Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105711
Revised: March 24, 2025
Accepted: April 25, 2025
Published online: June 15, 2025
Processing time: 117 Days and 8.6 Hours
Diabetic kidney disease (DKD) has a high incidence and mortality rate and lacks effective preventive and therapeutic methods. Apoptosis is one of the main reasons for the occurrence and development of DKD. Mesenchymal stem cells (MSCs) have shown great promise in tissue regeneration for DKD treatment and have protective effects against DKD, including decreased blood glucose and urinary protein levels and improved renal function. MSCs can directly differentiate into kidney cells or act via paracrine mechanisms to reduce apoptosis in DKD by modulating signaling pathways. MSC-derived extracellular vesicles (MSC-EVs) mitigate apoptosis and DKD-related symptoms by transferring miRNAs to target cells or organs. However, studies on the regulatory mechanisms of MSCs and MSC-EVs in apoptosis in DKD are insufficient. This review comprehensively examines the mechanisms of apoptosis in DKD and research progress regarding the roles of MSCs and MSC-EVs in the disease process.
Core Tip: Diabetic kidney disease (DKD) represents a prevalent and serious complication of diabetes. Mesenchymal stem cells (MSCs) and their extracellular vesicles (MSC-EVs) show promise for treating DKD by regulating apoptosis; however, challenges such as a lack of standardization, hinder their clinical application. This review examines the research progress on the roles of MSCs and MSC-EVs in this disease.
- Citation: Nie P, Qin W, Nie WC, Li B. Progress in the application of mesenchymal stem cells to attenuate apoptosis in diabetic kidney disease. World J Diabetes 2025; 16(6): 105711
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105711.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105711
Diabetic kidney disease (DKD) results from long-term chronic disorders of glucose and lipid metabolism and is a prevalent chronic microvascular complication of diabetes[1].
In 2021, 10.5% of the global population (536.6 million individuals) had diabetes. By 2045, this figure is projected to increase to 12.2% (780 million patients), with type 2 diabetes accounting for approximately 90% of the total incidence[2]. Approximately 20%-40% of patients with diabetes develop DKD[3], which is the primary cause of end-stage renal disease[4,5].
DKD can be caused by multiple factors, such as impaired insulin signaling and metabolic disorders, which can oxidize mitochondria and produce substantial quantities of reactive oxygen species (ROS)[6]. When ROS accumulate in renal cells, they trigger apoptosis of glomerular and tubular cells, leading to matrix reorganization and tissue fibrosis, thus facilitating the development of DKD[7]. Currently, effective methods for preventing and delaying DKD progression are lacking[8]. In recent years, many researchers have suggested that apoptosis or imbalanced death of renal parenchymal cells is one of the main causes of DKD development[9].
Mesenchymal stem cells (MSCs), a type of adult stem cell endowed with a remarkable self-renewal capacity and multi-directional differentiation potential, possess biological characteristics such as immune regulation, low immunogenicity, inflammatory chemotaxis, and tissue repair[10]. They are promising for tissue regeneration in the treatment of DKD and have certain protective effects, including reduced blood glucose and urinary protein levels and improved renal function[11]. Extracellular vesicles (EVs) derived from stem cells alleviate apoptosis and DKD symptoms by transferring miRNAs to target cells or organs, thus making them safer and more efficacious than cell transplantation[12]. However, research on the regulatory mechanisms of MSCs and their EVs in apoptosis in DKD is insufficient. Therefore, this study summarizes the mechanisms of apoptosis in DKD and research progress regarding the functions of MSCs and their EVs in the aforementioned process.
To systematically evaluate the mechanisms by which MSCs regulate apoptosis in DKD, we searched the literature published in PubMed, Web of Science, Embase, and the Cochrane Library from January 2000 to January 2025. We constructed Boolean operator-based search formulas using keywords and synonyms: (“MSCs” or “mesenchymal stem cells”) and (“diabetic kidney disease” or “DKD” or “diabetic nephropathy”) and (“apoptosis” or “programmed cell death”) and (“therapeutic mechanism” or “exosome” or “miRNA”). Search terms were expanded to include MeSH terms, such as “mesenchymal stem cell” (MeSH), in PubMed and free-text variants, including “caspase-3”, “Bax/Bcl2 ratio”, “TUNEL assay”, and other biomarkers related to apoptosis. The inclusion criteria were as follows: Original research explicitly investigating the regulation of apoptotic mechanisms in MSCs or extracellular vesicle-mediated DKD cells, studies on apoptosis-related signaling pathways or biomarkers, and peer-reviewed articles with full texts in English.
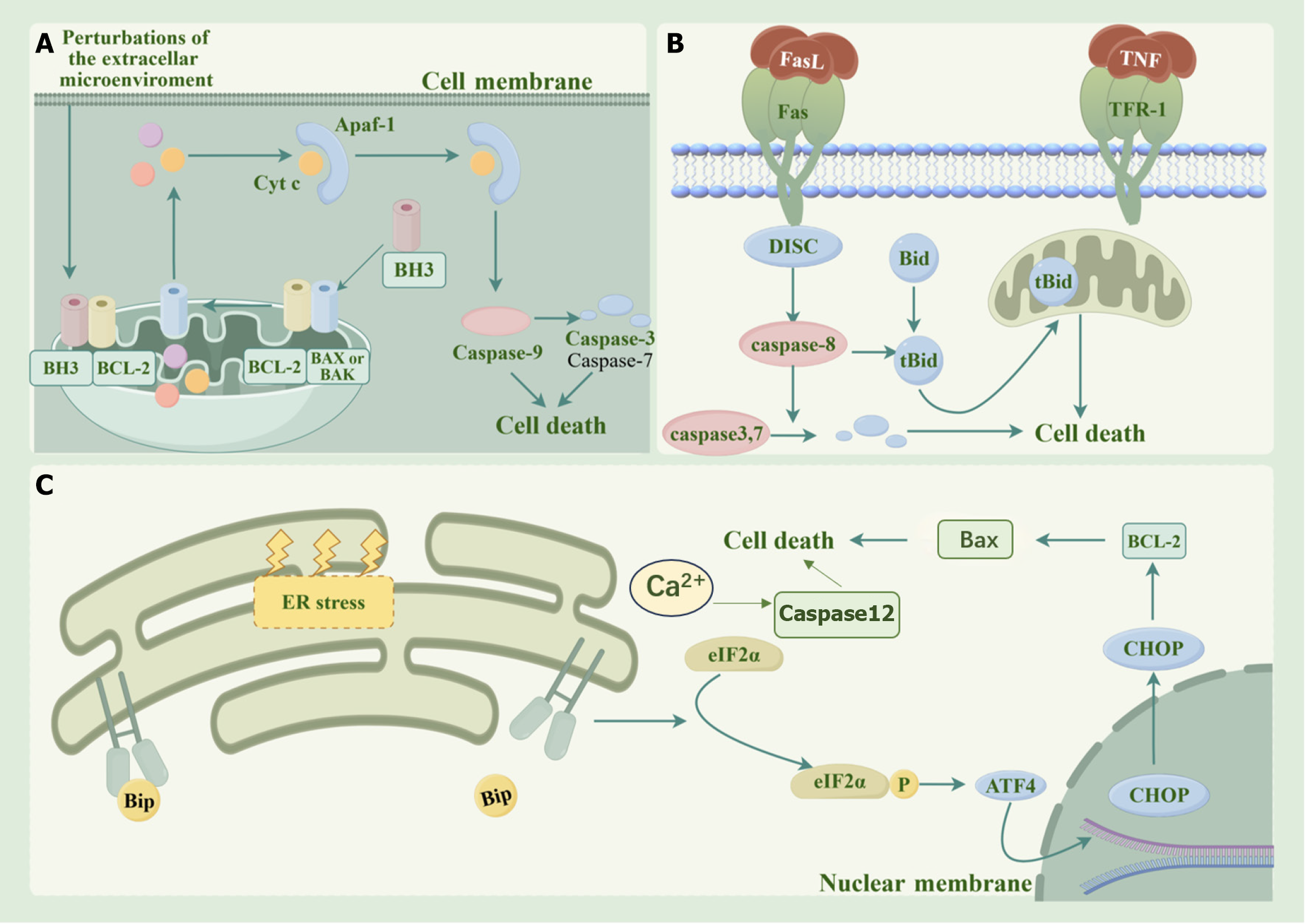
Apoptosis, also known as programmed cell death, is pivotal in DKD initiation and progression. This is a highly regulated process. Apoptosis primarily occurs through endogenous, exogenous, or endoplasmic reticulum (ER) stress-induced pathways, mediated by cysteine proteases (caspases)[13] (Figure 1). The overproduction of advanced glycation end products and ROS caused by hyperglycemia can trigger these three pathways, which interact with each other through the mitochondria, thus reflecting an important mechanism that leads to DKD[14,15]. Common signaling pathways that affect apoptosis in DKD include phosphatidylinositol 3-kinases (PI3K)/protein kinase B (AKT)/mammalian target of ra
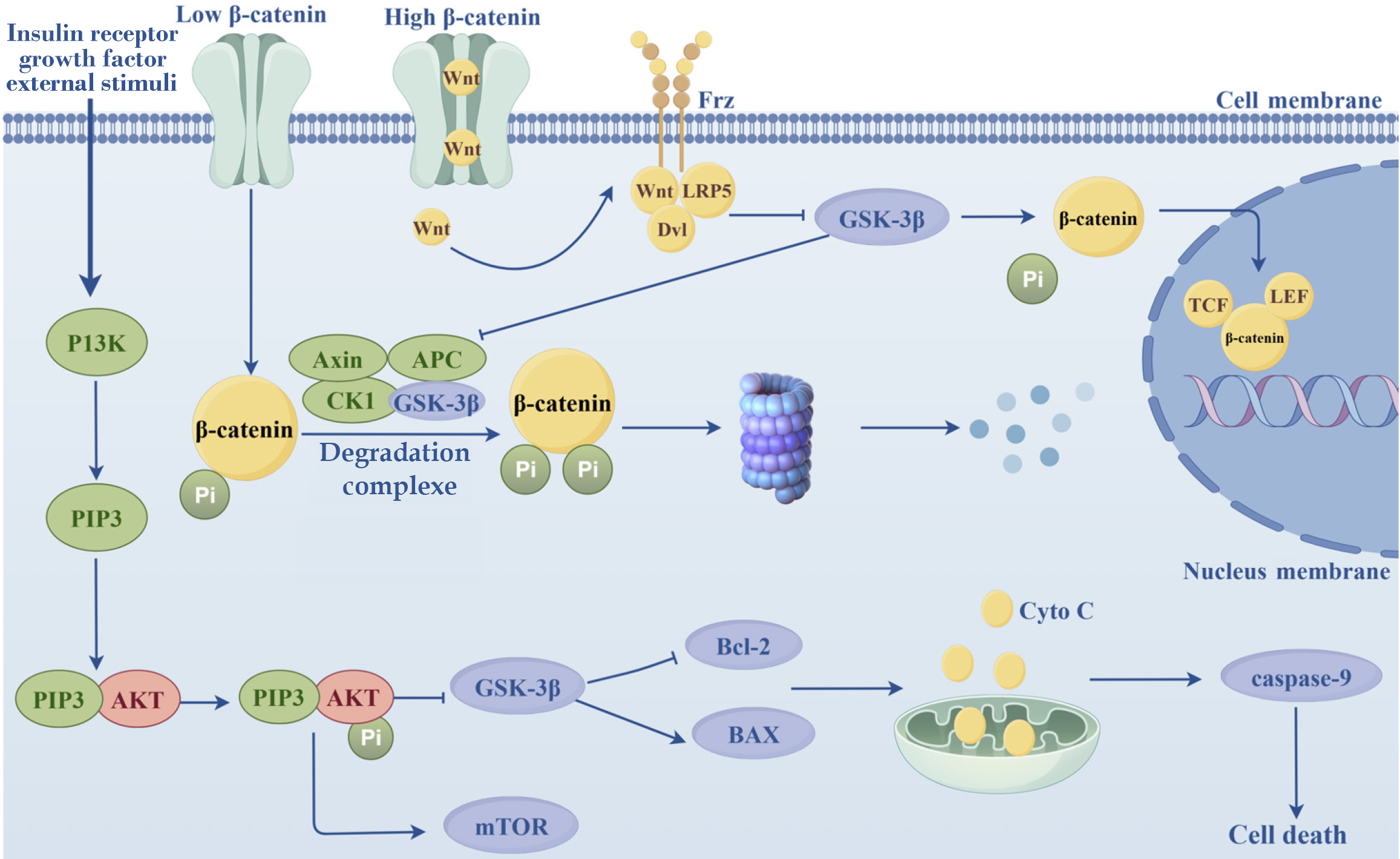
The activation of the PI3K/AKT/mTOR signaling pathway suppresses apoptosis and stimulates cell differentiation and survival, and this pathway plays crucial roles in the prevention and treatment of diabetes[16]. PI3Ks are a family of intracellular lipid-protein kinases that regulate cell proliferation, differentiation, apoptosis, and glucose transport[17-19]. PI3Ks can be activated by insulin receptors, growth factors, and external stimuli to generate the second messenger PIP3, which recognizes and binds to Akt, thus changing its conformation. Akt phosphorylation in turn activates the mTOR[20]. Glycogen synthase kinase-3β (GSK-3β) is a downstream protein of Akt that is widely involved in the regulation of mitochondrial function[21]. In DKD, Akt phosphorylation is impeded, leaving GSK-3β in an activated state[22]. Once activated, GSK-3β modulates the Bax/Bcl2 ratio, which subsequently affects mitochondrial membrane permeability and promotes the release of cytochrome C (Cytc) from the mitochondria, which ultimately plays a role in regulating apoptosis[23,24]. Cytoplasmic Cytc initiates apoptosis by activating the apoptosis promoter caspase 9, which cleaves and hydro
The Wnt/β-catenin axis is a well-studied signaling pathway that affects apoptosis in DKD[26]. When β-catenin protein levels are low, the Wnt pathway is shut down and β-catenin gets phosphorylated by the β-catenin degradation complex[27]. The complex is composed of adenomatous polyposis coli gene protein, axin, GSK-3β, and casein kinase 1[28]. When β-catenin levels increase, the Wnt pathway is activated. The Wnt protein then binds to Fzd and LRP5/6 and recruits the cellular cytoplasmic protein dishevelled, which inhibits GSK-3β activity and prevents the formation of degradation complexes[29]. β-catenin then undergoes dephosphorylation, accumulates in the cytoplasm, and then translocates into the nucleus, where it forms a complex with the nuclear T cell factor/Lymphoid enhancer binding factor and activates a series of downstream genes, such as cyclin D1 and c-myc. These genes regulate the expression of other genes and influence cellular activities, such as proliferation and apoptosis[29]. Previous studies have indicated that the level of β-catenin in the cytoplasm is extremely low in normal kidneys. However, renal injury can trigger the activation of β-catenin in tubular epithelial and mesangial cells[30]. The activation of β-catenin can alleviate apoptosis in DKD, and Nrf2 may be a crucial element in its regulatory route[31].
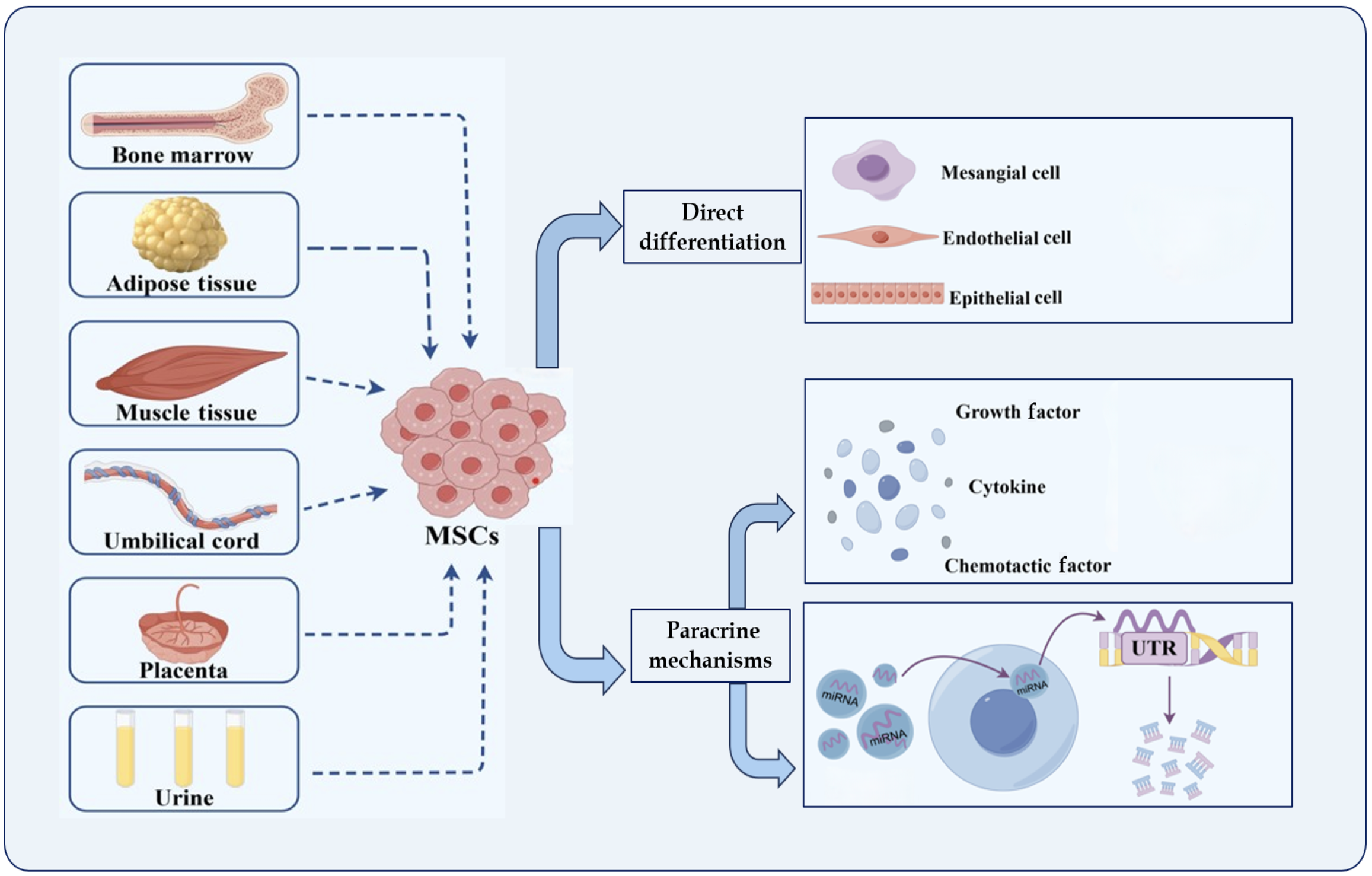
MSCs, which are multipotent stem cells that originate from the mesoderm during the early developmental stage, can be derived from various sources, including bone marrow (BM), placenta, adipose tissue, umbilical cord, and urine. MSCs possess remarkable self-renewal abilities, potential for multidirectional differentiation, and low immunogenicity[32]. Additionally, they can generate a diverse range of cytokines and growth factors that exhibit functions such as hematopoiesis support, immunomodulation, and anti-inflammation[33]. MSCs are promising candidates for tissue regeneration in treating DKD[34]. Numerous studies have shown the therapeutic effects of MSCs on DKD, including the attenuation of oxidative damage[35] and inflammatory responses[36,37], inhibition of apoptosis[38] and fibrosis[39,40], modulation of autophagy[41,42], and maintenance of pedicle cell morphology[43]. Currently, MSCs are believed to act on DKD in various ways, including direct differentiation into damaged tissue cells and induction of paracrine effects.
Prior studies have shown that MSCs have a selective homing effect and can differentiate into various kidney cell types[44], including mesangial cells[45], endothelial cells[46], and epithelial cells[47], thus improving injured kidney tissue and delaying the progression of renal fibrosis[48]. Yokoo et al[49] reported that human BM-derived MSCs (BM-MSCs) could to differentiate into mature kidney structures after being implanted into rat embryos and transplanted into rats. Lee et al[50] found that while undetectable in the lungs, liver, spleen, and other organs, BM-MSCs could colonize the pancreas and kidneys of experimental mice; 11% of the cells migrated to the kidneys. This attenuated the proliferation of glomerular thylakoid membranes and decreased macrophage infiltration.
Furthermore, Ito et al[45] discovered that BM-MSCs can differentiate into glomerular mesangial cells in mice. Additionally, Wong et al[51] co-cultured MSCs with oxidatively damaged glomerular mesangial cells in vitro, analyzed their contractility using flow cytometry, and found that MSCs co-cultured with damaged glomerular mesangial cells had a morphology similar to that of glomerular mesangial cells and contracted upon exposure to Ang II. The CD54 (-) CD62E (+) expression on MSCs in this experiment was directly opposite to that of CD54 (+) CD62E (-) on pure MSCs; therefore, it was concluded that MSCs could differentiate into glomerular mesangial cells.
Biologically active molecules secreted by MSCs: One of the most crucial mechanisms by which MSCs improve renal injury is through paracrine function[52]. Chen et al[53] suggested that BM-MSCs mitigate renal injury by secreting related factors that inhibit inflammatory responses and oxidative damage and enhance vascular regeneration through a paracrine mechanism. Park et al[54] discovered that upregulation of the extracellular matrix and epithelial-mesenchymal transition (EMT) could be influenced by providing conditioned medium to rat kidney cells (NRK-52E) without contact with human umbilical cord blood stem cells (hUCB-MSCs), suggesting that hUCB-MSCs could prevent and control DKD through paracrine effects. Numerous studies have shown that MSCs can secrete various biologically active molecules that can be primarily grouped into three categories: Growth factors, cytokines, and chemokines[55,56]. These molecules, including epidermal growth factor (EGF), hepatocyte growth factor, bone morphogenetic protein-7 (BMP-7), and vascular endo
MSCs secrete EVs: Paracrine secretion by MSCs, of which EVs are the main carriers, has been well documented[61]. MSCs secrete EVs (MSC-EVs) protect the kidneys against damage via multiple mechanisms, including autonomous targeting and anti-apoptotic, anti-inflammatory, antioxidant, and antifibrotic mechanisms[62,63]. Exosomes, a type of EVs with diameters ranging from 30 to 150 nm that are generated within intracellular vesicles, incorporate 350-400 proteins derived from the cytoplasmic membrane[64], and represent a unique mechanism for intercellular communication[65,66]. EVs are found in all types of biological fluids (including blood, cerebrospinal fluid, urine, and tear fluid)[66]. Under physiological conditions, EVs are regulated by donor cells, exchange or transmit information to recipient cells in healthy tissues[67] and influence disease progression[68]. EVs can uniquely transmit and exchange intracellular chemical information, interact with recipient cells, and have been applied in the treatment of various diseases[69,70].
The contents of EVs include signaling molecules and proteins, such as mRNA, miRNA, and other small molecules[71,72], which can be delivered to specific cells, regulate intercellular communication, and induce genetic and epigenetic changes in the target cells[73]. miRNAs are important mediators of EVs that perform distinct biological functions[72]. MSC-EVs contain miRNAs that are closely related to cell survival, differentiation, and immune regulation, including miR-223, miR-564, and miR-451[60]. Notably, these miRNAs exert their biological effects by altering the activity of target cells through various mechanisms[74].
MSC-EVs share similar properties with the MSCs from which they are derived and can perform unique biological functions, such as inducing anti-inflammatory, regenerative, and immunomodulatory activities to promote angiogenesis[75]. MSC-EVs express tetrameric molecules (including CD9, CD63, CD81, and CD107) and MSC markers (CD29, CD73, CD44, and CD105), which are intimately linked to the function of MSC-EVs and are capable of interacting with numerous cell types at neighboring/distant sites and eliciting appropriate cellular responses[76]. MSC-EVs are vital for facilitating the capacity of MSCs to function as matrix-supporting cells, maintain tissue homeostasis, and react to external stimuli[77]. Compared to MSCs, MSC-EVs have the following advantages: Smaller size, stronger targeting, lower cytotoxicity, and potential as a new type of nanomedicine carrier for the treatment of various diseases[78]. MSC-EVs cannot self-replicate; hence, the risk of endogenous tumor formation is remarkably reduced. They also exhibit lower immunogenicity and produce fewer embolisms after intravenous injection, making them safer than MSCs[15]. Furthermore, MSC-EVs possess longer circulating half-lives, high permeability, good biocompatibility, and easier quantification and maintenance of biological activity during storage and transportation. MSC-EVs also have important clinical applications in the treatment of various human diseases (Figure 3).
In recent years, several studies have confirmed that MSCs from different sources can protect renal structures and improve renal function by preventing damaged tubular epithelial cells and podocytes from undergoing abnormal apoptosis (Table 1).
| Cell types | Animal models/cell | Dose | Injection method | Frequency/time | Outcomes | Ref. |
| BM-MSCs | STZ-induced SD rats | 1 × 106 cells | Tail vein | Once | ↑VEGF, Bcl2; ↓TNF-α, TGF-β, Bax | [59] |
| BM-MSCs | Podocytes | Transfected with miR-124a | Co-culture | 24 hours | ↓Apoptosis rate, ↓Bax, caspase-3, ↑Bcl2 | [79] |
| BM-MSCs | STZ-induced rats | 3 × 106 cells with miR-124a 4 ng/mm3 | Tail vein | Once | ↓Bax, caspase-3, ↑Bcl2 | [79] |
| BM-MSCs | STZ-induced male C57BL/6 mice | 1 × 104 cells | Tail vein | Twice | ↓Apoptotic cells, Bax, ROS; ↑ Bcl2 | [82] |
| BM-MSCs | STZ-induced SD rats | 2 × 107 cells | Intraperitoneally | One time at 4 weekly intervals | ↑ATF3, Bcl2; ↓Fas, FasL, P53, caspase-3, BAX,BAX/BCL -2 | [83] |
| BM-MSCs | High-fat diet and STZ administration | 2 × 106 cells | Tail vein | ↑LAMP2; ↓β-cell apoptosis | [84] | |
| AD-MSCs | STZ-induced SD rats | 1 × 107 cells | Tail vein | Once | ↑Bcl2, klotho, ↓Bax, Wnt1, Wnt3a, Snail, β-catenin | [38] |
| AD-MSCs | STZ-induced SD rats | 5 × 106 cells | Tail vein | Five times at 4 weekly intervals | ↑WT1, ↓disruption of the tubules and interstitium | [85] |
| AD-MSCs | MPC5 | 1 × 105 cells/cm2 | Cultured with hAd-MSC-conditioned medium | 24, 48, 72 hours | ↓Cleaved caspase-3; Maintain the normal arrangement of synaptopodin and nephrin in podocytes | [43] |
| hUC-MSCs | STZ-induced SD rats | 2 × 106 cells | Tail vein | Once a week for 2 weeks | ↓Apoptosis rate, ↓Bax, hioredoxin-interacting protein, ↑Bcl2 | [89] |
| hUC-MSCs | STZ-induced SD rats | 2 × 106 cells | Tail vein | three times every 10 days | ↓TUNEL + cells, ↓caspase-3, ↑Bcl2/ Bax | [90] |
BM-MSCs were the first and most frequently used cells to study apoptosis in DKD. Abdel Aziz et al[59] employed a rat model of STZ-induced diabetes and showed that BM-MSCs improved kidney function, regenerated kidney tissues in rats, reduced the expression of the apoptotic gene Bax, and boosted the expression of the anti-apoptotic gene Bcl2. Furthermore, Sun et al[79] injected mouse BM-MSCs via the tail vein into diabetic rats and found that BM-MSCs combined with miR-124 significantly upregulated the expression of nephrin, podocin, CD2AP, and Bcl2 and inhibited the expression of caspase 3 and Bax. These findings suggest that BM-MSCs combined with miR-124 promote podocyte proliferation, inhibit apoptosis, and suppress the Notch signaling pathway in podocytes. VEGF is one of the most significant factors contributing to podocyte integrity[80]. Researchers have previously applied MSC treatment to an animal model of puromycin-induced podocyte injury by intraperitoneal injection for 60 days and found that BM-MSCs administration restored VEGF expression in the kidneys of mice[81]. Konari et al[82] found that BM-MSCs could ameliorate apoptosis in DKD via mitochondrial translocation, and BM-MSC-derived isolated mitochondria augmented the expression of Bcl2 and suppressed ROS generation in vitro. After the intraperitoneal injection of BM-MSCs into type 1 diabetic mice, Khamis et al[83] observed that BM-MSCs led to a significant reduction in the expression of the pro-apoptotic markers Fas, FasL, and Bax and remarkably increased the expression of the anti-apoptotic marker Bcl2. Additionally, Zhao et al[84] reported a significant increase in the co-localization of mitochondria and LC3-positive autophagosomes after treating INS-1 cells with BM-MSCs using immunofluorescence analysis. This observation suggests that BM-MSCs can improve mitochondrial function by removing damaged mitochondria from chronically hyperglycemic INS-1 cells.
Adipose-derived MSCs (AD-MSCs) are increasingly used in DKD research. In STZ-induced diabetic rat kidneys, AD-MSCs decreased the elevated expression of Bax, Wnt1, Wnt3a, and β-catenin, enhanced the expression of Bcl2, and ameliorated renal cell apoptosis by modulating the Wnt/β-catenin signaling pathway[38]. AD-MSCs were subsequently co-cultured with high glucose-stimulated rat renal tubular epithelial cells (NRK-52E), and it was found that AD-MSCs mitigated apoptosis through the inhibition of the Wnt/β-catenin pathway[38]. Zhang et al[85] reported that multiple intravenous injections of AD-MSCs markedly reduced glomerular hypertrophy and decreased urinary protein levels in diabetic rats. Further studies have confirmed that AD-MSCs attenuate HG-induced damage to podocytes and reduce podocyte apoptosis.
Owing to their terminally differentiated state, podocytes possess a restricted capacity for cell division and have limited regeneration following injury, depletion, and senescence. Furthermore, excessive podocyte loss makes patients more prone to glomerular diseases[86]. MSCs have become potential podocyte-protective agents for cell-based therapies to treat podocyte injury in DKD or other chronic kidney diseases owing to their regenerative and paracrine characteristics[87]. Li et al[43] treated mouse podocytes (MPC5) with human AD-MSCs and found that the high level of EGF in the conditioned medium of MSCs was a key factor in preventing damage and apoptosis of podocytes, reducing the level of cleaved caspase 3 and maintaining the normal arrangement of synaptopodin and nephrin in podocytes. Additionally, when an anti-EGF antibody was added to the conditioned medium of MSCs, it impeded their protective effect on podocytes.
Human umbilical cord MSCs (hUC-MSCs) are regarded as a prospective cell type in regenerative medicine because of their availability, low immunogenicity, absence of ethical disputes, and high proliferative potential[88]. Chen et al[89] assessed the impact of hUC-MSCs on diabetic rats and discovered that hUC-MSCs decreased the apoptotic rate of renal cells. Notably, this outcome was achieved by enhancing Bcl-xL expression and activating apoptosis signal-regulated kinase 1 and p38 MAPK. In our previous study, we treated STZ-induced diabetic Sprague Dawley (SD) rats with hUC-MSC via tail vein injection and found that hUC-MSCs could lower the expression of caspase 3 and Bax/Bcl2 and upregulate the expression of Nrf2 and its downstream factors in diabetic rat kidney tissues. We also found that hUC-MSCs reduced DKD-related oxidative damage and apoptosis by activating Nrf2 in vitro[90].
Several studies have shown that autophagy and ER stress are critical for allowing podocytes to cope with injury. Therefore, podocyte autophagy has emerged as a promising therapeutic target in DKD treatment. Liu et al[91] found that placenta-derived MSCs (P-MSCs) have significantly upregulated expression of the autophagy-related regulators, SIRT1, FOXO 1, LC3 II, and beclin 1, in rat renal tissues. This occurred after 8 consecutive weeks of tail vein injection of P-MSCs into type I diabetic rats, which was attenuated by the autophagy inhibitor 3-MA.
In summary, MSCs from different sources inhibit apoptosis in DKD.
Recent studies have found that MSCs contribute to disease amelioration, mainly through the paracrine pathway of EVs, particularly through MSC-derived exosomes. These exosomes can transfer renoprotective miRNAs to damaged mesangial cells and podocytes, thereby exerting beneficial effects in DKD[70]. The relevant studies associated with these findings are presented in Table 2.
| Exosome source | Animal models | miRNA | Dose | Injection method | Frequency/time | Outcomes | Ref. |
| BM-MSCs | Renal tubular epithelial cells | 5.3 × 107 | Co-culture | 96 hours | ↓TGF-β 1, ↓Apotpsis, ↑ZO-1 | [92] | |
| BM-MSCs | HUVECs | miR-146a-5p | 100 μL | Co-ulture | 48 hours | ↑IL-10, ↓apoptosis rate, NF-KB | [93] |
| AD-MSCs | MPC5 | miR-486 | 25 μg/mL | Co-culture | 48 hours | Cleaved caspase-3↓ | [94] |
| AD-MSCs | db/db | miR-486 | Tail vein | 12 weeks | ↓Cleaved caspase-3, ↓p-mTOR | [94] | |
| AD-MSCs | MPC 5 | miR-15b-15p | 25 μg/mL | Co-culture | 24 hours | ↑Caspase3, Bcl2, ↓cleaved caspase3 | [96] |
| AD-MSCs | SD rats with type 1 diabetes | MiR-125a | 50 μg | Tail vein | Twice a week for 3 weeks | ↓Bax, ↑Bcl2, ET1↓ | [97] |
| AD-MSCs | MP5 cells | miR-26a-5p | 25 mg/mL | Co-culture | 48 hours | ↓Cleaved caspase-3, Bax↓ Bcl2↑ | [98] |
| AD-MSCs | db/db mice | miR-26a-5p | Tail vein | Once at 13 weeks | ↓TUNEL + cells; ↓cleaved caspase-3, Bax; ↑Bcl2 | [98] | |
| HUC-MSCs | Podocytes | 30 μg/mL | Co-culture | 48 hours | ↑Podocytes cell viability, ↓apoptosis of podocytes | [99] | |
| HUC-MSCs | HK2 cells | miR-424-5p | 50 μg/mL | Co-culture | 48 hours | ↓Apoptosis rate, ↓TUNEL + cells, ↓Bax,cleaved caspase-3/caspase-3, ↑Bcl2 | [100] |
| HUC-MSCs | db/db | miR-424-5p | 10 mg/kg bw | Tail vein | twice a week for 6 weeks | ↓Bax, cleaved caspase-3/total caspase-3, ↑Bcl2 | [100] |
| HUC-MSCs | STZ-induced SD rat | Mi-R-146a-5p | 2 × 106 | Tail vein | Once a week for 2 weeks | ↑IL-10, M2 macrophage marker; ↓IL-1β, IL-6 and TNF-αmRNA, TRAF6, M1 macrophage marker | [101] |
| HUC-MSCs | Type 2 diabetic BKS. db/db mice | MiR-22-3p | Exo 20, μg/mL | Tail Vein | 3 times in the first week, twice a week in the next 3 weeks | ↑wnt-1; ↓ Caspase-1, ASC | [102] |
| HUC-MSCs | Podocytes | MiR-22-3p | 20 μg/mL | Co-culture | 24 hours | ↑Podocytes cell viability; ↓apoptosis of podocytes; ↓Caspase-1 | [102] |
| USCs | STZ-induced SD rat | VEGF, TGF-β1, angiogenin, BMP-7 | 100 μg | Tail vein | Weekly for 12 weeks | ↓Apoptosis rate; ↓TUNEL + cells; ↓caspase-3 | [103] |
| USCs | HPDCs | miR-16-5p | 0.4 × 105 cells | Co-culture | 4-5 days | ↑Podocyte viability; ↓apoptosis rate, Bax, Caspase-3, α-SMA, VEGFA | [104] |
| USCs | STZ induced SD rat | miR-16-5p | 100 μg | Tail vein | once a week for 12 weeks | ↓VEGFA, MCP-1, TGF-β1, TNF-α | [104] |
| USCs | STZ-induced SD rat | 2 × 106 cells | Tail vein | Six times every other week | ↓TUNEL + cells; ↓caspase-3 | [105] |
Notably, Nagaishi et al[92] administered BM-MSCs and MSC-conditioned medium (MSC-CM) to diabetic mice and found that both interventions reduced proteinuria. EVs purified from the conditioned medium of BM-MSCs exhibited anti-apoptotic effects and safeguarded the tight junction structure of renal tubular epithelial cells, indicating that MSC therapy holds promise as a means of preventing DKD through paracrine effects. Furthermore, Chen et al[93] found that AS-IV treatment promoted MSC-exo secretion and augmented the expression of miR-146a-5p. When MSCs-EVs with high miR-146a-5p expression were transferred to HG-damaged human umbilical vein endothelial cells (HUVECs), an improvement in cell viability and tube-forming ability was noted. Simultaneously, the apoptosis rate and inflammatory response decreased. After HUVECs were treated with the MSC miR-146a-5p inhibitor, the mRNA and protein levels of tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6) and NF-κB were markedly increased. These observations indicate that miR-146a-5p restrains TRAF6 and phosphorylated NF-κB expression. Additionally, Ebrahim et al[42] reported that exosomes derived from BM-MSCs can trigger autophagy via the mTOR signaling pathway, thereby ameliorating renal damage in a type 1 DKD rat model.
Jin et al[94] found that AD-MSC-EVs suppressed the Smad1/mTOR signaling pathway in podocytes by elevating the expression of miR-486, thus leading to an increase in autophagy, decrease in podocyte apoptosis, and improvement in DKD symptoms. Furthermore, a recent study showed that AD-MSCs-EVs are capable of delivering miR-215-5p to podocytes and inhibiting high glucose-induced accumulation of ZEB2 by directly targeting the 3’-UTR of ZEB2, thus weakening HG-induced podocyte EMT and eliciting a sequence of pathological alterations that eventually relieve the symptoms of DKD[95]. Furthermore, Zhao et al[96] found that miR-15b-5p suppression counteracted the protective effect of AD-MSCs-EVs on podocytes in a high-glycemic milieu, suggesting that AD-MSCs-EVs protect podocytes in a high-glycemic milieu by enhancing miR-15b-5p expression. Additionally, PDK4 expression increased after inhibiting miR-15b-5p expression, and knockdown of PDK4 resulted in decreased apoptosis and reduced inflammation in podocytes in a high-glucose environment. These findings suggest that miR-15b-5p protects podocytes in a high-glucose environment by downregulating its target gene, PDK4. MiR-15b-5p modulates the expression of VEGF-A via PDK4[96]. Moreover, inhibition of VEGFA can mitigate the injury of MPC5 cells in a high-glucose environment by decreasing inflammation and apoptosis[96]. Hao et al[97] showed that AD-MSC-EVs regulate apoptosis in type 1 DKD cells through miRNA-125a. Duan et al[98] found that AD-MSC-EVs reduce the apoptosis of podocytes induced by high glucose stimulation. After applying them to db/db mice, it was found that AD-MSCs-EVs rich in miR-26a-5p could target TLR4 and inhibit the NF-κB/VEGFA pathway, thus suppressing cell apoptosis and alleviating the damage to podocytes in db/db mice.
Additionally, Wang et al[99] using both in vivo and in vitro studies, discovered that elevated glucose levels could induce apoptosis and simultaneously activate the nucleotide-binding and oligomerization domain-containing protein 2 (NOD2) signaling pathway in podocytes. HUC-MSC-EVs inhibit the NOD2 signaling pathway by inhibiting the phosphorylated forms of its downstream effector molecules, namely phosphorylated receptor-interacting protein 2 and NF-κB p65. Thus, they alleviate the apoptosis and inflammation induced by high glucose. Cui et al[100] found that hUC-MSC-EVs inhibit hyperglycemia-induced apoptosis and EMT in HK2 cells and db/db mice and express miR-424-5p. They transfected HUC-MSCs with an miR-424-5p inhibitor and then extracted exosomes. They also found that miR-424-5p expression was reduced and could not reduce apoptosis, EMT, and the expression of Yes-associated protein 1 (YAP1). Their research presented fresh perspectives on the protective function mediated by hUC-MSC-EVs in DKD, where hUC-MSC-EVs were able to suppress high-glucose-induced apoptosis and EMT by miR-424-5p targeting of YAP1.
Zhang et al[101] injected hUC-MSCs into STZ-treated male SD rats through the tail vein and found that they restored miR-146a-5p expression in rat kidneys. MiR-146a-5p derived from hUC-MSCs inhibits renal inflammation and restores renal function by targeting the TRAF6-STAT1 pathway and promoting M2 macrophage polarization. Additionally, Wang et al[102] showed that hUC-MSC-EVs attenuate podocyte apoptosis, as well as the levels of NLRP3 and proteins in its downstream signaling pathway, in high-glucose-treated samples and diabetic mice. When miR-22-3p was knocked down in MSC-EVs, its anti-apoptotic and anti-inflammatory functions were eliminated both in vitro and in vivo, suggesting that EVs containing miR-22-3p derived from hUC-MSCs could be targeted to suppress the activation of NLRP3 signaling and reduce apoptosis and inflammation of podocytes in DKD.
Jiang et al[103] used an STZ-induced diabetic rat model to demonstrate that urothelial stem cells (USCs) reduced urinary protein content, decreased caspase 3 expression, and attenuated apoptosis of glomerular podocytes. They also showed that USC intervention could inhibit podocyte apoptosis in a cellular model of podocyte injury induced by high-glucose culture medium. The researchers further examined the expression of cytokines in USCs-conditioned medium and USCs-exo and found that the expression of VEGF, transforming growth factor-β, angiopoietin, and BMP-7 was notably higher in USCs-conditioned medium than in the control medium in which USCs were not cultured. USC-exo has been shown to inhibit VEGFA by overexpressing miR-16-5p, promoting podocyte proliferation, and inhibiting podocyte apoptosis, thus protecting podocytes from DKD damage[104]. Dong et al[105] used to STZ-induced diabetic rats and found that USC intervention improved urinary protein and renal function, reduce caspase 3 expression, and attenuated apoptosis in the renal tissues of diabetic rats.
Collectively, MSC-derived EVs from heterogeneous sources exert regulatory effects on apoptotic pathways in diabetes models, with their therapeutic efficacy largely attributable to miRNA-dependent mechanisms.
Although this study highlights MSC-mediated suppression of apoptosis, recent findings suggest that pyroptosis[106] and ferroptosis[107] may contribute to DKD progression. Further work should explore whether MSC-derived exosomes modulate these pathways to achieve broader protective effects against DKD.
Despite the promising preclinical evidence, multiple obstacles impede the clinical use of MSC therapy for DKD[108]. First, up to now, the majority of studies have been carried out on rodent models, with limited data from large animal studies, such as primates or pigs, that better mimic human renal physiology. Second, although MSCs exhibit low immunogenicity, their long-term safety profile remains controversial. Pretreatment of tumor cells with a human MSC-CM was sufficient to promote tumor growth, compared to the in vivo co-injection of MSCs in a mouse xenograft model[109]. These findings highlight the importance of long-term tumor surveillance in future clinical trials. Finally, standardized protocols for MSCs isolation, expansion, and delivery are lacking, which affects efficacy outcomes[110]. The translation of preclinical research findings into reliable, efficacious, and secure therapies for patients remains restricted[111], and the best way to perform in vivo implantations, the optimal dosage and duration of treatment, and how to improve the culture conditions of the cells to further enhance the viability and functionality of stem cells in the body is unclear. These issues underscore the need for rigorous trials before making further clinical recommendations.
To enhance the stability and quality of MSCs and MSC-EVs, novel strategies have emerged, including pretreatment technologies or genetic manipulation to optimize the secretory profile of MSCs and integration of MSCs with advanced biomaterials to improve tissue-specific engraftment[112] of synthetic nanoparticles that stabilize MSCs and amplify therapeutic efficacy[113].
In conclusion, advancing the translational applications of MSCs in large animal studies requires the optimization of experimental models, standardization of protocols for manufacturing and characterization, and multidisciplinary collaborations that bridge regenerative medicine and bioengineering.
MSCs and their EVs can reduce apoptosis and slow DKD progression. As a novel cell-free therapeutic approach, MSC-EVs can transport miRNAs (such as miR-16-5p and miR-22-3p) to target cells or organs. They can relieve DKD symptoms by regulating apoptosis-related signaling pathways, making them safer and more effective than cell transplantation. However, as a new mode of stem cell application, challenges such as limited large-animal data, controversial long-term safety, and a lack of standardized protocols impede their clinical use. With continuous advancements in technology, MSC-EVs are believed to become an effective strategy for alleviating DKD symptoms in the future.
We thank our colleagues for suggestions on this manuscript.
| 1. | Navarro-González JF, Sánchez-Niño MD, Donate-Correa J, Martín-Núñez E, Ferri C, Pérez-Delgado N, Górriz JL, Martínez-Castelao A, Ortiz A, Mora-Fernández C. Effects of Pentoxifylline on Soluble Klotho Concentrations and Renal Tubular Cell Expression in Diabetic Kidney Disease. Diabetes Care. 2018;41:1817-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Claude Mbanya J, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. Erratum to "IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045" [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res Clin Pract. 2023;204:110945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 3. | Zou LX, Sun L. Global diabetic kidney disease research from 2000 to 2017: A bibliometric analysis. Medicine (Baltimore). 2019;98:e14394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Sagoo MK, Gnudi L. Diabetic Nephropathy: An Overview. Methods Mol Biol. 2020;2067:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 5. | Młynarska E, Buławska D, Czarnik W, Hajdys J, Majchrowicz G, Prusinowski F, Stabrawa M, Rysz J, Franczyk B. Novel Insights into Diabetic Kidney Disease. Int J Mol Sci. 2024;25:10222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | Coughlan MT, Sharma K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016;90:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Selby NM, Taal MW. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 9. | Erekat NS. Programmed Cell Death in Diabetic Nephropathy: A Review of Apoptosis, Autophagy, and Necroptosis. Med Sci Monit. 2022;28:e937766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 10. | Lou S, Duan Y, Nie H, Cui X, Du J, Yao Y. Mesenchymal stem cells: Biological characteristics and application in disease therapy. Biochimie. 2021;185:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Zhang C, Guo R, Wu D, Shi J, Li L, Chu Y, Yuan X, Gao J. Mesenchymal Stem Cells: An Overview of Their Potential in Cell-Based Therapy for Diabetic Nephropathy. Stem Cells Int. 2021;2021:6620811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Wang SY, Hong Q, Zhang CY, Yang YJ, Cai GY, Chen XM. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res Ther. 2019;10:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39:BSR20180992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 609] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 14. | Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Díaz AG. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int J Endocrinol. 2018;2018:1875870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Xu L, Fan Q, Wang X, Zhao X, Wang L. Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 2016;7:e2445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Zhang P, Liu Z, Ma G, Wang J, Shao J, Ma C, Wang L, Ma C. Huaiqihuang (HQH) protects podocytes from high glucose-induced apoptosis and inflammation response by regulating PI3K/AKT/mTOR pathway. Arch Physiol Biochem. 2024;1-8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Zhao L, Yang XR, Han X. MicroRNA-146b induces the PI3K/Akt/NF-κB signaling pathway to reduce vascular inflammation and apoptosis in myocardial infarction by targeting PTEN. Exp Ther Med. 2019;17:1171-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Wang XM, Yao M, Liu SX, Hao J, Liu QJ, Gao F. Interplay between the Notch and PI3K/Akt pathways in high glucose-induced podocyte apoptosis. Am J Physiol Renal Physiol. 2014;306:F205-F213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Ren Q, You Yu S. CD2-associated protein participates in podocyte apoptosis via PI3K/Akt signaling pathway. J Recept Signal Transduct Res. 2016;36:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Zhou L, Xue H, Yuan P, Ni J, Yu C, Huang Y, Lu LM. Angiotensin AT1 receptor activation mediates high glucose-induced epithelial-mesenchymal transition in renal proximal tubular cells. Clin Exp Pharmacol Physiol. 2010;37:e152-e157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Yang K, Chen Z, Gao J, Shi W, Li L, Jiang S, Hu H, Liu Z, Xu D, Wu L. The Key Roles of GSK-3β in Regulating Mitochondrial Activity. Cell Physiol Biochem. 2017;44:1445-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Chen M, Fang Y, Ge Y, Qiu S, Dworkin L, Gong R. The redox-sensitive GSK3β is a key regulator of glomerular podocyte injury in type 2 diabetic kidney disease. Redox Biol. 2024;72:103127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Liu H, Peng H, Chen S, Liu Y, Xiang H, Chen R, Chen W, Zhao S, Chen P, Lu H. S1PR2 antagonist protects endothelial cells against high glucose-induced mitochondrial apoptosis through the Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2017;490:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Al-Damry NT, Attia HA, Al-Rasheed NM, Al-Rasheed NM, Mohamad RA, Al-Amin MA, Dizmiri N, Atteya M. Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3β and p38α/MAPK in a rat model of diabetic cardiomyopathy. Biomed Pharmacother. 2018;107:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Ying C, Mao Y, Chen L, Wang S, Ling H, Li W, Zhou X. Bamboo leaf extract ameliorates diabetic nephropathy through activating the AKT signaling pathway in rats. Int J Biol Macromol. 2017;105:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Chen X, Tan H, Xu J, Tian Y, Yuan Q, Zuo Y, Chen Q, Hong X, Fu H, Hou FF, Zhou L, Liu Y. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/β-catenin signaling. Kidney Int. 2022;102:506-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 1217] [Article Influence: 405.7] [Reference Citation Analysis (0)] |
| 28. | Bose M, Almas S, Prabhakar S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med. 2017;65:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39:474-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Yang Z, Sun L, Nie H, Liu H, Liu G, Guan G. Connective tissue growth factor induces tubular epithelial to mesenchymal transition through the activation of canonical Wnt signaling in vitro. Ren Fail. 2015;37:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Wang S, Nie P, Lu X, Li C, Dong X, Yang F, Luo P, Li B. Nrf2 participates in the anti-apoptotic role of zinc in Type 2 diabetic nephropathy through Wnt/β-catenin signaling pathway. J Nutr Biochem. 2020;84:108451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Zhao Y, Guo Y, Jiang Y, Zhu X, Liu Y, Zhang X. Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem Biophys Res Commun. 2017;494:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 34. | Hamza AH, Al-Bishri WM, Damiati LA, Ahmed HH. Mesenchymal stem cells: a future experimental exploration for recession of diabetic nephropathy. Ren Fail. 2017;39:67-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Sávio-Silva C, Soinski-Sousa PE, Simplício-Filho A, Bastos RMC, Beyerstedt S, Rangel ÉB. Therapeutic Potential of Mesenchymal Stem Cells in a Pre-Clinical Model of Diabetic Kidney Disease and Obesity. Int J Mol Sci. 2021;22:1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 37. | Lv S, Liu G, Sun A, Wang J, Cheng J, Wang W, Liu X, Nie H, Guan G. Mesenchymal stem cells ameliorate diabetic glomerular fibrosis in vivo and in vitro by inhibiting TGF-β signalling via secretion of bone morphogenetic protein 7. Diab Vasc Dis Res. 2014;11:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 38. | Ni W, Fang Y, Xie L, Liu X, Shan W, Zeng R, Liu J, Liu X. Adipose-Derived Mesenchymal Stem Cells Transplantation Alleviates Renal Injury in Streptozotocin-Induced Diabetic Nephropathy. J Histochem Cytochem. 2015;63:842-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 39. | Bai Y, Wang J, He Z, Yang M, Li L, Jiang H. Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease via Lipoxin A4 by Targeting Transforming Growth Factor β (TGF-β)/smad Pathway and Pro-Inflammatory Cytokines. Med Sci Monit. 2019;25:3069-3076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Lang H, Dai C. Effects of Bone Marrow Mesenchymal Stem Cells on Plasminogen Activator Inhibitor-1 and Renal Fibrosis in Rats with Diabetic Nephropathy. Arch Med Res. 2016;47:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 41. | Cai X, Zou F, Xuan R, Lai XY. Exosomes from mesenchymal stem cells expressing microribonucleic acid-125b inhibit the progression of diabetic nephropathy via the tumour necrosis factor receptor-associated factor 6/Akt axis. Endocr J. 2021;68:817-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells. 2018;7:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 43. | Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B, Fu B, Shaoyuan C, Zhang W, Xuefeng S, Li R, Chen X. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 238] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 46. | Rookmaaker MB, Smits AM, Tolboom H, Van 't Wout K, Martens AC, Goldschmeding R, Joles JA, Van Zonneveld AJ, Gröne HJ, Rabelink TJ, Verhaar MC. Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am J Pathol. 2003;163:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 434] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 48. | Li J, Deane JA, Campanale NV, Bertram JF, Ricardo SD. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells. 2007;25:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, Hosoya T, Okabe M, Kobayashi E. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol. 2006;17:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438-17443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 549] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 51. | Wong CY, Tan EL, Cheong SK. In vitro differentiation of mesenchymal stem cells into mesangial cells when co-cultured with injured mesangial cells. Cell Biol Int. 2014;38:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Li X, Li X, Yang J, Lin J, Zhu Y, Xu X, Cui W. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small. 2023;19:e2207211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 53. | Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 54. | Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Chou YH, Pan SY, Yang CH, Lin SL. Stem cells and kidney regeneration. J Formos Med Assoc. 2014;113:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Li H, Rong P, Ma X, Nie W, Chen C, Yang C, Zhang J, Dong Q, Wang W. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018;215:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 57. | Hickson LJ, Eirin A, Conley SM, Taner T, Bian X, Saad A, Herrmann SM, Mehta RA, McKenzie TJ, Kellogg TA, Kirkland JL, Tchkonia T, Saadiq IM, Tang H, Jordan KL, Zhu X, Griffin MD, Rule AD, van Wijnen AJ, Textor SC, Lerman LO. Diabetic Kidney Disease Alters the Transcriptome and Function of Human Adipose-Derived Mesenchymal Stromal Cells but Maintains Immunomodulatory and Paracrine Activities Important for Renal Repair. Diabetes. 2021;70:1561-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, Hussein RE, Abdelaziz M. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Bochon B, Kozubska M, Surygała G, Witkowska A, Kuźniewicz R, Grzeszczak W, Wystrychowski G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int J Mol Sci. 2019;20:2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Wu P, Tang Y, Jin C, Wang M, Li L, Liu Z, Shi H, Sun Z, Hou X, Chen W, Xu W, Qian H. Neutrophil membrane engineered HucMSC sEVs alleviate cisplatin-induced AKI by enhancing cellular uptake and targeting. J Nanobiotechnology. 2022;20:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 62. | Lu Y, Wang L, Zhang M, Chen Z. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles: A Novel Approach for Kidney Disease Treatment. Int J Nanomedicine. 2022;17:3603-3618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 63. | Huang W, Hong S, Zhu X, Alsaeedi MH, Tang H, Krier JD, Gandhi D, Jordan KL, Saadiq IM, Jiang Y, Eirin A, Lerman LO. Obesity Blunts the Effect of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Kidney Int Rep. 2023;8:1841-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (1)] |
| 64. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4132] [Article Influence: 413.2] [Reference Citation Analysis (0)] |
| 65. | Henning RJ. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J Cardiovasc Transl Res. 2021;14:195-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 66. | Wu Y, Chen W, Guo M, Tan Q, Zhou E, Deng J, Li M, Chen J, Yang Z, Jin Y. Metabolomics of Extracellular Vesicles: A Future Promise of Multiple Clinical Applications. Int J Nanomedicine. 2022;17:6113-6129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 67. | Gurunathan S, Kang MH, Song H, Kim NH, Kim JH. The role of extracellular vesicles in animal reproduction and diseases. J Anim Sci Biotechnol. 2022;13:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Perez Hurtado EC, Henao Agudelo JS, Foganholi da Silva RA, Viração TA, Fernandes CJDC. The role of extracellular vesicles in cancer. Curr Top Membr. 2024;94:247-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Yang X, Gao X, Jiang X, Yue K, Luo P. Targeting capabilities of engineered extracellular vesicles for the treatment of neurological diseases. Neural Regen Res. 2025;20:3076-3094. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Peng L, Chen Y, Shi S, Wen H. Stem cell-derived and circulating exosomal microRNAs as new potential tools for diabetic nephropathy management. Stem Cell Res Ther. 2022;13:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 71. | Kim H, Dusabimana T, Kim SR, Je J, Jeong K, Kang MC, Cho KM, Kim HJ, Park SW. Supplementation of Abelmoschus manihot Ameliorates Diabetic Nephropathy and Hepatic Steatosis by Activating Autophagy in Mice. Nutrients. 2018;10:1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Hu M, Shen X, Zhou L. Role of Extracellular Vesicle-Derived Noncoding RNAs in Diabetic Kidney Disease. Kidney Dis (Basel). 2024;10:303-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 73. | Pontrelli P, Oranger A, Barozzino M, Conserva F, Papale M, Gesualdo L. The pathological role of the ubiquitination pathway in diabetic nephropathy. Minerva Med. 2018;109:53-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 74. | Hu R, Li X, Peng C, Gao R, Ma L, Hu J, Luo T, Qing H, Wang Y, Ge Q, Wang Z, Wu C, Xiao X, Yang J, Young MJ, Li Q, Yang S. miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Hade MD, Suire CN, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021;10:1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 76. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1230] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 77. | Padinharayil H, Varghese J, Wilson C, George A. Mesenchymal stem cell-derived exosomes: Characteristics and applications in disease pathology and management. Life Sci. 2024;342:122542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 78. | Song Y, Wu J, Liu Y, Xu N, Bai H, Wang L, Ai J, Li K. The remodeling of ovarian function: targeted delivery strategies for mesenchymal stem cells and their derived extracellular vesicles. Stem Cell Res Ther. 2024;15:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Sun J, Zhao F, Zhang W, Lv J, Lv J, Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med. 2018;22:4840-4855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol. 2012;32:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Ornellas FM, Ramalho RJ, Fanelli C, Garnica MR, Malheiros DMAC, Martini SV, Morales MM, Noronha IL. Mesenchymal Stromal Cells Induce Podocyte Protection in the Puromycin Injury Model. Sci Rep. 2019;9:19604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Konari N, Nagaishi K, Kikuchi S, Fujimiya M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9:5184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 83. | Khamis T, Abdelkhalek A, Abdellatif H, Dwidar N, Said A, Ahmed R, Wagdy K, Elgarhy R, Eltahan R, Mohamed H, Said Amer E, Hanna M, Ragab T, Kishk A, Wael J, Sarhan E, Saweres L, Reda M, Elkomy S, Mohamed A, Samy A, Khafaga A, Shaker Y, Yehia H, Alanazi A, Alassiri M, Tîrziu E, Bucur IM, Arisha AH. BM-MSCs alleviate diabetic nephropathy in male rats by regulating ER stress, oxidative stress, inflammation, and apoptotic pathways. Front Pharmacol. 2023;14:1265230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Zhao K, Hao H, Liu J, Tong C, Cheng Y, Xie Z, Zang L, Mu Y, Han W. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death Dis. 2015;6:e1885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Zhang L, Li K, Liu X, Li D, Luo C, Fu B, Cui S, Zhu F, Zhao RC, Chen X. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22:3074-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Zhan P, Zhang Y, Shi W, Liu X, Qiao Z, Wang Z, Wang X, Wu J, Tang W, Sun Y, Zhang Y, Zhen J, Shang J, Liu M, Yi F. Myeloid-derived growth factor deficiency exacerbates mitotic catastrophe of podocytes in glomerular disease. Kidney Int. 2022;102:546-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 87. | Meng J, Gao X, Liu X, Zheng W, Wang Y, Wang Y, Sun Z, Yin X, Zhou X. Effects of xenogeneic transplantation of umbilical cord-derived mesenchymal stem cells combined with irbesartan on renal podocyte damage in diabetic rats. Stem Cell Res Ther. 2024;15:239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Hsiao PJ, Kao WY, Sung LC, Lin CY, Tsou LL, Kao YH, Chou CL, Lee KT. The Role of Mesenchymal Stem Cells in Treating Diabetic Kidney Disease: Immunomodulatory Effects and Kidney Regeneration. Int J Med Sci. 2025;22:1720-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Chen L, Xiang E, Li C, Han B, Zhang Q, Rao W, Xiao C, Wu D. Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Nephrocyte Injury and Proteinuria in a Diabetic Nephropathy Rat Model. J Diabetes Res. 2020;2020:8035853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Nie P, Bai X, Lou Y, Zhu Y, Jiang S, Zhang L, Tian N, Luo P, Li B. Human umbilical cord mesenchymal stem cells reduce oxidative damage and apoptosis in diabetic nephropathy by activating Nrf2. Stem Cell Res Ther. 2021;12:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Liu H, Wang J, Yue G, Xu J. Placenta-derived mesenchymal stem cells protect against diabetic kidney disease by upregulating autophagy-mediated SIRT1/FOXO1 pathway. Ren Fail. 2024;46:2303396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 92. | Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 93. | Chen J, Chen J, Li Q, Hu M, Zhong X, Yu L, Zhang X, Huang H, Liu J, Huang Z, Liu X, Xiong W. Astragaloside promotes the secretion of MSC-derived exosomal miR-146a-5p by regulating TRAF6/NF-κB pathway to attenuate inflammation in high glucose-impaired endothelial cells. In Vitro Cell Dev Biol Anim. 2025;61:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 95. | Jin J, Wang Y, Zhao L, Zou W, Tan M, He Q. Exosomal miRNA-215-5p Derived from Adipose-Derived Stem Cells Attenuates Epithelial-Mesenchymal Transition of Podocytes by Inhibiting ZEB2. Biomed Res Int. 2020;2020:2685305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 96. | Zhao T, Jin Q, Kong L, Zhang D, Teng Y, Lin L, Yao X, Jin Y, Li M. microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis. J Bioenerg Biomembr. 2022;54:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Hao Y, Miao J, Liu W, Cai K, Huang X, Peng L. Mesenchymal Stem Cell-Derived Exosomes Carry MicroRNA-125a to Protect Against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. Diabetes Metab Syndr Obes. 2021;14:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 98. | Duan Y, Luo Q, Wang Y, Ma Y, Chen F, Zhu X, Shi J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J Biol Chem. 2020;295:12868-12884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 99. | Wang Y, Lu D, Lv S, Liu X, Liu G. Mesenchymal stem cell-derived exosomes ameliorate diabetic kidney disease through NOD2 signaling pathway. Ren Fail. 2024;46:2381597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 100. | Cui C, Zang N, Song J, Guo X, He Q, Hu H, Yang M, Wang Y, Yang J, Zou Y, Gao J, Wang L, Wang C, Liu F, He F, Hou X, Chen L. Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J. 2022;36:e22517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Le X, Zheng S, Zhang K, He J, Liu M, Tu C, Rao W, Du H, Ouyang Y, Li C, Wu D. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res Ther. 2022;13:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 102. | Wang Y, Liu J, Wang H, Lv S, Liu Q, Li S, Yang X, Liu G. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Diabetic Kidney Disease Through the NLRP3 Signaling Pathway. Stem Cells. 2023;41:368-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 103. | Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 104. | Duan YR, Chen BP, Chen F, Yang SX, Zhu CY, Ma YL, Li Y, Shi J. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J Cell Mol Med. 2021;25:10798-10813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 105. | Dong X, Zhang T, Liu Q, Zhu J, Zhao J, Li J, Sun B, Ding G, Hu X, Yang Z, Zhang Y, Li L. Beneficial effects of urine-derived stem cells on fibrosis and apoptosis of myocardial, glomerular and bladder cells. Mol Cell Endocrinol. 2016;427:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 106. | Zheng F, Ma L, Li X, Wang Z, Gao R, Peng C, Kang B, Wang Y, Luo T, Wu J, Yang Y, Gong L, Li Q, Yang S, Hu J. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes. 2022;71:2739-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 107. | Kim S, Kang SW, Joo J, Han SH, Shin H, Nam BY, Park J, Yoo TH, Kim G, Lee P, Park JT. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 108. | Eirin A, Lerman LO. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles for Chronic Kidney Disease: Are We There Yet? Hypertension. 2021;78:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 109. | Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X, Xu WR. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10:3198-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Deszcz I. Stem Cell-Based Therapy and Cell-Free Therapy as an Alternative Approach for Cardiac Regeneration. Stem Cells Int. 2023;2023:2729377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 111. | Peired AJ, Sisti A, Romagnani P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016;2016:4798639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 112. | Vizoso FJ, Costa LA, Eiro N. Mesenchymal Stem Cells and Their Derived Products in Ageing and Diseases. Int J Mol Sci. 2024;25:6979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 113. | Altalhi SA, Shati AA, Alfaifi MY, Al-Salmi FA, Elbehairi SEI, Alqahtani LS, Fayad E, Elshaarawy RFM, Nasr AM. Therapeutic potential and protection enhancement of mesenchymal stem cell against cisplatin-induced nephrotoxicity using hyaluronic acid-chitosan nanoparticles as an adjuvant. Int J Pharm. 2023;640:123023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |