Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105496
Revised: April 7, 2025
Accepted: May 20, 2025
Published online: June 15, 2025
Processing time: 140 Days and 6.3 Hours
The relationship between low physical activity and cognitive impairment in type 2 diabetes mellitus (T2DM) patients remains unclear.
To explore this association and identify risk factors for cognitive impairment in elderly T2DM patients.
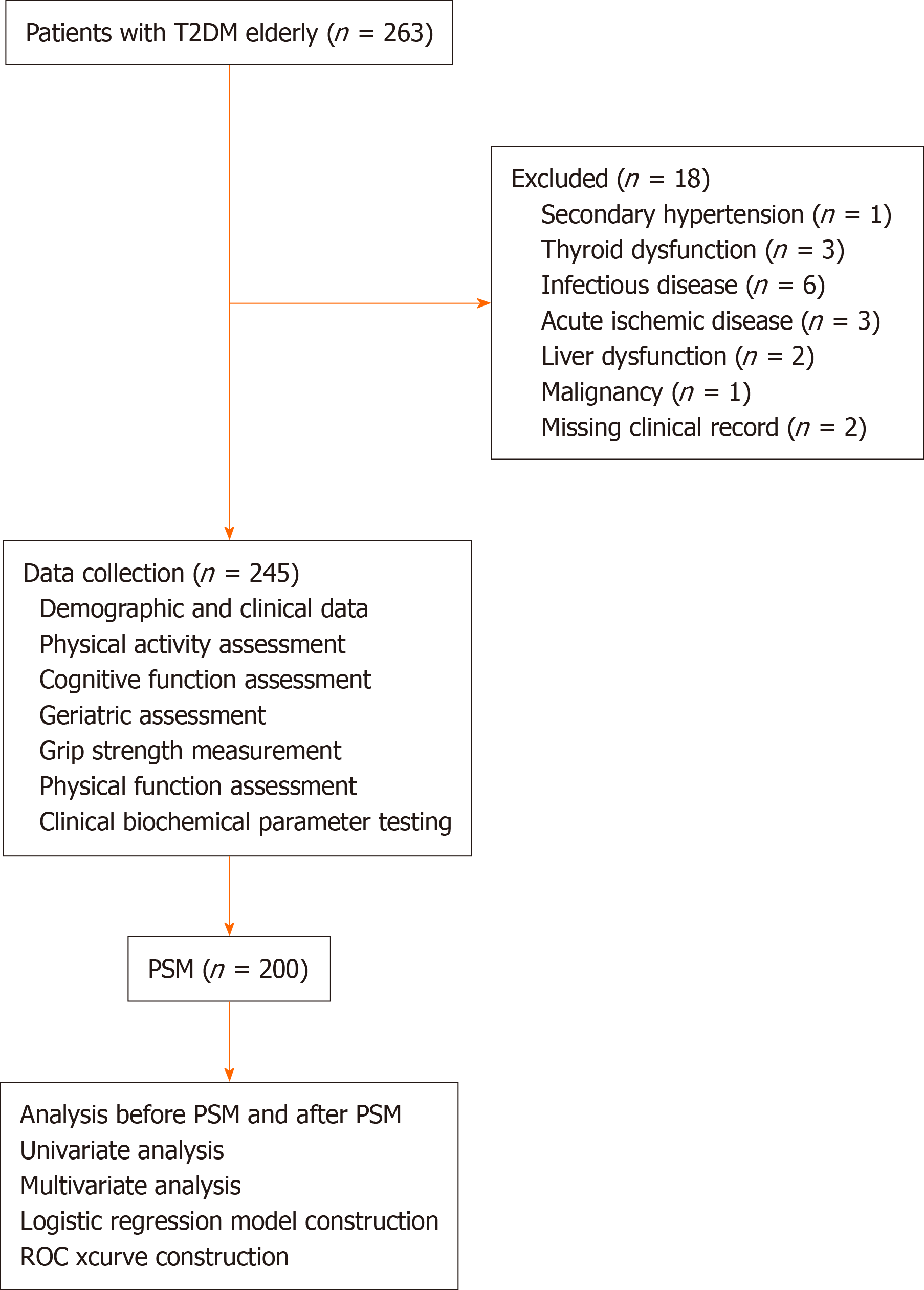
A retrospective analysis was conducted on 245 elderly T2DM patients treated at Xuanwu Hospital, Beijing, in 2023. Patients were categorized into low physical activity (n = 126) and non-low physical activity (n = 119) groups. After propensity score matching (PSM) of 100 pairs, univariate and binary logistic regression analyses identified risk factors for cognitive impairment. A predictive model was constructed and evaluated using receiver operating characteristic curve analysis.
Before PSM, the percentage of cognitive impairment was higher in the low physical activity group (P < 0.05), but after PSM, this difference was not signi
Low physical activity was not associated with cognitive impairment in our study population. Some results differed before and after PSM analysis, indicating that PSM supports objective assessment of risk factors by controlling for selection bias and confounding factors related to population characteristics. The constructed cognitive risk model provides insight for the development of a clinical tool for early prevention of cognitive impairment in elderly T2DM patients.
Core Tip: The relationship between physical activity and cognitive impairment in elderly diabetes patients remains controversial. For this investigation of risk factors for cognitive impairment in these patients, we applied propensity score matching between groups with low and non-low levels of physical activity. After adjusting for confounders and balancing baseline differences, we surprisingly found no significant association between low physical activity and cognitive decline in this population, challenging prior research. These results emphasize the complexity of factors affecting cognitive decline in diabetes. Potential contributors include social environment, sarcopenia, frailty, and multifactorial elements, offering new perspectives on managing cognitive health in diabetic populations.
- Citation: Ma YX, Li J, Si SC, Zhao H, Liu J, Lv LF, Yang K, Yang W. Does low physical activity cause cognitive decline in elderly type 2 diabetes patients: A propensity score matching analysis. World J Diabetes 2025; 16(6): 105496
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105496.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105496
With continued aging of the global population, the burden of chronic diseases among the elderly is rapidly increasing, with type 2 diabetes mellitus (T2DM) specifically posing a major public health concern. According to the International Diabetes Federation, the global number of diabetes patients exceeded 537 million in 2023 and is projected to reach 783 million by 2045[1]. T2DM not only impacts the daily life of elderly patients but is also associated with various severe complications, including cardiovascular, gastrointestinal, and neurological disorders, all of which further exacerbate the disease burden and mortality risk in this population. In recent years, researchers have begun to investigate the close relationship between T2DM and cognitive decline in the elderly. In 2021, Whitelock et al[2] found that elderly diabetic patients have a 1.5-fold greater risk of developing Alzheimer’s disease than their non-diabetic counterparts. The latest Lancet Commission report identified 14 modifiable risk factors for dementia, including physical inactivity, diabetes, and hypertension, each of which is associated with a 2% reduction in dementia risk if eliminated[3]. Several global cohort studies have supported this finding, providing evidence that the prevalence rates of mild cognitive impairment and dementia are significantly higher among diabetes patients than in age-matched control populations[4]. However, despite the well-established association between diabetes and cognitive decline, the specific mechanisms underlying this relationship remain unclear. Current research suggests that multiple pathophysiological processes may be involved, including metabolic dysfunction, neuroinflammation, and vascular damage.
Due to declining physical function and reduced social participation, the elderly are particularly prone to adopting a sedentary lifestyle characterized by low physical activity. While this trend is widespread globally, it is especially common in highly urbanized areas. Unfortunately, low physical activity has been shown to have negative health effects via multiple mechanisms. Low physical activity is closely linked to chronic endocrine disorders, which exacerbate insulin resistance and make glycemic control more challenging in elderly diabetes patients, thereby increasing the risks of diabetes-related complications[5]. Moreover, low physical activity is recognized as an important risk factor for cognitive decline. Research to date has shown that the impact of low physical activity on the nervous system may accelerate cognitive deterioration through multiple pathways[6]. Despite evidence from multiple studies that increasing physical activity can improve overall health in the elderly, with well-established benefits particularly in the cardiovascular and endocrine systems, other studies have reported no significant improvement in cognitive function with increased physical activity[7]. Due to these conflicting research findings, the relationship between physical activity and cognitive function remains uncertain[8,9]. Therefore, research is urgently needed to determine whether low physical activity affects cognitive function in diabetic populations and to elucidate the causes and risk factors for cognitive impairment in elderly diabetes patients.
To further explore the association between low physical activity and cognitive impairment in elderly patients with T2DM, we conducted the present study to examine the clinical characteristics of elderly T2DM patients with low physical activity and determine whether low physical activity affects their cognitive function. Our results provide evidence for the pathological basis of diabetes-related cognitive impairment and also provide insight for the development of new clinical intervention strategies. With the use of propensity score matching (PSM) to control for selection bias and confounding factors, we objectively evaluated the risk factors influencing cognitive impairment in elderly T2DM patients and developed a corresponding cognitive risk model. This risk model can provide a tool for early detection and a basis for early prevention of cognitive impairment in elderly T2DM patients.
The protocol for this study was approved by the ethics committee of Xuanwu Hospital of Capital Medical University and adhered to the principles outlined in the Declaration of Helsinki.
This retrospective, single-center case study included 245 elderly patients with T2DM who were admitted to the Department of Geriatrics at Xuanwu Hospital, Capital Medical University, between January 2023 and December 2023. Permission for this study was granted by our institutional ethics committee.
The inclusion criteria were: Age ≥ 60 years; diagnosis of T2DM according to the Clinical Guidelines for Prevention and Treatment of Type 2 Diabetes Mellitus in the Elderly in China (2022 edition).
The exclusion criteria were: Presence of any other type of diabetes; diagnosis of secondary hypertension, thyroid dysfunction, infectious disease, acute ischemic disease, liver dysfunction, or malignancy; missing clinical record; and refusal to consent to the utilization of medical records for research purposes. All patients and their families were informed of the study objectives and provided written informed consent.
Demographic and clinical data of the patients were collected, including detailed records of sex, age, educational background, years of education, marital status, physical exercise habits, occupational type (mental or physical labor), social activities, nighttime sleep duration and quality, height, weight, mid-upper arm circumference, smoking history, and alcohol consumption history. Body mass index (BMI) was calculated as follows: BMI = body weight (kg)/height2 (m2). Mid-upper arm circumference was measured as follows: With the patient standing naturally or seated upright with their upper body straight and arms relaxed and hanging naturally. A soft measuring tape was used to encircle the thickest part of the biceps brachii muscle on the upper arm. The circumference was measured once for each arm, and the maximum value was recorded, accurate to 0.1 cm. Additionally, information regarding each patient’s medical history was collected, including their history of falls, physical activity level, comorbidities, and details of diabetes diagnosis and treatment, such as duration of diabetes, treatment methods, and chronic diabetic complications.
Physical activity was categorized using the International Physical Activity Questionnaire as either low physical activity or non-low physical activity according to the calculated energy expenditure for physical activity per week. Metabolic equivalent (MET) minutes per week (MET-min/week) values were calculated as follows: Weekly energy expenditure = MET corresponding to the level of physical activity × daily duration of activity × number of activity days per week. Low physical activity was defined by a value of < 600 MET-min/week[10].
Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) scale, which includes multiple components, such as delayed memory, visuospatial abilities, executive function, verbal abstraction, calculation, attention, language skills, and orientation to time and place. The maximum total score for the MoCA is 30 points, and scores < 26 indicate the presence of cognitive impairment[11].
Two trained healthcare professionals administered the fatigue, resistance, ambulation, illness, and loss of weight (FRAIL) scale to assess frailty and the inpatient Mini Nutritional Assessment-Short Form (MNA-SF) as well as assessments of instrumental activities of daily living (IADL) for the enrolled patients. The FRAIL scale consists of five components: Fatigue, endurance, walking ability, illnesses, and weight loss. Patients were classified as pre-frail if they met any two criteria and as frail if they met three or more criteria. The MNA-SF includes six components: BMI, weight loss over the previous 3 months, dietary changes, stress or acute illness, mobility, and neuropsychological problems. The MNA-SF scoring is as follows: A total score of 12–14 indicates normal nutritional status, 8–11 indicates a risk of malnutrition, and 0–7 indicates malnutrition. The IADL scale assesses eight activities: Telephone use, shopping, meal preparation, housekeeping, laundry, public transportation use, medication management, and financial management. IADL disability is defined as impairment in one or more of these activities.
Grip strength was assessed using a handgrip dynamometer (WCS-100). During the measurement, participants stood upright with their feet naturally apart and arms hanging relaxed by their side. The participant held the dynamometer in their dominant hand and exerted maximum force to squeeze it. The value displayed on the dynamometer was recorded as the grip strength. Grip strength was measured twice for each participant, and the highest value was recorded for analysis. Additionally, grip strength of the dominant hand was measured three times using an electronic hand dy
Physical function was assessed using the 6-minute walk test (6MWT). The distance walked during the 6MWT in meters was recorded as 6-minute walk distance (6MWD). An average value was calculated from two trials for each participant.
Peripheral venous blood samples (5 mL) were collected from all participants in the morning after an overnight fast. Serum samples were obtained via centrifugation of the blood samples. An automated biochemical analyzer and immunoturbidimetric methods were used to measure fasting blood glucose, postprandial blood glucose, and glycated hemoglobin. Lipid levels, including total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol, also were assessed and measured. The levels of β2-microglobulin (β2-MG), high-sensitivity C-reactive protein (hsCRP), and interleukin (IL) -6 in peripheral blood were determined by enzyme-linked immunosorbent assay.
Statistical analyses were performed using SPSS version 26.0. Continuous variables are expressed as mean ± SD, while categorical variables are presented as frequencies or proportions. Comparisons between groups were conducted using the χ² test or Fisher’s exact test, as appropriate. Multivariate analysis was performed using logistic regression. Variables identified from the univariate analysis (P < 0.10) were included in a multivariate logistic regression model using a backward stepwise selection method to identify factors independently associated with cognitive decline. PSM was carried out using the extended bundle feature in SPSS 26.0, using 1:1 nearest neighbor matching to minimize baseline imbalances and enhance comparability between groups. A significance level of α = 0.10 was set for univariate analysis, and a significance level of α = 0.05 was applied for all other tests. After matching, the dataset was randomly divided into a training set (two-thirds of the sample) and a test set (one-third of the sample). The training set was used for model development, and the test set was used to assess the model’s generalizability. A risk model was constructed based on the regression coefficients. The model’s performance was evaluated by receiver-operating characteristic (ROC) curve analysis to determine the area under the curve (AUC), sensitivity, and specificity. Values of P < 0.05 indicated statistical significance.
A total of 245 elderly patients with T2DM were included in this study. A flow diagram of the study is presented in Figure 1. The demographic characteristics of the participants are presented in Table 1. Notably, 51.4% (n = 126) of the participants were classified as having a low physical activity level. The demographic and clinical characteristics of the patients in the low physical activity and non-low physical activity groups are compared in Table 2. These comparisons showed that the low physical activity group had an older age, a greater likelihood of residing in urban areas, a lower prevalence of cardiovascular disease, a higher prevalence of hearing impairment, a smaller mid-upper arm circumference, a shorter 6WMT, lower grip strength, a lower LDL-C level, and higher levels of hsCRP, β2-MG, and IL-6 (all P < 0.05). Additionally, the low physical activity group had higher prevalence rates of frailty, cognitive impairment [MOCA score, nutritional risk (MNA-SF score)], and IADL-based disability (all P < 0.05).
| Characteristics | Overall (n = 245) |
| Age | 70.9 ± 7.8 |
| Male | 152 (51.4) |
| Residence in urban area | 194 (79.2) |
| Education | 10.3 ± 4.3 |
| Occupational category | |
| Light physical work | 46 (18.8) |
| Heavy physical work | 36 (14.7) |
| Mental work | 134 (54.7) |
| Other | 29 (11.8) |
| Marital status | |
| Married | 177 (72.2) |
| Unmarried/divorced | 68 (27.8) |
| Living condition | |
| Building with elevator | 126 (51.4) |
| Building without elevator | 73 (29.8) |
| Bungalow | 14 (5.7) |
| Low physical activity | 126 (51.4) |
| Characteristic | Before PSM | t/χ2 | P value | After PSM | t/χ2 | P value | ||
| Low physical activity group | Non-low physical activity group | Low physical activity group | Non-low physical activity group | |||||
| Age (years) | 72.1 ± 0.7 | 69.9 ± 0.7 | -2.31 | 0.01 | 72.1 ± 8.1 | 70.2 ± 7.4 | -0.8 | 0.42 |
| Male | 52 (41.3) | 62 (52.1) | 2.82 | 0.08 | 44 (44.0) | 46 (46.1) | 0.08 | 0.77 |
| Smoking | 50 (39.7) | 49 (41.2) | 0.05 | 0.81 | 36 (36) | 33 (33) | 0.19 | 0.65 |
| Drinking | 11 (15.2) | 16 (21.0) | 1.39 | 0.23 | 17 (17.2) | 9 (9.0) | 2.93 | 0.08 |
| Hypertension | 100 (79.4) | 87 (73.7) | 1.92 | 0.38 | 81 (81.0) | 74 (74.0) | 1.89 | 0.38 |
| Cardiovascular disease | 73 (57.9) | 87 (73.7) | 6.73 | < 0.01 | 37 (37) | 30 (30) | 1.1 | 0.29 |
| Stroke | 105 (83.3) | 105 (88.2) | 1.20 | 0.27 | 15 (15.0) | 13 (13.0) | 0.16 | 0.68 |
| Hearing impairment | 9 (6.3%) | 0 (0%) | 9.931 | < 0.01 | 4 (4.0) | 0 (0.0) | 4.08 | 0.04 |
| Hyperlipidemia | 60 (42.0%) | 71 (46.4) | 0.593 | 0.441 | 42 (42.0) | 47 (47.0) | 0.50 | 0.47 |
| Education level (years) | 9.8 ± 0.4 | 10.3 ± 0.5 | 0.71 | 0.46 | 10.8 ± 3.9 | 10.3 ± 4.8 | -0.18 | 0.85 |
| Married status | 1.28 | 0.25 | 0.23 | 0.63 | ||||
| Married/cohabitating | 95 (75.4) | 82 (68.9) | 75 (75) | 72 (72) | ||||
| Divorced/single/widowed | 31 (24.6) | 37 (31.1) | 25 (25) | 28 (28) | ||||
| Residential region | 6.71 | 0.01 | 0.77 | 0.38 | ||||
| Urban | 108 (85.7) | 86 (72.3) | 82 (82) | 77 (77) | ||||
| Rural | 18 (14.3) | 33 (27.7) | 18 (18) | 23 (23) | ||||
| Night sleep time | 1.31 | 0.25 | 0.07 | 0.78 | ||||
| ≤ 6 h/day | 43 (37.4) | 29 (29.9) | 30 (33.7) | 27 (31.8) | ||||
| > 6 h/day | 72 (62.6) | 68 (70.1) | 59 (66.3) | 58 (68.2) | ||||
| BMI (kg/m2) | 26.1 ± 0.3 | 25.4 ± 0.3 | -1.43 | 0.15 | 26.8 ± 3.8 | 25.9 ± 3.4 | -1.67 | 0.09 |
| Mid-UAC (mm) | 26.5 ± 3.9 | 27.8 ± 3.7 | 2.51 | 0.01 | 26.8 ± 3.4 | 27.2 ± 3.3 | 2.14 | 0.03 |
| 6MWT (m) | 460.8 ± 40 | 540.5 ± 82 | -6.63 | < 0.01 | 482.3 ± 61 | 560.4 ± 80 | -5.92 | < 0.01 |
| Grip strength (kg) | 24.2 ± 11.8 | 29.3 ± 14.5 | 2.74 | < 0.01 | 24.1 ± 12.9 | 27.3 ± 11.2 | 1.72 | 0.08 |
| FBG (mmol/L) | 8.2 ± 3.5 | 8.1 ± 3.1 | -1.13 | 0.86 | 8.0 ± 3.1 | 7.9 ± 3.5 | 0.14 | 0.88 |
| HbA1C (%) | 8.4 ± 2.3 | 8.1 ± 2.2 | -0.96 | 0.35 | 8.4 ± 2.1 | 8.2 ± 2.2 | -0.82 | 0.41 |
| hsCRP (mg/L) | 6.8 ± 5.6 | 2.7 ± 5.5 | -2.72 | < 0.01 | 6.8 ± 5.5 | 4.5 ± 3.2 | -2.44 | 0.01 |
| β2-MG (mg/L) | 3.7 ± 2.2 | 3.2 ± 1.4 | -1.97 | 0.04 | 3.6 ± 2.2 | 3.2 ± 1.6 | -1.60 | 0.11 |
| IL-6 (pg/mL) | 8.3 ± 5.9 | 5.3 ± 4.7 | -2.22 | 0.02 | 5.08 ± 2.0 | 6.25 ± 3.1 | -2.01 | 0.04 |
| LDL-C (mmol/L) | 2.2 ± 0.9 | 2.6 ± 1.0 | 2.14 | 0.03 | 2.2 ± 0.9 | 2.6 ± 0.9 | 1.22 | 0.22 |
| TG (mmol/L) | 1.9 ± 1.8 | 1.7 ± 1.2 | -1.24 | 0.22 | 1.8 ± 1.6 | 1.7 ± 1.1 | -1.19 | 0.23 |
| TCH (mmol/L) | 4.1 ± 1.1 | 4.3 ± 1.2 | 1.20 | 0.20 | 4.2 ± 1.1 | 4.3 ± 1.2 | 0.45 | 0.65 |
| HDL-C (mmol/L) | 1.2 ± 0.5 | 1.2 ± 0.3 | 0.44 | 0.65 | 1.2 ± 0.3 | 1.3 ± 0.4 | 0.26 | 0.78 |
| MOCA-based cognitive impairment | 32 (30.5) | 25 (21.0) | 2.63 | 0.03 | 25 (25.0) | 22 (22.0) | 1.44 | 0.22 |
| MNA-SF-based malnutrition | 21 (20.0) | 12 (11.1) | 5.93 | 0.04 | 6 (6.0) | 10 (10.0) | 1.09 | 0.58 |
| IADL-based disability | 62 (49.2) | 35 (29.4) | 10.02 | < 0.01 | 48 (48.0) | 32 (32.0) | 5.33 | 0.02 |
| FRAIL-based frailty phenotype | 32 (31.1) | 4 (3.4) | 32.31 | < 0.01 | 22 (22.0) | 4 (4.0) | 20.5 | < 0.01 |
PSM was then conducted to balance the relevant baseline characteristics between the low physical activity and non-low physical activity groups, including age, gender, medical history, and living environment. This process resulted in the successful matching of 100 pairs of patients. The two matched groups no longer showed significant between-group differences in age, prevalence of cardiovascular disease, residential region, grip strength, and LDL-C level (all P > 0.05; Table 2). Additionally, after PSM, the groups no longer showed a difference in cognitive function based on MOCA score or in nutritional status based on MNA-SF score (both P > 0.05). After PSM, significant differences between the low physical activity and non-low physical activity groups remained in the prevalence of hearing impairment, mid-upper arm circumference, 6MWT, hs-CRP and IL-6 levels, frailty phenotype, and IADL-based disability (log-rank test, all P < 0.05).
Model 1, variables with a univariate analysis result of P < 0.1 were further analyzed using binary logistic regression. The analysis revealed that a history of stroke, grip strength, 6WMT, low MNA-SF score (malnutrition), IADL-based disability, and low physical activity were significant factors influencing the development of cognitive impairment in elderly T2DM patients (all P < 0.05; Table 3). After PSM of 200 patients and subsequent stepwise analysis, Model 2 was constructed. The results indicated that age, occupational status, history of stroke, MNA-SF score, and IADL-based disability were independent factors influencing the occurrence of cognitive impairment in elderly T2DM patients after PSM. However, low physical activity was not identified as a significant factor in this context (Table 3).
| Variable | Model 1 | Model 2 | ||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | |
| Age | 0.066 | 1.068 (1.036-1.102) | < 0.01 | 0.068 | 1.081 (1.032-1.131) | < 0.01 | 0.075 | 1.079 (1.028-1.132) | 0.02 | |||
| Male | -0.643 | 0.526 (0.299-0.923) | 0.02 | 0.227 | 1.255 (0.786-1.987) | 0.51 | ||||||
| Education level | -0.08 | 0.923 (0.862-0.987) | 0.02 | 0.043 | 1.004 (0.449-1.760) | 0.285 | ||||||
| Mental work | -0.572 | 0.564 (0.223-0.812) | 0.178 | -0.909 | 0.403 (0.197-0.824) | 0.01 | -1.248 | 0.287 (0.127-0.651) | < 0.01 | |||
| Residential region (urban) | -0.122 | 0.886 (0.734-0.992) | 0.70 | 0.381 | 1.464 (1.098-1.803) | 0.34 | ||||||
| Smoking | -0.57 | 1.99 (0.013-3.211) | 0.05 | -0.366 | 0.693 (0.228-1.091) | 0.31 | ||||||
| Drinking | 0.075 | 1.078 (0.978-1.232) | 0.56 | 0.075 | 1.078 (0.332-2.597) | 0.87 | ||||||
| Hypertension | 0.457 | 1.579 (1.343-1.923) | 0.18 | 0.282 | 1.326 (1.021-1.643) | 0.486 | ||||||
| Cardiovascular disease | 0.489 | 1.631 (0.983-2.922) | 0.10 | 0.657 | 1.932 (0.332-3.594) | 0.06 | ||||||
| Hearing impairment | 2.378 | 10.78 (2.10-54.8) | < 0.01 | 2.227 | 9.273 (0.940-91.436) | 0.04 | ||||||
| Stroke | 1.068 | 2.91 (1.251-6.770) | 0.01 | 1.415 | 4.118 (1.40, 12.109) | 0.01 | 1.130 | 3.096 (1.220-7.858) | 0.02 | 1.527 | 4.604 (1.506-14.076) | < 0.01 |
| Hyperlipidemia | -0.048 | 0.954 (0.322-1.940) | 0.86 | 0.122 | 1.129 (0.756-1.911) | 0.72 | ||||||
| BMI | 0.001 | 1.001 (0.921-1.344) | 0.97 | 0.046 | 1.047 (0.334-2.019) | 0.29 | ||||||
| Mid-UAC | -0.029 | 0.972 (0.187-3.009) | 0.54 | -0.007 | 0.993 (0.033-2.870) | 0.9 | ||||||
| 6MWT | 0.269 | 1.308 (1.131-1.513) | < 0.01 | 0.175 | 1.191 (1.024, 1.385) | 0.02 | 0.26 | 1.297 (1.084-1.553) | < 0.01 | |||
| Grip strength | -0.063 | 0.93 (0.90-0.987) | < 0.01 | -0.055 | 0.947 (0.911, 0.984) | < 0.01 | -0.053 | 0.948 (0.911-0.988) | 0.01 | |||
| FBG | -0.04 | 0.996 (0.541-1.982) | 0.935 | -0.023 | 0.977 (0.334-2.081) | 0.67 | ||||||
| HbA1C | 0.106 | 1.12 (0.711-1.593) | 0.09 | 0.098 | 1.103 (0.321-3.017) | 0.199 | ||||||
| hsCRP | 0.07 | 1.007 (0.448-1.831) | 0.61 | 0.07 | 1.007 (0.409-1.910) | 0.646 | ||||||
| β2-MG | 0.24 | 1.273 (1.051-1.542) | 0.013 | 0.285 | 1.330 (1.036-1.714) | 0.028 | ||||||
| IL-6 | 0.02 | 1.02 (0.772-1.406) | 0.21 | 0.014 | 1.014 (0.119-4.557) | 0.508 | ||||||
| TCH | -0.309 | 0.734 (0.569-0.948) | 0.012 | -0.206 | 0.814 (0.286-1.337) | 0.173 | ||||||
| TG | 0.073 | 1.076 (0.471-2.696) | 0.44 | 0.012 | 1.012 (0.553-3.469) | 0.902 | ||||||
| LDL-C | -0.263 | 0.77 (0.224-1.927) | 0.07 | -0.267 | 0.766 (0.468-1.370) | 0.126 | ||||||
| HDL-C | 0.068 | 1.071 (0.443-3.042) | 0.82 | 0.056 | 1.057 (0.427-3.285) | 0.871 | ||||||
| MNA-SF-based malnutrition | 1.063 | 2.895 (1.438-5.830) | < 0.01 | 1.075 | 2.930 (1.218, 7.046) | 0.02 | 0.902 | 2.465 (1.040-5.839) | 0.04 | 0.912 | 2.908 (1.01-4.913) | 0.044 |
| IADL-based disability | 1.333 | 3.793 (2.117-6.796) | < 0.01 | 0.896 | 2.449 (1.160, 5.172) | 0.01 | 1.171 | 3.226 (1.624-6.405) | < 0.01 | 0.856 | 2.354 (1.062-5.215) | 0.035 |
| FRAIL-based frailty phenotype | -0.380 | 1.463 (0.563-1.980) | 0.42 | 0.914 | 2.493 (0.462-5.707) | 0.157 | ||||||
| Low physical activity | 0.621 | 1.860 (1.053-3.285) | 0.03 | 0.407 | 1.502 (0.978-3.750) | 0.23 | ||||||
We additionally examined potential interaction effects between cognitive function, low physical activity, and age within the regression model; however, no statistically significant interactions were found (all P > 0.05).
The logistic regression model for early detection of cognitive impairment in elderly T2DM patients was constructed as follows: logit(P) = -6.339 + 0.075 × age – 1.248 × mental labor + 1.527 × history of stroke + 0.912 × abnormal MNA-SF + 0.856 × abnormal IADL, where logit(P) = ln[P/(1–P)] represents the probability of cognitive impairment.
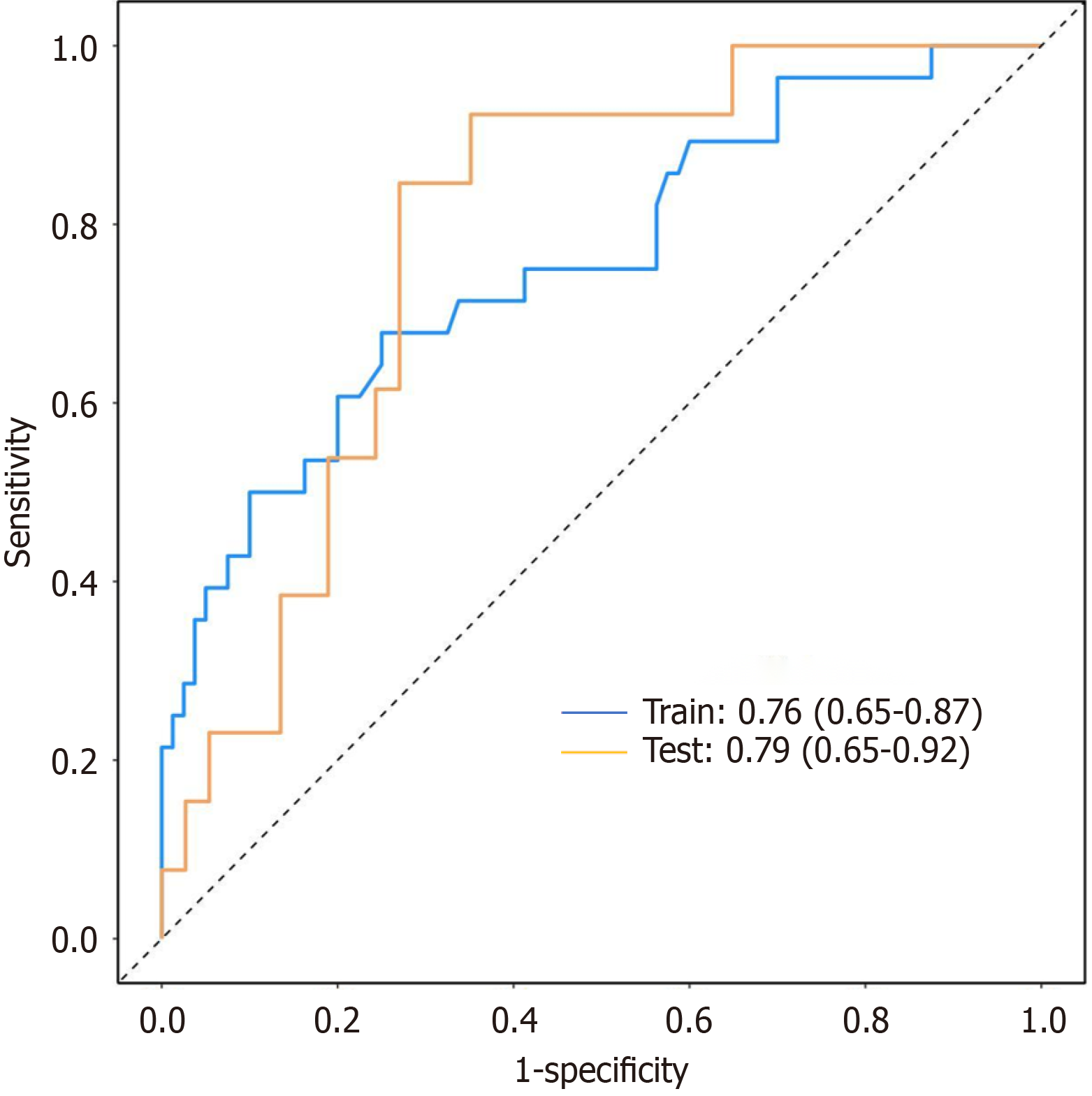
Baseline characteristics between the two sets showed no statistically significant differences (Table 4, all P > 0.05), indicating good balance after randomization. Multivariate logistic regression analyses performed in both the training and test sets confirmed that the identified risk factors, including age, occupational status, history of stroke, MNA-SF score, and IADL-based disability, were consistent with those identified in the post-PSM model (Table 5, all P < 0.05). The model demonstrated good discriminatory ability in both datasets, with AUC values of 0.76 (95%CI: 0.65–0.87) in the training set and 0.79 (95%CI: 0.65–0.92) in the test set (Figure 2).
| Variables | Total (n = 200) | Training set (n = 140) | Test set (n = 60) | Statistic | P value |
| Age (years) | 70.87 ± 7.77 | 70.93 ± 7.84 | 70.73 ± 7.66 | t = -0.16 | 0.871 |
| Male | 90 (45.00) | 65 (46.43) | 25 (41.67) | χ² = 0.38 | 0.535 |
| Smoking | 69 (34.50) | 46 (32.86) | 23 (38.33) | χ² = 0.56 | 0.455 |
| Drinking | 26 (13.07) | 20 (14.29) | 6 (10.17) | χ² = 0.62 | 0.431 |
| Hypertension | 155 (77.89) | 112 (80.58) | 43 (71.67) | χ² = 0.32 | 0.141 |
| Cardiovascular disease | 67 (33.50) | 51 (36.43) | 16 (26.67) | χ² = 1.80 | 0.180 |
| Stroke | 28 (14.00) | 21 (15.00) | 7 (11.67) | χ² = 0.39 | 0.534 |
| Hearing impairment | 4 (2.00) | 4 (2.86) | 0 (0.00) | χ² = 0.60 | 0.440 |
| Hyperlipidemia | 89 (44.50) | 65 (46.43) | 24 (40.00) | χ² = 0.70 | 0.402 |
| Hypertension | 155 (77.89) | 112 (80.58) | 43 (71.67) | χ²= 0.32 | 0.141 |
| Education level (years) | 10.02 ± 4.39 | 10.09 ± 4.35 | 9.85 ± 4.50 | t = -0.33 | 0.740 |
| BMI (kg/m2) | 25.83 ± 3.66 | 25.85 ± 3.84 | 25.77 ± 3.22 | t = -0.24 | 0.513 |
| Mid-UAC (mm) | 27.20 ± 3.73 | 26.73 ± 3.73 | 28.26 ± 3.53 | t = 2.50 | 0.113 |
| 6MWT (m) | 6.45 ± 2.43 | 6.57 ± 2.35 | 6.18 ± 2.60 | t = -0.97 | 0.335 |
| Grip strength (kg) | 26.53 ± 13.73 | 26.44 ± 13.91 | 26.75 ± 13.44 | t = 0.13 | 0.893 |
| FBG (mmol/L) | 7.99 ± 3.29 | 7.85 ± 3.26 | 8.31 ± 3.37 | t = 0.90 | 0.368 |
| HbA1C (%) | 8.19 ± 2.30 | 8.21 ± 2.34 | 8.17 ± 2.23 | t =-0.11 | 0.914 |
| hsCRP (mg/L) | 4.78 ± 11.62 | 5.21 ± 12.89 | 3.72 ± 7.60 | t = -0.76 | 0.451 |
| β2-MG (mg/L) | 3.42 ± 1.63 | 3.48 ± 1.72 | 3.27 ± 1.37 | t = -0.77 | 0.440 |
| IL-6 (pg/mL) | 6.59 ± 10.32 | 6.06 ± 9.51 | 7.93 ± 12.12 | t = 1.06 | 0.291 |
| TCH (mmol/L) | 4.24 ± 1.20 | 4.26 ± 1.30 | 4.21 ± 0.93 | t = -0.34 | 0.738 |
| TG (mmol/L) | 1.80 ± 1.67 | 1.86 ± 1.69 | 1.65 ± 1.63 | t = -0.84 | 0.403 |
| LDL-C (mmol/L) | 2.35 ± 1.03 | 2.37 ± 1.07 | 2.30 ± 0.93 | t = -0.45 | 0.654 |
| HDL-C (mmol/L) | 1.28 ± 0.48 | 1.27 ± 0.50 | 1.32 ± 0.41 | t = 0.64 | 0.524 |
| Mental work | 109 (60.56) | 76 (60.80) | 33 (60.00) | χ² = 0.01 | 0.919 |
| Residential region, urban | 159 (79.50) | 109 (77.86) | 50 (83.33) | χ² = 0.77 | 0.379 |
| MOCA-based cognitive impairment | 47 (23.50) | 32 (22.90) | 15 (25.00) | χ² = 0.61 | 0.413 |
| MNA-SF-based malnutrition | 26 (14.21) | 18 (14.29) | 8 (14.04) | χ2 = 0.00 | 0.964 |
| IADL-based disability | 80 (40.00) | 62 (44.29) | 18 (30.00) | χ² = 3.57 | 0.059 |
| FRAIL-based frailty phenotype | 26 (14.29) | 17 (13.71) | 9 (15.52) | χ² = 0.31 | 0.858 |
| Variable | Training | Test | ||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | |
| Age | 0.062 | 1.063 (1.011-1.124) | 0.01 | 1.612 | 1.083 (1.012- 1.151) | 0.01 | 0.077 | 1.080 (0.990-1.167) | 0.05 | 0.161 | 1.175 (1.039-1.330) | 0.01 |
| Male | 0.169 | 1.245 (0.883-1.862) | 0.06 | 0.233 | 1.258 (0.901-1.983) | 0.23 | ||||||
| Education level | -0.042 | 0.959 (0.872-1.055) | 0.39 | -0.114 | 0.814 (0.449-0.760) | 0.28 | ||||||
| Mental work | -0.062 | 0.640 (0.013-0.739) | 0.01 | -0.066 | 0.610 (0.47-1.22) | 0.03 | -0.893 | 0.509 (0.097-0.894) | 0.02 | -1.303 | 0.233 (0.065-0.794) | < 0.01 |
| Residential region (urban) | -0.122 | 0.886 (0.734-0.992) | 0.70 | 0.41 | 1.575 (0.961-1.912) | 0.51 | ||||||
| Smoking | 0.301 | 1.704 (1.311-2.163) | 0.48 | 0.496 | 1.132 (0.908-1.801) | 0.69 | ||||||
| Drinking | 0.127 | 1.308 (0.293-2.643) | 0.82 | 0.087 | 1.181 (0.764-1.597) | 0.74 | ||||||
| Hypertension | 0.068 | 1.071 (0.391-2.934) | 0.19 | 0.522 | 1.347 (0.990-1.814) | 0.41 | ||||||
| Cardiovascular disease | 0.721 | 2.057 (0.922-4.588) | 0.06 | 0.471 | 1.843 (0.891-2.914) | 0.09 | ||||||
| Hearing impairment | 2.395 | 10.31 (3.10-46.8) | 0.04 | 2.124 | 5.173 (1.302-13.232) | 0.04 | ||||||
| Stroke | 1.561 | 4.762 (1.573-14.419) | < 0.01 | 1.611 | 5.010 (1.27-12.908) | 0.02 | 0.984 | 1.292 (0.213-4.858) | 0.02 | 1.134 | 1.456 (0.831-4.762) | 0.02 |
| Hyperlipidemia | 0.043 | 1.044 (0.473-2.305) | 0.81 | 0.312 | 1.541 (0.451-2.211) | 0.73 | ||||||
| BMI | 0.037 | 1.038 (0.940-1.146) | 0.46 | 0.031 | 1.542 (0.411-2.897) | 0.21 | ||||||
| Mid-UAC | 0.012 | 1.012 (0.894-1.145) | 0.84 | -0.014 | 0.323 (0.033-2.870) | 0.87 | ||||||
| 6MWT | 0.149 | 1.161 (0.971-1.389) | 0.10 | 0.213 | 1.245 (1.084-1.913) | 0.08 | ||||||
| Grip strength | -0.033 | 0.967 (0.928-1.008) | 0.11 | -0.413 | 0.899 (0.414-1.184) | 0.11 | ||||||
| FBG | -0.032 | 0.969 (0.852-1.101) | 0.62 | -0.051 | 0.914 (0.245-2.181) | 0.82 | ||||||
| HbA1C | 0.008 | 1.035 (0.873-1.227) | 0.38 | 0.144 | 1.243 (0.324-3.197) | 0.43 | ||||||
| hsCRP | 0.07 | 1.008 (0.979-1.039) | 0.59 | 0.114 | 1.303 (0.441-1.910) | 0.65 | ||||||
| β2-MG | 0.094 | 1.099 (0.869-1.389) | 0.43 | 0.34 | 1.311 (1.046-2.09) | 0.51 | ||||||
| IL-6 | -0.013 | 0.987 (0.927-1.052) | 0.69 | 0.011 | 1.017 (0.213-3.511) | 0.81 | ||||||
| TCH | -0.259 | 0.772 (0.553-1.076) | 0.12 | -0.099 | 0.974 (0.423-1.037) | 0.17 | ||||||
| TG | 0.003 | 1.097 (0.886-1.264) | 0.97 | 0.013 | 1.041 (0.351-2.916) | 0.78 | ||||||
| LDL-C | -0.293 | 0.746 (0.502-1.109) | 0.14 | 0.012 | 1.016 (0.688-1.349) | 0.31 | ||||||
| HDL-C | 0.193 | 1.213 (0.572-2.570) | 0.61 | 0.091 | 1.133 (0.387-3.465) | 0.83 | ||||||
| MNA-SF-based malnutrition | 1.063 | 2.895 (1.438-5.830) | < 0.01 | 0.75 | 1.680 (1.01-2.146) | 0.04 | 0.931 | 3.465 (1.34-8.331) | 0.02 | 1.021 | 3.193 (0.944-5.523) | 0.04 |
| IADL-based disability | 0.987 | 2.683 (1.188-6.061) | 0.02 | 1.170 | 3.203 (1.189-6.06) | 0.02 | 0.606 | 1.833 (0.539-6.294) | < 0.01 | 0.833 | 2.104 (1.187-6.051) | 0.03 |
| FRAIL-based frailty phenotype | 1.110 | 3.033 (0.622-14.785) | 0.17 | 0.911 | 2.933 (0.462-5.097) | 0.22 | ||||||
| Low physical activity | 0.371 | 1.57 (1.290-2.987) | 0.41 | 0.432 | 1.033 (0.478-3.250) | 0.51 | ||||||
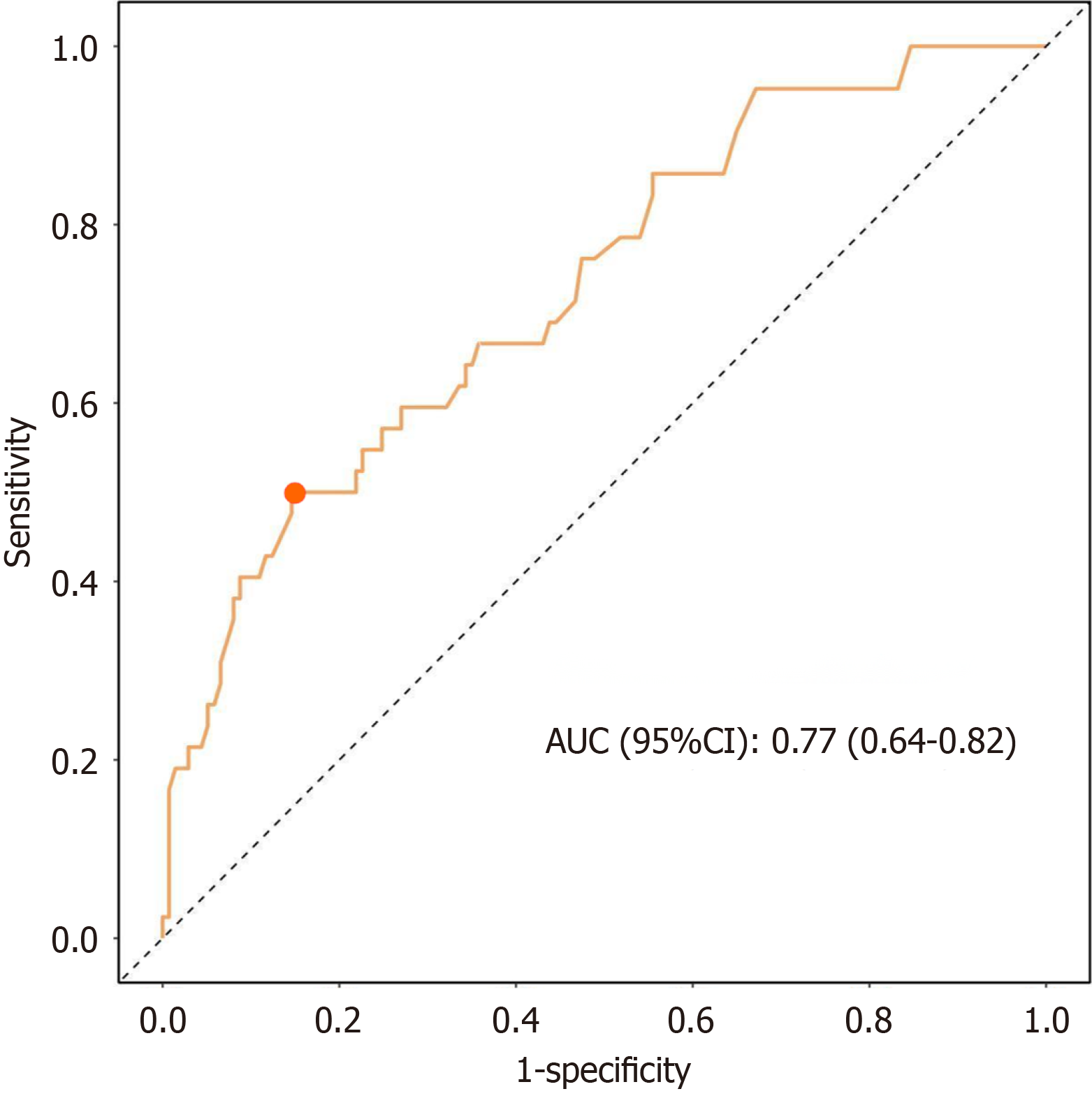
ROC curve analysis of the performance of this risk model revealed that the AUC value for identifying patients with cognitive impairment was 0.77, with a 95%CI of 0.64–0.82. The optimal cutoff value for the predicted probability was 0.40, at which the Youden index reached its maximum of 0.36. At this point the sensitivity and specificity of the model were 0.85 and 0.50, respectively (Figure 3).
This retrospective study was designed to investigate the impact of physical activity as well as other factors on cognitive function among T2DM patients aged 60 years and older. Through careful categorization of the physical activity levels of participants, we were able to analyze and compare the clinical characteristics of elderly T2DM patients with differing activity levels. To provide a comprehensive assessment, physical activity was analyzed from the perspectives of frequency, duration, and intensity. Participants were asked detailed questions regarding the number of days they engaged in physical activity, the duration of each session, and the intensity of the activities. These metrics allowed us to calculate the total energy expenditure and categorize physical activity levels accordingly, and also to further explore the relationship between these types of activities and cognitive function. After implementation of PSM to eliminate relevant confounding and bias factors, the present study found that cognitive function in the elderly population with T2DM was not significantly associated with physical activity levels. Instead, cognitive function in these patients showed correlations with variables such as age, nature of employment, nightly sleep duration, history of stroke, nutritional status, and disability status. From these variables, we constructed a risk prediction model for cognitive impairment and evaluated its predictive efficacy. The calculated AUC for the model was 0.77, which indicates good predictive performance and suggests the potential clinical value of this model in the care of elderly T2DM patients.
Previous studies on the impact of low physical activity on cognitive function have suggested that physical activity may have a positive effect on cognitive function in elderly patients with T2DM[12,13]. However, controversy and incon
To minimize the impact of confounding factors, the present study employed PSM to control for baseline characteristics such as age, sex, years of education, and prior medical history. After PSM, we reanalyzed the factors influencing cognitive function in elderly T2DM patients with different levels of physical activity. The data showed no significant difference in cognitive function between T2DM patients with low vs non-low physical activity after controlling for these confounders. This finding contrasts with the conclusions of some previous studies and even systematic reviews that increased physical activity, particularly with high-intensity or regular physical activity, can significantly improve cognitive function in elderly diabetic patients. However, the results of the present study are plausible for several reasons. First, it is possible that the level of physical activity among the included patients did not reach the threshold necessary to elicit im
Both the preliminary analysis and post-PSM analysis in the present study identified a history of stroke, poor nutritional status, and disability as significant risk factors for cognitive impairment in elderly T2DM patients. These findings are partially consistent with previous research[6,23,24]. Stroke, as a critical cardiovascular and cerebrovascular event, often leads to insufficient blood and oxygen supply to the brain, resulting in a rapid decline in cognitive function[25,26]. Previous studies have demonstrated that individuals with a history of stroke have a significantly higher risk of cognitive impairment compared to those without such a history[27,28]. Moreover, malnutrition, which is highly prevalent among the elderly, not only affects the body’s metabolic functions but may also negatively impact brain health by exacerbating inflammatory responses and oxidative stress. Disability, which is commonly associated with reduced physical activity and decreased social interaction, also can further accelerate cognitive decline. Therefore, these risk factors identified in the present study align closely with findings in the existing literature, and thus, the present study provides additional evidence that a history of stroke, poor nutritional status, and disability contribute to the deterioration of cognitive function in elderly T2DM patients.
Notably, after adjustment for confounding factors and baseline characteristics through PSM, our logistic regression analysis also did not identify physical activity as an independent risk factor for cognitive impairment in elderly T2DM patients included in this study. This finding challenge traditional views and suggests that the mechanisms underlying cognitive impairment in the diabetic population may be more complex than previously understood. For diabetes patients, engagement in physical activity could influence cognitive function through various mechanisms and biomarkers. Although the exact mechanisms linking diabetes and cognitive impairment remain unclear, several hypotheses have been proposed. First, insulin resistance is closely related to central nervous system function. Insulin not only regulates glucose utilization in the brain but also plays a role in neuronal growth and synaptic plasticity[15,29,30]. Physical activity can enhance peripheral insulin sensitivity and improve glucose metabolism; however, in patients with advanced-stage diabetes, insulin receptor desensitization in the central nervous system may have already reached a pathological steady state[31]. In such cases, alterations in blood–brain barrier permeability may impair the ability of exercise to modulate central insulin signaling pathways, thereby attenuating its neuroprotective effects on cognitive function. Second, physical activity can reduce chronic inflammatory responses within the body. Nevertheless, patients with diabetes often present with a persistent low-grade inflammatory state that may exhibit characteristics of “inflammatory resistance”. Chronic hyperglycemia can lead to prolonged microglial activation in the brain. As a result, even if physical activity reduces peripheral inflammatory cytokines, the neuroinflammatory microenvironment may remain active[32]. Elevated levels of inflammatory markers, such as CRP and IL-6, have been detected in elderly diabetes patients and shown to correlate with cognitive impairment[33]. Reducing the levels of these inflammatory markers through physical activity may help protect brain function. Evidence from multiple studies suggests that advanced glycation end-products, which are produced with prolonged hyperglycemia, play a crucial role in promoting oxidative stress and inflammation, further exacerbating neuronal damage[34-36]. Furthermore, another critical biomarker, brain-derived neurotrophic factor (BDNF), is essential for neuronal survival, differentiation, and plasticity, and physical activity has been shown to increase BDNF expression, thereby promoting the maintenance and improvement of cognitive function[37].
While previous studies have provided preliminary evidence supporting the role of physical activity in mitigating cognitive impairment in T2DM patients, many questions remain unanswered due to the limited scope of existing research. For instance, Radler et al[38] found that the relationship between low physical activity and cognitive decline is significantly influenced by the metabolic health status of patients[8]. Two additional studies identified the presence of metabolic syndrome, particularly hyperglycemia and insulin resistance, as a key factor exacerbating cognitive dysfunction[39,40]. Similarly, Mehta et al[41] reported that chronic inflammation serves as a crucial mediator of the cognitive impairment observed in diabetes patients with low physical activity levels. Despite the general consensus among researchers that an association exists between low physical activity and cognitive impairment in T2DM patients, the specific underlying mechanisms remain a subject of considerable debate. A wide variety of patient characteristics, such as the duration of diabetes, presence of complications, educational history, socioeconomic status, and depressive symptoms, may influence the interpretation of the research results. These variables are crucial in understanding the complex relationship between diabetes and cognitive impairment in the elderly, underscoring the need for further investigation that accounts for these confounding factors. With the findings of the present study, it remains unclear whether physical activity influences cognitive function indirectly by affecting nutritional status or the degree of disability in elderly T2DM patients. This observation opens new avenues for future research, particularly in exploring how physical activity, as an indirect factor, might influence cognitive function through its impact on other physiological or psychological health indicators. Further investigation of these potential mediating mechanisms could provide valuable theoretical foundations for the development of more effective prevention and intervention strategies. By advancing our understanding of how physical activity interacts with various health determinants to influence cognitive outcomes, future research could lead to more targeted and comprehensive approaches to managing cognitive impairment in elderly T2DM patients.
Although physical activity level was not identified as a significant risk factor for cognitive impairment in our study population, several other factors were, and we used these to construct a prediction model for cognitive impairment that demonstrated good performance. These findings provide insight into additional factors that influence cognitive function in elderly T2DM patients and the possibility of using a risk prediction model to facilitate early intervention in these patients.
This study employed a retrospective design to investigate the impact of physical activity on cognitive function in T2DM patients aged 60 years and older. By analyzing the clinical characteristics of T2DM patients with two different levels of physical activity, we aimed to uncover the potential relationship between physical activity and cognitive function. To ensure the accuracy of our analysis, we utilized PSM to control for differences in baseline characteristics such as age, gender, education level, and medical history, thereby reducing the influence of confounding factors on the study outcomes. A multivariate analysis was conducted to construct a regression model for predicting cognitive impairment risk in elderly T2DM patients, and the model demonstrated good predictive performance. However, this study has several limitations that warrant consideration. First, as a single-center study with a relatively small sample size, the generalizability of the risk prediction model may be limited. Specifically, the inclusion of participants from a single hospital may introduce geographical and institutional limitations. Although PSM was applied to control for confounding factors, the relatively homogeneous sample source may have led to potential selection bias. This limitation may affect the generalizability of the findings to elderly T2DM patients in other regions or socioeconomic settings. To enhance the model’s external validity, multicenter studies with larger sample sizes are needed to validate and optimize the model. Moreover, the observed nonsignificant association between low physical activity and cognitive impairment after PSM adjustment should be interpreted with caution. To enhance the model’s external validity, multicenter studies with larger sample sizes are needed to validate and optimize the model. Second, this study did not include an in-depth analysis of the categorization of cognitive function or the nature and classification of physical activity. Different types and intensities of physical activity may have varying effects on specific aspects of cognitive function, which could be more pronounced in specific subgroups. Third, recall bias is an inherent limitation in studies relying on self-reported data, such as the assessment of physical activity using the International Physical Activity Questionnaire. Participants may overestimate or underestimate their activity levels due to memory inaccuracies or social desirability bias. This limitation may influence the observed associations between physical activity and cognitive impairment. Therefore, future research should explore the specific impacts of various types and intensities of physical activity on different forms of cognitive function in elderly T2DM patients, to facilitate further refinement of the existing risk prediction model.
In summary, this retrospective study assessed the impact of physical activity specifically on cognitive function in elderly T2DM patients and investigated other risk factors for cognitive impairment in these patients. PSM was used to effectively control for confounding factors and to perform a detailed examination of cognitive performance in patients with two different levels of physical activity. Although some findings from this study diverge from previous research, their plausibility is supported by multiple hypotheses. Additionally, our results confirmed that a history of stroke, poor nutritional status, and disability are significant risk factors for cognitive impairment in elderly T2DM patients. Based on these findings, we developed a risk prediction model for cognitive impairment in elderly T2DM patients, which showed good predictive performance. Future research should aim to validate and refine this model on a larger scale and to explore the specific mechanisms by which physical activity may influence cognitive function in elderly T2DM patients. Such efforts are essential for providing more precise guidance for clinical interventions.
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Claude Mbanya J, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. Erratum to "IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045" [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res Clin Pract. 2023;204:110945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 2. | Whitelock V, Rutters F, Rijnhart JJM, Nouwen A, Higgs S. The mediating role of comorbid conditions in the association between type 2 diabetes and cognition: A cross-sectional observational study using the UK Biobank cohort. Psychoneuroendocrinology. 2021;123:104902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, Ames D, Banerjee S, Burns A, Brayne C, Fox NC, Ferri CP, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Nakasujja N, Rockwood K, Samus Q, Shirai K, Singh-Manoux A, Schneider LS, Walsh S, Yao Y, Sommerlad A, Mukadam N. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;404:572-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 691] [Article Influence: 691.0] [Reference Citation Analysis (0)] |
| 4. | Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 2019;19:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 5. | Cho JA, Park SH, Cho J, Kim JO, Yoon JH, Park E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients. 2020;12:1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Ma F, Zhang Q, Shi J, Li S, Wu L, Zhang H. Risk factors for cognitive dysfunction and glycemic management in older adults with type 2 diabetes mellitus: a retrospective study. BMC Endocr Disord. 2023;23:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 813] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 8. | Zhai L, Yang Y, Zhang J, Hou W, Yang Y, Ding D, Li C, Zhu Y. Association between cognitive dysfunction and diabetes in patients over 65 years old: a cross-sectional study using propensity score matching. J Rehabil Med. 2024;56:jrm18372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Meng J, Yan R, Zhang C, Bai X, Yang X, Yang Y, Feng T, Liu X. Dipeptidyl peptidase-4 inhibitors alleviate cognitive dysfunction in type 2 diabetes mellitus. Lipids Health Dis. 2023;22:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Bauman A, Ainsworth BE, Sallis JF, Hagströmer M, Craig CL, Bull FC, Pratt M, Venugopal K, Chau J, Sjöström M; IPS Group. The descriptive epidemiology of sitting. A 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011;41:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 11. | Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11622] [Cited by in RCA: 15963] [Article Influence: 798.2] [Reference Citation Analysis (0)] |
| 12. | Gerten S, Engeroff T, Fleckenstein J, Füzéki E, Matura S, Pilatus U, Vogt L, Pantel J, Banzer W. Deducing the Impact of Physical Activity, Sedentary Behavior, and Physical Performance on Cognitive Function in Healthy Older Adults. Front Aging Neurosci. 2021;13:777490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Oliveira JJ, Ribeiro AGSV, de Oliveira Silva JA, Barbosa CGR, Silva ASE, Dos Santos GM, Verlengia R, Pertille A. Association between physical activity measured by accelerometry and cognitive function in older adults: a systematic review. Aging Ment Health. 2023;27:2089-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Erlenbach E, McAuley E, Gothe NP. The Association Between Light Physical Activity and Cognition Among Adults: A Scoping Review. J Gerontol A Biol Sci Med Sci. 2021;76:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Leischik R, Schwarz K, Bank P, Brzek A, Dworrak B, Strauss M, Litwitz H, Gerlach CE. Exercise Improves Cognitive Function-A Randomized Trial on the Effects of Physical Activity on Cognition in Type 2 Diabetes Patients. J Pers Med. 2021;11:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Colberg SR, Somma CT, Sechrist SR. Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J Am Med Dir Assoc. 2008;9:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Zhao RR, O'Sullivan AJ, Fiatarone Singh MA. Exercise or physical activity and cognitive function in adults with type 2 diabetes, insulin resistance or impaired glucose tolerance: a systematic review. Eur Rev Aging Phys Act. 2018;15:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognition in women with type 2 diabetes. Am J Epidemiol. 2009;170:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Shellington EM, Reichert SM, Petrella RJ. Commentary on: "Effects of Regular Physical Activity on the Cognitive Performance of Type 2 Diabetic Patients: A Systematic Review" by Podolski et al. (Metab Syndr Relat Disord 2017;15:481-493). Metab Syndr Relat Disord. 2018;16:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 20. | Wohlwend M, Olsen A, Håberg AK, Palmer HS. Exercise Intensity-Dependent Effects on Cognitive Control Function during and after Acute Treadmill Running in Young Healthy Adults. Front Psychol. 2017;8:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Dowllah IM, Lopez-Alvarenga J, Maestre GE, Karabulut U, Lehker M, Karabulut M. Relationship Between Cognitive Performance, Physical Activity, and Socio-Demographic/Individual Characteristics Among Aging Americans. J Alzheimers Dis. 2023;92:975-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Xu W, Hu X, Zhang X, Ling C, Wang C, Gao L. Cognitive Impairment and Related Factors Among Middle-Aged and Elderly Patients with Type 2 Diabetes from a Bio-Psycho-Social Perspective. Diabetes Metab Syndr Obes. 2021;14:4361-4369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Suain Bon R, Ariaratnam S, Mat Saher Z, Mohamad M, Lee FS. Cognitive Impairment and Its Associated Risk Factors in the Elderly With Type 2 Diabetes Mellitus. Front Psychiatry. 2021;12:669725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Tao C, Hu X, Li H, You C. White Matter Injury after Intracerebral Hemorrhage: Pathophysiology and Therapeutic Strategies. Front Hum Neurosci. 2017;11:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Xu M, Qian L, Wang S, Cai H, Sun Y, Thakor N, Qi X, Sun Y. Brain network analysis reveals convergent and divergent aberrations between mild stroke patients with cortical and subcortical infarcts during cognitive task performing. Front Aging Neurosci. 2023;15:1193292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Zhang X, Bi X. Post-Stroke Cognitive Impairment: A Review Focusing on Molecular Biomarkers. J Mol Neurosci. 2020;70:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Alashram AR, Annino G, Padua E. Rehabilitation interventions for cognitive deficits in stroke survivors: A systematic review of randomized controlled trials. Appl Neuropsychol Adult. 2025;32:262-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Coll-Padrós N, León M, Valech N, Ros E, Vidal J, Estruch R, Fitó M, Salas-Salvadó J, Corella D, Molinuevo JL, Rami L. Physical activity is associated with better global cognition and frontal function in overweight/obese older adults with metabolic syndrome. Eur Rev Aging Phys Act. 2019;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Wang R, Yan W, Du M, Tao L, Liu J. The effect of physical activity interventions on cognition function in patients with diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2021;37:e3443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Hosseini K, Khalaji A, Behnoush AH, Soleimani H, Mehrban S, Amirsardari Z, Najafi K, Fathian Sabet M, Hosseini Mohammadi NS, Shojaei S, Masoudkabir F, Aghajani H, Mehrani M, Razjouyan H, Hernandez AV. The association between metabolic syndrome and major adverse cardiac and cerebrovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Sci Rep. 2024;14:697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 32. | Yang X, Xu Y, Gao W, Wang L, Zhao X, Liu G, Fan K, Liu S, Hao H, Qu S, Dong R, Ma X, Ma J. Hyperinsulinemia-induced microglial mitochondrial dynamic and metabolic alterations lead to neuroinflammation in vivo and in vitro. Front Neurosci. 2022;16:1036872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Papagianni G, Panayiotou C, Vardas M, Balaskas N, Antonopoulos C, Tachmatzidis D, Didangelos T, Lambadiari V, Kadoglou NPE. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2023;164:156157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Borriello M, Iannuzzi C, Sirangelo I. Pinocembrin Protects from AGE-Induced Cytotoxicity and Inhibits Non-Enzymatic Glycation in Human Insulin. Cells. 2019;8:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Chellappa RC, Palanisamy R, Swaminathan K. RAGE Isoforms, its Ligands and their Role in Pathophysiology of Alzheimer's Disease. Curr Alzheimer Res. 2020;17:1262-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Aida Y, Kamide T, Ishii H, Kitao Y, Uchiyama N, Nakada M, Hori O. Soluble receptor for advanced glycation end products as a biomarker of symptomatic vasospasm in subarachnoid hemorrhage. J Neurosurg. 2021;134:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Shekarchian M, Peeri M, Azarbayjani MA. Physical activity in a swimming pool attenuates memory impairment by reducing glutamate and inflammatory cytokines and increasing BDNF in the brain of mice with type 2 diabetes. Brain Res Bull. 2023;201:110725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Radler KH, Chapman S, Zdrodowska MA, Dowd HN, Liu X, Huey ED, Cosentino S, Louis ED. Physical Activity as a Predictor of Cognitive Decline in an Elderly Essential Tremor Cohort: A Prospective, Longitudinal Study. Front Neurol. 2021;12:658527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Díaz-Camargo E, Hernández-Lalinde J, Sánchez-Rubio M, Chaparro-Suárez Y, Álvarez-Caicedo L, Fierro-Zarate A, Gravini-Donado M, García-Pacheco H, Rojas-Quintero J, Bermúdez V. NHANES 2011-2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations. Int J Environ Res Public Health. 2023;20:5257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 40. | Zhang S, Zhang Y, Wen Z, Yang Y, Bu T, Bu X, Ni Q. Cognitive dysfunction in diabetes: abnormal glucose metabolic regulation in the brain. Front Endocrinol (Lausanne). 2023;14:1192602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 41. | Mehta BK, Singh KK, Banerjee S. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int J Neurosci. 2019;129:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |