Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.103685
Revised: March 21, 2025
Accepted: April 17, 2025
Published online: June 15, 2025
Processing time: 175 Days and 3.9 Hours
Erianin is a natural bibenzyl compound extracted from Dendrobium chrysotoxum and is known for its anti-inflammatory and antioxidant properties.
To explore the possible therapeutic mechanisms of erianin and determine if it can reduce cardiac damage in mice with type 2 diabetes.
High-fat diet and intraperitoneal injections of streptozotocin were used to induce type 2 diabetes mellitus in C57BL/6 mice. Mice were divided into different groups including control, model, and treatment with various doses of erianin (10, 20, and 40 mg/kg) as well as ML-385 + erianin group.
Erianin reduced oxidative stress and inflammation and alleviated diabetic cardiomyopathy through the activation of the adenosine monophosphate-acti
Erianin can effectively alleviate myocardial injury in type 2 diabetic mice by activating the AMPK-Nrf2-HO-1 pathway.
Core Tip: This study investigates the cardioprotective effects of erianin, a natural compound from Dendrobium chrysotoxum, in a type 2 diabetic mouse model. Erianin administration significantly reduced myocardial damage and improved metabolic parameters by activating the adenosine monophosphate-activated protein kinase/nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling pathway. These findings suggest that erianin may serve as a potential therapeutic agent to mitigate cardiac injury and inflammation in diabetes, offering new insights into managing diabetic cardiomyopathy.
- Citation: Chen JH, Dai XC, Quan ZJ, Liu XY. Erianin mitigates diabetic cardiomyopathy via adenosine monophosphate-activated protein kinase-nuclear factor erythroid 2-related factor 2-heme oxygenase-1 pathway activation. World J Diabetes 2025; 16(6): 103685
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/103685.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.103685
Diabetes is a leading cause of cardiovascular diseases worldwide, and diabetic cardiomyopathy (DCM) is a significant complication that has limited therapeutic options. More than 450 million individuals are affected by diabetes worldwide, and this number is projected to reach 693 million by 2045 according to the International Diabetes Federation[1]. This growing epidemic poses a serious threat to public health because it can cause extensive damages to multiple target organs, including the heart. In Framingham’s study, when other confounding factors are excluded, people with diabetes are two to five times more likely to develop heart failure than normal people[2]. More than 90% of all diabetes cases in China are type 2 diabetes mellitus (T2DM). Data from the Chinese Medical Association’s Diabetes Branch revealed that the incidence rates of complications in T2DM are as follows: Cardiovascular disease at 17.1%, cerebrovascular disease at 12.6%, hypertension at 34.2%, and lower limb vascular disease at 5.2%. DCM refers to a condition in which patients with diabetes experience impaired cardiac contraction and relaxation functions independently of coronary artery disease, hypertension, and other heart-related ailments. The development of DCM progresses through early-stage diastolic dysfunction and late-stage systolic impairment, and it culminates in the eventual onset of heart failure[3]. Despite nearly five decades of advancements in our understanding of DCM in modern medicine, effective treatment strategies remain elusive, with glycemic control still being the primary approach. Cardiovascular benefits vary widely among numerous medications used in clinical diabetes management[4]. Traditional Chinese medicine boasts a rich heritage in addressing diabetes and its associated complications. In recent years, research has identified several Chinese herbal medicines and their constituents with potential therapeutic effects on DCM[5-7].
Nuclear factor erythroid 2-related factor 2 (Nrf2), as a potent endogenous antioxidant factor, plays a major role in the defense of the body against the damaging effects of diabetes. Numerous studies have proven that Nrf2 can ameliorate diabetic myocardial injury. Chen et al[8] reported that diabetic people and animal models exhibited a considerable reduction in Nrf2 expression. The reduced expression of Nrf2 may exacerbate insulin resistance, abnormal angiogenesis, and endothelial dysfunction, leading to cardiac injury. Conversely, increasing Nrf2 expression can protect myocardial cells and the heart from the effects of a high-glucose environment[9]. Therapeutic strategies targeting Nrf2 can sig
Erianin, derived from Dendrobium orchid, is a lignan compound known for its diverse biological effects, including anti-inflammatory, antioxidant, and anti-tumor properties[13]. Dendrobium can decrease fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) levels, enhance insulin sensitivity, and improve insulin resistance in patients with T2DM[14-17]. Dendrobium not only has a therapeutic effect on diabetes but also shows considerable therapeutic potential for DCM. Iron-bark Dendrobium possesses potential to ameliorate DCM through mechanisms involving enhanced lipid transportation, insulin resistance inhibition, and epithelial-mesenchymal transition suppression[18]. Considering the protective effects of Dendrobium on T2DM and cardiovascular health, its main component, erianin, may serve as an adjuvant therapeutic agent for myocardial injury. This study presents an initial exploration of how erianin alleviates myocardial damage in T2DM mice and its potential mechanism.
Under license number SCXK (Beijing, China) 2019-0010, 51 male C57BL/6 mice with specific pathogen-free grade and a weight of 18-22 g were acquired from Sipeifu (Beijing, China) Biotechnology Co., Ltd. The mice were allowed to and had unrestricted access to food and water at 25 ± 2 °C for 1 week. All experimental procedures were approved by the Animal Ethics Committee of Jinzhou Medical University (No. 2023062) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Forty-three C57BL/6 mice were given a diet heavy in fat and sugar (MS1606, MKBio, China) containing 10% lard, 20% sucrose, 2.5% cholesterol, 1% cholic acid, and 66.5% basic diet for 3 weeks to induce insulin resistance and hyperglycemia. Plasma insulin levels were measured using an ELISA kit (ab277390, Abcam, United Kingdom) to confirm the presence of insulin resistance. Hyperlipidemia was assessed by measuring total cholesterol (TC) (RF8213, Ruifan, China) and triglyceride (TG) (RF8037, Ruifan, China) levels using commercial kits. After a 12-hour fast, the mice were injected intraperitoneally with 1% streptozotocin (STZ) (40 mg/kg body weight) (No. 20131211, Sigma-Aldrich, United States) solution for 3 consecutive days[19]. Following another 12-hour fast, FBG level of ≥ 11.1 mmol/L and appearance of symptoms such as polydipsia, polyphagia, and polyuria confirmed the success of the model. Three mice were selected randomly, and their heart tissues were sampled and observed by hematoxylin-eosin (HE) staining (No. C0105S, Beyotime, China). Our results confirmed that the mice had symptoms of myocardial injury. The successfully modeled mice were randomized into five groups: Three erianin intervention groups of eight mice each, receiving oral administration of erianin (10, 20, and 40 mg/kg, dissolved in corn oil; No. YT62752, Ita Biological Technology Co., Ltd., China) for 6 weeks. The ML-385 (a specific Nrf2 inhibitor) intervention group was given ML-385 (20 mg/kg; No. 846557-71-9, Abcam, the United Kingdom) and erianin (40 mg/kg) for 6 weeks. The model group received an equivalent volume of corn oil through oral administration for 6 weeks. The control group was normally fed and received intraperitoneal injection of an equivalent volume of citrate solution for 3 days, following the same dosing schedule as the erianin intervention groups. They were also orally administered with an equivalent volume of corn oil for 6 weeks. Following STZ injections, all the mice remained on high-fat diet until study termination to sustain diabetic metabolic derangements. Metabolic parameters including insulin resistance and lipid profile alterations were monitored longitudinally.
Animal groups are summarized in Supplementary Table 1. The research design flowchart for this study is shown in Supplementary Figure 1.
The mice were observed at different intervals, and blood glucose and body weight were recorded each week. The mice were then given 1% pentobarbital sodium intraperitoneally to induce anesthesia. Blood samples were obtained from the orbital sinus, and the serum was separated and stored at -80 °C. The heart was removed and stored in 4% paraformaldehyde at -80 °C.
Cardiac function was assessed using the Vevo 2100 high-resolution ultrasound system (VisualSonics, Canada) under 2% isoflurane anesthesia. Left ventricular dimensions and functional parameters including left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular internal dimension in diastole (LVIDd), and left ventricular internal dimension in systole (LVIDs) were measured in parasternal long-axis views following established protocols.
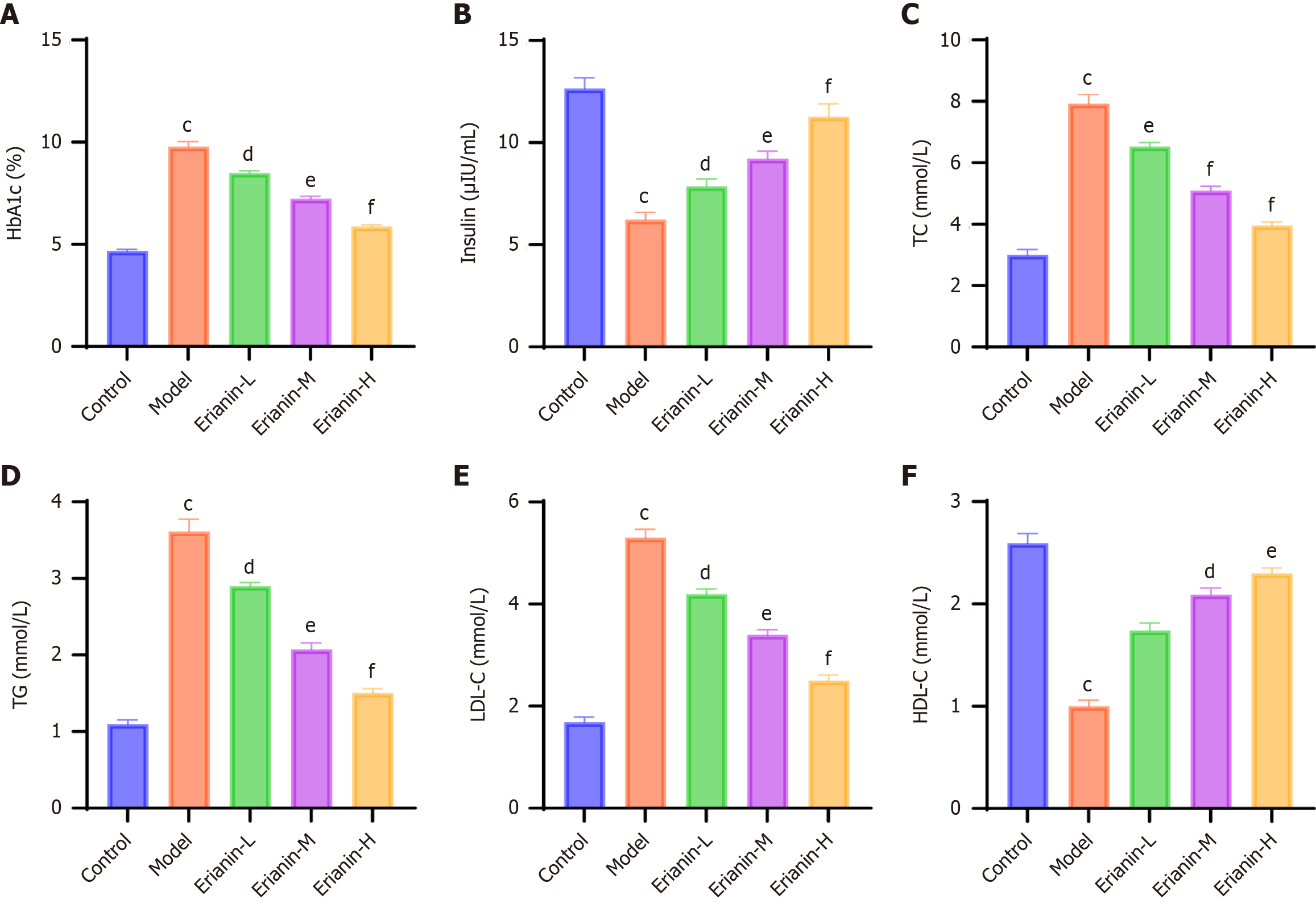
HbA1c levels were quantified using a commercial kit (No. A056-2, Nanjing Jiancheng Bioengineering Institute, China). Plasma insulin concentrations were measured using an ELISA kit (ab 285341, Abcam, United States). TC (ab 65390, Abcam, United States) and TG (MAK266, Sigma-Aldrich, United States) levels were determined by enzymatic assays. Low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were analyzed using enzymatic assay kits (specific catalog numbers for LDL-C and HDL-C should be provided here if available). High-fat diet administration continued throughout STZ injection and subsequent interventions to maintain metabolic challenge.
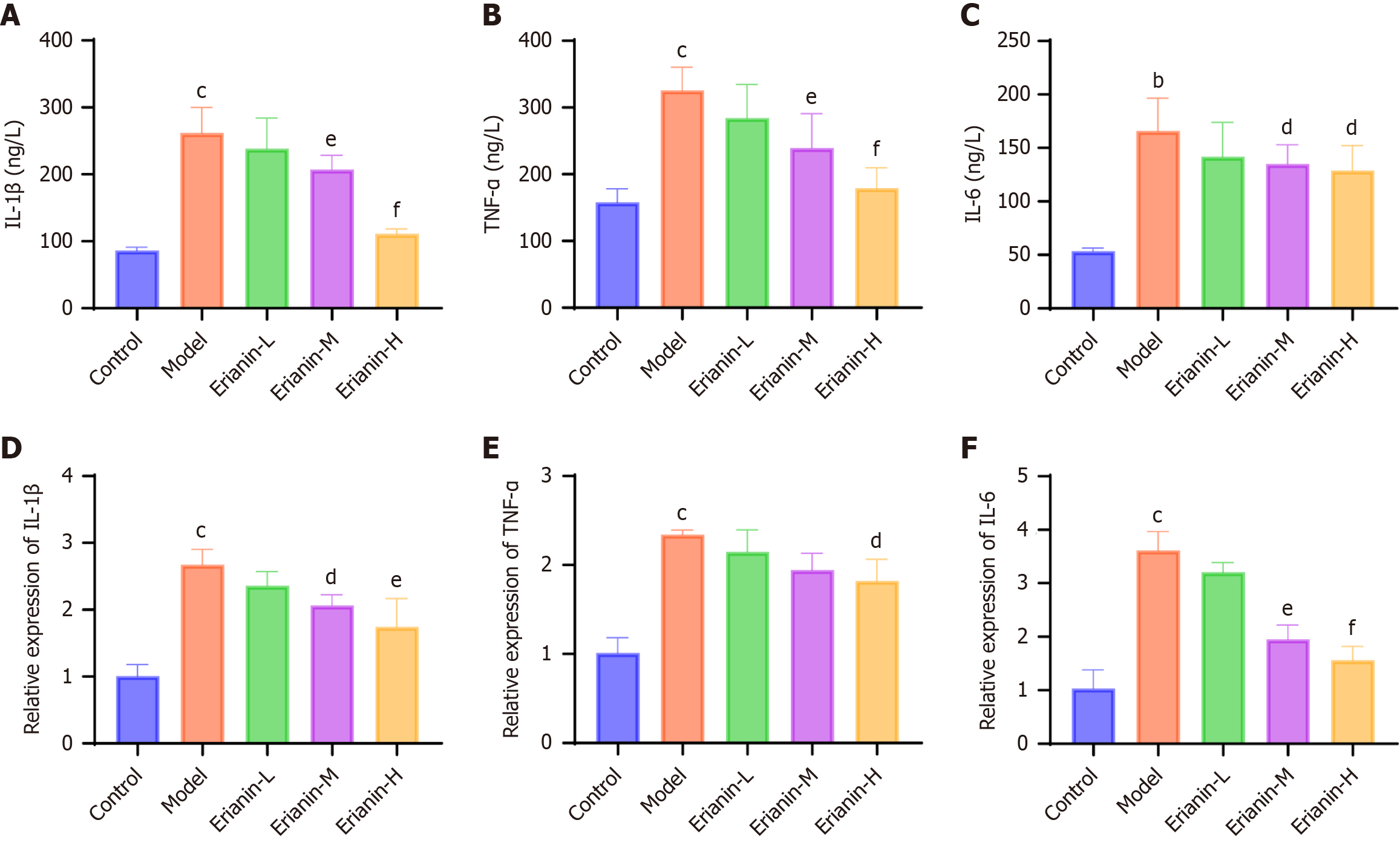
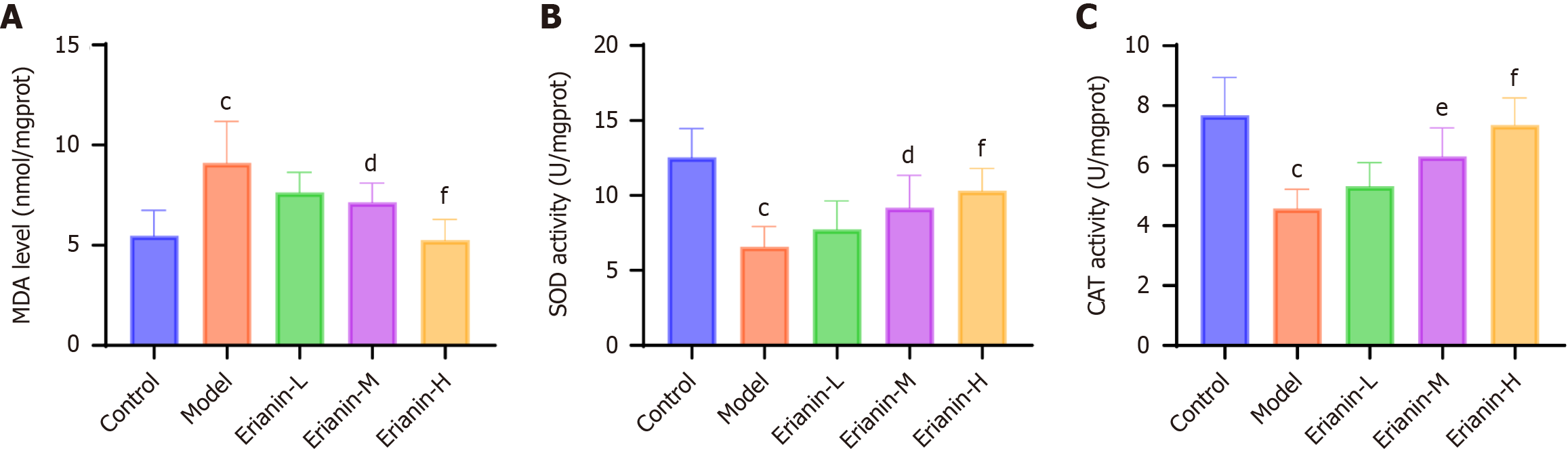
Commercial kits (No. A032-1-1, No. H197-1-1, No. H149-2-2, No. H002-1-2, No. H052-1-2, No. H007-1-1, No. A003-1-2, No. A001-1-2, No. A007-1-1, Nanjing Jiancheng Bioengineering Institute, China) were acquired for analyses. The levels of creatine kinase (CK), CK isoenzymes (CK-MB), cardiac troponin I (cTnI), interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), IL-6, malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were determined as directed in the kits.
The cardiac tissues were embedded in paraffin and treated in 4% paraformaldehyde to create 4 μm-thick slices. For light microscopy viewing and photography, the paraffin slices from each group were deparaffinized to water, stained with HE, dried, and cover slipped.
Cardiac tissues were subjected to RNA extraction using Thermo Fisher Scientific’s Trizol reagent (AM9738, United States). Reverse transcription was carried out using a reverse transcription-polymerase chain reaction test kit (RR014A and RR037A, Takara, Japan) and the β-actin gene as an internal control. The 2ΔΔct technique was employed to calculate the relative levels of mRNA. Table 1 lists the primer sequences.
| Forward sequence | Reverse sequence | |
| TNF-α | ACCACGCTCTTCTGTCTACT | AGGAGGTTGACTTTCTCCTG |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| IL-6 | CACATGTTCTCTGGGAAATCGTGGA | TCTCTCTGAAGGACTCTGGCTTTGT |
| β-actin | CGTGGGCCGCCCTAGGCACCA | TTGGCCTTAGGGTTCAGGGGG |
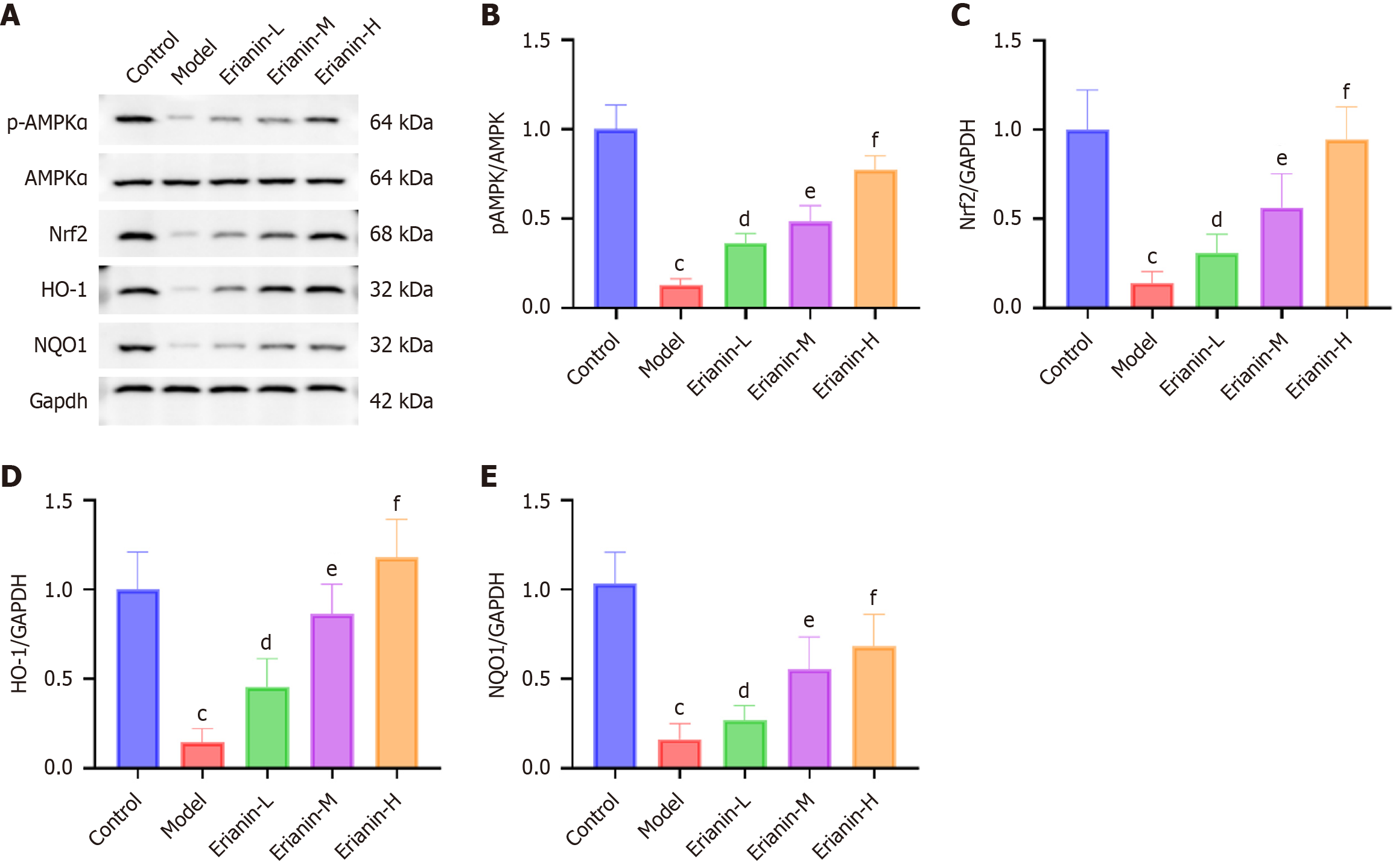
The frozen cardiac tissues from each group were retrieved, minced, and added to tissue lysis buffer (P0013, Beyotime, China). After being thoroughly homogenized and centrifuged, the supernatant was collected as the total protein solution. Protein concentration was determined using the kit (042820200917, Beyotime, China). Subsequently, 5 × loading buffer was added and thoroughly mixed. The mixture was boiled to denature the proteins, which were electrophoretically separated and transferred onto a membrane. The primary antibody [1:1000, p-adenosine monophosphate-activated protein kinase (AMPK)-α ab92701, AMPKα ab32047, Nrf2 ab62352, HO-1 ab305290, NAD(P)H:quinone oxidoreductase 1 (NQO1) ab80588, Abcam, United States) and secondary antibody (1:10000, ab205718, Abcam, United States) were used for incubation. After washing the membrane, enhanced chemiluminescence solution was used for visualization.
A priori power analysis (α = 0.05, power = 0.8) was conducted using GPower 3.1.9.7 based on preliminary data from cardiac function parameters (LVEF and LVFS). The calculated effect size (Cohen’s f = 0.35) indicated that a minimum sample size of six mice per group would be sufficient to detect significant differences. Our final sample size (n = 8 per group) exceeded the requirement to ensure adequate statistical power. The Shapiro-Wilk test confirmed the normal distribution of data, thereby justifying the use of parametric tests (one-way ANOVA with LSD post-hoc analysis). These methodological choices enhanced the validity of our conclusions by minimizing Types I and II errors.
Statistical analysis was conducted by GraphPad Prism 5, and results were expressed as mean ± SE. The Shapiro-Wilk test was used for continuous variable to confirm normal distribution. One-way analysis of variance was performed for comparisons among multiple groups, whereas least significant difference method was used for pairwise comparisons. Statistical significance was set at P < 0.05.
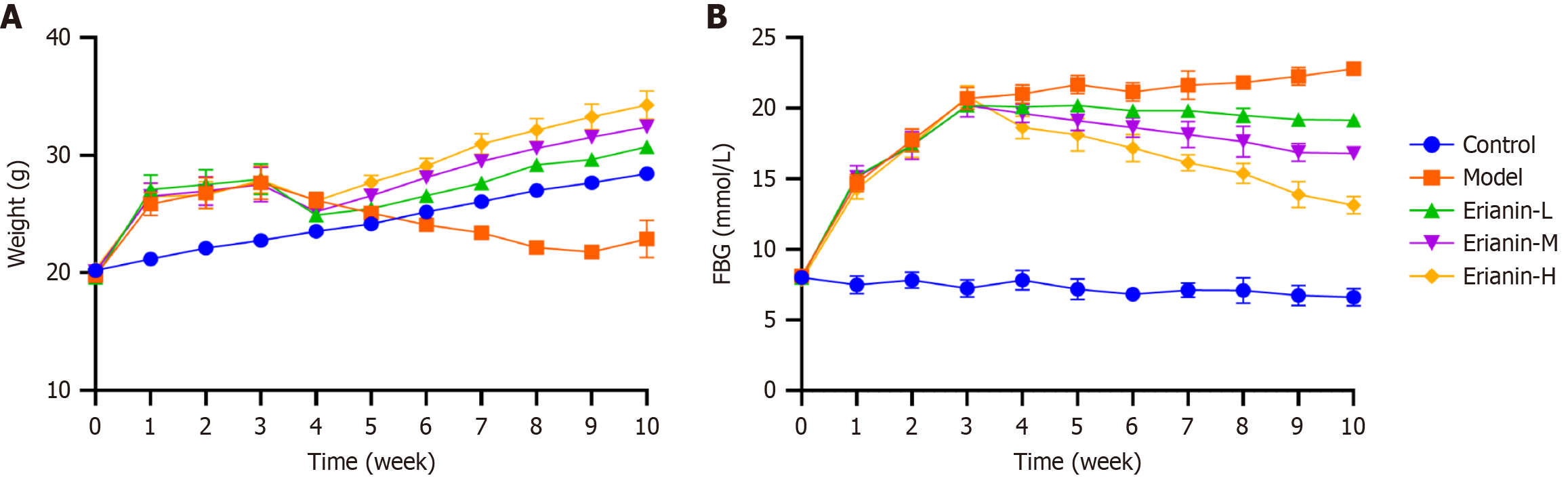
The weight of mice in the model group increased dramatically following high-fat diet administration and then gradually decreased following STZ injection. The body weight of mice in the erianin group increased slowly compared with that in the model group (Figure 1A). The FBG level in the model group considerably increased compared with that in the control, whereas the FBG level in the erianin group significantly decreased compared with that in the model (Figure 1B). The findings indicate that erianin effectively mitigates the negative impact of a high-fat diet and STZ induction on body weight and FBG levels in type 2 diabetic mice. The erianin-treated groups exhibited a controlled rate of weight gain and significantly reduced FBG levels than the model group, which showed substantial increases. Hence, erianin might contribute to the regulation of glucose metabolism and weight management under diabetic conditions.
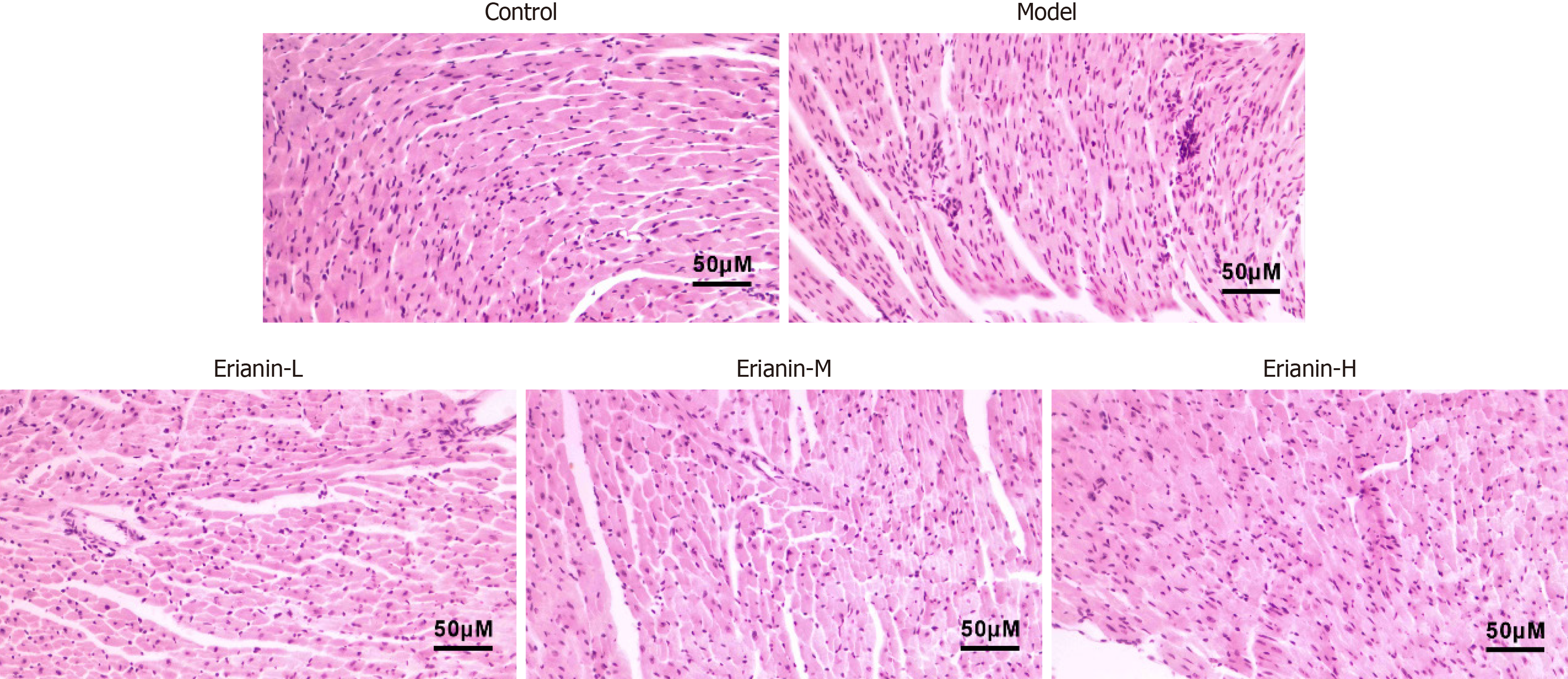
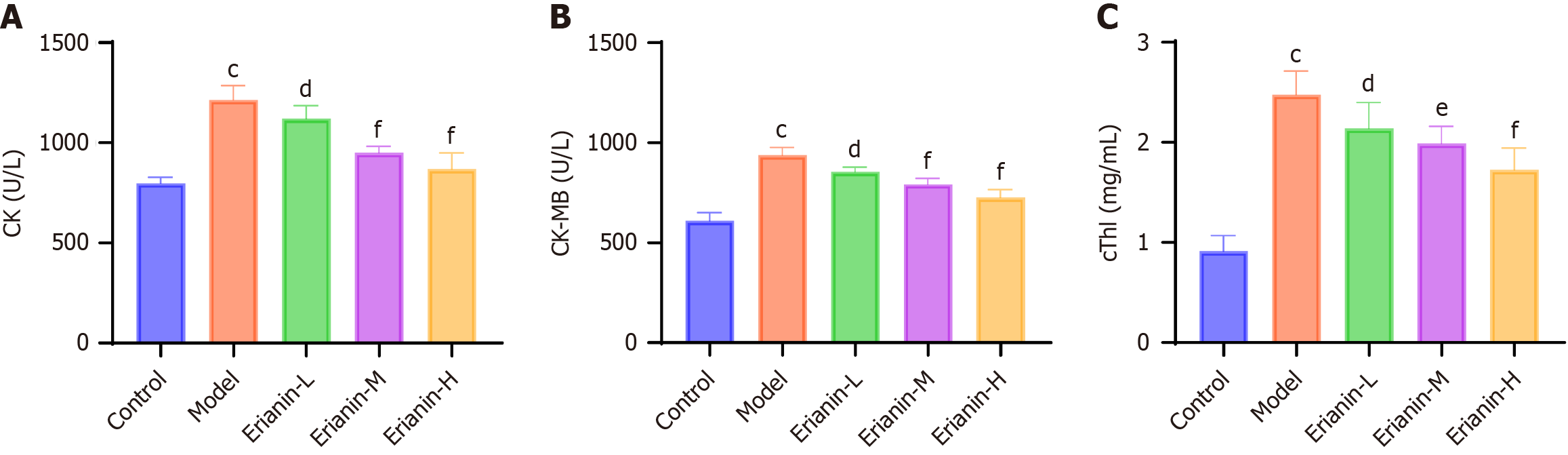
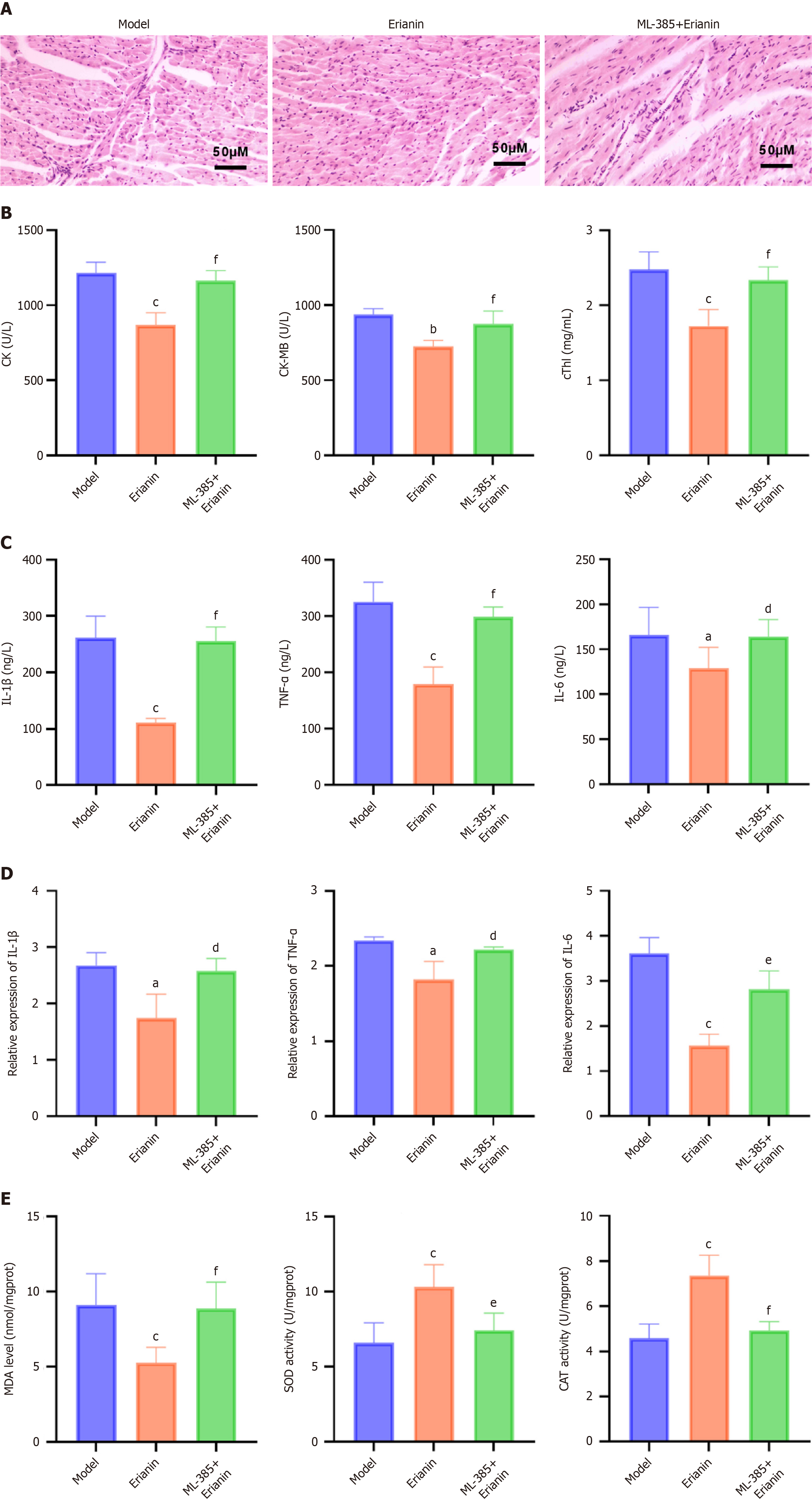
The myocardial fibers of control mice were arranged neatly and uniformly, and infiltration of inflammatory cells or significant cellular degeneration or necrosis were not observed (Figure 2). In the model group, mouse heart muscle fibers were disorganized and irregularly arranged, with localized occurrences of inflammatory cell infiltration, cellular degeneration, and necrosis. Compared with the model, the erianin-L and erianin-H groups exhibited well-organized cell arrangement in mouse cardiac tissues and improved myoplasmic dissolution. In the erianin-H group, the myocardial tissue pathology improved. As illustrated in Figure 3, the erianin-treated groups demonstrated significant reductions in the levels of serum markers of myocardial damage compared with the model group (CK: 1214.0 ± 71.7 U/L; CK-MB: 937.7 ± 39.2 U/L; cTnI: 2.5 ± 0.2 mg/mL). Specifically, the erianin-H group exhibited marked decreases in CK (868.9 ± 80.6 U/L, P < 0.001), CK-MB (726.8 ± 39.2 U/L, P < 0.001), and cTnI (1.7 ± 0.2 mg/mL, P < 0.001). Erianin confers protective effects against myocardial damage in type 2 diabetic mice. The improvements in myocardial fiber arrangement and the reductions in serum markers of myocardial injury in the erianin-treated groups highlight its potential to ameliorate heart tissue pathology and reduce myocardial damage. Hence, erianin may play a beneficial role in maintaining cardiac structure and function under diabetic conditions.
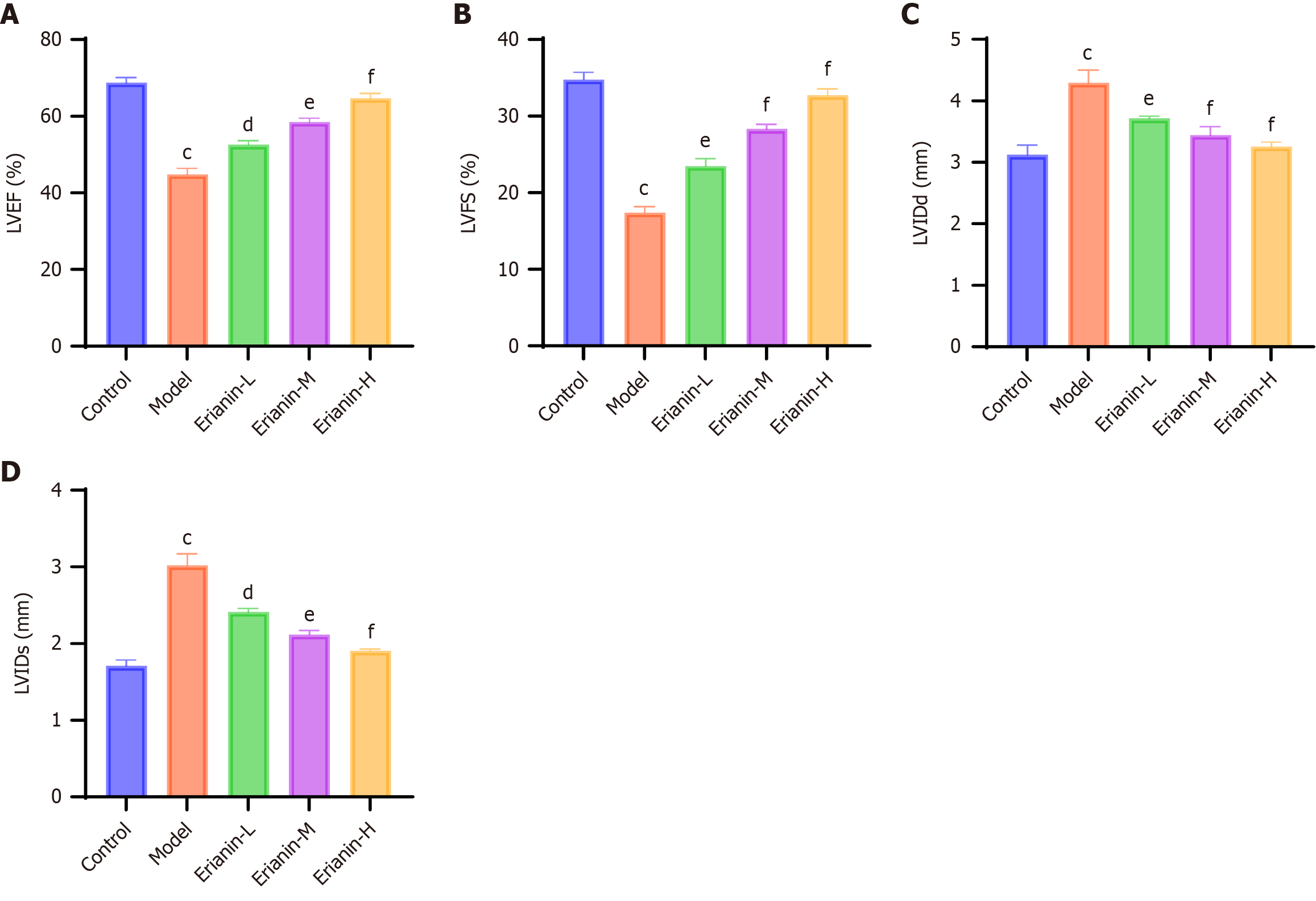
As shown in Figure 4, echocardiographic assessment revealed significant cardiac dysfunction in the model group compared with the controls (P < 0.001 for all parameters). Treatment with erianin dose-dependently improved left ventricular systolic function, particularly in the high-dose group where LVEF and LVFS approached normal levels (P < 0.001 vs model). Ventricular remodeling parameters such as LVIDd and LVIDs were significantly restored in the erianin-treated groups (P < 0.05 vs model).
Comprehensive metabolic profiling demonstrated that erianin intervention significantly ameliorated diabetes-associated dysregulation. Glycemic control improved progressively across treatment groups, with high-dose erianin achieving near-physiological HbA1c levels (P < 0.001 vs model; Figure 5A). Plasma insulin levels increased dose-dependently (P < 0.05), indicating enhanced β-cell function (Figure 5B). Lipid metabolism analysis revealed that erianin decreased the levels of TC, TG, and LDL-C (P < 0.05 vs model) and restored the level of HDL-C (P < 0.01; Figure 5C-F).
The serum and cardiac tissue mRNA levels of IL-1β, TNF-α, and IL-6 in the model group (Figure 6, respectively) were higher than those in the control (P < 0.05). However, their expression was significantly reduced in the erianin group (P < 0.05), and the effect was dose dependent. Our observations suggest that erianin significantly attenuates inflammatory responses in the myocardium of type 2 diabetic mice. The marked decrease in the serum and cardiac tissue levels of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 after erianin treatment underscores its anti-inflammatory potential. Hence, erianin may be integrated into therapeutic strategies aimed at reducing inflammation-associated cardiac damage in diabetes.
The MDA levels and the activities of SOD and CAT in mouse cardiac tissues were measured using assay kits (Figure 7). Compared with the control group (MDA: 5.48 ± 1.26 nmol/mg; SOD: 12.54 ± 1.93 U/mg; CAT: 7.69 ± 1.26 U/mg), the model group (MDA: 9.11 ± 2.08 nmol/mg; SOD: 6.60 ± 1.34 U/mg; CAT: 4.58 ± 0.63 U/mg) exhibited a significant increase in MDA levels and a decrease in the activities of SOD and CAT (P < 0.05). The MDA levels and the activities of SOD and CAT in the erianin-L group (MDA: 7.64 ± 1.01 nmol/mg; SOD: 7.75 ± 1.90 U/mg; CAT: 5.31 ± 0.80 U/mg) had no statistically significant difference compared with the model group. In the erianin-M group (MDA: 7.14 ± 0.96 nmol/mg; SOD: 9.18 ± 2.16 U/mg; CAT: 6.31 ± 0.95 U/mg) and the erianin-H group (MDA: 5.26 ± 1.03 nmol/mg; SOD: 10.30 ± 1.51 U/mg; CAT: 7.37 ± 0.89 U/mg), the MDA levels decreased (P < 0.05) and the activities of SOD and CAT increased to some extent (P < 0.05). The erianin-H group showed the most pronounced changes (P < 0.001). Erianin shows promising antioxidative properties in the cardiac tissues of type 2 diabetic mice, as evidenced by the decrease in MDA levels and the increase in SOD and CAT activities, particularly in the higher dose groups. Hence, erianin effectively reduces oxidative stress, contributing to the preservation of myocardial integrity and function in diabetic environments.
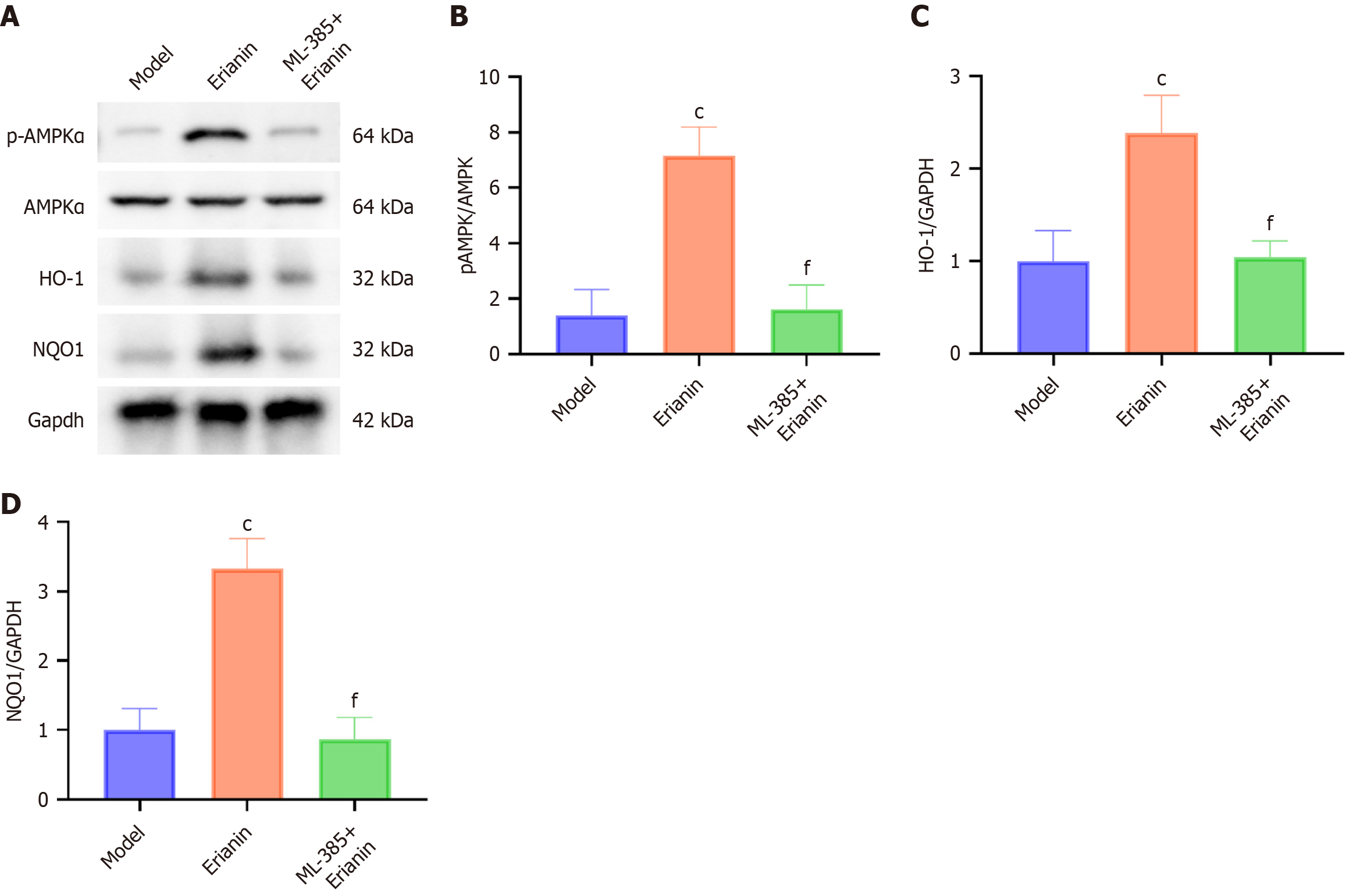
The model group exhibited significantly lower protein levels of p-AMPKα, Nrf2, HO-1, and NQO1 than the control (P < 0.05; Figure 8). The protein levels of p-AMPKα, Nrf2, HO-1, and NQO1 in all intervention groups with erianin were significantly higher than those in the model, and this increase indicated a dose-dependent response to erianin (P < 0.05). As such, erianin has a notable effect on enhancing the AMPK/Nrf2/HO-1 signaling pathway in the myocardium, thereby inducing an improved adaptive response to oxidative and inflammatory stresses in type 2 diabetic mice. Hence, erianin might serve as a modulator of protective cellular pathways. The results offer a mechanistic insight into the cardioprotective function of erianin in diabetes.
Myocardial tissues from the model group, erianin group (40 mg/kg), and ML-385 (20 mg/kg) + erianin group (40 mg/kg) were stained by HE. Mice in the ML-385+erianin group had improved myocardial pathological damage compared with those in the model group; nevertheless, myocardial fiber disarray, irregularity, and inflammatory cell infiltration were not observed (Figure 9A). The serum markers of myocardial injury were significantly elevated in the ML-385 + erianin group compared with those in the erianin group (P < 0.05; Figure 9B). Proinflammatory cytokines TNF-α, IL-1β, and IL-6 had higher blood levels (Figure 9C) and myocardial tissue mRNA levels (Figure 9D) in the ML-385+erianin group than in the erianin group (P < 0.05). The ML-385 + erianin group showed significantly higher levels of MDA (P < 0.05) and lower SOD and CAT activities (P < 0.05) in the cardiac tissues than the erianin group (Figure 9E).
Western blot detection revealed significantly higher levels of p-AMPK α, HO-1, and NQO1 in the erianin group than in the ML-385 + erianin group (Figure 10).
Adverse outcomes in patients with diabetes are mainly associated with various complications caused by long-term disturbances in glucose and lipid metabolism and can lead to damage and dysfunction in multiple organs such as the heart, kidneys, and brain[20]. Diabetic myocardial injury is one of the deadliest complications for people with diabetes. Overall, studies involving type 2 diabetic mice indicated that erianin may mitigate the detrimental effects of T2DM. Animals treated with erianin demonstrated a consistent weight response, alleviated myocardial pathological damage, and reduced levels of FBG, myocardial damage markers, proinflammatory factors, and oxidative stress. These changes were probably linked to the increased expression of Nrf2, HO-1, NQO1, and p-AMPKα and the activation of Nrf2 regulatory mechanisms. Importantly, these findings highlight the potential of erianin as an adjuvant therapy for managing DCM. Further clinical validation is needed to translate preclinical benefits into human applications.
In the T2DM model, mice subjected to the high-fat diet exhibited a notable weight gain and a slight increase in blood glucose levels; on the basis of obesity in mice, low-dose STZ injection induced a late-stage T2DM model, characterized by symptoms such as weight loss and high blood sugar[21]. The present study demonstrated that erianin could alleviate the rapid decline in mouse body weight and reduce blood glucose levels. The results suggested that erianin exerted a regulatory effect on the body weight and blood glucose levels of mice. The improvement in metabolic parameters including HbA1c, lipid profiles, and insulin levels reflects the function of erianin in dual moderation of AMPK-mediated glucose uptake and lipid metabolism[21], thereby restoring cardiac energy homeostasis and function.
The functional decline of the heart in DCM is commonly associated with structural changes in the myocardium and is characterized by the accumulation of extracellular collagen and remodeling of the matrix. Excessive collagen formation and the progression of myocardial fibrosis contribute to increased myocardial stiffness and subsequently affect cardiac contractile function[22,23]. The serum levels of cardiac markers including CK, CK-MB, and cTnI are sensitive indicators of myocardial tissue damage. The elevated levels of CK, CK-MB, and cTnI in the body indicate myocardial tissue injury[24]. Pathological examination of cardiac tissue slices revealed varying degrees of deformation, myolysis, and disruptions in cardiac fibers in mice subjected to a prolonged high-fat diet[25]. A high-fat diet can induce a certain degree of myocardial fibrosis, and the degree of fibrosis increases in individuals with T2DM[26]. Erianin dose-dependently decreased the myocardial infarct area and the levels of serum indicators of myocardial damage (CK, CK-MB, and cTnI) in a rat is
Inflammation plays a crucial role in the progression of diabetes and the development of its complications. The high expression of inflammatory factors leads to the aggravation of the disease and the increase in the incidence of complications[28]. IL-1β is the main circulating form of IL-1 and, along with TNF-α, is a hallmark inflammatory factor associated with vascular inflammation. Individuals with diabetes have considerably higher expression levels of TNF-α, IL-1β, and IL-6 than their normal counterparts[29,30]. In diabetic animal models, alleviating inflammatory responses can lead to myocardial protection[31]. Erianin exhibits certain anti-inflammatory effects, namely, reducing NF-κB transcription activation[32] and increasing TNF-α expression[33]. The present study demonstrated that inflammatory factor levels were elevated in DCM mice, and erianin reduced the levels of IL-1β, IL-6, and TNF-α. The interplay between inflammation and oxidative stress is bidirectional, that is, inflammatory cytokines, such as TNF-α and IL-6, can exacerbate ROS production by impairing mitochondrial function[34], while oxidative stress amplifies NF-κB-driven inflammation[35]. The role of erianin in simultaneous suppression of both pathways likely disrupts this vicious cycle, as evidenced by reduced MDA levels (a lipid peroxidation marker) and restored SOD/CAT activity, which can mitigate myocardial cell apoptosis and fibrosis. Inflammation can damage endothelial cells, enhance autophagy, accelerate apoptosis, and promote fibrosis in myocardial cells. The long-term presence of chronic inflammation in the body stimulates metabolic disorders in cells, thereby exacerbating insulin resistance, forming a vicious cycle. Erianin possibly alleviates myocardial damage caused by inflammation by inhibiting autophagy, apoptosis, and metabolic disorders.
Oxidative stress is caused by the excessive production of ROS, and this phenomenon is one of the main mechanisms underlying the occurrence of DCM. Lipid peroxidation products and advanced glycation end-products accumulate as a result of imbalances in the metabolism of fat and glucose. This disturbance throws off the balance between the production and elimination of ROS, which then damages cardiac cells through oxidative stress[34]. In type 2 diabetic mice, the disruption of glucose metabolism leads to a large amount of ROS through processes, such as glucose auto-oxidation and metabolic stress. Moreover, the antioxidant enzyme activity in the body decreases, and the ability to clear free radicals significantly declines. These changes are evident in the markedly increased content of MDA and the significant decrease in the levels of SOD and CAT[35]. Chen et al[36] found that erianin may act as an antioxidant by inhibiting ROS/MAPK/NF-κB signaling, thereby reducing oxidative damage induced by high glucose in renal tubular epithelial cells. In the present experiment, the content of the lipid metabolism product MDA in the erianin group was significantly reduced. As such, erianin intervention helped improve pancreatic islet cell function and reduced oxidative stress in type 2 diabetic mice. The activity levels of SOD and CAT significantly increased, suggesting that erianin may exert its antioxidant stress effects by enhancing the activity levels of antioxidant enzymes. This antioxidant effect likely synergizes with anti-inflammatory actions because SOD and CAT not only neutralize ROS but also suppress NLRP3 inflammasome activation, thereby attenuating IL-1β and IL-18 production[35].
AMPK, a protein kinase activated by adenosine monophosphate, comprises a heterotrimeric complex composed of catalytic subunits α and β as well as regulatory subunit γ. The biological activity of AMPK is triggered when serine 72 on the α subunit is phosphorylated to form p-AMPKα, which regulates downstream signaling molecules. AMPK plays a key role in regulating glucose, lipid, and energy metabolism in the body. AMPK activation can suppress inflammatory responses and oxidative stress reactions[37]. Nrf2, a pivotal regulator of antioxidant factor transcription, plays a vital role in the Nrf2/HO-1 signaling pathway, which is essential for sustaining antioxidant response. Nrf2 can be activated by AMPK[38-40]. Previous studies indicated that erianin regulated Nrf2, NQO1, and HO-1 in human lung cancer A549 cells[41]. In the present experimental study, we observed that p-AMPKα, Nrf2, HO-1, and NQO1 were significantly reduced in the heart of mice in the model group. In T2DM mice, AMPK activation was inhibited, and the Nrf2/HO-1 signaling pathway was suppressed. After erianin intervention, the levels of p-AMPKα, Nrf2, HO-1, and NQO1 were significantly upregulated, which indicated that the AMPK/Nrf2/HO-1 pathway inhibited by T2DM was activated by erianin. This mechanistic interplay explains the observed improvements in metabolic parameters (e.g., HbA1c, lipid profiles) and echocardiographic indices (e.g., LVEF, LVIDd); AMPK activation enhances myocardial glucose uptake and mitochondrial biogenesis, while Nrf2/HO-1 signaling reduces oxidative injury[25,40].
Nrf2 is a crucial regulator of oxidative stress and a transcription factor that primarily exerts antioxidant and protective effects in various cells by transcriptionally regulating the expression of antioxidant stress proteins[42]. In the present experiment, Nrf2-inhibited mice treated with erianin showed no significant decrease in serum blood urea nitrogen and serum creatinine; HE staining revealed persistent swelling, congestion, and severe damage to kidney tissues. Hence, Nrf2 may be a crucial mechanism for erianin to exert its protective and therapeutic effects on myocardial injury. After Nrf2 inhibition in mice treated with erianin, the levels of p-AMPKα and AMPKα did not significantly increase, confirming the involvement of Nrf2 in the upregulation of p-AMPKα and AMPKα expression induced by erianin. Nevertheless, further comprehensive investigations are necessary to determine the precise mechanism by which Nrf2 controls p-AMPKα and AMPKα.
This study provides robust evidence for the cardioprotective effects of erianin via the AMPK/Nrf2/HO-1 pathway, but it is limited by its focus on a single signaling axis. Other pathways implicated in DCM, such as NF-κB, MAPK, and PI3K/Akt, were not explored, leaving potential cross-talk mechanisms unaddressed. Future studies should employ multi-omics approaches to map the broad regulatory snetwork of erianin. In clinical settings, the findings position erianin as a promising candidate for adjunctive therapy in diabetic patients with early-stage cardiac dysfunction. The dual anti-inflammatory and antioxidant properties of erianin as well as its metabolic benefits could complement existing glucose-lowering regimens. However, rigorous pharmacokinetic and safety studies in larger animal models (e.g., diabetic primates) and human trials are imperative to validate its translational potential.
Erianin has the potential to mitigate the effects of oxidative stress and inflammation on cardiac tissues, which might lead to a reduction in myocardial injury in mice with T2DM. The underlying mechanism could be connected to the activation of the AMPK/Nrf2/HO-1 signaling pathway. This work offers a novel approach to the therapeutic prevention and management of cardiac damage in T2DM.
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4389] [Article Influence: 627.0] [Reference Citation Analysis (0)] |
| 2. | Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 684] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 4. | Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17:585-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (35)] |
| 5. | Du F, Huang H, Cao Y, Ran Y, Wu Q, Chen B. Notoginsenoside R1 Protects Against High Glucose-Induced Cell Injury Through AMPK/Nrf2 and Downstream HO-1 Signaling. Front Cell Dev Biol. 2021;9:791643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Wu X, Zhang T, Lyu P, Chen M, Ni G, Cheng H, Xu G, Li X, Wang L, Shang H. Traditional Chinese Medication Qiliqiangxin Attenuates Diabetic Cardiomyopathy via Activating PPARγ. Front Cardiovasc Med. 2021;8:698056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Hayden MR, Tyagi SC. Is type 2 diabetes mellitus a vascular disease (atheroscleropathy) with hyperglycemia a late manifestation? The role of NOS, NO, and redox stress. Cardiovasc Diabetol. 2003;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J. 2014;38:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Wu X, Huang L, Liu J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review). Exp Ther Med. 2021;22:678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Luo J, Yan D, Li S, Liu S, Zeng F, Cheung CW, Liu H, Irwin MG, Huang H, Xia Z. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J Cell Mol Med. 2020;24:1760-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Sathibabu Uddandrao VV, Brahmanaidu P, Nivedha PR, Vadivukkarasi S, Saravanan G. Beneficial Role of Some Natural Products to Attenuate the Diabetic Cardiomyopathy Through Nrf2 Pathway in Cell Culture and Animal Models. Cardiovasc Toxicol. 2018;18:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 1268] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 13. | Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu WL, Shi JY, Jia Q, Li YM. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin. 2017;38:157-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | Wu HS, Xu JH, Chen LZ, Sun JJ. [Studies on anti-hyperglycemic effect and its mechanism of Dendrobium candidum]. Zhongguo Zhong Yao Za Zhi. 2004;29:160-163. [PubMed] |
| 15. | Mi WJ, Chen SH, Lv GY, Li Y, Zhang YL. [Study on the hypoglycemic effect of Dendrobium officinale root extract on type 2 diabetes model mice]. Zhongyao Yaoli Yu Linchuang. 2015;31:125-129. |
| 16. | Zhao M, Han J. Dendrobium Officinale Kimura et Migo Ameliorates Insulin Resistance in Rats with Diabetic Nephropathy. Med Sci Monit Basic Res. 2018;24:84-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Wu J, Zhuang HH, Mao ZT, Wu Jun, Li TM, Li W, Wu HQ. [Comparison of the clinical efficacy of Dendrobium officinale in the treatment of type 2 diabetes]. Jiangxi Zhongyiyao. 2017;48:45-47. |
| 18. | Zeng J, Li D, Li Z, Zhang J, Zhao X. Dendrobium officinale Attenuates Myocardial Fibrosis via Inhibiting EMT Signaling Pathway in HFD/STZ-Induced Diabetic Mice. Biol Pharm Bull. 2020;43:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos MPD, Kawashita NH, França SA. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad Bras Cienc. 2019;91:e20180314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8:e2100275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 21. | Andonova M, Dzhelebov P, Trifonova K, Yonkova P, Kostadinov N, Nancheva K, Ivanov V, Gospodinova K, Nizamov N, Tsachev I, Chernev C. Metabolic Markers Associated with Progression of Type 2 Diabetes Induced by High-Fat Diet and Single Low Dose Streptozotocin in Rats. Vet Sci. 2023;10:431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Zhao X, Liu S, Wang X, Chen Y, Pang P, Yang Q, Lin J, Deng S, Wu S, Fan G, Wang B. Diabetic cardiomyopathy: Clinical phenotype and practice. Front Endocrinol (Lausanne). 2022;13:1032268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 23. | Li YP, Ma YT. [Advances in research on therapeutic strategies for diabetic cardiomyopathy]. Xinxueguanbingxue Jinzhan. 2022;43:795-798. [DOI] [Full Text] |
| 24. | Fu F, Dong H, Song C. [Clinical significance of indexes of myocardial injury and inflammation in patients with acute myocardial infarction]. Zhonghua Linchuang Shiyanshiguanli Dianzi Zazhi. 2021;9:160-163. [DOI] [Full Text] |
| 25. | Hu B, Li DC. [Effects of high-fat diet on myocardial injury in diabetic rats]. Sichuan Yixue. 2013;34:1296-1298. |
| 26. | Zhang ZG. [Protective effect of sulforaphane on myocardial injury in type 2 diabetic mice]. PhD Thesis, Jilin University. 2016. Available from: https://kns.cnki.net/kcms2/article/abstract?v=sMQVub3UVPjqGsmWz5Vr6rBhqeJ6zyLZ7PPFsGeTuoCBjrSnU6eEpd66YFnvbz8UD8_MHvyFfF502e6HN-Mw6XSgjwRIgMEtcF65IVr7YLPQBGKXWkTVQ1uqQ8jYVhmMEOpgxWEiiKoeH5fK2dDx7bspJ2IrL_dMBEIL0RzVAJWkz54zRHSs53riexmxed5144JHqZPYNh318_gAUSJYsfE3Xb2g0mGveuqlyV1z7Q55FE6njL4Im_I3LeUGCcWAGPx7BI3khCDe1-0NW0qx4g==&uniplatform=NZKPT&language=CHS. |
| 27. | Yuan PL, Liu G, Ma LX, Ren F, Hu JK, Huang H, Guan J. [Maulanin attenuates myocardial ischemia-reperfusion injury by inhibiting NLRP3 inflammasome-mediated cell death]. Shanxi Yike Daxue Xuebao. 2023;54:1200-1207. [DOI] [Full Text] |
| 28. | Zheng ST, Fu QY, Yang SJ, Chen FL, Xia XL. [Study on the relationship between inflammatory and immunological indexes and renal pathological changes in diabetic patients]. Jianyan Yixue Yu Linchuang. 2018;15:44-49. [DOI] [Full Text] |
| 29. | Mostafavi E, Nargesi AA, Asbagh FA, Ghazizadeh Z, Heidari B, Mirmiranpoor H, Esteghamati A, Vigneron C, Nakhjavani M. Abdominal obesity and gestational diabetes: the interactive role of magnesium. Magnes Res. 2015;28:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Cucak H, Hansen G, Vrang N, Skarsfeldt T, Steiness E, Jelsing J. The IL-1β Receptor Antagonist SER140 Postpones the Onset of Diabetes in Female Nonobese Diabetic Mice. J Diabetes Res. 2016;2016:7484601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Elia E, Ministrini S, Carbone F, Montecucco F. Diabetic cardiomyopathy and inflammation: development of hostile microenvironment resulting in cardiac damage. Minerva Cardiol Angiol. 2022;70:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Yu Z, Zhang T, Gong C, Sheng Y, Lu B, Zhou L, Ji L, Wang Z. Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway. Sci Rep. 2016;6:34306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Zhang T, Ouyang H, Mei X, Lu B, Yu Z, Chen K, Wang Z, Ji L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. 2019;33:11776-11790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 34. | Feng W, Lei T, Wang Y, Feng R, Yuan J, Shen X, Wu Y, Gao J, Ding W, Lu Z. GCN2 deficiency ameliorates cardiac dysfunction in diabetic mice by reducing lipotoxicity and oxidative stress. Free Radic Biol Med. 2019;130:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Xie FZ, Shi DY, Xiao L, Liu JD, Liu SL. [Changes in glucose stress and antioxidant compensation in type 2 diabetes]. Fudan Xuebao. 2009;36:23-27. [DOI] [Full Text] |
| 36. | Chen MF, Liou SS, Kao ST, Liu IM. Erianin protects against high glucose-induced oxidative injury in renal tubular epithelial cells. Food Chem Toxicol. 2019;126:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Huang BP, Lin CH, Chen HM, Lin JT, Cheng YF, Kao SH. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF-κB signaling in murine macrophages. DNA Cell Biol. 2015;34:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Li X, Wu D, Tian Y. Fibroblast growth factor 19 protects the heart from oxidative stress-induced diabetic cardiomyopathy via activation of AMPK/Nrf2/HO-1 pathway. Biochem Biophys Res Commun. 2018;502:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Peng M, Qiang L, Xu Y, Li C, Li T, Wang J. Inhibition of JNK and activation of the AMPK-Nrf2 axis by corosolic acid suppress osteolysis and oxidative stress. Nitric Oxide. 2019;82:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Deng TX, Wang ML, Wen WJ, Yuan L. [Maolansu induces apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway]. Zhongguo Binglishengli Zazhi. 2019;35:1457-1462. [DOI] [Full Text] |
| 42. | Nazima B, Manoharan V, Miltonprabu S. Retraction: Grape seed proanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem Cell Biol. 2020;98:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |