Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.104937
Revised: February 22, 2025
Accepted: March 14, 2025
Published online: May 15, 2025
Processing time: 108 Days and 21.4 Hours
Diabetic wounds represent a significant challenge in the medical field, sig
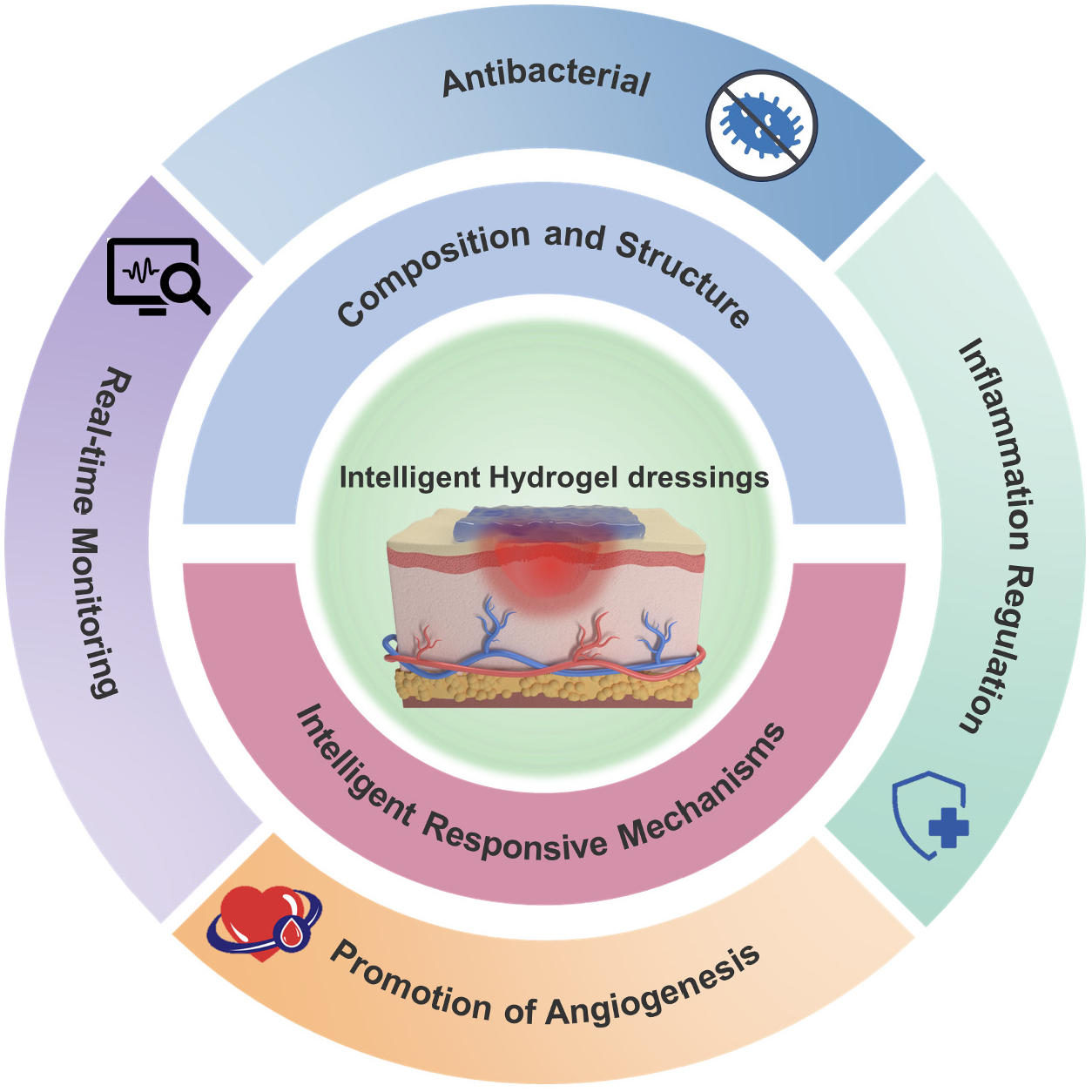
Core Tip: This review provides an in-depth analysis of intelligent hydrogel dressings for diabetic wound treatment, emphasizing their unique compositions, responsive mechanisms, and diverse applications. It highlights their advantages in maintaining a moist wound environment, precise drug delivery, and real-time monitoring, while addressing challenges and future directions. This comprehensive overview aims to inspire innovation and guide the development of next-generation wound care solutions.
- Citation: Liu H, He L. Intelligent hydrogel-based dressings for treatment of chronic diabetic wounds. World J Diabetes 2025; 16(5): 104937
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/104937.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.104937
Diabetes mellitus has become a pressing global public health crisis, with its prevalence skyrocketing at an alarming rate[1-3]. According to the International Diabetes Federation, around 537 million adults globally had diabetes in 2021, and this figure is forecasted to surge to 643 million by 2030 and a staggering 783 million by 2045[1,2]. In China, the situation is equally disconcerting, as the diabetes prevalence in adults has continuously climbed from 5.5% in 2000 to 11.2% in 2018[3]. This upward trend not only poses a significant threat to public health but also places a heavy financial and medical burden on individuals and healthcare systems[3]. Diabetic wounds, a common and persistent complication of diabetes, stem from a series of physiological disruptions caused by the disease[4-6]. Chronic hyperglycemia is like a "toxic tumor" that undermines various bodily functions, being a fundamental factor in diabetic wound development[4-6]. Hy
In the field of diabetic wound treatment, traditional methods have been dominant for a long time, with gauze and bandages being commonly used dressings[16]. However, these conventional dressings are far from sufficient in meeting the complex needs of diabetic wounds. Their ability to manage wound exudate is extremely limited. They are like leaky sieves, unable to effectively absorb and handle the fluid from the wound[17]. Exudate accumulates at the wound site, creating a moist and nutrient-rich environment that is ideal for bacterial growth, greatly increasing the risk of infection[17,18]. Moreover, traditional dressings adhere strongly to the wound[19,20]. During dressing changes, patients ex
Fortunately, with the rapid progress in materials science and biotechnology, intelligent hydrogel dressings have emerged as a promising new treatment option[21-23]. Intelligent hydrogels are polymeric materials with a unique three-dimensional network structure[23,24]. They can absorb large amounts of water without dissolving, which makes them suitable for wound dressings[23,24]. Their most remarkable feature is their ability to respond to subtle changes in the wound microenvironment, such as pH, temperature, and glucose concentration[25-27]. They can adjust their properties accordingly, acting like precise pharmacists to deliver drugs accurately based on different wound-healing stages[25,26]. This not only maximizes the therapeutic effect but also minimizes side effects[25,26]. Additionally, they can maintain the optimal moisture balance at the wound site and monitor the wound status in real time, providing crucial information for healthcare providers to adjust treatment plans[27,28]. This intelligent response mechanism helps create a favorable environment for wound healing, promotes cell proliferation, tissue regeneration, and infection prevention, and ultimately improves the prognosis and quality of life of diabetic patients[3,29,30].
This review aims to comprehensively summarize the application progress of intelligent hydrogel dressings in diabetic wound treatment. We will analyze their action mechanisms, different types, and the advantages and challenges in clinical applications (Figure 1). By synthesizing relevant research, we hope to offer useful insights for researchers and healthcare professionals, aiming to promote further research and clinical application of these dressings, and ultimately improve the treatment of diabetic wounds.
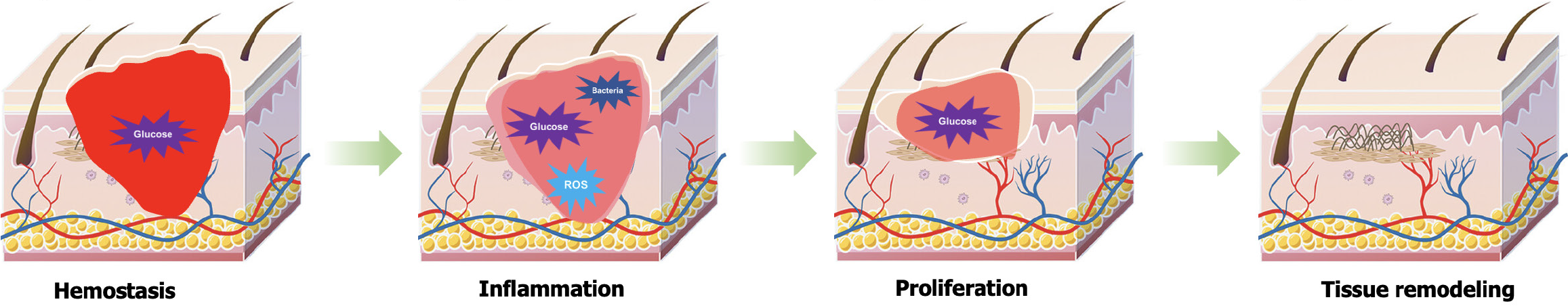
The hyperglycemic environment induced by diabetes significantly disrupts the normal physiological process of wound healing, exerting profound and complex negative impacts on wound repair. Normal wound healing is a highly precise and orderly biological process that encompasses four consecutive stages: Hemostasis, inflammation, proliferation, and tissue remodeling (Figure 2). These stages are intricately interconnected, requiring precise regulation and collaboration among various cell types (such as platelets, macrophages, fibroblasts, and endothelial cells), multiple growth factors [such as platelet-derived growth factor, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β)], and components of the extracellular matrix (ECM)[16,31]. However, the persistent hyperglycemic state in diabetic patients acts like a "tumor", triggering a series of chain reactions that severely disrupt this delicate balance.
During the hemostasis phase, platelet function is primarily affected. Under normal conditions, platelets rapidly aggregate and form clots to effectively stop bleeding upon vascular injury[32,33]. However, the hyperglycemic environment interferes with the normal physiological functions of platelets, impairing their aggregation and adhesion capabilities, which in turn affects the smooth progression of the coagulation process[34]. Studies have shown that hyperglycemia can lead to abnormal functioning of platelet surface receptors and disordered intraplatelet signaling pathways, thereby weakening the clotting activity of platelets and significantly compromising the initial hemostatic effect at the wound site. In the inflammatory phase, macrophage function undergoes significant abnormalities under the "erosion" of hyperglycemia[35,36]. As an essential component of the immune system, macrophages play a crucial role in clearing pathogens and necrotic tissues, and regulating inflammatory responses during wound healing. However, the hyperglycemic environment in diabetic patients impairs their functionality, preventing timely and effective clearance of pathogens and necrotic tissues at the wound site, leading to prolonged inflammatory responses. More critically, the excessive release of pro-inflammatory cytokines [such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α)] creates a vicious cycle, further exacerbating the inflammatory response and imposing more severe obstacles to wound healing[37,38].
As the wound healing process progresses into the proliferative stage, a series of issues triggered by diabetes persistently follow. Hyperglycemia leads to severe dysregulation in the secretion of growth factors, significantly inhibiting the proliferation and migration of fibroblasts and endothelial cells[3,39]. Fibroblasts, responsible for synthesizing collagen and other ECM components, see their proliferation and migration hampered, directly affecting the renewal and repair of ECM at the wound site. Concurrently, the decreased proliferation and migration of endothelial cells slow down neovascularization, resulting in inadequate oxygen and nutrient supply to the wound area, thus greatly hindering the tissue repair process[40]. Furthermore, hyperglycemia severely disrupts the balance between synthesis and degradation of the ECM. Normally, the ECM provides vital structural support to cells and undergoes dynamic remodeling during wound healing[41]. However, excessively high glucose levels in diabetic patients alter the expression and activity of ECM-related proteins, reducing collagen deposition and compromising the structural integrity and stability of the ECM. This disruption affects the structural support and tissue remodeling required for wound healing.
These abnormal changes caused by diabetes intertwine and influence each other, forming a tightly woven "web" that makes diabetic wounds difficult to heal naturally, presenting significant challenges for clinical treatment[42,43]. Therefore, a deep understanding of the mechanisms by which diabetes affects the wound healing process is crucial for developing targeted therapeutic strategies and novel wound dressings.
Natural polymer hydrogel dressings have attracted significant attention due to their unique properties. Chitosan and hyaluronic acid, as the main components, exhibit excellent characteristics and remarkable therapeutic potential. Chitosan, a natural polysaccharide extracted from abundant biomass resources such as crab shells and shrimp shells, contains cationic amine groups in its structure. This feature enables it to interact electrostatically with the negatively charged phospholipids on the bacterial cell membrane. This interaction disrupts the integrity of the bacterial cell membrane, leading to membrane decomposition and cell disintegration, thereby effectively inhibiting bacterial growth[44]. Additionally, chitosan can bind to the essential metal ions required for bacterial survival, interfering with their normal metabolic processes and further enhancing its antibacterial effect. Moreover, chitosan also inhibits bacterial mRNA synthesis and protein synthesis, fundamentally impeding bacterial proliferation and exerting its antibacterial efficacy in multiple dimensions. The antibacterial activity of chitosan is closely related to its molecular structure. Low molecular weight chitosan has smaller molecular chains, which possess better mobility, attraction, and ionic interaction capabilities, allowing it to bind more effectively to the bacterial cell membrane and exhibit stronger antibacterial activity. Meanwhile, a lower degree of acetylation also contributes to the improved antibacterial performance of chitosan[45,46].
In addition to its direct antibacterial effect, chitosan also plays a crucial role as an inflammation regulator during the healing process of diabetic wounds. In the inflammatory response, the activity of immune cells and the balance of cytokines are vital for wound healing[47]. Chitosan can stimulate immune cells to release a variety of inflammatory cytokines, chemokines, growth factors, and bioactive lipids. Studies have shown that in the diabetic wound environment, chitosan can significantly enhance the polarization of macrophages, promoting the transformation of macrophages from the pro-inflammatory phenotype (M1 type) to the anti-inflammatory phenotype (M2 type). M2 macrophages secrete more anti-inflammatory cytokines, such as IL-4 and TGF-β1, which can effectively suppress the inflammatory response, reduce the adverse effects of inflammation on wound healing, and promote tissue repair and regeneration[48,49]. Hyaluronic acid, as a key component of the ECM, plays a unique role in wound healing with its excellent hydrophilicity and water retention properties. Its molecular structure enables it to absorb and retain a large amount of water, creating a moist microenvironment at the wound site. This moist environment is crucial for cell migration and proliferation, as it provides a favorable medium for cell movement and division. Research indicates that hyaluronic acid binds to specific receptors on the cell surface, activating a series of intracellular signaling pathways, thereby promoting the proliferation and migration of various cells, such as fibroblasts and keratinocytes. Fibroblasts are responsible for synthesizing ECM components, such as collagen, during the wound healing process, while keratinocytes participate in epithelial regeneration. The promoting effect of hyaluronic acid on these cells contributes to the acceleration of new tissue formation, filling the wound defect and facilitating the wound healing process[50,51].
In addition to chitosan and hyaluronic acid, other natural polymers such as alginate and collagen also play important roles in the preparation of hydrogel dressings. Alginate has excellent water absorption properties and can quickly absorb wound exudate, forming a gel-like substance that provides a physical barrier to prevent the invasion of external bacteria and maintain a moist environment at the wound, which is conducive to cell growth and tissue repair[52]. Collagen, as the main structural protein of the skin, provides an ideal adhesion scaffold for cells. During the wound healing process, cells can attach to the collagen fibers, proliferate, differentiate, and migrate, orderly constructing new tissue. Collagen can also interact with other ECM components to regulate cell behavior and promote the smooth progress of each stage of wound healing.
In addition to natural polymers, synthetic polymer hydrogel dressings play a crucial role. Polyacrylic acid (PAA), poly(N-isopropylacrylamide) (PNIPAM), polyacrylamide (PAM), polylactic acid (PLA), and polyvinyl alcohol (PVA), as well as their modified polymers, have attracted significant attention due to their unique characteristics, offering various possibilities to meet the diverse needs of diabetic wound treatment. PAA exhibits excellent water absorption capacity. The carboxyl groups on its molecular chains can interact with water molecules through hydrogen bonds, enabling it to absorb and retain a large amount of water. This high water-absorbing property allows PAA hydrogels to effectively absorb wound exudate in diabetic wound treatment, maintaining a moist environment at the wound site and promoting cell migration and proliferation. Through chemical modification, such as the introduction of other functional groups, its physicochemical properties can be further regulated. For example, by incorporating certain antibacterial groups into the PAA molecular chain, a hydrogel dressing with both antibacterial and water-absorbing properties can be prepared, effectively preventing wound infection and accelerating wound healing.
PNIPAM is a temperature-sensitive polymer with a unique lower critical solution temperature (LCST). Below the LCST, the PNIPAM molecular chains are in an extended state, and the hydrogel swells; when the temperature rises above the LCST, the molecular chains rapidly contract, leading to a phase transition of the hydrogel, with a decrease in volume and the release of the encapsulated substances. This temperature-sensitive property can be utilized in a drug controlled-release system for diabetic wound treatment. At normal body temperature, the drug-loaded PNIPAM hydrogel remains swollen, and the drug is encapsulated within it; when the local temperature at the wound increases due to inflammation or other reasons, the hydrogel contracts, accelerating the drug release rate, thus achieving intelligent drug release and enhancing the therapeutic effect. Additionally, modifying PNIPAM, such as by blending or grafting with other biocompatible polymers, can improve its biocompatibility and mechanical properties, making it more suitable for diabetic wound dressings. PAM has good water solubility and chemical stability. The amide groups on its molecular chains can form hydrogen bonds, endowing the hydrogel with a certain degree of strength and toughness. In diabetic wound treatment, PAM hydrogels can serve as drug carriers, loading drug molecules through physical adsorption or chemical bonding. By modifying PAM, such as introducing cationic or anionic groups, the interaction between the hydrogel and drug molecules or wound tissue can be enhanced. For instance, after introducing cationic groups, the PAM hydrogel can interact with the negatively charged bacterial cell membrane, exerting an antibacterial effect and simultaneously promoting cell adhesion, which is beneficial for tissue regeneration during the wound healing process.
PLA is a biodegradable polyester with good biocompatibility and biodegradability. In diabetic wound treatment, PLA hydrogels can gradually degrade during the wound healing process, eliminating the need for a second surgical removal. The degradation product of PLA, lactic acid, is non-toxic to the human body and has a certain anti-inflammatory effect, which can reduce the inflammatory response at the wound. By copolymerization or blending modification methods, the degradation rate and physical properties of PLA can be adjusted. For example, blending with other flexible polymers can improve the flexibility of PLA hydrogels, enabling them to better conform to the wound surface and provide a suitable healing environment for the wound, promoting cell growth and tissue repair. PVA is a water-soluble polymer with good film-forming ability and biocompatibility. The hydroxyl groups on its molecular chains can form hydrogen bonds, giving the hydrogel a certain mechanical strength. In diabetic wound treatment, PVA hydrogels can act as a physical barrier, preventing external bacteria from invading the wound while maintaining wound moisture. Modifying PVA through chemical cross-linking or physical blending can further enhance its properties. For example, chemically cross-linked PVA hydrogels have better stability and anti-swelling properties, allowing them to maintain their structural integrity at the wound site for an extended period; blending with other bioactive substances, such as antibacterial agents or growth factors, can produce multifunctional hydrogel dressings with enhanced antibacterial and wound-healing capabilities.
The hydrogel and nanomaterial composite dressings have emerged as a highly promising therapeutic approach. By combining nanomaterials (such as silver nanoparticles, gold nanoparticles, and zinc oxide nanoparticles) with hydrogels, not only are the antibacterial properties of the dressings significantly enhanced, but also a variety of other functions are imparted, creating more favorable conditions for the healing of diabetic wounds. Nanomaterials exhibit excellent antibacterial activity due to their unique small-size effect and surface effect. Silver nanoparticles, as a commonly used nano-antibacterial material, mainly exert their antibacterial mechanism based on the release of silver ions. These silver ions can interact with sulfur-and nitrogen-containing biomolecules within bacterial cells, interfering with the bacterial respiratory enzyme system and disrupting the bacterial cell membrane structure, thereby leading to bacterial death. Research has shown that silver nanoparticles have a significant inhibitory and bactericidal effect on various common pathogenic bacteria in diabetic wounds, such as Staphylococcus aureus and Escherichia coli. When combined with hydrogels, silver nanoparticles can be uniformly dispersed in the hydrogel network. As the hydrogel comes into contact with the wound, silver ions are slowly released, continuously exerting antibacterial effects and effectively reducing the risk of wound infection.
Gold nanoparticles also possess certain antibacterial properties, and their antibacterial mechanism may be related to the surface plasmon resonance effect. When gold nanoparticles are irradiated with a specific wavelength of light, a local enhanced electromagnetic field effect is generated, which can disrupt the bacterial cell membrane and inhibit bacterial growth[53]. In addition, gold nanoparticles have good biocompatibility and chemical stability, and can serve as drug carriers or binding sites for bioactive molecules in composite hydrogels, further expanding the functions of the composite dressings. Zinc oxide nanoparticles are also an attractive antibacterial nanomaterial. They can release zinc ions, which can bind to the phospholipids on the bacterial cell membrane, changing the permeability of the membrane and causing the leakage of bacterial contents, thus achieving antibacterial purposes[54]. At the same time, zinc oxide nanoparticles also have a certain photocatalytic activity. Under light irradiation conditions, they can generate reactive oxygen species with strong oxidizing properties, such as hydroxyl radicals and superoxide anions, which can further enhance the bactericidal effect. By incorporating zinc oxide nanoparticles into hydrogels, photo-responsive antibacterial composite dressings can be prepared, which have potential application value in diabetic wound treatment. In addition to the strong antibacterial properties, the combination of nanomaterials and hydrogels also has a positive impact on the physical properties of hydrogels. In terms of mechanical strength, the addition of nanomaterials can enhance the mechanical properties of hydrogels. For example, some nanoparticles can interact with the polymer chains of hydrogels, forming physical cross-linking points or chemical cross-linking bonds, thereby improving the tensile and compressive mechanical properties of hydrogels. This enables the composite hydrogel dressings to better maintain their shape and structural integrity during use, be less prone to rupture or deformation, and effectively conform to the wound surface, providing stable protection and support for the wound[55].
In terms of conductivity, the introduction of certain nanomaterials (such as conductive nanoparticles or nanowires) can endow hydrogels with a certain conductivity. During the healing process of diabetic wounds, conductive hydrogels can mimic the bioelectrical environment of the human body, facilitating signal transduction and substance exchange between cells. Studies have found that appropriate electrical stimulation can promote the proliferation, migration, and collagen synthesis of fibroblasts, accelerate angiogenesis, and thus improve the healing speed and quality of wounds[56]. In addition, conductive hydrogels can be combined with external electrical stimulation devices to achieve precise regulation of the wound healing process, providing new ideas and methods for diabetic wound treatment.
To form a stable three-dimensional network structure, physical cross-linking and chemical cross-linking are the two main methods. Physical cross-linking usually depends on relatively weak interactions such as hydrogen bonds and van der Waals forces. This cross-linking method is relatively mild and can better preserve the original properties of the polymer, but the stability of the hydrogel may be relatively low. For example, a physically cross-linked PVA hydrogel prepared by the freeze-thaw cycle method forms ice crystals at low temperatures, and the pores left after the ice crystals melt and the entanglement between polymer chains constitute a three-dimensional network structure[57]. This hydrogel has certain elasticity and water absorption, but may experience excessive swelling or even dissolution in a high-concentration solution. Chemical cross-linking connects polymer chains together through covalent bonds to form a more stable network structure, endowing the hydrogel with higher stability and strength. Common chemical cross-linking agents include glutaraldehyde, genipin, and some compounds containing double bonds, etc. They can react with the active groups on the polymer to form stable chemical bonds. However, the chemical cross-linking process requires strict control of the amount of cross-linking agent and reaction conditions to avoid excessive cross-linking that leads to an increase in the brittleness of the hydrogel, affecting its practical effect in wound dressing applications[58].
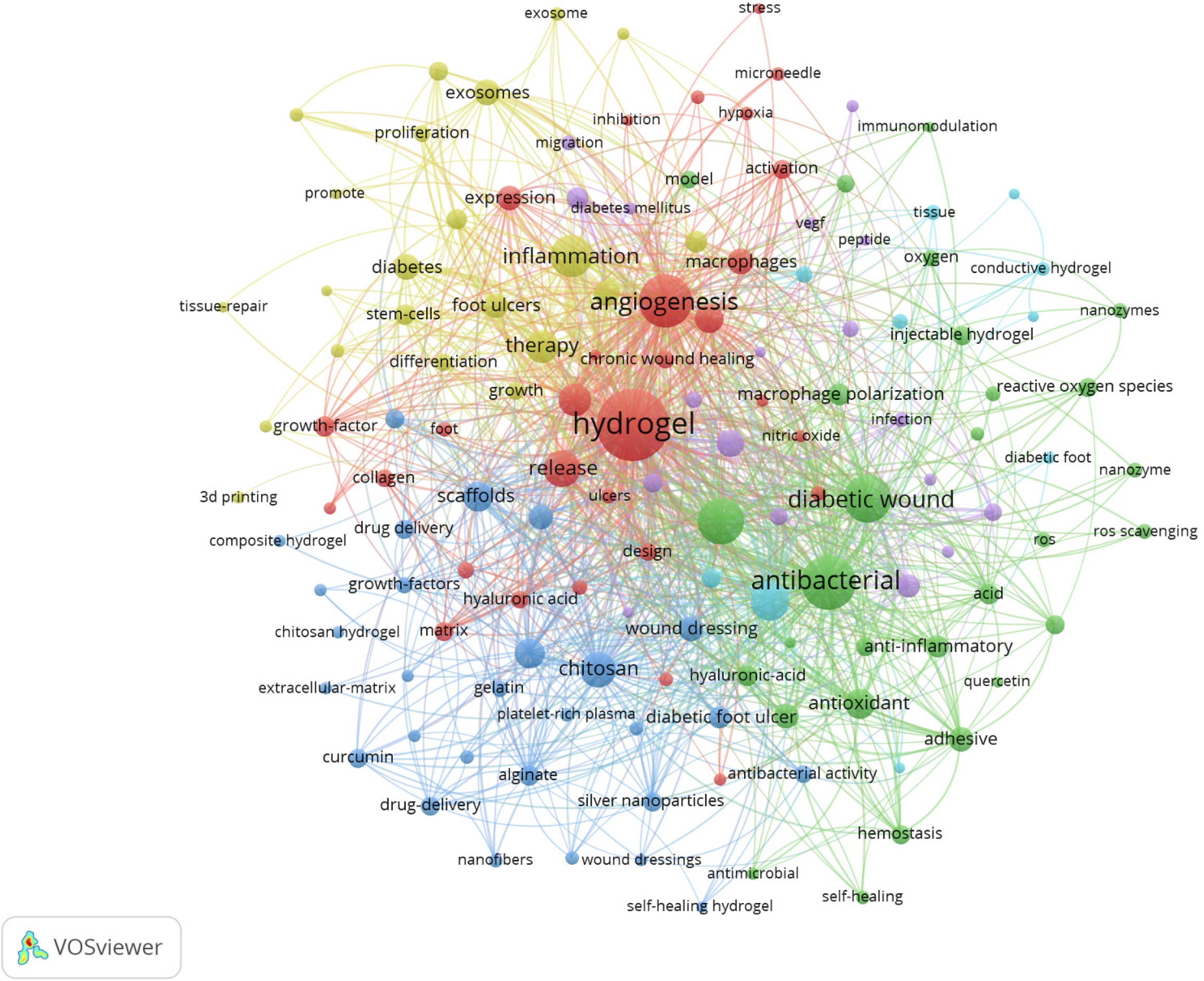
To further illustrate the significance and broad research scope of hydrogels in diabetic wound treatment, a VOS map is generated from the analysis of 1043 papers using the keywords “hydrogel” and “diabetic wound” (Figure 3). This map visually presents the knowledge structure of related research and is of crucial significance for organizing and comprehending the research status in this complex field. In the map, nodes represent keywords, with the size of the nodes being directly proportional to the frequency of keyword occurrence. That is, the higher the frequency, the larger the node. Lines between nodes denote the co-occurrence relationship of keywords, and the thicker the line, the stronger the co-occurrence intensity. The prominent “hydrogel” node, which is centrally located and relatively large in size, underlines the central position of hydrogels in the research of diabetic wound treatment. Surrounding “hydrogel”, a multitude of closely related keywords form a complex yet orderly network. For instance, keywords such as “diabetic wound”, “wound dressing”, and “drug delivery” are directly connected to hydrogel, clearly revealing the application directions of hydrogels in diabetic wound treatment, mainly serving as wound dressings and drug carriers. Keywords like “antibacterial”, “anti-inflammatory”, “angiogenesis”, and “tissue regeneration” reflect the core functional mechanisms of hydrogels in promoting the healing of diabetic wounds, indicating that the research focuses on how hydrogels can improve the wound microenvironment damaged by diabetes through these functions, thereby facilitating the healing process. Meanwhile, the connections between “nanoparticles”, “nanocomposites”, and the hydrogel node illustrate the research trend of combining nanomaterials with hydrogels to enhance performance. For example, nanoparticles such as silver nanoparticles can significantly enhance the antibacterial ability of hydrogels. Keywords like “growth factors” and “cytokines” reflect the promoting effect of combining bioactive components with hydrogels on wound healing, as growth factors can effectively stimulate cell proliferation and angiogenesis. In addition, the presence of nodes representing special types of hydrogels such as “self-healing hydrogel” and “conductive hydrogel” showcases the exploration directions of new hydrogel materials in diabetic wound treatment.
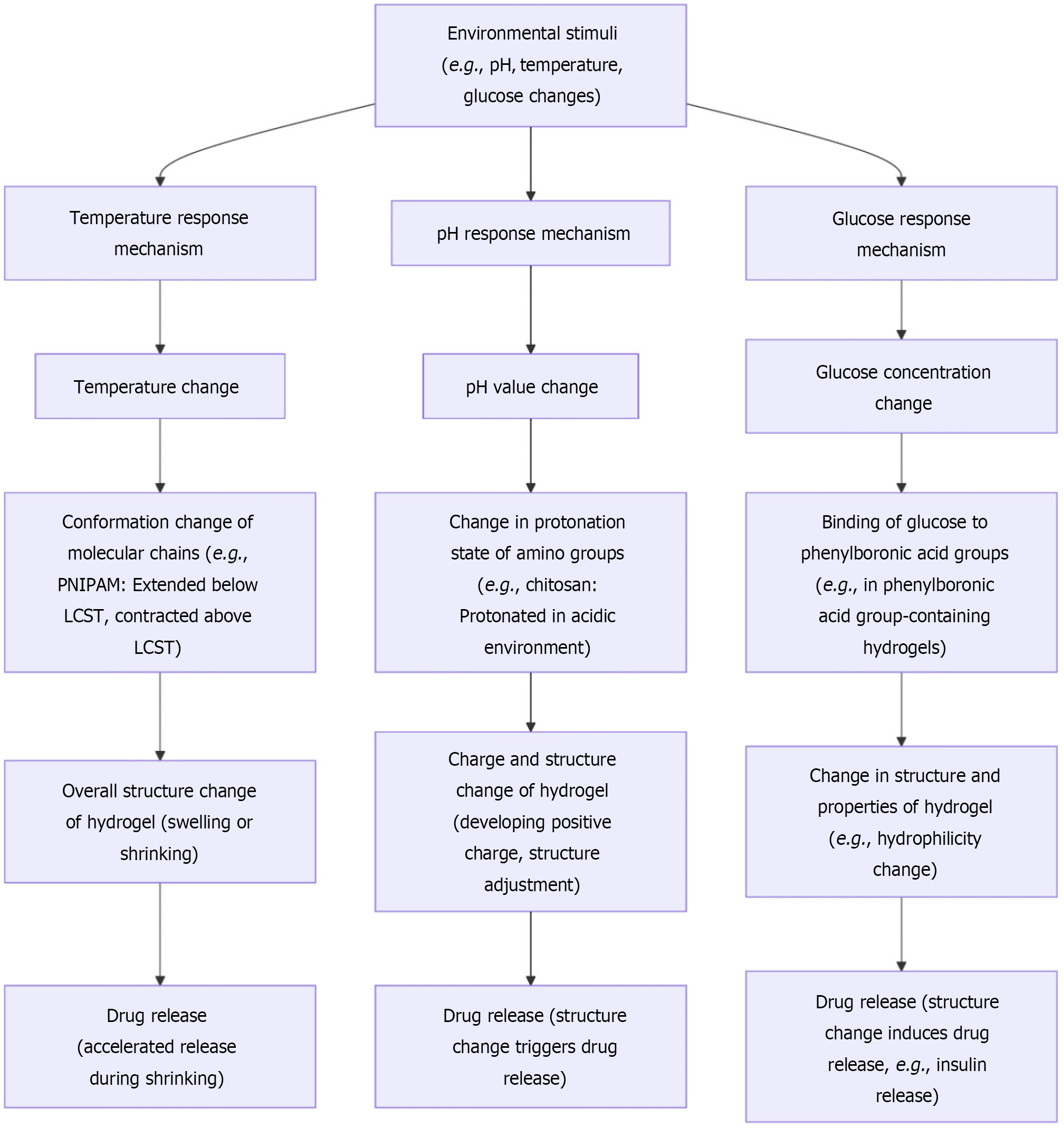
Hydrogel-based intelligent dressings are capable of responding to a variety of external stimuli, endowing them with unique advantages in the treatment of diabetic wounds. The detailed mechanisms of these responses are visually presented in Figure 4. Regarding temperature responsiveness, certain hydrogels exhibit thermosensitive properties. For instance, PNIPAM-based hydrogels undergo a phase transition near a critical temperature. When the ambient temperature is below the critical temperature, the hydrogel is in a swollen state, capable of absorbing a large amount of water; conversely, when the temperature rises above the critical temperature, the hydrogel rapidly shrinks and expels water. This temperature-responsive characteristic can be exploited for controlled drug release. In the treatment of diabetic wounds, drugs can be encapsulated within the hydrogel[59,60]. When the local temperature of the wound increases (such as due to the inflammatory response), the hydrogel contracts and releases the drug, achieving on-demand drug delivery. This mechanism not only helps to increase the drug concentration at the wound site, enhancing the therapeutic effect, but also reduces the distribution of the drug in normal tissues, minimizing side effects.
pH responsiveness is another crucial mechanism of hydrogel intelligence. Chitosan hydrogels possess different charge states under varying pH conditions. In an acidic environment (which is often present in diabetic wounds), the amino groups of chitosan become protonated, endowing it with a positive charge. This leads to alterations in the hydrogel structure, such as changes in swelling degree or the formation of specific pore structures, thereby triggering drug release[61]. For example, some antibiotic-loaded chitosan hydrogels release antibiotics in the acidic wound environment, effectively inhibiting bacterial growth. Simultaneously, pH changes can also influence the interaction between the hydrogel and cells, promoting cell adhesion, proliferation, and other processes beneficial for wound healing. Glucose-responsive hydrogels offer a precise means of regulating blood glucose levels in the treatment of diabetic wounds[62]. These hydrogels typically contain groups that can specifically bind to glucose, such as phenylboronic acid groups. In a hyperglycemic environment, glucose binds to the phenylboronic acid groups in the hydrogel, inducing changes in the hydrogel's structure and properties, which in turn leads to the release of insulin or other hypoglycemic drugs. This not only helps to maintain local blood glucose balance at the wound site, reducing the adverse effects of hyperglycemia on wound healing, but also avoids the risk of systemic hypoglycemia. For example, insulin encapsulated in a glucose-responsive hydrogel is released when the glucose concentration at the wound increases, lowering the local blood glucose level, promoting cellular glucose uptake and utilization, and accelerating wound healing. Light-responsive hydrogels utilize light of specific wavelengths as a stimulus to control drug release or modulate hydrogel properties. Upon exposure to light, the photosensitive groups within the hydrogel undergo isomerization or chemical reactions, resulting in changes to the hydrogel structure[63]. For instance, hydrogels containing azobenzene groups experience isomerization of the azobenzene molecules under ultraviolet light irradiation, altering the hydrophilicity or spatial structure of the hydrogel and triggering drug release. This light-controlled release mechanism offers the advantages of non-invasiveness and remote manipulation. In the treatment of diabetic wounds, external light irradiation can be used to precisely control the release time and dosage of drugs at the wound site, enhancing the flexibility and effectiveness of treatment.
Intelligent hydrogel dressings have emerged as a promising solution for managing chronic diabetic wounds. To provide a comprehensive overview of how intelligent hydrogel dressings are applied in diabetic wound care, Table 1 summarizes key applications and highlights specific examples of these innovative materials in action[64-88]. In addition, many gel products for treating diabetic wounds have been tested in clinical trials. For example, the Fitostimoline hydrogel, which has been tested in phase 4 clinical trials for diabetic foot treatment, has demonstrated its potential in promoting wound healing. Additionally, the SANTYL hydrogel, used for DFUs and diabetic foot wounds, has also been tested in phase 4 clinical trials successfully. These advanced materials are engineered to respond to changes in the wound microenvironment, providing targeted therapy and real-time monitoring capabilities that enhance the healing process.
| Components | Structural features | Intelligent responsiveness mechanisms | Application advantages | Ref. |
| Using GelMA and chitosan methacrylate (CMCSMA) as the GACo MPNs and phenol red | Double cross-linked network structure, cross-linked by photoinitiation, with good injectability, shape adaptability, and mechanical strength | pH response, visual pH monitoring | Antibacterial, anti-inflammatory, angiogenic, real-time pH monitoring | [64] |
| Based on LAMC, loaded with CDs, ceria oxide-molybdenum disulfide nanoparticles (C@M) and PDA coating (C@M@P) | LAMC is formed by amidation reaction, and nanoparticles are loaded by physical blending, with an interconnected porous structure | pH response, temperature response (photothermal property) | Antibacterial, antioxidant, anti-inflammatory, real-time pH and temperature monitoring | [65] |
| Composed of different modules, including glucose-responsive hydrogel, pH-responsive hydrogel, and temperature-responsive hydrogel | The glucose-responsive hydrogel is formed by radical polymerization and hydrogen bonding, and combined with the PC structure | Glucose response, pH response, temperature response | Real-time multi-biomarker monitoring (glucose, pH, temperature) | [17] |
| OHA, borax, gelatin, GOx, Cu2-xSe-BSA nanozyme | Form a network structure through Schiff base bond and borate ester bond dual dynamic crosslinking | Glucose-responsive, triggered by acidic environment | Generate antibacterial substances, regulate immune microenvironment, and promote angiogenesis | [66] |
| ε-PL, SilMA, TA, Cu²+, Zn²+ | Form a concentric circular structure with dual-drug delivery system and different release kinetics through MPN | Cu@TA is released first, followed by Zn@TA | Exert antibacterial, antioxidant, immunomodulatory, and angiogenic effects in sequence | [67] |
| N-Carboxyethyl chitosan-grafted-phenylboronic acid, oxidized dextran, ε-polylysine-coated manganese dioxide, β-cyclodextrin-reduced graphene oxide/N,N-di-sec-butyl-N,N-dinitroso-1,4-phenylenediamine | Construct a network through Schiff base reaction and phenylboronate ester bond, with porous structure | Responsive to NIR and glucose stimulation, release NO and O2 | Conductive, self-healing, antibacterial, antioxidant, and promote wound healing | [68] |
| Hyaluronic acid, pectin, MnCoO@ε-PL, PTA | Dynamic acylhydrazone and imine bonds, physical cross-linking | pH and ROS dual-responsive | Antibacterial, antioxidant, promote cell survival and proliferation, accelerate wound healing | [69] |
| Polyacrylamide, polydopamine-doped polypyrrole | Incorporation of conductive polymer nanofibrils | Electro-responsive | Real-time monitoring, on-demand drug delivery, antibacterial, moist and visible environment, promote wound healing | [70] |
| Heparin, ultrasmall nanozymes | Incorporation of metal nanoparticles | Enzyme-responsive release of metal ions | Inhibit macrophage-driven inflammation, scavenge ROS, accelerate wound healing | [71] |
| OPLL, CMCS, GOx, exosomes | Crosslinked by Schiff base covalent bond | pH-responsive drug release | Regulate microenvironment, promote angiogenesis | [72] |
| CMCS, oligoprocyanidins, oxide dextran, deferoxamine | Crosslinked by imine and hydrogen bonding | pH-triggered drug release | Antioxidant, anti-inflammatory, promote angiogenesis | [73] |
| GelMA, cerium dioxide loaded with taurine (@Tau) | Microneedle structure | N/A | Antioxidant, anti-inflammatory, anti-aging | [74] |
| Gelatin hydrogel micropattern and gelatin electrospun membrane | Gelatin hydrogel micropattern is photocrosslinked and further crosslinked with genipin, embedded in gelatin electrospun membrane | N/A | Good integration with regenerated tissue, protect ADSCs, promote DW healing | [75] |
| HA-PBA-ALD, PVA, ADH, PBNPs, VEGF | Formed by crosslinking HA-PBA-ALD with PVA/ADH through boronate ester bonds and acylhydrazone bonds, PBNPs and VEGF are physically loaded | Responsive to inflammation (pH and ROS-sensitive dynamic covalent bonds) | Controlled release, anti-inflammatory, antioxidant, promote angiogenesis, accelerate wound healing | [76] |
| PLL-grafted Glu, HA, AAm, TPA, UB | The inner layer is formed by crosslinking PLL-Glu with TPA, the middle layer is a double-network structure of PLL, HA, and UB, the outer layer is crosslinked PLL and AAm | ROS-responsive (degradation of the ROS-responsive layer in the presence of ROS) | Good biocompatibility, adhesion, mechanical strength, antioxidative action, promote angiogenesis, reduce senescence and ROS production, accelerate wound closure | [77] |
| HA, PBA, tea polyphenol-stabilized silver nanoparticles (TP@Ag nps) | Crosslinked via borate ester bonds between PBA and HA, with TP@Ag NPs incorporated | Glucose-responsive degradation and release of TP@Ag NPs | Antioxidant, antibacterial, anti-inflammatory, accelerated wound healing | [78] |
| Chitosan, glycidyl trimethylammonium chloride, dextran, sodium Periodate, 1,8-dihydroxynaphthalene, aniline, ammonium persulfate, curcumin | Schiff base reaction between oxidized dextran and quaternized chitosan grafted polyaniline | NIR responsive release of curcumin | Antioxidant, antibacterial, promote nerve regeneration and modulate neuro-immune microenvironment | [79] |
| CMC, precoordinated europium-ethylenediaminetetraacetic acid complexes | Crosslinked by metal-carboxyl coordination interaction | pH-responsive fluorescence for wound state monitoring | Promote angiogenesis, excellent biocompatibility, self-healing | [80] |
| Xanthan gum, sodium alginate, HBD peptide nanoparticles, silver ions | Crosslinked with calcium ions and silver ions, forming a 3D dual-network skeleton structure | Ultrasound-responsive, releasing drugs and degrading hydrogel under ultrasound stimulation | Antibacterial, antioxidant, promoting angiogenesis, achieving deep wound penetration | [18] |
| Thiolated hyaluronic acid, methacrylate gelatin, poly(L-lactic acid) nanoparticles loaded with TGF-β receptor antagonists | Interpenetrating hydrogel scaffold, photocrosslinked | Regulating macrophage polarization and inhibiting scar formation in different stages | Modulating immune microenvironment, remodeling skin tissue, preventing scar formation | [81] |
| Astragalus polysaccharide, carboxymethyl chitosan, sodium alginate, PPy-PDA-MnO2 nanoparticles loaded with resveratrol | Crosslinked by Schiff base reaction and hydrogen bond, with multiple-network structure | Conductive, can monitor muscle function | Enhancing wound healing, antioxidant, tissue adhesive, conducting electromyography monitoring | [82] |
| CMC, TA, and quercetin-loaded zeolitic imidazolate framework-8 nanoparticles modified with HA | Crosslinked by dynamic ionic bonds and hydrogen bonds | N/A | Antibacterial, anti-oxidative stress, anti-apoptosis, angiogenesis promotion, ER stress relief | [83] |
| PVA and BPNS | Doped with BPNS and modified with NaCl | NIR light-responsive photothermal conversion | Electrical stimulation, photothermal effect for antibacterial and wound healing acceleration | [84] |
| O-CMCS, gelatin methacryloyl, and spermidine | Double network with dynamic imine bonds and non-dynamic photo-crosslinked bonds | N/A | Reduce inflammation, promote macrophage polarization, enhance acute and diabetic wound healing | [83] |
| Chitosan nanoparticles, carboxymethyl chitosan, bioactive glass, titanium dioxide, MSC-derived exosomes | Chitosan nanoparticles encapsulate exosomes, and bioactive glass and titanium dioxide are loaded in carboxymethyl chitosan hydrogel | The dressing continuously releases bioactive substances to enhance angiogenesis and collagen deposition | Antibacterial, anti-inflammatory, angiogenic, promotes collagen deposition | [85] |
| Catechol-functionalized chitosan, acrylic acid, catechol functional methacryloyl chitosan-silver nanoparticles, vanillin | Synthesized by one-step thermal polymerization, with aldehyde and phenolic hydroxyl groups forming crosslinks | N/A | Antibacterial, antioxidant, anti-inflammatory, regulates macrophage polarization | [86] |
| Polyacrylamide, sodium alginate, Chlorella | Chlorella is loaded in a semi-interpenetrating network formed by crosslinking acrylamide with alginate chains dispersed | Chlorella photosynthesizes to produce oxygen and bioelectricity in response to light | Produces oxygen and bioelectricity, antibacterial, promotes cell proliferation and migration | [87] |
| Laponite, poly (acrylic acid), LL37, NanoFlares | Laponite and poly (acrylic acid) form hydrogel matrix, incorporating LL37 and NanoFlares | NanoFlares detect and quantify mRNA biomarkers for real-time wound monitoring | Antimicrobial, monitors wound healing status in real time | [88] |
From the perspective of physical structure, the unique three-dimensional network structure of hydrogels is crucial for preventing bacterial invasion. The pore size within the hydrogel acts as a natural physical barrier, similar to a fine-mesh sieve, effectively blocking the entry path of bacteria. As detailed in the research by Liu et al[89] and others, certain intelligent hydrogels possess a special topological microstructure that makes it difficult for bacteria to adhere and colonize on their surface. This physical barrier function not only hinders the invasion of external bacteria but also limits the contact between the wound and harmful substances in the external environment to some extent, creating a relatively safe microenvironment for the wound. The most prominent advantage of intelligent hydrogel dressings lies in their intelligent response mechanism, which enables precise control of the release of antibacterial drugs based on the real-time status of wound infections, significantly enhancing the antibacterial effect[90]. In the early stage of wound infection, when the number of bacteria begins to increase and the inflammatory response intensifies, the hydrogel can sensitively detect these changes and rapidly initiate the drug release mechanism. For example, some hydrogels contain functional groups that are sensitive to inflammation-related biomarkers. When the concentration of these biomarkers rises, it triggers changes in the internal structure of the hydrogel, thereby accelerating the release of antibacterial drugs. This precise drug release can promptly inhibit the growth and reproduction of bacteria in the early stage of infection, effectively controlling the spread of the infection. When the wound infection is effectively controlled and the inflammatory response gradually subsides, the drug release rate of the hydrogel automatically adjusts according to the environmental changes, avoiding excessive drug release and reducing potential side effects, such as the development of drug resistance and unnecessary damage to normal tissues.
The realization of this intelligent response mechanism depends on the high sensitivity of hydrogels to changes in various factors in the wound microenvironment. Some intelligent hydrogels can accurately respond to changes in pH value in the wound microenvironment[91]. During an infection-induced inflammatory response, the local pH value of the wound decreases. Specific components in the hydrogel (such as pH-sensitive polymers) can sense this change, triggering alterations in their own structure or chemical properties, and thus accelerating the release of antibacterial drugs. In addition, changes in temperature and enzyme concentration can also be detected by certain intelligent hydrogels. For instance, some hydrogels contain temperature-sensitive components. When the local temperature of the wound rises due to the inflammatory response, these temperature-sensitive components undergo phase transition or structural adjustment, which in turn affects the drug release rate[92]. Changes in enzyme concentration can also serve as a signal to trigger the response of the hydrogel. When the activity of enzymes related to inflammation or infection at the wound site increases, the enzyme-responsive groups in the hydrogel react specifically with them, triggering the drug release mechanism. A notable example is the study by Shang et al[93] who synthesized a composite hydrogel dressing (CMCS-CEBT) composed of mesenchymal stem cell-derived exosomes, chitosan nanoparticles, bioactive glass, and titanium dioxide. The experimental results showed that this dressing could accelerate the healing process of full-thickness skin defects, diabetic wounds, and burn skin injuries, stimulate angiogenesis, and increase collagen deposition and the expression of anti-inflammatory factors. It exhibited good cell compatibility in vitro, could stimulate endothelial cell adhesion and proliferation, and had anti-inflammatory, angiogenic, and antibacterial activities. Another recent study by Zhang et al[94] designed a novel self-healing conductive hydrogel (PEG/Ag/CNT-M + E hydrogel). They crosslinked four-armed SH-PEG with Ag+ and coordinated Ag-S to produce a dynamic PEG hydrogel. Meanwhile, they introduced multiwalled carbon nanotubes with good conductivity to form hydrogen bonds with thiol groups, constructing a stable three-dimensional structure for loading exosomes and metformin. In vivo experiments demonstrated that this hydrogel could promote wound healing, trigger cell proliferation and angiogenesis, and relieve peritraumatic inflammation and vascular injury. The mechanism involved reducing the level of ROS by interfering with mitochondrial fission, thereby protecting F-actin homeostasis and alleviating microvascular dysfunction.
The inflammatory response is a natural defense mechanism of the body against injury. However, in diabetic patients, due to factors such as the hyperglycemic environment, the inflammatory response is often imbalanced, presenting as an excessive and persistent inflammatory state[95]. This excessive inflammation results in the release of a large amount of inflammatory mediators, such as IL-1β and TNF-α, which further damage the surrounding tissues and impede the wound healing process. Smart hydrogel dressings precisely regulate the inflammatory response through multiple pathways. Some smart hydrogel dressings can actively release an appropriate amount of anti-inflammatory factors, such as IL-4 and IL-10, in the early stage of inflammation. These anti-inflammatory factors can inhibit the excessive activation of inflammatory cells and reduce the release of inflammatory mediators, thereby alleviating the damage to tissues caused by excessive inflammation activation[96]. In the later stage of inflammation, the functional state of macrophages plays a crucial role in tissue repair. Smart hydrogel dressings can promote the transformation of macrophages into the repair-promoting phenotype (M2 type) through various means. Jiang et al[97] demonstrated that specific bioactive molecules released by some hydrogels can activate the signaling pathways within macrophages, inducing their polarization into M2 macrophages. M2 macrophages have stronger phagocytic and tissue repair-promoting abilities. They can clear cell debris and pathogens at the wound site and secrete various growth factors and ECM components, such as TGF-β and VEGF, accelerating the tissue repair process and promoting collagen deposition and angiogenesis. For instance, Xu et al[98] developed a smart hydrogel that can release extracellular vesicles derived from foreskin mesenchymal stem cells. These vesicles can regulate the polarization state of macrophages, transforming them into M2 macrophages, thereby promoting the healing of wounds in diabetic mice. In the experiment, a significant acceleration in wound healing and a notable reduction in the inflammatory response were observed.
The immunomodulatory effect of smart hydrogel dressings is not limited to the regulation of the inflammatory response but also involves the modulation of the overall activity and function of immune cells. In the diabetic wound environment, immune cell function is abnormal, leading to a decreased ability of the body to resist infection. Meanwhile, excessive immune responses may cause damage to the body's own tissues[99,100]. Smart hydrogel dressings can enhance the ability of immune cells to recognize and eliminate pathogens by regulating the expression of immune cell surface receptors and affecting intracellular signal transduction, thereby improving the body's anti-infection ability. At the same time, they can avoid the autoimmune damage caused by the over-activation of immune cells, ensuring that the wound healing process occurs in a suitable immune environment. At the cellular level, smart hydrogel dressings can regulate the chemotaxis of immune cells, attracting more immune cells to gather at the wound site and enhancing local immune defense[101]. At the molecular level, they can adjust the types and quantities of cytokines and chemokines secreted by immune cells, optimizing the interaction network among immune cells and promoting the restoration of immune balance. For example, Wang et al[102] designed a smart hydrogel that can release specific bioactive molecules in response to the pH, ROS, and glucose levels in the wound microenvironment, regulating the function of immune cells. In a diabetic wound model, it showed good promoting effects on wound healing, with reduced infiltration of inflammatory cells and enhanced angiogenesis and collagen deposition.
Angiogenesis is crucial for the healing of diabetic wounds as it supplies necessary nutrients and oxygen to the wound, while also removing metabolic waste products, and facilitating cell proliferation, migration, and tissue remodeling[103]. In diabetic patients, factors such as hyperglycemia and oxidative stress inhibit the angiogenesis process, resulting in reduced formation of new blood vessels, which severely hinders the normal wound healing process. Smart hydrogel dressings promote angiogenesis through multiple mechanisms, with one key strategy being the release of angiogenesis-related growth factors[104]. For example, VEGF is a potent angiogenic stimulator. Smart hydrogels can encapsulate VEGF and achieve precise release based on the changes in the wound microenvironment. When the hydrogel detects hypoxia or other angiogenic signals at the wound site, it slowly releases VEGF, stimulating the proliferation and migration of endothelial cells and promoting the formation of new blood vessels. Studies have shown that the application of VEGF-containing smart hydrogels in diabetic wound models significantly increased the vascular density at the wound site, improved blood supply, and accelerated the wound healing process[105].
The physical properties of smart hydrogels themselves also provide favorable conditions for angiogenesis and tissue regeneration. Their 3D porous structure, similar to the ECM, offers a suitable environment for cell growth and migration. It allows endothelial cells and other relevant cells to aggregate, proliferate, and interact within it, facilitating the formation of new vascular structures. Hydrogels can also absorb wound exudate, maintaining a moist wound environment, which is essential for cell activity and the tissue regeneration process[106]. A moist environment helps maintain normal cell metabolism, promotes cell migration and proliferation, and is conducive to the diffusion and action of bioactive molecules such as growth factors at the wound site. Some smart hydrogels can also promote angiogenesis and tissue regeneration by regulating cell signaling pathways. For example, by activating specific signaling pathways such as the PI3K/Akt/mTOR pathway, they can upregulate the expression of related genes, thereby promoting the proliferation, migration of endothelial cells, and the formation of vascular lumens. The regulation of these signaling pathways can also enhance the function of fibroblasts, promoting the synthesis and remodeling of the ECM, further driving the tissue regeneration process.
In experimental studies, Wu et al[107] developed a chitosan-based thermosensitive hydrogel loaded with self-assembled multifunctional nanoparticles for antibacterial and angiogenic effects. The nanoparticles named CIZ, composed of chlorogenic acid, indocyanine green, and Zn2+, were incorporated into a chitosan-β-glycerophosphate hydrogel. This formulation enabled rapid gel formation under photothermal effects and demonstrated potent antioxidative and anti-inflammatory effects while enhancing the expression of VEGF and platelet endothelial cell adhesion molecule-1 (CD31), promoting angiogenesis both in vitro and in vivo. Moreover, Deng et al[64] fabricated an on-demand detachable adhesive hydrogel based on dual dynamic covalent cross-linking with near-infrared/pH dual-responsive properties. The GelMA/CMCSMAP-GACo 0.5 hydrogel significantly downregulated the expression of matrix metalloproteinase 2, which is known to degrade ECM proteins. By effectively enhancing ECM deposition, this hydrogel accelerated the healing process of diabetic wounds. Additionally, it was equipped with machine learning-enabled visual monitoring capabilities that allowed for real-time assessment of the wound environment, including pH changes, providing valuable insights into the healing progress.
The real-time monitoring and personalized treatment functions of intelligent hydrogel dressings in the treatment of diabetic wounds have brought new breakthroughs in precision medicine[108]. The healing process of diabetic wounds is complex and variable, with differences existing in the wound conditions of different patients and even in different stages of the same patient. Therefore, it is crucial to monitor the wound status in real time and accurately and implement personalized treatment. The pH value is an important indicator reflecting the changes in the wound microenvironment. The pH-sensitive elements in intelligent hydrogel dressings can accurately monitor it. During the healing process of diabetic wounds, the pH value fluctuates with factors such as the degree of inflammation and cell metabolism. The normal physiological pH range is conducive to the normal function of cells and tissue repair[109,110]. When the pH value deviates from the normal range, it may indicate infection, intensified inflammation, or tissue hypoxia. By monitoring the pH value in real time, the changing trend of the wound microenvironment can be detected in time, providing a basis for adjusting the treatment plan. Humidity monitoring is also crucial, as appropriate humidity is essential for wound healing. Intelligent hydrogel dressings can sense the humidity level of the wound to prevent it from being too dry or too wet. Excessive dryness will hinder cell migration and tissue regeneration, while excessive wetness may increase the risk of infection.
Monitoring of inflammatory markers is another important function of intelligent hydrogel dressings[111]. For example, changes in the levels of inflammatory markers such as C-reactive protein and IL-6 can directly reflect the degree of wound inflammation. By detecting these markers, intelligent hydrogel dressings can provide timely feedback on the inflammatory state, helping medical staff to evaluate the intensity of the inflammatory response during the wound healing process and adjust the anti-inflammatory treatment strategy. For the monitoring of bacterial growth, intelligent hydrogels can use biosensor technology to detect the types and quantities of bacteria at the wound site. Bacterial infection is a common complication in the healing of diabetic wounds. Early and accurate detection of bacterial growth is helpful for timely selection of appropriate antibacterial drugs for treatment to prevent the spread of infection. For example, Sun et al[112] developed an intelligent hydrogel that can monitor the bacterial growth in the wound microenvironment. When an increase in the number of bacteria is detected, it releases antibacterial components, effectively inhibiting bacterial proliferation and creating favorable conditions for wound healing.
The formulation of a personalized treatment plan is a major advantage of intelligent hydrogel dressings. Taking the drug release rate as an example, based on the real-time monitoring data of the wound, if the level of inflammatory markers is high, the intelligent hydrogel dressing can automatically increase the release speed of anti-inflammatory drugs to quickly reduce the inflammatory response; when the inflammation is under control, the drug release rate is gradually reduced to avoid over-treatment[113]. In terms of promoting angiogenesis, if it is detected that the local blood supply of the wound is insufficient, the release amount of angiogenesis-related growth factors can be increased to stimulate the formation of new blood vessels and improve the nutritional supply. In electric field treatment, some intelligent hydrogel dressings can generate an electric field to promote cell migration and tissue regeneration. By monitoring the wound state in real time, the electric field intensity can be precisely adjusted. For wounds with low cell activity in the initial stage of healing, the electric field intensity can be appropriately increased to enhance cell migration ability; as the wound healing process progresses, the electric field intensity is gradually reduced to maintain a level suitable for promoting tissue remodeling[114].
In experimental studies, Hu et al[115] designed a renewable electroconductive hydrogel that not only accelerated diabetic wound healing but also allowed for motion monitoring. This innovative dressing incorporated graphene oxide nanosheets and polyaniline within a PAM matrix, creating an injectable hydrogel with mild photothermal-controlled oxygen release capabilities. The system effectively promoted angiogenesis and enhanced wound healing in diabetic mice by increasing VEGF expression and reducing ROS. Additionally, the hydrogel's electroconductivity enabled it to monitor patient movements, providing valuable data on how physical activity impacts the healing process. Moreover, Jeong et al[88] developed a sprayable hydrogel integrated with optical mRNA nanosensors for real-time monitoring and healing of diabetic wounds. This advanced hydrogel system was capable of dynamically sensing and responding to changes in the wound microenvironment by detecting specific mRNA biomarkers associated with inflammation and tissue repair. The nanosensors provided continuous feedback on the expression levels of key genes involved in wound healing, such as those encoding VEGF and IL-6, enabling precise adjustments in therapy. Importantly, the sprayable nature of this hydrogel facilitated easy application and ensured uniform coverage over irregular wound surfaces, enhancing its practical utility in clinical settings. The combination of real-time molecular diagnostics and therapeutic intervention demonstrated significant improvements in wound closure rates and overall healing quality in diabetic mouse models.
Intelligent hydrogel dressings have demonstrated significant potential in diabetic wound treatment, yet several challenges must be addressed to fully realize their clinical application. One major issue is the limited responsiveness of current dressings to low-concentration key indicators in the wound microenvironment, such as specific inflammatory factors or bacterial toxins. For example, Chen et al[116] reported that certain hydrogels exhibit delayed responses to low-concentration inflammatory factors, hindering timely anti-inflammatory action. Enhancing sensitivity and accuracy requires the development of new responsive materials, optimization of sensor microstructures, and improvements in signal conversion techniques. Long-term stability and biocompatibility are also critical concerns. The complex physiological environment can lead to material degradation, functional decline, or adverse immune reactions, which may compromise the safety and efficacy of the dressings[117,118]. To address this, future research should focus on selecting materials with high stability and biocompatibility, optimizing their chemical composition and physical structure, and thoroughly investigating their long-term interactions with cells and tissues.
Integrating multiple functions, such as antibacterial, anti-inflammatory, and angiogenesis-promoting properties, remains a significant challenge due to material compatibility issues and functional interference. Developing new materials with compatible multifunctional properties and optimizing dressing structures are essential to achieving synergistic effects. Additionally, while intelligent hydrogel dressings exhibit antibacterial properties, they face challenges in preventing sepsis, particularly against high-virulence bacterial strains. Future research should focus on developing novel antibacterial components and optimizing dressing structures to enhance antibacterial persistence[41]. Cost is another barrier to the widespread adoption of intelligent hydrogel dressings. The complex production process and expensive raw materials contribute to high prices, limiting accessibility. Strategies to reduce costs include optimizing synthesis methods, using more affordable materials, and achieving large-scale production. Supportive pricing strategies and medical insurance policies can further improve cost-effectiveness and promote clinical adoption. The lack of standardized clinical validation criteria and rigorous trials also hinders the promotion of intelligent hydrogel dressings. Currently, there are few relevant clinical studies, and the evaluation criteria are not unified, making it difficult to compare results across studies[42,119]. Future efforts should establish unified evaluation standards and conduct multicenter, large-sample clinical trials to comprehensively assess the safety and efficacy of these dressings.
Looking ahead, the integration of emerging technologies, such as 3D printing, personalized medicine, and smart monitoring systems, holds immense promise for advancing intelligent hydrogel dressings. For instance, 3D printing can enable the customization of dressings based on patient-specific wound characteristics, while smart monitoring systems can provide real-time data on wound status. Additionally, self-healing hydrogels based on dynamic chemical bonds can extend dressing lifespan, and nanobiotechnology can enable precise drug delivery and cell behavior regulation. Interdisciplinary collaboration among materials scientists, biologists, engineers, and clinicians is essential to accelerate the translation of intelligent hydrogel dressings from the laboratory to clinical practice. By addressing these challenges and leveraging emerging technologies, intelligent hydrogel dressings can revolutionize diabetic wound treatment, significantly improving healing outcomes and patients' quality of life.
Intelligent hydrogel-based dressings represent a transformative approach to treating chronic diabetic wounds. By mimicking the physiological moist environment, they maintain optimal moisture levels essential for cell activities and tissue regeneration while effectively absorbing wound exudate. This dual functionality prevents fluid accumulation and removes harmful substances, creating an ideal microenvironment for healing. Despite their potential, significant challenges remain. These include improving responsiveness to low-concentration wound indicators, ensuring long-term stability and biocompatibility, integrating multifunctional properties, reducing production costs, and establishing standardized clinical validation criteria. Additionally, increasing acceptance among healthcare workers and patients is crucial for widespread adoption. To bridge the gap between laboratory studies and clinical applications, future research should focus on several key areas. First, developing more sensitive and responsive materials to detect subtle changes in the wound microenvironment is essential. Second, optimizing the design of multifunctional hydrogels to ensure compatibility and synergy among different properties will enhance their therapeutic efficacy. Third, cost-effective manufacturing processes, such as scalable synthesis methods and the use of affordable raw materials, must be prioritized to improve accessibility. Fourth, rigorous multicenter clinical trials with standardized evaluation criteria are needed to comprehensively assess the safety and efficacy of these dressings. The integration of emerging technologies, such as 3D printing, personalized medicine, and smart monitoring systems, holds immense promise for advancing intelligent hydrogel dressings. By addressing these challenges and leveraging emerging technologies, intelligent hydrogel dressings can revolutionize diabetic wound treatment, significantly improving healing outcomes and patients' quality of life.
| 1. | MacDonald C, Bennekou M, Midtgaard J, Langberg H, Lieberman D. Why exercise may never be effective medicine: an evolutionary perspective on the efficacy versus effectiveness of exercise in treating type 2 diabetes. Br J Sports Med. 2025;59:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Udler MS. Genetic insights into global heterogeneity of type 2 diabetes. Nat Med. 2025;31:35-36. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Huang L, Chen H, Nie J, Zhao Y, Miao J. Advanced dressings based on novel biological targets for diabetic wound healing: A review. Eur J Pharmacol. 2025;987:177201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Sloan L, Cheng AYY, Escalada J, Haluzík M, Mauricio D. The role of basal insulins in the treatment of people with type 2 diabetes and chronic kidney disease: A narrative review. Diabetes Obes Metab. 2024;26:1157-1170. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Verde L, Di Lorenzo T, Savastano S, Colao A, Barrea L, Muscogiuri G. Chrononutrition in type 2 diabetes mellitus and obesity: A narrative review. Diabetes Metab Res Rev. 2024;40:e3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Zhou Z, Li C, Zeng Y, Huang T, Jiang X, Yu DG, Wang K. Natural polymer nanofiber dressings for effective management of chronic diabetic wounds: A comprehensive review. Int J Biol Macromol. 2024;282:136688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | He YF, Hu XD, Liu JQ, Li HM, Lu SF. Bariatric surgery and diabetes: Current challenges and perspectives. World J Diabetes. 2024;15:1692-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Reference Citation Analysis (3)] |
| 8. | Qu M, Xu W, Zhou X, Tang F, Chen Q, Zhang X, Bai X, Li Z, Jiang X, Chen Q. An ROS-Scavenging Treg-Recruiting Hydrogel Patch for Diabetic Wound Healing. Adv Funct Materials. 2024;34:2314500. [DOI] [Full Text] |
| 9. | Tenda ED, Henrina J, Cha JH, Triono MR, Putri EA, Aristy DJ, Tahapary DL. Obstructive sleep apnea: Overlooked comorbidity in patients with diabetes. World J Diabetes. 2024;15:1448-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Tseng SL, Kang L, Li ZJ, Wang LQ, Li ZM, Li TH, Xiang JY, Huang JZ, Yu NZ, Long X. Adipose-derived stem cells in diabetic foot care: Bridging clinical trials and practical application. World J Diabetes. 2024;15:1162-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Veeranki V, Prasad N. Utilising continuous glucose monitoring for glycemic control in diabetic kidney disease. World J Diabetes. 2024;15:2006-2009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 12. | Zhang JJ, Ni P, Song Y, Gao MJ, Guo XY, Zhao BQ. Effective protective mechanisms of HO-1 in diabetic complications: a narrative review. Cell Death Discov. 2024;10:433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Rotbei S, Tseng WH, Merino-barbancho B, Haleem MS, Montesinos L, Pecchia L, Fico G, Botta A. Evaluating impact of movement on diabetes via artificial intelligence and smart devices systematic literature review. Expert Syst Appl. 2024;257:125058. [DOI] [Full Text] |
| 14. | Chuanboding, Wang N, He H, Sun X, Bi X, Li A, Sun P, Li J, Yan L, Gao Y, Shen L, Ting Z, Zhang S. Advances in the treatment of type 2 diabetes mellitus by natural plant polysaccharides through regulation of gut microbiota and metabolism: A review. Int J Biol Macromol. 2024;274:133466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Yang S, Zhang H, Sun X, Bai J, Zhang J. 3D-Printed Liquid Metal-in-Hydrogel Solar Evaporator: Merging Spectrum-Manipulated Micro-Nano Architecture and Surface Engineering for Solar Desalination. ACS Nano. 2024. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Zhong S, Meng F, Cui X. Multi-Functional hydrogels to promote diabetic wound Healing: A review. Chem Eng J. 2024;497:154855. [DOI] [Full Text] |
| 17. | Yang X, Chai L, Huang Z, Zhu B, Liu H, Shi Z, Wu Y, Guo L, Xue L, Lei Y. Smart photonic crystal hydrogels for visual glucose monitoring in diabetic wound healing. J Nanobiotechnology. 2024;22:618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Zong L, Teng R, Zhang H, Liu W, Feng Y, Lu Z, Zhou Y, Fan Z, Li M, Pu X. Ultrasound-Responsive HBD Peptide Hydrogel with Antibiofilm Capability for Fast Diabetic Wound Healing. Adv Sci (Weinh). 2024;11:e2406022. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Naomi R, Fauzi MB. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics. 2020;12:881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Arbab S, Ullah H, Muhammad N, Wang W, Zhang J. Latest advance anti-inflammatory hydrogel wound dressings and traditional Lignosus rhinoceros used for wound healing agents. Front Bioeng Biotechnol. 2024;12:1488748. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Zhao Y, Zhao Y, Xu B, Liu H, Chang Q. Microenvironmental dynamics of diabetic wounds and insights for hydrogel-based therapeutics. J Tissue Eng. 2024;15:20417314241253290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Bei Z, Ye L, Tong Q, Ming Y, Yang T, Zhu Y, Zhang L, Li X, Deng H, Liu J, Chen W, Chu B, Qian Z. Thermostimulated shrinking and adhesive hydrogel dressing for treating chronic diabetic wounds. Cell Rep Phys Sci. 2024;5:102289. [DOI] [Full Text] |
| 23. | Vartanian A. Diabetic wound dressings. Nat Rev Mater. 2024;9:92-92. [DOI] [Full Text] |
| 24. | Zhao H, Wu Y, Xie Y, Li Y, Chen C, Li C, Yang F, Zhang D, Wang Y, Yuan J. Hydrogel dressings for diabetic foot ulcer: A systematic review and meta-analysis. Diabetes Obes Metab. 2024;26:2305-2317. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Li Y, Leng Y, Liu Y, Zhong J, Li J, Zhang S, Li Z, Yang K, Kong X, Lao W, Bi C, Zhai A. Advanced multifunctional hydrogels for diabetic foot ulcer healing: Active substances and biological functions. J Diabetes. 2024;16:e13537. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Khattak S, Ullah I, Sohail M, Akbar MU, Rauf MA, Ullah S, Shen J, Xu H. Endogenous/exogenous stimuli-responsive smart hydrogels for diabetic wound healing. Aggregate. 2025;6:e688. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Kammona O, Tsanaktsidou E, Kiparissides C. Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings. Gels. 2024;10:147. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Guo W, Ding X, Zhang H, Liu Z, Han Y, Wei Q, Okoro OV, Shavandi A, Nie L. Recent Advances of Chitosan-Based Hydrogels for Skin-Wound Dressings. Gels. 2024;10:175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Zhao J, Gao C, Guo W, Zhang B, Ren S, Wu S, Guo J, Qu W. Conductive hydrogels as an “innovative healer” for the treatment of diabetic wounds. Mater Chem Front. 2024;8:2944-2977. [DOI] [Full Text] |
| 30. | Huan Z, Li J, Luo Z, Yu Y, Li L. Hydrogel-Encapsulated Pancreatic Islet Cells as a Promising Strategy for Diabetic Cell Therapy. Research (Wash D C). 2024;7:0403. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Liao Y, Zhang Z, Zhao Y, Zhang S, Zha K, Ouyang L, Hu W, Zhou W, Sun Y, Liu G. Glucose oxidase: An emerging multidimensional treatment option for diabetic wound healing. Bioact Mater. 2025;44:131-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 32. | Vargas Guerrero M, Aendekerk FMA, de Boer C, Geurts J, Lucchesi J, Arts JJC. Bioactive-Glass-Based Materials with Possible Application in Diabetic Wound Healing: A Systematic Review. Int J Mol Sci. 2024;25:1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Yan L, Wang Y, Feng J, Ni Y, Zhang T, Cao Y, Zhou M, Zhao C. Mechanism and application of fibrous proteins in diabetic wound healing: a literature review. Front Endocrinol (Lausanne). 2024;15:1430543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Tricou LP, Al-Hawat ML, Cherifi K, Manrique G, Freedman BR, Matoori S. Wound pH-Modulating Strategies for Diabetic Wound Healing. Adv Wound Care (New Rochelle). 2024;13:446-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Barkhordari S, Shahi F, Khonakdar HA. Advanced Drug Delivery Systems for Topical Insulin in Diabetic Wound Healing. ChemistrySelect. 2024;9:e202403847. [DOI] [Full Text] |
| 36. | Mgwenya TN, Abrahamse H, Houreld NN. Photobiomodulation studies on diabetic wound healing: An insight into the inflammatory pathway in diabetic wound healing. Wound Repair Regen. 2025;33:e13239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mahmoud NN, Hamad S, Shraim S. Inflammation-Modulating Biomedical Interventions for Diabetic Wound Healing: An Overview of Preclinical and Clinical Studies. ACS Omega. 2024;9:44860-44875. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Sharma A, Dheer D, Puri V, Alsayari A, Wahab S, Kesharwani P. Insights of biopolymeric blended formulations for diabetic wound healing. Int J Pharm. 2024;656:124099. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Li Y, Zhu Z, Li S, Xie X, Qin L, Zhang Q, Yang Y, Wang T, Zhang Y. Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing. J Nanobiotechnology. 2024;22:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 40. | Song J, Wu Y, Chen Y, Sun X, Zhang Z. Epigenetic regulatory mechanism of macrophage polarization in diabetic wound healing (Review). Mol Med Rep. 2025;31:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Jain N, Singh Y, Nouri A, Garg U, Pandey M. Assessment of healing capacity of glucose-responsive smart gels on the diabetic wound: A comprehensive review. J Drug Deliv Sci Technol. 2024;93:105403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Chhillar A, Jaiswal A. Hyaluronic Acid-Based Self-Healing Hydrogels for Diabetic Wound Healing. Adv Healthc Mater. 2025;14:e2404255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Deng LE, Qiu Y, Zeng Y, Zou J, Kumar A, Pan Y, Nezamzadeh-Ejhieh A, Liu J, Liu X. Current and promising applications of MOF composites in the healing of diabetes wounds. RSC Med Chem. 2024;15:2601-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
| 44. | Wang M, Deng Z, Guo Y, Xu P. Engineering functional natural polymer-based nanocomposite hydrogels for wound healing. Nanoscale Adv. 2022;5:27-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Rajinikanth B S, Rajkumar DSR, K K, Vijayaragavan V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus. 2024;16:e55193. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Rao SS, Venkatesan J, Prabhu A, Rekha P. Natural polymeric biomaterials in growth factor delivery for treating diabetic foot ulcers. J Drug Deliv Sci Technol. 2020;55:101385. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Raina N, Rani R, Pahwa R, Gupta M. Biopolymers and treatment strategies for wound healing: an insight view. Int J Polym Materi Polym Biomater. 2022;71:359-375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C Mater Biol Appl. 2020;114:110994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 49. | Li X, Bai L, Zhang X, Fang Q, Chen G, Xu G. Application of Bletilla striata polysaccharide hydrogel for wound healing among in diabetes. Colloids Surf B Biointerfaces. 2024;241:114033. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Hu S, Cai X, Qu X, Yu B, Yan C, Yang J, Li F, Zheng Y, Shi X. Preparation of biocompatible wound dressings with long-term antimicrobial activity through covalent bonding of antibiotic agents to natural polymers. Int J Biol Macromol. 2019;123:1320-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Huang X, Zheng Y, Ming J, Ning X, Bai S. Natural polymer-based bioadhesives as hemostatic platforms for wound healing. Int J Biol Macromol. 2024;256:128275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 52. | Hasan S, Hasan MA, Hassan MU, Amin M, Javed T, Fatima L. Biopolymers in diabetic wound care management: A potential substitute to traditional dressings. Eur Polym J. 2023;189:111979. [RCA] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 53. | Alven S, Aderibigbe BA. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers (Basel). 2021;13:2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Ahmadian Z, Adiban H, Rashidipour M, Eskandari MR. Bioactive Natural and Synthetic Polymers for Wound Repair. Macromol Res. 2022;30:495-526. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Aderibigbe BA. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers (Basel). 2022;14:3806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Abuhamad AY, Masri S, Fadilah NIM, Alamassi MN, Maarof M, Fauzi MB. Application of 3D-Printed Bioinks in Chronic Wound Healing: A Scoping Review. Polymers (Basel). 2024;16:2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Bhuyan MM, Jeong JH. Gels/Hydrogels in Different Devices/Instruments-A Review. Gels. 2024;10:548. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Bayer IS. A Review of Sustained Drug Release Studies from Nanofiber Hydrogels. Biomedicines. 2021;9:1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Kesharwani P, Bisht A, Alexander A, Dave V, Sharma S. Biomedical applications of hydrogels in drug delivery system: An update. J Drug Deliv Sci Technol. 2021;66:102914. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 60. | Tehrany PM, Rahmanian P, Rezaee A, Ranjbarpazuki G, Sohrabi Fard F, Asadollah Salmanpour Y, Zandieh MA, Ranjbarpazuki A, Asghari S, Javani N, Nabavi N, Aref AR, Hashemi M, Rashidi M, Taheriazam A, Motahari A, Hushmandi K. Multifunctional and theranostic hydrogels for wound healing acceleration: An emphasis on diabetic-related chronic wounds. Environ Res. 2023;238:117087. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Loh XJ, Chee PL, Owh C. Biodegradable Thermogelling Polymers. Small Methods. 2019;3:1800313. [DOI] [Full Text] |
| 62. | Liu H, Cao X, Liu B, He L. Thermoresponsive in situ forming self-healing hydrogel dressings with pH/glucose dual responsive curcumin release for diabetic wound healing. J Mater Sci. 2024;59:17573-17592. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Liu J, Yi X, Zhang J, Yao Y, Panichayupakaranant P, Chen H. Recent Advances in the Drugs and Glucose-Responsive Drug Delivery Systems for the Treatment of Diabetes: A Systematic Review. Pharmaceutics. 2024;16:1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Deng D, Liang L, Su K, Gu H, Wang X, Wang Y, Shang X, Huang W, Chen H, Wu X, Wong W, Li D, Zhang K, Wu P, Wu K. Smart hydrogel dressing for machine learning-enabled visual monitoring and promote diabetic wound healing. Nano Today. 2025;60:102559. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 65. | Wang Y, Liu K, Wei W, Dai H. A Multifunctional Hydrogel with Photothermal Antibacterial and AntiOxidant Activity for Smart Monitoring and Promotion of Diabetic Wound Healing. Adv Funct Materials. 2024;34:2402531. [DOI] [Full Text] |
| 66. | Jiang S, Xie D, Hu Z, Song H, Tang P, Jin Y, Xia J, Ji Y, Xiao Y, Chen S, Fu Q, Dai J. Enhanced diabetic wound healing with injectable hydrogel containing self-assembling nanozymes. J Control Release. 2024;372:265-280. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Zhang Y, Tang Z, Chen L, Yang M, Zeng Y, Bai X, Zhang B, Zhou J, Zhang W, Tang S. Intelligent sequential degradation hydrogels by releasing bimetal-phenolic for enhanced diabetic wound healing. J Control Release. 2025;378:961-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | He J, Li Z, Chen J, Wang J, Qiao L, Guo B, Hu J. NIR/glucose stimuli-responsive multifunctional smart hydrogel wound dressing with NO/O2 dual gas-releasing property promotes infected diabetic wound healing. Chem Eng J. 2024;492:152249. [DOI] [Full Text] |
| 69. | Li H, Wang Y, Che X, Guo L, Huang L, Li X, Gao W. A novel pH/ROS dual responsive engineering hydrogels based on poly(tannic acid)-assisted surface deposition of nano-enzymes with efficient antibacterial and antioxidant activity for diabetic wound healing. Chem Eng J. 2024;496:153370. [DOI] [Full Text] |
| 70. | Gong X, Yang J, Zheng Y, Chen S, Duan H, Gao J, Haick H, Yi C, Jiang L. Polymer Hydrogel-Based Multifunctional Theranostics for Managing Diabetic Wounds. Adv Funct Materials. 2024;34:2315564. [DOI] [Full Text] |
| 71. | Chen J, Luo M, Chen Y, Xing Z, Peng C, Li D. Smart macrophage-targeting wound dressings accelerate diabetic wound healing. Chem Eng J. 2024;500:156860. [DOI] [Full Text] |
| 72. | Yang P, Ju Y, Shen N, Zhu S, He J, Yang L, Lei J, He X, Shao W, Lei L, Fang B. Exos-Loaded Gox-Modified Smart-Response Self-Healing Hydrogel Improves the Microenvironment and Promotes Wound Healing in Diabetic Wounds. Adv Healthc Mater. 2024;e2403304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 73. | Ding C, Liu X, Zhang S, Sun S, Yang J, Chai G, Wang N, Ma S, Ding Q, Liu W. Multifunctional hydrogel bioscaffolds based on polysaccharide to promote wound healing: A review. Int J Biol Macromol. 2024;259:129356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 74. | Tian S, Mei J, Zhang L, Wang S, Yuan Y, Li J, Liu H, Zhu W, Xu D. Multifunctional Hydrogel Microneedle Patches Modulating Oxi-inflamm-aging for Diabetic Wound Healing. Small. 2024;20:e2407340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Zang H, Jiang H, Huang J, El Akkawi MM, Yan L, Liang K, Lin Z, Zhu Z, Li Y. Bilayer micropatterned hydrogel scaffolds loaded with ADSCs improved integration with regenerated tissue and diabetic wound healing. Chem Eng J. 2024;489:151342. [DOI] [Full Text] |
| 76. | He F, Xu P, Zhu Z, Zhang Y, Cai C, Zhang Y, Shao J, Jin F, Li Q, You J, Zhou H, Zhang W, Wei J, Hong X, Zhang Z, Han C, Zhang Y, Gu Z, Wang X. Inflammation-Responsive Hydrogel Accelerates Diabetic Wound Healing through Immunoregulation and Enhanced Angiogenesis. Adv Healthc Mater. 2025;14:e2400150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 77. | Li J, Shen Y, Wang X, Wu T, Huang Q, Shen M, Xu S, Li Y. A multilayer hydrogel incorporating urolithin B promotes diabetic wound healing via ROS scavenging and angiogenesis. Chem Eng J. 2024;496:153661. [DOI] [Full Text] |
| 78. | Han X, Yang Y, Xu Y, Hong X, Tang Z, Zhang H, Liu N, Li M, Wang Z, Zheng A. 3D micro-nano printing technology as a transformative tool apply for microneedle drug delivery. J Drug Deliv Sci Technol. 2024;96:105709. [DOI] [Full Text] |
| 79. | Bi S, He C, Zhou Y, Liu R, Chen C, Zhao X, Zhang L, Cen Y, Gu J, Yan B. Versatile conductive hydrogel orchestrating neuro-immune microenvironment for rapid diabetic wound healing through peripheral nerve regeneration. Biomaterials. 2025;314:122841. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Xie H, Wang Z, Wang R, Chen Q, Yu A, Lu A. Self-Healing, Injectable Hydrogel Dressing for Monitoring and Therapy of Diabetic Wound. Adv Funct Materials. 2024;34:2401209. [DOI] [Full Text] |
| 81. | Yang J, Lai M, Ma Y, Wu J, Zhang C, Yuan H, Liang G, Meng C, Su Y, Luan B, Gu L, Wang Y. Spatiotemporal modulation of immune microenvironment via composite hydrogel brakes for diabetic wound healing. Chem Eng J. 2024;493:152251. [DOI] [Full Text] |
| 82. | Tang L, Xie S, Wang D, Wei Y, Ji X, Wang Y, Zhao N, Mou Z, Li B, Sun WR, Wang PY, Basmadji NP, Pedraz JL, Vairo C, Lafuente EG, Ramalingam M, Xiao X, Wang R. Astragalus polysaccharide/carboxymethyl chitosan/sodium alginate based electroconductive hydrogels for diabetic wound healing and muscle function assessment. Carbohydr Polym. 2025;350:123058. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Gong J, Wang H, Xie C, Dai Y, Wang Y, Guo W. A multifunctional injectable hydrogel for boosted diabetic wound healing assisted by Quercetin-ZIF system. Chem Eng J. 2024;495:153425. [DOI] [Full Text] |
| 84. | Ge X, Mao M, Yu H, Liu J, Kong J, Sun Y, Huang W, Wang DY, Wang Y. Application of self powered black phosphorus nanosheets-based hydrogel skin patch in wound healing of diabetes. Nano Energy. 2024;131:110289. [DOI] [Full Text] |
| 85. | Lyu S, Liu Q, Yuen HY, Xie H, Yang Y, Yeung KW, Tang CY, Wang S, Liu Y, Li B, He Y, Zhao X. A differential-targeting core-shell microneedle patch with coordinated and prolonged release of mangiferin and MSC-derived exosomes for scarless skin regeneration. Mater Horiz. 2024;11:2667-2684. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 86. | Wei X, Liu C, Li Z, Gu Z, Yang J, Luo K. Chitosan-based hydrogel dressings for diabetic wound healing via promoting M2 macrophage-polarization. Carbohydr Polym. 2024;331:121873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 87. | Wu Y, Li M, He R, Xiao L, Liu S, Chen K, Qiang H, Ji K, Li L, Yin Y, Yuan X, Li M, Gao J, Li Y. Photosynthetic live microorganism-incorporated hydrogels promote diabetic wound healing via self-powering and oxygen production. Chem Eng J. 2024;485:149545. [DOI] [Full Text] |
| 88. | Jeong D, Jang SY, Roh S, Choi JH, Seo IJ, Lee JH, Kim J, Kwon I, Jung Y, Hwang J, Jang WY, Yoo J. Sprayable hydrogel with optical mRNA nanosensors for Real-Time monitoring and healing of diabetic wounds. Chem Eng J. 2024;493:152711. [DOI] [Full Text] |
| 89. | Liu G, Zhou Y, Xu Z, Bao Z, Zheng L, Wu J. Janus hydrogel with dual antibacterial and angiogenesis functions for enhanced diabetic wound healing. Chin Chem Lett. 2023;34:107705. [RCA] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 90. | Lin Z, Fan D, Li G, He L, Qin X, Zhao B, Wang Q, Liang W. Antibacterial, Adhesive, and Conductive Hydrogel for Diabetic Wound Healing. Macromol Biosci. 2023;23:e2200349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 91. | Ji JY, Ren DY, Weng YZ. Efficiency of Multifunctional Antibacterial Hydrogels for Chronic Wound Healing in Diabetes: A Comprehensive Review. Int J Nanomedicine. 2022;17:3163-3176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 92. | Chengwei W, Yihao L, Xiaoxiao Y, Wentao L, Xianhao Z, Ya R, Changru Z, Han Y, Weiqing K, Jinwu W, Haoyi N. In-situ forming hydrogel incorporated with reactive oxygen species responsive and antibacterial properties for diabetic infected chronic wound healing. Chem Eng J. 2022;450:138077. [DOI] [Full Text] |
| 93. | Shang S, Zhuang K, Chen J, Zhang M, Jiang S, Li W. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact Mater. 2024;34:298-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 94. | Zhang Y, Li M, Wang Y, Han F, Shen K, Luo L, Li Y, Jia Y, Zhang J, Cai W, Wang K, Zhao M, Wang J, Gao X, Tian C, Guo B, Hu D. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact Mater. 2023;26:323-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 95. | Zhang K, Yang C, Cheng C, Shi C, Sun M, Hu H, Shi T, Chen X, He X, Zheng X, Li M, Shao D. Bioactive Injectable Hydrogel Dressings for Bacteria-Infected Diabetic Wound Healing: A "Pull-Push" Approach. ACS Appl Mater Interfaces. 2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 96. | Zahoor S, Tahir HM, Ali S, Ali A, Muzamil A, Murtaza Z, Zahoor N. Diabetic wound healing potential of silk sericin protein based hydrogels enriched with plant extracts. Int J Biol Macromol. 2023;242:125184. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 97. | Jiang L, Yang X, Zhang Y, He D, Gao Y, Lu K, Hao Y, Gao Y, Lu D, Jin X, Li C. Novel ROS-scavenging hydrogel with enhanced anti-inflammation and angiogenic properties for promoting diabetic wound healing. Biomater Adv. 2023;144:213226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 98. | Xu C, Cao JF, Pei Y, Kim Y, Moon H, Fan CQ, Liao MC, Wang XY, Yao F, Zhang YJ, Zhang SH, Zhang J, Li JZ, Kim JS, Ma L, Xie ZJ. Injectable hydrogel harnessing foreskin mesenchymal stem cell-derived extracellular vesicles for treatment of chronic diabetic skin wounds. J Control Release. 2024;370:339-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Shivam K, Selvam A, Sangam S, Majood M, Pahari S, Patra R, Sharma AK, Mukherjee M. Graphene quantum dots-hybrid hydrogel as an avant-garde biomimetic scaffold for diabetic wound healing. Biomater Adv. 2023;149:213395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 100. | Liu W, Lei L, Ma F, Zhan M, Zhu J, Khan MZH, Liu X. A Dioscorea opposita Polysaccharide-Calcium Carbonate Microsphere-Doped Hydrogel for Accelerated Diabetic Wound Healing via Synergistic Glucose-Responsive Hypoglycemic and Anti-Inflammatory Effects. ACS Biomater Sci Eng. 2025;11:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 101. | Chen J, Mu Z, Chen D, Huang C, Jin T, Li L, Zeng Y, Zhou Q, Zhang Y, Mao H, Deng H, Shen X, Yang H, Cai X. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem Eng J. 2023;469:143985. [DOI] [Full Text] |
| 102. | Wang W, Zheng J, Hong X, Zhou J, Xiong Y, Yang H, Li S, Chen G, Su Q, Li W, Cheng B, Fu J, Wu T. Micro-environment triple-responsive hyaluronic acid hydrogel dressings to promote antibacterial activity, collagen deposition, and angiogenesis for diabetic wound healing. J Mater Chem B. 2024;12:4613-4628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 103. | Su Z, Zhang W, Mo Z, Zhang P, Wu Z, Huang H, Fan X. Novel asymmetrical double-layer structural adhesive hydrogels with synergetic neuroprotection and angiogenesis effect for diabetic wound healing. Chem Eng J. 2025;505:159081. [DOI] [Full Text] |
| 104. | Xiong W, Zhang X, Hu J, Zou X, Huang H, Qu W, Cai S, Li C, Wei Y, Zhong X, Cai Z, Huang Z. PF-PEG@ASIV-EXO Hydrogel Accelerates Diabetic Wound Healing by Ferroptosis Resistance and Promoting Angiogenesis. ACS Biomater Sci Eng. 2024;10:6263-6285. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Jia Z, Chen L, Gu D, Li X, Wen T, Li W. Lentinan-loaded GelMA hydrogel accelerates diabetic wound healing through enhanced angiogenesis and immune microenvironment modulation. Int J Biol Macromol. 2024;264:130716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 106. | Dai F, Zhang J, Chen F, Chen X, Lee CJ, Liang H, Zhao L, Tan H. A Multi-Responsive Hydrogel Combined With Mild Heat Stimulation Promotes Diabetic Wound Healing by Regulating Inflammatory and Enhancing Angiogenesis. Adv Sci (Weinh). 2024;11:e2408783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 107. | Wu Z, Wu W, Zhang C, Zhang W, Li Y, Ding T, Fang Z, Jing J, He X, Huang F. Enhanced diabetic foot ulcer treatment with a chitosan-based thermosensitive hydrogel loaded self-assembled multi-functional nanoparticles for antibacterial and angiogenic effects. Carbohydr Polym. 2025;347:122740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 108. | Yan R, Sun Z, Du W, Liu N, Sun Q, Li P, Liang F, Zhu Y, Huang W, Yu H. An Electrostatic-Induction-Enabled Anti-Touching Hydrogel Dressing for Chronic Wound Care. Adv Funct Materials. 2024;34:2402217. [DOI] [Full Text] |
| 109. | Sun L, Wang Z, Kang H, Luo P, Su J, Wei W, Zhou P, Yu A, Dai H. A flexibility self-powered Band-Aid for diabetes wound healing and skin bioelectronics. Chem Eng J. 2024;481:148096. [DOI] [Full Text] |
| 110. | Tian L, Liu T, Jiang Y, He B, Hao H. Multifunctional hydrogel sensor with Tough, self-healing capabilities and highly sensitive for motion monitoring and wound healing. Chem Eng J. 2024;497:154890. [DOI] [Full Text] |
| 111. | Si R, Wang Y, Yang Y, Wu Y, Wang M, Han B. A multifunctional conductive organohydrogel as a flexible sensor for synchronous real-time monitoring of traumatic wounds and pro-healing process. Chem Eng J. 2024;489:151419. [DOI] [Full Text] |
| 112. | Sun J, Jia W, Qi H, Huo J, Liao X, Xu Y, Wang J, Sun Z, Liu Y, Liu J, Zhen M, Wang C, Bai C. An Antioxidative and Active Shrinkage Hydrogel Integratedly Promotes Re-Epithelization and Skin Constriction for Enhancing Wound Closure. Adv Mater. 2024;36:e2312440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 113. | Liu R, Bi S, Zhang L, Li X, Dai K, Wang H, Zhang Z, Gu J. Flexible and antibacterial conductive hydrogels based on silk fibroin/polyaniline/AgNPs for motion sensing and wound healing promotion under electrical stimulation. J Mater Chem B. 2024;12:10346-10356. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 114. | Chen C, Wang Y, Zhang H, Zhang H, Dong W, Sun W, Zhao Y. Responsive and self-healing structural color supramolecular hydrogel patch for diabetic wound treatment. Bioact Mater. 2022;15:194-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 115. | Hu XQ, Zhu JZ, Hao Z, Tang L, Sun J, Sun WR, Hu J, Wang PY, Basmadji NP, Pedraz JL, Vairo C, Lafuente EG, Ramalingam M, Xie S, Wang R. Renewable Electroconductive Hydrogels for Accelerated Diabetic Wound Healing and Motion Monitoring. Biomacromolecules. 2024;25:3566-3582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 116. | Chen Y, Wang X, Tao S, Wang Q, Ma PQ, Li ZB, Wu YL, Li DW. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil Med Res. 2023;10:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 117. | Wang H, Zhang L. Intelligent biobased hydrogels for diabetic wound healing: A review. Chem Eng J. 2024;484:149493. [DOI] [Full Text] |
| 118. | Zhao J, Liu J, Hu Y, Hu W, Wei J, Qian H, Sun Y. Research advances in hydrogel-based wound dressings for diabetic foot ulcer treatment: a review. J Mater Sci. 2024;59:8059-8084. [DOI] [Full Text] |
| 119. | Cheng C, Zhong H, Zhang Y, Gao X, Wang J, Liu J, Han X. Bacterial responsive hydrogels based on quaternized chitosan and GQDs-ε-PL for chemo-photothermal synergistic anti-infection in diabetic wounds. Int J Biol Macromol. 2022;210:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |