Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.104787
Revised: February 20, 2025
Accepted: March 11, 2025
Published online: May 15, 2025
Processing time: 114 Days and 12.1 Hours
Monogenic diabetes is a heterogeneous disorder characterized by hyperglycemia arising from defects in a single gene. Maturity-onset diabetes of the young (MODY) is the most common type with 14 subtypes, each linked to specific mutations affecting insulin synthesis, secretion and glucose regulation. Common traits across MODY subtypes include early-onset diabetes, a family history of autosomal dominant diabetes, lack of features of insulin resistance, and absent islet cell autoimmunity. Many cases are misdiagnosed as type 1 and type 2 dia
Core Tip: Monogenic diabetes is an uncommon form of diabetes caused by single gene mutations. Depending on the type of underlying gene mutation, the disease can present at any age with diverse clinical presentations. The diagnostic workup includes genetic testing and may involve tests like C-peptide assay and autoantibody screening to exclude other forms of diabetes. Next-generation sequencing technologies such as targeted gene panels focusing on known monogenic diabetes genes, and whole-exome sequencing that analyze the protein-coding regions of the genome are replacing older methods like Sanger sequencing. Pitfalls in workup can include misdiagnosis as type 1 or type 2 diabetes, leading to inappropriate treatment and potential complications. This evidence-based review updates the current clinical approach to monogenic diabetes.
- Citation: Bhattacharya S, Fernandez CJ, Kamrul-Hasan ABM, Pappachan JM. Monogenic diabetes: An evidence-based clinical approach. World J Diabetes 2025; 16(5): 104787
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/104787.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.104787
Monogenic diabetes refers to a heterogeneous group of disorders characterized by diabetes mellitus arising from a defect in a single gene. Although the disease accounts for about 1%-2% of the global diabetes burden, only a small proportion receives the correct and timely diagnosis[1-3]. The two most common forms are maturity-onset diabetes of the young (MODY) and neonatal diabetes mellitus (NDM). However, several other subtypes are also described, developing from gene mutations affecting insulin resistance, autoimmunity, or mitochondrial function[4,5].
While many monogenic diabetes have distinct clinical features, others closely resemble type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM)[6-8]. An accurate diagnosis is essential for implementing precision treatment, monitoring disease progression, and providing genetic counseling[9,10]. Recent advancements and broader availability of genetic testing have increased the scope of diagnosis of monogenic diabetes[11,12]. This article aims to explore the best practices in diagnosing and treating monogenic diabetes, with a special focus on the latest scientific advancements, empowering clinicians to enhance outcomes.
A comprehensive literature search was conducted using PubMed, EMBASE, Scopus, Web of Science, Cochrane Library, and Google Scholar. A combination of Medical Subject Headings terms and free-text keywords were used, including “monogenic diabetes”, “maturity-onset diabetes of the young”, “neonatal diabetes”, “monogenic autoimmune diabetes”, “insulin resistance syndromes”, “lipodystrophy”, “mitochondrial diabetes” and “syndromic diabetes”. The specific genetic subtypes such as “GCK-MODY”, “HNF1A-MODY”, “adenosine triphosphate-sensitive potassium (KATP) channel mutations”, “ABCC8 mutations”, “INS gene mutations” and other reported genetic mutations were also searched. Additional terms related to diagnosis and testing, such as “genetic testing”, “molecular diagnosis”, “next generation sequencing”, “Sanger sequencing”, “methylation analysis”, “in silico analysis”, “functional studies” and other related terms were included.
Monogenic diabetes comprises a range of clinically, metabolically, and genetically diverse conditions caused by gene defects that affect insulin production, secretion, or action[4,5]. Table 1 outlines the different categories, including MODY, NDM, other monogenic forms with defective insulin synthesis and secretion, monogenic autoimmune diabetes syn
| Types | Subtypes/varieties | Gene/protein | Clinical features | Inheritance |
| MODY | MODY 1 to 14 | See Table 2 | See Table 2 | |
| Neonatal diabetes | Permanent NDM | See Table 2 | See Table 2 | |
| Transient NDM | See Table 2 | See Table 2 | ||
| Other monogenic diabetes with defective insulin synthesis and secretion | Mitochondrial DM | m.3243A > G | Maternally inherited diabetes and deafness | Maternal |
| Wolfram syndrome 1 or 2 | WFS1 or CISD2 | DIDMOAD 1 or 2 diabetes insipidus, diabetes mellitus, optic atrophy and deafness | AR | |
| Rogers syndrome | SLC19A2 | Thiamine-responsive megaloblastic anaemia, deafness, and diabetes | AR | |
| H or PHID syndrome | SLC29A3 | H syndrome: Hyperpigmentation, hypertrichosis, hepatosplenomegaly, heart anomaly, hearing loss, low height, hypogonadism and hyperglycaemia; PHID syndrome | AR | |
| Monogenic autoimmune diabetes syndromes | IPEX syndrome | FOXP3 | IPEX syndrome | XR |
| LRBA | Common variable immunodeficiency 8 | AR | ||
| STAT3 | Infancy-onset multi-system autoimmune disease type 1 | AD | ||
| APECED syndrome | AIRE | Autoimmune polyendocrinopathy syndrome type 1 | AR | |
| IL2RA | Immunodeficiency 41 with lymphoproliferation and autoimmunity | AR | ||
| IPEX-like syndrome | STAT1 | IPEX-like syndrome | AD | |
| STAT5B | Growth defect, primary immunodeficiency, and endocrine abnormalities | AR | ||
| Insulin resistance syndromes | Congenital generalized lipodystrophy (Seip-Berardinelli syndrome) | AGPAT2 | CGL1-acromegalic features | AR |
| BSCL2 | CGL2-cardiomyopathy, polyneuropathy, mental retardation | AR | ||
| CAV1 | CGL3-short stature, pulmonary arterial hypertension, and vitamin D resistance | AR | ||
| PTRF | CGL4-congenital myopathy, pyloric stenosis, cardiomyopathy, atlantoaxial instability | AR | ||
| Partial lipodystrophy | LMNA | FPLD2-fat loss from limbs; fat deposition in face, neck and perineum (Dunnigan variety) | AD | |
| PPARG | FPLD3-loss of subcutaneous fat from distal extremities | AD | ||
| PLIN1 | FPLD4-loss of subcutaneous fat from extremities | AD | ||
| AKT2 | AKT2 linked FPLD-loss of subcutaneous fat from extremities | AD | ||
| CIDEC | FPLD5-fat loss of lower limbs and abdomen, and multilocular lipid droplets | AR | ||
| LIPE | FPLD6-upper body pseudo-lipomatosis, fat loss from limbs, and muscle atrophy in some cases | AR | ||
| LMNA | Mandibuloacral dysplasia A syndrome-skeletal anomaly, loss of extremity fat, neuropathy, premature ageing | AR | ||
| ZMPST24 | Mandibuloacral dysplasia B syndrome-skeletal anomaly, loss of fat, premature renal failure, progeroid | AR | ||
| PIK3R1 | SHORT syndrome | AD | ||
| POLD1 | MDPL syndrome | De novo | ||
| WRN | Werner syndrome-progeria and cataract | AR |
Monogenic diabetes presents at various stages of life, with specific types more likely at particular phases. Hypergly
MODY is the prototypical form of monogenic diabetes, usually manifesting in individuals younger than 25 years with autosomal dominant inheritance. It has 14 known subtypes linked to gene mutations affecting insulin production and glucose regulation[16,19]. MODY types were initially classified numerically, but genetic defects associated with each subtype are currently used to subtype MODY[36]. The mutations involved in the pathogenesis of MODY and clinical features are enumerated in Table 2[16-19,30,37-62]. The subtypes differ in the age of onset, progression and complication rate, treatment response, and extra-pancreatic manifestations. The most common mutations are HNF1A (MODY3), GCK (MODY2), HNF4A (MODY1), and HNF1B (MODY5)[63].
| Subtype | Gene | Frequency | Gene-disease | Clinical feature | Inheritance | |
| MODY | MODY 1 | HNF4A | 14% | Classical MODY | Fetal macrosomia and/or neonatal hypoglycemia, respond to low-dose SU initially, progressive β-cell failure, require insulin later, risks of complications | AD |
| MODY 2 | GCK | 22% | Mild fasting hyperglycemia does not require treatment except during gestation determined by the GCK mutation status of the foetus | AD, rarely AR | ||
| MODY 3 | HNF1A | 33% | Disproportionate glucosuria (low renal threshold), response to low-dose SU initially, progressive β-cell failure, require insulin later, risks of complications | AD, rarely AR | ||
| MODY 4 | IPF1/PDX1 | < 1% | A heterozygous mutation causing MODY or T2DM or homozygous mutation causing PNDM (see below) | AD | ||
| MODY 5 | HNF1B | 6% | Syndromic MODY | Renal cysts and diabetes, pancreas hypoplasia, exocrine insufficiency, β-cell defect, low magnesium, gout, altered LFT, and autism; Require insulin; Risks of complications; 40% are de novo | AD | |
| MODY 6 | NEUROD1 | 1% | Classical MODY | A heterozygous mutation causing MODY or homozygous mutation causing NDM (not mentioned below) | AD | |
| MODY 7 | KLF11 | < 1% | Evidence refuted | Potentially causing T2DM in the presence of obesity rather than causing MODY | AD | |
| MODY 8 | CEL | < 1% | Syndromic MODY | Diabetes and pancreatic exocrine dysfunction; Pancreatic cysts may be present | AD | |
| MODY 9 | PAX4 | < 1% | Evidence refuted | Potentially causing T2DM in the presence of obesity rather than causing MODY | AD | |
| MODY 10 | INS | 2% | Classical MODY | Mild defects can present as MODY whereas severe defects can present as TNDM or PNDM (as below), insulin treatment preserves β-cell mass and insulin secretion | AD | |
| MODY 11 | BLK | < 1% | Evidence refuted | Potentially causing T2DM in the presence of obesity rather than causing MODY | AD | |
| MODY 12 | ABCC8 | 4% | Classical MODY | Manifest as relapse following TNDM or as isolated MODY with no history of TNDM, respond to low-dose SU | AD | |
| MODY 13 | KCNJ11 | 2% | Manifest as relapse following TNDM or as isolated MODY with no history of TNDM, respond to low-dose SU | AD | ||
| MODY 14 | APPL1 | < 1% | Evidence weak | Delayed onset MODY with low penetrance and less severity; Potentially causing T2DM in the presence of obesity rather than causing MODY | AD | |
| RFX6-MODY | RFX6 | < 1% | Classical MODY | Significantly low penetrance; Likely to respond to DPP4 inhibitors or GLP-1 receptor agonists (low GIP levels are present in these patients) | AD | |
| NDM | TNDM 45% NDM | ZAC and HYMAI | 70% TNDM | TNDM1 | 6q24 abnormal uniparental disomy 40%, paternal duplication 40%, maternal hypomethylation 20%, macroglossia, umbilical hernia, cardiac/renal defect, hypothyroidism | Sporadic or AD |
| ABCC8 | 15% TNDM | TNDM2 | TNDM (early infancy), remission (early childhood), and/or relapse of diabetes (in adulthood), mild developmental features may be seen, marked response to low-dose SU | Sporadic or AD | ||
| KCNJ11 | 10% TNDM | TNDM (early infancy), remission (early childhood), and/or relapse of diabetes (in adulthood), mild developmental features may be seen, marked response to low-dose SU | Sporadic or AD | |||
| INS | 5% TNDM | IUGR; Doesn’t respond to SU but responds to insulin therapy | AD, rarely AR | |||
| HNF1B | Renal cyst and pancreatic hypoplasia | AD | ||||
| SLC2A2 | TNDM, PNDM (rare), or Fanconi-Bickel syndrome (Fanconi syndrome, short stature, rickets, growth retardation, hepatomegaly, and glucose/galactose intolerance) | AR | ||||
| PNDM 45% NDM | KCNJ11 | 50% PNDM | DEND (developmental delay, epilepsy, NDM) or iDEND syndrome (mild developmental delay, no epilepsy), severe hyperglycemia, DKA frequent, response to high-dose SU | Sporadic or AD | ||
| INS | 30% PNDM | IUGR; Doesn’t respond to SU but responds to insulin therapy | AD | |||
| ABCC8 | 15% PNDM | DEND (developmental delay, epilepsy, NDM) or iDEND syndrome (mild developmental delay, no epilepsy), severe hyperglycemia, DKA frequent, response to high-dose SU | Sporadic, AD or AR | |||
| GCK | 3% PNDM | IUGR; Homozygous mutations causing PNDM requiring lifelong insulin therapy | AR | |||
| IPF1/PDX1 | 2% PNDM | Pancreatic hypoplasia causing PNDM (homozygous mutation) | AR | |||
| HNF1B | Renal cyst and pancreatic hypoplasia | AD | ||||
| Syndromic NDM; 10% NDM | EIF2AK3 | Rare | PNDM with spondyloepiphyseal dysplasia, and renal anomalies (Wolcott-Rallison syndrome) | AR | ||
| FOXP3 | IPEX syndrome | XR | ||||
| GATA4/6 | Permanent neonatal diabetes with pancreatic agenesis and congenital heart defects | AD | ||||
| RFX6 | Neonatal diabetes, pancreatic hypoplasia, gallbladder agenesis, intestinal atresia (Mitchell-Riley syndrome) | AR | ||||
| GLIS3 | Congenital hypothyroidism, glaucoma, hepatic fibrosis, polycystic kidneys, developmental delay | AR | ||||
| PTF1A | Pancreatic and cerebellar hypoplasia | AR |
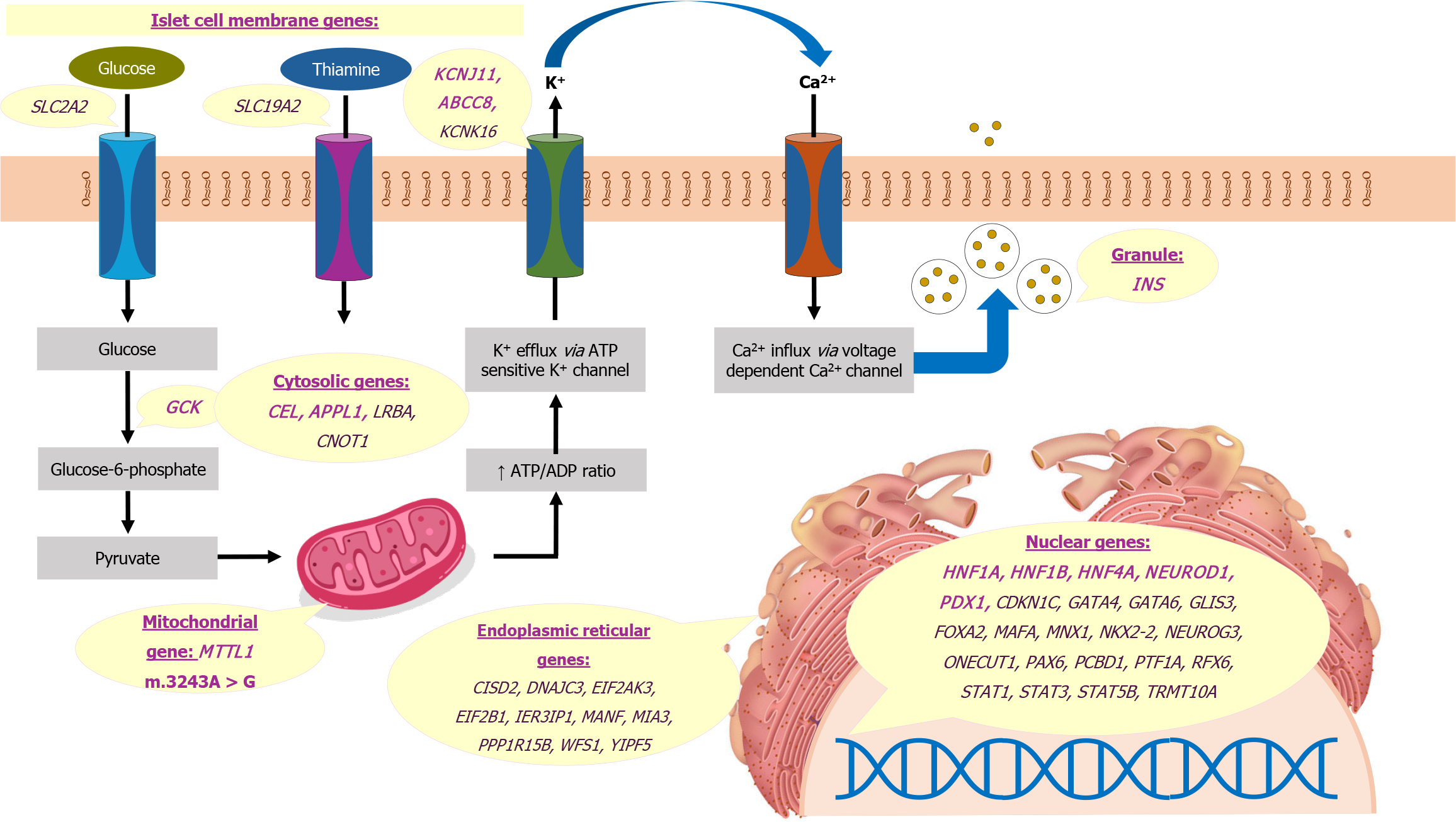
The mutations in MODY affect transcription, glucose sensing, protein folding, ion channel function, and signal transduction[19]. Most gene mutations causing MODY, including HNF4A, HNF1A, and HNF1B, result in defects in transcriptional regulation. The hepatic nuclear factors are expressed in various tissues, including the liver, pancreas, and kidney[64]. The HNF1A gene is crucial for pancreatic β-cell development and function, regulating genes related to insulin synthesis, secretion, and glucose metabolism. Heterozygous mutations in HNF1A lead to progressive β-cell dysfunction, reduced insulin secretion in response to glucose, and a lower renal threshold for glucose[37,65]. Similarly, the HNF4A gene, along with transcription factors like HNF1A and HNF1B, regulate insulin gene expression and control genes involved in insulin secretion, glucose transport, and metabolism[19,20]. HNF1B is expressed during embryonic development in various tissues, including the kidney. Mutations in HNF1B, in addition to diabetes mellitus, also result in renal cysts, renal dysplasia, and urinary tract malformations[41]. The other transcription factors PDX1, NEUROD1, KLF11, PAX4, and BLK also play critical roles in β-cell development, insulin biosynthesis, and secretion[19].
Mutations in the GCK gene lead to altered glucose sensing, raising the threshold for glucose-stimulated insulin secretion. This results in mild, stable hyperglycemia without the risk of complications[39]. Carboxyl-ester lipase or CEL-MODY, characterized by endocrine and exocrine pancreatic insufficiency, occurs due to misfolding and cytotoxic aggregation of carboxyl-ester lipase[66]. Mutations in the INS gene are characterized by misfolding and intracellular accumulation of proinsulin, β-cell apoptosis, and progressive insulin secretory defect[67]. ABCC8 and KCNJ11 mutations represent ion channel disorders that are also responsible for NDM[68]. Lastly, the APPL1 protein propagates the insulin signal within β-cells, and heterozygous loss-of-function mutations in this gene lead to defective glucose-stimulated insulin secretion and reduced survival of β-cells[69]. The genes affected in MODY and other monogenic diabetes are shown in Figure 3[4]. These genes are depicted based on their subcellular localization including the nucleus, endoplasmic reticulum, mitochondria, cytosol, islet cell plasma membrane, and insulin secretory granule.
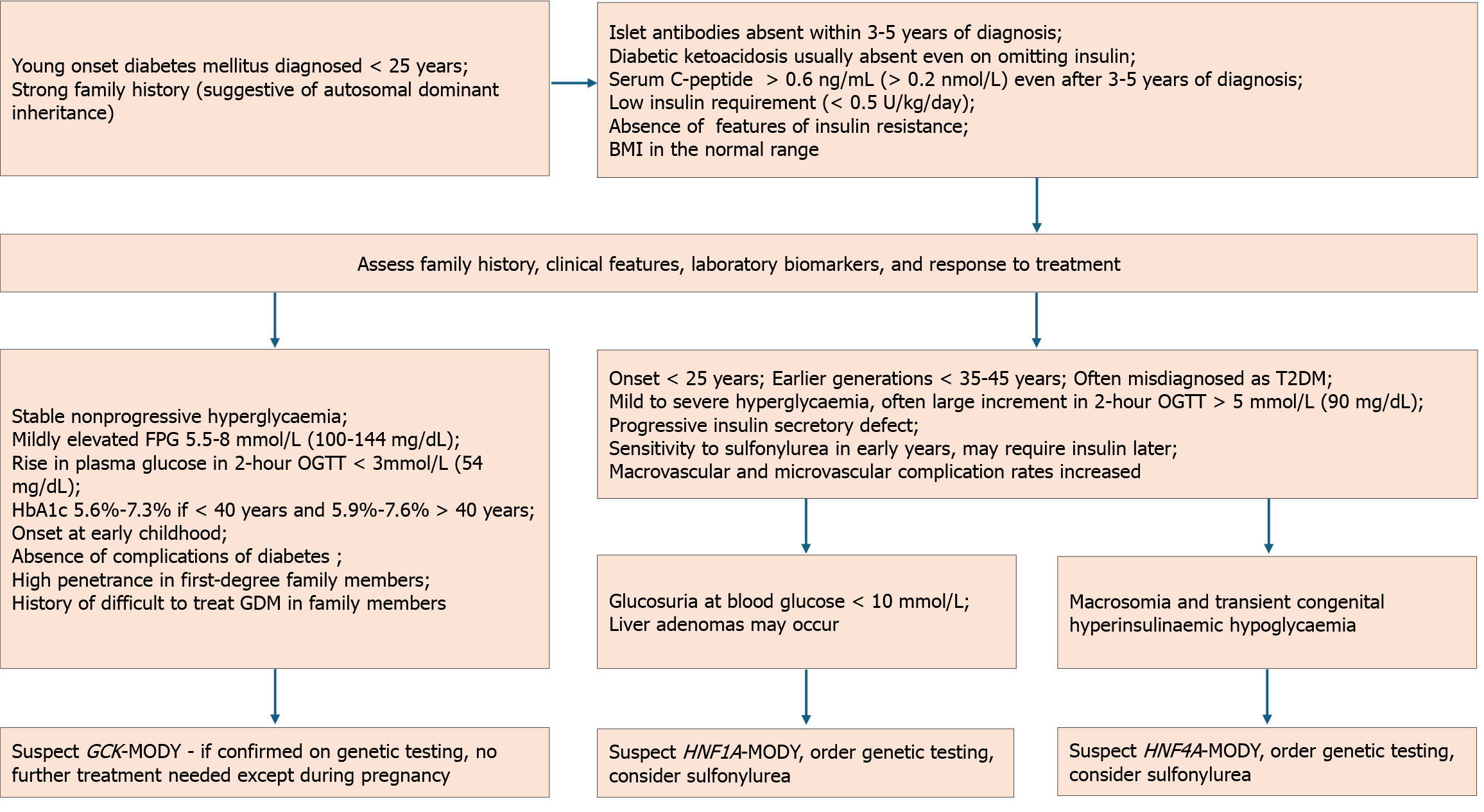
Clinical manifestations can vary widely depending on the genetic mutation. However, some common unifying features are observed across different MODY subtypes. MODY should be suspected in individuals with early-onset diabetes, a family history of autosomal dominant diabetes, and in the absence of typical features of T1DM and T2DM[16,19].
T1DM is characterized by absolute dependence on insulin, low serum C-peptide and the presence of autoantibodies. Usually, a panel of five antibodies comprising glutamic acid decarboxylase-65, islet antigen 2, zinc transporter 8, islet cell and insulin antibodies are used for demonstrating autoimmunity in T1DM. The absence of the antibodies and presence of normal C-peptide in serum or urine in a child or young adult should raise doubts about the diagnosis of T1DM[5]. T2DM is increasingly being recognized in adolescents and young adults and is typically associated with features of insulin resistance.
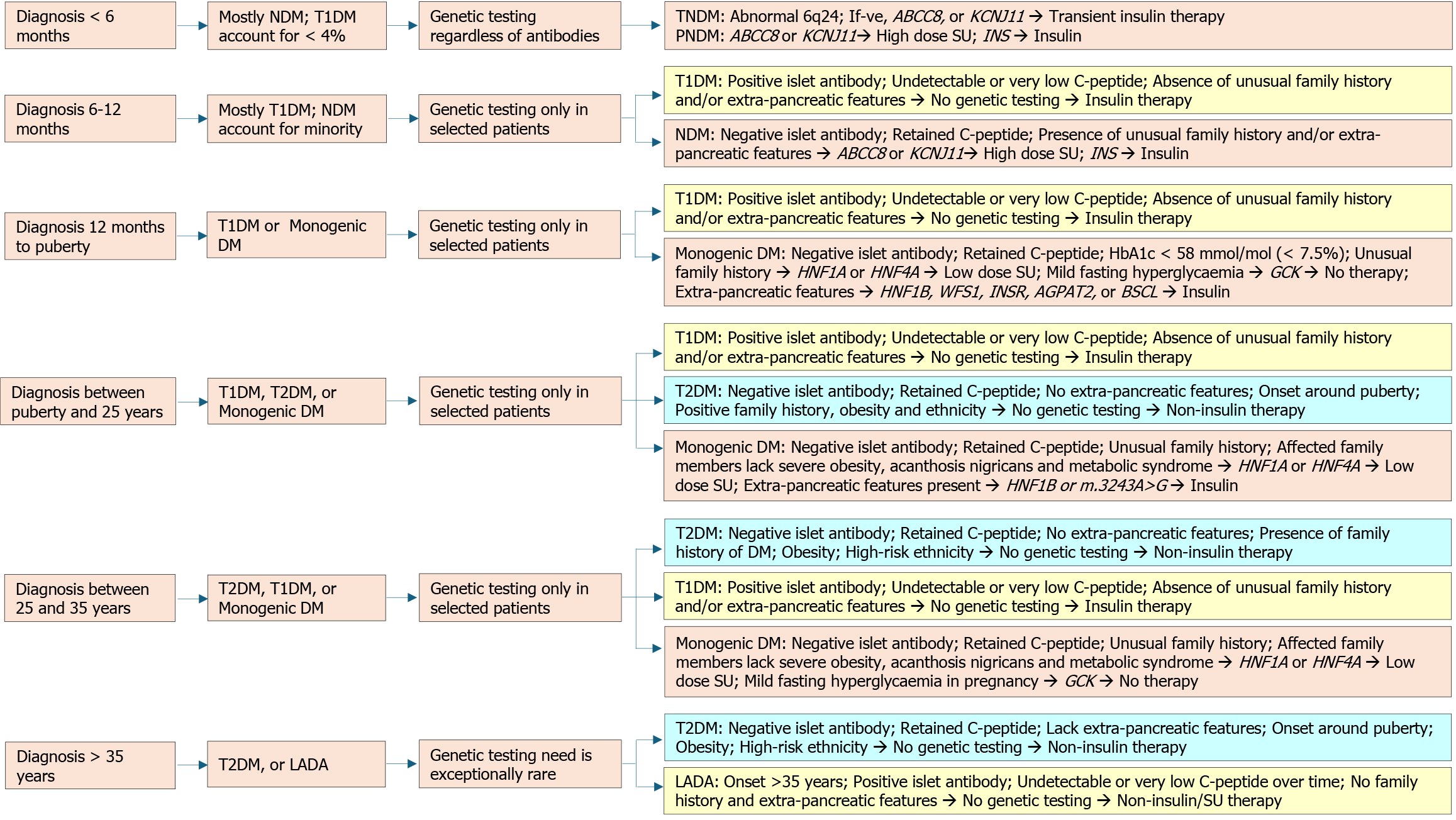
The onset of MODY is typically before the age of 25 years, though mild fasting hyperglycemia in GCK-MODY is present since birth but remains asymptomatic[16,39]. The average age of diagnosis of GCK-MODY is 14 years, but can vary according to the population being studied and the diagnostic practices in place[70]. Individuals with HNF1A mutations, the commonest form of MODY in many countries, develop diabetes by 25 years in 63%, by 35 years in 79%, and by 55 years in 96%[38]. Those harboring truncating mutations manifest earlier with a median age of diagnosis at 18 years, compared to those having missense mutations whose median age was 22 years[71]. According to a multinational registry from four European countries, the median age of diagnosis is 13.8 years for HNF4A-MODY and 13.5 years for HNF1B-MODY[42].
A strong family history is usually evident, but that is often the case with T2DM, highlighting the need for genetic testing[72]. Though individuals with MODY have normal body mass index (BMI) or are only mildly overweight, 4% of youth with obesity or overweight with presumed T2DM have mutations for MODY[73]. GCK-MODY is characterized by mild fasting hyperglycemia and does not require treatment except during gestation[39]. Mutations in HNF4A, HNF1A, and HNF1B genes manifest as progressive β-cell failure, with a risk of macrovascular as well as microvascular complications similar to T2DM[38,41,65]. Certain subtypes have specific characteristics such as renal developmental disease or cysts in HNF1B-MODY, macrosomia and/or neonatal hypoglycemia mostly in HNF4A-MODY and rarely in HNF1A-MODY, and exocrine pancreatic dysfunction or pancreatic cysts in CEL-MODY[19,74-76].
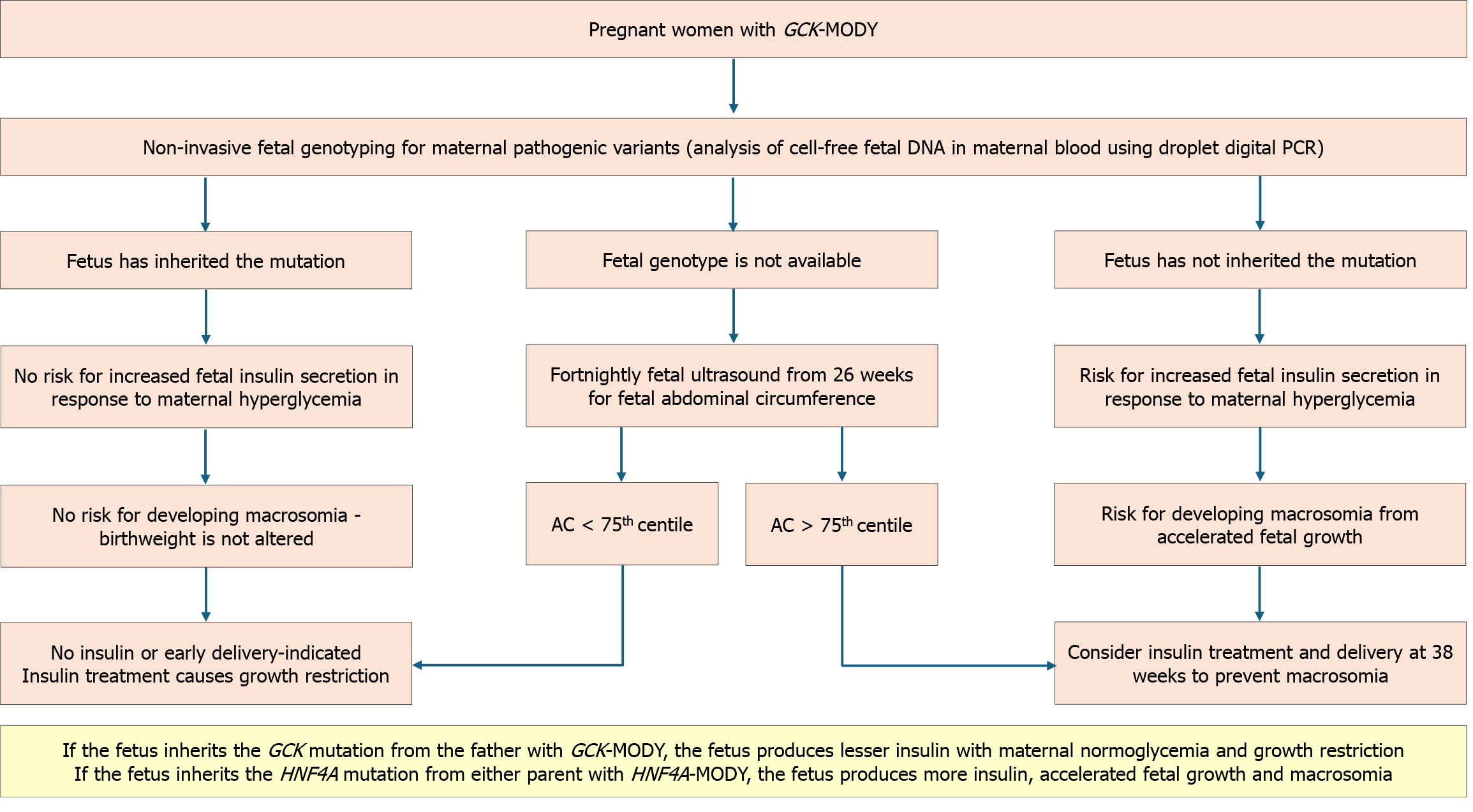
The clinical profile initially raises suspicion of MODY, which can be corroborated by biomarkers. Confirmation requires genetic testing. Detailed information should be obtained, including family history spanning three or more generations, the age and habitus at diagnosis, insulin dependence, response to medications used, and hypoglycemia history. Figure 4 represents the diagnostic algorithm for the three common MODY variants with onset of diabetes below 25 years, a strong family history of diabetes, and the presence of clinical features, biomarkers, and response to treatment.
While several biomarkers have been explored as diagnostic adjuncts, their clinical utility is limited. The wider availability of genetic testing via next-generation sequencing (NGS) has reduced the reliance on biomarkers. At best, biomarkers can be considered a supportive tool to guide the need for genetic analysis in resource-limited settings.
High-sensitivity C-reactive protein: A few studies suggest that high-sensitivity C-reactive protein (hs-CRP) levels are lower in HNF1A-MODY compared to other types of diabetes[77,78]. Deactivating mutations in HNF1A could result in reduced hs-CRP levels, given the presence of HNF1A binding sites in the promoter region of the CRP gene[79]. Other studies found an overlap in hs-CRP levels between HNF1A-MODY and different types of diabetes, suggesting that its role as a biomarker could be limited[80,81].
The hs-CRP assays used in a study from the United Kingdom and Croatia employed a wide-range latex-enhanced immunoturbidometric assay on an ADVIA 2400 analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany) with a quantification limit of 0.01 mg/L and Abbott hs-CRP method with a quantification limit of 0.1 mg/L, respectively. Both methods had a reproducibility coefficient of variation below 10.5%[82]. Platforms in another large multi-center European study included ADVIA 2400 analyzer (Siemens Healthcare Diagnostics, Frimley, United Kingdom), Konelab 60i analyzer (Thermo Fisher Scientific, Vantaa, Finland) and BN ProSpec analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany), with lower limits of detection of 0.03 mg/L, 0.25 mg/L and 0.16 mg/L respectively. Criteria combining low hsCRP (≤ 0.25 mg/L for the first assay and ≤ 0.5 mg/L for the second two assays) and the age of diagnosis ≤ 25 years resulted in 90% sensitivity for detection of HNF1A-MODY with specificity ranging from 65% to 81% across assays. The authors noted that despite the statistically significant differences, the findings were not relevant practically for diagnostic purposes[83].
Glycosuria: HNF1A-MODY can be associated with defects in the promoter region of sodium-glucose cotransporter 2 (SGLT2) and demonstrates a low renal threshold because of decreased transporter expression[84]. Disproportionate glycosuria is present in 30%-40% of the cases of HNF1A-MODY, and the presence of glycosuria at blood glucose < 10 mmol/L (180 mg/dL) could serve as an indicator of this MODY subtype[37,85]. However, the studies have been heterogeneous and used both quantitative tests[85,86] and qualitative assessments utilizing dipstick[87].
Apolipoproteins and high-density lipoproteins: HNF1A and HNF4A genes play key roles in the transcription of proteins regulating apolipoprotein and high-density lipoprotein (HDL) levels[37,88]. HDL levels are elevated in HNF1A-MODY compared to T2DM[37,89]. Individuals with HNF4A mutations, on the other hand, have reduced HDL-cholesterol, apolipoprotein A1, apolipoprotein A2, and triglyceride, while low-density lipoprotein-cholesterol can be raised[40,90].
Studies from the United Kingdom and Croatia revealed higher HDL-cholesterol among individuals with HNF1A-MODY. Those with the mutations had a median HDL-cholesterol of 1.33 mmol/L (interquartile range 0.51), compared to 1.15 mmol/L (interquartile range 0.40) in those without the mutations[82]. Additionally, individuals with HNF1A-MODY had lower plasma triglycerides (1.36 mmol/L vs 1.93 mmol/L, P = 0.07) than those with T2DM. HDL-cholesterol above 1.12 mmol/L was 75% sensitive and 64% specific in differentiating HNF1A-MODY from T2DM[89].
The HDL assay platforms used in different studies include an automatic biochemical analyzer (AU5800; Beckman Coulter Inc., Brea, CA, United States)[91] and Architect ci4100 device (Abbott Diagnostics, Wiesbaden, Germany)[92]. The number of participants in most studies was less than 50 to 70[91,93] with only one series with more than 500 participants[89]. There is currently insufficient evidence to consider HDL as a biomarker for MODY.
Urinary C-peptide creatinine ratio: In adults with diabetes for over five years, a 2-hour post-meal (largest meal) urinary C-peptide creatinine ratio (UCPCR) ≥ 0.2 nmol/mmol differentiated HNF1A-MODY or HNF4A-MODY from T1DM with 97% sensitivity and 96% specificity. Urinary C-peptide was measured by electrochemiluminescence immunoassay on a Roche Diagnostics (Mannheim, Germany) E170 analyzer. UCPCR was predictable and lower in T1DM than HNF1A/HNF4A MODY [median (interquartile range)] [< 0.02 nmol/mmol (< 0.02 nmol/mmol to < 0.02 nmol/mmol) vs 1.72 nmol/mmol (0.98-2.90 nmol/mmol)][94]. With current available evidence, UCPCR can’t be recommended to distinguish between monogenic diabetes and T2DM[95].
Other biomarkers: Other proposed biomarkers for HNF1A-MODY include fucosylated GP30 plasma glycans[80], microRNA-244[96], ghrelin[97], apolipoprotein M[98], and response to sulfonylurea[37,99]. Though the biomarkers can be a valuable clue, none can reliably diagnose MODY or distinguish between its subtypes. Genetic testing through NGS offers high throughput, low cost, and accurate diagnosis of monogenic diabetes and is recommended in suspected cases of MODY.
Several risk calculators have been developed to identify individuals who may have MODY based on clinical and laboratory features. One such calculator is the MODY risk calculator (MRC) developed and validated by the University of Exeter group for GCK-MODY, HNF1A-MODY, and HNF4A-MODY in 1 to 35-year-old Caucasians. It can be accessed online at https://www.diabetesgenes.org/exeter-diabetes-app/ModyCalculator and can assess the need for genetic testing in suspected cases[100]. MRC incorporates various factors such as gender, age at diabetes diagnosis, BMI, treatment history (diet, oral hypoglycemic agents, or insulin), glycated hemoglobin (HbA1c), family history of diabetes, ethnicity, and specific clinical characteristics. While initially validated for Caucasians, studies from China[101], Portugal[102], Australia[103], and Brazil[104,105] have found it to be applicable across different populations. In non-white populations, the probability is likely to be lower due to the higher prevalence of youth-onset T2DM[100]. The eight parameters used in the logistic regression model distinguished the three common monogenic diabetes subtypes collectively from T1DM or T2DM with a c-statistic of 0.98 and 0.95, respectively, in the Caucasian population[36].
The HNF1A-MODY clinical screening strategy utilizes four biomarkers: BMI < 28 kg/m2, hs-CRP < 0.75 mg/L, fasting insulin < 102 pmol/L, and HDL-cholesterol > 1.12 mmol/L. In a Chinese population with young-onset diabetes, meeting three of the four criteria resulted in a sensitivity of 90.5% and specificity of 73.6% for identifying HNF1A-MODY. A sensitivity of 88.9% and specificity of 89.6% were attained when all four criteria were present[106]. However, this score has not been validated in other ethnicities. The authors did not specify the laboratory method used for insulin analysis in this study. The wide variability of commercially available insulin assays must be considered when interpreting results using different platforms.
The HNF1B score is calculated using 17 items, including factors such as antenatal discovery, family history, structural and pathological involvement of the kidneys, electrolyte or uric acid abnormalities (hypomagnesemia, hypokalemia, and early onset of gout), as well as the involvement of the pancreas, genital tract, and liver. The optimal cut-off threshold to exclude HNF1B mutations was 8, resulting in a sensitivity of 98.2%, specificity of 41.1%, and negative predictive value of 99%[107].
HNF1A-MODY: HNF1A-MODY can lead to microvascular complications like retinopathy, nephropathy, and neuropathy, underscoring the importance of early diagnosis and treatment[108]. The phenotype of HNF1A-MODY ranges from mild nonprogressive hyperglycemia to progressive hyperglycemia and extra-pancreatic features[109]. Dietary management is often sufficient in individuals with mild hyperglycemia, while pharmacological interventions are necessary in progressive cases[5,63,110]. A randomized crossover trial demonstrated high sensitivity to oral sulfonylureas compared to metformin[111]. Decreased hepatic clearance of sulfonylurea derivatives, leading to increased serum levels and improved drug efficacy, probably increases the response to the drug[112].
International Society for Pediatric and Adolescent Diabetes guidelines recommend low-dose formulations like 20-40 mg/day gliclazide as the first-line therapy due to superior glycemic control and minimal risk of treatment failure[5]. In an observational study, many initially presumed T1DM cases could be shifted from insulin to sulfonylurea after HNF1A-MODY was diagnosed. Shorter diabetes duration, lower HbA1c, and normal or low BMI improve the chance of successful transition. For those with diabetes > 11 years and poor glycemic control, sulfonylurea can be considered as an adjunct to insulin rather than a replacement, especially in the presence of overweight or obesity[113].
Other treatment options include meglitinides, dipeptidyl peptidase-4 inhibitors[114], and glucagon-like peptide-1 receptor agonists[115]. In a randomized controlled trial from Denmark, the combination of glimepiride and linagliptin did not improve the mean amplitude of glycemic excursions (mean difference of -0.7 mmol/L, P = 0.1540) compared to glimepiride alone. However, reductions in the coefficient of variation on continuous glucose monitoring (-3.6%, P = 0.0401), HbA1c (-0.5%, P = 0.0048), and the dose of glimepiride (-0.7 mg/day, P = 0.0099) were demonstrated[114].
The safety of SGLT2 inhibitors in HNF1A-MODY remains unclear. Severe insulin deficiency, reduced expression of SGLT2 in the proximal tubules, and glycosuria cast doubts on the safety and efficacy of SGLT2 inhibitors[110,116]. Caution should be exerted, as diabetic ketoacidosis (DKA) has been reported in two HNF1A-MODY patients with the use of SGLT2 inhibitors[117].
HNF4A-MODY: Individuals with HNF4A-MODY typically exhibit normal glucose tolerance in the first or second decade but experience a gradual decline in insulin secretion after that. Complication rates are akin to those seen in T1DM and T2DM[40]. Sulfonylurea is the recommended treatment when lifestyle adjustments are insufficient. To avoid hypogly
GCK-MODY: Individuals with GCK-MODY do not require pharmacological agents to control the mild nonprogressive fasting hyperglycemia other than during pregnancy[5,39]. Women are often misdiagnosed as gestational diabetes mellitus (GDM). In pregnancies with a known GCK-MODY mutation, treatment recommendations are based on the fetal genotype and growth[72]. If the mother has a heterozygous GCK mutation but the fetus has a normal genotype, insulin treatment is necessary to prevent fetal macrosomia. Conversely, if the fetus is affected by GCK-MODY, treatment is not required. In the absence of fetal genotyping facilities, starting serial ultrasounds from 26 weeks onward is recommended. If the fetal abdominal circumference exceeds the 75th percentile, it indicates the absence of the fetal GCK mutation. Insulin is needed to prevent macrosomia in such situations. The insulin dosage required to achieve normoglycemia is higher than in GDM[119,120]. The algorithm for managing GCK-MODY in pregnancy is given in Figure 5[120-123].
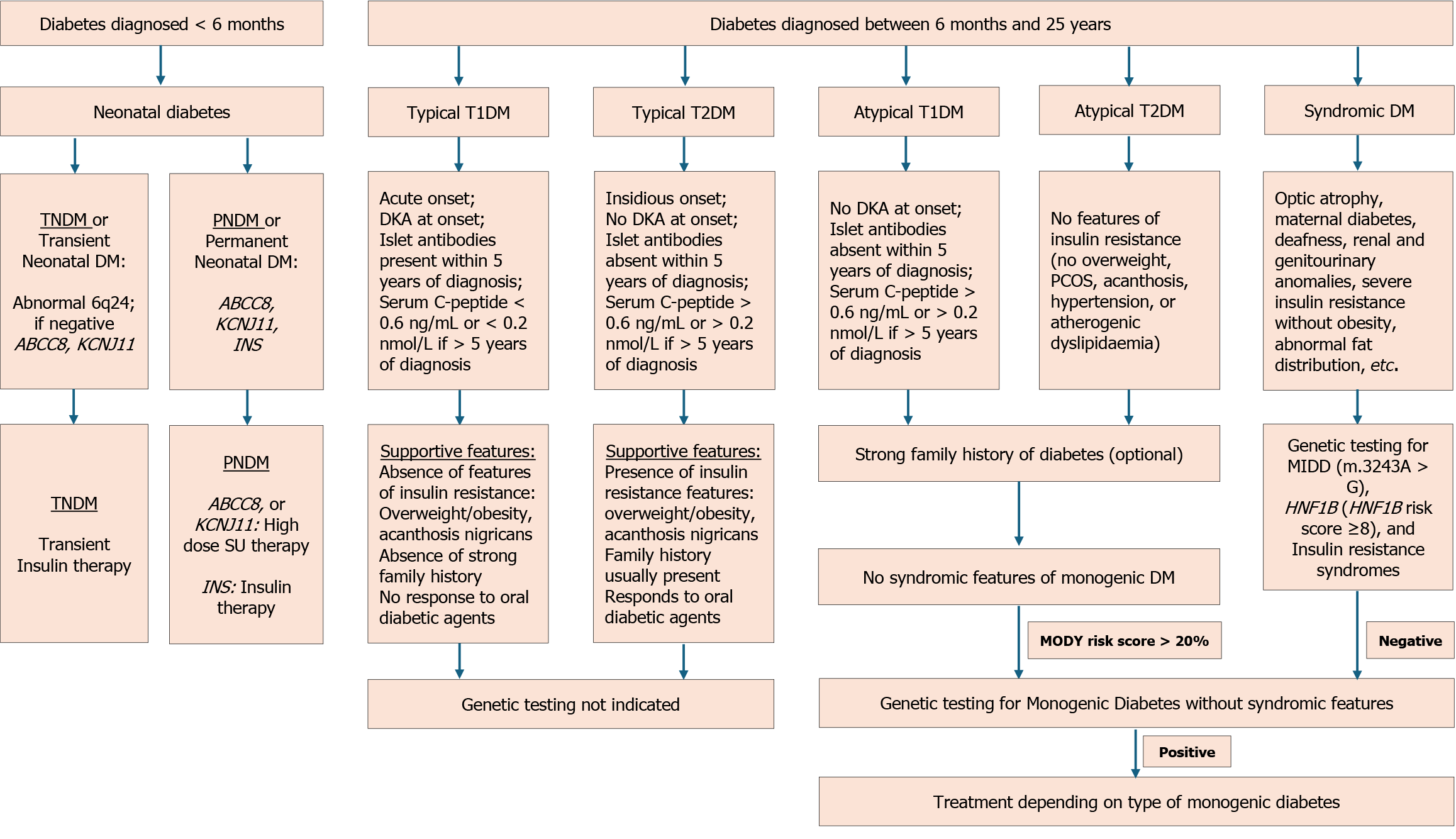
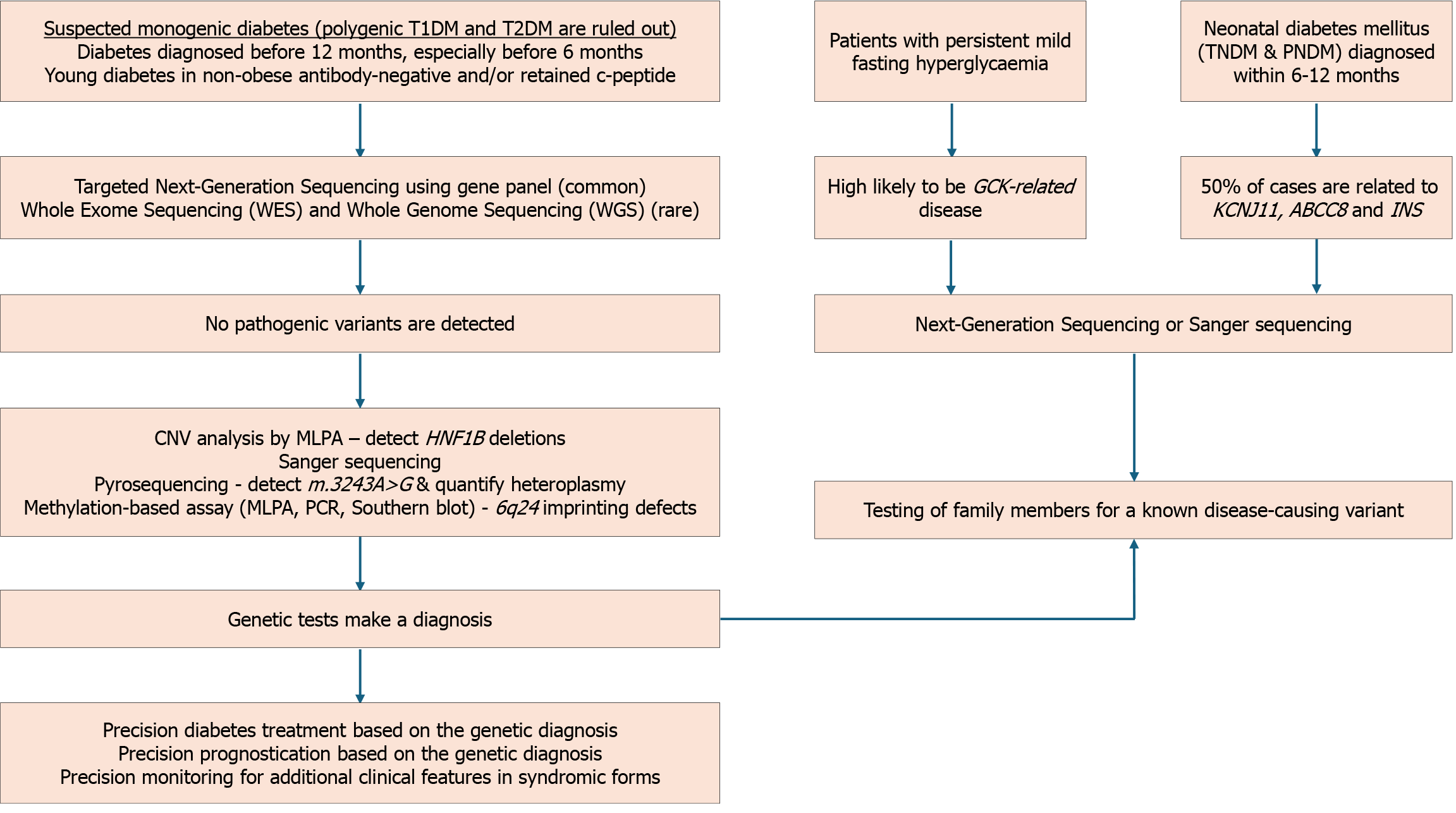
NDM is a rare condition affecting 1:300000-400000 live births[17]. All infants diagnosed under 6 months should undergo genetic testing for a monogenic cause, regardless of their islet autoantibody status[5]. While T1DM is rare before 6 months, it can still occur in 4% of cases[124]. Some instances of NDM may be diagnosed between 6 and 12 months, although most infants in this age group have T1DM[51,125]. Reasons for considering genetic testing between 6-12 months include negative autoantibody results, extra-pancreatic features like gastrointestinal anomalies or congenital defects, unusual family history, or the development of multiple autoimmune disorders[5].
NDM is a diverse group of diseases with several reported genetic alterations. It can be attributed to two main groups of mechanisms: Malformation of the pancreas, which affects the development or survival of insulin-secreting cells, or abnormal functioning of the existing pancreatic β-cells[17,18]. In a series of 174 index cases of NDM, 47 had no detectable genetic defect, 40 (23%) had 6q24 abnormalities, 43 (25%) had mutations in KCNJ11, 31 (21%) had mutations in ABCC8, and 13 (7.4%) had mutations in INS[126]. About half of NDM cases require lifelong treatment and are classified as permanent NDM (PNDM)[126]. In contrast, transient NDM (TNDM) cases experience remission within a few weeks or months, though it may recur later in life[18,51,125].
PNDM is a rare form of diabetes that typically manifests during infancy, with around 90% of cases appearing within the first six months of life[126]. The majority of infants with PNDM carry activating mutations in either of the two genes encoding the KATP channel (KCNJ11 or ABCC8) or the INS gene. The mode of inheritance is autosomal dominant for mutations of KCNJ11, autosomal dominant or autosomal recessive for mutations of ABCC8 and INS, and autosomal recessive for mutations of GCK and PDX1[52].
Activating mutations in KCNJ11 or ABCC8, which code for the inner subunit (Kir6.2) and the outer subunit (sulfonylurea receptor 1), respectively, prevent KATP channel closure. The resultant hyperpolarized membrane state prevents calcium channel activation and insulin secretion[53,127]. KATP NDM typically presents in the first month of life as excessive urination, thirst, poor growth, dehydration, ketosis, and developmental delays[68,127]. The median age at diagnosis of PNDM is 9.6 weeks[128]. DKA was reported in 85% of cases[127]. Around one-fifth of children with KCNJ11 mutations exhibit neurological symptoms from affection of KATP channels expressed in neurons and muscles. Severe mutations may result in the triad of developmental delay, early-onset epilepsy, and NDM, while milder mutations lead to less severe developmental disorders without epilepsy[129]. Intrauterine growth restriction (IUGR) is present in most cases and relates to decreased fetal insulin release[18]. Dominant heterozygous mutations in INS are the next common cause of NDM. Neurological manifestations are usually absent. Presentation can be delayed in some cases and can occur after 6 months.
NDM, due to INS gene mutation, should be managed with insulin[54,130]. Most children with activating mutations in the KATP channel genes can be switched from insulin to sulfonylurea tablets or suspensions. Treatment with sulfonylurea stabilizes glycemic control, carries minimal risk of hypoglycemia, and benefits neurological manifestations[131,132].
The insulin output deficit in TNDM may stem from delayed maturation of pancreatic islets and β-cells due to altered gene expression on chromosome 6. Overexpression of the chromosome region 6q24 is the primary cause of TNDM1, the most common subtype of TNDM[133,134]. The region houses two major TNDM gene candidates, ZAC (zinc finger, apoptosis, and cell cycle) and HYMA1 [(Fe) hydrogenase subunit HymA][135,136]. Normally, the 6q24 locus is imprinted, allowing only paternal allele expression, but in 6q24-TNDM, in about 70% of cases, loss of imprinting and consequent overexpression of both genes occur[55]. The overexpression can arise from paternal uniparental disomy of chromosome 6, duplication of the TNDM1 region of the paternal chromosome 6, or maternal hypomethylation of the TNDM1 region[17]. The rest of the cases of TNDM involve mutations in KCNJ11 and ABCC8 genes encoding the KATP channel and rarely HNF1B gene mutations[17,137-139].
The infant typically presents in the first few weeks after birth. Neonates harboring 6q24 mutations are diagnosed at a median age of one week. The presentation in those with KATP mutations occurs slightly later, at around 4 weeks[140]. DKA is less common compared to PNDM, but IUGR is more frequent[53]. Remission occurs before 4 months, but 70% of cases recur within the second decade[125,141]. Patients with a 6q24 locus abnormality may have developmental defects such as macroglossia, umbilical hernia, cardiac, renal, and urinary malformations, nonautoimmune anemia, hypothy
Mutations in many genes (see Table 1) have been linked to autoimmune syndromes leading to diabetes in infancy with pancreatic islet autoantibodies[5,144]. These monogenic conditions, akin to pediatric T1DM, have been considered rare among early infancy T1DM cases[4,145]. Mutations in the FOXP3 gene are responsible for immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome[29]. Other gene mutations like CTLA4, ITCH, IL2RA, and LRBA also cause autoimmune conditions with diabetes, enteropathy, hypothyroidism, and hemolytic anemia[146-149].
Several rare forms of monogenic diabetes have been described, including insulin resistance syndromes, mitochondrial diabetes, and syndromic forms. Three syndromes of monogenic insulin resistance are Donohue syndrome (leprechau
Monogenic lipodystrophy is associated with diabetes and results from the complete or partial absence of adipose tissues. Monogenic lipodystrophy is classified into two main types: Congenital generalized lipodystrophy (CGL) and familial partial lipodystrophy (FPLD). CGL, often caused by AGPAT2 or BSCL2 gene mutations, shows generalized fat loss along with diabetes and dyslipidemia. FPLD results from LMNA or PPARG mutations and presents as fat loss in specific areas like limbs. Leptin replacement therapy, like metreleptin, benefits individuals with FPLD and CGL, improving glycemia and cardiometabolic outcomes[25,26].
The presence of diabetes and multi-organ involvement, especially early-onset deafness with a maternal inheritance pattern, should raise the suspicion of a mitochondrial disorder. Mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes, and maternally inherited diabetes and deafness are the primary mitochondrial diabetes disorders, often attributed to the m.3243A > G mutation[21,150]. Mitochondrial diabetes typically manifests in adulthood, with earlier onset occurring in the presence of a higher proportion of mutated mitochondrial genomes[151]. The clinical details of the different forms of monogenic diabetes are summarized in Table 2.
Genetic screening for monogenic diabetes has been transformed by NGS technologies, replacing labor-intensive and costly methods like Sanger sequencing[152]. NGS encompasses two primary approaches: Targeted gene panels focusing on known monogenic diabetes genes and whole-exome sequencing (WES) analyzing the protein-coding regions of the genome[153]. Targeted gene panels are efficient and provide specific information. In contrast, WES offers comprehensive data, albeit with bioinformatics challenges[154-156].
Given the unfeasibility of universal genetic screening for all cases of youth-onset diabetes, a combination of phenotypic and biochemical variables offers the best method to identify candidates for genetic testing[4]. A MRC to identify candi
NGS: Targeted gene panels using NGS are the preferred technique for confirming the diagnosis of monogenic diabetes[9]. Owing to their targeted nature, they rapidly and cost-effectively provide specific information with a lower risk of secondary information and data complexity[11,158]. While targeted gene panels require periodic redesign and validation as new genes are identified, WES doesn’t have this limitation. However, WES generates large datasets that can be challenging to analyze, but it also allows for the discovery of pathogenic variants in genes not initially considered. Effective use of WES contributes to ongoing research and identifying new monogenic diabetes genes[9,52,159,160]. Several large series have confirmed the clinical utility of NGS for diagnosing MODY and other forms of monogenic diabetes[161-163].
Detection of copy number variations: Multiplex ligation-dependent probe amplification (MLPA) is used for detecting copy number variations (CNVs) such as deletions or duplications of one or more exons seen in HNF1B-MODY, GCK-MODY, or in mitochondrial diabetes with m.3243A > G mutation[9,164-166]. Conventionally, NGS cannot detect CNVs. However, CNV calling, a new bioinformatic tool, can assess CNVs using NGS output data without requiring additional sequencing[9,167]. MODY occurring due to CNVs in GCK and HNFB1 have been detected by this technique[168,169]. A quantitative method based on pyrosequencing to analyze the heteroplasmy of m.3243A > G has been demonstrated to be accurate and reliable[170].
Sanger sequencing: Sanger sequencing, though largely replaced by NGS, remains an accurate and reliable method for detecting point mutations, small insertions/deletions (indels), and known variants. It is especially valuable for con
Detection of hypomethylation: The diagnosis of 6q24-TNDM may require a demonstration of relative hypomethylation within the 6q24 differentially methylated region by DNA methylation analysis. Southern blot, methylation-specific-MLPA, or methylation-specific polymerase chain reaction can confirm hypomethylation. DNA methylation studies can diagnose 6q24-TNDM caused by any genetic defect but don’t establish a specific mechanism[171,172].
Diagnosing monogenic diabetes presents several challenges. The limited awareness about this condition among healthcare providers can result in underdiagnosis or misdiagnosis in many parts of the world[7]. In the United Kingdom, several initiatives, including the genetic diabetes nurse project, the MODY probability calculator, and targeted NGS, have improved the detection of MODY by threefold over 10 years[173].
The clinical and genetic heterogeneity of monogenic diabetes, along with its overlap with common T1DM and T2DM, makes it challenging to distinguish based solely on clinical criteria[74]. The presence of mosaic patterns occasionally confounds the diagnostic process[9]. Not all individuals with pathogenic variants develop the disease because of the reduced penetrance and variable expressivity. Additionally, a wide range of symptoms and age of onset in those who manifest the disease can further complicate the diagnosis. Furthermore, GCK-MODY and T2DM can sometimes co-exist, complicating the management[174]. Understanding the genetic modifiers that influence penetrance and expressivity can reveal protective genetic variants, offering insights into more common forms of diabetes and potential new therapeutic strategies.
Genetic testing for MODY relies heavily on the depth and coverage of sequencing. Sequence depth refers to the number of times a nucleotide is read during the sequencing process, and higher depth increases the confidence in detecting variants, especially those that are rare or occur at low frequencies. Coverage, on the other hand, pertains to the proportion of the target region (in this case, the MODY genes) that is successfully sequenced. Comprehensive coverage ensures that all potential mutations within the MODY genes are identified. For accurate diagnosis and differentiation between MODY subtypes, as well as between MODY and other forms of diabetes like T2DM, both high sequence depth and extensive coverage are critical. Insufficient depth or coverage could lead to missed mutations or false-negative results, undermining the reliability of genetic testing[159,175].
Another challenge in clinical practice is negative genetic test results in a person with a strong clinical phenotype of MODY. It’s important to re-evaluate the clinical diagnosis to ensure it aligns with MODY characteristics. Reassessing laboratory and clinical data, such as C-peptide levels and antibody testing, alongside a detailed family history, is crucial. Further genetic testing in cases with high probability, including comprehensive panels, such as WES or whole genome sequencing, may be necessary. Additionally, techniques like MLPA should be used to check for large deletions or duplications. Seeking expert consultation from specialists in monogenic diabetes or geneticists can provide valuable insights. Longitudinal follow-ups to monitor the individual over time and enrolling them in research or clinical trials for advanced diagnostics can aid in accurate diagnosis[12,176].
NGS has revolutionized genetic testing for monogenic diabetes, making it more accessible and cost-effective. NGS has expanded the reach of diagnosis, including those with other single-gene disorders. However, with increased testing, there’s also a rise in variants of unknown significance (VUS). These variants pose a challenge in distinguishing disease-causing mutations from harmless genetic variations[177].
The American College of Medical Genetics and Genomics and the Association for Molecular Pathology published a consensus statement outlining recommendations for the interpretation of sequence variants. The statement suggested using standardized terminology like ‘pathogenic’, ‘likely pathogenic’, ‘uncertain significance’, ‘likely benign’, and ‘benign’ based on population data, computational analyses, functional studies, and segregation patterns to define variants[178].
National Institutes of Health-funded resources like Clingen and Clinvar curate and publicize evidence on gene-disease and gene-variant relationships and refine practice guidelines periodically[179]. The formation of expert panels like the Clingen Monogenic Diabetes Gene Curation Expert Panel and the Monogenic Diabetes Variant Curation Expert Panel represents a significant step in establishing guidelines and standards for determining the causative genes and variants in monogenic diabetes[180,181]. Assessment of variant pathogenicity in exome-based sequencing is complex and relies on computational models, in vitro analyses, and clinical information[182].
In silico models: In silico models for monogenic diabetes are computational tools used to simulate genetic processes and interactions associated with disease mutations. These models can help predict the effects of genetic variants on protein structure, function, and overall disease phenotype. They incorporate data from various sources, such as genomic sequences, protein databases, and functional assays, to provide insights into the pathogenicity of specific genetic variants and their contribution to disease development[178,183-185].
Functional studies: Functional studies help to assess the pathogenicity of genetic variants. These studies examine if the genetic variants impact the protein structure, function, and cellular processes related to glucose metabolism and insulin physiology. Both in vitro and in vivo models, including cell lines, patient-derived induced pluripotent stem cells (iPSCs), and animal models (e.g., knockout or transgenic mice) have been employed. In vitro assays using β-cell lines or iPSC-derived β-like cells help assess the effect of specific mutations on insulin secretion, ion channel activity, and gene expression. Animal models provide insights into the systemic effects of mutations, such as glucose intolerance and beta-cell mass regulation. Advanced techniques like CRISPR-Cas9 genome editing, single-cell transcriptomics, and high-throughput functional screening have broadened the scope of functional studies[9,176,182].
Clinical and biomarker data: Finally, the clues towards pathogenicity are often garnered from studying the proband and their family members in detail and scrutinizing relevant biomarkers. Analysis of the presence or absence of the variant in affected and unaffected individuals within the family, observing patterns of inheritance, and assessing whether the variant co-segregates with the disease phenotype across generations provides crucial information. Conclusive proof often involves observing the co-segregation of the variant from a different kindred[182,186].
The availability of genetic testing raises vital ethical issues. Informed consent is mandatory to make the individual and family members understand the implications of testing, including the potential for discovering VUS. Privacy and confidentiality are significant issues, given the sensitivity of genetic information. Additionally, the familial implications of test results can create additional dilemmas. There is a risk of discrimination based on genetic information, underscoring the need for legal protection[187]. Lastly, the psychological impact of receiving test results can be a challenge and might necessitate counselling and support. These ethical considerations highlight the need for a coordinated approach in implementing genetic testing to prioritize the rights and well-being of the care-seeker[188].
Access to genetic testing may be restricted in resource-limited settings as inadequate infrastructure, lack of trained personnel, and financial constraints can be critical barriers. Shortage of genetic counsellors can be an additional constraint. The geographic variation in genetic and clinical spectrum of monogenic diabetes can further complicate management. Increasing the awareness among healthcare providers about genetic testing is important. The available resources should be optimally used based on algorithms that consider clinical information and logistic boundaries. Finally, policy changes should be instituted, promoting funding in low-resource settings for advocating cost-effective methods[187].
Many cases of monogenic diabetes remain undiagnosed due to limited awareness among healthcare providers, clinical heterogeneity, overlapping with more common types of diabetes, limited access to genetic testing, cost barriers, complex interpretation of genetic results, and low clinical suspicion in asymptomatic or mildly symptomatic cases[7]. Screening tools must be developed and validated across various ethnicities to identify candidates for genetic testing in youth-onset diabetes, as population-based genetic testing is impractical.
NGS has made genetic testing accessible, but handling the data generated from extensive testing and the interpretation of the results remains an evolving process. The causal associations of many variants are generally accurate, yet false assignments are not uncommon and can have significant consequences for patients and resources[189]. Though the cost of genetic testing has come down, significant barriers persist, such as insurance coverage and diagnostic inertia, stemming from complex procedures for ordering genetic tests.
The process of evaluating evidence for variant implication involves two key steps. Firstly, the overall evidence for the involvement of a gene is assessed, primarily focusing on statistical support from genetic analyses and possibly augmented by data from informatics and functional studies. Secondly, a comprehensive evaluation of the genetic, experimental, and informatics-based support for individual candidate variants is conducted[183]. Despite advances in functional studies, the precise molecular mechanisms underlying many monogenic diabetes variants are not fully elucidated. Further research is needed to understand these mechanisms and develop targeted therapies.
The variability in disease penetrance and phenotypic expression of pathogenic variants can complicate management. While some individuals may remain asymptomatic, others may experience severe clinical symptoms, making it challenging to predict the disease course and determine appropriate interventions. Understanding the phenotypic spectrum of variants in dominant genes, from benign to disease-causing, is essential. For example, common variants in genes like KCNJ11 and GCK can cause minor changes in glucose regulation, while less frequent variants with a more pronounced functional impact are associated with MODY and neonatal diabetes[39,190].
Monogenic diabetes models have advanced our understanding of diabetes pathophysiology and the genetics of common diabetes. Insights from these studies also help to interpret genome-wide association study data for T1DM and T2DM. The targets of major anti-diabetic drugs like sulfonylurea and thiazolidinedione are linked to two genes involved in the pathogenesis of monogenic diabetes, KCNJ11 and the peroxisome proliferator-activated receptor γ, respectively.
Translating knowledge from monogenic diabetes research into effective treatments remains challenging, with direct drug development remaining elusive. However, iPSCs from monogenic diabetes represent a significant breakthrough. Differentiating iPSCs into β-like cells allows researchers to study disease mechanisms like impaired insulin secretion in a controlled laboratory setting[191]. This understanding can pave the way for newer treatments in the broader context of diabetes management[192]. The various ways in which monogenic diabetes is misdiagnosed, and its impact are given in Table 3[7,74,118,193,194].
| Stage of life | Misdiagnosis | Clinical clues | Impact of accurate diagnosis on the management plans | |
| HNF1A and HNF4A related diabetes | Neonate and infancy | Congenital hyperinsulinism | Transient neonatal hypoglycaemia (diazoxide discontinued in the first decade in the majority). Progresses to hyperglycemia (usually < 25 years); Association with Fanconi syndrome; Family history of diabetes; Macrosomia independent of glycaemic control (HNF4A-MODY is a more likely cause than HNF1A-MODY) | Treatment with diazoxide; Natural history differs from other causes of congenital hyperinsulinism |
| Childhood and adolescence | T1DM | Islet antibodies absent within 3-5 years of diagnosis | Stop insulin; Treat with low-dose sulfonylurea (respond initially); use other antidiabetics as necessary; avoid SGLT2i in HNF1A-MODY | |
| Diabetic ketoacidosis is usually absent even when omitting insulin; serum C-peptide > 0.6 ng/mL (> 0.2 nmol/L) after 3-5 years of onset; low insulin requirement (< 0.5 U/kg/day) | ||||
| Adults | T2DM | Absence of features of insulin resistance; BMI in the normal range | Treat with low dose SU (respond initially); use other antidiabetics as necessary; avoid SGLT2i in HNF1A-MODY; Aim to reduce CVD risk despite normal lipids in HNF1A-MODY | |
| Pregnancy | GDM | Insulin is recommended therapy, consider additional glyburide if control inadequate, especially in 1st trimester | Treat with insulin, fetal growth surveillance from 26 weeks | |
| GCK related diabetes | Childhood and adolescence | T1DM | Stable nonprogressive mild fasting hyperglycemia; islet antibodies absent within 3-5 years of diagnosis; diabetic ketoacidosis absent on omitting insulin; strong family history | Stop insulin; pharmacological intervention is not needed |
| Adults | T2DM | Stable nonprogressive mild fasting hyperglycemia; no features of insulin resistance or obesity; strong family history | Pharmacological intervention is not needed; screening for microvascular and macrovascular complications is not indicated | |
| Pregnancy | GDM | Stable nonprogressive mild fasting hyperglycemia; Minimal rise in plasma glucose levels after oral glucose or food | Insulin if the fetus does not have the mutation; no treatment if the fetus carries the mutation | |
| Neonatal diabetes mellitus | Neonate and infancy | T1DM | Negative autoantibody testing; Extra-pancreatic features (gastrointestinal anomalies, congenital defects, and neurological disorders); unusual family history; small for gestational age | Should be transitioned to high-dose sulfonylurea from insulin |
| Mitochondrial diabetes | Adults | T2DM | Maternal inheritance; associated sensorineural hearing loss or progressive external ophthalmoplegia at an early age | Oral antidiabetics; may require insulin later; avoid metformin |
Monogenic diabetes encompasses a diverse range of conditions stemming from single-gene defects. The condition is often misdiagnosed and mismanaged. Diagnosing monogenic diabetes is hindered by limited awareness among healthcare providers, clinical and genetic complexities, and cost barriers to genetic testing. MODY, the common form of monogenic diabetes, is suspected based on early-onset diabetes, family history, and biomarkers, with confirmation usually through NGS. NDM, affecting infants typically before 6 months of age, necessitates early genetic testing for accurate diagnosis and tailored management. Management of monogenic diabetes opens the window for precision medicine, largely made possible by broader availability and accessibility of NGS. Targeted gene panels offer specificity and cost-effectiveness, although WES offers wider opportunities for gene discovery. NGS has increased the accessibility of genetic testing but also has led to the identification of VUS. Advanced computational models, functional studies, and segregation analysis for pathogenicity assessment are required to understand the significance of VUS. Increased awareness, accessibility to genetic testing, and continued research are pivotal for overcoming challenges and providing precision care in monogenic diabetes mellitus.
We thank Ms Fernandez-Jerrin J for providing the audio clip for the core tip of this article.
| 1. | Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, Moudiotis C, Smith R, Fraser B, Robertson S, Greene S, Ellard S, Pearson ER, Hattersley AT; UNITED Team. Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes. Diabetes Care. 2016;39:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, Topol EJ, Seufferlein T, Boehm BO. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med. 2017;15:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | IDF Diabetes Atlas. IDF Diabetes Atlas Reports. [cited March 04, 2025]. Available from: https://diabetesatlas.org/atlas-reports/. |
| 4. | Bonnefond A, Unnikrishnan R, Doria A, Vaxillaire M, Kulkarni RN, Mohan V, Trischitta V, Froguel P. Monogenic diabetes. Nat Rev Dis Primers. 2023;9:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (1)] |
| 5. | Greeley SAW, Polak M, Njølstad PR, Barbetti F, Williams R, Castano L, Raile K, Chi DV, Habeb A, Hattersley AT, Codner E. ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1188-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Owen KR. Monogenic diabetes: old and new approaches to diagnosis. Clin Med (Lond). 2013;13:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 8. | Yeung RO, Hannah-Shmouni F, Niederhoffer K, Walker MA. Not quite type 1 or type 2, what now? Review of monogenic, mitochondrial, and syndromic diabetes. Rev Endocr Metab Disord. 2018;19:35-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Murphy R, Colclough K, Pollin TI, Ikle JM, Svalastoga P, Maloney KA, Saint-Martin C, Molnes J; ADA/EASD PMDI, Misra S, Aukrust I, de Franco E, Flanagan SE, Njølstad PR, Billings LK, Owen KR, Gloyn AL. The use of precision diagnostics for monogenic diabetes: a systematic review and expert opinion. Commun Med (Lond). 2023;3:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Harris AG, Letourneau LR, Greeley SAW. Monogenic diabetes: the impact of making the right diagnosis. Curr Opin Pediatr. 2018;30:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Campbell MR. Review of current status of molecular diagnosis and characterization of monogenic diabetes mellitus: a focus on next-generation sequencing. Expert Rev Mol Diagn. 2020;20:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Vaxillaire M, Froguel P, Bonnefond A. How Recent Advances in Genomics Improve Precision Diagnosis and Personalized Care of Maturity-Onset Diabetes of the Young. Curr Diab Rep. 2019;19:79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zammouri J, Vatier C, Capel E, Auclair M, Storey-London C, Bismuth E, Mosbah H, Donadille B, Janmaat S, Fève B, Jéru I, Vigouroux C. Molecular and Cellular Bases of Lipodystrophy Syndromes. Front Endocrinol (Lausanne). 2021;12:803189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Iqbal J, Jiang HL, Wu HX, Li L, Zhou YH, Hu N, Xiao F, Wang T, Xu SN, Zhou HD. Hereditary severe insulin resistance syndrome: Pathogenesis, pathophysiology, and clinical management. Genes Dis. 2023;10:1846-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Balasubramanyam A. Defining and Classifying New Subgroups of Diabetes. Annu Rev Med. 2021;72:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Antal Z. Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines. 2021;2:485-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Polak M, Cavé H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J Rare Dis. 2007;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Beltrand J, Busiah K, Vaivre-Douret L, Fauret AL, Berdugo M, Cavé H, Polak M. Neonatal Diabetes Mellitus. Front Pediatr. 2020;8:540718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Nkonge KM, Nkonge DK, Nkonge TN. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin Diabetes Endocrinol. 2020;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 20. | Yahaya TO, Ufuoma SB. Genetics and Pathophysiology of Maturity-onset Diabetes of the Young (MODY): A Review of Current Trends. Oman Med J. 2020;35:e126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Yee ML, Wong R, Datta M, Fazlo TN, Ebrahim MM, Mcnamara EC, De Jong G, Gilfillan C. Mitochondrial disease: an uncommon but important cause of diabetes mellitus. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Urano F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr Diab Rep. 2016;16:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Lu H, Lu H, Vaucher J, Tran C, Vollenweider P, Castioni J. [Thiamine-responsive megaloblastic anemia or Rogers syndrome: A literature review]. Rev Med Interne. 2019;40:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Senniappan S, Hughes M, Shah P, Shah V, Kaski JP, Brogan P, Hussain K. Pigmentary hypertrichosis and non-autoimmune insulin-dependent diabetes mellitus (PHID) syndrome is associated with severe chronic inflammation and cardiomyopathy, and represents a new monogenic autoinflammatory syndrome. J Pediatr Endocrinol Metab. 2013;26:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Lightbourne M, Brown RJ. Genetics of Lipodystrophy. Endocrinol Metab Clin North Am. 2017;46:539-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Patni N, Garg A. Lipodystrophy for the Diabetologist-What to Look For. Curr Diab Rep. 2022;22:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Semple RK, Savage DB, Cochran EK, Gorden P, O'Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32:498-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Perge K, Capel E, Villanueva C, Gautheron J, Diallo S, Auclair M, Rondeau S, Morichon R, Brioude F, Jéru I, Rossi M, Nicolino M, Vigouroux C. Ciliopathy due to POC1A deficiency: clinical and metabolic features, and cellular modeling. Eur J Endocrinol. 2024;190:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2489] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 30. | Ben-Skowronek I. IPEX Syndrome: Genetics and Treatment Options. Genes (Basel). 2021;12:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Clin Chem. 2023;69:808-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 33. | Riddle MC, Philipson LH, Rich SS, Carlsson A, Franks PW, Greeley SAW, Nolan JJ, Pearson ER, Zeitler PS, Hattersley AT. Monogenic Diabetes: From Genetic Insights to Population-Based Precision in Care. Reflections From a Diabetes Care Editors' Expert Forum. Diabetes Care. 2020;43:3117-3128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Hattersley AT. Laboratory Guidelines Are Needed for Diagnostic Genetic Testing for Monogenic Diabetes. Clin Chem. 2023;69:788-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Shimada A, Kawasaki E, Abiru N, Awata T, Oikawa Y, Osawa H, Kajio H, Kozawa J, Takahashi K, Chujo D, Noso S, Fukui T, Miura J, Yasuda K, Yasuda H, Imagawa A, Ikegami H. New diagnostic criteria (2023) for slowly progressive type 1 diabetes (SPIDDM): Report from Committee on Type 1 Diabetes of the Japan Diabetes Society (English version). J Diabetes Investig. 2024;15:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:200-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 37. | Valkovicova T, Skopkova M, Stanik J, Gasperikova D. Novel insights into genetics and clinics of the HNF1A-MODY. Endocr Regul. 2019;53:110-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Gardner DS, Tai ES. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes Metab Syndr Obes. 2012;5:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Hulín J, Škopková M, Valkovičová T, Mikulajová S, Rosoľanková M, Papcun P, Gašperíková D, Staník J. Clinical implications of the glucokinase impaired function - GCK MODY today. Physiol Res. 2020;69:995-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Shih DQ, Dansky HM, Fleisher M, Assmann G, Fajans SS, Stoffel M. Genotype/phenotype relationships in HNF-4alpha/MODY1: haploinsufficiency is associated with reduced apolipoprotein (AII), apolipoprotein (CIII), lipoprotein(a), and triglyceride levels. Diabetes. 2000;49:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Gambella A, Kalantari S, Cadamuro M, Quaglia M, Delvecchio M, Fabris L, Pinon M. The Landscape of HNF1B Deficiency: A Syndrome Not Yet Fully Explored. Cells. 2023;12:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 42. | Warncke K, Kummer S, Raile K, Grulich-Henn J, Woelfle J, Steichen E, Prinz N, Holl RW. Frequency and Characteristics of MODY 1 (HNF4A Mutation) and MODY 5 (HNF1B Mutation): Analysis From the DPV Database. J Clin Endocrinol Metab. 2019;104:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Abreu GM Miss, Tarantino RM, da Fonseca ACP, de Souza RB, Soares CAPD, Cabello PH, Rodacki M, Zajdenverg L, Zembrzuski VM, Campos Junior M. PDX1-MODY: A rare missense mutation as a cause of monogenic diabetes. Eur J Med Genet. 2021;64:104194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 44. | Horikawa Y, Enya M. Genetic Dissection and Clinical Features of MODY6 (NEUROD1-MODY). Curr Diab Rep. 2019;19:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Laver TW, Wakeling MN, Knox O, Colclough K, Wright CF, Ellard S, Hattersley AT, Weedon MN, Patel KA. Evaluation of Evidence for Pathogenicity Demonstrates That BLK, KLF11, and PAX4 Should Not Be Included in Diagnostic Testing for MODY. Diabetes. 2022;71:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 46. | Sun S, Gong S, Li M, Wang X, Wang F, Cai X, Liu W, Luo Y, Zhang S, Zhang R, Zhou L, Zhu Y, Ma Y, Ren Q, Zhang X, Chen J, Chen L, Wu J, Gao L, Zhou X, Li Y, Zhong L, Han X, Ji L. Clinical and genetic characteristics of CEL-MODY (MODY8): a literature review and screening in Chinese individuals diagnosed with early-onset type 2 diabetes. Endocrine. 2024;83:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Ovsyannikova AK, Rymar OD, Shakhtshneider EV, Klimontov VV, Koroleva EA, Myakina NE, Voevoda MI. ABCC8-Related Maturity-Onset Diabetes of the Young (MODY12): Clinical Features and Treatment Perspective. Diabetes Ther. 2016;7:591-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Bonnefond A, Philippe J, Durand E, Dechaume A, Huyvaert M, Montagne L, Marre M, Balkau B, Fajardy I, Vambergue A, Vatin V, Delplanque J, Le Guilcher D, De Graeve F, Lecoeur C, Sand O, Vaxillaire M, Froguel P. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS One. 2012;7:e37423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Ivanoshchuk DE, Shakhtshneider EV, Rymar OD, Ovsyannikova AK, Mikhailova SV, Orlov PS, Ragino YI, Voevoda MI. Analysis of APPL1 Gene Polymorphisms in Patients with a Phenotype of Maturity Onset Diabetes of the Young. J Pers Med. 2020;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Şimşek E, Çilingir O, Şimşek T, Kocagil S, Erzurumluoğlu Gökalp E, Demiral M, Binay C. Screening of Mutations in Maturity-onset Diabetes of the Young-related Genes and RFX6 in Children with Autoantibody-negative Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol. 2024;16:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Shield JP, Gardner RJ, Wadsworth EJ, Whiteford ML, James RS, Robinson DO, Baum JD, Temple IK. Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed. 1997;76:F39-F42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Kocova M. Genetic Spectrum of Neonatal Diabetes. Balkan J Med Genet. 2020;23:5-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Shimomura K, Maejima Y. K(ATP) Channel Mutations and Neonatal Diabetes. Intern Med. 2017;56:2387-2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI; Neonatal Diabetes International Collaborative Group. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040-15044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 412] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 55. | Mackay DJ, Temple IK. Transient neonatal diabetes mellitus type 1. Am J Med Genet C Semin Med Genet. 2010;154C:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis. 2010;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Shaw-Smith C, De Franco E, Lango Allen H, Batlle M, Flanagan SE, Borowiec M, Taplin CE, van Alfen-van der Velden J, Cruz-Rojo J, Perez de Nanclares G, Miedzybrodzka Z, Deja G, Wlodarska I, Mlynarski W, Ferrer J, Hattersley AT, Ellard S. GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes. 2014;63:2888-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Yue X, Luo Y, Wang J, Huang D. Monogenic Diabetes with GATA6 Mutations: Characterization of a Novel Family and a Comprehensive Analysis of the GATA6 Clinical and Genetics Traits. Mol Biotechnol. 2024;66:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Passone CGB, Vermillac G, Staels W, Besancon A, Kariyawasam D, Godot C, Lambe C, Talbotec C, Girard M, Chardot C, Berteloot L, Hachem T, Lapillonne A, Poidvin A, Storey C, Neve M, Stan C, Dugelay E, Fauret-Amsellem AL, Capri Y, Cavé H, Ybarra M, Chandra V, Scharfmann R, Bismuth E, Polak M, Carel JC, Pigneur B, Beltrand J. Mitchell-Riley Syndrome: Improving Clinical Outcomes and Searching for Functional Impact of RFX-6 Mutations. Front Endocrinol (Lausanne). 2022;13:802351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | London S, De Franco E, Elias-Assad G, Barhoum MN, Felszer C, Paniakov M, Weiner SA, Tenenbaum-Rakover Y. Case Report: Neonatal Diabetes Mellitus Caused by a Novel GLIS3 Mutation in Twins. Front Endocrinol (Lausanne). 2021;12:673755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Wen X, Yang Y. Emerging roles of GLIS3 in neonatal diabetes, type 1 and type 2 diabetes. J Mol Endocrinol. 2017;58:R73-R85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Demirbilek H, Cayir A, Flanagan SE, Yıldırım R, Kor Y, Gurbuz F, Haliloğlu B, Yıldız M, Baran RT, Akbas ED, Demiral M, Ünal E, Arslan G, Vuralli D, Buyukyilmaz G, Al-Khawaga S, Saeed A, Al Maadheed M, Khalifa A, Onal H, Yuksel B, Ozbek MN, Bereket A, Hattersley AT, Hussain K, De Franco E. Clinical Characteristics and Long-term Follow-up of Patients with Diabetes Due To PTF1A Enhancer Mutations. J Clin Endocrinol Metab. 2020;105:e4351-e4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Delvecchio M, Pastore C, Giordano P. Treatment Options for MODY Patients: A Systematic Review of Literature. Diabetes Ther. 2020;11:1667-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 64. | Lau HH, Ng NHJ, Loo LSW, Jasmen JB, Teo AKK. The molecular functions of hepatocyte nuclear factors - In and beyond the liver. J Hepatol. 2018;68:1033-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 65. | Miyachi Y, Miyazawa T, Ogawa Y. HNF1A Mutations and Beta Cell Dysfunction in Diabetes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Torsvik J, Johansson S, Johansen A, Ek J, Minton J, Raeder H, Ellard S, Hattersley A, Pedersen O, Hansen T, Molven A, Njølstad PR. Mutations in the VNTR of the carboxyl-ester lipase gene (CEL) are a rare cause of monogenic diabetes. Hum Genet. 2010;127:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Molven A, Ringdal M, Nordbø AM, Raeder H, Støy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, Undlien DE, Joner G, Søvik O; Norwegian Childhood Diabetes Study Group, Bell GI, Njølstad PR. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | De Franco E, Saint-Martin C, Brusgaard K, Knight Johnson AE, Aguilar-Bryan L, Bowman P, Arnoux JB, Larsen AR, Sanyoura M, Greeley SAW, Calzada-León R, Harman B, Houghton JAL, Nishimura-Meguro E, Laver TW, Ellard S, Del Gaudio D, Christesen HT, Bellanné-Chantelot C, Flanagan SE. Update of variants identified in the pancreatic β-cell K(ATP) channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum Mutat. 2020;41:884-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 69. | Cheng KK, Lam KS, Wu D, Wang Y, Sweeney G, Hoo RL, Zhang J, Xu A. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc Natl Acad Sci U S A. 2012;109:8919-8924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Carmody D, Naylor RN, Bell CD, Berry S, Montgomery JT, Tadie EC, Hwang JL, Greeley SA, Philipson LH. GCK-MODY in the US National Monogenic Diabetes Registry: frequently misdiagnosed and unnecessarily treated. Acta Diabetol. 2016;53:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Bellanné-Chantelot C, Carette C, Riveline JP, Valéro R, Gautier JF, Larger E, Reznik Y, Ducluzeau PH, Sola A, Hartemann-Heurtier A, Lecomte P, Chaillous L, Laloi-Michelin M, Wilhem JM, Cuny P, Duron F, Guerci B, Jeandidier N, Mosnier-Pudar H, Assayag M, Dubois-Laforgue D, Velho G, Timsit J. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes. 2008;57:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Broome DT, Pantalone KM, Kashyap SR, Philipson LH. Approach to the Patient with MODY-Monogenic Diabetes. J Clin Endocrinol Metab. 2021;106:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 73. | Kleinberger JW, Copeland KC, Gandica RG, Haymond MW, Levitsky LL, Linder B, Shuldiner AR, Tollefsen S, White NH, Pollin TI. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet Med. 2018;20:583-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes. 2019;12:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 75. | Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 76. | Cromer SJ, Sella AC, Rosenberg E, Scully K, McDonnell M, Abreu AP, Weil M, Bernstein SN, Quinn M, Powe C, Mitchell DM, Udler MS. Report of Prolonged Neonatal Hypoglycemia in Three Infants of Mothers With Variants in HNF1A. AACE Clin Case Rep. 2022;8:224-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Owen KR, Thanabalasingham G, James TJ, Karpe F, Farmer AJ, McCarthy MI, Gloyn AL. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010;33:1919-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | McDonald TJ, Shields BM, Lawry J, Owen KR, Gloyn AL, Ellard S, Hattersley AT. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011;34:1860-1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Toniatti C, Demartis A, Monaci P, Nicosia A, Ciliberto G. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990;9:4467-4475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Szopa M, Klupa T, Kapusta M, Matejko B, Ucieklak D, Glodzik W, Zapala B, Sani CM, Hohendorff J, Malecki MT, Skupien J. A decision algorithm to identify patients with high probability of monogenic diabetes due to HNF1A mutations. Endocrine. 2019;64:75-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Bellanné-Chantelot C, Coste J, Ciangura C, Fonfrède M, Saint-Martin C, Bouché C, Sonnet E, Valéro R, Lévy DJ, Dubois-Laforgue D, Timsit J; Monogenic Diabetes Study Group of the Société Francophone du Diabète (SFD). High-sensitivity C-reactive protein does not improve the differential diagnosis of HNF1A-MODY and familial young-onset type 2 diabetes: A grey zone analysis. Diabetes Metab. 2016;42:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Juszczak A, Pavić T, Vučković F, Bennett AJ, Shah N, Pape Medvidović E, Groves CJ, Šekerija M, Chandler K, Burrows C, Rojnić Putarek N, Vučić Lovrenčić M, Ćuća Knežević J, James TJ, Gloyn AL, Lauc G, McCarthy MI, Owen KR, Gornik O. Plasma Fucosylated Glycans and C-Reactive Protein as Biomarkers of HNF1A-MODY in Young Adult-Onset Nonautoimmune Diabetes. Diabetes Care. 2019;42:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, Ellard S, Farmer AJ, McCarthy MI, Owen KR. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 84. | Pontoglio M, Prié D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, Friedlander G. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000;1:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 85. | Menzel R, Kaisaki PJ, Rjasanowski I, Heinke P, Kerner W, Menzel S. A low renal threshold for glucose in diabetic patients with a mutation in the hepatocyte nuclear factor-1alpha (HNF-1alpha) gene. Diabet Med. 1998;15:816-820. [PubMed] [DOI] [Full Text] |
| 86. | Bingham C, Ellard S, Nicholls AJ, Pennock CA, Allen J, James AJ, Satchell SC, Salzmann MB, Hattersley AT. The generalized aminoaciduria seen in patients with hepatocyte nuclear factor-1alpha mutations is a feature of all patients with diabetes and is associated with glucosuria. Diabetes. 2001;50:2047-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Stride A, Ellard S, Clark P, Shakespeare L, Salzmann M, Shepherd M, Hattersley AT. Beta-cell dysfunction, insulin sensitivity, and glycosuria precede diabetes in hepatocyte nuclear factor-1alpha mutation carriers. Diabetes Care. 2005;28:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4α is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 89. | McDonald TJ, McEneny J, Pearson ER, Thanabalasingham G, Szopa M, Shields BM, Ellard S, Owen KR, Malecki MT, Hattersley AT, Young IS. Lipoprotein composition in HNF1A-MODY: differentiating between HNF1A-MODY and type 2 diabetes. Clin Chim Acta. 2012;413:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, Wierzbicki AS, Clark PM, Lebl J, Pedersen O, Ellard S, Hansen T, Hattersley AT. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Ping F, Fu J, Xiao X. Distinguishing the lipid profile of GCK-MODY patients and its correlation with hsCRP levels. Front Endocrinol (Lausanne). 2022;13:1024431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Fendler W, Borowiec M, Antosik K, Szadkowska A, Deja G, Jarosz-Chobot P, Mysliwiec M, Wyka K, Pietrzak I, Skupien J, Malecki MT, Mlynarski W. HDL cholesterol as a diagnostic tool for clinical differentiation of GCK-MODY from HNF1A-MODY and type 1 diabetes in children and young adults. Clin Endocrinol (Oxf). 2011;75:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Fendler W, Rizzo M, Borowiec M, Malachowska B, Antosik K, Szadkowska A, Banach M, Urbanska-Kosinska M, Szopa M, Malecki M, Mlynarski W. Less but better: cardioprotective lipid profile of patients with GCK-MODY despite lower HDL cholesterol level. Acta Diabetol. 2014;51:625-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Besser RE, Shepherd MH, McDonald TJ, Shields BM, Knight BA, Ellard S, Hattersley AT. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care. 2011;34:286-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Wang Y, Gao Y, Cai X, Chen L, Zhou L, Ma Y, Gong S, Han X, Ji L. Clinical Implications of Urinary C-Peptide Creatinine Ratio in Patients with Different Types of Diabetes. J Diabetes Res. 2019;2019:1747684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Angelescu MA, Andronic O, Dima SO, Popescu I, Meivar-Levy I, Ferber S, Lixandru D. miRNAs as Biomarkers in Diabetes: Moving towards Precision Medicine. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 97. | Nowak N, Hohendorff J, Solecka I, Szopa M, Skupien J, Kiec-Wilk B, Mlynarski W, Malecki MT. Circulating ghrelin level is higher in HNF1A-MODY and GCK-MODY than in polygenic forms of diabetes mellitus. Endocrine. 2015;50:643-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Mughal SA, Park R, Nowak N, Gloyn AL, Karpe F, Matile H, Malecki MT, McCarthy MI, Stoffel M, Owen KR. Apolipoprotein M can discriminate HNF1A-MODY from Type 1 diabetes. Diabet Med. 2013;30:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Firdous P, Nissar K, Masoodi SR, Ganai BA. Biomarkers: Tools for Discriminating MODY from Other Diabetic Subtypes. Indian J Endocrinol Metab. 2022;26:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012;55:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 101. | Zhao J, Chen Y, Ma F, Shu H, Zheng L, Liu Y, Li X, Xu T, Zhou Z, Zhou K. MODY Probability Calculator Is Suitable for MODY Screening in China: A Population-based Study. J Endocr Soc. 2024;8:bvae047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 102. | da Silva Santos T, Fonseca L, Santos Monteiro S, Borges Duarte D, Martins Lopes A, Couto de Carvalho A, Oliveira MJ, Borges T, Laranjeira F, Couce ML, Cardoso MH. MODY probability calculator utility in individuals' selection for genetic testing: Its accuracy and performance. Endocrinol Diabetes Metab. 2022;5:e00332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 103. | De Sousa SMC, Wu KHC, Colclough K, Rawlings L, Dubowsky A, Monnik M, Poplawski N, Scott HS, Horowitz M, Torpy DJ. Identification of monogenic diabetes in an Australian cohort using the Exeter maturity-onset diabetes of the young (MODY) probability calculator and next-generation sequencing gene panel testing. Acta Diabetol. 2024;61:181-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 104. | Tarantino RM, Abreu GM, Fonseca ACP, Kupfer R, Pereira MFC, Campos Júnior M, Zajdenverg L, Rodacki M. MODY probability calculator for GCK and HNF1A screening in a multiethnic background population. Arch Endocrinol Metab. 2020;64:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Santomauro AC Jr, Magalhães ÁLF, Motta FT, de Santana LS, Franco PC, de Freitas SM, Sanchez JJD, Costa-Riquetto AD, Teles MG. The performance of the MODY calculator in a non-Caucasian, mixed-race population diagnosed with diabetes mellitus before 35 years of age. Diabetol Metab Syndr. 2023;15:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 106. | Ma Y, Gong S, Wang X, Cai X, Xiao X, Gu W, Yang J, Zhong L, Xiao J, Li M, Liu W, Zhang S, Zhou X, Li Y, Zhou L, Zhu Y, Luo Y, Ren Q, Huang X, Gao X, Zhang X, Zhang R, Chen L, Wang F, Wang Q, Hu M, Han X, Ji L. New clinical screening strategy to distinguish HNF1A variant-induced diabetes from young early-onset type 2 diabetes in a Chinese population. BMJ Open Diabetes Res Care. 2020;8:e000745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 108. | Isomaa B, Henricsson M, Lehto M, Forsblom C, Karanko S, Sarelin L, Häggblom M, Groop L. Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia. 1998;41:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009;26:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 110. | Naylor RN, Patel KA, Kettunen JLT, Männistö JME, Støy J, Beltrand J, Polak M; ADA/EASD PMDI, Vilsbøll T, Greeley SAW, Hattersley AT, Tuomi T. Systematic Review of Treatment of Beta-Cell Monogenic Diabetes. medRxiv. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 111. | Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003;362:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 393] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 112. | Boileau P, Wolfrum C, Shih DQ, Yang TA, Wolkoff AW, Stoffel M. Decreased glibenclamide uptake in hepatocytes of hepatocyte nuclear factor-1alpha-deficient mice: a mechanism for hypersensitivity to sulfonylurea therapy in patients with maturity-onset diabetes of the young, type 3 (MODY3). Diabetes. 2002;51 Suppl 3:S343-S348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Shepherd MH, Shields BM, Hudson M, Pearson ER, Hyde C, Ellard S, Hattersley AT, Patel KA; UNITED study. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia. 2018;61:2520-2527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 114. | Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 115. | Østoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, Knop FK, Vilsbøll T. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care. 2014;37:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 116. | Hohendorff J, Szopa M, Skupien J, Kapusta M, Zapala B, Platek T, Mrozinska S, Parpan T, Glodzik W, Ludwig-Galezowska A, Kiec-Wilk B, Klupa T, Malecki MT. A single dose of dapagliflozin, an SGLT-2 inhibitor, induces higher glycosuria in GCK- and HNF1A-MODY than in type 2 diabetes mellitus. Endocrine. 2017;57:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Pruhova S, Dusatkova P, Neumann D, Hollay E, Cinek O, Lebl J, Sumnik Z. Two cases of diabetic ketoacidosis in HNF1A-MODY linked to severe dehydration: is it time to change the diagnostic criteria for MODY? Diabetes Care. 2013;36:2573-2574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 119. | Dickens LT, Naylor RN. Clinical Management of Women with Monogenic Diabetes During Pregnancy. Curr Diab Rep. 2018;18:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Timsit J, Ciangura C, Dubois-Laforgue D, Saint-Martin C, Bellanne-Chantelot C. Pregnancy in Women With Monogenic Diabetes due to Pathogenic Variants of the Glucokinase Gene: Lessons and Challenges. Front Endocrinol (Lausanne). 2021;12:802423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Jeeyavudeen MS, Murray SR, Strachan MWJ. Management of monogenic diabetes in pregnancy: A narrative review. World J Diabetes. 2024;15:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 122. | Caswell RC, Snowsill T, Houghton JAL, Chakera AJ, Shepherd MH, Laver TW, Knight BA, Wright D, Hattersley AT, Ellard S. Noninvasive Fetal Genotyping by Droplet Digital PCR to Identify Maternally Inherited Monogenic Diabetes Variants. Clin Chem. 2020;66:958-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 123. | Rudland VL. Diagnosis and management of glucokinase monogenic diabetes in pregnancy: current perspectives. Diabetes Metab Syndr Obes. 2019;12:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 124. | Johnson MB, Patel KA, De Franco E, Hagopian W, Killian M, McDonald TJ, Tree TIM, Domingo-Vila C, Hudson M, Hammersley S, Dobbs R; EXE-T1D Consortium, Ellard S, Flanagan SE, Hattersley AT, Oram RA. Type 1 diabetes can present before the age of 6 months and is characterised by autoimmunity and rapid loss of beta cells. Diabetologia. 2020;63:2605-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 125. | von Mühlendahl KE, Herkenhoff H. Long-term course of neonatal diabetes. N Engl J Med. 1995;333:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 126. | Busiah K, Drunat S, Vaivre-Douret L, Bonnefond A, Simon A, Flechtner I, Gérard B, Pouvreau N, Elie C, Nimri R, De Vries L, Tubiana-Rufi N, Metz C, Bertrand AM, Nivot-Adamiak S, de Kerdanet M, Stuckens C, Jennane F, Souchon PF, Le Tallec C, Désirée C, Pereira S, Dechaume A, Robert JJ, Phillip M, Scharfmann R, Czernichow P, Froguel P, Vaxillaire M, Polak M, Cavé H; French NDM study group. Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study [corrected]. Lancet Diabetes Endocrinol. 2013;1:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | Hashimoto Y, Dateki S, Hirose M, Satomura K, Sawada H, Mizuno H, Sugihara S, Maruyama K, Urakami T, Sugawara H, Shirai K, Yorifuji T. Molecular and clinical features of K(ATP) -channel neonatal diabetes mellitus in Japan. Pediatr Diabetes. 2017;18:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Letourneau LR, Carmody D, Wroblewski K, Denson AM, Sanyoura M, Naylor RN, Philipson LH, Greeley SAW. Diabetes Presentation in Infancy: High Risk of Diabetic Ketoacidosis. Diabetes Care. 2017;40:e147-e148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Svalastoga P, Sulen Å, Fehn JR, Aukland SM, Irgens H, Sirnes E, Fevang SKE, Valen E, Elgen IB, Njølstad PR. Intellectual Disability in K(ATP) Channel Neonatal Diabetes. Diabetes Care. 2020;43:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Polak M, Dechaume A, Cavé H, Nimri R, Crosnier H, Sulmont V, de Kerdanet M, Scharfmann R, Lebenthal Y, Froguel P, Vaxillaire M; French ND (Neonatal Diabetes) Study Group. Heterozygous missense mutations in the insulin gene are linked to permanent diabetes appearing in the neonatal period or in early infancy: a report from the French ND (Neonatal Diabetes) Study Group. Diabetes. 2008;57:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 131. | Bowman P, Sulen Å, Barbetti F, Beltrand J, Svalastoga P, Codner E, Tessmann EH, Juliusson PB, Skrivarhaug T, Pearson ER, Flanagan SE, Babiker T, Thomas NJ, Shepherd MH, Ellard S, Klimes I, Szopa M, Polak M, Iafusco D, Hattersley AT, Njølstad PR; Neonatal Diabetes International Collaborative Group. Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study. Lancet Diabetes Endocrinol. 2018;6:637-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 132. | Babiker T, Vedovato N, Patel K, Thomas N, Finn R, Männikkö R, Chakera AJ, Flanagan SE, Shepherd MH, Ellard S, Ashcroft FM, Hattersley AT. Successful transfer to sulfonylureas in KCNJ11 neonatal diabetes is determined by the mutation and duration of diabetes. Diabetologia. 2016;59:1162-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 133. | Cavé H, Polak M, Drunat S, Denamur E, Czernichow P. Refinement of the 6q chromosomal region implicated in transient neonatal diabetes. Diabetes. 2000;49:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 134. | Gardner RJ, Mackay DJ, Mungall AJ, Polychronakos C, Siebert R, Shield JP, Temple IK, Robinson DO. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet. 2000;9:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 135. | Hoffmann A, Spengler D. Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism. World J Biol Chem. 2015;6:95-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Arima T, Drewell RA, Arney KL, Inoue J, Makita Y, Hata A, Oshimura M, Wake N, Surani MA. A conserved imprinting control region at the HYMAI/ZAC domain is implicated in transient neonatal diabetes mellitus. Hum Mol Genet. 2001;10:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 138. | Pezzino G, Ruta R, Rapini N, Chiodo DC, Mucciolo M, Tomaselli L, Cianfarani S, Barbetti F. A rare cause of transient neonatal diabetes mellitus: Spontaneous HNF1B splice variant. Diabet Med. 2024;41:e15202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 139. | Patch AM, Flanagan SE, Boustred C, Hattersley AT, Ellard S. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007;9 Suppl 2:28-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Bonfanti R, Iafusco D, Rabbone I, Diedenhofen G, Bizzarri C, Patera PI, Reinstadler P, Costantino F, Calcaterra V, Iughetti L, Savastio S, Favia A, Cardella F, Lo Presti D, Girtler Y, Rabbiosi S, D'Annunzio G, Zanfardino A, Piscopo A, Casaburo F, Pintomalli L, Russo L, Grasso V, Minuto N, Mucciolo M, Novelli A, Marucci A, Piccini B, Toni S, Silvestri F, Carrera P, Rigamonti A, Frontino G, Trada M, Tinti D, Delvecchio M, Rapini N, Schiaffini R, Mammì C, Barbetti F; Diabetes Study Group of ISPED. Differences between transient neonatal diabetes mellitus subtypes can guide diagnosis and therapy. Eur J Endocrinol. 2021;184:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 141. | Yorifuji T, Matsubara K, Sakakibara A, Hashimoto Y, Kawakita R, Hosokawa Y, Fujimaru R, Murakami A, Tamagawa N, Hatake K, Nagasaka H, Suzuki J, Urakami T, Izawa M, Kagami M. Abnormalities in chromosome 6q24 as a cause of early-onset, non-obese, non-autoimmune diabetes mellitus without history of neonatal diabetes. Diabet Med. 2015;32:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 142. | Docherty LE, Kabwama S, Lehmann A, Hawke E, Harrison L, Flanagan SE, Ellard S, Hattersley AT, Shield JP, Ennis S, Mackay DJ, Temple IK. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia. 2013;56:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 143. | Gunes SO, Calisici E, Arslan M, Akin O, Karagol BS. Transient Neonatal Diabetes Mellitus and Seizure with an Unknown Etiology. J Pediatr Genet. 2023;12:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 144. | Johnson MB, Hattersley AT, Flanagan SE. Monogenic autoimmune diseases of the endocrine system. Lancet Diabetes Endocrinol. 2016;4:862-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 145. | Strakova V, Elblova L, Johnson MB, Dusatkova P, Obermannova B, Petruzelkova L, Kolouskova S, Snajderova M, Fronkova E, Svaton M, Lebl J, Hattersley AT, Sumnik Z, Pruhova S. Screening of monogenic autoimmune diabetes among children with type 1 diabetes and multiple autoimmune diseases: is it worth doing? J Pediatr Endocrinol Metab. 2019;32:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Hossen MM, Ma Y, Yin Z, Xia Y, Du J, Huang JY, Huang JJ, Zou L, Ye Z, Huang Z. Current understanding of CTLA-4: from mechanism to autoimmune diseases. Front Immunol. 2023;14:1198365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 147. | Kleine-Eggebrecht N, Staufner C, Kathemann S, Elgizouli M, Kopajtich R, Prokisch H, Lainka E. Mutation in ITCH Gene Can Cause Syndromic Multisystem Autoimmune Disease With Acute Liver Failure. Pediatrics. 2019;143:e20181554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Borysewicz-Sańczyk H, Sawicka B, Wawrusiewicz-Kurylonek N, Głowińska-Olszewska B, Kadłubiska A, Gościk J, Szadkowska A, Łosiewicz A, Młynarski W, Kretowski A, Bossowski A. Genetic Association Study of IL2RA, IFIH1, and CTLA-4 Polymorphisms With Autoimmune Thyroid Diseases and Type 1 Diabetes. Front Pediatr. 2020;8:481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 149. | Kardelen AD, Kara M, Güller D, Ozturan EK, Abalı ZY, Ceylaner S, Kıykım A, Cantez S, Torun SH, Poyrazoglu S, Bas F, Darendelıler F. LRBA deficiency: a rare cause of type 1 diabetes, colitis, and severe immunodeficiency. Hormones (Athens). 2021;20:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 150. | Murphy R, Turnbull DM, Walker M, Hattersley AT. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med. 2008;25:383-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 151. | Koenig MK. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol. 2008;38:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 152. | Hu T, Chitnis N, Monos D, Dinh A. Next-generation sequencing technologies: An overview. Hum Immunol. 2021;82:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 413] [Article Influence: 103.3] [Reference Citation Analysis (1)] |
| 153. | Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 154. | Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, Houghton JA, Shepherd M, Hattersley AT, Weedon MN, Caswell R. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56:1958-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 155. | Zmysłowska A, Jakiel P, Gadzalska K, Majos A, Płoszaj T, Ben-Skowronek I, Deja G, Glowinska-Olszewska B, Jarosz-Chobot P, Klonowska B, Kowalska I, Mlynarski W, Mysliwiec M, Nazim J, Noczynska A, Robak-Kontna K, Skala-Zamorowska E, Skowronska B, Szadkowska A, Szypowska A, Walczak M, Borowiec M. Next- generation sequencing is an effective method for diagnosing patients with different forms of monogenic diabetes. Diabetes Res Clin Pract. 2022;183:109154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 156. | Kheriji N, Dallali H, Gouiza I, Hechmi M, Mahjoub F, Mrad M, Krir A, Soltani M, Trabelsi H, Hamdi W, Bahlous A, Ben Ahmed M, Jamoussi H, Kefi R. Whole-exome sequencing reveals novel variants of monogenic diabetes in Tunisia: impact on diagnosis and healthcare management. Front Genet. 2023;14:1224284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 157. | Misra S, Shields B, Colclough K, Johnston DG, Oliver NS, Ellard S, Hattersley AT. South Asian individuals with diabetes who are referred for MODY testing in the UK have a lower mutation pick-up rate than white European people. Diabetologia. 2016;59:2262-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 158. | Xue Y, Ankala A, Wilcox WR, Hegde MR. Solving the molecular diagnostic testing conundrum for Mendelian disorders in the era of next-generation sequencing: single-gene, gene panel, or exome/genome sequencing. Genet Med. 2015;17:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 159. | Johansson S, Irgens H, Chudasama KK, Molnes J, Aerts J, Roque FS, Jonassen I, Levy S, Lima K, Knappskog PM, Bell GI, Molven A, Njølstad PR. Exome sequencing and genetic testing for MODY. PLoS One. 2012;7:e38050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 160. | Ali Khan I. Do second generation sequencing techniques identify documented genetic markers for neonatal diabetes mellitus? Heliyon. 2021;7:e07903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 161. | Özdemir TR, Kırbıyık Ö, Dündar BN, Abacı A, Kaya ÖÖ, Çatlı G, Özyılmaz B, Acar S, Koç A, Güvenç MS, Kutbay YB, Erdoğan KM. Targeted next generation sequencing in patients with maturity-onset diabetes of the young (MODY). J Pediatr Endocrinol Metab. 2018;31:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Aydogan HY, Gul N, Demirci DK, Mutlu U, Gulfidan G, Arga KY, Ozder A, Camli AA, Tutuncu Y, Ozturk O, Cacina C, Darendeliler F, Poyrazoglu S, Satman I. Precision Diagnosis of Maturity-Onset Diabetes of the Young with Next-Generation Sequencing: Findings from the MODY-IST Study in Adult Patients. OMICS. 2022;26:218-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 163. | Donath X, Saint-Martin C, Dubois-Laforgue D, Rajasingham R, Mifsud F, Ciangura C, Timsit J, Bellanné-Chantelot C; Monogenic Diabetes Study Group of the Société Francophone du Diabète. Next-generation sequencing identifies monogenic diabetes in 16% of patients with late adolescence/adult-onset diabetes selected on a clinical basis: a cross-sectional analysis. BMC Med. 2019;17:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 164. | Sztromwasser P, Michalak A, Małachowska B, Młudzik P, Antosik K, Hogendorf A, Zmysłowska A, Borowiec M, Młynarski W, Fendler W. A cross-sectional study of patients referred for HNF1B-MODY genetic testing due to cystic kidneys and diabetes. Pediatr Diabetes. 2020;21:422-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 165. | Dotto RP, Mathez ALG, Franco LF, de Sá JR, Weinert LS, Silveiro SP, de Mello Almada Giuffrida F, da Silva MRD, Reis AF. Improving the identification of mody mutations by using mlpa technique in the molecular diagnostics routine. Diabetol Metab Syndr. 2015;7:A246. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 166. | Loos MA, Gomez G, Mayorga L, Caraballo RH, Eiroa HD, Obregon MG, Rugilo C, Lubieniecki F, Taratuto AL, Saccoliti M, Alonso CN, Aráoz HV. Clinical and molecular characterization of mitochondrial DNA disorders in a group of Argentinian pediatric patients. Mol Genet Metab Rep. 2021;27:100733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 167. | Lavrichenko K, Johansson S, Jonassen I. Comprehensive characterization of copy number variation (CNV) called from array, long- and short-read data. BMC Genomics. 2021;22:826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 168. | Berberich AJ, Wang J, Cao H, McIntyre AD, Spaic T, Miller DB, Stock S, Huot C, Stein R, Knoll J, Yang P, Robinson JF, Hegele RA. Simplifying Detection of Copy-Number Variations in Maturity-Onset Diabetes of the Young. Can J Diabetes. 2021;45:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 169. | Berberich AJ, Huot C, Cao H, McIntyre AD, Robinson JF, Wang J, Hegele RA. Copy Number Variation in GCK in Patients With Maturity-Onset Diabetes of the Young. J Clin Endocrinol Metab. 2019;104:3428-3436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 170. | Yan JB, Zhang R, Xiong C, Hu C, Lv Y, Wang CR, Jia WP, Zeng F. Pyrosequencing is an accurate and reliable method for the analysis of heteroplasmy of the A3243G mutation in patients with mitochondrial diabetes. J Mol Diagn. 2014;16:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 171. | Touati A, Errea-Dorronsoro J, Nouri S, Halleb Y, Pereda A, Mahdhaoui N, Ghith A, Saad A, Perez de Nanclares G, H'mida Ben Brahim D. Transient neonatal diabetes mellitus and hypomethylation at additional imprinted loci: novel ZFP57 mutation and review on the literature. Acta Diabetol. 2019;56:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 172. | Temple IK, Mackay DJ. Diabetes Mellitus, 6q24-Related Transient Neonatal. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editor. GeneReviews®. Seattle (WA): University of Washington, Seattle 1993. [PubMed] |
| 173. | Pang L, Colclough KC, Shepherd MH, McLean J, Pearson ER, Ellard S, Hattersley AT, Shields BM. Improvements in Awareness and Testing Have Led to a Threefold Increase Over 10 Years in the Identification of Monogenic Diabetes in the U.K. Diabetes Care. 2022;45:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 174. | Stanik J, Kusekova M, Huckova M, Valentinova L, Masindova I, Stanikova D, Ferenczova J, Gasperikova D, Klimes I. Impact of Type 2 diabetes on Glucokinase diabetes (GCK-MODY) phenotype in a Roma (Gypsy) family - case report. Endocr Regul. 2012;46:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 175. | Kwak SH, Jung CH, Ahn CH, Park J, Chae J, Jung HS, Cho YM, Lee DH, Kim JI, Park KS. Clinical whole exome sequencing in early onset diabetes patients. Diabetes Res Clin Pract. 2016;122:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 176. | Zhang H, Colclough K, Gloyn AL, Pollin TI. Monogenic diabetes: a gateway to precision medicine in diabetes. J Clin Invest. 2021;131:e142244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 177. | Burke W, Parens E, Chung WK, Berger SM, Appelbaum PS. The Challenge of Genetic Variants of Uncertain Clinical Significance : A Narrative Review. Ann Intern Med. 2022;175:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 178. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22547] [Article Influence: 2254.7] [Reference Citation Analysis (0)] |
| 179. | Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, Ledbetter DH, Maglott DR, Martin CL, Nussbaum RL, Plon SE, Ramos EM, Sherry ST, Watson MS; ClinGen. ClinGen--the Clinical Genome Resource. N Engl J Med. 2015;372:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1010] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 180. | ClinGen. Monogenic Diabetes Gene Curation Expert Panel. [cited March 04, 2025]. Available from: https://clinicalgenome.org/affiliation/40016/. |
| 181. | ClinGen. Monogenic Diabetes Variant Curation Expert Panel. [cited March 04, 2025]. Available from: https://clinicalgenome.org/affiliation/50016/. |
| 182. | Misra S, Owen KR. Genetics of Monogenic Diabetes: Present Clinical Challenges. Curr Diab Rep. 2018;18:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 183. | MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 934] [Cited by in RCA: 973] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 184. | Peixoto-Barbosa R, Calliari LE, Crispim F, Moisés RS, Dib SA, Reis AF, Giuffrida FMA. Clinical screening for GCK-MODY in 2,989 patients from the Brazilian Monogenic Diabetes Study Group (BRASMOD) and the Brazilian Type 1 Diabetes Study Group (BrazDiab1SG). Arch Endocrinol Metab. 2024;68:e230314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 185. | Ushijima K, Narumi S, Ogata T, Yokota I, Sugihara S, Kaname T, Horikawa Y, Matsubara Y, Fukami M, Kawamura T; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. KLF11 variant in a family clinically diagnosed with early childhood-onset type 1B diabetes. Pediatr Diabetes. 2019;20:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 186. | Stankute I, Kazlauskiene M, Blouin JL, Schwitzgebel VM, Verkauskiene R. Co-segregation analysis and functional trial in vivo of candidate genes for monogenic diabetes. BMJ Open Diabetes Res Care. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 187. | Ascencio-Carbajal T, Saruwatari-Zavala G, Navarro-Garcia F, Frixione E. Genetic/genomic testing: defining the parameters for ethical, legal and social implications (ELSI). BMC Med Ethics. 2021;22:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 188. | Ferreira A, Gotschall JW, Grant-Kels JM. Ethical concerns of direct-to-consumer genetic testing. J Am Acad Dermatol. 2024;90:1117-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 189. | Xue Y, Chen Y, Ayub Q, Huang N, Ball EV, Mort M, Phillips AD, Shaw K, Stenson PD, Cooper DN, Tyler-Smith C; 1000 Genomes Project Consortium. Deleterious- and disease-allele prevalence in healthy individuals: insights from current predictions, mutation databases, and population-scale resequencing. Am J Hum Genet. 2012;91:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 190. | He B, Li X, Zhou Z. Continuous spectrum of glucose dysmetabolism due to the KCNJ11 gene mutation-Case reports and review of the literature. J Diabetes. 2021;13:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 191. | Heller S, Melzer MK, Azoitei N, Julier C, Kleger A. Human Pluripotent Stem Cells Go Diabetic: A Glimpse on Monogenic Variants. Front Endocrinol (Lausanne). 2021;12:648284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 192. | Burgos JI, Vallier L, Rodríguez-Seguí SA. Monogenic Diabetes Modeling: In Vitro Pancreatic Differentiation From Human Pluripotent Stem Cells Gains Momentum. Front Endocrinol (Lausanne). 2021;12:692596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 193. | Tung JY, Boodhansingh K, Stanley CA, De León DD. Clinical heterogeneity of hyperinsulinism due to HNF1A and HNF4A mutations. Pediatr Diabetes. 2018;19:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 194. | Juszczak A, Pryse R, Schuman A, Owen KR. When to consider a diagnosis of MODY at the presentation of diabetes: aetiology matters for correct management. Br J Gen Pract. 2016;66:e457-e459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |