Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.99576
Revised: February 17, 2025
Accepted: March 21, 2025
Published online: May 15, 2025
Processing time: 163 Days and 0.2 Hours
Managing refractory sudden sensorineural hearing loss (RSSHL) in patients with diabetes mellitus (DM) presents significant therapeutic challenges, highlighting the importance of identifying effective treatment strategies.
To analyze the therapeutic effectiveness of intratympanic injection plus retroauricular injection for RSSHL complicated with DM.
This study included 84 patients with RSSHL complicated with DM from April 2021 to April 2024, all receiving routine treatment. Participants were categorized into the control group (40 cases), receiving an intratympanic injection of methylprednisolone sodium succinate (MPSS), and the research group (44 cases), treated with retroauricular MPSS injection the next day in addition to the treatment administered in the control group. The efficacy, adverse reactions (tympanic membrane perforation, middle ear infections, burning sensation, vertigo, and tinnitus), blood glucose (BG) [fasting BG (FBG), 2-hour postprandial BG (2hPBG), and glycosylated hemoglobin (HbA1c)], hearing thresholds at different frequencies (250 Hz, 500 Hz, and 1000 Hz), serum biochemical indexes [interleukin (IL)-6, C-reactive protein (CRP), and procalcitonin (PCT)], and quality of life assessed by the short-form 36 item health survey (SF-36) were comparatively analyzed.
The research group demonstrated a markedly higher total effectiveness rate (81.82% vs 60.00%, P = 0.027) and a comparable incidence of total adverse reactions than the control group. Further, the research group exhibited notably reduced FBG, 2hPBG, HbA1c, IL-6, CRP, and PCT post-treatment (P < 0.01), which were lower compared with the pre-treatment levels and the control group (P < 0.05), as well as reduced hearing thresholds at different frequencies (250 Hz, 500 Hz, and 1000 Hz, P < 0.05). Furthermore, the post-treatment SF-36 scores of the research group in terms of energy, social functioning, role functioning, physical functioning, mental health, and overall health were all significantly improved than the pre-treatment levels and the control group (P < 0.05).
The above results indicate that intratympanic plus retroauricular injections of MPSS are effective in treating RSSHL complicated with DM without increasing the incidence of adverse reactions, which has a health promotion value.
Core Tip: Studies on the efficacy of combined intratympanic and retroauricular injection of methylprednisolone sodium succinate (MPSS) for refractory sudden sensorineural hearing loss (RSSHL) complicated with diabetes mellitus (DM) remains limited. Herein, we comprehensively assessed treatment outcomes, adverse effects, blood glucose (BG) levels, hearing thresholds across various frequencies, serum biochemical markers, and quality of life. The results indicate that intratympanic injection combined with retroauricular administration of MPSS significantly improves therapeutic efficacy in patients with RSSHL complicated with DM. This approach not only improves BG control and suppresses systemic inflammation but also enhances quality of life without increasing the overall risk of adverse effects. These results provide valuable information and a potential framework for optimizing treatment strategies in this patient population.
- Citation: Li D, Qiao F, Dai J, Xu M, Gong HY, Yang HM, Li JC, Huai D. Therapeutic effectiveness of intratympanic and retroauricular methylprednisolone sodium succinate for refractory sudden sensorineural hearing loss in diabetic patients. World J Diabetes 2025; 16(5): 99576
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/99576.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.99576
Sudden sensorineural hearing loss (SSHL) is a prevalent emergency in otology that typically persists beyond 72 hours and is characterized by a hearing decline of over 30 decibels in at least three consecutive frequencies[1]. Relevant statistics indicated that SSHL affects 5-30 out of every 100000 Americans annually, with approximately 4000 new cases emerging each year and an increasing risk on a global scale[2,3]. The risk of the disease increases as people age, and the etiological factors are intricate, with virus infections, microvascular damage, and immune-related reasons as widely known causes[4,5]. Systemic corticosteroid therapy, which effectively improves the hearing of nearly half of patients, is the standard therapeutic approach for SSHL. However, approximately 30%-50% of cases may demonstrate an insufficient response to this treatment modality[6]. Patients with diabetes mellitus (DM) are prone to inner ear microcirculation damage due to higher blood viscosity, thereby triggering SSHL[7]. Dealing with refractory SSHL (RSSHL) in presence of DM poses a challenge, and selecting the appropriate medication and administration method is crucial[8]. Hence, this study aims to conduct relevant analyses to provide more potent therapeutic alternatives for managing patients with RSSHL com
Intraauricular injections, also known as intratympanic injections, are a topical treatment scheme in which an injected corticosteroid penetrates the inner ear through the round window membrane and then distributes in the spiral ligament, basement membrane, cortical organs, and spiral ganglia[9,10]. This therapy exerts the same curative effect as systemic administration and contains a significantly higher steroid exolymph concentration than intravenous injection or oral administration while reducing the risk of side effects related to systemic absorption to some extent[11,12]. Retroauricular injections, as in intra-auricular injections, are salvage treatments. In this therapy corticosteroids are absorbed into cir
Considering the lack of research on the efficacy of intratympanic plus retroauricular injections in treating RSSHL complicated with DM, this study aimed to conduct relevant clinical analyses from multiple perspectives such as efficacy, adverse reactions, blood glucose (BG), hearing thresholds at different frequencies, serum biochemical indicators, and quality of life, to provide more useful evidence for the treatment of RSSHL complicated by DM.
The ethics committee of The Second People’s Hospital of Huai’an approved this retrospective study, and the participants signed written informed consent. This study included 84 patients with RSSHL complicated with DM admitted from April 2021 to April 2024. The control group (n = 40) received intratympanic MPSS injections, and the research group (n = 44) was treated with retroauricular MPSS injections the next day in addition to intratympanic MPSS. Clinical and laboratory comparability in baseline data was not significantly different between the two patient cohorts (P > 0.05). Refractory cases are those demonstrating a poor therapeutic response, characterized by either a lack of significant hearing improvement after 2 weeks of standardized treatment or frequent recurrences (i.e., repeated episodes within a short timeframe, such as 6 months, with inadequate treatment response after each episode). Based on hearing recovery criteria, treatment is considered ineffective if the average hearing threshold at 0.5, 1, 2, and 4 kHz improved by ≤ 15 dB or if the speech recognition rate increased by ≤ 15%. The specific design flowchart of the research is presented in Figure 1.
Inclusion criteria: Patients (age range: 18-80 years) meeting the diagnostic criteria for RSSHL[16] and DM[17]; all patients having first-onset sudden deafness within a course of < 2 weeks and no other treatment; patients who failed to respond to vasodilation, microcirculation improvement, inner ear nerve nutrition, inner ear hair cell repair, thrombolysis, hyperbaric oxygen, and hormone shock therapy for 2 weeks in the early stage, as well as those with sudden deafness with no obvious symptom improvements.
Exclusion criteria: Non-refractory sudden deafness; the presence of middle ear lesions, inner ear malformations, and retrocochlear space-occupying lesions during examinations; absolute contraindications to glucocorticoid use; hearing loss due to acoustic neuroma, secretory otitis media, Meniere’s disease, and other diseases; history of middle ear surgery; children or pregnant or lactating women; severe heart, lung, kidney, or endocrine system disorders; mental illness or epilepsy.
Both groups received routine treatment of alprostadil (Beijing BioLab Biotechnology Co., Ltd., BP1808-AYX) of 10 μg added with 0.9% sodium chloride (Wenzhou Kemiao Biotechnology Co., Ltd., KM11259869) injection of 100 mL for intravenous drip, once daily; oral betahistine mesylate (Wenzhou Kemiao Biotechnology Co., Ltd., KM11102340) 12 mg, three times daily; mecobalamin (Wenzhou Kemiao Biotechnology Co., Ltd., KM11126869) 500 μg, three times daily; a diabetic diet. The control group received an intratympanic MPSS (Wuhan AmyJet Scientific Inc., MDK-592988-1.0 g) injection of 40 mg injected into the tympanic cavity after tympanic membrane puncture, once every two days. The research group also received retroauricular MPSS injections of 40 mg, injected subcutaneously into the posterior ethmoid area of the affected ear the next day. A 10-day treatment was completed.
Therapeutic effectiveness: The therapeutic effectiveness of the two groups was compared 10 days after treatment. ‘Cured’ is when the hearing of the affected ear recovers to the premorbid level or that of the healthy ear. ‘Markedly effective’ is an improvement of > 30 dB in the hearing of the affected ear. ‘Considered effective’ is an improvement of 15-30 dB in the hearing of the affected ear. ‘Considered ineffective’ is an improvement of < 15 dB in the hearing of the affected ear. The total effectiveness rate was the percentage of the sum of the number of cured, markedly effective, and effective cases in the total number of cases.
Adverse reactions: The number of adverse events, such as tympanic membrane perforation (TMP), middle ear infections (MEI), burning sensation, vertigo, and tinnitus, that occurred during treatment was observed and recorded, and the corresponding percentages were calculated for assessment.
BG: The BG levels of the two groups before treatment and following 10 days of therapy were compared, including fasting BG (FBG), 2-hour postprandial BG (2hPBG), and glycosylated hemoglobin (HbA1c) levels.
Healing: Pure tone audiometry (PTA) was compared between the two groups at baseline and on day 10 of treatment. PTA was tested using an air conduction pure tone audiogram, covering three frequencies of 250 Hz, 500 Hz, and 1000 Hz, and the average level of the thresholds measured at these frequencies was obtained.
Serum biochemical indexes: Prior to treatment and following 10 days of therapy, 3 mL of fasting venous blood was collected in the early morning, and the serum was obtained after centrifugation to identify interleukin (IL)-6, C-reactive protein (CRP), and procalcitonin (PCT) levels by enzyme-linked immunosorbent assays (ELISAs). The experimental procedures were strictly conducted following the instructions provided with the respective human ELISA kits (Wuhan EnkiLife Sciences Co., Ltd., EH10020, EH10076; Wuhan Bioyears Biotechnology Co., Ltd., TD711203).
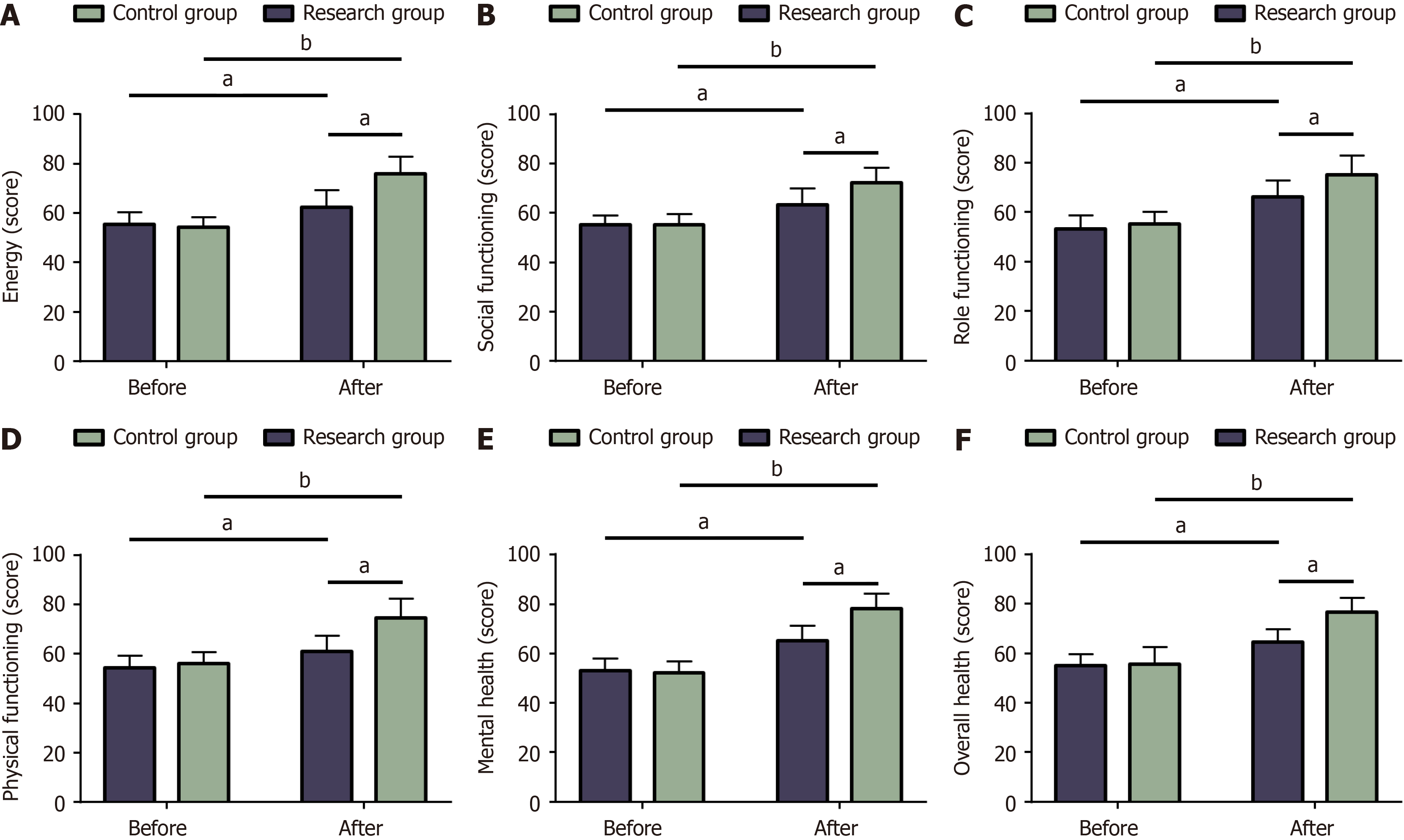
Quality of life: Quality of life was assessed prior to the treatment and half a year following the treatment. The short-form 36 item health survey (SF-36) was employed, assessing patients’ energy, social functioning, role functioning, physical functioning, mental health, and overall health. The score ranges from 0 to 100 points, with a higher score indicating a better quality of life.
The mean ± SE of the mean was used to express the measurement data, using independent sample t-test and paired t-test for inter-group and intra-group comparisons, respectively. Count data were expressed as a rate (percentage), and comparisons between the two groups of counting data were performed using the χ2 test. Statistical software Statistical Package for the Social Sciences version 22.0 was used for data analysis. A P value of < 0.05 indicated statistical sig
No statistical difference was determined in the inter-group comparison of baseline data such as age, sex, DM duration, mean arterial pressure, body mass index, and heart rate (P > 0.05) (Table 1).
| Data | Control group (n = 40) | Research group (n = 44) | χ2/t | P value |
| Age (years old) | 56.75 ± 7.28 | 55.75 ± 8.44 | 0.579 | 0.564 |
| Sex (male/female) | 24/16 | 23/21 | 0.508 | 0.476 |
| Diabetes duration (years) | 7.35 ± 2.13 | 6.68 ± 1.64 | 1.624 | 0.108 |
| Mean arterial pressure (mmHg) | 97.22 ± 11.40 | 99.84 ± 12.29 | 1.010 | 0.316 |
| Body mass index (kg/m2) | 22.32 ± 2.18 | 22.66 ± 3.58 | 0.519 | 0.605 |
| Heart rate (beats/min) | 78.45 ± 9.36 | 80.00 ± 7.33 | 0.849 | 0.398 |
The total effectiveness rates of the control and research groups were 60.00% and 81.82%, respectively. By comparison, the total effectiveness rate of treatment was statistically higher in the research group than in the control group (P < 0.05) (Table 2).
| Curative effect | Control group (n = 40) | Research group (n = 44) | χ2 | P value |
| Cured | 5 (12.50) | 9 (20.45) | ||
| Markedly effective | 12 (30.00) | 18 (40.91) | ||
| Effective | 7 (17.50) | 9 (20.45) | ||
| Ineffective | 16 (40.00) | 8 (18.18) | ||
| Total effectiveness | 24 (60.00) | 36 (81.82) | 4.887 | 0.027 |
The study indicated that TMP, MEI, burning sensation, vertigo, and tinnitus were observed in 0, 0, 1, 2, and 1 cases in the research group and 1, 1, 2, 1, and 1 cases in the control group, respectively. The research group demonstrated a comparable total incidence of adverse reactions with the control group (9.09% vs 15.00%, P < 0.05) (Table 3).
| Adverse reactions | Control group (n = 40) | Research group (n = 44) | χ2 | P value |
| Tympanic membrane perforation | 1 (2.50) | 0 (0.00) | ||
| Middle ear infections | 1 (2.50) | 0 (0.00) | ||
| Burning sensation | 2 (5.00) | 1 (2.27) | ||
| Vertigo | 1 (2.50) | 2 (4.55) | ||
| Tinnitus | 1 (2.50) | 1 (2.27) | ||
| Total | 6 (15.00) | 4 (9.09) | 0.698 | 0.404 |
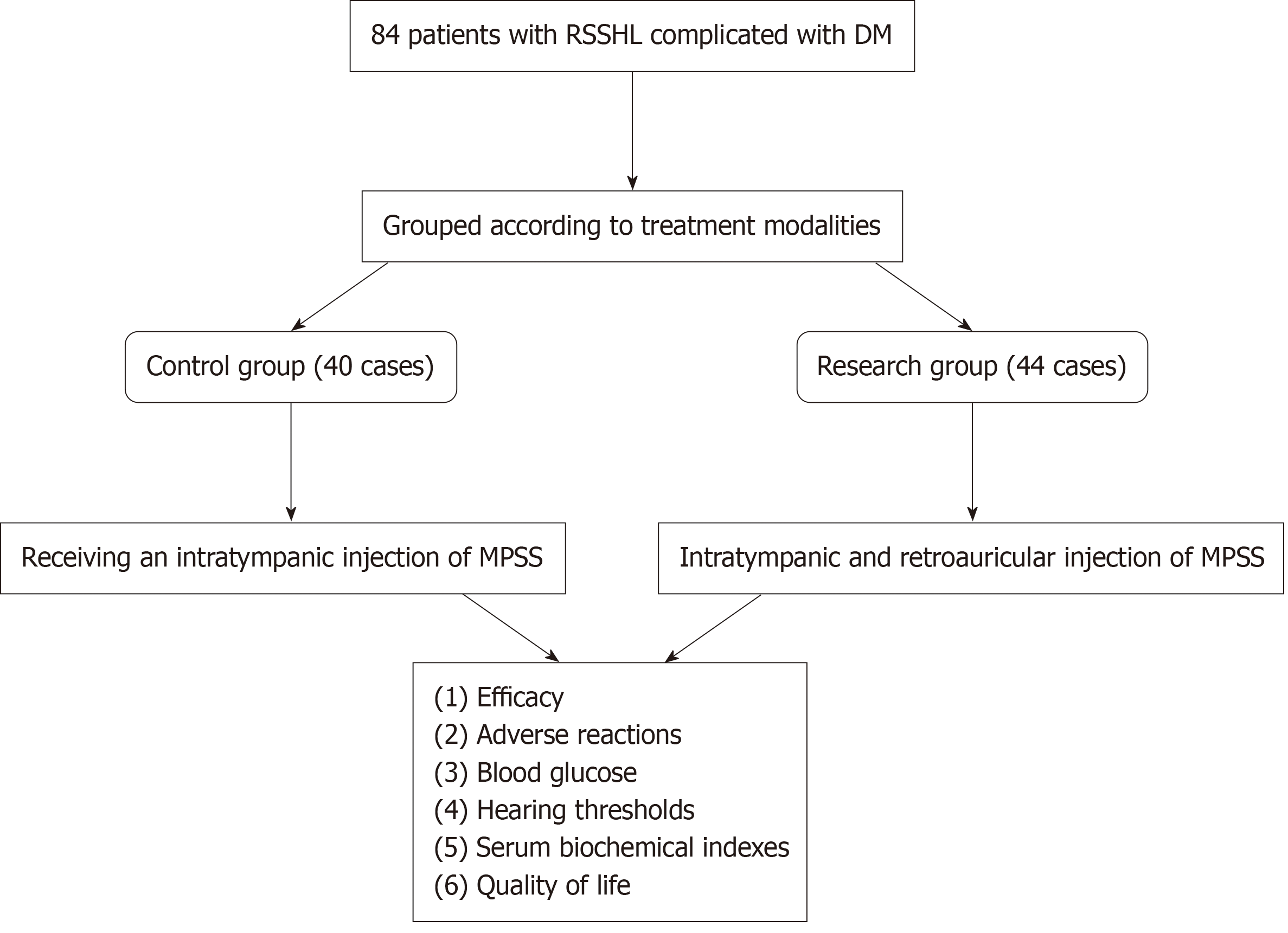
Pre-treatment, the FBG levels of the control group were 8.73 ± 1.50 mmol/L, whereas 8.79 ± 1.13 mmol/L in the research group. After the treatment regimen completion, the FBG levels in the control group decreased to 7.23 ± 0.95 mmol/L, whereas the research group demonstrated a more pronounced reduction, reaching 5.73 ± 0.99 mmol/L. The control and research groups presented 10.12 ± 1.63 mmol/L and 10.57 ± 1.24 mmol/L 2hPBG pre-treatment, respectively. The 2hPBG levels in the control and research groups were reduced to 9.15 ± 1.37 mmol/L and 7.86 ± 1.08 mmol/L post-treatment, respectively. The HbA1c levels pre-treatment in the control and research groups were 11.58% ± 2.15% and 12.00% ± 2.46%, respectively. Post-treatment, HbA1c levels were reduced to 9.90% ± 1.46% in the control group and 8.00% ± 1.49% in the research group. BG analysis revealed similar FBG, 2hPBG, and HbA1c levels in the two groups pre-treatment (P > 0.05). The above indicators in both groups were decreased post-treatment (P < 0.05), with more significant decreases in the research group (P < 0.05) (Figure 2).
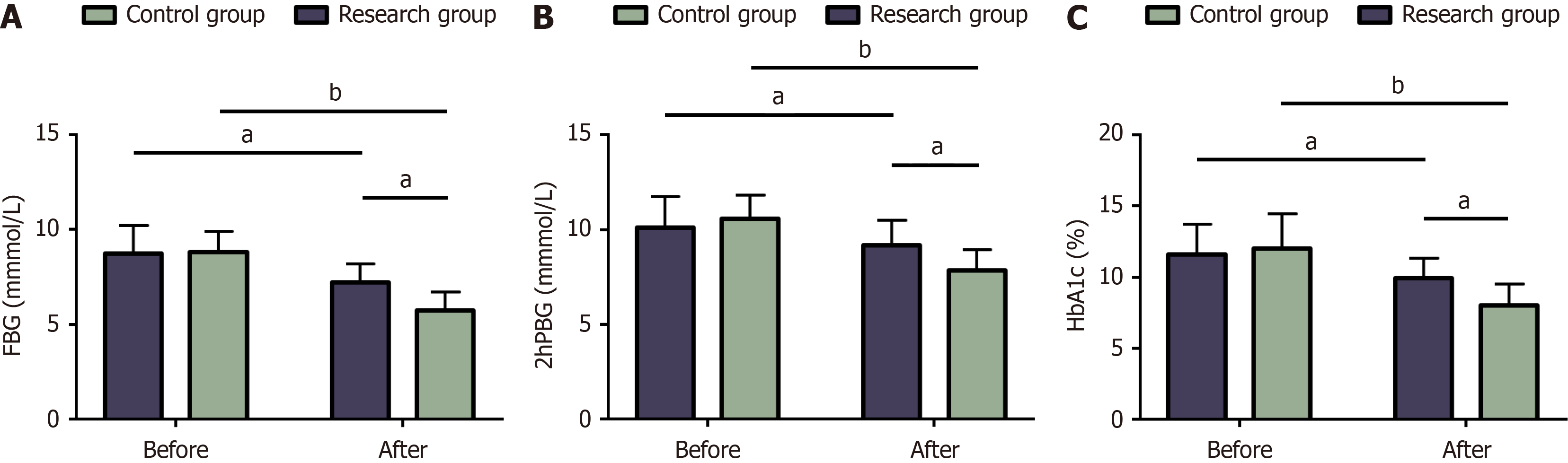
The hearing thresholds at 250 Hz in the control and research groups were 30.2 ± 7.75 dB and 22.82 ± 10.71 dB, respectively. The control and research groups demonstrated a hearing threshold of 34.95 ± 10.93 and 20.02 ± 9.54 in terms of 500 Hz frequency, respectively. The control and research groups registered a hearing threshold of 44.88 ± 14.34 and 32.82 ± 12.25 for the 1000-Hz frequency, respectively. The hearing thresholds of the two groups at different frequencies, such as 250 Hz, 500 Hz, and 1000 Hz, were statistically significant (P < 0.05), with markedly lower hearing thresholds in the research group compared to the control group (P < 0.05) (Figure 3).
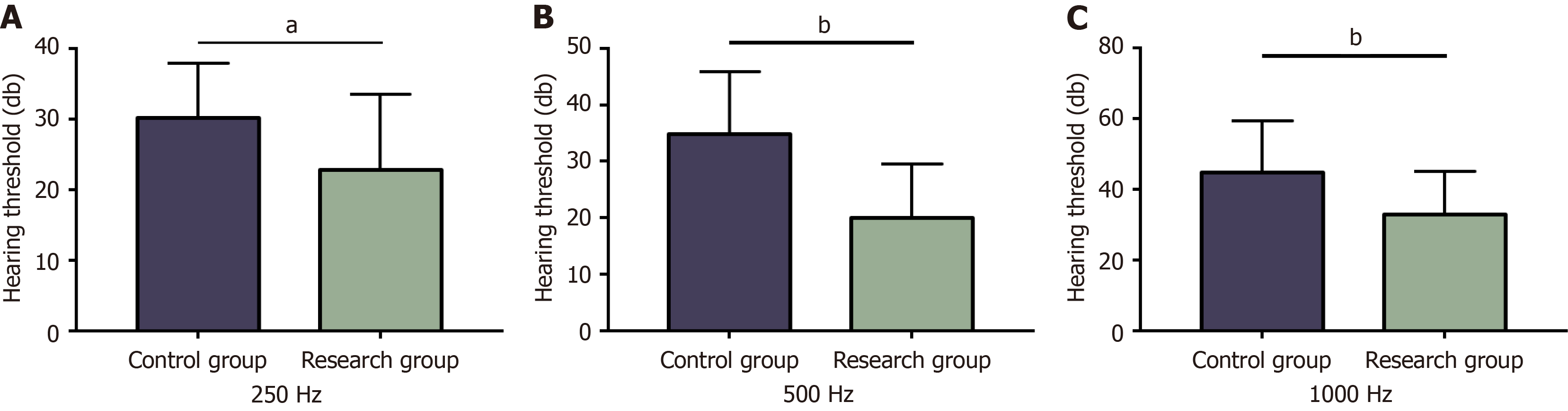
The IL-6 levels of the control and research groups were 67.1 ± 17.9 pg/mL and 65.86 ± 16.82 pg/mL pre-treatment, which decreased to 45.32 ± 12.06 pg/mL and 28.75 ± 8.38 pg/mL upon treatment completion, respectively. The control and research groups demonstrated pre-treatment CRP levels of 107.48 ± 9.58 mg/L and 104.95 ± 10.12 mg/L, which decreased post-treatment to 75.90 ± 6.69 mg/L and 47.23 ± 5.39 mg/L, respectively. Pre-treatment PCT levels in the control and research groups were 9.45 ± 6.22 ng/L and 10.80 ± 9.59 ng/L, which reduced post-treatment to 3.40 ± 1.18 ng/L and 1.84 ± 0.71 ng/L, respectively. Post-testing, we revealed no notable inter-group differences in pre-treatment IL-6, CRP, and PCT (P > 0.05). All these serum biochemical indexes were reduced statistically in both groups post-treatment (P < 0.05), with even lower IL-6, CRP, and PCT levels in the research group (P < 0.05) (Figure 4).
Pre-treatment energy scores of the control and research groups were 55.48 ± 5.01 points and 54.39 ± 4.02 points, which increased post-treatment to 62.45 ± 6.72 points and significantly increased to 75.75 ± 7.04 points, respectively. Pre-treatment social function scores of the control and research groups were 55.35 ± 3.71 and 55.11 ± 4.33 points, which increased upon treatment completion to 63.32 ± 6.61 points and 72.39 ± 5.86 points, respectively. Pre-treatment role function scores of the control and research groups were 53.4 ± 5.27 points and 55.39 ± 4.87 points, which increased post-treatment to 66.12 ± 6.76 points and 75.11 ± 7.96 points, respectively. Pre-treatment physical function scores of the control and research groups were 54.35 ± 4.91 and 56.07 ± 4.69 points, which increased post-treatment to 61.1 ± 6.17 points and 74.45 ± 8.02 points, respectively. Pre-treatment mental health scores of the control and research groups were 53.08 ± 4.97 points and 52.23 ± 4.76 points, which improved post-treatment to 65.18 ± 6.09 points and 78.18 ± 6.11 points, respectively. Finally, pre-treatment overall health scores of the control and research groups were 55.02 ± 4.72 points and 55.66 ± 6.86 points, which increased post-treatment to 64.62 ± 5.11 points and 76.75 ± 5.78 points, respectively. The two groups were not statistically different in SF-36 scores in terms of energy, social functioning, role functioning, physical functioning, mental health, and general health pre-treatment (P > 0.05). The SF-36 scores of the above aspects in both groups sig
Hypertension, stress, sleep disturbances, and DM are known to increase the risk of SSHL[18]. RSSHL complicated by DM may increase the incidence of clinical manifestations, such as tinnitus, vertigo, and vomiting in patients, which to some extent limit their normal life and add to treatment difficulty[19]. Delayed treatment or inappropriate approaches cause permanent hearing loss of the affected ear and even other sequelae[20]. This study primarily investigates the treatment optimization approaches for such patients, hoping to contribute to improving the therapeutic efficacy of patients with RSSHL complicated with DM.
The treatment modality of intratympanic plus retroauricular MPSS injections received by the research group boasted higher efficacy when compared to the sole intratympanic MPSS injection. Ren et al[21] revealed that retroauricular MPSS injection demonstrated a superior therapeutic outcome compared to not undergoing any steroid treatment, with the overall effective prognosis rate increasing from 10.00% to 48.48%, indicating the effectiveness of retroauricular MPSS injection as a salvage therapy for patients with RSSHL. Lv et al[22] emphasized that both intratympanic and retroauricular steroid injections exert considerable therapeutic efficiency for patients with RSSHL and significantly improve their hearing. Additionally, Gao et al[23] highlighted that the combination of local retroauricular injection and oral MPSS administration yielded better outcomes for patients with flat-type sudden hearing loss compared to oral MPSS administration alone. This indicates that the combined use of these two delivery methods may synergistically improve the
This study has several limitations that warrant further improvement. First, the study failed to comprehensively consider factors such as anti DM medications and comorbid conditions. Incorporating such relevant information would be conducive to circumventing the potential effect of these elements on the research outcomes. Second, no fundamental research has been conducted on the associated mechanisms, thereby hindering the in-depth exploration of the specific treatment mechanisms. Third, enlarging the sample size in multi-center prospective trials would be instrumental in further improving the accuracy of the research results. In the future, improvements will be actively pursued from the aforementioned perspectives to enhance the research’s robustness and validity.
Altogether, intratympanic plus retroauricular MPSS injections are effective in improving therapeutic effectiveness in patients with RSSHL complicated with DM, controlling BG levels, suppressing serum inflammatory reactions, and promoting the quality of life without increasing the risk of total adverse reactions.
| 1. | Tripathi P, Deshmukh P. Sudden Sensorineural Hearing Loss: A Review. Cureus. 2022;14:e29458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Schreiber BE, Agrup C, Haskard DO, Luxon LM. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Michel O; Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. [The revised version of the german guidelines "sudden idiopathic sensorineural hearing loss"]. Laryngorhinootologie. 2011;90:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Xiong W, Dai Q, Wang Y, Hou Z, Lu K, Sun X, Duan F, Wang H, Zhang D, Wang M. Idiopathic Sudden Sensorineural Hearing Loss in Different Ages: Prognosis of Patients With Initial Total Hearing Loss. Front Psychol. 2022;13:818967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Yamada S, Kita J, Shinmura D, Nakamura Y, Sahara S, Misawa K, Nakanishi H. Update on Findings about Sudden Sensorineural Hearing Loss and Insight into Its Pathogenesis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Lin CY, Chang CH, Chang CJ, Ko JY, Wu SY, Kuo PH. Salvage therapy for refractory sudden sensorineural hearing loss (RSSNHL): a systematic review and network meta-analysis. Int J Audiol. 2025;64:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Cavallaro G, Pantaleo A, Pontillo V, Barbara F, Murri A, Quaranta N. Endothelial Dysfunction and Metabolic Disorders in Patients with Sudden Sensorineural Hearing Loss. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Tajdini A, Karimi Yazdi A, Ravand H, Sahebi L. The Use of Herbal Medicine in Sudden Sensorineural Hearing Loss in Diabetic Patients. Iran J Otorhinolaryngol. 2023;35:207-215. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Miwa T, Kanai R, Kanemaru SI. Long-term exposure to high-concentration dexamethasone in the inner ear via intratympanic administration. Steroids. 2023;189:109152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Xie W, Karpeta N, Liu J, Peng H, Li C, Zhang Z, Liu Y, Duan M. Efficacy of intratympanic or postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid in the treatment of sudden sensorineural hearing loss: A prospective randomized study. Front Neurol. 2023;14:1138354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 11. | Choi JW, Lee CK, Kim SB, Lee DY, Ko SC, Park KH, Choi SJ. Potential benefits of salvage intratympanic dexamethasone injection in profound idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2020;277:2219-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, Goebel JA, Hammerschlag PE, Harris JP, Isaacson B, Lee D, Linstrom CJ, Parnes LS, Shi H, Slattery WH, Telian SA, Vrabec JT, Reda DJ. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Han JS, Kim YL, Yu HJ, Park JM, Kim Y, Park SY, Park SN. Safety and Efficacy of Intratympanic Alpha-Lipoic Acid Injection in a Mouse Model of Noise-Induced Hearing Loss. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Mühlmeier G, Tisch M. [Tolerance and acceptance of intratympanic injections]. HNO. 2022;70:685-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Deng HS, Hou YW, Zhang JN, Yang T. Postauricular versus systemic use of steroids for sudden hearing loss: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2023;102:e34494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Chen N, Karpeta N, Ma X, Ning X, Liu X, Song J, Jiang Z, Ma X, Liu X, Zhong S, Sun Q, Liu J, Chen G, Duan M, Yu L. Diagnosis, differential diagnosis, and treatment for sudden sensorineural hearing loss: Current otolaryngology practices in China. Front Neurol. 2023;14:1121324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 17. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1956] [Article Influence: 326.0] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Chen Q, Xu Y. Research progress in refractory sudden hearing loss: steroid therapy. J Int Med Res. 2020;48:300060519889426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Nakagawa T, Kumakawa K, Usami S, Hato N, Tabuchi K, Takahashi M, Fujiwara K, Sasaki A, Komune S, Sakamoto T, Hiraumi H, Yamamoto N, Tanaka S, Tada H, Yamamoto M, Yonezawa A, Ito-Ihara T, Ikeda T, Shimizu A, Tabata Y, Ito J. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014;12:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Wang H, Zhao Z, Chen S. Local vs Systemic Use of Steroids for Sudden Deafness with Diabetes: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2023;1455613231170090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Ren G, Xu J, Lan L, Ma B, Zhang Q. Postauricular injection of methylprednisolone sodium succinate as a salvage treatment for refractory sudden sensorineural hearing loss. Ir J Med Sci. 2021;190:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lv L, Gao Z, Liu J, Zhuang Y, Hou J, Zhu W, Liu Z, Bai Z, She W. Comparison between postauricular steroid injection and intratympanic steroid perfusion for refractory severe and profound sudden sensorineural hearing loss. Am J Otolaryngol. 2022;43:103189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Gao Y, Wang CL, Zheng Y. [Clinical curative effect analysis of postauricular topical injection combining with oral hormone in the treatment of the flat type of sudden hearing loss]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31:1639-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Kim YH, Lee DY, Lee DH, Oh S. Tympanic Membrane Perforation After Intratympanic Steroid Injection: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2022;166:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Qiu K, Mao M, Deng D, Jiang C, Li L, Zheng Y, Ren J, Zhao Y. Is postauricular injection a systemic or a topical route for inner ear drug delivery? Hear Res. 2022;422:108570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Wang X, Liu XD, Zhao B, Yang SY, Cao YR, Zhang W, Liu H. [Treatment of sudden deafness with type 2 diabetesmellitus by post-auricular subperiosteal injection of methylprednisolone]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32:1719-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Meng XD, Li TT, Deng LM. Therapeutic efficacy of methylprednisolone sodium succinate via diverse administration routes for mid- to high-frequency sudden sensorineural hearing loss. World J Clin Cases. 2024;12:3321-3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Yao Y, Li L. Analysis of Therapeutic Options for Noise-Induced Hearing Loss: Retroauricular Injection of Methylprednisolone Sodium Succinate Combined with Hyperbaric Oxygenation. Noise Health. 2024;26:370-375. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Qin Y, Zhao W, Jia Z, Bauman WA, Peng Y, Guo XE, Chen Z, He Z, Cardozo CP, Wang D, Qin W. Neuroprotective macromolecular methylprednisolone prodrug nanomedicine prevents glucocorticoid-induced muscle atrophy and osteoporosis in a rat model of spinal cord injury. Nanomedicine. 2024;62:102773. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Gundogan O, Pinar E, Imre A, Ozturkcan S, Cokmez O, Yigiter AC. Therapeutic efficacy of the combination of intratympanic methylprednisolone and oral steroid for idiopathic sudden deafness. Otolaryngol Head Neck Surg. 2013;149:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Li X, Zhang XY, Wang QJ, Wang DY. Efficacy of methylprednisolone sodium succinate for injection (postotic injection) on the auditory threshold and speech recognition rate of sudden deafness patients. Int J Clin Exp Med. 2015;8:14110-14114. [PubMed] |
| 32. | Bashir H, Ahmad Bhat S, Majid S, Hamid R, Koul RK, Rehman MU, Din I, Ahmad Bhat J, Qadir J, Masood A. Role of inflammatory mediators (TNF-α, IL-6, CRP), biochemical and hematological parameters in type 2 diabetes mellitus patients of Kashmir, India. Med J Islam Repub Iran. 2020;34:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Cheng S, Gao W, Xu X, Fan H, Wu Y, Li F, Zhang J, Zhu X, Zhang Y. Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res Bull. 2016;127:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |