Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.106890
Revised: May 7, 2025
Accepted: June 6, 2025
Published online: July 15, 2025
Processing time: 127 Days and 11.8 Hours
The incidence of type 2 diabetes mellitus (T2DM) in children and adolescents is increasing, yet there is limited information on the available pharmacological interventions to combat T2DM and prevent associated comorbidities.
To assess the effectiveness of current pharmacological treatments in managing T2DM in children and adolescents. The protocol of the study was registered in PROSPERO (CRD42022382165).
Searches were performed in PubMed, EMBASE, Scopus, and ClinicalTrials.gov for publications between 1990 to September 2024 without language restrictions. Randomized control trials (RCTs) of pharmacotherapy in children and adolescents with T2DM (aged < 19 years) were included. The primary outcome was a change in glycated hemoglobin (HbA1c) from baseline to follow-up. Secondary outcomes were changes in body weight, body mass index (BMI), total cholesterol, triglycerides, high density lipoprotein, and low-density lipoprotein from baseline, and incidence of adverse events during study periods. Screening, full-text review, data extraction, and assessments of risk of bias were done by two reviewers. Conflicts on each step were resolved by a third reviewer. Data analysis was performed using Review Manager Version 6.5 (RevMan 6.5) and ‘R’ software via RStudio, ‘meta’ and ‘netmeta’.
A total of 12 studies having low to moderate risk of bias with 1658 participants, and follow-up duration 12-52 weeks were included. In our network meta-analysis, compared to control(s), the reduction of HbA1c was sig
Pharmacotherapy of T2DM with dulaglutide, dapagliflozin, liraglutide, empagliflozin, exenatide, and linagliptin in children is associated with modest reduction of HbA1c. Larger RCTs with longer follow-up durations are needed to guide better therapeutic decision making.
Core Tip: Although the prevalence of obesity and type 2 diabetes are increasing among children, there is only limited evidence on pharmacotherapeutic interventions to address the issue. Twelve studies including 1658 participants (follow-up: 12-52 weeks) in this meta-analysis revealed that dulaglutide, dapagliflozin, liraglutide, empagliflozin, exenatide, and linagliptin treatment resulted in mean glycated hemoglobin reductions of -1.20%, -0.94%, -0.91%, -0.87%, -0.59% and
- Citation: Gagnon CA, Buchanan K, Deaver JM, Schmitt JA, Lahart IM, Shetty S, Ashraf AP, Pappachan JM. Pharmacological management of type 2 diabetes mellitus in children and adolescents: A systematic review and network meta-analysis. World J Diabetes 2025; 16(7): 106890
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/106890.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.106890
The incidence of type 2 diabetes mellitus (T2DM) in children and adolescents has been increasing worldwide since the early 1990s, paralleling the rise in childhood obesity. The Systematic Evaluation and Research on Children and Health for Diabetes in Youth study revealed that the annual incidence of T2DM among children and young adults increased steadily by 5.31% from 2002 to 2018[1], and this trend is expected to worsen in the coming years. A recent study from the United Kingdom showed a T2DM prevalence of 8.1% among obese children[2]. Similar prevalence figures likely exist in other global regions also due to the obesity pandemic. T2DM in children and adolescents is characterized by an accelerated decline in beta cell function and a higher risk of premature complications[3]. In the Treatment Options for Type 2 Diabetes in Adolescents and Youth follow up study[3], individuals diagnosed with T2DM at an average age of 13 years exhibited early onset microvascular complications by an average age of 26. Specifically, 67% of these participants had hypertension, 50% experienced dyslipidemia, nearly half of the participants had diabetic kidney disease and 30% suffered from neuropathy. It is crucial to treat children and adolescents with T2DM optimally to prevent premature micro and macrovascular diseases and comorbidities.
T2DM in children and adolescents is multifactorial, often arising in the context of childhood obesity, genetic predisposition, physical inactivity, and adverse dietary habits. Management should primarily target reduction in body weight and body mass index (BMI). Intense health behavioral and lifestyle changes involving ≥ 26 hours of face-to-face contact, have proven effective, even though it may not be practical in most situations[4]. The prevalence of childhood and adolescent obesity[5], and diabesity (diabetes resulting from obesity)[6] continues to rise. Consequently, pharmacotherapy with antidiabetic agents often becomes imperative for optimal management.
Developing pharmacotherapeutic interventions for children is challenging due to regulatory hurdles, difficulties in obtaining consent for clinical trials, and maintaining appropriate follow-up visits from participants. For many years, only metformin and insulin were approved for treating T2DM in children and adolescents. While insulin is essential for managing severe hyperglycemia, it is not weight neutral. While metformin can help to improve insulin resistance, the Restoring Insulin SEcretion study involving youth with recently diagnosed T2DM found that metformin did not improve β-cell function. In fact, β-cell function and glycemia deteriorated during metformin treatment[7]. The newer agents such as the glucagon like peptide receptor agonists (GLP-1RA) offer better insulin sensitization due to their remarkable effect on weight loss, appetite suppression, improved satiety, and delayed gastric emptying. Moreover, the GLP-1RA group of antidiabetic agents also enhances endogenous insulin secretion. Recently, liraglutide (2019), exenatide (2021), and dulaglutide (2022) have been added to the list of approved medications for management of T2DM in children and adolescents in the United States.
With the steady increase in prevalence of pediatric and adolescent diabesity, primary and secondary care medical providers are likely to encounter children and adolescents with T2DM in their daily clinical practice. Therefore, it is important to evaluate the efficacy and safety of available pharmacotherapeutic agents for treating pediatric patients with T2DM to inform evidence-based medical practice. This review adds to the previous studies by including the findings from recent randomized controlled trials (RCTs). By investigating the latest study results, we aim to assess the clinical benefits of these pharmacological interventions in treating pediatric T2DM, providing contemporary insights into which medications are most effective for the children affected by T2DM.
This systematic review included RCTs investigating the therapeutic efficacy and safety of various antidiabetic me
Our primary research question was how pharmacological interventions in children and adolescents with T2DM influence glycated hemoglobin (HbA1c) levels from baseline to follow-up. The primary outcome measure was the change in HbA1c from baseline to follow-up. The secondary outcome measures were the changes in body weight, BMI, total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), and low-density lipoprotein (LDL) from baseline to follow-up as well as the incidence of adverse events with these pharmacological interventions.
Search strategy: Four electronic databases, PubMed, EMBASE, Scopus and ClinicalTrials.gov were searched systematically to screen literature published between January 1, 1990 and September 30, 2024, without language restrictions, to identify potential studies for inclusion. Grey literature also was searched but restricted to using key terms on the clinical trial registration database (ClinicalTrials.gov) looking for trials relevant to this review at any stage of completion. Reference lists in recent review articles that were identified during the search and finally included studies were also checked to identify other additional potentially eligible studies (Supplementary material for full search strategy).
Inclusion criteria: For this systematic review, studies were eligible if they included children and adolescents (age < 19 years old) with T2DM randomized to receive any pharmacological treatment for diabetes. Studies that administered one single pharmacological therapy or in combination with other medications, in any dosing regime, over any amount of time were included. RCTs, published in any language between January 1, 1990 to September 30, 2024 were considered for inclusion. To be eligible, studies needed to include more than 10 participants in each arm, evaluate a change in HbA1c at ≥ 12 weeks, and have a comparison group of children and adolescents who received no intervention, placebo, or an alternative treatment.
Exclusion criteria: Observational studies, qualitative studies, case reports, case series, conference abstracts, and review articles were excluded. Those studies with less than 10 participants to avoid the potential for statistical errors from smaller sample sizes[9], and those without data available for the primary outcome of interest (change in HbA1c level) were also excluded.
Two reviewers (Gagnon CA and Buchanan K) screened articles independently by title and abstract, then by full text, to determine if they were eligible for inclusion. At every stage of screening, any differences between reviewers were resolved with a third and fourth reviewer (Schmitt JA, Lahart IM). In cases where multiple publications were associated with the same RCT, one key paper was selected for each RCT, and associated articles were used as supplementary information during the data extraction process. Gagnon CA, Buchanan K, Lahart IM, Shetty S and Pappachan JM performed data extraction independently from the final selection of articles using a preformed extraction table. From each study selected, data was collected on metabolic and/or other parameters such as change in HbA1c, change in weight, change in BMI, change in lipid profile, and number of hypoglycemic events or other adverse events. Any differences between the reviewers during data extraction were discussed, and a consensus decision was made for each data point.
Data extraction included the following variables: Study characteristics (year of publication, country, intervention duration), study design (RCTs), population characteristics (setting, sample size, demographic characteristics, diagnostic criteria for T2DM), interventions (drug, dose) and outcomes (primary and secondary outcomes). Since some RCTs had differing duration times and multiple data points, we identified the “follow-up” data as the data point with the longest period from the baseline. If the study was of a crossover RCT design, then we chose the last data point before the crossover occurred or unblinding. If there was an intervention in the study that was not strictly pharmacological, we did not include those data points.
Finally, all extracted information was transferred to RevMan web and Statistical Software (version 6.5; R Core Team 2021) via RStudio (version 2024.4.2.764). Network meta-analyses were performed via the ‘meta’ and ‘netmeta’ R packages.
Assessment of risk of bias: Quality appraisal was conducted on each full-text article using the Cochrane risk of bias 2 tool[10], which included domains of the randomization process, deviations from the intended intervention (protocol violation), missing outcome data (attrition bias), measurement of the outcome, and selective reporting of outcomes. Each domain was judged as low, having some concerns, or high risk of bias. Quality appraisal was assessed with supplementary documents including other papers from the same RCT, protocols, and information from clinical trial registries. Gagnon CA, Buchanan K, Schmitt JA and Shetty S performed quality appraisal independently with any differences between reviewers resolved with Pappachan JM and Ashraf AP.
Quantitative synthesis: The analysis was performed in line with the recommendations from the Cochrane Collaboration, and the Quality of Reporting of Meta-Analyses guidelines[11,12]. Continuous parameters were calculated and analyzed using weighted mean difference/mean difference (WMD/MD) changes from baseline along with the 95% confidence interval (CI). Dicho
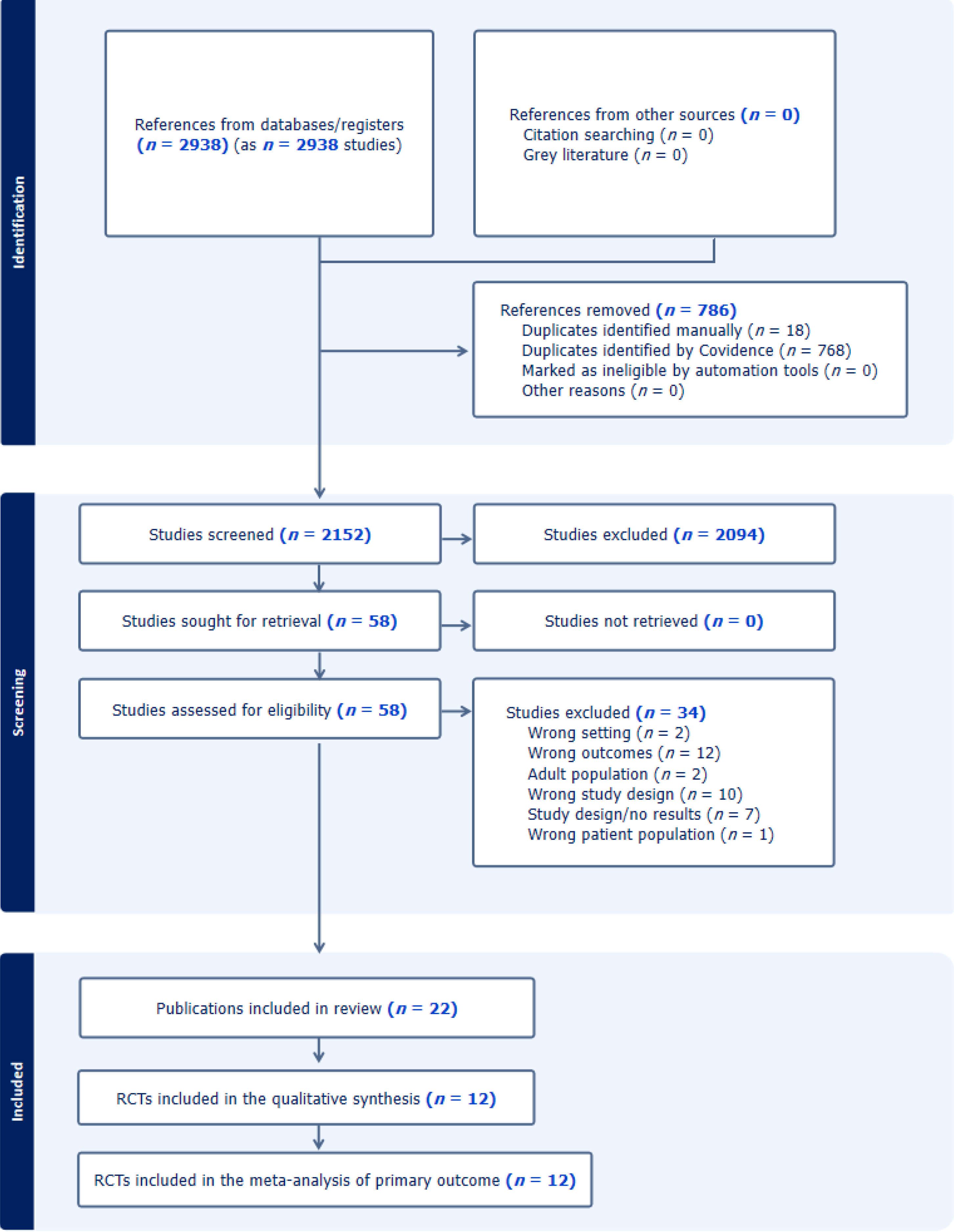
A total of 2750 studies were identified by the literature search. After removal of 685 duplicates, 2065 studies were screened by title and abstracts, and 50 full-text studies were assessed for eligibility from which 12 RCTs were included for analysis (Figure 1)[13-24]. The number of participants in the included studies and the duration of follow up were relatively low for a lifelong and common disease like T2DM that would reduce the overall quality of the evidence obtained from this review.
In total, there were 1658 participants included. Ten studies were double-blinded, one was an open label study, and one was single-blinded (Table 1). The largest RCT included 263 children with T2DM[13], while the smallest had 24 par
| Ref. | Year | Blinding setting | Study period (weeks) | Interventions | Sample | Mean age (years) | Baseline HbA1c (%) | Baseline weight (kg) | Baseline BMI (kg/m2) | Baseline TC (mg/dL) | Baseline TG (mg/dL) | Baseline HDL (mg/dL) | Baseline LDL (mg/dL) |
| Gottschalk et al[13] | 2007 | Single blind | 24 | Glimepiride | 132 | 13.8 ± 2.3 | 8.52 ± 1.58 | 82.60 ± 25.60 | 31.57 ± 8.48 | ||||
| Metformin | 131 | 13.8 ± 2.3 | 8.54 ± 1.57 | 83.83 ± 27.47 | 31.60 ± 8.17 | ||||||||
| Tamborlane et al[20] | 2018 | Double blind | 12 | Linagliptin-1 mg | 10 | 14.0 ± 1.9 | 8.22 ± 0.93 | 75.3 ± 19.3 | 28.0 ± 5.2 | ||||
| Linagliptin-5 mg | 14 | 14.3 ± 2.1 | 7.87 ± 0.98 | 84.8 ± 25.1 | 33.0 ± 8.0 | ||||||||
| Placebo | 15 | 13.7 ± 2.0 | 7.60 ± 0.92 | 78.2 ± 21.8 | 29.2 ± 6.0 | ||||||||
| Tamborlane et al[18] | 2019 | Double blind | 26 | Liraglutide + metformin | 66 | 14.57 ± 1.73 | 7.87 ± 1.35 | 93.3 ± 31.0 | 34.55 ± 10.87 | ||||
| Metformin | 68 | 14.57 ± 1.73 | 7.69 ± 1.34 | 89.8 ± 22.1 | 33.27 ± 7.36 | ||||||||
| Tamborlane et al[19] | 2022 | Double blind | 24 | Dapagliflozin 10 mg | 39 | 16.1 ± 3.3 | 7.95 ± 1.59 | 89.2 ± 25.7 | 31.3 ± 7.5 | ||||
| Placebo | 33 | 16.2 ± 3.6 | 7.85 ± 1.19 | 92.5 ± 31.9 | 33.6 ± 8.8 | ||||||||
| Tamborlane et al[15] | 2022 | Double blind | 24 | Exenatide -2 mcg | 58 | 14.9 ± 1.88 | 8.13 ± 1.2 | 102.18 ± 30.1 | 36.86 ± 9.28 | 167.44 ± 42.54 | 161.2 ± 104.52 | 41.76 ± 12.76 | 94.74 ± 36.74 |
| Placebo | 24 | 15.6 ± 1.66 | 8.28 ± 1.5 | 96.7 ± 22.7 | 35.14 ± 6.58 | ||||||||
| Jalaludin et al[22] | 2022 | Double blind | 54 | Sitagliptin 100 mg + metformin | 107 | 14.4 ± 2.2 | 8.0 ± 1.1 | 81.9 ± 25.4 | 31.2 ± 8.1 | ||||
| Metformin | 113 | 13.9 ± 1.8 | 8.1 ± 1.1 | 79.8 ± 24.8 | 30.6 ± 8.5 | ||||||||
| Shankar et al[14] | 2022 | Double blind | 54 | Sitagliptin | 95 | 14.3 ± 2.0 | 7.4 ± 1.0 | 89.1 ± 25.3 | 33.3 ± 7.7 | ||||
| Placebo | 95 | 13.7 ± 1.9 | 7.6 ± 1.1 | 81.9 ± 24.8 | 31.2 ± 7.7 | ||||||||
| Arslanian et al[16] | 2022 | Double blind | 26 | Dulaglutide 0.75 mg | 51 | 14.7 ± 2.21 | 7.92 ± 1.27 | 90 ± 28.3 | 33.6 ± 9 | 164.5 | 167.5 | 40.9 | 91.4 |
| Dulaglutide 1.5 mg | 52 | 14.7 ± 1.8 | 8.16 ± 1.39 | 92.6 ± 21.6 | 34.3 ± 7 | 166.1 | 162.7 | 41.8 | 95.3 | ||||
| Placebo | 51 | 14.2 ± 2.1 | 8.14 ± 1.12 | 88.9 ± 29.4 | 34.3 ± 10.2 | 169.9 | 189.4 | 41.8 | 93.5 | ||||
| Laffel et al[21] (DINAMO) | 2023 | Double blind | 26 | Empagliflozin 10 mg | 52 | 14.4 ± 1.9 | 8.0 ± 1.29 | 98.66 ± 24.35 | 35.54 ± 7.17 | ||||
| Linagliptin 5 mg | 52 | 14.6 ± 1.9 | 8.05 ± 1.11 | 102.76 ± 26.40 | 35.55 ± 7.55 | ||||||||
| Placebo | 53 | 14.6 ± 1.8 | 8.05 ± 1.23 | 96.38 ± 29.55 | 33.3 ± 7.7 | ||||||||
| Shehadeh et al[22](T2N OW) | 2023 | Double blind | 26 | Dapagliflozin 5 mg | 81 | 14.4 ± 2.0 | 8.22 ± 1.46 | 78.8 ± 18.1 | Z-score 17 ± 0.7 | ||||
| Saxagliptin 2.5 mg | 88 | 14.5 ± 1.8 | 8.02 ± 1.43 | 82.5 ± 24.0 | Z-score 18 ± 0.7 | ||||||||
| Placebo | 76 | 14.7 ± 1.6 | 7.96 ± 1.63 | 76.2 ± 23.1 | Z-score 15 ± 0.8 | ||||||||
| NCT00658021[24] | 2020 | Double blind | 28 | Exenatide 5 mcg BD | 40 | 13.7 ± 1.94 | NR (HbA1 range: 6.5 - 10.5) | NR | NR | ||||
| Exenatide 10 mcg BD | 38 | 14.0 ± 1.95 | NR | NR | NR | ||||||||
| Placebo | 42 | 14.4 ± 1.82 | NR | NR | NR | ||||||||
| Dietsche et al[23] | 2023 | Open label | 12 | Liraglutide+ metformin | 11 | 15.0 ± 2.1 | 7.3 ± 0.8 | 114 ± 24.4 | 38.6 ± 8.3 | ||||
| Metformin | 13 | 15.6 ± 2.1 | 6.3 ± 0.8 | 117 ± 25.0 | 41.1 ± 7.6 | ||||||||
| Studies excluded from meta-analysis of primary outcomes | |||||||||||||
| RISE[29] | 2019 | Open label | 52 | Insulin then metformin | 44 | 14.9 ± 2.0 | 5.7 ± 0.6 | 102.0 ± 25.7 | 36.5 ± 6.4 | 98.32 | 38.67 ± 11.6 | 88.94 ± 30.94 | |
| Metformin | 47 | 13.9 ± 2.1 | 5.7 ± 0.6 | 97.7 ± 23.3 | 36.9 ± 6.4 | 103.63 | 38.67 ± 7.73 | 81.21 ± 23.2 | |||||
| Wheeler et al[28] | 2018 | Open label | 26 | Insulin determir + metformin | 20 | 15 ± 2.1 | 8.72 ± 0.86 | 75.9 ± 16.6 | 28.7 ± 4.8 | ||||
| NPH insulin + metformin | 22 | 15 ± 2.2 | 8.95 ± 1.05 | 73.2 ± 23.4 | 27.7 ± 6.6 | ||||||||
| Jones et al[26] | 2002 | Double blind | 8 | Metformin | 42 | 13.9 ± 1.8 | 8.3 ± 1.3 | 92.8 ± 31.8 | 34.2 ± 10.6 | 174.0 ± 38.67 | 150.57 ± 115.1 | 42.5 ± 11.6 | 100.5 ± 30.9 |
| Placebo | 40 | 13.6 ± 1.8 | 9.0 ± 1.4 | 90.3 ± 38.1 | 33.9 ± 12.7 | 189.5 ± 38.67 | 203.7 ± 194.9 | 42.5 ± 11.6 | 112.1 ± 27.1 | ||||
| Klein et al[25] | 2014 | Double blind | 5 | Liraglutide | 14 | 14.4 ± 2.2 | 8.3 ± 1.4 | 112.7 ± 37.3 | 40 ± 10.3 | 165.12 ± 39.06 | 153.1 ± 97.35 | 39.44 ± 9.28 | 95.51 ± 27.84 |
| Placebo | 7 | 15.6 ± 2.1 | 7.8 ± 0.9 | 114.2 ± 34.7 | 39.9 ± 6.8 | 185.61 ± 33.26 | 176.99 ± 85.84 | 39.44+8.89 | 110.98 ± 27.84 | ||||
| Barrientos-Pérez et al[27] | 2022 | Double blind | 6 | Lixisenatide | 18 | 15.6 ± 1.0 | 8.16 ± 0.93 | 91.3 ± 18.8 | 33.2 ± 4.8 | ||||
| Placebo | 5 | 15.4 ± 1.5 | 8.14 ± 1.58 | 98 ± 14.7 | 37.4 ± 3.6 | ||||||||
Five studies[25-29] were excluded from analysis due to reasons as follows: Three studies[25-27] had follow-up duration of < 12 weeks only (Table 1), making it difficult to conclude a meaningful judgement on effects of the drug intervention on HbA1c change as specified in our study protocol, One study was on the comparative efficacy of two different types of intermediate acting insulin in the management of T2DM compared to metformin[28], and one study[29] was on the effect of insulin treatment for a period followed by metformin compared to metformin from beginning for children with T2DM.
None of the studies were deemed to have a high risk of bias in any of the five domains (Supplementary Figure 1). One study had some concerns regarding deviations from intended intervention[23]. Four studies had some concerns re
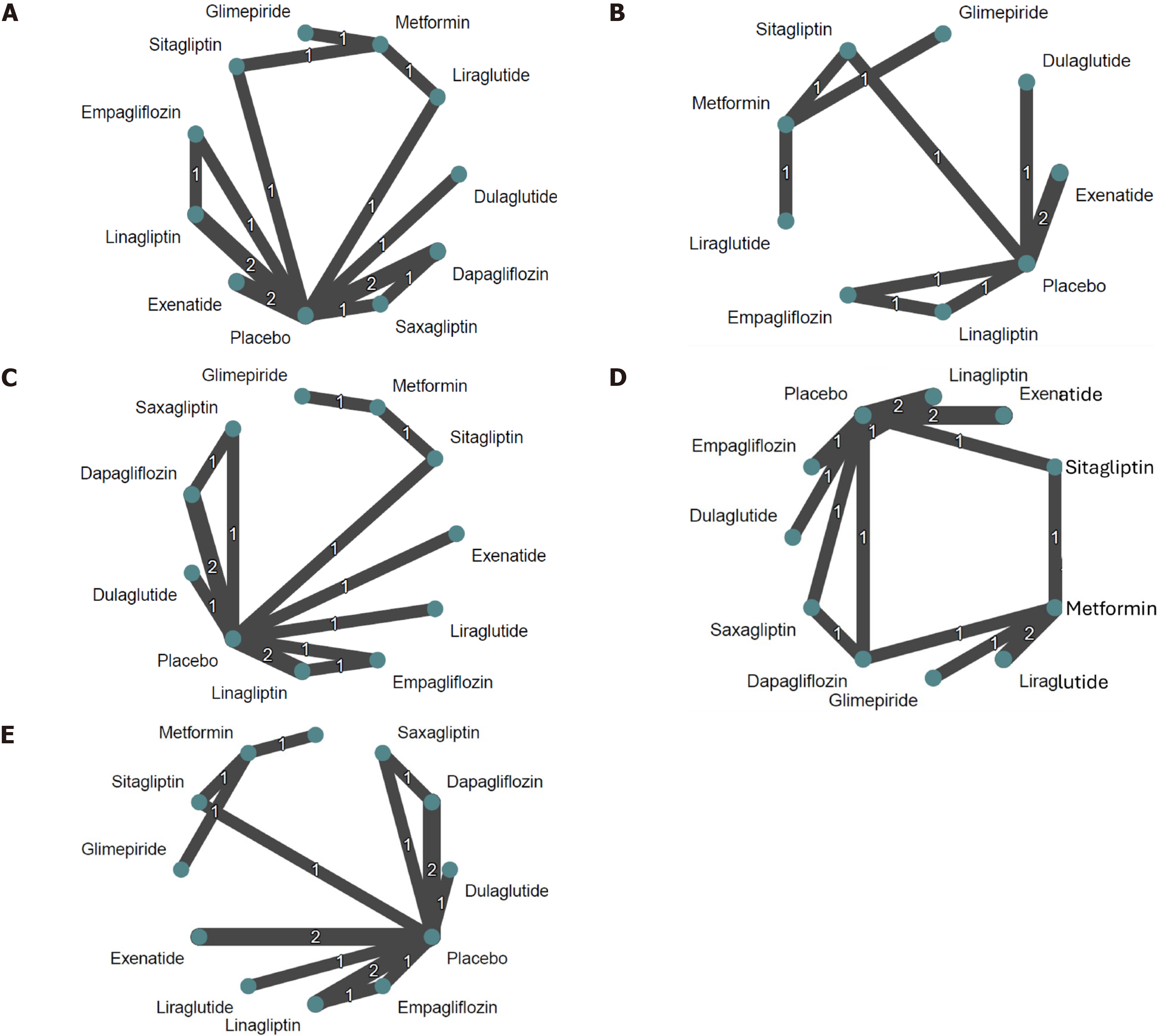
Network meta-analysis: We obtained HbA1c change from baseline data from 12 trials (1658 participants)[13-24]. These trials compared a total of 12 treatments (and 6 drug classes) and our network comprised 19 pairwise comparisons. Our dataset contains two multi-arm studies Laffel et al[21] and Shehadeh et al[22] These two studies each contain three comparisons, while all other studies contain only one comparison each. Figure 2A presents the network map for HbA1c.
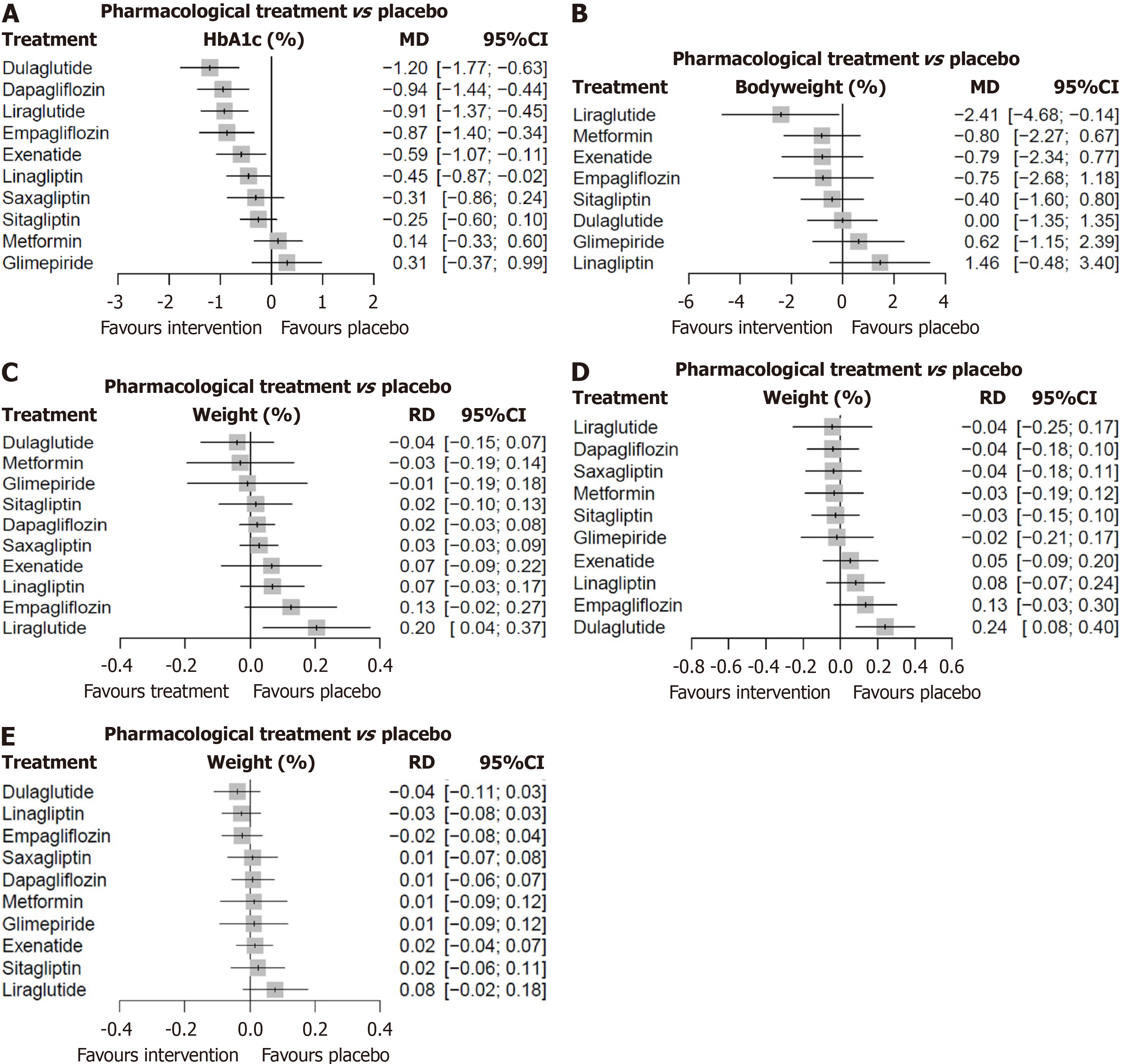
The forest plot of the network meta-analysis for various pharmacological therapeutic agents vs placebo is depicted in Figure 3A.
Compared with placebo, dulaglutide (MD -1.20), dapagliflozin (-0.94), liraglutide (-0.91), empagliflozin (-0.87), exenatide (0.59) and linagliptin (0.45) all demonstrated statistically significant reductions in HbA1c (Figure 3A). Pairwise meta-analyses show the effects of each investigational drug in the RCT compared to placebo (Supplementary Figures 2 and 3), the drug vs metformin (Supplementary Figure 4), and the individual drug classes vs placebo (Supplementary Figure 5) on HbA1c outcomes.
Supplementary Figure 6 shows the league table [direct estimates (upper-right triangle) and mixed estimates (lower-left triangle)] and Supplementary Figure 7 the direct evidence plots for HbA1c changes with various drug molecules.
Node-splitting revealed evidence of inconsistency between indirect and direct effects only for the metformin vs placebo loop (P = 0.03; Supplementary Figure 8). This was consistent with our network heat map that revealed important inconsistency within the metformin, placebo, and liraglutide treatment loop (Supplementary Figure 9). Baseline dif
The comparison-adjusted funnel plot for HbA1c appeared symmetrical (Supplementary Figure 10). This symmetry was corroborated by a non-statistically significant Egger’s test (P = 0.935). Therefore, there is no indication that there are small-study effects in our network, at least not because new treatments with superior effects are more likely to be found in published literature.
A subgroup analysis for follow-up HbA1c and P value ranking of individual drugs are shown in Supplementary Figures 11 and 12 respectively.
A subgroup analysis by drug classes revealed that compared to placebo, SGLT-2 inhibitors (MD: -0.90, 95%CI: -1.25 to -0.55, P value = 0.89) ranked first (according to P value) for reducing HbA1c, followed by GLP-1 agonists (MD: -0.89, 95%CI: -1.17 to -0.60, P value = 0.87) and DPP-4 inhibitors (MD: -0.32, 95%CI: -0.55 to -0.08, P value = 0.47; Supplementary Figure 13). Metformin and sulfonylureas were ranked lowest among the treatments, and there was no evidence of an effect compared to placebo.
Network meta-analysis: Eight RCTs[13,15,16-19,21,24] provided bodyweight change from baseline data (n = 999), comprising 9 treatments (and 5 drug classes) and 10 pairwise comparisons. The body weight dataset contained one multi-arm study, Tamborlane et al[19] (3 comparisons). Figure 2B presents the network map for body weight and Figure 3B shows the network forest plot for body weight changes.
Pairwise meta-analysis for body weight changes as per RCTs, drug classes compared to placebo, RCTs comparing metformin are shown in Supplementary Figures 14-16. P-score ranking showed that only liraglutide possessed a sig
We found no evidence of a pharmacological effect on body weight compared with placebo, with non-statistical heterogeneity/inconsistency that might not be considered important. There was no significant difference between drug classes and placebo but a tendency for weight gain for glimepiride compared to metformin (Supplementary Figure 16).
The comparison-adjusted funnel plot and a non-statistically significant Egger’s test (P = 0.821) indicated that small study effects were unlikely (Supplementary Figure 18). Subgroup analysis by drug classes revealed no evidence of a statistical effect on body weight compared to placebo (Supplementary Figures 19 and 20).
Change in BMI from baseline: Only two trials[14,16] provided BMI change from baseline data (n = 343), comprising three treatments (and 2 drug classes) and two pairwise comparisons. Therefore, network meta-analysis was not possible for BMI dataset. There was no statistically significant difference in the BMI during follow-up as shown in Supplementary Figures 21 and 22 in pairwise comparisons.
Change in blood lipids from baseline: Network meta-analysis or pairwise analysis were not possible for TC, TG, LDL, and HDL as only one trial[19] reported these outcomes.
Hypoglycemia events: In our analysis, we included 8 studies[14-16,18-22] with 1057 participants and 143 level 1 hy
In our network meta-analysis, we found that liraglutide (RD 0.20, 95%CI: 0.04-0.37) may increase the risk of level 1 hypoglycemic events (Figures 2C and 3C). No other drug or drug class was found to increase hypoglycemic event risk. No pharmacological treatment was found to increase the risk of level 2 hypoglycemic events compared to placebo (Supplementary Figures 23-27). Similarly, when treatments were grouped by drug class, none were found to influence the risk of level 2 hypoglycemic event.
Adverse events: We included 12 studies[13-24] reporting any adverse events (1658 participants) in our analysis. Figure 2D shows the network map and Figure 3D the network forest plot for minor adverse events.
Our network meta-analysis revealed that except for dulaglutide (RD: 0.24, 95%CI: 0.08-0.40), no other drug treatment was associated with significantly increased risk of minor adverse events compared to placebo (Figure 3D). However, no drug or drug class was found to be associated with excess risk of serious adverse events (Figures 2E and 3E, Supplementary Figures).
No other drug or drug class was found to increase the risk of minor or major adverse events (Supplementary Figures 28-36). No pharmacological treatment (individual agents or drug class) was found to increase the risk of serious adverse events compared to placebo or metformin.
This systematic review included 12 RCTs with moderate to low overall risk of bias and analyzed treatment outcomes of a total of 1658 participants with T2DM managed by various antidiabetic agents. These trials using 12 pharmacotherapeutic interventions and 19 pairwise comparisons with the measurement of changes in HbA1c from baseline found statistically significant reductions with dulaglutide (-1.20%), followed by dapagliflozin (-0.94%), liraglutide (-0.91%), empagliflozin (-0.87%), exenatide (-0.59%) and linagliptin (-0.45%). All other drugs had little or no effect on HbA1c compared to placebo at the end of study period. As drug classes, SGLT-2i, GLP-1RA, and DPP-4i medications were associated with significant HbA1c reduction (Supplementary Figure 13) while sulfonylureas and metformin appear to have no significant effects of HbA1c.
The overall treatment effect of each drug and drug classes from the studies reporting body weight changes was statistically not significant except for liraglutide treatment (-2.41 kg). Only 2 studies reporting changes from baseline revealed no significant reduction in the BMI. The dulaglutide dose used in the clinical trial was 0.75 mg and 1.5 mg once weekly (Table 1). Unfortunately, due to the limited number of studies and the variability in dosing regimens, a formal dose-response analysis was not feasible within this network meta-analysis. It must be noted that higher doses are studied for weight loss (3.0 mg and 4.5 mg once weekly). Similarly, the highest liraglutide dose used in clinical trials among children was 1.8 mg for glycemic control, whereas the weight loss effect is higher at 3.0 mg once daily. Moreover, the study duration of most RCTs had been of short duration (12-52 weeks) only for a lifelong ailment like T2DM that could have skewed the results of our review. Therefore, the effects on body weight and BMI observed in this review from various studies should be interpreted with caution, considering the data from large RCTs investigating treatment effects of some of these agents in the adult populations with T2DM, which showed either a weight loss potential or weight neutrality[30-33].
Though a previous systematic review and network meta-analysis reported markedly therapeutic outcomes of ma
The rapid changes in body weight and BMI during adolescent growth spurt would also have contributed to the above observations. Moreover, pathophysiology and adipocyte biology in childhood and adolescent obesity and diabesity differ significantly from adults, potentially explaining some discrepancy in the therapeutic effects observed in this study[35]. Rapid growth spurt with marked changes in hormonal and behavioral milieu during the adolescent stage would worsen insulin resistance, another reason for the differences observed in the therapeutic effects of various antidiabetic agents with disease modifying properties in T2DM (especially, GLP-1RA and SGLT-2i) compared to that in adult populations.
Analyses of other cardiometabolic parameters such as changes in TC, TG, HDL, and LDL were not possible as only one study reported such outcomes during follow-up. Also, the favorable changes in cardiometabolic parameters typically associated with weight loss may not have been evident because these participants did not experience significant weight loss except in one study which did not report these cardiometabolic benefits.
Although our network meta-analyses showed statistically higher risk of level 1 hypoglycemia with liraglutide treatment (RD: 0.20), none of the other drugs or drug classes reveled any higher risk compared to placebo or metformin. However, neither any drug nor any classes showed statistically higher risk of level 2 hypoglycemia. These findings are reasonably reassuring in managing children who can be more prone to hypoglycemia with antidiabetic drug therapy owing to their high metabolic turnover and higher physical activities compared to adults. Reassuringly, most drug classes used in the RCTs such as GLP-1RA, SGLT-2i, DPP-4i and metformin are generally not associated with hypoglycemic tendency.
Similarly, minor adverse events [except dulaglutide treatment associated with statistically higher risk (RD: 0.24)] and serious adverse events were comparable between drug intervention and comparator arms, supporting the safety of the investigational drugs assessed enabling clinicians to use them with reasonable confidence in their medical practice. Though higher incidence of genital thrush and urinary infections are observed among adults treated with SGLT-2i, we didn’t observe a statistically higher incidence of adverse events compared to placebo among children and young adults as observed in a recent systematic review[36]. Moreover, the efficacy in HbA1c reduction in this study (MD: -0.93, 95%CI:
Limitations: Most of the reported studies were of small to medium size, limiting the statistical significance of several of the treatment outcomes. Future research should include larger, multicenter, and potentially multinational RCTs to address this issue. As T2DM is a lifelong disease, long-term studies examining both primary and secondary outcomes are essential in making meaningful judgements regarding the interventions. Unfortunately, the studies included were of only 12-52 weeks duration, making it impossible to interpret the long-term treatment effects, a serious limitation of RCTs included in this review. Moreover, due to the limited number of studies and the variability in dosing regimens, a formal dose-response analysis of various drugs was not feasible within this network meta-analysis. There were significant differences in the baseline characteristics of the participants such as HbA1c, body weight and BMI which makes comparison between individual interventions difficult. Moreover, only one study reported the cardiometabolic pa
Newer GLP-1RA agents like Semaglutide[37] and the glucagon-like peptide/glucose dependent insulinotropic po
Strengths: This review brings forth a comprehensive assessment of efficacy and safety of T2DM pharmacotherapeutic agents currently available for use in children to inform clinical practice decisions. This systematic review highlights the variability in efficacy and safety among different antidiabetic agents in managing T2DM, particularly with respect to HbA1c reduction, body weight, and adverse event profiles. These findings underscore the importance of individualized treatment plans, especially in pediatric and adolescent populations, where developmental physiology and metabolic dynamics significantly differ from those of adults and can influence therapeutic outcomes.
Given the rapidly evolving landscape of pediatric T2DM across the globe, this review serves to familiarize clinicians with existing evidence regarding both the effectiveness and risks of various antidiabetic agents. As the reported studies were RCTs with a moderate to low risk of bias, the generated evidence from the review is of moderate strength, despite the relatively smaller sample sizes. Moreover, the primary outcome such as HbA1c reduction in several key studies (e.g., dulaglutide[16], liraglutide[18], dapagliflozin[15] and empagliflozin[21]) included in this review were comparable to large-scale studies in adults[39-43]. Sensitivity analyses, including subgroup analyses, to assess the consistency of evidence further strengthens the robustness of our findings.
Despite the limitations of small sample sizes and short duration of follow-up, this review offers reasonably robust evidence supporting the use of antidiabetic agents such as dulaglutide, liraglutide, dapagliflozin, exenatide, linagliptin, and empagliflozin to improve diabesity outcomes in children and adolescents. These agents demonstrated statistically significant reductions in HbA1c from baseline to follow-up.
Future studies should address uncertainties regarding the cardiometabolic and body weight reduction related outcomes with pharmacotherapy of T2DM in children. Larger, muti-center, and multinational studies with longer clinical and biochemical follow up durations are essential to refine best evidence-based strategies for managing for early onset T2DM and diabesity in the pediatric populations. Future research should also explore the optimal dosing regimens tailored to children and adolescents with T2DM such as the impact of pubertal growth and development o metabolism, differences in adipocyte biology compared to adults, and higher baseline insulin resistance observed in youth, all of which might influence drug efficacy and safety. Given the promising benefits of GLP-1RA and SGLT-2i classes observed in this review, with potential disease modifying potential in pediatric T2DM, more rigorous and comprehensive evidence should emerge in future research.
Newer antidiabetic agents such as dulaglutide, liraglutide, dapagliflozin, exenatide, linagliptin, and empagliflozin appear to improve diabesity outcomes in children and adolescents with significant reductions in HbA1c. Marked variability in the availability of medical resources, lifestyle factors, economic levels, and racial/ethnic backgrounds across different global regions may limit the generalizability of our findings. We acknowledge that our review has limitations in ad
We thank Dr Marina G Kudiyirickal, MSc, MJDF-RCS, PhD for providing the voice clip for the audio core tip of this paper.
| 1. | Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, Dolan LM, Shah AS, Bellatorre A, Sauder K, Marcovina S, Reynolds K, Pihoker C, Imperatore G, Divers J; SEARCH for Diabetes in Youth study. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023;11:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 2. | Hawton K, Apperley L, Parkinson J, Owens M, Semple C, Canvin L, Holt A, Easter S, Clark K, Lund K, Clarke E, O'Brien J, Giri D, Senniappan S, Shield JPH. Complications of excess weight seen in two tier 3 paediatric weight management services: an observational study. Arch Dis Child. 2025;110:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 4. | Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, Avila Edwards KC, Eneli I, Hamre R, Joseph MM, Lunsford D, Mendonca E, Michalsky MP, Mirza N, Ochoa ER, Sharifi M, Staiano AE, Weedn AE, Flinn SK, Lindros J, Okechukwu K. Executive Summary: Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics. 2023;151:e2022060641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Hannon TS, Arslanian SA. Obesity in Adolescents. N Engl J Med. 2023;389:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, Khunti K, Magliano DJ, Luk A. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11:768-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | RISE Consortium. Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care. 2014;37:780-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46988] [Article Influence: 2936.8] [Reference Citation Analysis (0)] |
| 9. | Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc Sci Med. 2022;292:114523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 1530] [Article Influence: 382.5] [Reference Citation Analysis (0)] |
| 10. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 14926] [Article Influence: 2487.7] [Reference Citation Analysis (0)] |
| 11. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16737] [Article Influence: 669.5] [Reference Citation Analysis (0)] |
| 12. | Clarke M, Horton R. Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet. 2001;357:1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Shankar RR, Zeitler P, Deeb A, Jalaludin MY, Garcia R, Newfield RS, Samoilova Y, Rosario CA, Shehadeh N, Saha CK, Zhang Y, Zilli M, Scherer LW, Lam RLH, Golm GT, Engel SS, Kaufman KD. A randomized clinical trial of the efficacy and safety of sitagliptin as initial oral therapy in youth with type 2 diabetes. Pediatr Diabetes. 2022;23:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, Karoly E, Troja T, Doehring O, Carter D, Monyak J, Sjöström CD. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care. 2022;45:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, Bismuth E, Dib S, Cho JI, Cox D; AWARD-PEDS Investigators. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med. 2022;387:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 17. | Jalaludin MY, Deeb A, Zeitler P, Garcia R, Newfield RS, Samoilova Y, Rosario CA, Shehadeh N, Saha CK, Zhang Y, Zilli M, Scherer LW, Lam RLH, Golm GT, Engel SS, Kaufman KD, Shankar RR. Efficacy and safety of the addition of sitagliptin to treatment of youth with type 2 diabetes and inadequate glycemic control on metformin without or with insulin. Pediatr Diabetes. 2022;23:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 19. | Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, Karlsson C, Norjavaara E. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Tamborlane WV, Laffel LM, Weill J, Gordat M, Neubacher D, Retlich S, Hettema W, Hoesl CE, Kaspers S, Marquard J. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr Diabetes. 2018;19:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, Neubacher D, Marquard J; DINAMO Study Group. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 22. | Shehadeh N, Barrett T, Galassetti P, Karlsson C, Monyak J, Iqbal N, Tamborlane WV. Dapagliflozin or Saxagliptin in Pediatric Type 2 Diabetes. NEJM Evid. 2023;2:EVIDoa2300210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Dietsche KB, Magge SN, Dixon SA, Davis FS, Krenek A, Chowdhury A, Mabundo L, Stagliano M, Courville AB, Yang S, Turner S, Cai H, Kasturi K, Sherman AS, Ha J, Shouppe E, Walter M, Walter PJ, Chen KY, Brychta RJ, Peer C, Zeng Y, Figg W, Cogen F, Estrada DE, Chacko S, Chung ST. Glycemia and Gluconeogenesis With Metformin and Liraglutide: A Randomized Trial in Youth-onset Type 2 Diabetes. J Clin Endocrinol Metab. 2024;109:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | AstraZeneca. Safety and Efficacy of Exenatide as Monotherapy and Adjunctive Therapy to Oral Antidiabetic Agents in Adolescents With Type 2 Diabetes. [accessed 2024 Sep 30]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT00658021 ClinicalTrials.gov Identifier: NCT00658021. |
| 25. | Klein DJ, Battelino T, Chatterjee DJ, Jacobsen LV, Hale PM, Arslanian S; NN2211-1800 Study Group. Liraglutide's safety, tolerability, pharmacokinetics, and pharmacodynamics in pediatric type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Technol Ther. 2014;16:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Barrientos-Pérez M, Hsia DS, Sloan L, Nell H, Mungur O, Hovsepian L, Schmider W, Spranger R, Yang N, Niemoeller E. A study on pharmacokinetics, pharmacodynamics and safety of lixisenatide in children and adolescents with type 2 diabetes. Pediatr Diabetes. 2022;23:641-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Wheeler MD, Barrientos-Perez M, Lo FS, Liang B, Lunsford A, Thórisdóttir Ó, Zuckerman-Levin N. A 26-week, randomized trial of insulin detemir versus NPH insulin in children and adolescents with type 2 diabetes (iDEAt2). Eur J Pediatr. 2018;177:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 29. | RISE Consortium; RISE Consortium Investigators. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on β-Cell Function: Comparison of Responses In Youth And Adults. Diabetes. 2019;68:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Du Q, Wu B, Wang YJ, Yang S, Zhao YY, Liang YY. Comparative effects of sitagliptin and metformin in patients with type 2 diabetes mellitus: a meta-analysis. Curr Med Res Opin. 2013;29:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH, Chen YJ, Li XX, Zhang JY, Yang KH. The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials. Acta Diabetol. 2019;56:249-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Dai D, Mao Y, Jin H, Zhang W. Efficacy and hypoglycemic risk of sitagliptin in obese/overweight patients with type 2 diabetes compared with GLP-1 receptor agonists: A meta-analysis. Medicine (Baltimore). 2019;98:e17081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Li Y, Gong X, Găman MA, Hernández-Wolters B, Velu P, Li Y. The effect of subcutaneous dulaglutide on weight loss in patients with Type 2 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Eur J Clin Invest. 2024;54:e14125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Wu S, He Y, Wu Y, Ji Y, Hou L, Liu X, Ge Y, Yu Y, Yu Y, Wei Y, Qian F, Luo Q, Feng Y, Feng Y, Wang J, Huo M, Li H, Xue F, Liu Y. Comparative efficacy and safety of glucose-lowering drugs in children and adolescents with type 2 diabetes: A systematic review and network meta-analysis. Front Endocrinol (Lausanne). 2022;13:897776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Pappachan JM, Fernandez CJ, Ashraf AP. Rising tide: The global surge of type 2 diabetes in children and adolescents demands action now. World J Diabetes. 2024;15:797-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (3)] |
| 36. | Dos Santos Borges R, Conegundes AF, Haikal de Paula L, Lara Santos R, Alves SN, Machado RA, Bussolaro Viana I, Simões E Silva AC. Efficacy and Safety of SGLT2 Inhibitors in Pediatric Patients and Young Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pediatr Diabetes. 2024;2024:6295345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 142] [Reference Citation Analysis (10)] |
| 38. | Karagiannis T, Malandris K, Avgerinos I, Stamati A, Kakotrichi P, Liakos A, Vasilakou D, Kakaletsis N, Tsapas A, Bekiari E. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia. 2024;67:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 39. | Qie S, Li X, Wang X, Liu Y, Li J, Liu G. Efficacy and safety of long-acting glucagon-like peptide-1 receptor agonist dulaglutide in patients with type 2 diabetes: a systematic review and meta-analysis of 21 randomized controlled trials. Endocrine. 2020;68:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Tan J, Wang Y, Liu S, Shi Q, Zhou X, Zhou Y, Yang X, Chen P, Li S. Long-Acting Metformin Vs. Metformin Immediate Release in Patients With Type 2 Diabetes: A Systematic Review. Front Pharmacol. 2021;12:669814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann Intern Med. 2020;173:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 42. | Wang L, Xin Q, Wang Y, Chen Z, Yuan R, Miao Y, Zhang G, Cong W. Efficacy and safety of liraglutide in type 2 diabetes mellitus patients complicated with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2021;171:105765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Zheng H, Sigal RJ, Coyle D, Bai Z, Johnston A, Elliott J, Hsieh S, Kelly SE, Chen L, Skidmore B, Toupin-April K, Wells GA. Comparative efficacy and safety of antihyperglycemic drug classes for patients with type 2 diabetes following failure with metformin monotherapy: A systematic review and network meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2022;38:e3515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |