Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.103468
Revised: March 21, 2025
Accepted: June 3, 2025
Published online: July 15, 2025
Processing time: 237 Days and 15.9 Hours
Cognitive decline in type 2 diabetes mellitus (T2DM) occurs years before the onset of clinical symptoms. Early detection of this incipient cognitive decline stage, which is T2DM without mild cognitive impairment, is critical for clinical inter
To identify structural changes in the brains of T2DM patients without cognitive impairment to gain insights into the early-stage cognitive decline.
Using diffusion tensor imaging (DTI), we constructed structural brain networks in 47 T2DM patients and 47 age-/sex-matched healthy controls. Machine learning models incorporating connectivity features were developed to classify T2DM brains and predict disease duration.
T2DM patients exhibited reduced global/local efficiency and small-worldness, alongside weakened connectivity in cortical regions but enhanced subcortical-frontal connections, suggesting compensatory mechanisms. A classification model leveraging 18 connectivity features achieved 92.5% accuracy in distinguishing T2DM brains. Structural connectivity patterns further predicted disease onset with an error of ± 1.9 years.
Our findings reveal early-stage brain network reorganization in T2DM, highlighting subcortical-frontal con
Core Tip: This study sheds light on the early-stage cognitive decline in type 2 diabetes mellitus (T2DM) by identifying disrupted brain connectivity and developing a classification model with a promising accuracy of 92.5%. Deep subcortical nuclei showed increased connectivity with the frontal lobe in T2DM patients without cognitive impairment. The diabetic brain exhibited measurable characteristics before patients presented with detectable cognitive impairments. The high-accuracy models demonstrate the potential of diffusion tensor imaging-based biomarkers for detecting preclinical cognitive decline and monitoring disease progression.
- Citation: Li YF, Wei Y, Li MR, Sun ZZ, Xie WY, Li QF, Xie CH, Xiang JY, Tan X, Qiu SJ, Liang Y. Detect the disrupted brain structural connectivity in type 2 diabetes mellitus patients without cognitive impairment. World J Diabetes 2025; 16(7): 103468
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/103468.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.103468
Type 2 diabetes mellitus (T2DM) is a common metabolic disorder characterized by chronically elevated blood glucose levels, and long-term hyperglycemia damages the brain and contributes to cognitive decline[1]. T2DM-related cognitive decline is characterized by a prolonged prodromal and preclinical phase in which silent pathologic changes develop initially, followed by the onset of mild cognitive impairment (MCI), and ultimately culminating in dementia. Early detection of this incipient cognitive decline stage, which is T2DM without MCI, is critical for clinical intervention, yet it remains elusive and challenging to identify[2]. Therefore, timely recognition of preclinical phase of cognitive decline in T2DM patients assumes paramount importance for optimal patient care.
Emerging evidence suggests that disrupted white matter connectivity, rather than gray matter atrophy, may serve as the earliest biomarker of incipient cognitive decline in T2DM[3,4]. In one of our previous studies, we found interesting compensatory recruitment of the pons and the left temporal pole with increased local diffusion homogeneity in T2DM without MCI compared to the healthy controls (HCs), which illuminated the white matter was already altered before MCI developed[5]. Neurological studies show that disruption in white matter tract diffusion characteristics of many regions[6] (e.g., the connections in the temporal lobe and hippocampus, connections between frontal and temporal lobes, and connections with thalamus) is associated with impaired glucose metabolism and cognitive dysfunction. However, most studies focus on T2DM patients with measurable cognitive impairment, leaving a critical gap in understanding structural network reorganization during the asymptomatic phase[6]. Cognitive screening tools lack sensitivity for preclinical stages, structural connectivity metrics derived from diffusion tensor imaging (DTI) may enable early identification of at-risk T2DM patients, facilitating timely interventions to delay dementia progression. More specifically, it is worth exploring whether the diabetes brain without clinical cognitive loss can be identified from normal healthy brain based on structural images.
In addition, previous studies have predominantly focused on investigating either regional gray matter or white matter changes to understand the effects of diabetes on the brain[3,4,7-10]. However, it remains unclear whether gray matter alterations or white matter fiber connections are more informative in distinguishing the diabetic brain. In this study, we utilized DTI to construct a quantitative white matter structural network in individuals with T2DM who did not have MCI. We then employed machine learning techniques to explore novel approaches for automatically diagnosing the diabetic brain and predicting the duration of the disease. Additionally, we investigated the specific features that contributed the most to the classification and prediction models, aiming to identify the most vulnerable brain regions and white matter fiber tracts that characterize a typical diabetic brain. This study aims to: (1) Identify distinct structural connectivity patterns in T2DM patients without cognitive impairment using DTI-derived networks; (2) Develop machine learning models to classify T2DM brains and predict disease duration; and (3) Test the hypothesis that white matter connectivity features outperform gray matter volumetry in detecting preclinical T2DM-related brain alterations.
In this study, a total of 47 right-handed T2DM patients and 47 sex- and age-matched HCs were recruited (Supplementary Figure 1). The study was approved by the Ethics Committee of the institutional review board of Guangzhou University of Chinese Medicine, and all subjects provided informed consent. T2DM diagnosis was based on the diagnostic criteria of the American Diabetes Association. Physical and neurological examinations were conducted by neurologists to rule out clinically manifested cognitive derangements, including checking the cranial nerves, motor system, and presence of any cerebellar signs, for all participants. Then, each subject underwent the Montreal Cognitive Assessment test, and a score of 26 or higher was considered indicative of normal cognitive function without MCI. The T2DM subjects included in this study received oral metformin and insulin via pump or subcutaneous injection while in the hospital to maintain stable blood glucose levels. Exclusion criteria for participants were as follows: (1) Complications associated with T2DM (e.g., fundus hemorrhage, peripheral neuropathy); (2) Intracranial injury (e.g., intracranial organic lesions, history of severe head trauma, history of stroke); (3) Psychiatric or neurological disorders (e.g., epilepsy, depression); (4) Microvascular and macrovascular diseases; (5) Self-reported history of alcohol or drug abuse; and (6) Contraindications to MR. Demographic data, medical measurements which contained blood pressure, hemoglobin A1C levels, body mass index, systolic blood pressure, diastolic blood pressure, fasting blood glucose, triglyceride, total cholesterol, low density lipoprotein, and T2DM duration were recorded for each participant, detailed in Table 1.
| T2DM subjects, (n = 47) | Healthy controls, (n = 47) | P value | |
| Demographics | |||
| Age (years) | 57.36 ± 8.04 | 55.62 ± 8.36 | 0.305 |
| Gender (male/female) | 18/29 | 25/22 | 0.147 |
| Clinical data | |||
| BMI (kg/m2) | 24.52 ± 2.09 | 20.78 ± 1.40 | 0.0001 |
| SBP (mmHg) | 124.15 ± 9.66 | 114.51 ± 6.67 | 0.0001 |
| DBP (mmHg) | 75.62 ± 6.54 | 70.36 ± 5.14 | 0.0001 |
| HbA1c (%) | 8.03 ± 1.53 | N/A | N/A |
| FBG (mmol/L) | 6.73 ± 2.15 | N/A | N/A |
| TG (mmol/L) | 1.65 ± 0.42 | N/A | N/A |
| TC (mmol/L) | 4.93 ± 1.03 | N/A | N/A |
| HDL (mmol/L) | 1.00 ± 0.20 | N/A | N/A |
| LDL (mmol/L) | 2.59 ± 0.53 | N/A | N/A |
| Course of disease (years) | 6.60 ± 3.41 | N/A | N/A |
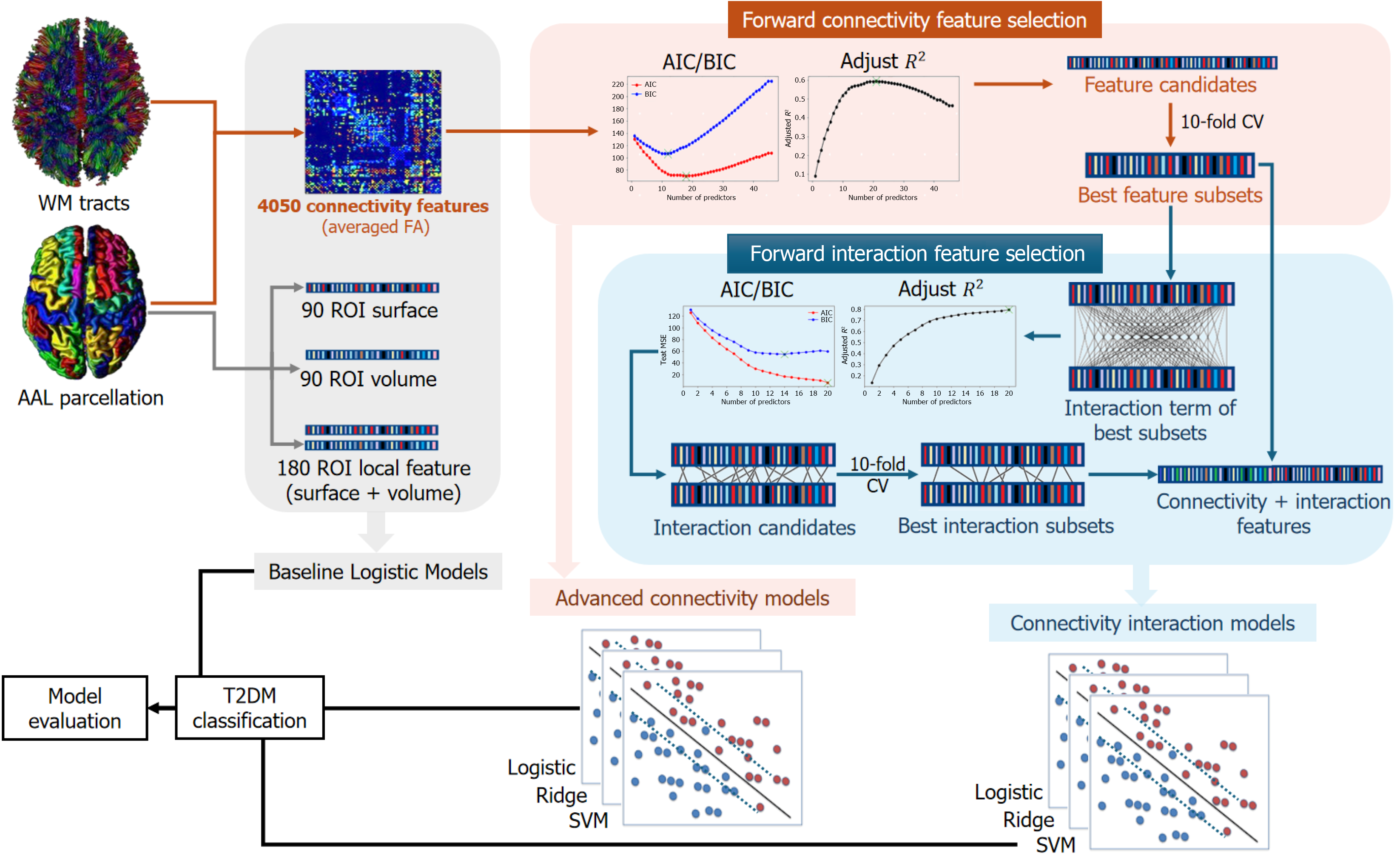
Structural magnetic resonance imaging (MRI) data were obtained using an 8-channel head coil on a 3T GE scanner, including T1-weighted, T2-weighted, FLAIR sequences for lesion screening, followed by DTI scans with parameters matching previous studies[5]. The detailed preprocessing of MRI data followed previous works[6,11] and utilized the PANDA pipeline toolbox[12]. Diffusion MRI data were preprocessed to correct distortions and motion artifacts, followed by computation of fractional anisotropy (FA). Using the automated anatomical labelling atlas, the brain was parcellated into 90 regions of interest (ROIs) for structural network analysis. White matter tracts were reconstructed via spherical deconvolution-based tractography with FA thresholds, and connections' strengths between ROIs were determined by averaging FA values of fibers between regions. This process produced a weighted 90 × 90 connectivity matrix for each participant, where each element represented the strength of the connection between a pair of ROIs (Figure 1).
Baseline models: Initially, we constructed baseline models using Logistic regression to classify T2DM and HC. The four baseline models utilized the following features: (1) Connectivity baseline model: All structural connectivity features (total: 9090/2 = 4050 connections); (2) Local surface model: 90 ROI surface size features; (3) Local volume model: 90 ROI volume size features; and (4) Local baseline model: 90 ROI surface size features + 90 ROI volume size features.
Advanced connectivity model with feature selection: To construct more effective models for predicting T2DM using brain connectivity features, feature selection techniques were performed before model building. First, we utilized a permutation test to select structural connection features that exhibited significant differences between the T2DM group and the HC group. Subsequently, using these significant connections, we employed the forward stepwise selection strategy to identify the best subset of features for classification. For subsets with different numbers of predictors, we evaluated the Akaike information criterion (AIC), Bayesian information criterion (BIC), and adjusted-R2 to choose the best model among the subsets (Supplementary Figure 2). To compare the performance of different machine learning methods, three different classification models (Logistic classification, ridge classification with hyper-parameter α tuning (which controls the penalty to the model size), and support vector machine (SVM) were built based on the selected subset of features.
Connectivity interaction model: To further enhance the classification performance, we developed a Connectivity Interaction Model that incorporates interaction terms, taking into consideration the potential synergistic effects of predictors on the response variable. Previous studies have indicated that in the context of T2DM, the connectivity between different brain regions may be influenced synergistically[3,4,7]. Thus, we employed a Logistic regression function that includes interaction terms to capture this type of mechanism. To determine the most relevant interaction features, we utilized the forward stepwise selection method with the same procedure in 2.3.2 (Supplementary Figure 3). Similarly, three different classification models (Logistic regression, Ridge Regression, SVM) were built based on the selected subset of features.
Baseline models: Similar to the classification models, we initially constructed four baseline linear regression models using different sets of features. These models included: (1) Connectivity baseline model: All of 4050 structural con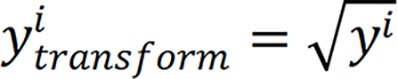 , where represents the original duration value for observation i.
, where represents the original duration value for observation i.
Connectivity advanced model with feature selection: Similar to the classification model, we aimed to build a predictive regression model with superior performance. We selected the brain connectivity features that passed the permutation test and employed the forward stepwise feature selection method. Subsequently, we used metrics such as AIC, BIC, and adjusted-R2 to identify the optimal subsets of features (Supplementary Figure 4).
Classification performance was evaluated using stratified 10-fold cross-validation, with metrics averaged and their variances reported for comprehensive assessment. Disease duration prediction performance was assessed using Leave-One-Out Cross-Validation (LOOCV) with Root Mean Square Error (RMSE) and its variance utilized to evaluate model performance (Supplementary material).
There is no evidence of differences in demographics between two groups (Table 1). As illustrated in the table, the average duration of T2DM patients is 6.6 ± 3.4 years (Figure 1).
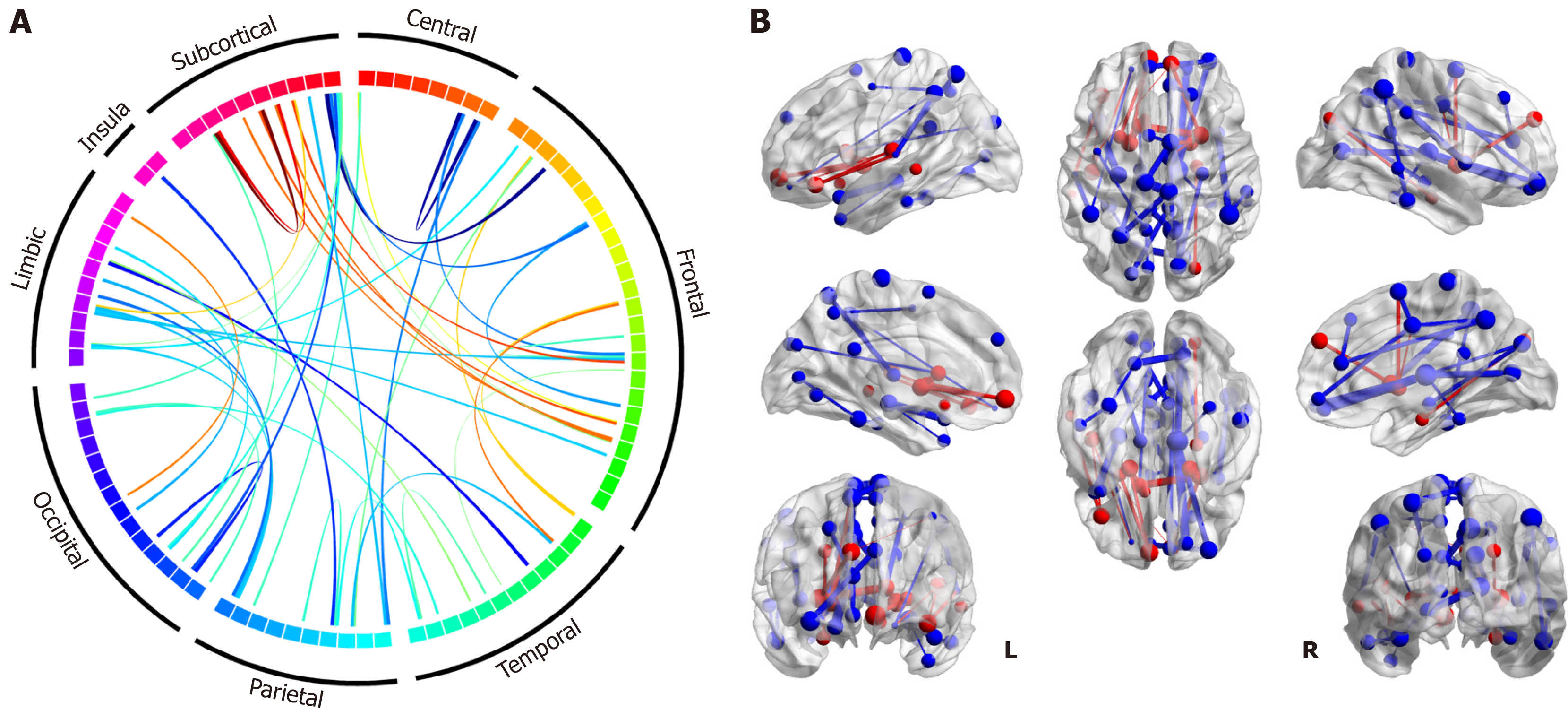
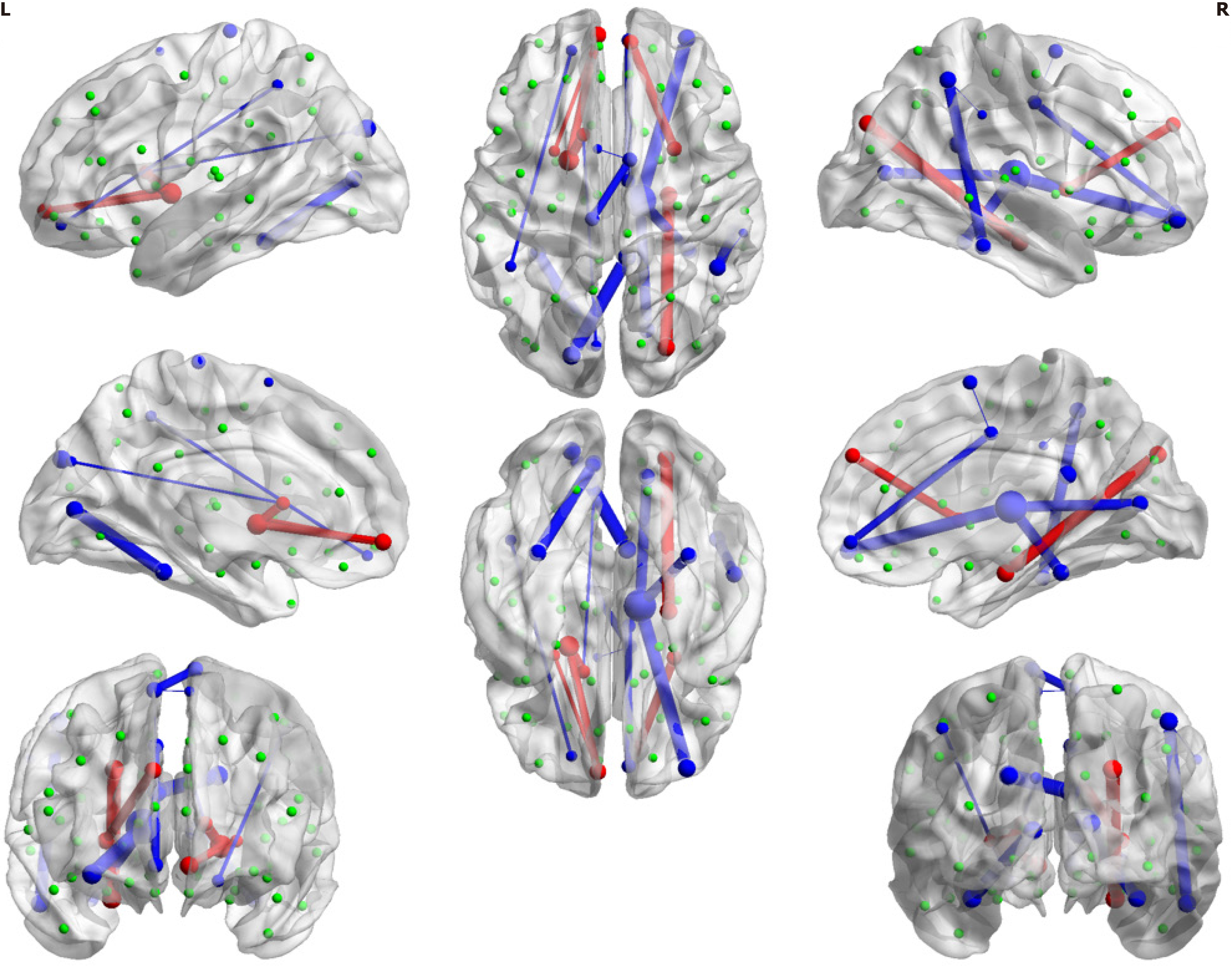
To examine the structural connectivity difference between the T2DM group and HC group, we compare the FA strength of each connection between two groups by permutation tests with 5000 randomizations following the procedures in previous work[13] using the DPABI toolbox. Multiple comparisons are corrected with FDR correction (P < 0.025). The connections with significant difference between the two groups are illustrated using Circos[14] and 3D figures, which are generated by the BrainNet Viewer[15].
There are a total of 47 connections with significant intergroup differences. As shown in Figure 2, the differences primarily manifest in decreased structural connectivity in the T2DM patient group, with FA weight significantly weaker than that in the HC group. However, there are also a few connections to subcortical and frontal regions that exhibited en
The performance results of all models are presented in Table 2. These results indicate that the baseline models do not achieve satisfactory performance for the classification task. Nevertheless, when comparing the performance between the baseline models, it suggests that utilizing brain region connectivity features may provide more valuable predictions for T2DM patients compared to relying solely on the volume size and surface size of the brain regions.
| Models | Accuracy | Precision | Recall | F1-score |
| Connectivity baseline model | 0.5011 (0.0064) | 0.5009 (0.0085) | 0.5333 (0.0195) | 0.5104 (0.0105) |
| Local surface model | 0.4678 (0.0056) | 0.4840 (0.0103) | 0.4733 (0.0161) | 0.4649 (0.0056) |
| Local volume model | 0.3614 (0.0057) | 0.3478 (0.0111) | 0.3844 (0.0274) | 0.3610 (0.0179) |
| Local baseline model | 0.3824 (0.0066) | 0.3616 (0.0129) | 0.4044 (0.0376) | 0.3772 (0.0226) |
| Advanced connectivity models | ||||
| Logistic | 0.8099 (0.0071) | 0.7983 (0.0072) | 0.8311 0.0162) | 0.8104 (0.0083) |
| Ridge (α = 0.9) | 0.8304 (0.0058) | 0.8694 (0.0066) | 0.7867 (0.0189) | 0.8185 (0.0089) |
| SVM | 0.8093 (0.0026) | 0.8127 (0.0035) | 0.8088 (0.0106) | 0.8065 (0.0038) |
| Connectivity interaction models | ||||
| Logistic | 0.8204 (0.0071) | 0.8011 (0.0076) | 0.8511 (0.0125) | 0.8232 (0.0081) |
| Ridge (α = 0.2) | 0.9251 (0.0007) | 0.9377 (0.0026) | 0.9155 (0.0018) | 0.9249 (0.0006) |
| SVM | 0.8204 (0.0049 | 0.8350 (0.0089) | 0.8088 (0.00106) | 0.8170 (0.0059) |
According to the results of forward feature selection, the model evaluation metrics (AIC, BIC, and adjusted-R2) indicated the models with 10-20 predictors would be optimal (Supplementary Figure 2). We tested the models under these subsets and found that the model with the 18 selected features provided the best performance during cross-validation (Figure 3 and Supplementary Figure 3). Three models were subsequently built using Logistic classification, ridge classification, and SVM, using the selected features. The performance of these models was significantly better than the baseline model that included all connectivity features (Table 2). This indicates the preserved 18 connection features (Table 3) are more discriminative in identifying whether the person is a T2DM patient. As indicated in the table, the observed connections primarily involve deep subcortical nuclei (caudate, pallidum, putamen and thalamus), as well as the connections between these subcortical nuclei and the frontal and occipital lobes.
| Connections | Ridge regression coefficients |
| Parietal_Inf_R-SupraMarginal_R | 3.3436 |
| Supp_Motor_Area_R-Cingulum_Mid_R | 3.1377 |
| Cuneus_L-Caudate_L | 2.0824 |
| Frontal_Sup_Orb_L-Parietal_Inf_L | 1.7015 |
| Supp_Motor_Area_L-Supp_Motor_Area_R | 1.5698 |
| Frontal_Mid_Orb_R-Thalamus_R | 1.5043 |
| Calcarine_R-Thalamus_R | 1.3564 |
| Supp_Motor_Area_R-Paracentral_Lobule_L | 1.3261 |
| Frontal_Med_Orb_R-Cingulum_Mid_R | 1.1464 |
| Parietal_Inf_R-Temporal_Inf_R | 0.9017 |
| ParaHippocampal_R-Occipital_Sup_R | 0.8107 |
| Frontal_Med_Orb_L-Pallidum_L | 0.8031 |
| Calcarine_L-Fusiform_L | 0.7449 |
| Caudate_L-Pallidum_L | 0.6747 |
| Frontal_Sup_Medial_R-Putamen_R | 0.5617 |
| Frontal_Med_Orb_L-Putamen_L | 0.5418 |
| Fusiform_R-Thalamus_R | 0.4652 |
| Cingulum_Post_R-Occipital_Sup_L | 0.3565 |
By employing the original 18 connectivity features with different subsets of interaction terms to build the model, we discovered that the model, which included 14 selected interaction terms, provided the best classification performance (Table 2 and Supplementary Figure 3). The incorporation of second-order interaction terms significantly improved the model performance, particularly for the ridge regression model, which improved its accuracy from 83.04% to 92.51%. The other metrics, including precision, recall, and F1-score, were also outstanding when compared to the previous models.
The performance of four baseline models is presented in Table 4. Similar to classification models, the results indicate that structural connectivity provides more useful information for predicting the time course when compared to models constructed using surface and volume size features. Nevertheless, the performance of these baseline models was not satisfactory. The high variance of RMSE indicates that the model prediction is unstable.
| Models | RMSE | Var (RMSE) |
| Connectivity baseline model | 3.03 | 5.35 |
| Local surface model | 4.38 | 12.20 |
| Local volume model | 4.41 | 8.95 |
| Local baseline model | 4.13 | 8.67 |
| 1.94 | 2.13 |
In order to build the advanced connectivity model, similar to the procedure applied with classification model, we use the 47 brain connectivity features selected from the permutation test and then adapt the forward stepwise feature selection. The AIC, BIC and adjusted-R2 for each subset with different amounts of predictors are given in Supplementary Figure 4. Based on these model metrics, a subset of six brain connectivity features was selected. The final model achieved an RMSE of 1.94 and the variance of RMSE is 2.13 in the LOOCV, indicating significantly improved stability in the predictions (Table 4).
We further check whether the obtained regression function satisfies the five assumptions of linear regression, and the details are given in Supplementary Figures 5-7. Since the fitted linear regression model with the selected six brain connectivity features satisfies all the five assumptions and has stable performance in the LOOCV, we believe it is reliable for predicting the disease duration.
In this study, our findings exhibit highly promising performance in both characterizing the diabetic brain without cognitive impairment and predicting the onset time of T2DM. In this section, we discuss three major findings and provide suggestions for future work: (1) Characterizing the diabetic brain without cognitive impairment; (2) The progressively increasing brain damage accompanying the duration of T2DM; and (3) The benefits of using connectivity features.
The optimal classification model for the diabetic brain was constructed using the 18 connection features that passed the permutation test and forward feature selection. The final model achieved a highly promising accuracy rate in cross-validation (92.5%), implying that patients with T2DM can develop distinguishable microstructural brain damage that characterizes the diabetic brain even before the onset of overt cognitive impairment.
Furthermore, the classification results have confirmed the crucial role of the 18 selected connections in distinguishing the diabetic brain. Most of those selected connections involve deep subcortical nuclei (including the pallidum, putamen, and thalamus) connecting with the frontal and occipital lobes. These findings enrich and refine the imaging features of the subclinical T2DM brain without cognitive impairment. Additionally, many of the connections were weaker in the T2DM patient group (Figure 3), indicating impaired fiber connections in T2DM patients. Some impaired connections align with previous findings in T2DM populations with cognitive impairment (e.g., connections to the thalamus, visual regions, frontal lobes, and temporal lobes), which supports the idea that the disease may impact these brain regions even before the onset of cognitive impairment. For instance, the thalamus is a crucial candidate that responds to cognitive impairment in Alzheimer's disease (AD). The thalamus serves as the primary gateway for sensory input and relays it to the temporal, occipital and frontal cortex[7]. The fronto-striato-thalamic circuits in type 2 diabetes are closely linked to memory, executive function, learning, and attention. Notably, three of the 18 critical connections in the model are associated with the right thalamus (Figure 3). This suggests that brain damage in critical regions related to memory and cognitive loss already exists in these patients, even though they do not exhibit clinical cognitive impairment. Structural scans appear to be more sensitive to the development of the diabetic brain than clinical cognitive diagnosis.
The enhanced connectivity between deep subcortical nuclei (e.g., thalamus, pallidum) and frontal regions (Figure 2) in T2DM patients without cognitive impairment aligns with emerging evidence of adaptive neuroplasticity in preclinical metabolic disorders. For instance, studies in prediabetic cohorts have reported increased functional connectivity in specific local regions, hypothesized to counteract early glucose toxicity and preserve cognitive performance[16]. Similarly, in AD, hyperactivation of the prefrontal cortex during prodromal stages is interpreted as a compensatory response to brain dysfunction[17]. Biologically, this compensatory mechanism may involve synaptic reorganization driven by insulin signaling dysregulation. However, this compensatory capacity may be time-limited. Longitudinal studies in T2DM suggest that prolonged metabolic stress eventually overwhelms adaptive plasticity, leading to network collapse and cognitive decline[18]. Our observation of weakened cortical connections alongside subcortical-frontal hyperconnectivity supports this biphasic model, where early compensation precedes later decompensation. Additionally, further analysis examining the association between white matter connectivity network and clinical data (Supplementary Figure 8) demonstrated a positive correlation between the nodal connectivity efficiency (e.g., nodal betweenness, nodal degree centrality) of deep subcortical nuclei (bilateral pallidum) and blood pressure as well as blood glucose levels. These findings provide additional support for the possibility of early compensation in T2DM individuals.
Another noteworthy discovery is that we can predict the onset time of T2DM with relatively small errors (± 1.9 years) using information collected from current structural MRI scans in leave-one-out cross-validation. The optimal model employs only six selected connections from the 47 connections that passed the permutation tests. This finding supports the notion that the brain undergoes progressive changes in response to the duration of T2DM. Even in patients without cognitive impairment, it is still feasible to detect and monitor their brain disruption development during regular brain scans.
The structural connectivity features based on white matter fiber tracts outperform the regional features based on gray matter volume and surface size in all conditions. As reported in Table 2, the baseline model with connectivity features exhibited superior performance in all assessment metrics (accuracy, precision, recall, and F1-score). Since the baseline models do not penalize model size, and connectivity features have more dimensions than regional features, we further explored how forward feature selection for regional features would improve its performance in classifying diabetic brains. The results are reported in Supplementary Figures 9 and 10 and Supplementary Tables 1 and 2. As observed, the best model using regional features remains inferior to the selected model using connectivity features. Moreover, the connectivity features exhibit lower RMSE and a smaller variance of LOO cross-validation performance in predicting T2DM duration. These results support the notion that structural connections are more sensitive to hyperglycemia than gray matter atrophy measured by voxel-based methods.
The robust performance of white matter connectivity features suggests their potential as biomarkers for early detection of neurological complications in T2DM. Future studies could develop simplified MRI protocols (e.g., optimized DTI sequences) to quantify key fiber tracts as part of routine clinical assessments. This may enable identification of high-risk patients before overt cognitive or motor symptoms emerge. Future work should explore machine learning frameworks that integrate structural connectivity data with metabolic, genetic, and inflammatory markers to build comprehensive risk stratification models.
In this study, we investigated the disrupted brain connectivity in T2DM patients. Initially, we attempted to construct a classification model to distinguish the diabetic brain from the healthy brain. The results indicate that the brain in T2DM becomes characterized before patients exhibit measurable cognitive impairment. This is supported by the high accuracy (92.5%) achieved by the classification model built using brain region connectivity features. To further investigate how brain damage progresses with the duration of T2DM, we developed a regression model utilizing connectivity features to predict the time course of T2DM patients. The final linear regression model achieved an error of ± 1.9 years in predicting the onset of T2DM. During model construction, we employed various feature selection techniques and discovered that structural connections based on white matter fiber tracts are better at distinguishing the diabetic brain than regional gray matter information which is novel and noteworthy.
We are deeply grateful to Prof. Xiao-Hong Yuan (Biostatistic Service, Shandong Linyi People's Hospital) for her rigorous statistical analysis and validation of the results.
| 1. | Behfar Q, Behfar SK, von Reutern B, Richter N, Dronse J, Fassbender R, Fink GR, Onur OA. Graph Theory Analysis Reveals Resting-State Compensatory Mechanisms in Healthy Aging and Prodromal Alzheimer's Disease. Front Aging Neurosci. 2020;12:576627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 3. | Fang F, Zhan YF, Zhuo YY, Yin DZ, Li KA, Wang YF. Brain atrophy in middle-aged subjects with Type 2 diabetes mellitus, with and without microvascular complications. J Diabetes. 2018;10:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Guo WB, Liu F, Chen JD, Xu XJ, Wu RR, Ma CQ, Gao K, Tan CL, Sun XL, Xiao CQ, Chen HF, Zhao JP. Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Jing J, Liu C, Zhu W, Pan Y, Jiang J, Cai X, Zhang Z, Li Z, Zhou Y, Meng X, Cheng J, Wang Y, Li H, Jiang Y, Zheng H, Wang S, Niu H, Wen W, Sachdev PS, Wei T, Liu T, Wang Y. Increased Resting-State Functional Connectivity as a Compensatory Mechanism for Reduced Brain Volume in Prediabetes and Type 2 Diabetes. Diabetes Care. 2023;46:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 6. | Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6594] [Cited by in RCA: 7507] [Article Influence: 469.2] [Reference Citation Analysis (0)] |
| 7. | Lei H, Hu R, Luo G, Yang T, Shen H, Deng H, Chen C, Zhao H, Liu J. Altered Structural and Functional MRI Connectivity in Type 2 Diabetes Mellitus Related Cognitive Impairment: A Review. Front Hum Neurosci. 2021;15:755017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Li M, Li Y, Liu Y, Huang H, Leng X, Chen Y, Feng Y, Ma X, Tan X, Liang Y, Qiu S. Altered Hippocampal Subfields Volumes Is Associated With Memory Function in Type 2 Diabetes Mellitus. Front Neurol. 2021;12:756500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Liang Y, Zhang H, Tan X, Liu J, Qin C, Zeng H, Zheng Y, Liu Y, Chen J, Leng X, Qiu S, Shen D. Local Diffusion Homogeneity Provides Supplementary Information in T2DM-Related WM Microstructural Abnormality Detection. Front Neurosci. 2019;13:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Luo T, Tu YF, Huang S, Ma YY, Wang QH, Wang YJ, Wang J; Alzheimer's Disease Neuroimaging Initiative. Time-dependent impact of type 2 diabetes mellitus on incident prodromal Alzheimer disease: A longitudinal study in 1395 participants. Eur J Neurol. 2023;30:2620-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Münch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 12. | Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 13. | Vanacore N, Di Pucchio A, Lacorte E, Bacigalupo I, Mayer F, Grande G, Cesari M, Canevelli M. [From mild cognitive impairment to dementia: what is the role of public health?]. Recenti Prog Med. 2017;108:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM. Faster permutation inference in brain imaging. Neuroimage. 2016;141:502-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2883] [Cited by in RCA: 2720] [Article Influence: 226.7] [Reference Citation Analysis (0)] |
| 16. | Xiong Y, Tian T, Fan Y, Yang S, Xiong X, Zhang Q, Zhu W. Diffusion Tensor Imaging Reveals Altered Topological Efficiency of Structural Networks in Type-2 Diabetes Patients With and Without Mild Cognitive Impairment. J Magn Reson Imaging. 2022;55:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Yao L, Yang C, Zhang W, Li S, Li Q, Chen L, Lui S, Kemp GJ, Biswal BB, Shah NJ, Li F, Gong Q. A multimodal meta-analysis of regional structural and functional brain alterations in type 2 diabetes. Front Neuroendocrinol. 2021;62:100915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Yu R, Zhang H, An L, Chen X, Wei Z, Shen D. Connectivity strength-weighted sparse group representation-based brain network construction for MCI classification. Hum Brain Mapp. 2017;38:2370-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |