Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105080
Revised: March 27, 2025
Accepted: May 22, 2025
Published online: June 15, 2025
Processing time: 154 Days and 5.4 Hours
The impact of varying degrees of pregnancy-induced hypertension (PIH) on the risk of developing diabetes later in life is currently unknown.
To assess the long-term risks of type 2 diabetes mellitus (T2DM), prediabetes, and mortality that are associated with hypertensive disorders of pregnancy.
This retrospective cohort study used the TriNetX United States Collaborative Network to examine outcomes, especially T2DM, prediabetes and mortality, related to hypertensive disorders of pregnancy in females aged 21-45. Participants had no history of hypertension or diabetes before pregnancy or before 20 weeks of gestation. Propensity score matching was applied to balance covariates such as gestational diabetes, polycystic ovarian syndrome, chronic kidney disease, hy
This study included 318544 females aged 21-45 with and without PIH. Females with PIH had higher risks of T2DM [hazard ratio (HR): 1.907, 95% confidence interval (CI): 1.821-1.998), prediabetes (HR: 1.610, 95%CI: 1.537-1.687), and mortality (HR: 1.501, 95%CI: 1.361-1.655) over a follow-up of up to 18 years. Incidence rates for T2DM, prediabetes, and mortality were 3.2%, 2.7%, and 0.6%, respectively. Subgroup analyses showed that the presence of gestational hypertension, preeclampsia, and eclampsia increased risks across all outcomes. Persistent hypertension beyond 12 weeks postpartum was linked to more than a 3-fold increase in mortality. Preventative aspirin use during pregnancy did not reduce the risks of T2DM, prediabetes, or mortality among those with PIH.
PIH significantly increases the long-term risks of T2DM, prediabetes, and mortality, highlighting the urgent need for improved long-term management strategies to enhance overall health in such individuals.
Core Tip: This study highlighted the increased risks of type 2 diabetes mellitus (T2DM), prediabetes, and mortality in females with a history of pregnancy-induced hypertension (PIH). These risks were further amplified in cases of persistent postpartum hypertension. Gestational hypertension, preeclampsia, and eclampsia were associated with elevated risks, particularly for mortality and T2DM. Preventative aspirin use during pregnancy did not appear to mitigate these long-term risks. Our findings underscored the importance of enhanced monitoring and preventive strategies for females who experienced PIH and especially in those who had persistent hypertension during the postpartum period.
- Citation: Shih YH, Yang CY, Lung CC. Long-term risk of diabetes following hypertensive disorders of pregnancy: A retrospective cohort study. World J Diabetes 2025; 16(6): 105080
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105080.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105080
Hypertensive disorders of pregnancy impact 10% of pregnancies globally and include chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and chronic hypertension with superimposed preeclampsia[1]. Gestational hypertension and preeclampsia/eclampsia often develop after 20 weeks of gestation[1]. Preeclampsia, in addition to hypertension, is also accompanied by signs of maternal organ or uteroplacental dysfunction or proteinuria. It is a significant contributor to maternal morbidity and is linked to several adverse fetal outcomes including intrauterine growth restriction, preterm birth, placental abruption, fetal distress, and fetal death in utero[1].
Females with preeclampsia often experience insulin resistance during pregnancy that is independent of obesity and glucose intolerance[2]. Research suggests that insulin resistance may precede the onset of preeclampsia, indicating that it may contribute to preeclampsia development[2,3]. Therefore, the presence of insulin resistance with preeclampsia may serve as a marker for future diabetes risk, even in the absence of gestational diabetes mellitus.
Previous research have suggested that females with a history of preeclampsia or eclampsia are at an increased risk of developing type 2 diabetes[1,4-6]. A population-based cohort study found that the incidence rate of diabetes is higher in females with preeclampsia than in those with gestational hypertension[4]. However, the risk of developing diabetes later in life in females who experienced pregnancy-induced hypertension (PIH), including gestational hypertension, pr
The present study addressed this research gap by conducting a retrospective analysis using extensive data from the TriNetX database. We specifically focused on examining patients who developed hypertensive disorders after 20 weeks of gestation and their association with the subsequent development of type 2 diabetes mellitus (T2DM) over a follow-up period of up to 18 years.
We performed a retrospective analysis using data from the United States Collaborative Network, which includes information from 80 healthcare organizations within the TriNetX Research Network. TriNetX primarily collects data from structured electronic health record systems and encompasses demographics, diagnoses, procedures, and medications and employs natural language processing to extract information from clinical documents. The TriNetX database consists of de-identified electronic health records that undergo extensive preprocessing to address missing values. The platform has standardized data across healthcare organizations, ensuring consistency and allowing uniform querying. Additionally, natural language processing is used to extract relevant clinical information from unstructured records.
While TriNetX applies data harmonization techniques to improve data completeness, missing data were managed through the inherent standardization process of the platform. This ensures a robust and comprehensive dataset for analysis[7]. The utilization of TriNetX data in scholarly research has demonstrated consistent growth since 2015. Between 2018 and 2021, the annual publication output more than doubled, culminating in 248 peer-reviewed publications and abstracts by 2022. This sustained expansion as well as the established role of the network in facilitating clinical trial partnerships and supporting published research underscore its viability as a robust, secure, and sustainable framework for maintaining research-oriented data networks across academic and industry sectors[7].
Ethical considerations were thoroughly addressed including obtaining an informed consent waiver due to the anonymous nature of the data. The study complied with regulatory guidelines such as the Health Insurance Portability and Accountability Act and the General Data Protection Regulation. Approval was granted by the Institutional Review Board of Taichung Veterans General Hospital (CE24431B).
The study utilized data from the TriNetX database from January 1, 2006 to December 31, 2020. Two cohorts were established: The PIH cohort that included females aged 21-45 years with PIH (defined as gestational hypertension, preeclampsia with or without severe features, and eclampsia) and no history of hypertension or diabetes before pregnancy or before 20 weeks of gestation; and the non-PIH cohort that consisted of females aged 21-45 who delivered without complications and had no prior hypertension or diabetes.
The age range of 21-45 was selected to focus on individuals in their reproductive years while minimizing confounding factors associated with younger or older age groups. Females younger than 21 were excluded due to potential differences in risk profiles and healthcare access in adolescent pregnancies. Females older than 45 were excluded to reduce the influence of perimenopausal changes and age-related comorbidities, which could confound associations between PIH and long-term metabolic risks.
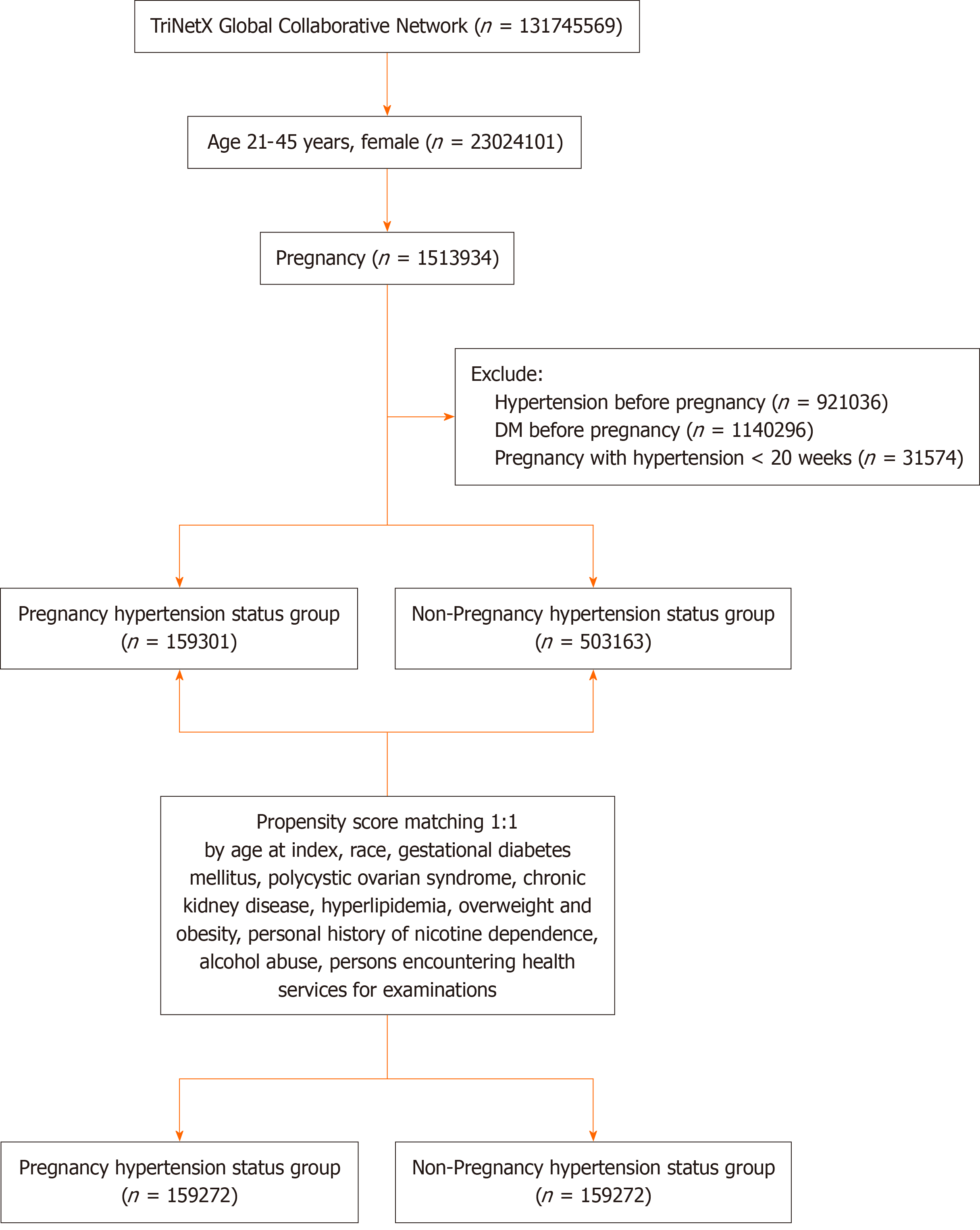
Blood pressure records in the database are based on the measurements taken during patient medical visits. Propensity score matching (PSM) was used to balance covariates such as gestational diabetes mellitus, polycystic ovarian syndrome, chronic kidney disease, hyperlipidemia, overweight/obesity, nicotine dependence, alcohol abuse, and healthcare utilization. The risk of T2DM, prediabetes, and mortality was evaluated for an 18-year follow-up period. Figure 1 illustrates the study flowchart. Supplementary Table 1 lists the diseases discussed in this article along with their corresponding codes.
In this investigation, we utilized PSM through the integrated TriNetX tool to establish 1: 1 matched groups characterized by similar baseline traits. PSM was applied selectively to covariates without missing values, and distinct matching procedures were employed for subsequent stratified analyses involving a constrained set of covariates. The PSM process seamlessly integrated with the TriNetX system and employed a greedy nearest neighbor algorithm with a 0.1 caliper of pooled standard deviations. The assessment of balance relied on the standardized mean difference and utilized proprietary technology safeguarded by trade secrets.
For time-to-event outcomes, Cox proportional hazards regression models were employed to estimate hazard ratios (HR) and 95% confidence intervals (CI). These models were used to assess the association between PIH and the risk of developing T2DM, prediabetes, and mortality over the follow-up period. Subsequent to PSM HR were computed using χ2 tests for measures of association. Cumulative probability was evaluated through Kaplan-Meier curves, incorporating log-rank tests and HR for cohort comparisons. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were executed on the TriNetX online platform.
The initial dataset included 662464 patients, with 159301 in the PIH cohort and 503163 in the non-PIH cohort. After applying PSM, each cohort was balanced to include 159272 patients. The average age at index was 27.5 years, with the cohort comprising 57.2% White, 18.5% Black or African American, and 2.6% Asian individuals (Table 1).
| Before PSM | After PSM | |||||||
| Pregnancy-induced hypertension (n = 159301) | Non-pregnancy hypertension (n = 503163) | SMD | P value | Pregnancy-induced hypertension (n = 159272) | Non-pregnancy hypertension (n = 159272) | SMD | P value | |
| Age at index (mean ± SD) | 27.7 ± 5.6 | 26.9 ± 5.5 | 0.147 | < 0.001 | 27.7 ± 5.6 | 27.7 ± 5.6 | 0.001 | 0.898 |
| Race | ||||||||
| White | 91079 (57.2) | 261926 (52.1) | 0.103 | < 0.001 | 91061 (57.2) | 91025 (57.2) | 0.001 | 0.897 |
| Black or African American | 29538 (18.5) | 74161 (14.7) | 0.102 | < 0.001 | 29527 (18.5) | 29673 (18.6) | 0.002 | 0.506 |
| Asian | 4128 (2.6) | 20490 (4.1) | 0.083 | < 0.001 | 4128 (2.6) | 4112 (2.6) | 0.003 | 0.858 |
| Lifestyle | ||||||||
| Personal history of nicotine dependence | 5366 (3.4) | 11826 (2.4) | 0.061 | < 0.001 | 5361 (3.4) | 5344 (3.4) | 0.001 | 0.867 |
| Alcohol abuse | 94 (0.1) | 259 (0.1) | 0.003 | 0.256 | 94 (0.1) | 72 (0.1) | 0.006 | 0.088 |
| Persons encountering health services for examinations | 64133 (40.3) | 199470 (39.6) | 0.013 | < 0.001 | 64120 (40.3) | 64196 (40.3) | 0.001 | 0.784 |
| Comorbidities | ||||||||
| Gestational Diabetes mellitus | 12509 (7.9) | 25732 (5.1) | 0.111 | < 0.001 | 12486 (7.8) | 12450 (7.8) | 0.001 | 0.812 |
| Polycystic ovarian syndrome | 3870 (2.4) | 6079 (1.2) | 0.091 | < 0.001 | 3845 (2.4) | 3796 (2.4) | 0.002 | 0.57 |
| Chronic kidney disease | 298 (0.2) | 460 (0.1) | 0.026 | < 0.001 | 295 (0.2) | 276 (0.2) | 0.003 | 0.426 |
| Hyperlipidemia | 1368 (0.9) | 3777 (0.5) | 0.048 | < 0.001 | 1362 (0.9) | 1314 (0.8) | 0.003 | 0.351 |
| Overweight and obesity | 26168 (16.4) | 44970 (8.9) | 0.226 | < 0.001 | 26139 (16.4) | 26169 (16.4) | 0.001 | 0.886 |
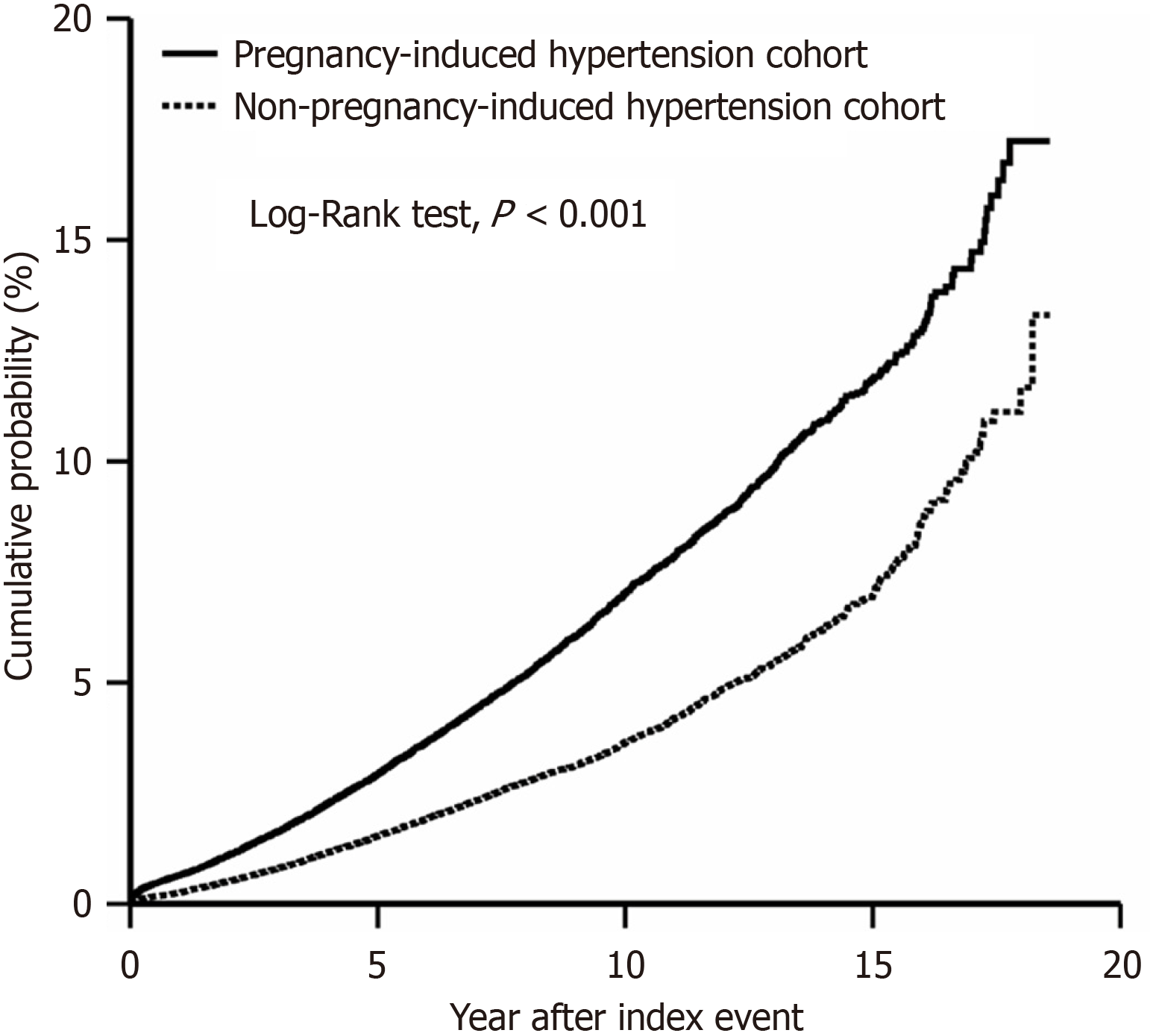
We observed a maximum follow-up of 18 years and an average follow-up of 8.3 years. Our analysis revealed that individuals with PIH had a significantly elevated risk of T2DM compared to those without PIH. The HR was 1.907 (95%CI: 1.821-1.998), indicating almost a 2-fold increase in risk (Figure 2). The risk of prediabetes (HR: 1.601, 95%CI: 1.537-1.687) and overall mortality (HR: 1.501, 95%CI: 1.361-1.655) was also significantly higher. The incidence rates of T2DM, prediabetes, and mortality among individuals with a history of PIH were 3.2%, 2.7%, and 0.6%, respectively (Table 2).
| DM | Pre DM | Deceased | |
| Pregnancy-induced hypertension status (n = 159272)1 | |||
| Yes | 5050 (3.2%) | 4457 (2.7%) | 981 (0.6%) |
| No | 2797 (1.8%) | 2932 (1.8%) | 681 (0.4%) |
| HR (95%CI) | 1.907 (1.821, 1.998) | 1.610 (1.537, 1.687) | 1.501 (1.361, 1.655) |
Subgroup analyses comparing the gestational hypertension cohort with the non-PIH cohort revealed that gestational hypertension was associated with a significantly increased risk of developing T2DM (HR: 1.936, 95%CI: 1.844-2.033), prediabetes (HR: 1.658, 95%CI: 1.579-1.742), and mortality (HR: 1.315, 95%CI: 1.185-1.459) (Table 3).
| DM | Pre DM | Deceased | |
| Gestational hypertension status (n = 145012) | |||
| Yes | 4539 | 4001 | 797 |
| No | 2527 | 2624 | 647 |
| HR (95%CI) | 1.936 (1.844, 0.033) | 1.658 (1.579, 1.742) | 1.315 (1.185, 1.459) |
| Preeclampsia without severe features (n = 11660) | |||
| Yes | 540 | 405 | 123 |
| No | 226 | 228 | 47 |
| HR (95%CI) | 1.971 (1.687, 2.303) | 1.429 (1.214, 1.681) | 2.133 (1.523, 2.988) |
| Preeclampsia with severe features (n = 12546) | |||
| Yes | 483 | 419 | 98 |
| No | 258 | 281 | 44 |
| HR (95%CI) | 1.837 (1.579, 2.137) | 1.465 (1.260, 1.705) | 2.160 (1.513, 3.083) |
| Eclampsia (n = 6628) | |||
| Yes | 258 | 199 | 87 |
| No | 137 | 154 | 26 |
| HR (95%CI) | 1.772 (1.440, 2.180) | 1.181 (0.957, 1.458) | 3.118 (2.011, 4.834) |
All forms of preeclampsia, including that without severe features, with severe features, or eclampsia, were linked to higher risks of T2DM, prediabetes, and mortality. The only exception was eclampsia, which did not show a statistically significant increased risk for prediabetes (HR: 1.181; 95%CI: 0.957-1.458) (Table 3).
We also explored a cohort of patients with PIH during pregnancy that persisted for up to 12 weeks postpartum. This group was defined as having chronic hypertension[8]. These individuals had a significantly increased likelihood of developing T2DM (HR: 2.543, 95%CI: 2.144-3.016) and prediabetes (HR: 2.073, 95%CI: 1.756-2.448) compared with those in the non-PIH cohort. Additionally, these patients had more than a 3-fold increased risk of mortality with HR of 3.143 (95%CI: 1.983-4.982) (Table 4).
| DM | Pre DM | Deceased | |
| Pregnancy hypertension status with postpartum hypertension | |||
| Yes | 457 | 419 | 74 |
| No | 186 | 209 | 24 |
| HR (95%CI) | 2.543 (2.144, 3.016) | 2.073 (1.756, 2.448) | 3.143 (1.983, 4.982) |
| Pregnancy hypertension status with aspirin prevention (n = 4374) | |||
| Yes | 170 | 195 | 21 |
| No | 126 | 145 | 14 |
| HR (95%CI) | 1.736 (1.369, 2.201) | 1.783 (1.429, 2.225) | 2.133 (1.056, 4.311) |
Our study found that the risks of T2DM, prediabetes, and overall mortality remained high even with preventative aspirin use. Among patients with PIH who used aspirin (50-150 mg) during pregnancy, the risk of developing T2DM (HR: 1.736, 95%CI: 1.369-2.201), prediabetes (HR: 1.783, 95%CI: 1.429-2.225), and overall mortality (HR: 2.133, 95%CI: 1.056-4.311) was increased (Table 4).
Additionally, we examined the impact of preventative aspirin use on these risks among patients with PIH. The results showed no significant differences in the risk of T2DM (HR: 1.075, 95%CI: 0.844-1.369) or mortality (HR: 0.950, 95%CI: 0.479-1.884) between those who used aspirin and those who did not. However, aspirin use was associated with an increased risk of developing prediabetes (HR: 1.344, 95%CI: 1.106-1.634).
When stratified by the PIH subtypes, gestational hypertension, preeclampsia (with or without severe features), and eclampsia, aspirin use did not reduce the risk of T2DM or mortality. However, an increased risk of prediabetes was observed in patients with gestational hypertension who used aspirin (HR: 1.599) and in patients with eclampsia who used aspirin (HR: 3.820) (Supplementary Table 2).
Our study, with a follow-up period of up to 18 years, highlighted the significant long-term health risks associated with PIH. Individuals with a history of PIH were at a significantly higher risk of T2DM (HR: 1.907), prediabetes (HR: 1.610), and mortality (HR: 1.501) compared with those without PIH. Subgroup analyses further revealed that gestational hypertension was linked to increased risks of T2DM, prediabetes, and mortality. Preeclampsia in various forms also elevated these risks, although eclampsia did not appear to significantly impact the risk of prediabetes. Specifically, individuals with chronic postpartum hypertension had even greater risks, including a more than 3-fold increase in mortality (HR: 3.143) and significantly higher likelihood of developing T2DM (HR: 2.543) and prediabetes (HR: 2.073). Notably, preventative aspirin use during pregnancy did not decrease the risk of T2DM, prediabetes, and mortality in patients with PIH compared with patients without PIH.
Gestational hypertension and preeclampsia are significant contributors to maternal, fetal, and neonatal mortality and morbidity[9]. Preeclampsia primarily results from abnormal placentation and is marked by defective cytotrophoblast invasion of spiral arteries and an impaired differentiation process[10]. This dysfunction disrupts the nitric oxide pathway, leading to increased uterine arterial resistance and chronic placental ischemia[10]. Concurrently, oxidative stress releases harmful substances into the maternal circulation, resulting in endothelial dysfunction, vascular hyperpermeability, and hypertension[11]. Insulin resistance is also hypothesized to play a role in the pathophysiology of preeclampsia. Those who develop preeclampsia exhibit higher levels of insulin resistance prior to pregnancy, during the first and second trimesters, and even years after pregnancy than patients who do not experience preeclampsia. This association is linked to the many overlapping risk factors for preeclampsia and insulin resistance, such as obesity, advanced maternal age, non-White race, chronic hypertension, diabetes, and gestational diabetes[12].
A cohort study in Scotland found that 10.4% of mothers who had preeclampsia subsequently developed T2DM. Logistic regression analysis revealed an adjusted odds ratio of 1.40 (95%CI: 1.12-1.75) for future T2DM in the cohort[5]. Similarly, a retrospective cohort study in Canada reported that females who experienced preeclampsia during pregnancy had twice the risk of developing diabetes in the 16.5 years following delivery compared with females without preeclampsia, with an HR of 2.08 (95%CI: 1.97-2.19)[4]. However, not all studies have found a consistent link between preeclampsia and an increased risk of diabetes. For instance, a cohort study in Finland compared females with preeclampsia or eclampsia to normotensive females. They reported an HR of 1.42 (95%CI: 0.92-2.19) for developing T2DM over a 40-year follow-up period, indicating no statistically significant difference in risk between the two groups[6]. In our study, we found that females who experienced PIH had a nearly 2-fold increased risk of developing T2DM, which aligns with the findings of the aforementioned Canadian retrospective study.
Although varying degrees of PIH may impact the risk of developing diabetes later in life, there is currently no comprehensive research addressing this issue. A previous study from Finland found that gestational hypertension significantly increased the risk of T2DM, with an HR of 1.52 (95%CI: 1.21-1.89). In contrast, the HR for preeclampsia/eclampsia did not show a statistically significant difference, a finding that may seem counterintuitive[6]. Another cohort study reported that both gestational hypertension and preeclampsia approximately doubled the risk of developing T2DM. The HR for gestational hypertension was 1.95 (95%CI: 1.83-2.07), while the HR for preeclampsia/eclampsia was 2.08 (95%CI: 1.97-2.19)[4].
Our findings indicated that the risk of developing T2DM or prediabetes was consistently elevated across different levels of PIH, including gestational hypertension, preeclampsia with or without severe features, and eclampsia. However, an exception was observed in eclampsia, which did not significantly increase the risk of prediabetes (HR: 1.181, 95%CI: 0.957-1.458). This seemingly contradictory result may be explained by several factors. Females with a history of eclampsia may bypass the intermediate stage of prediabetes and progress more rapidly to T2DM. Additionally, the database may not accurately capture prediabetes diagnoses because prediabetes is often undiagnosed or not formally coded for management, leading to potential underrepresentation in the data. In contrast, T2DM is more likely to be formally recorded once diagnosed. Furthermore, the HR for prediabetes suggests that there may be a trend toward increased risk, but the study may not have had sufficient power to detect a significant association. Future research should incorporate more detailed metabolic analyses and long-term follow-up to further explore the underlying mechanisms.
Most females who experience PIH will see their blood pressure return to normal within 12 weeks postpartum[13]. The American College of Obstetricians and Gynecologists recommends that all females affected by PIH have an initial contact with an obstetric care provider within 3 weeks postpartum, followed by a comprehensive postpartum visit by 12 weeks postpartum[14]. This is to ensure that the patient’s blood pressure has returned to normal and to recommend further treatment if needed. No previous study has focused on the risk of T2DM in females with preeclampsia and persistent postpartum hypertension beyond 12 weeks. Our findings indicate that if a patient experiences PIH and hypertension beyond 12 weeks postpartum, her risk of developing T2DM is 2.5 times higher compared with females without PIH. This risk is significantly higher than the 1.9-fold increase associated with PIH alone.
A meta-analysis suggested that aspirin is highly effective in preventing preterm preeclampsia when administered to high-risk females and started before 16 weeks of gestation, reducing its incidence by more than 60%[15]. The American College of Obstetricians and Gynecologists also recommends aspirin prophylaxis for females at high risk of preeclampsia to prevent the condition[16]. However, the current literature has not explored the impact of aspirin use in patients who develop preeclampsia on the subsequent risk of T2DM. One double-blind, placebo-controlled trial found that daily oral low-dose aspirin can reduce the incidence of T2DM, but this study focused on individuals who were at least 65 years of age and free of cardiovascular disease, physical disabilities, or dementia[17]. Our findings suggest that aspirin use during pregnancy does not reduce the risk of T2DM or mortality in patients with PIH. Surprisingly, it may even be associated with an increased risk of developing prediabetes, particularly among those with gestational hypertension and eclampsia. These results highlighted the need for further research to assess the long-term metabolic impacts of aspirin use in PIH patients.
While the previously discussed studies focused solely on the risk of T2DM, they did not specifically analyze the risks of prediabetes or mortality. Our findings indicated that both prediabetes and mortality risks are almost universally increased. Specifically, different degrees of PIH appear to have a similar impact on the risk of prediabetes. However, the risk of mortality is notably higher with more severe forms of PIH. The increased mortality risk associated with severe PIH may be influenced by several factors. For instance, preeclampsia is known to significantly increase the risk of car
While our study was robust, certain limitations should be acknowledged. The absence of specific coding in the dataset restricted detailed reporting of key metrics, including the gestational age at preeclampsia diagnosis, week of delivery, time to blood pressure normalization postpartum, and aspirin dose, duration, and adherence. Additionally, unmeasured confounders such as socioeconomic status, lifestyle behaviors, and medication adherence may have influenced the observed associations. Future research should investigate the mechanisms linking preeclampsia to diabetes risk and explore potential confounding factors.
Our retrospective cohort study using the TriNetX database provided robust evidence of an increased long-term risk of T2DM in patients who developed hypertension after 20 weeks of gestation. The severity of preeclampsia appeared to have a similar impact on diabetes risk. PIH with chronic postpartum hypertension lasting more than 12 weeks further amplified these risks. Additionally, preventative aspirin use during pregnancy did not mitigate the risks of T2DM, prediabetes, nor mortality in patients with preeclampsia.
| 1. | Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Sierra-Laguado J, García RG, Celedón J, Arenas-Mantilla M, Pradilla LP, Camacho PA, López-Jaramillo P. Determination of insulin resistance using the homeostatic model assessment (HOMA) and its relation with the risk of developing pregnancy-induced hypertension. Am J Hypertens. 2007;20:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Parretti E, Lapolla A, Dalfrà M, Pacini G, Mari A, Cioni R, Marzari C, Scarselli G, Mello G. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, Hwee J, Booth GL. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10:e1001425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Libby G, Murphy DJ, McEwan NF, Greene SA, Forsyth JS, Chien PW, Morris AD; DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: an intergenerational study from the Walker cohort. Diabetologia. 2007;50:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Männistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, Suvanto E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Palchuk MB, London JW, Perez-Rey D, Drebert ZJ, Winer-Jones JP, Thompson CN, Esposito J, Claerhout B. A global federated real-world data and analytics platform for research. JAMIA Open. 2023;6:ooad035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 199] [Reference Citation Analysis (0)] |
| 8. | Countouris M, Mahmoud Z, Cohen JB, Crousillat D, Hameed AB, Harrington CM, Hauspurg A, Honigberg MC, Lewey J, Lindley K, McLaughlin MM, Sachdev N, Sarma A, Shapero K, Sinkey R, Tita A, Wong KE, Yang E, Cho L, Bello NA. Hypertension in Pregnancy and Postpartum: Current Standards and Opportunities to Improve Care. Circulation. 2025;151:490-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-Induced hypertension. Hormones (Athens). 2015;14:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019;124:1094-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 1213] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 11. | Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 12. | Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | Ghuman N, Rheiner J, Tendler BE, White WB. Hypertension in the postpartum woman: clinical update for the hypertension specialist. J Clin Hypertens (Greenwich). 2009;11:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Khosla K, Heimberger S, Nieman KM, Tung A, Shahul S, Staff AC, Rana S. Long-Term Cardiovascular Disease Risk in Women After Hypertensive Disorders of Pregnancy: Recent Advances in Hypertension. Hypertension. 2021;78:927-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguère Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 760] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 16. | . ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132:e44-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 414] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 17. | Zoungas S, Zhou Z, Owen AJ, Curtis AJ, Espinoza SE, Ernst ME, Woods RL, Orchard SG, McNeil JJ, Murray AM, Nelson MR, Reid CM, Ryan J, Wolfe R. Daily low-dose aspirin and incident type 2 diabetes in community-dwelling healthy older adults: a post-hoc analysis of efficacy and safety in the ASPREE randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2024;12:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 720] [Article Influence: 90.0] [Reference Citation Analysis (0)] |