Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.91200
Revised: December 18, 2024
Accepted: February 20, 2025
Published online: May 15, 2025
Processing time: 478 Days and 4.8 Hours
Diabetic encephalopathy (DE) is a common and serious complication of diabetes that can cause death in many patients and significantly affects the lives of in
To analyze literature on DE using scientometrics to provide a comprehensive pi
We reviewed studies on DE or cognitive impairment published between 2004 and 2023. The latter were used to identify the most frequent keywords in the keyword analysis and explore the hotspots and trends of DE.
Scientometric analysis revealed 1308 research papers on DE, a number that in
We identified the main inducing factors and comorbidities of DE, though other complex factors undoubtedly increase social and economic burdens. These findings provide vital references for future studies.
Core Tip: Here, we try to put forward the scientometric analysis method of diabetic encephalopathy (DE) and suggest a future research direction. The statistical results of keyword analysis were manually classified, including inducing factors, complications, pathogenesis, treatment, and animal model of DE. We combine scientometric analysis with historical review to find key evidence and highlight emerging DE trends. Exploring the solution to DE is an urgent problem to be solved. Therefore, there is more and more research in the field of DE. Because there is no research literature in this area, it is of great guiding significance to further explore DE’s prevention and control measures.
- Citation: Ye XW, Zhang HX, Li Q, Li CS, Zhao CJ, Xia LJ, Ren HM, Wang XX, Yang C, Wang YJ, Jiang SL, Xu XF, Li XR. Scientometric analysis and historical review of diabetic encephalopathy research: Trends and hotspots (2004-2023). World J Diabetes 2025; 16(5): 91200
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/91200.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.91200
Type 2 diabetes mellitus (T2DM) is a common chronic disease that threatens human health and labor. There were 463 million patients with T2DM worldwide in 2019, and this number is expected to reach 700 million by 2045, an increase of approximately 51%[1]. T2DM is characterized mainly by elevated blood glucose levels caused by insulin deficiency and poor cellular response to insulin. Evidence has shown that hyperglycemia can lead to circulatory, kidney, liver, peripheral nervous, and immune system disorders[2]. However, little attention has been paid to the impact of diabetes on cognitive function. In the 1950s, Reske-Nielsen and Lundbaek[3] proposed the concept of “diabetic encephalopathy” (DE), which is characterized by acquired cognitive impairment[4]. The main manifestations in this type of patient are impaired learning and memory, delayed executive function, slowed information processing speed, and neuropathology, which mainly manifest as neurodegeneration and the corresponding neurochemical and neurostructural abnormalities[5,6]. The degree of damage can be divided into three stages from mild to severe: Diabetes-related cognitive decline, mild cognitive impairment, and dementia[7]. Epidemiological evidence shows that patients with T2DM have a 2.8-fold greater risk of developing dementia than those without T2DM[8], and up to 20% of T2DM patients aged over 60 years may develop dementia[9]. Previous cross-sectional studies have shown that the prevalence of mild cognitive impairment in patients with T2DM is approximately 20%-30%, the incidence of dementia is approximately 17.3%[10], and the risk of developing Alzheimer’s disease (AD) in patients with diabetes is approximately 1.5 to 2.5 times greater than that in patients of the same age and sex without diabetes[11]. DE has been found to significantly reduces quality of life and increases mortality rates. Therefore, DE has received widespread attention in the medical community. Given that the etiology of DE is complex and its underlying mechanism is unclear, studying the development of DE will help prevent and reduce the loss of disease caused by DE[12,13].
Although extensive research has been conducted in the field of DE in recent decades, quantitative scientometric analyses for assessing the specific progress made in this field are lacking[14]. Scientometrics is a mathematical and statistical method for reviewing and calculating data correlations. This method can be used to evaluate specific research topics, predict emerging patterns, and identify research frontiers in the scientific field. Compared with traditional and systematic literature reviews, it can intuitively provide more information and views[15]. Scientometric analysis plays an essential role in research on schizophrenia[16], hyperactivity disorders[17], and esketamine[18]. These scientometric analysis studies summarize current research hotspots and provide future research directions in specific fields. Here, we propose a scientometric analysis method for DE and suggest future research directions (Figure 1). Statistical results including inducing factors, complications, pathogenesis, treatment, and animal models were manually classified. We combined scientometric analysis with a historical review to identify key evidence and highlight emerging DE trends.
Using the SCIE database as a data source ensured the quality of the literature data and improved the quality of this study. The terms “diabetic encephalopathy” were used as the topic, or the query “Query #1= ((TI = (diabetes mellitus)) OR TI = (hyperglycemia)) OR TI = (glucose intolerance); Query #2= ((((TS = (cognitive dysfunction)) OR TS = (cognitive impairment)) OR TS = (cognitive disorder)) OR TS = (cognitive decline)) OR TS = (cognitive); Query 3 = #2 AND #1 (TS = Topic, TI = Title)”. We searched the Web of Science Core Collection database for articles published through May 22, 2023. Articles and reviews were refined and used for scientometric analysis.
Raw data were obtained from Web of Science. The data were recorded in complete records and cited references. The file was in plain text format.
Raw data were analyzed using COOC (version 13.3) as previously described. First, we performed data cleaning and weight removal, merged synonyms, deleted meaningless words, transformed them into an appropriate text format, and then conducted a scientometric analysis. Primary information, including the publication number per year, country or region, journal, keyword, and literature title, was extracted using COOC. Data were visualized using CiteSpace (version 6.1. R6), VOSviewer (version 1.6.19), and OriginPro 2021.
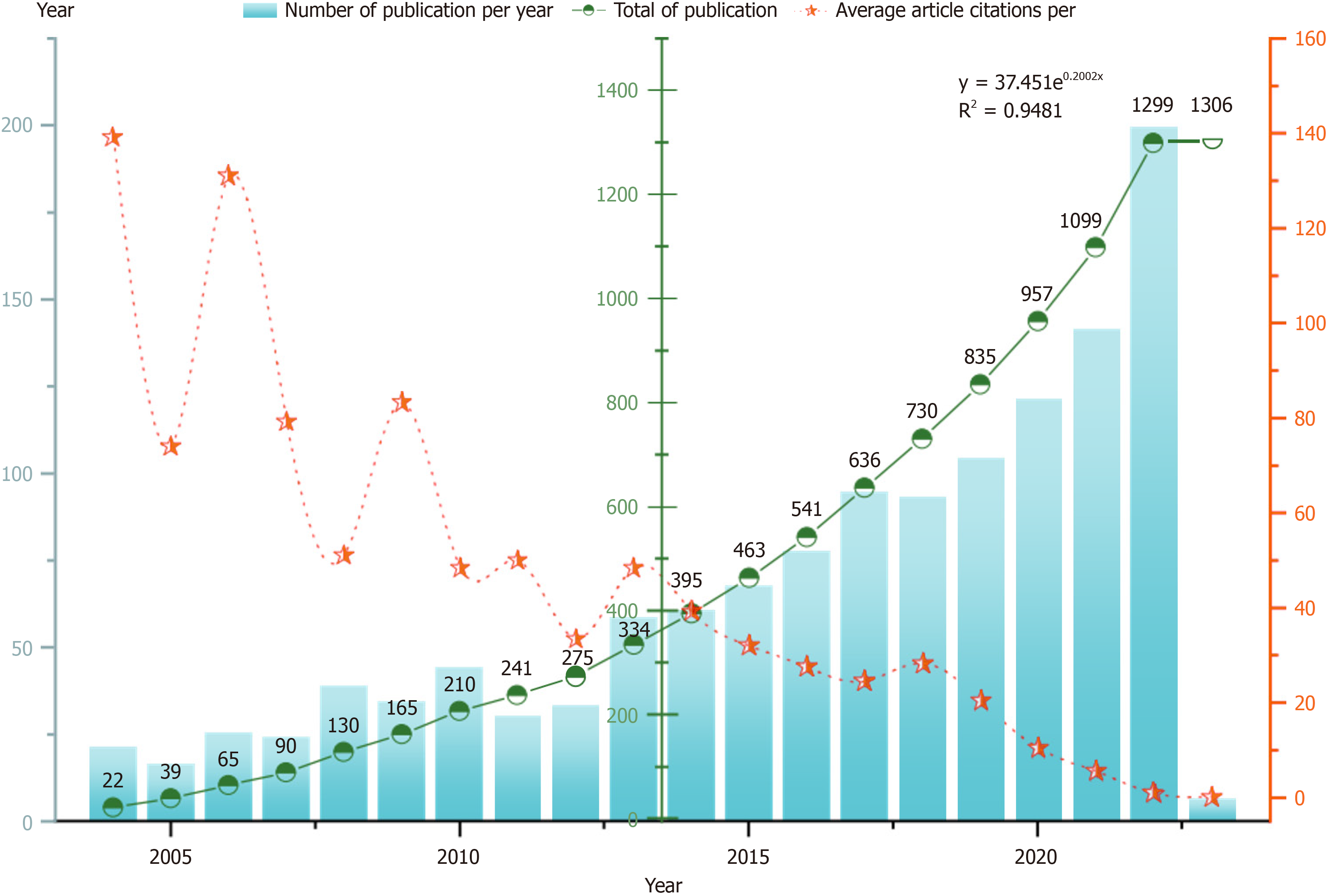
Before May 22, 2023, we downloaded 1306 publications about DE from the Web of Science Core Collection database. The average number of citations per document was 29.47. The term “diabetic encephalopathy” was first suggested by Danish scholar Reske-Nielsen and Lundbaek[3] in 1965. In 2006, Dutch scholar Mijnhout et al[19] reported that the concept of DE does not highlight changes in cognitive function in patients with diabetes. A new term, diabetes-associated cognitive decline, has been proposed for DE. The cumulative number of documents issued increased exponentially. The formula was y = 37.451e0.2002x, R2 = 0.9481, suggesting that the trend line adequately fits the annual growth trend in publication volume. Most articles were published in 2022, and the most cited year was 2004 (Figure 2). Research in the field of DE is ongoing.
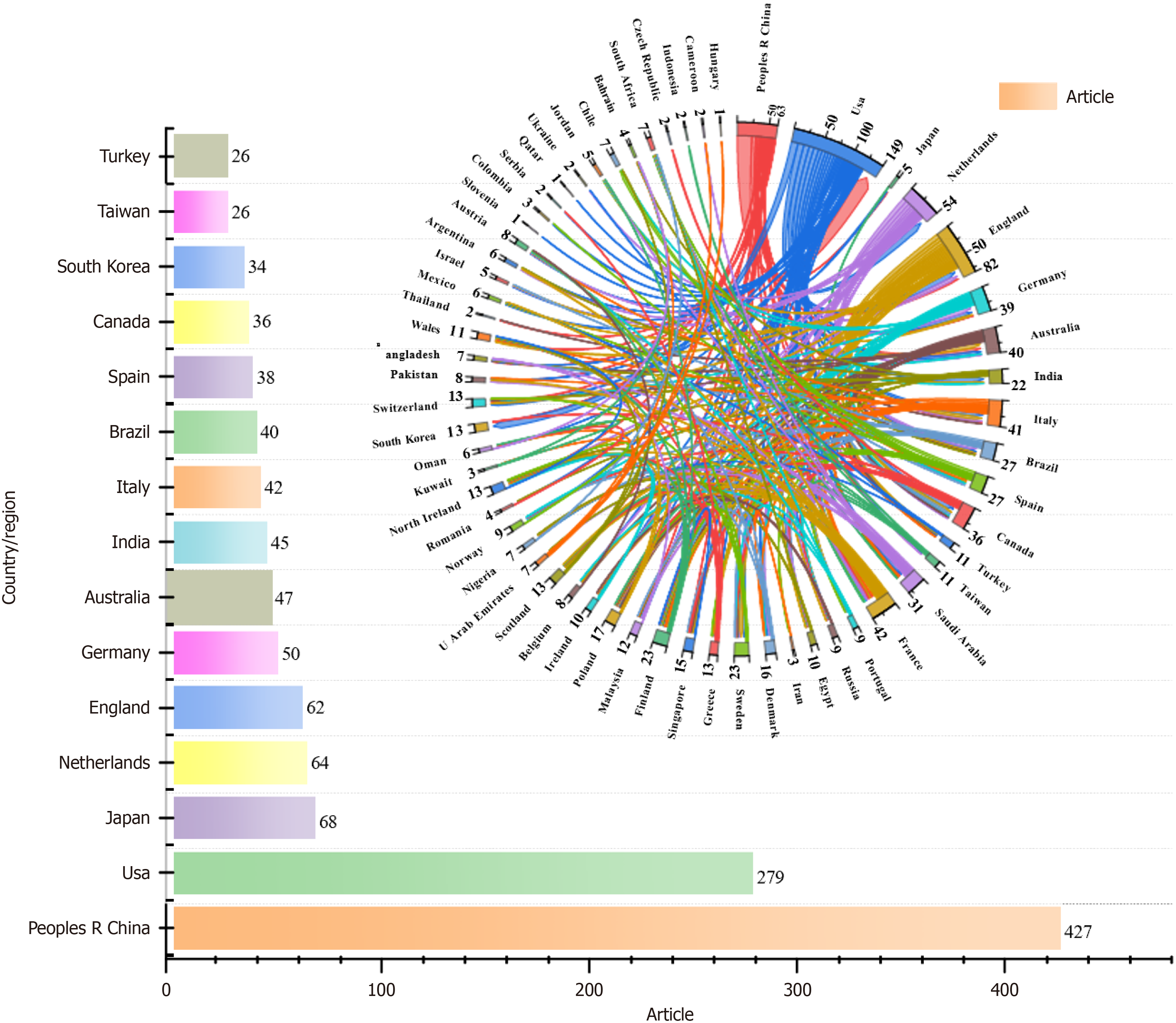
Seventy-five countries/regions contributed publications included in this study (Figure 3). China published the most articles, followed by the United States, Japan, the Netherlands, England, Germany, Australia, India, and Italy. Currently, China has the highest number of patients with diabetes in the world, accounting for more than a quarter of the total number worldwide, and approximately 140 million people aged 20-79 years are affected by the disease. These findings suggest that the degree of cognitive dysfunction caused by diabetes is also gradually increasing, and to solve this problem, relevant research investment is needed. Partnerships among countries demonstrated close cooperation. The United States, Netherlands, England, and China had the most research cooperation.
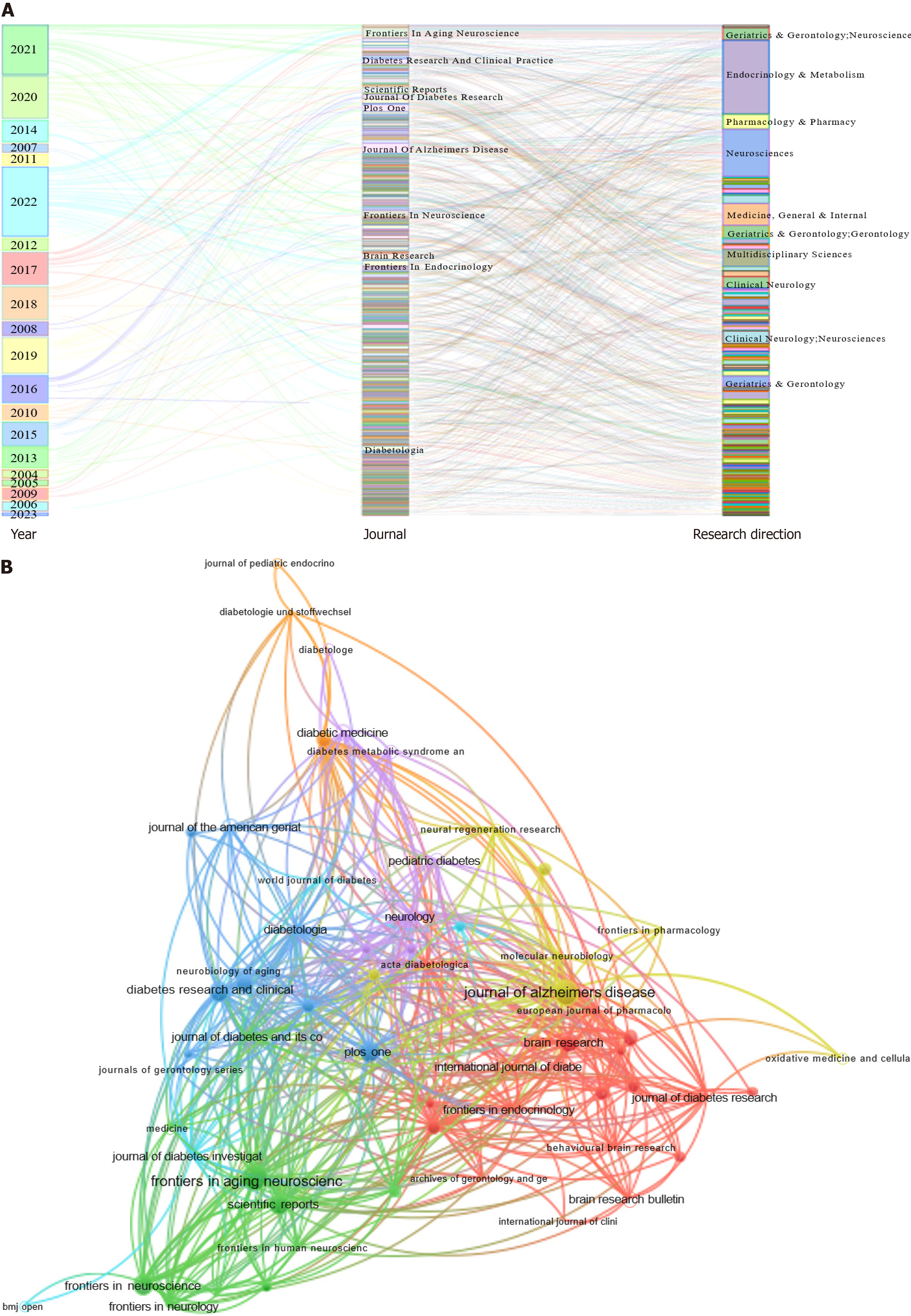
All publications were published in 533 journals (Figure 4). The citation counts of these journals indicated similar publication topics (Figure 4B). The top ten journals are shown in Figure 4A. The most relevant journal was Frontiers in Aging Neuroscience. Frontiers in Aging Neuroscience and the Journal of Alzheimer’s Disease had the highest number of publications, followed by PLoS One, Frontiers in Neuroscience, Diabetes Research and Clinical Practice, Scientific Reports, Brain Research, Journal of Diabetes Research, Frontiers in Endocrinology, and Diabetologia. The top ten research directions are shown in Figure 4A. The most relevant directions were endocrinology and metabolism, followed by neurosciences, medicine, general and internal, multidisciplinary sciences, pharmacology and pharmacy, geriatrics and gerontology, gerontology, geriatrics and gerontology, neurosciences, clinical neurology, neurosciences, clinical neurology, and geriatrics.
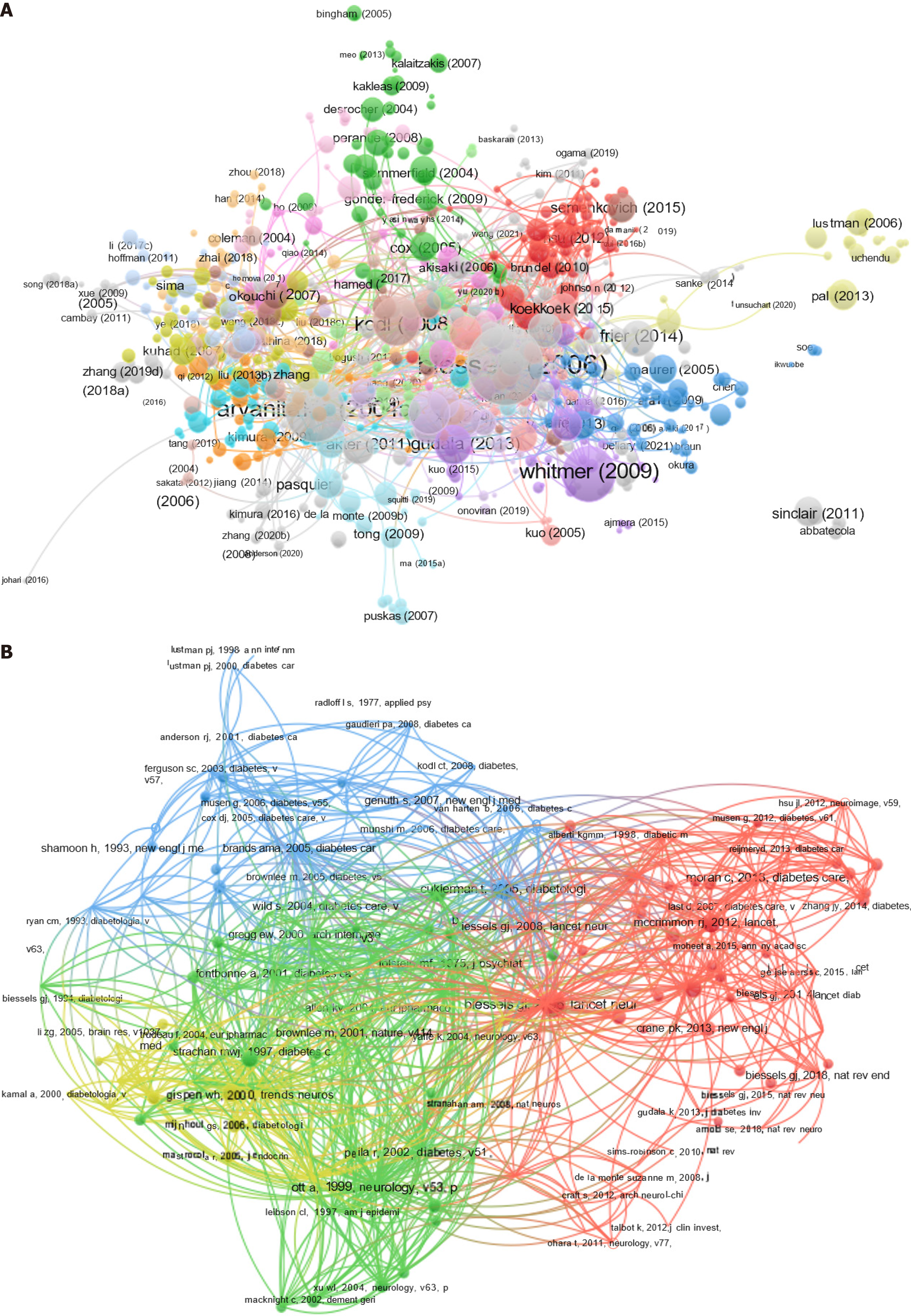
The top ten most-cited articles on DE are listed in Table 1. The themes of these articles were closely related (Figure 5A). The most-cited article reported that the incidence of dementia was greater in individuals with diabetes than in those without diabetes in seven of ten studies[20]. The second most-cited article, a longitudinal cohort study, showed that DM may be associated with an increased risk of developing AD and may differentially affect cognitive systems[21]. The third most cited article revealed that among older patients with T2DM, a history of severe hypoglycemic episodes was associated with a greater risk of dementia[22]. Details of the most frequently cited documents are presented in Table 2. The references were divided into four modules (Figure 5B). Arvanitakis[21], Ott[23], Biessels[20], and Cukierman[24] reported a clinical cohort study and confirmed that DM may be associated with an increased risk of developing dementia or cognitive decline, suggesting that diabetes may have contributed to the clinical syndrome in a substantial proportion of all patients with diabetes. However, some unrelated research references may have been included during the analyses. Nevertheless, they provided a direction for the study of DE (Figure 5).
| Year | Title | Country/region | Cited |
| 2004 | Diabetes mellitus and risk of alzheimer disease and decline in cognitive function | United States | 943 |
| 2004 | Diabetes mellitus and risk of dementia in the kungsholmen project - a 6-year follow-up study | Sweden | 336 |
| 2006 | Risk of dementia in diabetes mellitus: A Systematic review | England; Netherlands | 1491 |
| 2006 | Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit | United States | 261 |
| 2008 | Cognitive dysfunction and diabetes mellitus | United States | 582 |
| 2009 | Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus | United States | 723 |
| 2011 | Cognitive function, dementia and type 2 diabetes mellitus in the elderly | England; Scotland | 305 |
| 2013 | Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies | England; India | 351 |
| 2014 | Hypoglycaemia in diabetes mellitus: Epidemiology and clinical implications | Scotland | 298 |
| 2018 | Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications | Netherlands; United States | 431 |
| Cluster | Link | strength | Citations | Year | Author | Journal | DOI |
| Red | 161 | 1156 | 137 | 2006 | Biessels GJ | Lancet Neurol | 10.1016/S1474-4422(05)70284-2 |
| Blue | 156 | 746 | 87 | 2005 | Cukierman T | Diabetologia | 10.1007/s00125-005-0023-4 |
| Green | 154 | 1198 | 124 | 1999 | Ott A | Neurology | 10.1212/WNL.53.9.1937 |
| Yellow | 145 | 721 | 82 | 2004 | Arvanitakis Z | Arch Neurol-Chicago | 10.1001/archneur.61.5.661 |
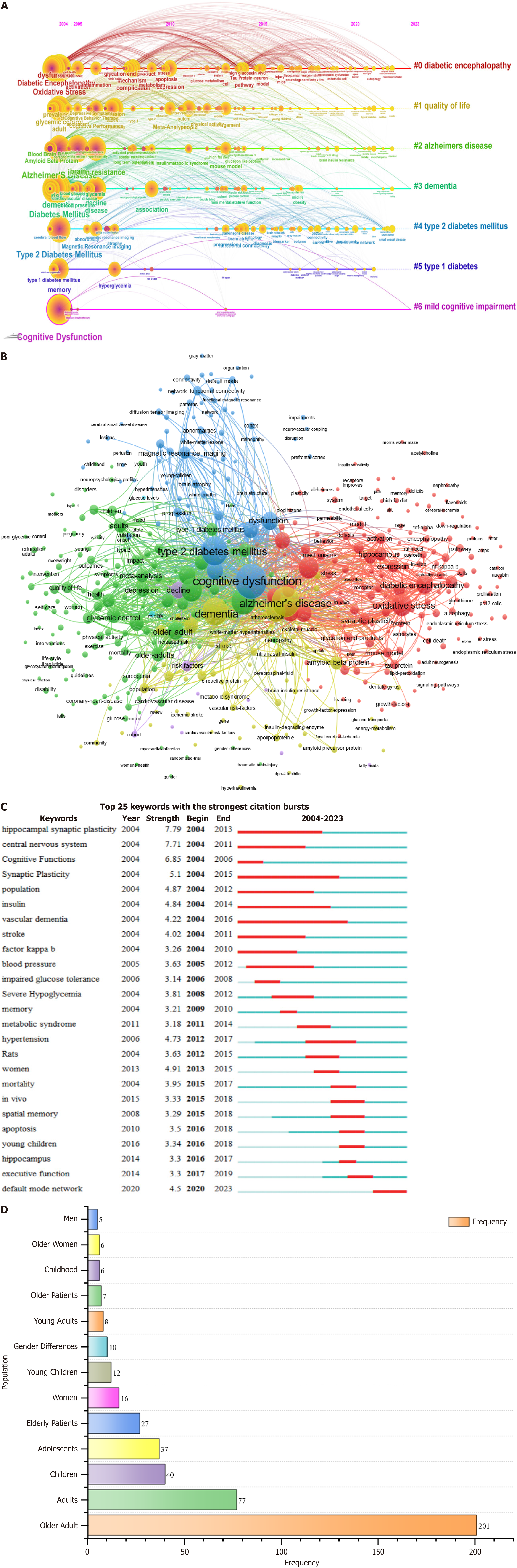
Scientometric theory holds that keywords reflect research trends and hotspots in a research field[14,25]. This review presents keyword visualization as a timeline and a co-occurrence network (Figure 6). The timeline graph shows the relationships between the clusters and the historical spans of the keywords in the clusters. Nodes of the same group were arranged on the same horizontal line in chronological order. The time axis was located above the view and was distributed from left to right. As shown in Figure 6A, the frequency of the keywords increased annually, and “Diabetic Encephalopathy” was the most relevant topic, followed by “Quality of Life”, “Alzheimer’s Disease”, “Dementia”, “Type 2 Diabetes Mellitus”, “Type 1 Diabetes Mellitus”, and “Mild Cognitive Impairment”. These keywords occupy a vital position in the field, indicating that these clustering tags are key research content and form a stable direction. For the co-occurrence network, keywords were divided into various clusters represented by different colors based on correlation (Figure 6B). Remarkably, without considering “cognitive function”, “Alzheimer’s Disease” was a cross-word in these clusters, and the keyword “Risk Factors” suggested that DM may be associated with AD. There are more studies on cognitive function in individuals with type 2 diabetes than in those with type 1 diabetes, focusing on oxidative stress, insulin resistance, and age.
Keywords with citation bursts indicate that they have more citations in a given period; this metric can test whether a field of research is hot over a given period and highlights emerging topics[15]. Through the use of keywords such as “hippocampal synaptic plasticity”, “central nervous system”, “cognitive functions”, and “synaptic plasticity”, it was inferred that clinical research or animal experiments, particularly hippocampal studies in the human/rat brain, such as studies of amyloid beta protein, magnetic resonance imaging, blood-brain barrier (BBB), and brain atrophy, were popularized during the first ten years. Keywords such as stroke and vascular dementia indicated that diabetes is a metabolic disease associated with multiple diseases (Figure 6C). In addition, we used the keyword “population”, so we conducted a study on the distribution of patients and found that diabetes occurred in all age groups (Figure 6D). People with diabetes were mainly elderly, followed by adults and teenagers. There are sex-related differences in the incidence of diabetes. The percentages of men and women who died of T2DM were 2.7% and 3.2%, respectively, a slightly greater percentage among women. The overall probability of developing T2DM was similar between the sexes. Nevertheless, there are age-related differences; the possibility of developing T2DM in women is much greater than in men. This gap is reversed in middle-aged individuals and is nearly the same as in older individuals[26]. Improving the prevention and treatment of diabetes remains challenging.
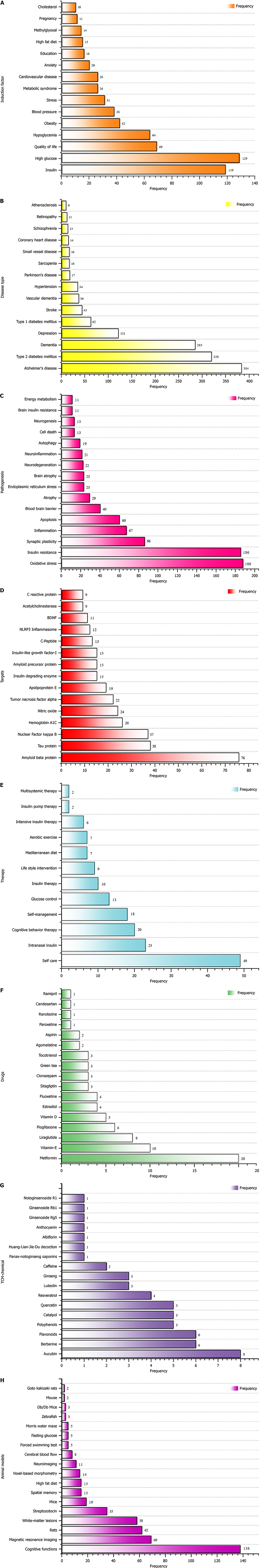
To reduce and mitigate the risk of relying solely on automated methods, we combine scientometric analysis with a narrative literature review to validate the primary findings. We have summarized the top 15 keywords for each classification based on keyword analysis to systematically understand DE and inform future research directions (Figure 7).
The risk factors for cognitive impairment in patients with diabetes can be categorized as uncontrollable or controllable (Figure 7A). Uncontrollable risk factors include age, sex, and genetics. By contrast, controllable risk factors include cardiovascular and cerebrovascular diseases, blood pressure, blood lipids, T2DM status, diet, smoking status, education level, and physical and mental activities[27]. Consistent evidence from observational studies has shown that the following common modifiable risk factors affect approximately 35% of people with dementia worldwide: diabetes, high blood pressure, obesity, middle-aged hearing loss, lack of exercise, depression, social isolation, smoking, and low level of education[28]. Diabetes and prediabetes are associated with an apparent decline in cognitive ability[29]. Distinguishing factors of diabetes (chronic hyperglycemia, recurrent hypoglycemia, and blood glucose fluctuations) and concomitant diabetes factors (obesity, hypertension, and dyslipidemia) are also involved in the occurrence and development of cognitive impairment in patients.
Insulin resistance is a typical feature of T2DM and a risk factor for AD[30]. Patients with insulin resistance exhibit poor cognitive function in several cognitive domains including orientation, memory delay, attention, and computation[31]. In T2DM, on the one hand, hypoxic metabolism caused by persistent hyperglycemia aggravates acidosis, damages hypoxic brain cells, and subsequently damages the central nervous system[32]. On the other hand, advanced glycation end products (AGEs) accumulate significantly in patients with T2DM and are toxic to nerve cells through a variety of mechanisms, resulting in cognitive impairment[33]. Chronic hyperglycemia can also induce the hyperphosphorylation of tau protein hyperphosphorylation[34]. Several studies have shown that hyperglycemia in diabetes can increase aldose reductase activity, activate the sorbitol pathway, cause intracellular hyperostosis and edema, damage the structure and function of neurons, and promote cognitive dysfunction[35]. In adult patients with T2DM, cognitive decline is accelerated after hypoglycemic attacks, and this cognitive decline can lead to severe hypoglycemic attacks[36].
Similarly, studies have shown a significant association among severe hypoglycemia, mild cognitive impairment, and dementia[37]. Therefore, there is a two-way relationship between hypoglycemia and cognitive impairment, which can increase the risk of dementia. In contrast, a decrease in cognitive function may also lead to an increase in the incidence of hypoglycemia. Compared with diabetes patients with normal blood pressure, patients with T2DM and hypertension experience more severe cognitive changes[38]. In addition, a 6-year logistic analysis of elderly patients with diabetes revealed that decreased high-density lipoprotein cholesterol levels and high diastolic blood pressure were significantly associated with cognitive decline[39]. Therefore, controlling diabetes-related metabolic disorders is important to maintain and delay cognitive dysfunction.
AD: AD is a neurodegenerative disease characterized by progressive cognitive impairment and impaired learning and memory (Figure 7B). Glucose metabolism disorders are closely related to the occurrence and development of AD and are important risk factors[40]. With improvements in societal development and economic status, eating habits have changed significantly. A high-saturated fat, high-sugar, high-animal protein, low-fiber diet, also known as the Western diet, is gaining popularity[41]. This diet not only increases the incidence of diabetes but also results in a lack of essential polyphenols, antioxidants, and omega-3 fatty acids related to brain maturation, which leads to decreased learning, memory, and cognitive function related to the hippocampus[42]. Epidemiological investigations have shown that 80% of patients with AD have diabetes or impaired glucose tolerance. Moreover, T2DM increases the risk of AD by 1.5 to 2.5 times, increases the risk of cognitive impairment compared with that of patients without diabetes, and gradually leads to sporadic AD[43,44]. AD is also known as type 3 diabetes, and the study of DE naturally intersects with AD. If the progression of T2DM to AD can be effectively alleviated, the medical burden on society can be reduced.
Depression: Depression is a complex mental disorder with a high prevalence, disability, and suicide rates due to great social harm, and is a severe threat to human life and health[45,46]. Diabetes often leads to depression. The incidence of depression in patients with diabetes is two to three times higher than that in people without diabetes[47]. The incidence of diabetes is also gradually increasing and is expected to increase to 10.2% (578 million) by 2030. This is expected to increase to 10.9% (700 million) by 2045. This indicates that the number of people with diabetes complicated with depression may gradually increase. Diabetes and depression are closely related, influencing and aggravating each other, seriously affecting patients’ body function and quality of life, increasing the risk of suicide, and further aggravating social harm[48,49]. An adequate response plan for patients with DM complicated with depression is urgently required.
Stroke: Stroke is a leading cause of death and long-term disability worldwide. Diabetes is associated with an increased risk of cardiovascular complications including stroke. Compared to people without diabetes, those with diabetes have a 1.5-2 times greater risk of stroke, which increases with the course of diabetes. These risks may also vary from sex to sex, and women are at greater risk than men[50]. Experimental evidence shows that diabetes not only affects the structure of the cerebral blood artery, leading to adverse remodeling, pathological neovascularization, and vascular regression, but also alters cerebrovascular function, resulting in an impaired myogenic response and endothelial dysfunction. Coupled with destruction of the integrity of the BBB, changes in blood flow and microbleeding into the brain can occur quickly. Neurovascular injury is more significant when ischemic damage is superimposed on the pathology. However, failure of the repair mechanism results in greater physical and cognitive defects[51]. In addition, an increase in blood viscosity caused by the consumption of greasy food and an increase in blood pressure caused by severe fatigue or emotional agitation can cause hemorrhagic strokes. Moreover, diabetes, hyperlipidemia, and hypertension are causes of ischemic stroke. Early diagnosis of stroke complications and tailored post-diabetes management are crucial to limit the burden of diabetes. Increasing neurologists’ awareness and participation in the treatment of diabetes and other cardiovascular risk factors is desirable to improve the effectiveness of stroke prevention and reperfusion therapy in patients with acute stroke-related diabetes[52].
Parkinson’s disease: Parkinson’s disease (PD) is a common neurodegenerative disorder. Its main clinical features include motor retardation, resting tremor, and ankylosis. Clinical studies have shown that the non-motor symptoms of PD-T2DM patients are more complicated and severe than those of patients with PD alone, mainly because of differences in limb sensation and cognition. Most of these are indifferent to emotional expression. The development of PD-T2DM is not a simple superposition of diseases. Nevertheless, T2DM can increase the risk of PD and the specificity of clinical symptoms. One study revealed that PD and T2DM share a common pathophysiological pathway that increases the risk of PD in patients with T2DM by 36% and makes them prone to Parkinson’s symptoms[53,54]. Another cross-sectional study excluding education, striatal dopamine denervation, and cortical choline denervation by covariance analysis revealed that the global cognitive Z score was -0.98 ± 1.01 in patients with PD with diabetes and that in patients without diabetes, it was -0.36 ± 0.91 (F = 8.39, P < 0.0001). It has been suggested that diabetes may be an independent risk factor for cognitive impairment in patients with PD[55].
Hypertension: Hypertension is an essential risk factor affecting the cardiovascular, renal, and peripheral arterial systems[56]. Hypertension is prevalent in the United States and affects nearly 111.6 million people, accounting for half of all American adults (https://www.cdc.gov). Moreover, the number of T2DM patients has reached almost 34.2 million (10% of the population)[57]. Hypertension often coexists with T2DM. 73.6% of adult patients with T2DM also have hypertension[58]. With increasing age, the risk of hypertension and T2DM increases significantly, and both are highest in the elderly population. Obesity is a significant driver of hypertension and T2DM owing to the effects of adipocyte-derived neurohormones on appetite, volume homeostasis, insulin resistance, and vascular inflammation. Therefore, the increase in the incidence of hypertension and T2DM is similar to that of obesity in the United States, and raises significant public health concerns[59]. Identifying the missing link between hypertension and the development of diabetes is crucial, as is identifying drugs that affect these two diseases and their pathological basis[60]. A core aspect of T2DM and hypertension management is the promotion of a healthy lifestyle. Moreover, nutritional therapy can help patients achieve blood sugar control and blood pressure goals[61]. Lifestyle changes, including regular exercise, diet, and smoking cessation, are at the forefront of treating diabetes and high blood pressure[62].
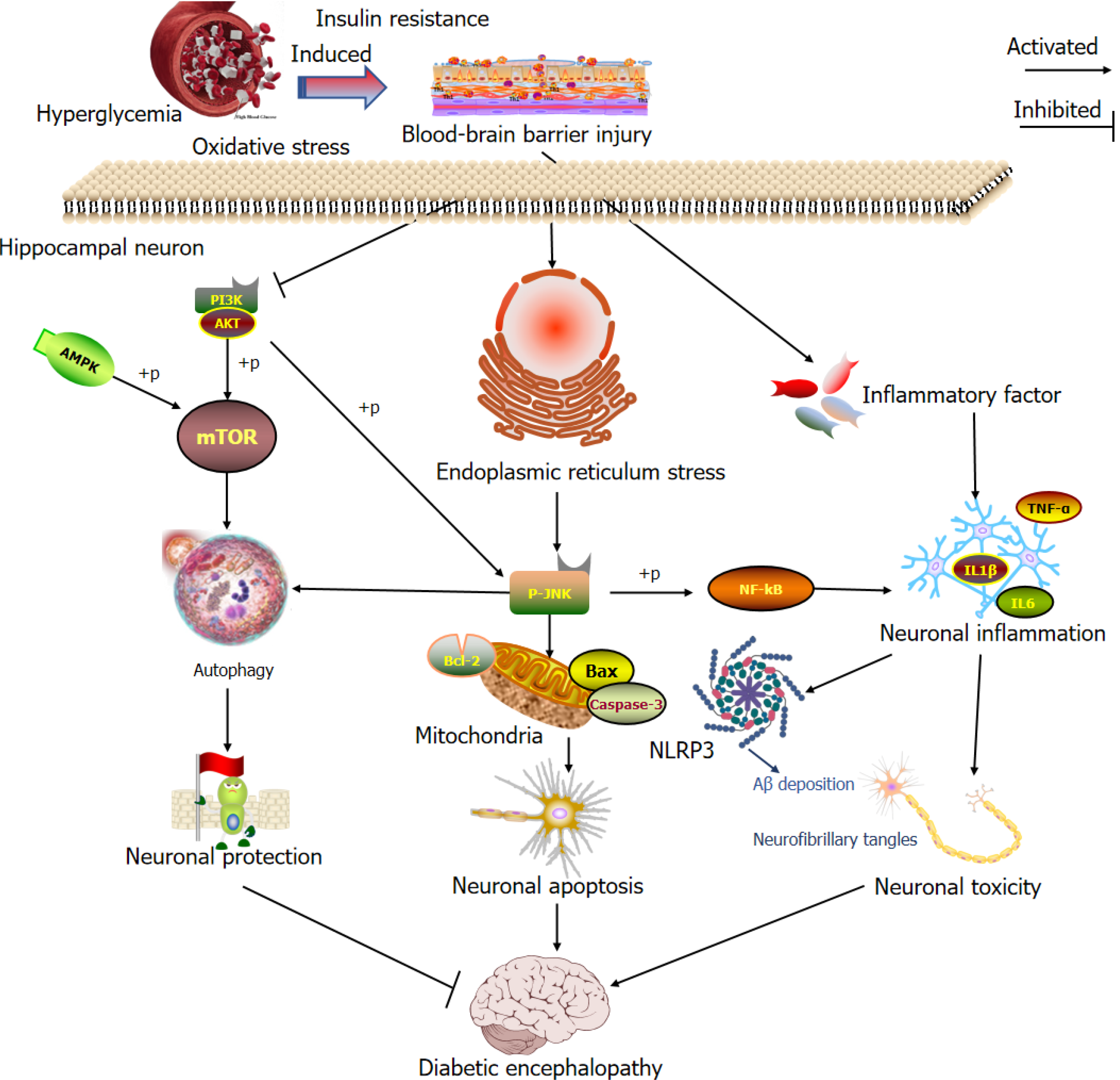
Insulin resistance: Insulin resistance is a core feature of T2DM and a risk factor for AD and related dementias (Figure 7C). Insulin resistance is present in the neurons of the brain in patients with DE. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity[63]. Insulin resistance has been observed in the hippocampi of Goto-Kakizaki rats. The insulin signaling pathway is weakened, resulting in the downregulation of memory-related and synaptic plasticity-related proteins and cognitive impairment. Drug interventions to activate the insulin signaling pathway in the brain can improve insulin resistance in hippocampal neurons and improve DE[64]. Laws et al[65]. showed that the insulin resistance index in patients with AD increased significantly. An increase in the insulin resistance index of the average cognitive population was related to a decrease in overall mental ability, verbal situational memory, and executive ability. Damage to the insulin signaling pathway is key to reducing learning and memory in a diabetic animal model and a mixed model of T2DM and AD. T2DM promotes pathological development of AD-related dementia[66]. Insulin receptors are widely distributed in the brain, and their density of insulin receptors is highest in the olfactory bulb, hypothalamus, hippocampus, cerebral cortex, striatum, and cerebellum. Insulin acts on neurons and glial cells mainly through the insulin-insulin receptor substrate 1-protein kinase B and mitogen-activated protein kinase signaling pathways[67]. On the one hand, central insulin resistance leads to the phosphorylation of serine in insulin receptor substrate 1 through a cascade reaction. It reduces the phosphorylation of c-Jun N-terminal kinase, which in turn affects downstream components of the insulin pathway, such as glycogen synthase kinase 3 beta and protein kinase A, and changes in the activity of these downstream components can stimulate tau protein hyperphosphorylation and neurofibrillary tangles. This results in impaired insulin signal transduction. On the other hand, central insulin resistance affects the clearance of amyloid-beta (Aβ) by increasing insulin levels and competitively binding insulin-degrading enzymes to Aβ[68]. Therefore, reducing insulin resistance is critical for inhibiting Aβ deposition and tau protein hyperphosphorylation.
Aβ deposition or tau hyperphosphorylation: Aβ deposition is the core pathological feature of AD and the main research topic in dementia (Figure 7D). Accumulation of islet amyloid peptides is a pathological feature of T2DM. Islet amyloid peptides can penetrate the BBB and form a heterodimer with Aβ42, resulting in conformational changes in the amyloid core region of the heterodimer, thus promoting Aβ42 aggregation and neuronal damage[69]. Studies have shown that spontaneous T2DM can significantly increase cholesterol levels in the media of the brains of nonhuman primates, which in turn destroys the degradation of lysosomes, exacerbates endocytosis, and leads to the deposition of amyloid precursor protein and Aβ in cells[70]. Insulin-degrading enzymes are critical proteins for the degradation of insulin and Aβ and act as bridges between T2DM and AD. Other studies have shown that the expression levels of insulin-degrading enzymes, peroxisome proliferator-activated receptor γ, and adenosine monophosphate-activated protein kinase are decreased in AD and T2DM mixed model mice. However, after the peroxisome proliferator-activated receptor γ/adenosine monophosphate-activated protein kinase signaling pathway was activated, the expression level of insulin-degrading enzymes increased significantly, and the accumulation of Aβ40 and Aβ42 decreased. Spatial learning and recognition impairments in mouse models were alleviated[71]. Identifying drugs that promote Aβ degradation or inhibit Aβ formation will be a challenge in the future treatment of AD or DE.
Tau plays an essential role in stabilizing the structure of neuronal microtubules. Neuronal fiber entanglement is one of the main pathological mechanisms of AD and is caused mainly by the abnormal phosphorylation of the tau protein. The phosphorylation of tau protein in the brains of db/db mice was significantly increased. The inhibition of the abnormal phosphorylation of tau protein can improve the learning and memory abilities of db/db mice and improve DE[72]. T1DM and T2DM induce tau protein phosphorylation and nerve fiber entanglement through direct and indirect induction[73]. Bi et al[74] found that SCR-1693 could improve the learning and memory abilities of streptozotocin (STZ)-induced diabetic rats in the Morris water maze test by increasing the activities of protein phosphatase and protein phosphatase 2A and reducing the level of tau protein phosphorylation[74]. Abnormal levels of tau protein are the main components of neurofibrillary tangles. SCR-1693 can reduce the accumulation of neurofibrillary tangles and prevent neuronal damage by regulating the posttranslational modification of tau protein, thus inhibiting its aggregation and promoting its degradation.
BBB injury: The BBB is composed of highly differentiated brain microvascular endothelial cells that are connected by tight junction (TJ) proteins and have low phagocytic activity and unique transporter expression. Recently, in T2DM animal models, memory impairment induced by BBB damage was shown to be closely related to neuroinflammation[75]. Inflammatory responses are essential mechanisms that mediate BBB injury. Inflammatory factors such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) can degrade TJ proteins and regulate their translocation, thus inhibiting these cytokines and protecting the TJs of brain microvascular endothelial cells[76]. High-glucose conditions upregulate the expression of many inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Diabetes can increase TNF-α and IL-6 levels, trigger the degradation of TJ proteins, inflammation, and leukocyte infiltration in the brain, promote the de
Inflammatory reactions: T2DM is a chronic inflammatory disease, and an inflammatory response accompanies many complications of T2DM. Neuroinflammation plays a vital role in DE development. Inflammatory factors induce neuronal damage and cognitive dysfunction[79]. Studies have shown that the blood levels of TNF-α and IL-6 are significantly increased in patients with DE and that these inflammatory factors are related to cognitive impairment[80]. Inflammatory reactions occur in the periphery and the hippocampus of db/db mice. The chronic inflammatory peripheral response can induce the activation of microglia, which can significantly increase the levels of IL-1β, TNF-α, and IL-6 in the hip
Oxidative stress: Oxidative stress, one of the most crucial pathogeneses of diabetes, is closely associated with protein glycosylation. Moreover, both these factors contribute to the progression of diabetes and its associated complications. Hyperglycemia can upregulate the markers of chronic inflammation and increase the production of reactive oxygen species, resulting in vascular dysfunction. Moreover, increased oxidative stress and inflammation can lead to insulin resistance and impaired insulin secretion[84]. Logan et al[85] reported that, compared with normal wild-type mice, superoxide dismutase-1 knockout mice presented impaired learning and working memory and significantly increased levels of 4-hydroxy-2-nonenal, IL-6, IL-1β, CD68, toll-like receptor, and monocyte chemoattractant protein-1 in the hippocampus. Gault et al[86] showed that sitagliptin induces antioxidant defense and reduces the cytotoxic effects of oxidative stress by increasing the expression of superoxide dismutase 2, nuclear transcription factor erythroid two and silencing regulatory information factor 1. Moreover, it can also increase insulin sensitivity and neurogenesis, and reverse memory impairment in mice fed a high-fat diet. Antioxidants can reduce insulin resistance, inflammation, and signaling pathway disorders caused by metabolic diseases such as diabetes; protect neurons; and ameliorate cognitive dysfunction in patients.
Endoplasmic reticulum stress: The endoplasmic reticulum (ER) is an organelle with various physiological functions. In neurons, it is mainly concentrated in axons, dendrites, and dendritic spines. It is responsible for synthesizing, folding, and processing lipids and proteins and for storing and transporting intracellular calcium. ER stress is an essential molecular mechanism in the occurrence and development of DM and its complications[87]. Studies have shown that diabetes can regulate the PERK and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways, thus inhibiting CREB activity, downregulating brain derived neurotrophic factor expression, and promoting cognitive impairment[88]. Sims-Robinson et al[89] explored the gene expression patterns in the hippocampi of db/db mice using microarray technology. The authors reported that ER stress genes were highly expressed in the hippocampus, indicating that ER stress was involved in the occurrence and development of DE. The ER stress inhibitor 4-phenylbutiric acid can protect primary hippocampal neurons from injury induced by high glucose levels by inhibiting the inflammatory response and neuronal apoptosis caused by ER stress and by improving the learning and memory ability of DE rats. ER stress is an essential target for improving DE[90].
Other: High glucose levels can significantly upregulate dynamin-related protein 1, which is involved in mitochondrial division; disrupt the balance between mitochondrial division and fusion, cause mitochondrial dysfunction, inhibit the expression of dynamin-related protein 1, and ameliorate mitochondrial dysfunction and DE in the hippocampus of db/db mice[91]. In addition, deposited Aβ can hinder insulin regulation of synaptic terminal mitochondria, resulting in energy metabolism disorders, affecting synaptic plasticity, and promoting cognitive dysfunction[92]. Owing to calcium overload in the hippocampus of db/db mice, the expression of Calcium-calmodulin-dependent protein kinase II is decreased, which leads to reduced synaptic plasticity, learning, and memory[93]. An in-depth analysis of the long-chain noncoding RNA plasmacytoma variant translocation 1 revealed that activating plasmacytoma variant translocation 1-mediated autophagy protected hippocampal neurons and ameliorated cognitive impairment in patients with diabetes[94]. Therefore, repairing mitochondrial damage, maintaining calcium homeostasis, enhancing synaptic plasticity, and reducing autophagy may be potential treatments for diabetes-related cognitive impairment.
Cognitive impairment has become one of the primary causes of death and disability in older adults, and the risk of cognitive impairment increases significantly in individuals with diabetes. The disability rate among individuals with cognitive impairment is high. Patients lose their ability to live independently in the late stages, ultimately requiring the care of others, resulting in a heavy economic and nursing burden on society and families. The early identification and intervention of risk factors for cognitive impairment, reasonable blood glucose control, selection of appropriate hypoglycemic drugs, and routine treatment of cognitive impairment are the main methods for treating diabetic cognitive impairment (Figure 7E).
Lifestyle intervention: Controlling diet and exercise may improve neuronal plasticity in brain regions related to cognitive function in patients with diabetic cognitive impairment[95]. Compared with low-fat diets, dietary interventions containing olive oil or nuts seem to improve cognitive ability[96]. A Finnish intervention study revealed that the risk of cognitive impairment and memory problems in elderly individuals was lower than in other older adults, including eating a healthy diet, exercising, training memory ability, and managing cardiovascular risk factors. Moreover, increasing mental activity (such as playing cards, reading, and learning new knowledge) can reduce the risk of AD by improving cognitive reserves[97]. The overall method of establishing a typical daily pattern in patients with ADs involves enhanced morning lighting and forced daytime activity. Fixed sleep time has produced encouraging results in the treatment of AD and is worthy of further study. Twenty clinical interventions based on cognitive impairment in patients with diabetes have been developed, mainly starting with behavioral exercise, dietary supplements, rehabilitation services, and drug treatment, and have achieved specific results (www.clinicaltrials.gov).
Blood glucose control and individualized treatment: Poor blood glucose control can lead to increased frequency of hospitalization in patients with cognitive impairment, especially in those with executive function impairment. Strict blood glucose control may increase the risk of hypoglycemia. The results of the ACCORD-MIND study revealed that, after a 40-month intervention, enhanced blood glucose management did not ameliorate cognitive decline[98]. Other studies, such as the ADVANCE Study, support this view[99]. Therefore, intensive hypoglycemic therapy is not re
Hypoglycemic drug therapy: Anti-diabetic drugs can improve hyperglycemia, insulin resistance, and cell metabolism and combat tissue inflammation and oxidative stress associated with insulin resistance. They may also positively affect cellular metabolism in the brain and improve cognitive ability (Figure 7F). Inhaled insulin can improve the mental state by increasing insulin concentration in the cerebrospinal fluid[100], and metformin and glucagon-like peptide-1 receptor agonist may enhance cognitive function and reduce the risk of dementia[101,102]. Sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, and sodium-dependent glucose transporter-2 inhibitors may have protective effects on cognitive function. However, clinical evidence is insufficient, and further research is needed to confirm these findings[100]. Therefore, under appropriate conditions, we can prioritize the use of these beneficial drugs for blood glucose management in diabetes patients with cognitive impairment and pay close attention to changes in cognitive function.
Compound prescription and monomer therapy in traditional Chinese medicine: Traditional Chinese medicine (TCM) and modern medicine have both advantages and disadvantages in the treatment of diabetes. Therefore, we should combine their benefits, overcome the bottleneck of diabetes treatment, and explore new measures for the prevention and treatment of diabetes by combining TCM syndromes with modern medicine. This is a worthwhile attempt[103]. Many TCMs are used to prevent and treat DE. Aucubin is an iridoid glycoside found in TCMs, such as Eucommia ulmoides and Plantago lanceolata[104,105]. Aucubin effectively controlled blood glucose levels, prevented complications, and improved the quality of life of diabetic rats. In DE, aucubin significantly rescues neurons in the hippocampal CA1 subfield and reduces working errors during behavioral testing[106]. Furthermore, aucubin can effectively inhibit apoptosis by modulating the expression of B cell leukemia/lymphoma 2 and B cell leukemia/lymphoma 2-associated X protein[107], significantly reducing lipid peroxide content, regulating the activities of antioxidant enzymes, and decreasing the activity of nitric oxide synthesis[108] in DE model rats. These findings indicate that aucubin is a potential neuroprotective agent.
Ginseng is one of the oldest Chinese herbal medicines and has been widely used in Asia for thousands of years, and as adjuvants for the treatment of diabetes in China[109]. By attenuating neuroinflammatory responses, Rg5 improved cognitive dysfunction and beta-amyloid deposition in STZ-induced memory-impaired rats[110]. Ginsenoside Rb1 ameliorated cognitive impairment caused by insulin resistance through the cyclin dependent kinase 5/p35-N-methyl-D-aspartate receptor-insulin degrading enzyme pathway[111]. Regulation of insulin secretion, glucose uptake, antioxidant stress, and the anti-inflammatory pathway may be the mechanisms underlying the antidiabetic effects of Panax ginseng[112].
Berberine, an isoquinoline alkaloid isolated from the Chinese herb, Coptidis rhizoma and other Berberis plants, exhibits a wide range of pharmacological properties. Berberine can be used to treat many diseases such as cancer and digestive, metabolic, cardiovascular, and neurological diseases[113]. Berberine can reduce tau protein phosphorylation by upregulating the PI3K/Akt/glycogen synthase kinase 3 beta signaling pathway and has a protective effect on neuronal axonal injury, thus improving the cognitive function of rats with type 2 DE[114]. Ginsenosides and alkaloids are potential hypoglycemic agents. However, the alkaloids from Coptidis rhizoma have apparent inhibitory effects. In contrast, the ginsenosides Rb1, Rg2, and Re have poor inhibitory effects on α-glucosidase[115]. Interestingly, there have been many reports on the use of Panax ginseng-Coptidis rhizoma couplet medicine (PCCM) for the treatment of diabetes. PCCM can treat T2DM by inhibiting liver glucose production, and its mechanism is related to the activation of the PI3K/Akt/forkhead box O1 signaling pathway[116]. PCCM had a dose-dependent effect on glucose metabolism, possibly through the Notch1/neurogenin 3 signaling pathway, to maintain β-cell characteristics and improve glucose metabolism in db/db mice[117]. Additionally, PCCM can play a hypoglycemic and β-cell protective role by regulating the intestinal flora, especially Bacteroides acids, and it is better than Panax ginseng or Coptidis rhizoma alone[118]. Therefore, we speculate that PCCM can be used to prevent and treat DE, which is promising (Figure 7G).
In diabetic models of cognitive impairment, a high-sugar high-fat diet combined with STZ is typically used to induce diabetes in rats. Individuals can spontaneously develop cognitive impairment or use transgenic mice such as Goto-Kakizaki rats and db/db mice directly[119,120]. Zebrafish T2DM models have been established using glucose immersion[121], diet induction[122], and gene knockout[123]. Blood glucose levels and behavioral parameters, including spatial learning ability and Morris water maze results, are common indicators. Magnetic resonance imaging revealed that the main pathological features of DE were atrophy of the gray matter, white matter, and hippocampal body; damage to synaptic plasticity; dysfunction of glial cells; and changes in cerebral vascular structure and function[124-127]. With multisite brain tissues as the object of study, these animal models of DE provide a vital tool for the development of more effective anti-DE agents (Figure 7H).
Bibliometrics can be used to analyze the scientific development trend of DE research and provide a comprehensive overview of the research topic, enabling researchers to quickly understand the information in the field, understand future research directions, and improve research efficiency. However, as with other scientometric studies, this study has several limitations. First, we searched only the SCI-E database from the World of Science and no other large medical databases, such as Scopus or EMBASE. In addition, we cannot analyze data simultaneously from multiple databases such as the Chinese National Knowledge Infrastructure. Second, all data were extracted using software, unlike systematic reviews or data summaries in which two or more reviewers are involved in manual removal. Third, the main keywords used in this study are related to the research objects. However, the keywords used in some related studies have not been stan
This review proposes a new method for exploring the current research status of DE through scientometric analysis to identify new research hotspots. According to the results of the scientometric analysis, an in-depth and comprehensive review was carried out. This study used scientometrics to analyze the literature in the SCI-E database. Our research results reveal the research hotspots, the most productive countries, institutions, categories, journals, references, and keywords that have focused on DE since 2004. In addition, the review was based on the keywords with the highest frequency in keyword analysis. This article summarizes the inducing factors, comorbidities, pathogenesis, treatment, and animal models of DE to provide a new and unique perspective on its treatment. For clinicians, reminding patients to control their diet effectively, maintain a good attitude, and have scientifically recommended work and rest can help delay the related encephalopathy caused by diabetes. Further exploration of prevention and control measures for DE is critical. These findings reveal the current research hotspots and the gray areas of further research.
The authors thank Beijing University of Chinese Medicine for their assistance in conducting this study.
| 1. | Magliano DJ, Chen L, Islam RM, Carstensen B, Gregg EW, Pavkov ME, Andes LJ, Balicer R, Baviera M, Boersma-van Dam E, Booth GL, Chan JCN, Chua YX, Fosse-Edorh S, Fuentes S, Gulseth HL, Gurevicius R, Ha KH, Hird TR, Jermendy G, Khalangot MD, Kim DJ, Kiss Z, Kravchenko VI, Leventer-Roberts M, Lin CY, Luk AOY, Mata-Cases M, Mauricio D, Nichols GA, Nielen MM, Pang D, Paul SK, Pelletier C, Pildava S, Porath A, Read SH, Roncaglioni MC, Lopez-Doriga Ruiz P, Shestakova M, Vikulova O, Wang KL, Wild SH, Yekutiel N, Shaw JE. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021;9:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3385] [Article Influence: 483.6] [Reference Citation Analysis (0)] |
| 3. | Reske-Nielsen E, Lundbaek K. Diabetic encephalopathy. Acta Neurol Scand. 2009;39:273-290. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Riederer P, Korczyn AD, Ali SS, Bajenaru O, Choi MS, Chopp M, Dermanovic-Dobrota V, Grünblatt E, Jellinger KA, Kamal MA, Kamal W, Leszek J, Sheldrick-Michel TM, Mushtaq G, Meglic B, Natovich R, Pirtosek Z, Rakusa M, Salkovic-Petrisic M, Schmidt R, Schmitt A, Sridhar GR, Vécsei L, Wojszel ZB, Yaman H, Zhang ZG, Cukierman-Yaffe T. The diabetic brain and cognition. J Neural Transm (Vienna). 2017;124:1431-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Parashar A, Mehta V, Malairaman U. Type 2 Diabetes Mellitus Is Associated with Social Recognition Memory Deficit and Altered Dopaminergic Neurotransmission in the Amygdala. Ann Neurosci. 2018;24:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 10. | Exalto LG, Biessels GJ, Karter AJ, Huang ES, Quesenberry CP Jr, Whitmer RA. Severe diabetic retinal disease and dementia risk in type 2 diabetes. J Alzheimers Dis. 2014;42 Suppl 3:S109-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract. 2017;124:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 12. | Luo A, Xie Z, Wang Y, Wang X, Li S, Yan J, Zhan G, Zhou Z, Zhao Y, Li S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci Biobehav Rev. 2022;137:104642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Ye XW, Liu MN, Wang X, Cheng SQ, Li CS, Bai YY, Yang LL, Wang XX, Wen J, Xu WJ, Zhang SY, Xu XF, Li XR. Exploring the common pathogenesis of Alzheimer’s disease and type 2 diabetes mellitus via microarray data analysis. Front Aging Neurosci. 2023;15:1071391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Ma W, Xu D, Zhao L, Yuan M, Cui YL, Li Y. Therapeutic role of curcumin in adult neurogenesis for management of psychiatric and neurological disorders: a scientometric study to an in-depth review. Crit Rev Food Sci Nutr. 2023;63:9379-9391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Xu D, Wang YL, Wang KT, Wang Y, Dong XR, Tang J, Cui YL. A Scientometrics Analysis and Visualization of Depressive Disorder. Curr Neuropharmacol. 2021;19:766-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Sabe M, Pillinger T, Kaiser S, Chen C, Taipale H, Tanskanen A, Tiihonen J, Leucht S, Correll CU, Solmi M. Half a century of research on antipsychotics and schizophrenia: A scientometric study of hotspots, nodes, bursts, and trends. Neurosci Biobehav Rev. 2022;136:104608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Cortese S, Sabé M, Chen C, Perroud N, Solmi M. Half a century of research on Attention-Deficit/Hyperactivity Disorder: A scientometric study. Neurosci Biobehav Rev. 2022;140:104769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Li X, Xiang P, Liang J, Deng Y, Du J. Global Trends and Hotspots in Esketamine Research: A Bibliometric Analysis of Past and Estimation of Future Trends. Drug Des Devel Ther. 2022;16:1131-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, Snoek FJ, Heine RJ. Diabetic encephalopathy: A concept in need of a definition. Diabetologia. 2006;49:1447-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1522] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 21. | Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 871] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 22. | Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565-1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 861] [Cited by in RCA: 738] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 23. | Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1484] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 24. | Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48:2460-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 692] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 25. | Li H, An H, Wang Y, Huang J, Gao X. Evolutionary features of academic articles co-keyword network and keywords co-occurrence network: Based on two-mode affiliation network. Physica A. 2016;450:657-669. [DOI] [Full Text] |
| 26. | Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1353] [Cited by in RCA: 1281] [Article Influence: 256.2] [Reference Citation Analysis (0)] |
| 27. | Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, Pan A, Zhang C, Jia J, Feng L, Kua EH, Wang YJ, Wang HF, Tan MS, Li JQ, Hou XH, Wan Y, Tan L, Mok V, Tan L, Dong Q, Touchon J, Gauthier S, Aisen PS, Vellas B. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 28. | Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 732] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 29. | Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 30. | Stoeckel LE, Arvanitakis Z, Gandy S, Small D, Kahn CR, Pascual-Leone A, Pawlyk A, Sherwin R, Smith P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res. 2016;5:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Zhong Y, Miao Y, Jia WP, Yan H, Wang BY, Jin J. Hyperinsulinemia, insulin resistance and cognitive decline in older cohort. Biomed Environ Sci. 2012;25:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 32. | Valente T, Gella A, Fernàndez-Busquets X, Unzeta M, Durany N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol Dis. 2010;37:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Yuan XY, Wang XG. Mild cognitive impairment in type 2 diabetes mellitus and related risk factors: a review. Rev Neurosci. 2017;28:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Huang R, Tian S, Zhang H, Zhu W, Wang S. Chronic hyperglycemia induces tau hyperphosphorylation by downregulating OGT-involved O-GlcNAcylation in vivo and in vitro. Brain Res Bull. 2020;156:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, Diamond DM. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes. 2008;9:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Feinkohl I, Aung PP, Keller M, Robertson CM, Morling JR, McLachlan S, Deary IJ, Frier BM, Strachan MW, Price JF; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care. 2014;37:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Lee AK, Rawlings AM, Lee CJ, Gross AL, Huang ES, Sharrett AR, Coresh J, Selvin E. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia. 2018;61:1956-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Petrova M, Prokopenko S, Pronina E, Mozheyko E. Diabetes type 2, hypertension and cognitive dysfunction in middle age women. J Neurol Sci. 2010;299:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Umegaki H, Iimuro S, Shinozaki T, Araki A, Sakurai T, Iijima K, Ohashi Y, Ito H; Japanese Elderly Diabetes Intervention Trial Study Group. Risk factors associated with cognitive decline in the elderly with type 2 diabetes: pooled logistic analysis of a 6-year observation in the Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. 2012;12 Suppl 1:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Cho S, Lee H, Seo J. Impact of Genetic Risk Factors for Alzheimer’s Disease on Brain Glucose Metabolism. Mol Neurobiol. 2021;58:2608-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Spencer SJ, Korosi A, Layé S, Shukitt-Hale B, Barrientos RM. Food for thought: how nutrition impacts cognition and emotion. NPJ Sci Food. 2017;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 42. | Suarez AN, Noble EE, Kanoski SE. Regulation of Memory Function by Feeding-Relevant Biological Systems: Following the Breadcrumbs to the Hippocampus. Front Mol Neurosci. 2019;12:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Salas IH, De Strooper B. Diabetes and Alzheimer’s Disease: A Link not as Simple as it Seems. Neurochem Res. 2019;44:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Chornenkyy Y, Wang WX, Wei A, Nelson PT. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019;29:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 45. | Subba R, Sandhir R, Singh SP, Mallick BN, Mondal AC. Pathophysiology linking depression and type 2 diabetes: Psychotherapy, physical exercise, and fecal microbiome transplantation as damage control. Eur J Neurosci. 2021;53:2870-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 47. | Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, Kim YN, Won MH, Park IS, Seong JK, Yoon YS. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010;40:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152-8161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 49. | Soliman E, Essmat N, Mahmoud MF, Mahmoud AAA. Impact of some oral hypoglycemic agents on type 2 diabetes-associated depression and reserpine-induced depression in rats: the role of brain oxidative stress and inflammation. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1391-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Mosenzon O, Cheng AY, Rabinstein AA, Sacco S. Diabetes and Stroke: What Are the Connections? J Stroke. 2023;25:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 51. | Wolf V, Abdul Y, Li W, Ergul A. Impact of diabetes and ischemic stroke on the cerebrovasculature: A female perspective. Neurobiol Dis. 2022;167:105667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Bradley SA, Spring KJ, Beran RG, Chatzis D, Killingsworth MC, Bhaskar SMM. Role of diabetes in stroke: Recent advances in pathophysiology and clinical management. Diabetes Metab Res Rev. 2022;38:e3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Zhang P, Liu B. Association between Parkinson’s Disease and Risk of Cancer: A PRISMA-compliant Meta-analysis. ACS Chem Neurosci. 2019;10:4430-4439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | de Pablo-Fernández E, Courtney R, Rockliffe A, Gentleman S, Holton JL, Warner TT. Faster disease progression in Parkinson’s disease with type 2 diabetes is not associated with increased α-synuclein, tau, amyloid-β or vascular pathology. Neuropathol Appl Neurobiol. 2021;47:1080-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Bohnen NI, Kotagal V, Müller ML, Koeppe RA, Scott PJ, Albin RL, Frey KA, Petrou M. Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Parkinsonism Relat Disord. 2014;20:1394-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Nie J, Zhang Y, Li J, Liang M, Wang G, Tian J, Liu C, Wang B, Cui Y, Wang X, Huo Y, Xu X, Hou FF, Qin X. Degree of Blood Pressure Control and Incident Diabetes Mellitus in Chinese Adults With Hypertension. J Am Heart Assoc. 2020;9:e017015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Jia G, Sowers JR. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension. 2021;78:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 58. | Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond). 2007;112:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 59. | Grossman A, Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc Diabetol. 2017;16:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 60. | Przezak A, Bielka W, Pawlik A. Hypertension and Type 2 Diabetes-The Novel Treatment Possibilities. Int J Mol Sci. 2022;23:6500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Simões Corrêa Galendi J, Leite RGOF, Banzato LR, Nunes-Nogueira VDS. Effectiveness of Strategies for Nutritional Therapy for Patients with Type 2 Diabetes and/or Hypertension in Primary Care: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19:4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Umeukeje EM, Washington JT, Nicholas SB. Etiopathogenesis of kidney disease in minority populations and an updated special focus on treatment in diabetes and hypertension. J Natl Med Assoc. 2022;114:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64:3927-3936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 64. | Li XH, Xin X, Wang Y, Wu JZ, Jin ZD, Ma LN, Nie CJ, Xiao X, Hu Y, Jin MW. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J Alzheimers Dis. 2013;34:755-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Laws SM, Gaskin S, Woodfield A, Srikanth V, Bruce D, Fraser PE, Porter T, Newsholme P, Wijesekara N, Burnham S, Doré V, Li QX, Maruff P, Masters CL, Rainey-Smith S, Rowe CC, Salvado O, Villemagne VL, Martins RN, Verdile G. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep. 2017;7:9766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Ramos-Rodriguez JJ, Jimenez-Palomares M, Murillo-Carretero MI, Infante-Garcia C, Berrocoso E, Hernandez-Pacho F, Lechuga-Sancho AM, Cozar-Castellano I, Garcia-Alloza M. Central vascular disease and exacerbated pathology in a mixed model of type 2 diabetes and Alzheimer’s disease. Psychoneuroendocrinology. 2015;62:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 67. | Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 1033] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 68. | Benedict C, Grillo CA. Insulin Resistance as a Therapeutic Target in the Treatment of Alzheimer’s Disease: A State-of-the-Art Review. Front Neurosci. 2018;12:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Ge X, Yang Y, Sun Y, Cao W, Ding F. Islet Amyloid Polypeptide Promotes Amyloid-Beta Aggregation by Binding-Induced Helix-Unfolding of the Amyloidogenic Core. ACS Chem Neurosci. 2018;9:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Takeuchi S, Ueda N, Suzuki K, Shimozawa N, Yasutomi Y, Kimura N. Elevated Membrane Cholesterol Disrupts Lysosomal Degradation to Induce β-Amyloid Accumulation: The Potential Mechanism Underlying Augmentation of β-Amyloid Pathology by Type 2 Diabetes Mellitus. Am J Pathol. 2019;189:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Li H, Wu J, Zhu L, Sha L, Yang S, Wei J, Ji L, Tang X, Mao K, Cao L, Wei N, Xie W, Yang Z. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer’s disease: possible mechanisms of IDE in T2D and AD. Biosci Rep. 2018;38:BSR20170862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Jung GY, Won SB, Kim J, Jeon S, Han A, Kwon YH. Betaine Alleviates Hypertriglycemia and Tau Hyperphosphorylation in db/db Mice. Toxicol Res. 2013;29:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | van der Harg JM, Eggels L, Bangel FN, Ruigrok SR, Zwart R, Hoozemans JJM, la Fleur SE, Scheper W. Insulin deficiency results in reversible protein kinase A activation and tau phosphorylation. Neurobiol Dis. 2017;103:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Bi A, An W, Wang C, Hua Y, Fang F, Dong X, Chen R, Zhang Z, Luo L. SCR-1693 inhibits tau phosphorylation and improves insulin resistance associated cognitive deficits. Neuropharmacology. 2020;168:108027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, Kolpakov MA, Bashkirova YV, Sabri AK, Persidsky Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol Neurobiol. 2019;56:1883-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 76. | Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010;30:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 77. | Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y, Chen Y, Chen S, Wang Y, Zhang Z, Yang GY. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front Aging Neurosci. 2018;10:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 78. | Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radic Biol Med. 2013;63:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Ni H, Guo Z, Wu Y, Wang J, Yang Y, Zhu Z, Wang D. The crucial role that hippocampus Cyclooxygenase-2 plays in memory. Eur J Neurosci. 2023;58:4123-4136. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Suzuki M, Umegaki H, Ieda S, Mogi N, Iguchi A. Factors associated with cognitive impairment in elderly patients with diabetes mellitus. J Am Geriatr Soc. 2006;54:558-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 82. | Ward R, Ergul A. Relationship of endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal cells in diabetes. Life Sci. 2016;159:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1538] [Cited by in RCA: 2112] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 84. | Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 167] [Reference Citation Analysis (0)] |
| 85. | Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, Deepa SS. Accelerated decline in cognition in a mouse model of increased oxidative stress. Geroscience. 2019;41:591-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 87. | Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular Stress, Excessive Apoptosis, and the Effect of Metformin in a Mouse Model of Type 2 Diabetic Embryopathy. Diabetes. 2015;64:2526-2536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Wu Y, Wu C, Ye L, Wang B, Yuan Y, Liu Y, Zheng P, Xiong J, Li Y, Jiang T, Li X, Xiao J. Exogenous fibroblast growth factor 1 ameliorates diabetes-induced cognitive decline via coordinately regulating PI3K/AKT signaling and PERK signaling. Cell Commun Signal. 2020;18:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Sims-Robinson C, Zhao S, Hur J, Feldman EL. Central nervous system endoplasmic reticulum stress in a murine model of type 2 diabetes. Diabetologia. 2012;55:2276-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Wang Z, Huang Y, Cheng Y, Tan Y, Wu F, Wu J, Shi H, Zhang H, Yu X, Gao H, Lin L, Cai J, Zhang J, Li X, Cai L, Xiao J. Endoplasmic reticulum stress-induced neuronal inflammatory response and apoptosis likely plays a key role in the development of diabetic encephalopathy. Oncotarget. 2016;7:78455-78472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 91. | Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, Hu G, Sosunov AA, McKhann GM, Yu H, Yan SS. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes. 2015;64:1728-1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 92. | Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 919] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 93. | Oomura Y, Aou S, Fukunaga K. Prandial increase of leptin in the brain activates spatial learning and memory. Pathophysiology. 2010;17:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Li Z, Hao S, Yin H, Gao J, Yang Z. Autophagy ameliorates cognitive impairment through activation of PVT1 and apoptosis in diabetes mice. Behav Brain Res. 2016;305:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 95. | Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE; For 2018 physical activity guidelines advisory committee*. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019;51:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 650] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 96. | Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MÁ. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 448] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 97. | Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003;58:P249-P255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Sullivan M, Horowitz KR, Ding J, Marcovina S, Lovato LC, Lovato J, Margolis KL, O’Connor P, Lipkin EW, Hirsch J, Coker L, Maldjian J, Sunshine JL, Truwit C, Davatzikos C, Bryan RN; ACCORD MIND investigators. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 99. | Tang X, Cardoso MA, Yang J, Zhou JB, Simó R. Impact of Intensive Glucose Control on Brain Health: Meta-Analysis of Cumulative Data from 16,584 Patients with Type 2 Diabetes Mellitus. Diabetes Ther. 2021;12:765-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Boccardi V, Murasecco I, Mecocci P. Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res Rev. 2019;54:100936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 101. | Chin-Hsiao T. Metformin and the Risk of Dementia in Type 2 Diabetes Patients. Aging Dis. 2019;10:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 102. | Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, García-Pérez LE, Lakshmanan M, Bethel A, Xavier D, Probstfield J, Riddle MC, Rydén L, Atisso CM, Hall S, Rao-Melacini P, Basile J, Cushman WC, Franek E, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 103. | Wang J, Ma Q, Li Y, Li P, Wang M, Wang T, Wang C, Wang T, Zhao B. Research progress on Traditional Chinese Medicine syndromes of diabetes mellitus. Biomed Pharmacother. 2020;121:109565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 104. | Lian X, Wang N, Ma L, Jiang H, Bai D, Xue H, Ma Q. Determination of aucubin by supramolecular solvent-based dispersive liquid-liquid microextraction and UPLC-MS/MS: Application to a pharmacokinetic study in rats with type 1 diabetes. J Pharm Biomed Anal. 2020;186:113301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Yang P, Zhang Q, Shen H, Bai X, Liu P, Zhang T. Research progress on the protective effects of aucubin in neurological diseases. Pharm Biol. 2022;60:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Xue HY, Lu YN, Fang XM, Xu YP, Gao GZ, Jin LJ. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep. 2012;39:9311-9318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Xue H, Jin L, Jin L, Zhang P, Li D, Xia Y, Lu Y, Xu Y. Neuroprotection of aucubin in primary diabetic encephalopathy. Sci China C Life Sci. 2008;51:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Xue HY, Jin L, Jin LJ, Li XY, Zhang P, Ma YS, Lu YN, Xia YQ, Xu YP. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res. 2009;23:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Bai L, Gao J, Wei F, Zhao J, Wang D, Wei J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front Pharmacol. 2018;9:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 110. | Chu S, Gu J, Feng L, Liu J, Zhang M, Jia X, Liu M, Yao D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int Immunopharmacol. 2014;19:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 111. | Yang R, Jiang X, He X, Liang D, Sun S, Zhou G. Ginsenoside Rb1 Improves Cognitive Impairment Induced by Insulin Resistance through Cdk5/p35-NMDAR-IDE Pathway. Biomed Res Int. 2020;2020:3905719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Chen W, Balan P, Popovich DG. Review of Ginseng Anti-Diabetic Studies. Molecules. 2019;24:4501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 113. | Ye XW, Wang HL, Cheng SQ, Xia LJ, Xu XF, Li XR. Network Pharmacology-Based Strategy to Investigate the Pharmacologic Mechanisms of Coptidis Rhizoma for the Treatment of Alzheimer’s Disease. Front Aging Neurosci. 2022;14:890046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Chen Q, Mo R, Wu N, Zou X, Shi C, Gong J, Li J, Fang K, Wang D, Yang D, Wang K, Chen J. Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front Pharmacol. 2017;8:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 115. | Li CL, Zhang KY, Du YJ, Lv JP, Yang XQ, Zhang H. [Component analysis of ginseng and golden thread as couplet medicines and its component effect on α-glucosidase]. Jilin Zhongyiyao. 2021;41:1102-1104. [DOI] [Full Text] |
| 116. | Wu F, Shao Q, Xia Q, Hu M, Zhao Y, Wang D, Fang K, Xu L, Zou X, Chen Z, Chen G, Lu F. A bioinformatics and transcriptomics based investigation reveals an inhibitory role of Huanglian-Renshen-Decoction on hepatic glucose production of T2DM mice via PI3K/Akt/FoxO1 signaling pathway. Phytomedicine. 2021;83:153487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 117. | Yuan F, Wang D, Ma L, Qin X, Gong J, Hu M, Su H, Dong H, Lu F. Effects of Huanglian-Renshen-Decoction, a Fixed Mixture of Traditional Chinese Medicine, on the Improvement of Glucose Metabolism by Maintenance of Pancreatic β Cell Identity in db/db Mice. Evid Based Complement Alternat Med. 2019;2019:1232913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Sun RX, Huang WJ, Xiao Y, Wang DD, Mu GH, Nan H, Ni BR, Huang XQ, Wang HC, Liu YF, Fu Q, Zhao JX. Shenlian (SL) Decoction, a Traditional Chinese Medicine Compound, May Ameliorate Blood Glucose via Mediating the Gut Microbiota in db/db Mice. J Diabetes Res. 2022;2022:7802107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 119. | Galán BSM, Serdan TDA, Rodrigues LE, Manoel R, Gorjão R, Masi LN, Pithon-Curi TC, Curi R, Hirabara SM. Reviewing physical exercise in non-obese diabetic Goto-Kakizaki rats. Braz J Med Biol Res. 2022;55:e11795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 120. | Hayden MR. The Mighty Mitochondria Are Unifying Organelles and Metabolic Hubs in Multiple Organs of Obesity, Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes: An Observational Ultrastructure Study. Int J Mol Sci. 2022;23:4820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Tanvir Z, Nelson RF, DeCicco-Skinner K, Connaughton VP. One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram. Dis Model Mech. 2018;11:dmm035220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 122. | Zang L, Shimada Y, Nishimura N. Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus. Sci Rep. 2017;7:1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 123. | Gong Y, Zhai G, Su J, Yang B, Jin J, Liu H, Yin Z, Xie S, Han D. Different roles of insulin receptor a and b in maintaining blood glucose homeostasis in zebrafish. Gen Comp Endocrinol. 2018;269:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 125. | Roomi AB, Mahdi Salih AH, Noori SD, Nori W, Tariq S. Evaluation of Bone Mineral Density, Serum Osteocalcin, and Osteopontin Levels in Postmenopausal Women with Type 2 Diabetes Mellitus, with/without Osteoporosis. J Osteoporos. 2022;2022:1437061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Roomi AB, Ali EA, Nori W, Rahmah MI. Asprosin is a Reliable Predictor of Osteoporosis in Type 2 Diabetic Postmenopausal Women: A Case-Control Study. Indian J Clin Biochem. 2025;40:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Shareef M, Roomi AB, Charfeddine B. Spexin is a Reliable Predictor of Diabetes Postmenopausal Iraqi Women: A Case-Control Study. Baghdad Sci J. 2024;22. [DOI] [Full Text] |