Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.106821
Revised: March 26, 2025
Accepted: May 22, 2025
Published online: July 15, 2025
Processing time: 129 Days and 20.2 Hours
Type 2 diabetes mellitus (T2DM) is a metabolic disorder linked to high blood glucose and gut dysbiosis. Probiotics like Lactobacillus rhamnosus LRa05 may im
To explore the impact of LRa05 with hypoglycemic medications on glycemic control and intestinal flora in T2DM patients with gut dysbiosis.
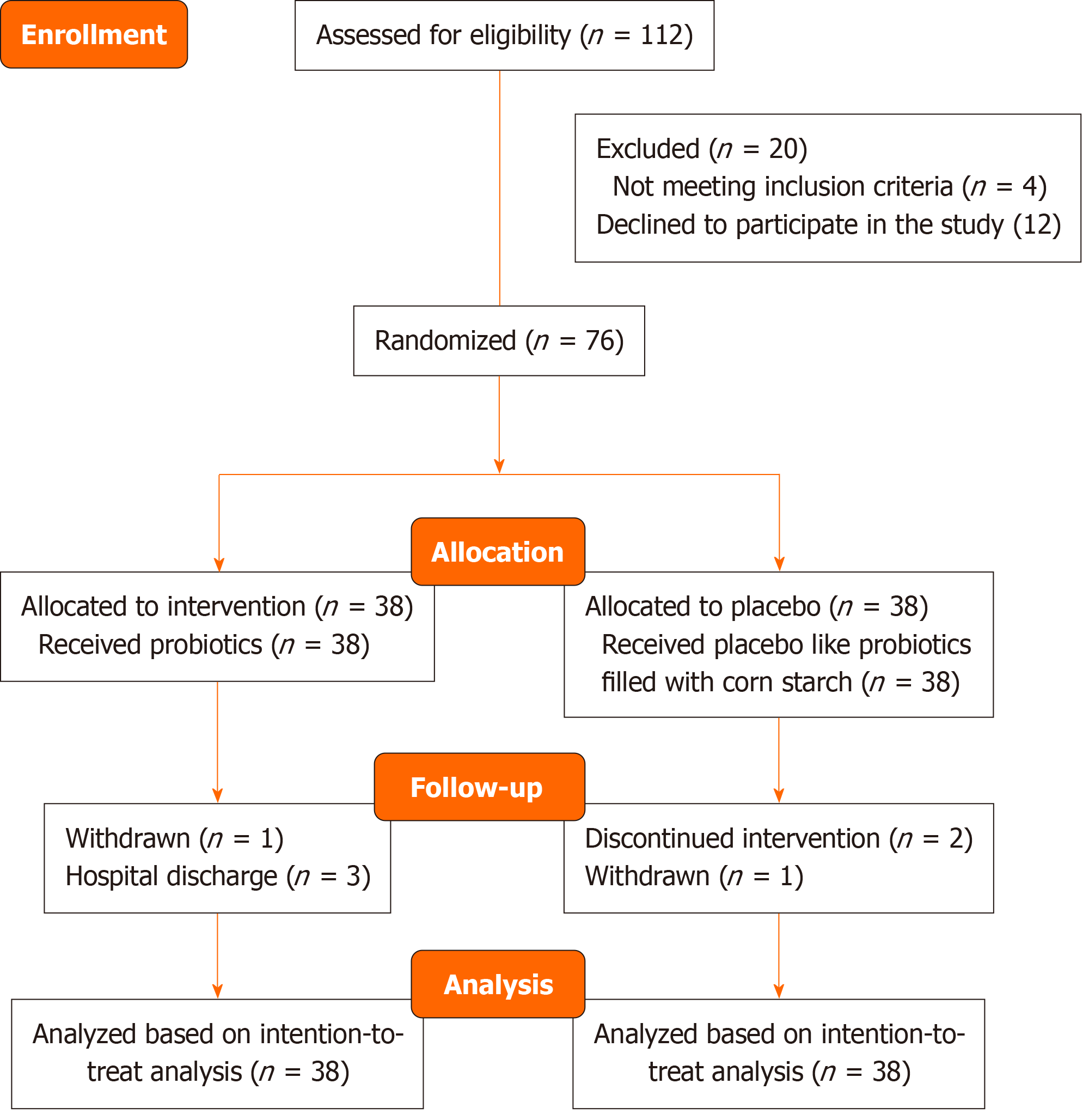
Seventy-six participants were randomly assigned to receive either LRa05 (0.1 g 2 × 1010 CFU) (n = 38) or a placebo (n = 38) for 12 weeks. Baseline characteristics were recorded, and changes in glycated hemoglobin, fasting blood glucose, and other biochemical indices were assessed using repeated measures one-way analysis of variance. Additionally, gut microbiota diversity was analyzed through species accumulation and alpha and beta diversity metrics.
The intervention group showed statistically significant improvements in lipid profiles, particularly in high-density lipoprotein cholesterol levels, which in
This study demonstrated that the combination of Lactobacillus rhamnosus LRa05 and hypoglycemic medications positively impacted glycemic control, specifically reflected in improved levels of high-density lipoprotein and fasting blood glucose. Additionally, significant alterations in gut microbiota composition were observed in patients with T2DM, indicating a potential synergistic effect between gut health and blood glucose regulation.
Core Tip: Lactobacillus rhamnosus LRa05 is a probiotic that has shown potential in managing type 2 diabetes by improving glycemic control and gut microbiota composition. In a study involving 76 participants over 12 weeks, those who consumed LRa05 experienced significant reductions in fasting blood glucose levels and improved lipid profiles, particularly an increase in high-density lipoprotein cholesterol. The probiotic also positively influenced gut health by promoting beneficial bacteria such as Bifidobacterium while reducing potentially harmful Bacillota levels. Although there were no notable changes in insulin sensitivity or inflammation markers, the overall findings suggest that LRa05 plays a crucial role in diabetes ma
- Citation: Geng L, Sun TT, Xia WB, Qin Y, Huo D, Qu GJ. Lactobacillus rhamnosus LRa05 on glycemic control and gut microbiota in patients with type 2 diabetes. World J Diabetes 2025; 16(7): 106821
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/106821.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.106821
Type 2 diabetes mellitus (T2DM) represents a chronic condition that affects the body with the inability to handle glucose, primarily due to insulin resistance. This leads to persistently high blood sugar levels with a disruption of fat metabolism[1]. It has become a significant global health challenge, with its incidence increasing due to rising obesity rates, sedentary lifestyles, and unhealthy dietary habits[2]. The consequences of this disease can be severe, contributing to complications like heart disease, kidney damage, neuropathy, and visual problems, all of which significantly impact quality of life and increase mortality rates[3]. Recent research has begun to uncover the fascinating role of gut microbiota in the de
The gut microbiota, comprising trillions of microorganisms, plays an essential role in host metabolism, immune regulation, and inflammation[7]. Dysbiosis, a disruption in the gut microbial community, is commonly observed in patients with T2DM, often characterized by reduced microbial diversity and an increased prevalence of pathogenic species[8]. Studies suggest that gut microbiota influences glucose metabolism through mechanisms such as short-chain fatty acid (SCFA) production, modulation of gut barrier integrity, and regulation of inflammatory pathways[9]. This growing body of evidence has led to an increased interest in probiotics as a therapeutic strategy for improving metabolic health in T2DM.
Probiotics are live microorganisms that confer health benefits when administered in adequate amounts[10]. Certain probiotic strains have demonstrated promising effects in enhancing glucose metabolism, improving insulin sensitivity, and modulating inflammatory responses[11]. Among these, Lactobacillus rhamnosus has emerged as a potential candidate due to its ability to strengthen gut barrier function, regulate immune responses, and produce bioactive metabolites beneficial for metabolic health[12]. Specifically, Lactobacillus rhamnosus LRa05 has been investigated for its metabolic benefits; however, previous research has primarily focused on isolated metabolic parameters[13,14], leaving a gap in understanding its comprehensive impact on both glycemic control and gut microbiota composition in individuals with T2DM.
This study aimed to explore how Lactobacillus rhamnosus LRa05 supplementation affects glycemic control and gut microbiota composition in individuals with T2DM. We hypothesized that adding LRa05 to standard blood sugar-lowering treatments will lead to significant improvements in key metabolic markers, such as glycated hemoglobin (HbA1c) and fasting blood glucose (FBG), while also positively influencing gut microbiota diversity. To test this hy
This study was designed as a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of Lactobacillus rhamnosus LRa05 in improving glycemic control and modulating gut microbiota among patients diagnosed with T2DM. The trial was registered at Chinese Clinical Trial Registry (https://www.chictr.org.cn/bin/home), number Chi
The study protocol received approval from the ethics committee of the First Affiliated Hospital of Harbin Medical University. All participants provided written informed consent, ensuring confidentiality and data protection throughout the study.
A sample size calculation was performed using G Power software (version 3.1.9.7) to determine the required number of participants, ensuring an 80% power to detect a clinically significant reduction of 0.5% in HbA1c levels with a two-sided significance level of 0.05, accounting for an estimated 10% dropout rate[15].
In this study participants were recruited from the outpatient clinic at the First Affiliated Hospital of Harbin Medical University. Prior to enrollment informed consent was obtained from all participants. The eligible participants included adults aged 25 to 65 years who had been diagnosed with T2DM in accordance with the World Health Organization criteria established in 2019. Additional eligibility requirements included HbA1c levels ranging between 6.5%-10.0% and FBG levels between 7.0-13.3 mmol/L. Participants were required to be on stable doses of antidiabetic medications, including metformin, sulfonylureas, or insulin, for at least 3 months prior to study initiation. Exclusion criteria included a history of type 1 diabetes or other significant chronic diseases such as cardiovascular or kidney disease, recent use of antibiotics, probiotics, or prebiotics within the prior 3 months, concurrent participation in other clinical trials, and pregnancy or lactation. These exclusion criteria were implemented to minimize potential confounding factors that could influence study outcomes. Participants were monitored for potential adverse events throughout the study, with safety assessments conducted at each follow-up visit. Any adverse effects related to the intervention were documented and reported to the ethics committee.
Participants were randomly assigned to either the intervention group receiving Lactobacillus rhamnosus LRa05 or the placebo group. The randomization sequence was generated using computer-generated sequence (Randomization.com) with a 1:1 allocation ratio, and allocation concealment was achieved by using sequentially numbered, opaque, sealed envelopes. Both participants and researchers remained blinded to group assignments throughout the study to eliminate bias and ensure objective assessment of outcomes.
Both participants and research personnel (including clinicians, laboratory staff, and statisticians) were blinded to treatment allocation throughout the study. The probiotic and placebo capsules were identical in appearance, texture, and taste, with blinding verified through sensory testing by an independent panel before study initiation.
Participants in the experimental group received a daily oral dose of Lactobacillus rhamnosus LRa05 powder (0.1 g 2 × 1010 CFU LRa05 powder + 2.9 g maltodextrin)/day, (Weikang Probiotics Co., Ltd., Production number: SC10632050900407), while the control group received a placebo identical in appearance and taste. The intervention lasted for 12 weeks, during which participants were instructed to maintain their usual dietary habits and physical activity levels to reduce confounding variables. Probiotic and placebo capsules were stored in a temperature-controlled environment (4 °C) to maintain viability and stability throughout the study period.
Primary outcome: The primary outcome was HbA1c and blood glucose, measured at baseline and weeks 6 and 12 and HbA1c tested using high-performance liquid chromatography to assess long-term glycemic control. Blood samples were collected at the clinic, with whole blood drawn and processed by centrifugation at 1500 × g for 25 min at 4 °C. After centrifugation the serum was separated, aliquoted into 1.5-mL tubes, and stored at -80 °C until analysis. To ensure consistency, frozen serum samples were batch-sent to Quest Diagnostics Laboratories for lipid panel measurements. Samples were analyzed in pairs, ensuring that corresponding participant samples from both intervention and placebo groups were examined together. All laboratory staff were blinded to treatment assignments to eliminate analytical bias.
Secondary outcomes: Secondary outcomes included a comprehensive lipid panel, assessing total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides using fasting serum samples. Biochemical analyses were performed at Quest Diagnostics utilizing spectrophotometry with the Beckman Olympus AU5400 Chemistry Analyzer. Non-HDL cholesterol was calculated by subtracting HDL-C from TC concentrations, while low-density lipoprotein cholesterol was determined using the Friedewald equation for participants with triglyceride levels below 400 mg/dL. Inter-assay coefficients of variation (CVs) were established for TC (1.4%), HDL-C (2.6%), and triglycerides (2.1%).
FBG levels were assessed using an enzymatic colorimetric assay, with intra-assay and inter-assay CVs of 1.5% and 2.7%, respectively. Serum insulin concentrations were measured using an ELISA kit (Cobas Integra 800 Autoanalyzer, Roche Diagnostics, Germany), with intra-assay and inter-assay CVs of 1.9% and 2.6%, respectively. Insulin resistance and beta cell function were evaluated using homeostasis model assessment indices (HOMA-IR). High-sensitivity C-reactive protein (CRP) was quantified via ELISA (LDN, Nordhorn, Germany), with intra-assay and inter-assay CVs of 2.6% and 4.5%, respectively. HbA1c was measured using reagents sourced from Biosystems S.A., with intra-assay and inter-assay CVs of 5.9% and 6.6%, respectively. Additional biochemical parameters, including uric acid and CRP, were measured at baseline and 12 weeks using standard biochemical methods.
Hematological assessments included WBC count, neutrophils (NEUT), lymphocytes, monocytes, RBC count, hemoglobin, and platelet count. Inflammatory indices, including the systemic inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio, were calculated to assess immune response trends. Biochemical assessments en
Body weight, height, and waist and hip circumferences were measured at baseline and post-intervention by a trained dietitian at the Endocrine Research Center. Standardized conditions were followed, with body weight recorded using a calibrated digital scale with 0.1 kg accuracy. A non-stretch tape measure was used to assess height and circumferences to the nearest 0.1 cm, ensuring minimal measurement variability.
Participants were instructed to maintain their usual diet and physical activity levels. Dietary intake was assessed at baseline and weeks 6 and 12 using a validated food frequency questionnaire and 24-h dietary recalls. Physical activity was monitored using the international physical activity questionnaire to detect significant deviations from baseline activity levels.
A total of two fecal samples were collected from participants before and after the intervention for gut microbiome analysis. Fecal samples were obtained using sterile, anaerobic collection kits, with participants following detailed instructions. The samples were transported in insulated, temperature-controlled containers and stored at -80 °C within 2 h of collection until DNA extraction. Genomic DNA was extracted using the CTAB/SDS method along with the QIAamp Fast DNA Fecal Mini Kit (Qiagen, Valencia, California, United States), following the manufacturer’s instructions. The targeted bacterial 16S rRNA gene V3-V4 region was amplified using the TransGen AP221-02 Kit (TransGen, Beijing, China). The library was sequenced on an Illumina NovaSeq platform, generating 250 bp paired-end reads.
Upon acquiring the raw reads, double-ended reads were separated into tags, which were then filtered to produce clean tags, eliminating any low-quality or biologically irrelevant data. Clustering was performed using the clean tags, and chimeric tags were removed to obtain the effective tags. Operational taxonomic units were generated from the effective tags, and operational taxonomic unit abundance data were calculated. Alpha diversity was assessed using Shannon, Simpson, Chao1, and ACE indices to measure species richness and evenness. Beta diversity was analyzed through principal coordinate analysis based on Bray-Curtis distance to compare microbiota composition between groups. Differential enrichment of the gut microbiome was evaluated using linear discriminant analysis effect size[16].
Statistical analyses were conducted using R software (version 4.2.0). Missing data were handled using multiple im
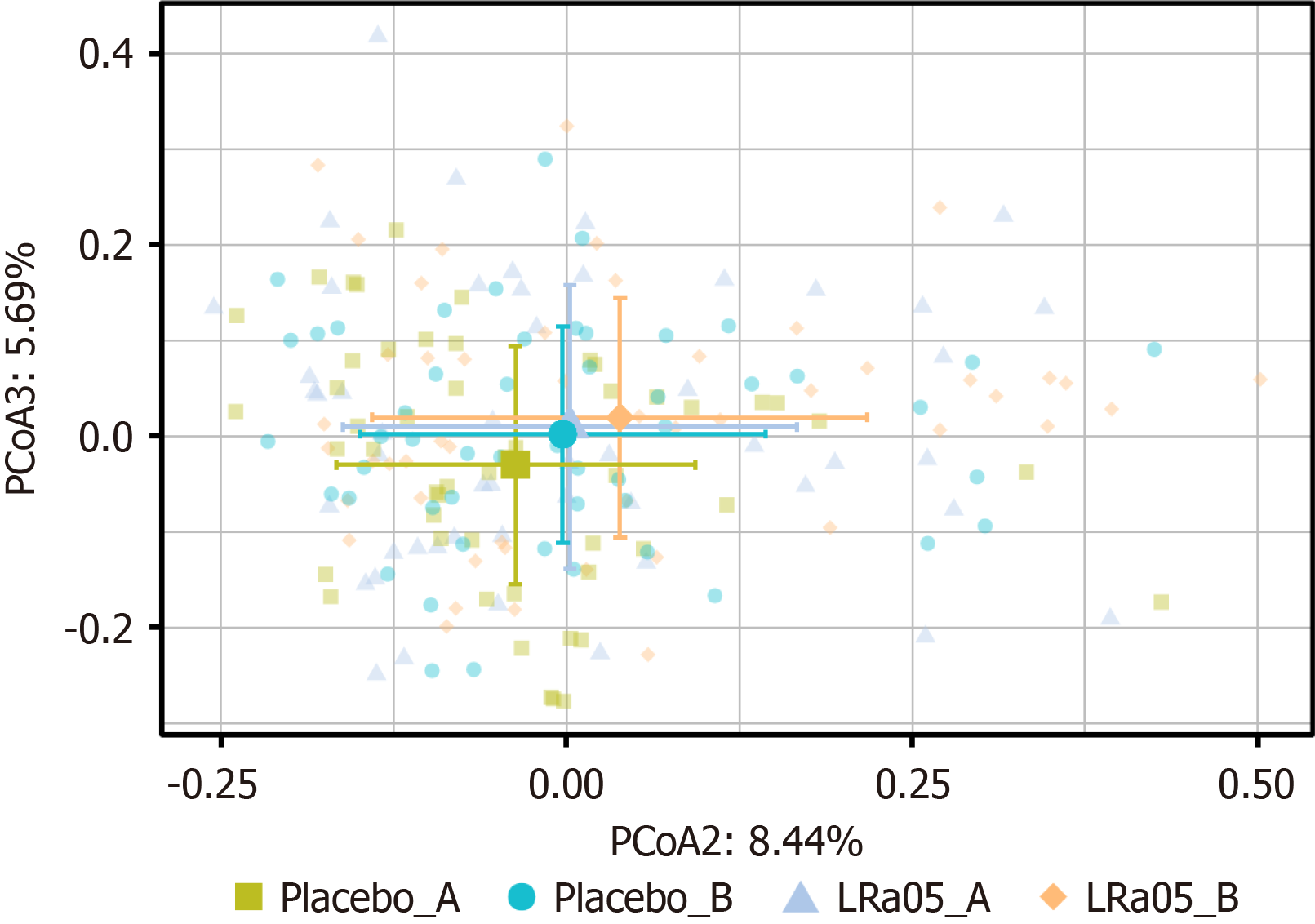
Table 1 summarizes the demographic and clinical characteristics of participants in both the placebo and intervention groups. The average age of participants in the intervention group (59.55 years) was slightly higher than that of the placebo group (54.68 years), with a P value of 0.0549, indicating a trend towards significance. Gender distribution was comparable between groups (P = 0.3651), suggesting no significant differences in sex composition. Body mass index and weight were similar across both groups, with P values of 0.6920 and 0.2964, respectively. Notably, the intervention group had a longer duration of diabetes (10.16 years) compared with the placebo group (7.18 years), with a statistically significant difference (P = 0.0255). Smoking status showed a trend towards significance, with a higher percentage of smokers in the placebo group (31.58%) compared with the intervention group (13.16%, P = 0.0540). Overall, these findings indicated that the groups were relatively well-matched, although some demographic differences existed that may warrant consideration in the analysis (Figure 1).
| Variables | Placebo group (n = 38) | Intervention group (n = 38) | P value |
| Age (years) | 54.68 (37.00-70.00) | 59.55 (35.00-75.00) | 0.054 |
| Gender | 0.365 | ||
| Male | 23 (60.5) | 19 (50.0) | |
| Female | 15 (39.5) | 19 (50.0) | |
| BMI, kg/m2 | 25.35 (2.9) | 25.61 (2.9) | 0.692 |
| Weight, kg | 78.10 (58.50-105.00) | 72.32 (55.00-102.50) | 0.296 |
| Smoking status | 12 (31.6) | 5 (13.2) | 0.054 |
| Drink Wine | 1 (2.6) | 6 (15.8) | 0.107 |
| Hypertension | 14 (36.8) | 17 (44.7) | 0.483 |
| Coronary heart disease | 3 (7.9) | 6 (15.7) | 0.286 |
| Diabetes duration (years) | 7.18 (1.00-20.00) | 10.16 (1.00-24.00) | 0.025 |
| Metformin use | 32 (84.0) | 31 (84.0) | 0.956 |
| Sulfonylureas use | 12 (31.0) | 15 (39.0) | 0.156 |
| Insulin use | 4 (10.5) | 5 (13.0) | 0.256 |
| Self-reported physical activity, MET (hours/week) | 46.71 ± 6.3 | 50.07 ± 6.1 | 0.332 |
Table 2 illustrates the blood indices and inflammatory markers at various time points throughout the study. The results indicated no significant changes in WBC counts or NEUT levels across groups over time, suggesting stable immune responses during the study period. This stability is important as it indicates that the intervention did not disrupt immune function. However, RBC counts showed a significant difference at baseline (P < 0.0001), indicating a potential baseline imbalance that should be considered when interpreting subsequent results. Hemoglobin levels also exhibited a notable difference between groups at baseline (P = 0.0094), but no significant changes were observed over time, suggesting that while initial differences existed, the intervention did not lead to further alterations. The systemic inflammation index and other inflammatory markers did not show significant fluctuations, suggesting that the intervention may not have had a pronounced impact on systemic inflammation, which is a critical factor in chronic disease management.
| Index | Placebo group (n = 38) | Intervention group (n = 38) | Time (P value)1 | Time group (P value)2 | T2 vs T0 (P value)3 | T3 vs T0 (P value)4 | ||
| mean ± SD | Median (Q1-Q3) | mean ± SD | Median (Q1-Q3) | |||||
| WBC | ||||||||
| Baseline | 6.86 ± 2.04 | 6.39 (5.41-8.12) | 6.52 ± 1.44 | 6.42 (5.66-7.02) | 0.767 | 0.840 | 0.810 | 0.762 |
| T2 | 6.71 ± 1.69 | 6.46 (5.43-7.99) | 6.44 ± 1.76 | 6.33 (5.01-7.29) | ||||
| T3 | 6.85 ± 1.87 | 6.51 (5.69-8.18) | 6.41 ± 1.53 | 6.34 (5.07-7.62) | ||||
| NEUT | ||||||||
| Baseline | 3.87 ± 1.42 | 3.40 (2.99-4.88) | 3.73 ± 1.18 | 3.51 (3.13-4.11) | 0.846 | 0.759 | 0.848 | 0.434 |
| T2 | 3.83 ± 1.16 | 3.65 (2.89-4.36) | 3.63 ± 1.46 | 3.59 (2.81-4.03) | ||||
| T3 | 3.93 ± 1.22 | 3.63 (3.28-4.54) | 3.60 ± 1.09 | 3.59 (2.70-4.25) | ||||
| LYMPH | ||||||||
| Baseline | 2.29 ± 0.76 | 2.30 (1.85-2.54) | 2.09 ± 0.60 | 2.11 (1.60-2.44) | 0.537 | 0.537 | 0.399 | 0.288 |
| T2 | 2.20 ± 0.81 | 2.11 (1.61-2.53) | 2.08 ± 0.7 | 2.03 (1.61-2.52) | ||||
| T3 | 2.20 ± 0.73 | 2.14 (1.77-2.43) | 2.10 ± 0.72 | 2.06 (1.49-2.40) | ||||
| MONO | ||||||||
| Baseline | 0.48 ± 0.16 | 0.46 (0.37-0.55] | 0.48 ± 0.15 | 0.48 (0.37-0.57) | 0.249 | 0.471 | 0.696 | 0.463 |
| T2 | 0.46 ± 0.12 | 0.47 (0.36-0.53) | 0.46 ± 0.15 | 0.45 (0.35-0.54) | ||||
| T3 | 0.49 ± 0.13 | 0.47 (0.39-0.57) | 0.46 ± 0.13 | 0.46 (0.39-0.59) | ||||
| Eosinophil | ||||||||
| Baseline | 0.15 ± 0.13 | 0.13 (0.08-0.17) | 0.17 ± 0.15 | 0.14 (0.09-0.21) | 0.120 | 0.691 | 0.350 | 0.607 |
| T2 | 0.15 ± 0.10 | 0.12 (0.08-0.20) | 0.19 ± 0.13 | 0.18 (0.08-0.24) | ||||
| T3 | 0.17 ± 0.12 | 0.13 (0.09-0.22) | 0.20 ± 0.19 | 0.15 (0.10-0.23) | ||||
| BASS | ||||||||
| Baseline | 0.05 ± 0.06 | 0.04 (0.03-0.06) | 0.03 ± 0.01 | 0.03 (0.02-0.04) | 0.413 | 0.253 | 0.224 | 0.258 |
| T2 | 0.04 ± 0.02 | 0.04 (0.02-0.05) | 0.04 ± 0.01 | 0.03 (0.03-0.04) | ||||
| T3 | 0.04 ± 0.02 | 0.04 (0.03-0.06) | 0.04 ± 0.02 | 0.03 (0.02-0.05) | ||||
| RBC | ||||||||
| Baseline | 5.09 ± 0.38 | 5.11 (4.90-5.35) | 5.09 ± 0.54 | 5.17 (4.86-5.45) | < 0.0001 | 0.140 | 0.145 | 0.763 |
| T2 | 4.97 ± 0.40 | 5.01 (4.71-5.26) | 5.05 ± 0.56 | 5.03 (4.76-5.39) | ||||
| T3 | 4.96 ± 0.39 | 4.92 (4.76-5.25) | 4.96 ± 0.54 | 5.04 (4.70-5.34) | ||||
| HGB | ||||||||
| Baseline | 155.47 ± 11.93 | 155.50 (147.00-164.00) | 150.47 ± 15.98 | 152.00 (139.00-162.00) | 0.009 | 0.229 | 0.152 | 0.986 |
| T2 | 152.92 ± 13.12 | 153.50 (142.00-160.00) | 150.37 ± 15.67 | 150.00 (139.00-164.00) | ||||
| T3 | 152.97 ± 11.67 | 152.00 (145.00-161.00) | 147.95 ± 15.78 | 150.00 (138.00-158.00) | ||||
| PLT | ||||||||
| Baseline | 226.68 ± 74.70 | 229.50 (168.00-269.00) | 225.55 ± 53.94 | 221.00 (197.00-253.00) | 0.983 | 0.658 | 0.634 | 0.360 |
| T2 | 224.97 ± 73.63 | 229.50 (169.00-267.00) | 226.34 ± 46.32 | 220.00 (202.00-250.00) | ||||
| T3 | 223.58 ± 68.32 | 234.00 (179.00-260.00) | 227.79 ± 52.87 | 218.00 (199.00-240.00) | ||||
| SII | ||||||||
| Baseline | 401.27 ± 177.04 | 356.88 (305.20-524.20) | 453.75 ± 301.90 | 379.10 (296.10-500.40) | 0.866 | 0.766 | 0.642 | 0.4350 |
| T2 | 424.75 ± 206.19 | 413.00 (237.20-519.03) | 451.33 ± 346.54 | 385.80 (288.10-460.60) | ||||
| T3 | 418.58 ± 174.58 | 405.50 (295.00-528.50) | 443.45 ± 319.82 | 367.70 (286.60-521.70) | ||||
| NLR | ||||||||
| Baseline | 1.77 ± 0.61 | 1.67 (1.35-2.23) | 1.94 ± 0.87 | 1.64 (1.40-2.25) | 0.773 | 0.735 | 0.622 | 0.378 |
| T2 | 1.90 ± 0.75 | 1.81 (1.31-2.46) | 1.95 ± 1.34 | 1.74 (1.29-2.11) | ||||
| T3 | 1.88 ± 0.58 | 1.86 (1.47-2.32) | 1.91 ± 1.00 | 1.69 (1.42-2.22) | ||||
| PLR | ||||||||
| Baseline | 102.87 ± 32.01 | 106.70 (84.06-121.73) | 117.05 ± 42.28 | 111.15 (91.70-144.44) | 0.245 | 0.939 | 0.734 | 0.960 |
| T2 | 108.27 ± 35.13 | 110.00 (77.70-141.90) | 120.48 ± 42.92 | 114.70 (93.30-145.20) | ||||
| T3 | 106.75 ± 33.37 | 108.60 (90.30-127.20) | 120.6 ± 48.47 | 113.50 (85.00-139.80) | ||||
| MLR | ||||||||
| Baseline | 0.22 ± 0.08 | 0.20 (0.17-0.26) | 0.25 ± 0.11 | 0.22 (0.18-0.28) | 0.977 | 0.314 | 0.869 | 0.132 |
| T2 | 0.22 ± 0.07 | 0.21 (0.17-0.26) | 0.25 ± 0.13 | 0.22 (0.17-0.29) | ||||
| T3 | 0.23 ± 0.07 | 0.22 (0.18-0.28) | 0.24 ± 0.09 | 0.23 (0.18-0.28) | ||||
| SIRI | ||||||||
| Baseline | 0.88 ± 0.51 | 0.79 (0.62-1.01) | 0.99 ± 0.78 | 0.84 (0.55-1.06) | ||||
| T2 | 0.88 ± 0.46 | 0.81 (0.56-1.13) | 0.99 ± 1.04 | 0.74 (0.48-1.06) | 0.865 | 0.494 | 0.979 | 0.189 |
| T3 | 0.92 ± 0.44 | 0.83 (0.65-0.95) | 0.89 ± 0.54 | 0.72 (0.55-1.08) | ||||
| NPR | ||||||||
| Baseline | 0.02 ± 0.01 | 0.02 (0.01-0.02) | 0.02 ± 0 | 0.02 (0.01-0.02) | 0.844 | 0.737 | 0.724 | 0.434 |
| T2 | 0.02 ± 0.01 | 0.02 (0.01-0.02) | 0.02 ± 0.01 | 0.02 (0.01-0.02) | ||||
| T3 | 0.02 ± 0.01 | 0.02 (0.01-0.02) | 0.02 ± 0.01 | 0.02 (0.01-0.02) | ||||
| PAR | ||||||||
| Baseline | 5.00 ± 1.63 | 5.24 (3.38-6.09) | 5.10 ± 1.28 | 4.98 (4.30-5.75) | 0.299 | 0.523 | 0.897 | 0.365 |
| T2 | 5.06 ± 1.63 | 5.02 (3.70-6.44) | 5.15 ± 1.10 | 5.09 (4.52-5.63) | ||||
| T3 | 5.05 ± 1.59 | 5.20 (3.76-6.21) | 5.27 ± 1.41 | 5.01 (4.52-5.56) | ||||
| CAR | ||||||||
| Baseline | 0.09 ± 0.05 | 0.06 (0.05-0.11) | 0.08 ± 0.08 | 0.06 (0.04-0.09) | 0.662 | 0.273 | 0.272 | 0.699 |
| T2 | 0.11 ± 0.19 | 0.07 (0.05-0.12) | 0.08 ± 0.04 | 0.07 (0.05-0.09) | ||||
| T3 | 0.09 ± 0.06 | 0.07 (0.04-0.10) | 0.09 ± 0.10 | 0.06 (0.04-0.10) | ||||
| CLR | ||||||||
| Baseline | 1.76 ± 1.02 | 1.47 (0.91-2.19) | 2.05 ± 2.18 | 1.13 (0.84-2.43) | 0.717 | 0.273 | 0.254 | 0.725 |
| T2 | 2.44 ± 4.03 | 1.46 (1.01-2.57) | 1.87 ± 1.48 | 1.35 (1.00-2.31) | ||||
| T3 | 1.79 ± 1.28 | 1.52 (0.98-2.22) | 2.26 ± 2.98 | 1.26 (0.89-2.07) | ||||
| TyG | ||||||||
| Baseline | 1.95 ± 0.74 | 1.84 (1.56-2.27) | 1.90 ± 0.64 | 1.79 (1.41-2.29) | 0.834 | 0.374 | 0.971 | 0.232 |
| T2 | 1.98 ± 0.83 | 2.00 (1.44-2.45) | 1.93 ± 0.57 | 1.96 (1.51-2.25) | ||||
| T3 | 2.03 ± 0.85 | 1.93 (1.39-2.62) | 1.83 ± 0.61 | 1.80 (1.47-2.19) | ||||
Table 3 provides a comprehensive overview of biochemical parameters, kidney function, and liver enzyme markers throughout the study. Notably, serum creatinine levels significantly differed between groups at baseline (P < 0.0001), suggesting a need for careful interpretation of kidney function results as this could indicate preexisting conditions that might affect outcomes. Urea and uric acid levels remained stable across the study duration, with no significant dif
| Index | Placebo group (n = 38) | Intervention group (n = 38) | Placebo group | Intervention group | T2 vs T0 (P value) | T3 vs T0 (P value) | ||
| mean ± SD | Median (Q1-Q3) | mean ± SD | Median (Q1-Q3) | |||||
| UREA | ||||||||
| Baseline | 5.77 ± 1.36 | 5.79 (4.67-6.6) | 5.76 ± 1.43 | 5.6 (4.81-6.42) | 0.317 | 0.761 | 0.614 | 0.461 |
| T2 | 5.78 ± 1.42 | 5.77 (4.85-6.77) | 5.93 ± 1.69 | 5.53 (4.89-6.9) | ||||
| T3 | 5.54 ± 1.54 | 5.48 (4.19-6.5) | 5.76 ± 1.68 | 5.42 (4.68-6.81) | ||||
| Cr | ||||||||
| Baseline | 63.14 ± 14.03 | 63.55 (53-74.7) | 62.33 ± 15.36 | 61.5 (51.7-69.8) | < 0.0001 | 0.445 | 0.202 | 0.706 |
| T2 | 67.03 ± 16.09 | 66.95 (56-77.7) | 64.41 ± 15.62 | 65.85 (54.1-72.4) | ||||
| T3 | 67.56 ± 15.11 | 66 (56.9-75.6) | 66.26 ± 15.16 | 65.6 (56.1-72.7) | ||||
| SG | ||||||||
| Baseline | 1.02 ± 0.01 | 1.02 (1.02-1.03) | 1.02 ± 0.01 | 1.02 (1.02-1.03) | 0.424 | 0.087 | 0.111 | 0.030 |
| T2 | 1.02 ± 0.01 | 1.02 (1.02-1.03) | 1.02 ± 0.01 | 1.02 (1.01-1.03) | ||||
| T3 | 1.03 ± 0.01 | 1.03 (1.02-1.03) | 1.02 ± 0.01 | 1.02 (1.01-1.03) | ||||
| Uric acid | ||||||||
| Baseline | 349.98 ± 82.4 | 347.75 (303.4-417.9) | 346.88 ± 71.75 | 347.3 (305.6-385.5) | 0.582 | 0.937 | 0.718 | 0.887 |
| T2 | 348.14 ± 71.62 | 344.7 (294.8-397.5) | 340.76 ± 66.08 | 344.7 (302-377.1) | ||||
| T3 | 354.41 ± 78.16 | 357.2 (302.9-414.5) | 349.36 ± 77.77 | 365.35 (280.2-406.5) | ||||
| Na | ||||||||
| Baseline | 140.49 ± 2.1 | 140.45 (138.9-142.3) | 141.01 ± 1.94 | 140.8 (139.6-142.1) | < 0.0001 | 0.007 | 0.005 | 0.840 |
| T2 | 140.13 ± 2.15 | 140.3 (138.9-141.9) | 139.34 ± 1.74 | 139.55 (138.3-140.4) | ||||
| T3 | 139.28 ± 2.63 | 139.55 (137.6-141) | 139.7 ± 1.78 | 139.8 (138.2-140.7) | ||||
| K | ||||||||
| Baseline | 4.34 ± 0.32 | 4.29 (4.14-4.49) | 4.25 ± 0.28 | 4.3 (4.04-4.4) | 0.248 | 0.126 | 0.851 | 0.048 |
| T2 | 4.34 ± 0.33 | 4.27 (4.12-4.51) | 4.26 ± 0.36 | 4.26 (4.01-4.44) | ||||
| T3 | 4.21 ± 0.26 | 4.2 (4.05-4.31) | 4.27 ± 0.36 | 4.21 (4.06-4.45) | ||||
| Cl | ||||||||
| Baseline | 104.64 ± 2.59 | 104.5 (103-106.2) | 104.64 ± 2.34 | 104.8 (102.9-105.9) | 0.002 | 0.295 | 0.216 | 0.988 |
| T2 | 104.31 ± 2.07 | 104.55 (102.7-105.5) | 103.65 ± 2.06 | 103.5 (102.6-104.6) | ||||
| T3 | 103.55 ± 2.74 | 104.2 (101.6-105.8) | 103.54 ± 2.57 | 103.55 (101.2-105.6) | ||||
| ALT | ||||||||
| Baseline | 28.5 ± 22.64 | 21.15 (15.9-33.2) | 25.32 ± 15.92 | 19.85 (16.3-28.9) | 0.610 | 0.481 | 0.473 | 0.273 |
| T2 | 28.32 ± 23.09 | 20.55 (17.3-31.2) | 27.67 ± 17.39 | 22.25 (17.3-33.2) | ||||
| T3 | 24.87 ± 11.6 | 22.25 (14.9-32.9) | 26.91 ± 21.95 | 21 (15.4-29.2) | ||||
| AST | ||||||||
| Baseline | 26.3 ± 14.98 | 21 (17.3-29.9) | 23.04 ± 8.84 | 20.35 (18.2-24.5) | 0.609 | 0.127 | 0.545 | 0.057 |
| T2 | 26.91 ± 18.3 | 20.5 (18.8-29.1) | 25.13 ± 8.54 | 22.8 (19.6-26.7) | ||||
| T3 | 24.4 ± 11.42 | 20.45 (17.6-28.1) | 27.29 ± 17.52 | 22.15 (18.2-29.4) | ||||
| ALB | ||||||||
| Baseline | 45.38 ± 3.28 | 45.5 (43.7-47.5) | 44.41 ± 2.59 | 44.4 (43-45.7) | 0.001 | 0.364 | 0.199 | 0.988 |
| T2 | 44.38 ± 2.6 | 44.25 (42.7-46.2) | 44.09 ± 2.44 | 43.6 (42.3-45.5) | ||||
| T3 | 44.44 ± 3.1 | 44.95 (42.4-46.6) | 43.46 ± 2.42 | 43.35 (42.3-44.5) | ||||
| TBIL | ||||||||
| Baseline | 16.97 ± 6.72 | 15.6 (12.72-18.62) | 15.84 ± 5.25 | 15.01 (12.25-17.85) | 0.197 | 0.614 | 0.324 | 0.806 |
| T2 | 15.84 ± 7.29 | 14 (11.3-18.18) | 15.66 ± 4.87 | 14.83 (12.26-18.15) | ||||
| T3 | 16.15 ± 8.76 | 13.73 (11.24-16.52) | 15.23 ± 4.91 | 13.96 (11.73-16.8) | ||||
| DBIL | ||||||||
| Baseline | 2.96 ± 1.36 | 2.62 (2.13-3.3) | 2.68 ± 0.86 | 2.5 (2.1-3.1) | 0.114 | 0.274 | 0.108 | 0.599 |
| T2 | 2.66 ± 1.17 | 2.5 (1.88-2.9) | 2.66 ± 0.92 | 2.4 (2.1-3.1) | ||||
| T3 | 2.77 ± 1.34 | 2.4 (1.96-3) | 2.57 ± 0.81 | 2.25 (1.96-3.2) | ||||
| IBIL | ||||||||
| Baseline | 14.01 ± 5.67 | 13.17 (10.76-15.55) | 13.16 ± 4.53 | 12.29 (10.11-15.05) | 0.269 | 0.710 | 0.412 | 0.866 |
| T2 | 13.17 ± 6.29 | 11.77 (9.5-15.46) | 13 ± 4.05 | 12.32 (10.02-15.57) | ||||
| T3 | 13.38 ± 7.66 | 11.3 (9.44-14.09) | 12.66 ± 4.24 | 11.26 (9.72-14.05) | ||||
Table 4 revealed significant findings regarding lipid profiles and insulin sensitivity measurements over time in both the placebo and intervention groups. Cholesterol levels showed a notable decrease over time, particularly in the intervention group, with a significant P value of 0.0164 indicating a time effect, although no significant interaction between time and group was observed (P = 0.7658). Triglyceride levels remained stable across both groups, suggesting no significant changes due to the intervention. In contrast, HDL-C levels improved significantly in the intervention group at all time points (P < 0.0001), highlighting the beneficial effects of the intervention. Low-density lipoprotein cholesterol also decreased significantly in the intervention group (P = 0.0022), indicating an improvement in lipid profiles.
| Index | Placebo group (n = 38) | Intervention group (n = 38) | Time (P value)1 | Time group (P value)2 | T2 vs T0 (P value) | T3 vs T0 (P value) | ||
| mean ± SD | Median (Q1-Q3) | mean ± SD | Median (Q1-Q3) | |||||
| CHOL | ||||||||
| Baseline | 5.17 ± 1.16 | 5.06 (4.48-6.04) | 5.16 ± 1.2 | 5.3 (4.26-5.93) | 0.016 | 0.765 | 0.797 | 0.656 |
| T2 | 4.92 ± 1.22 | 4.75 (4.12-5.67) | 4.85 ± 1.07 | 4.53 (4.2-5.37) | ||||
| T3 | 4.79 ± 1.09 | 4.74 (4.34-5.63) | 4.89 ± 0.88 | 4.89 (4.32-5.37) | ||||
| TG | ||||||||
| Baseline | 2.02 ± 1.95 | 1.51 (1.14-2.09) | 1.91 ± 1.31 | 1.33 (1.12-2.27) | 0.910 | 0.175 | 0.226 | 0.061 |
| T2 | 2.19 ± 2.36 | 1.63 (1.04-2.17) | 1.73 ± 0.96 | 1.48 (1.11-1.98) | ||||
| T3 | 2.31 ± 2.5 | 1.45 (1.1-2.37) | 1.71 ± 0.85 | 1.58 (0.97-2.06) | ||||
| HDL-C | ||||||||
| Baseline | 1.31 ± 0.32 | 1.30 (1.06-1.45) | 1.24 ± 0.19 | 1.23 (1.08-1.36) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| T2 | 1.27 ± 0.30 | 1.22 (1.07-1.47) | 1.36 ± 0.22 | 1.33 (1.21-1.55) | ||||
| T3 | 1.28 ± 0.28 | 1.27 (1.11-1.47) | 1.47 ± 0.24 | 1.46 (1.26-1.55) | ||||
| LDL-C | ||||||||
| Baseline | 3.3 ± 0.85 | 3.2 (2.86-3.98) | 3.35 ± 1 | 3.62 (2.66-3.89) | 0.002 | 0.806 | 0.816 | 0.517 |
| T2 | 3.07 ± 0.88 | 3.06 (2.59-3.72) | 3.17 ± 0.88 | 2.84 (2.62-3.69) | ||||
| T3 | 2.92 ± 0.85 | 2.84 (2.55-3.51) | 3.09 ± 0.72 | 3.13 (2.63-3.47) | ||||
| INS | ||||||||
| Baseline | 30.08 ± 51.6 | 11.7 (8-23.72) | 16.23 ± 18.4 | 10.98 (8.6-14.66) | 0.051 | 0.194 | 0.181 | 0.181 |
| T2 | 19.56 ± 23.26 | 10.4 (8.3-18.72) | 14.4 ± 15.39 | 10.12 (7.8-14.33) | ||||
| T3 | 19.59 ± 24.43 | 11.5 (8.3-18.16) | 14.12 ± 14.77 | 11 (8.7-13.75) | ||||
| CRP | ||||||||
| Baseline | 3.86 ± 2.24 | 3.06 (2.23-4.82) | 3.79 ± 2.1 0 | 3.03 (2.35-3.97) | 0.087 | 0.119 | 0.290 | 0.054 |
| T2 | 3.90 ± 2.70 | 3.19 (2.22-5.06) | 3.37 ± 1.88 | 2.94 (2.03-4.13) | ||||
| T3 | 3.80 ± 2.65 | 3.07 (1.95-4.42) | 2.78 ± 3.02 | 1.75 (1.17-3.19) | ||||
| HOMA-IR | ||||||||
| Baseline | 12.57 ± 21.45 | 4.53 (2.75-10.24) | 6.25 ± 7.38 | 3.93 (3.01-6.51) | 0.376 | 0.440 | 0.238 | 0.503 |
| T2 | 9.41 ± 14.74 | 4.51 (2.73-6.29) | 6 ± 6.26 | 3.79 (3.09-5.88) | ||||
| T3 | 9.82 ± 19.03 | 5.15 (2.87-7.8) | 5.39 ± 5.15 | 3.88 (2.77-5.79) | ||||
| FBG | ||||||||
| Baseline | 9.20 ± 2.26 | 8.81 (7.50-10.00) | 9.06 ± 1.38 | 9.06 (7.78-10.18) | < 0.0001 | < 0.0001 | 0.036 | 0.007 |
| T2 | 9.17 ± 2.52 | 8.71 (7.03-9.97) | 8.04 ± 1.91 | 7.83 (6.7-9.03) | ||||
| T3 | 9.20 ± 2.56 | 8.47 (7.41-10.29) | 7.34 ± 1.8 | 7.09 (6.04-8.29) | ||||
| HbA1c | ||||||||
| Baseline | 7.95 ± 1.48 | 7.40 (6.80-9.00) | 7.78 ± 1.44 | 7.60 (6.40-8.50) | 0.031 | 0.901 | 0.980 | 0.870 |
| T2 | 7.85 ± 1.47 | 7.65 (6.70-8.80) | 7.69 ± 1.09 | 7.75 (6.60-8.30) | ||||
| T3 | 7.64 ± 1.46 | 7.35 (6.70-8.30) | 7.44 ± 1.02 | 7.25 (6.80-8.10) | ||||
Insulin levels showed a trend toward reduction but did not reach statistical significance (P = 0.0518), suggesting stable insulin sensitivity. CRP levels remained unchanged, indicating stable inflammation levels. HOMA-IR values did not exhibit significant changes, indicating stable insulin resistance across both groups. FBG levels significantly decreased in the intervention group (P < 0.0001), suggesting improved glucose metabolism, while HbA1c levels exhibited a significant decrease over time in both groups (P = 0.031), there was no difference observed between the two groups. Overall, baseline measurements revealed similar HOMA-IR levels across both groups, with no significant changes over time. However, FBG levels showed a significant reduction in the intervention group, indicating improved glucose metabolism (P < 0.0001), while HbA1c levels were slightly higher in the placebo group (7.95 vs 7.78, P = 0.031) at baseline. Although both groups experienced minor reductions in HbA1c during the study, no significant interactions between time and group were observed.
Table 5 summarizes the glycemic indicators of participants in the probiotic and placebo groups before and after treatment, adjusted for confounders such as age, duration of diabetes, weight, and calorie intake. The results revealed that the probiotic group experienced a significant reduction in FBG levels, with a mean difference of -1.79 mmol/L (P = 0.001) post-treatment, while the placebo group showed no significant change (MD = -0.01, P = 0.991). Additionally, both groups had non-significant changes in HbA1c and insulin levels, although the probiotic group showed a trend towards improvement. Overall, the findings suggest that probiotics may effectively lower FBG, highlighting the importance of adjusting for confounding variables in the analysis.
| Variable | Probiotic group (n = 38) | Placebo group (n = 38) | MD (95%CI), P value |
| FBG (mmol/L) | |||
| Baseline | 9.06 ± 1.38 | 9.20 ± 2.26 | -0.14 (-0.99-0.70), 0.7422 |
| End | 7.34 ± 1.8 | 9.20 ± 2.56 | -1.85 (-2.80 to -0.80), 0.0013 |
| MD (95%CI), P value1 | -1.79 (-2.25 to -1.18), 0.001 | -0.01 (-0.82 to -0.82), 0.991 | |
| HbA1c (%) | |||
| Baseline | 7.78 ± 1.44 | 7.90 ± 1.48 | -0.16 (-0.80-0.60), 0.6312 |
| End | 7.44 ± 1.02 | 7.64 ± 1.46 | -0.20 (-0.78-0.36), 0.9453 |
| MD (95%CI), P value1 | -0.34 (-0.70-0.03), 0.071 | -0.30 (-0.73-0.13), 0.167 | |
| Insulin (mU/mL) | |||
| Baseline | 16.23 ± 18.40 | 30.08 ± 51.16 | -13.10 (-31.10-3.80), 0.1232 |
| End | 14.12 ± 14.77 | 19.59 ± 24.43 | -5.40 (-14.60-3.70), 0.0513 |
| MD (95%CI), P value1 | -2.11 (-4.64-0.31), 0.095 | -10.49 (-22.80-1.80), 0.093 | |
| HOMA-IR index | |||
| Baseline | 6.25 ± 7.38 | 12.57 ± 21.46 | -6.32 (-13.60-1.10), 0.0902 |
| End | 5.39 ± 5.15 | 9.82 ± 2.10 | -4.42 (3.10 to -13.70), 0.0553 |
| MD (95%CI), P value3 | -0.85 (-2.10-0.42), 0.182 | -2.75 (-8.30-2.08), 0.322 |
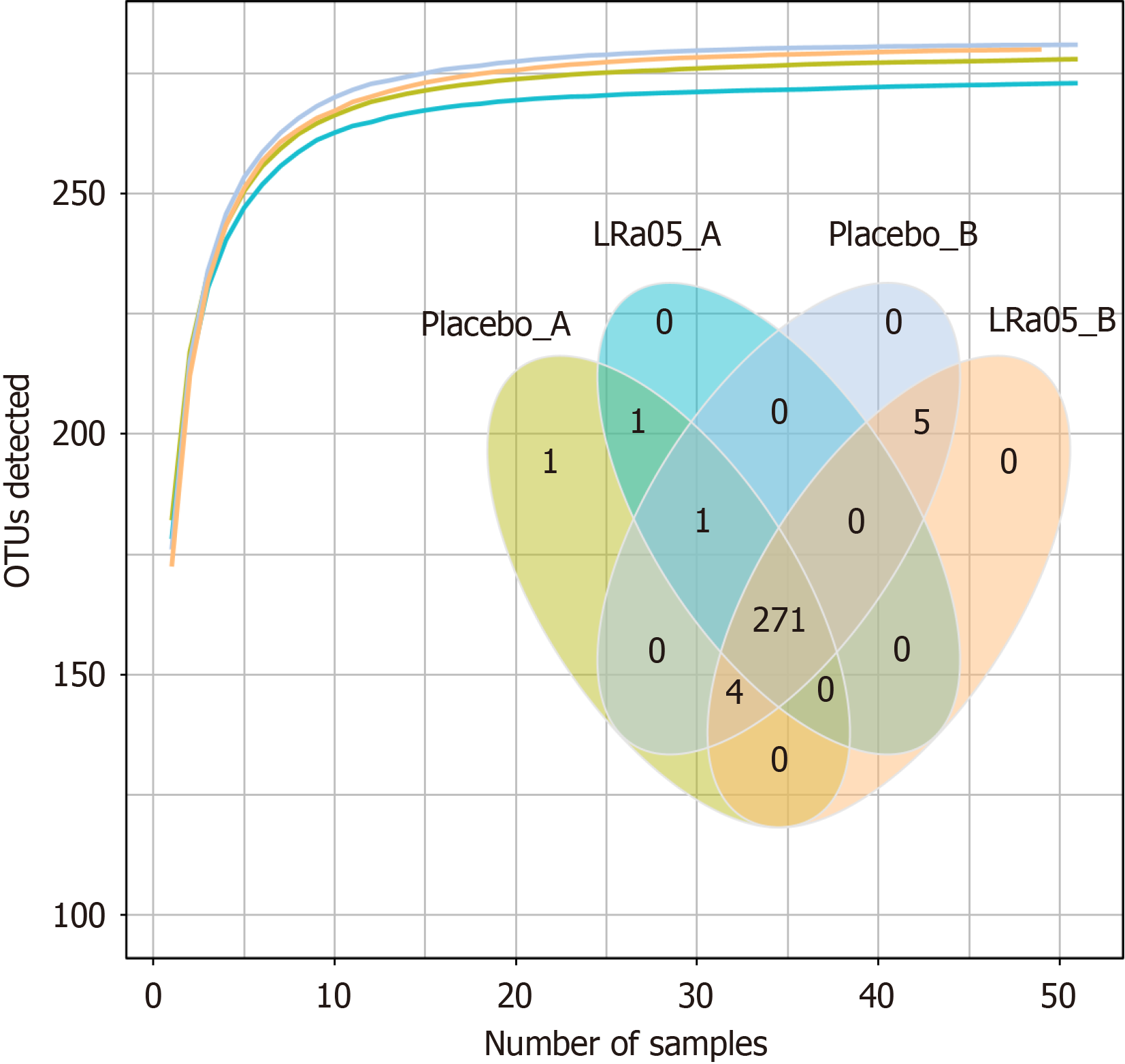
The species accumulation curve showed that species detection increased with sample size but eventually plateaued, indicating saturation. The Venn diagram (Figure 2) reveals 271 shared species between the placebo and LRa05 groups. Both interventions induced detectable changes in gut microbiota diversity.
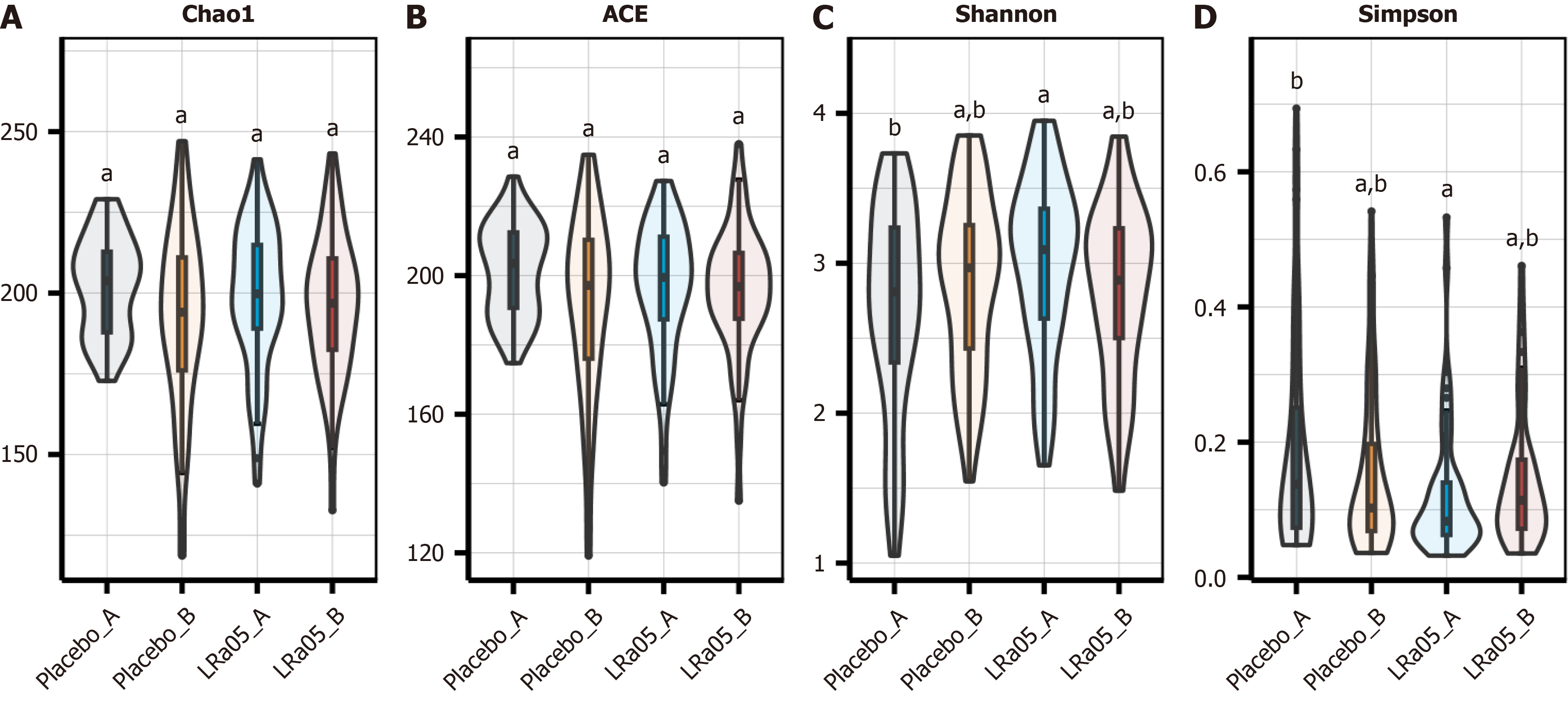
Microbial richness and diversity were assessed using Chao1, Ace, Shannon, and Simpson indices. Higher Chao1 and Ace values indicate greater richness, while higher Shannon values reflect increased diversity; conversely, higher Simpson values indicate lower diversity. Post-intervention, no significant differences were observed in bacterial richness (Chao1, Ace) or diversity (Shannon, Simpson) between or within groups (P > 0.05) (Figure 3).
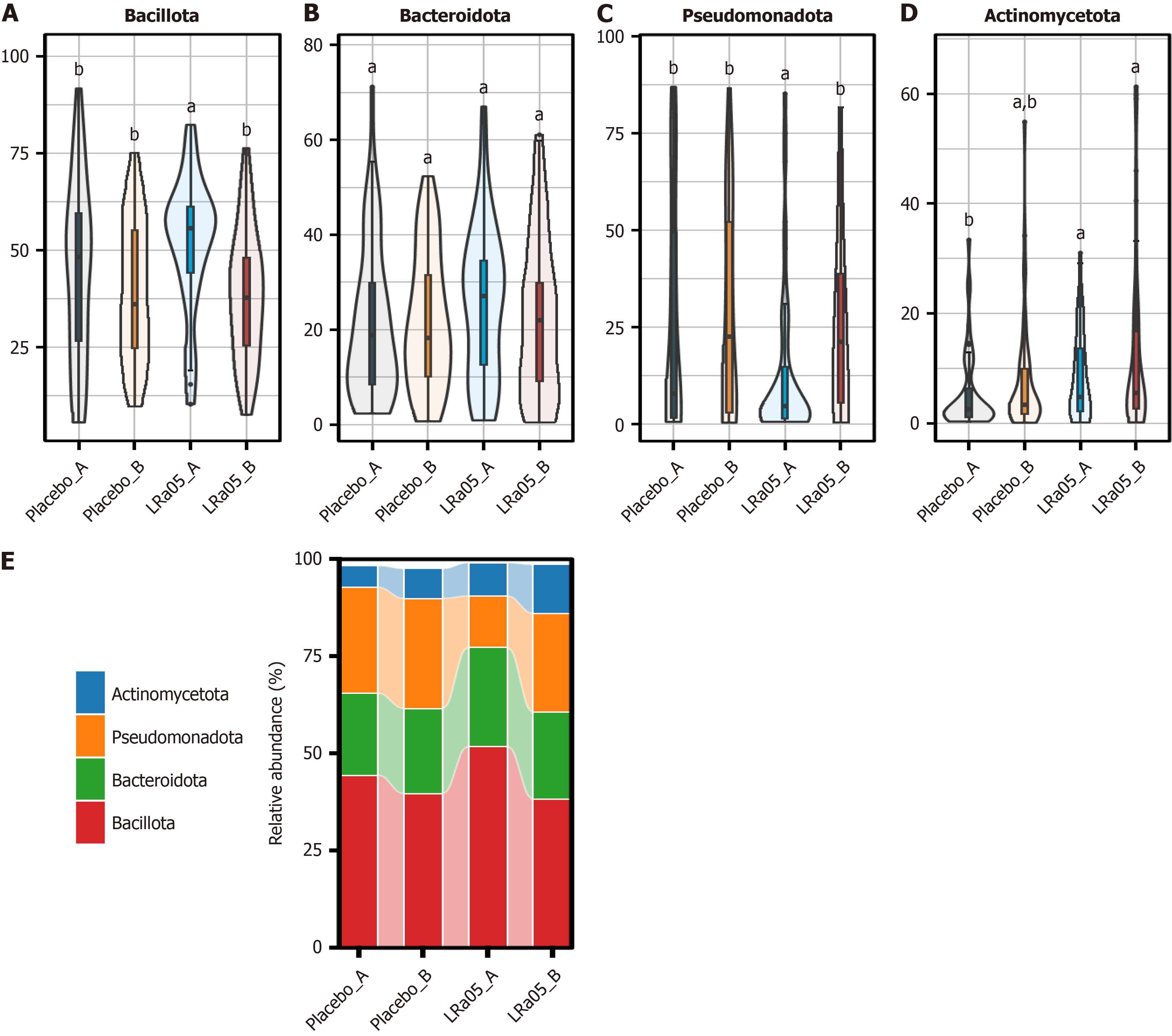
At the phylum level Bacillota, Bacteroidota, Pseudomonadota, and Actinomycetota were identified as the dominant bacterial phyla in the participants’ gut microbiota. Post-intervention analysis revealed a significant reduction in Bacillota abun
Beta diversity analysis indicated no statistically significant differences in gut microbiota composition between the placebo and LRa05 groups at time points A and B (P = 0.066 and P = 1.000, respectively). However, Adonis analysis demonstrated that the LRa05 intervention had a significant impact on microbial composition (P = 0.012), suggesting structural changes in the gut microbiota following treatment (Figure 5).
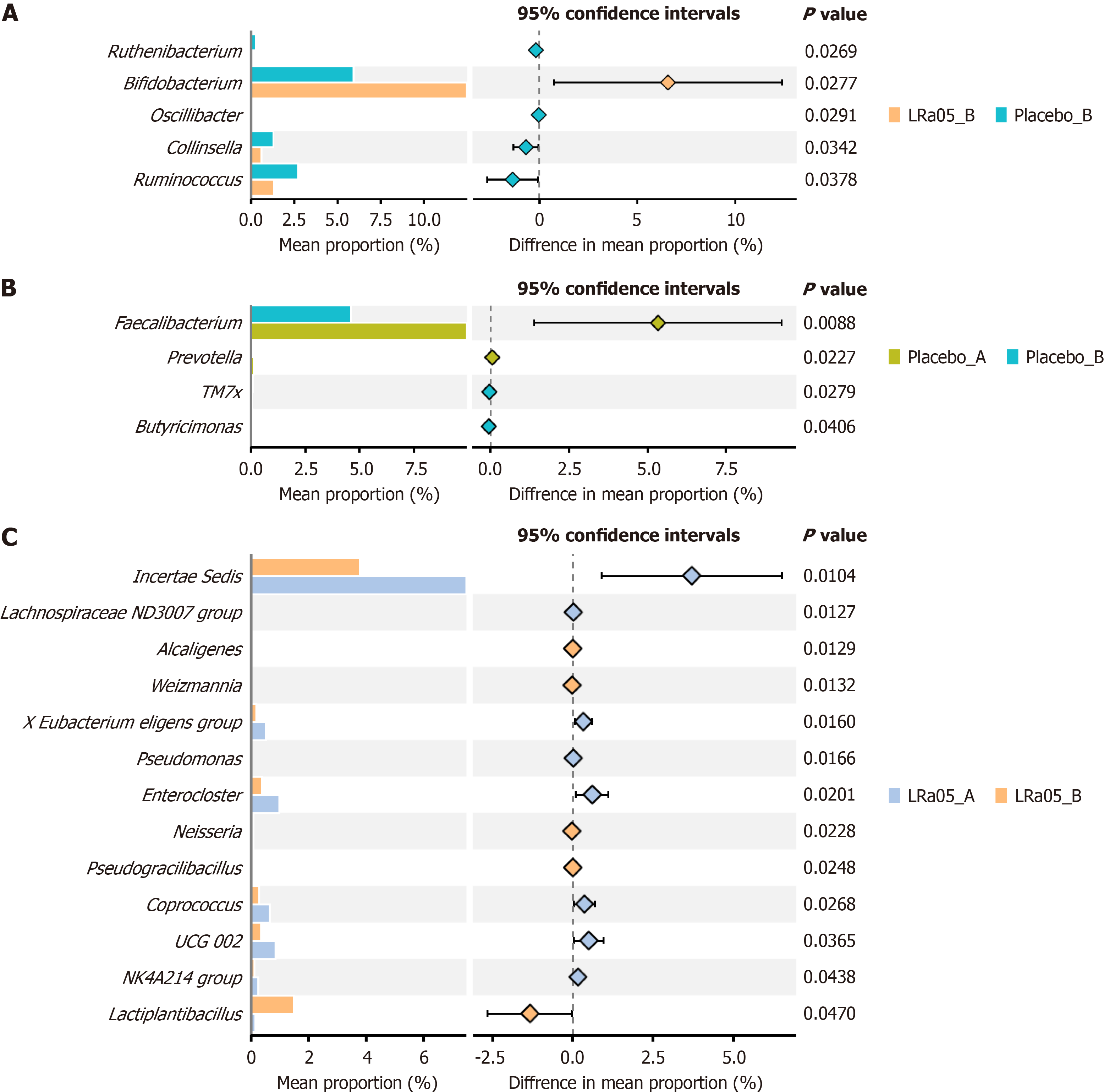
LRa05 treatment significantly increased Bifidobacterium abundance while reducing Ruminococcus and three other genera (P < 0.05) (Figure 6A). In the placebo group Butyricimonas and TM7x increased, while Faecalibacterium decreased (P < 0.05) (Figure 6B). LRa05 intervention significantly altered 13 genera, notably increasing Lactobacillus plantarum, Werzmannia, Pseudomonas, Enterocloster, and Coprococcus (P < 0.05).
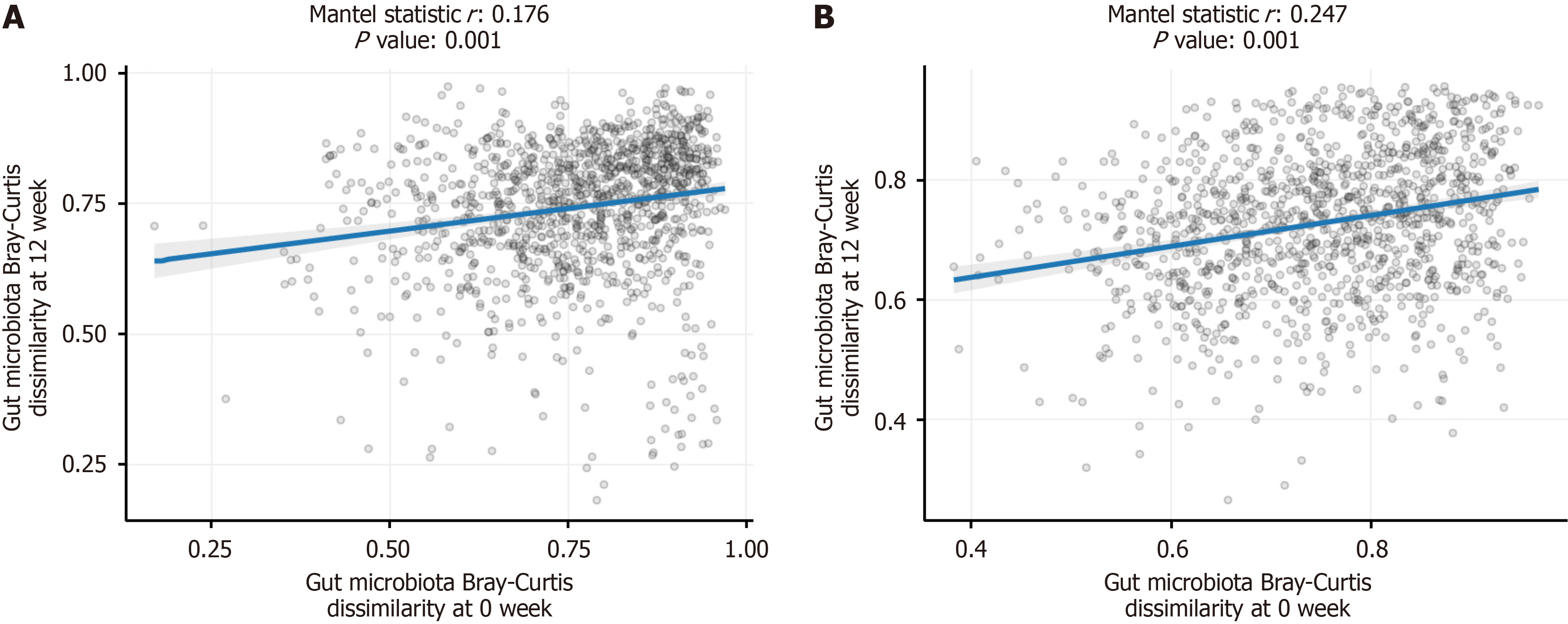
Mantel correlation analysis demonstrated a significant positive correlation in bacterial abundances before and after treatment in both the placebo and LRa05 groups (P = 0.001). However, the correlation coefficient was higher in the LRa05 group (r = 0.247) compared with the placebo group (r = 0.176), suggesting that LRa05 had a more pronounced impact on gut microbiota composition (Figure 7).
This study investigated the effects of Lactobacillus rhamnosus LRa05 supplementation on glycemic control, lipid me
Despite the hypothesized benefits of LRa05 on glucose metabolism, our results indicated that supplementation led to selective improvements rather than comprehensive enhancements in glycemic control. Notably, the FBG levels significantly decreased in the intervention group over time (P < 0.0001), suggesting an improvement in glucose me
The significant reduction in FBG within the intervention group supports the potential role of Lactobacillus rhamnosus in modulating glucose metabolism, possibly through mechanisms such as alterations in gut microbiota composition, enhanced SCFA production, and improved intestinal barrier function, all of which have been linked to better glucose homeostasis[17,18]. The findings of this study indicated a notable reduction in FBG levels; however, the absence of a corresponding significant change in HbA1c suggests that additional factors may have influenced the results. Specifically, the intervention duration and baseline metabolic variations warrant consideration. One possible explanation is that the 12-week intervention period may not have been sufficient to induce measurable changes in insulin sensitivity as adaptations in insulin resistance often require more prolonged interventions or higher probiotic doses[19,20]. Fur
While previous studies have demonstrated variable effects of probiotic supplementation on glycemic control, the current findings align with reports suggesting that probiotic efficacy depends on strain specificity, dosage, and baseline metabolic status[15,21-24]. Some studies have observed improvements in insulin sensitivity with Lactobacillus strains[25], while others have reported no significant effects[26]. The present study suggested that LRa05 may exert beneficial effects primarily on fasting glucose levels rather than on broader insulin sensitivity markers. Overall, the intervention demonstrated notable improvements in FBG and insulin resistance measurements, suggesting potential metabolic benefits. However, the lack of significant changes in insulin sensitivity and HOMA-IR underscores the complexity of probiotic-mediated metabolic modulation and highlights the need for longer intervention periods or adjunctive strategies to enhance insulin function. Future research should explore extended intervention durations and evaluate whether combining probiotics with dietary or pharmacological interventions could amplify the observed benefits on glycemic control and overall metabolic health.
A key finding of our study was the significant increase in HDL-C levels in the LRa05 group by the final time point (P < 0.0001). This aligns with previous evidence suggesting that probiotics, particularly Lactobacillus strains, can modulate lipid metabolism by influencing bile acid metabolism and reducing cholesterol absorption[27,28]. Research has shown that specific Lactobacillius strains can enhance reverse cholesterol transport and promote the excretion of cholesterol, thereby improving lipid profiles[29]. Although triglyceride levels did not show significant changes, a trend toward improvement was observed in the intervention group, suggesting potential long-term benefits that warrant further investigation. Given that dyslipidemia is a major cardiovascular risk factor in T2DM, the observed increase in HDL-C is clinically relevant and may contribute to cardiovascular risk reduction over time.
Our results indicated that LRa05 supplementation did not significantly alter systemic inflammatory markers, WBC counts, or NEUT levels. This suggests that while probiotics can modulate gut microbiota composition, their effects on systemic inflammation may require longer-term interventions or combination therapies. Previous studies have de
Gut microbiota analysis revealed significant structural changes in microbial composition following LRa05 supplementation, with notable alterations in 13 bacterial genera. The intervention was associated with a marked increase in the abundance of Bifidobacterium, a genus well-documented for its probiotic properties, including gut barrier reinforcement, metabolic regulation, and anti-inflammatory effects. Simultaneously, a reduction in Ruminococcus and three other genera was observed, suggesting a shift toward a more favorable microbiota profile that may contribute to improved metabolic outcomes. Additionally, the intervention group exhibited increased levels of Lactobacillus plantarum, Werzmannia, Pseudomonas, Enterocloster, and Coprococcus, further supporting the hypothesis that LRa05 supplementation enhances microbial diversity and promotes the expansion of beneficial species. These findings align with prior research indicating that probiotic interventions can selectively enrich health-promoting bacterial populations, which are often linked to improved metabolic homeostasis and reduced systemic inflammation[8,10,13,32]. The modulation of gut microbiota composition in this study underscores the potential of LRa05 in fostering a healthier microbial environment conducive to metabolic benefits.
Despite these compositional changes, overall alpha diversity metrics, including Chao1, Ace, Shannon, and Simpson indices, did not show significant differences between the intervention and placebo groups, suggesting that total microbial richness and evenness remained relatively stable. However, beta diversity analysis demonstrated a significant shift in microbial composition following LRa05 supplementation (P = 0.012), indicating that while absolute diversity remained unchanged, the structural composition of the gut microbiota underwent meaningful alterations. This finding is consistent with previous studies suggesting that probiotic supplementation can significantly impact microbial composition without necessarily affecting overall species richness. Furthermore, Mantel correlation analysis revealed a stronger correlation in bacterial abundances before and after treatment within the LRa05 group compared with the placebo group. This reinforces the notion that LRa05 had a more pronounced impact on gut microbiota stability and structural resilience over time. Such stabilization of microbial communities may be beneficial for long-term gut health as a well-balanced microbiota is associated with enhanced metabolic efficiency, improved glycemic control, and reduced chronic inflammation.
One possible explanation for the lack of significant shifts in alpha diversity metrics is the relatively short intervention duration (12 weeks), which may not have been sufficient to induce measurable changes in overall microbial richness[33,34]. Research indicates that longer durations of intervention are often necessary to observe substantial shifts in microbial communities as microbial ecosystems can take time to respond to dietary changes or supplementation[35]. However, the observed changes in specific bacterial taxa, coupled with the significant differences in beta diversity, suggest that LRa05 was effective in modulating microbial composition in a manner that could potentially lead to greater long-term benefits. Extending the duration of supplementation may yield more pronounced effects on microbial diversity and further enhance the metabolic benefits associated with gut microbiota modulation. In brief LRa05 supplementation led to meaningful changes in microbial composition, fostering the growth of beneficial bacteria while reducing potentially detrimental genera. While major shifts in overall diversity were not observed within the study period, the significant beta diversity differences and specific bacterial alterations indicate that LRa05 exerts a tangible effect on gut microbiota structure.
Overall, a growing body of evidence underscores the intricate relationship between gut microbiota metabolism, lipid regulation, and incretin-mediated metabolic effects. Gut microbiota play a pivotal role in host metabolism by modulating bile acid metabolism, SCFA production, and systemic inflammation, all of which contribute to lipid homeostasis and insulin sensitivity[9,13,17]. In the present study shifts in microbial taxa associated with enhanced SCFA production were observed and may have contributed to improvements in lipid profiles and glycemic control. SCFAs have been implicated in promoting lipid metabolism through bile acid modulation and cholesterol excretion, potentially explaining the significant increase in HDL-C levels following the intervention.
Furthermore, glucose-dependent insulinotropic polypeptide (GIP), a key incretin hormone, has been recognized for its role in lipid metabolism and insulinotropic effects. SCFAs have been shown to enhance incretin secretion, including GIP, which may contribute to improved insulin sensitivity and lipid homeostasis[36]. The interplay between SCFA production, GIP signaling, and lipid metabolism represents a potential mechanistic axis underlying the observed metabolic benefits[37]. Although direct assessment of GIP levels was not conducted in this study, the observed metabolic improvements align with this proposed mechanism. Future research incorporating targeted metabolomic and functional microbiome analyses is warranted to elucidate the complex interactions between gut microbiota-derived metabolites, incretin activity, and metabolic regulation.
Several limitations must be recognized. Firstly, baseline variations in the duration of diabetes and specific biochemical markers may have affected the outcomes, even after statistical adjustments were made. Secondly, the 12-week duration of the study might not have been long enough to detect significant changes in glycemic control or systemic inflammation. Additionally, dietary intakes that could influence glycemic control were not assessed. Lastly, the responses of gut microbiota to probiotic interventions are highly individualized. Future research should integrate metagenomics and metabolomics analyses to enhance the understanding of host-microbe interactions.
Supplementation with Lactobacillus rhamnosus LRa05 resulted in significant improvements in HDL-C levels and the composition of gut microbiota among patients with T2DM. Notably, a short-term effect was observed on FBG. However, there were no significant changes in glycemic control, insulin sensitivity, HbA1c, HOMA-IR, or inflammatory markers. These findings underscore the potential role of probiotics as adjunct therapies in managing T2DM, particularly in enhancing lipid metabolism and fostering a balanced gut microbiome. Further research is warranted to investigate the long-term effects and possible synergies with other therapeutic interventions.
| 1. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 797] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 2. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3406] [Article Influence: 486.6] [Reference Citation Analysis (0)] |
| 3. | Vaidya V, Gangan N, Sheehan J. Impact of cardiovascular complications among patients with Type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2015;15:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Baars DP, Fondevila MF, Meijnikman AS, Nieuwdorp M. The central role of the gut microbiota in the pathophysiology and management of type 2 diabetes. Cell Host Microbe. 2024;32:1280-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | Zhou Z, Sun B, Yu D, Zhu C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front Cell Infect Microbiol. 2022;12:834485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 6. | Moludi J, Maleki V, Jafari-Vayghyan H, Vaghef-Mehrabany E, Alizadeh M. Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics. Clin Exp Pharmacol Physiol. 2020;47:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Zhang L, Chu J, Hao W, Zhang J, Li H, Yang C, Yang J, Chen X, Wang H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediators Inflamm. 2021;2021:5110276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. Role of the Gastrointestinal Tract Microbiome in the Pathophysiology of Diabetes Mellitus. J Diabetes Res. 2017;2017:9631435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Muscogiuri G, Balercia G, Barrea L, Cignarelli A, Giorgino F, Holst JJ, Laudisio D, Orio F, Tirabassi G, Colao A. Gut: A key player in the pathogenesis of type 2 diabetes? Crit Rev Food Sci Nutr. 2018;58:1294-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Sanders ME, Merenstein D, Merrifield CA, Hutkins R. Probiotics for human use. Nutr Bull. 2018;43:212-225. [RCA] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab Syndr. 2018;12:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, Mitchell EA, Stanley TV, Purdie GL, Kang JM, Hood FE, Rowden JL, Barnes PK, Fitzharris PF, Crane J. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117:804-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Gai Z, Dong Y, Xu F, Zhang J, Yang Y, Wang Y. Changes in the gut microbiota composition of healthy young volunteers after administration of Lacticaseibacillus rhamnosus LRa05: A placebo-controlled study. Front Nutr. 2023;10:1105694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Gu J, Chen Y, Wang J, Gao Y, Gai Z, Zhao Y, Xu F. Lacticaseibacillus rhamnosus LRa05 alleviated liver injury in mice with alcoholic fatty liver disease by improving intestinal permeability and balancing gut microbiota. Benef Microbes. 2024;15:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Jafarnejad S, Saremi S, Jafarnejad F, Arab A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J Nutr Metab. 2016;2016:5190846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Baek C, Kim WJ, Moon J, Moon SY, Kim W, Hu HJ, Min J. Differences in the gut microbiome composition of Korean children and adult samples based on different DNA isolation kits. PLoS One. 2022;17:e0264291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY, Li HB. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021;13:3211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 18. | Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12:1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 670] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 19. | Mann JI. Can dietary intervention produce long-term reduction in insulin resistance? Br J Nutr. 2000;83 Suppl 1:S169-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Turner KM, Keogh JB, Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies. Nutr Metab Cardiovasc Dis. 2015;25:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS One. 2015;10:e0132121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Jiang H, Zhang Y, Xu D, Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J Clin Lab Anal. 2021;35:e23650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | López-Moreno A, Suárez A, Avanzi C, Monteoliva-Sánchez M, Aguilera M. Probiotic Strains and Intervention Total Doses for Modulating Obesity-Related Microbiota Dysbiosis: A Systematic Review and Meta-analysis. Nutrients. 2020;12:1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Szulińska M, Łoniewski I, van Hemert S, Sobieska M, Bogdański P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients. 2019;11:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38:38-43. [PubMed] |
| 27. | Li H, Liu F, Lu J, Shi J, Guan J, Yan F, Li B, Huo G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front Microbiol. 2020;11:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 28. | Yan F, Li N, Shi J, Li H, Yue Y, Jiao W, Wang N, Song Y, Huo G, Li B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019;10:5804-5815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 29. | Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Jarvenpaa S, Kautiainen H, Julkunen I, Vapaatalo H, Korpela R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008;14:2029-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 31. | Milajerdi A, Mousavi SM, Sadeghi A, Salari-Moghaddam A, Parohan M, Larijani B, Esmaillzadeh A. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. 2020;59:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 32. | Sachdeva A, Tomar T, Malik T, Bains A, Karnwal A. Exploring probiotics as a sustainable alternative to antimicrobial growth promoters: mechanisms and benefits in animal health. Front Sustain Food Syst. 2025;8:1523678. [DOI] [Full Text] |
| 33. | Lampe JW, Navarro SL, Hullar MA, Shojaie A. Inter-individual differences in response to dietary intervention: integrating omics platforms towards personalised dietary recommendations. Proc Nutr Soc. 2013;72:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11:2862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 505] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 35. | Fragiadakis GK, Wastyk HC, Robinson JL, Sonnenburg ED, Sonnenburg JL, Gardner CD. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am J Clin Nutr. 2020;111:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 36. | Srivastava S, Singh PR. Oral Administration of Lactobacillus casei and Bifidobacterium bifidum Improves Glucagon like Peptide-1(GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP) Level in Streptozotocin Induced Diabetic Rats. Curr Res Nutr Food Sci. 2021;9:431-440. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Lee EY, Zhang X, Miyamoto J, Kimura I, Taknaka T, Furusawa K, Jomori T, Fujimoto K, Uematsu S, Miki T. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J Endocrinol. 2018;239:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |