diabetes > 10 years, engaging in MET ≥ 1500/week of physical activity was significantly associated with reduced ESRD risk (Figure 2D).
Subgroup analysis for smoking and alcohol drinking: In participants with diabetes > 5 years, ≥ 1000 MET/week of physical activity significantly lowered ESRD risk, except in current smokers, where no risk reduction was observed regardless of activity level or diabetes duration (Figure 2E). A similar pattern was observed with alcohol intake: Physical activity reduced the risk of ESRD only in never or mild drinkers with diabetes lasting > 10 years, with no significant benefit in heavy drinkers (Figure 2F).
Subgroup analysis for CKD and proteinuria: Physical activity significantly reduced the risk of ESRD in all diabetes duration groups, except for new-onset DM, particularly in individuals without baseline CKD. Among individuals with CKD, only those with diabetes > 10 years and ≥ 1000 MET/week of physical activity showed reduced ESRD risk (Figure 2G). Similarly, in the subgroup analysis based on proteinuria, physical activity was associated with lower ESRD risk only in those without proteinuria (Figure 2H).
This nationwide population-based study aimed to investigate the relationship between exercise intensity, diabetes duration, and ESRD incidence among patients with diabetes. Our findings underscore the protective role of physical activity, particularly in those with long-standing diabetes, and highlight how diabetes duration, exercise intensity, and subgroup characteristics affect ESRD risk.
First, our study confirmed that longer diabetes duration significantly increases ESRD risk, consistent with previous research linking prolonged hyperglycemia to progressive kidney damage[12,13]. In our cohort, patients with diabetes > 10 years had a 2.6-fold higher ESRD risk (HR: 2.624) compared to those with newly diagnosed diabetes. This finding emphasizes the importance of early and sustained diabetes management to prevent long-term complications.
Another major finding is the association between physical activity and reduced ESRD risk. Participants who engaged in high-intensity exercise (≥ 1500 MET-min/week) had significantly lower ESRD risk (HR: 0.837) even after adjusting for confounders. This protective effect was observed across all diabetes duration groups but was stronger in those with longer disease duration. These findings support the “exercise as medicine” concept, suggesting that regular physical activity plays a crucial role in preventing diabetic kidney complications. A recent systematic review and meta-analysis also linked higher physical activity levels to reduced risk of CKD and ESRD, supporting our findings[14].
Subgroup analyses clarified how exercise impacts ESRD risk based on age, sex, BMI, hypertension, smoking, alcohol consumption, CKD, and proteinuria status. For instance, while physical activity did not reduce ESRD risk in new-onset diabetes regardless of age, those with diabetes lasting > 10 years consistently benefited across all age groups. These findings suggest that exercise is more effective in patients with a longer duration of diabetes, potentially due to its cumulative effects on metabolic and vascular health. Additionally, incorporating regular physical activity into multidisciplinary care may help slow CKD progression and prevent ESRD in this high-risk population[15]. These findings underscore the importance of sustained exercise and integrated care in managing diabetes-related renal complications.
Moreover, the results revealed sex differences. Women showed greater ESRD risk reduction from physical activity, likely attributed to sex-specific differences in body composition, hormones, and greater adherence to exercise[16]. However, increased physical activity lowered ESRD risk in both men and women with diabetes lasting > 10 years, highlighting the universal benefits of exercise across sexes.
Our analysis revealed that physical activity reduced ESRD risk in all patients with BMI ≥ 25, regardless of exercise intensity. In contrast, only those with BMI < 25 who exercised ≥ 500 MET-min/week had significant risk reduction. Hence, overweight and obese individuals may benefit more from physical activity due to higher baseline metabolic stress and inflammation. Recent studies support these findings, linking higher BMI to increased CKD and ESRD risk, which could be mitigated by physical activity[17]. Additionally, comprehensive reviews highlight the importance of physical activity and nutrition in managing CKD by reducing metabolic stress and inflammation in patients with higher BMI[18].
Hypertension status significantly influenced the relationship between exercise and ESRD risk, with stronger protective effects observed in individuals without hypertension. Given the major role of hypertension in ESRD progression, it may diminish the direct benefits of exercise in reducing ESRD risk. However, recent studies suggest that physical activity may be beneficial in managing resistant hypertension[19], highlighting the indirect role of exercise in reducing ESRD risk through improved blood pressure control.
Smoking and alcohol consumption also influenced the impact of exercise on ESRD risk. Notably, physical activity did not reduce ESRD risk in current smokers, regardless of diabetes duration. Smoking worsens kidney damage by increasing glomerular hypertension, inducing inflammation, and promoting oxidative stress, which accelerates kidney function decline and increases proteinuria[20].
This contrast highlights the detrimental effects of smoking on renal health, suggesting that smoking cessation is essential for patients with diabetes to fully benefit from exercise. Similarly, heavy drinkers showed no significant ESRD risk reduction with exercise, unlike never or mild drinkers, indicating that alcohol moderation is crucial in diabetes management. The renoprotective effects of physical activity likely stem from multiple mechanisms, such as reducing systemic inflammation and oxidative stress, improving insulin sensitivity and glucose metabolism[21,22], enhancing endothelial function and renal perfusion[5], and aiding blood pressure control. These physiological adaptations are crucial in diabetes, where metabolic dysregulation accelerates kidney damage, helping explain the inverse association between exercise intensity and ESRD risk observed in our study[14].
Regular physical activity reduces systemic inflammation and oxidative stress[23], which are key drivers of diabetic nephropathy. These benefits are clearer in individuals without vascular or metabolic stressors such as hypertension or smoking. Smoking is linked to sustained sympathetic activation, atherosclerosis, and oxidative damage[20], which may counteract the benefits of exercise. In contrast, non-smokers and non-hypertensive individuals may derive greater renoprotective benefits due to a more favorable baseline risk. Our findings align with prior studies suggesting that physical activity may help prevent kidney function decline[24,25]. Subgroup analysis suggests that the protective effects of physical activity on ESRD risk vary by baseline renal health. Specifically, in patients with strong risk factors such as CKD or proteinuria, the impact of exercise is less pronounced, while those without these factors gain greater protection, potentially delaying or preventing severe kidney disease. These findings highlight the importance of promoting physical activity, particularly in patients without established renal risk factors, to maximize its preventive benefits.
First, diabetes diagnosis relied on FBG levels ≥ 126 mg/dL or ICD-10 codes with anti-diabetic medication claims. Although common in epidemiology[26], using a single FBG measure may include borderline or pre-diabetic cases, reducing specificity.
Second, CKD was defined by a single eGFR measurement < 60 mL/min/1.73 m². Without repeated measures, we could not distinguish CKD from acute kidney injury. As a result, some patients with transient kidney dysfunction may have been misclassified as having CKD, potentially introducing misclassification bias.
Third, physical activity was self-reported via NHIS questionnaires, making it susceptible to recall bias and possible overestimation. Furthermore, the highest exercise category (≥ 1500 MET-min/week), corresponding to approximately 5 hours of vigorous activity weekly, may be unrealistic for elderly individuals or those with multiple comorbidities. In addition, healthier individuals might be more likely to engage in high-intensity physical activity, introducing selection bias that could partly explain the stronger protective effects observed in some subgroups.
Fourth, although subgroup analyses provided exploratory insights, no formal multiple-testing correction (e.g., Bonferroni) was applied. This increases the risk of false-positives; thus, these results should be interpreted cautiously.
Fifth, our dataset lacked important clinical variables such as HbA1c and quantitative proteinuria, limiting detailed assessment of glycemic control and kidney disease severity.
Sixth, despite the extended follow-up period, reverse causality cannot be ruled out, where individuals with undiagnosed or early-stage kidney dysfunction may have reduced physical activity levels due to subclinical symptoms. This likely influenced the observed associations between exercise and ESRD risk.
Lastly, as a retrospective observational study, while we identified relationships between exercise intensity and ESRD risk, causal inferences cannot be made. The observed associations indicate correlations that warrant further investigation.
Our study suggests that physical activity may reduce ESRD risk among patients with diabetes. Although exercise benefits were observed across various subgroups, their extent varies depending on factors such as diabetes duration, BMI, hypertension, smoking, alcohol consumption, CKD, and proteinuria status. These findings highlight support for incorporating tailored physical activity into diabetes management to protect renal health. Future research should investigate the mechanisms underlying the renal benefits of exercise and identify effective strategies to enhance exercise adherence in patients with diabetes.
| 1. | Wilkinson TJ, Shur NF, Smith AC. "Exercise as medicine" in chronic kidney disease. Scand J Med Sci Sports. 2016;26:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7148] [Cited by in RCA: 6609] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 3. | Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91:1128S-1132S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Aoike DT, Baria F, Kamimura MA, Ammirati A, de Mello MT, Cuppari L. Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol. 2015;47:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, Isbel NM. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Estimating the impact of body mass index on bladder cancer risk: Stratification by smoking status. Sci Rep. 2018;8:947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 731] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 9. | Kim YH, Kim SM, Han KD, Son JW, Lee SS, Oh SW, Lee WY, Yoo SJ; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Change in Weight and Body Mass Index Associated With All-Cause Mortality in Korea: A Nationwide Longitudinal Study. J Clin Endocrinol Metab. 2017;102:4041-4050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11824] [Article Influence: 454.8] [Reference Citation Analysis (0)] |
| 11. | Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, Chae IH. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40:3547-3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 12. | Finne P, Groop PH, Arffman M, Kervinen M, Helve J, Grönhagen-Riska C, Sund R. Cumulative Risk of End-Stage Renal Disease Among Patients With Type 2 Diabetes: A Nationwide Inception Cohort Study. Diabetes Care. 2019;42:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wu T, Ding L, Andoh V, Zhang J, Chen L. The Mechanism of Hyperglycemia-Induced Renal Cell Injury in Diabetic Nephropathy Disease: An Update. Life (Basel). 2023;13:539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 14. | Seidu S, Abdool M, Almaqhawi A, Wilkinson TJ, Kunutsor SK, Khunti K, Yates T. Physical activity and risk of chronic kidney disease: systematic review and meta-analysis of 12 cohort studies involving 1,281,727 participants. Eur J Epidemiol. 2023;38:267-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Hayashi A, Mizuno K, Shinkawa K, Sakoda K, Yoshida S, Takeuchi M, Yanagita M, Kawakami K. Effect of multidisciplinary care on diabetic kidney disease: a retrospective cohort study. BMC Nephrol. 2024;25:114. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Xu P, Chen JS, Chang YL, Wang X, Jiang X, Griffiths MD, Pakpour AH, Lin CY. Gender Differences in the Associations Between Physical Activity, Smartphone Use, and Weight Stigma. Front Public Health. 2022;10:862829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Nawaz S, Chinnadurai R, Al-Chalabi S, Evans P, Kalra PA, Syed AA, Sinha S. Obesity and chronic kidney disease: A current review. Obes Sci Pract. 2023;9:61-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 18. | Preda A, Carbone F, Tirandi A, Montecucco F, Liberale L. Obesity phenotypes and cardiovascular risk: From pathophysiology to clinical management. Rev Endocr Metab Disord. 2023;24:901-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 19. | Zhang W, Xu R, Cai Z, Zheng X, Zheng M, Ni C. Association between physical activity and resistant hypertension in treated hypertension patients: analysis of the national health and nutrition examination survey. BMC Cardiovasc Disord. 2023;23:289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Gündoğdu Y, Anaforoğlu İ. Effects of Smoking on Diabetic Nephropathy. Front Clin Diabetes Healthc. 2022;3:826383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, Smith AC. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol. 2014;25:2121-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147-e167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 957] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 23. | Watson EL, Baker LA, Wilkinson TJ, Gould DW, Xenophontos S, Graham-Brown M, Major RW, Ashford RU, Viana JL, Smith AC. Inflammation and physical dysfunction: responses to moderate intensity exercise in chronic kidney disease. Nephrol Dial Transplant. 2022;37:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Stump CS. Physical Activity in the Prevention of Chronic Kidney Disease. Cardiorenal Med. 2011;1:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169:2116-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, Jung CH, Lee KU, Ko KS; TaskForce Team for the Diabetes Fact Sheet of the Korean Diabetes Association. Past and Current Status of Adult Type 2 Diabetes Mellitus Management in Korea: A National Health Insurance Service Database Analysis. Diabetes Metab J. 2018;42:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |