Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.104311
Revised: February 8, 2025
Accepted: February 20, 2025
Published online: May 15, 2025
Processing time: 129 Days and 3.7 Hours
Diabetes mellitus (DM) and its associated complications are metabolic disorders characterized by hyperglycemia, leading to high morbidity and reduced quality of life worldwide. This global healthcare problem imposes substantial personal and social burdens that warrant comprehensive and in-depth investigation. Plantamajoside (PMS), a naturally bioactive ingredient derived from the traditional Chinese medicinal herb Plantaginis Herba, exhibits a range of pharmacological properties, including anti-inflammatory, antioxidative, and antitumor effects, and has been traditionally utilized in clinical applications such as removing phlegm and cl
Core Tip: Diabetes mellitus (DM) is a global health challenge impacting millions of lives, necessitating the urgent development of effective therapeutic agents. Wang et al observed that plantamajoside (PMS), a bioactive component derived from traditional Chinese medicine, alleviates pancreatic tissue damage and protects β-cells from apoptosis by upregulating DNAJC1. This research underscores the potential of PMS in DM treatment and suggests that other regulated cell death pathways may also be involved in DM progression as modulated by PMS. Further investigations are essential to elucidate the underlying mechanisms and to expand our understanding of PMS as a therapeutic agent for DM.
- Citation: Liu N, Yan WT, Xiong K. Plantamajoside: A potentially novel botanical agent for diabetes mellitus management. World J Diabetes 2025; 16(5): 104311
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/104311.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.104311
Diabetes mellitus (DM), mainly encompassing type 1 DM (T1DM) and T2DM, is a group of complex, chronic metabolic disorders characterized by persistent hyperglycemia, resulting from absolute or relative insulin deficiency, insulin resistance, or both[1,2]. Chronic hyperglycemia progressively induces damage to multiple organs, ultimately contributing to devastating complications, including diabetic cardiomyopathy, neuropathy, nephropathy and retinopathy[2]. As is reported by International Diabetes Federation, approximately 6.7 million people died from DM in 2021[3]. DM has emerged as a significant public health challenge and economic burden worldwide due to its increasing prevalence, high morbidity, reduced quality of life and multiple severe complications[4,5]. Currently, glycemic control and the prevention or mitigation of DM-associated complications remain the mainstay of DM management. Despite significant advances in the development of DM therapies, a subset of individuals continues to exhibit poor therapeutic responses, and some drugs are associated with severe adverse effects[6-8]. Investigating the pathophysiological mechanisms underlying DM progression, along with identifying novel therapeutic strategies and drug candidates to enhance glycemic control and reduce DM complications, remains continuous efforts with considerable implications for both social and economic development worldwide.
Traditional Chinese medicine (TCM), one of the oldest traditional medical systems around the world, has been practiced in China for thousands of years and emphasizes the balance of Yin and Yang while minimizing side effects[9,10]. TCM is gaining increasing attention worldwide and has been officially incorporated into the 11th version of International Statistical Classification of Diseases and Related Health Problems. From ancient archives to a formal endorsement by the World Health Organization as a recognized global healthcare system, TCM has established itself as a complementary and alternative medicine within the Western medical system and remains irreplaceable in the prevention and treatment of multiple diseases[11-13]. Plantamajoside (PMS), a naturally bioactive ingredient derived from the traditional Chinese medicinal herb Plantaginis Herba and related species, exhibits diverse pharmacological properties, including anti-inflammatory[14], antioxidant[15], antitumor[16], antifibrotic[17] and antimicrobial effects[18]. Growing evidence indicates that PMS exerts distinct bioactivities through various signaling pathways, presenting significant potential as a therapeutic agent (Table 1)[14,15,17,19-30]. For example, PMS has been shown to attenuate cardiac fibrosis via specifically binding with the receptor for advanced glycation end products (RAGE) to suppress the advanced glycation end products-activated RAGE/autophagy/endothelial-to-mesenchymal transition pathway, suggesting that PMS serves as an effective antifibrotic agent and a promising therapeutic candidate for chronic heart failure[19]. PMS also abates interleukin 1 beta-stimulated extracellular matrix degeneration and inflammatory responses by suppressing the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways, thereby alleviating osteoarthritis progression, highlighting its potential as a therapeutic candidate for osteoarthritis[20]. In addition, the safety of PMS was verified in an oral toxicity study[31]. Considering the versatility, safety, and availability for industrial synthesis[32], PMS represents a potential drug candidate that warrants further exploration in the treatment of various diseases. PMS reportedly possesses antiglycation activity, suggesting its potential relevance to DM[23]. Nevertheless, the therapeutic involvement of PMS in DM and the underlying mechanism is poorly studied, necessitating further investigation.
| Bioactivities | Mechanism of action | Specific diseases | Ref. |
| Antioxidative | Scavenge free radicals, reduce oxidative stress and decrease activities of SOD, catalase, and GSH-Px | Acute myocardial infarction | Zeng et al[15]; Choi et al[21]; Amakura et al[22] |
| Anti-inflammation | Inhibit PI3K/AKT signaling pathway and pro-inflammatory factors, such as COX-2, iNOS, IL-6, NO, IL-8, and TNF-α | Osteoarthritis, pulmonary inflammation | Liu et al[14]; Lin et al[20]; Wu et al[23] |
| Antifibrosis | Suppress the fibrotic process and downregulate fibrosis-related factors, such as α-SMA, TGF-β, and Col1α1 | Cardiac fibrosis, liver fibrosis | Wang and Yan[17]; Zhang et al[19] |
| Antitumor | Inhibit the growth and invasion of various tumor cells and induce apoptosis | Cervical cancer, hepatocellular carcinoma | Yin et al[24]; Zou et al[25] |
| Antimicrobial | Inhibit the growth of bacteria, fungi and virus via disrupting metabolic processes and inhibiting the formation of aggregates and biofilms | Bacterial infections, fungal infections, viral infections | Zhakipbekov et al[26]; Chen et al[27] |
| Neuroprotective | Reduce neurons death, inhibit microglia polarization, alleviate substantia nigra damage | Acute spinal cord injury, Alzheimer’s disease, Parkinson’s disease | Darwish et al[28]; Guo et al[29]; Živković et al[30] |
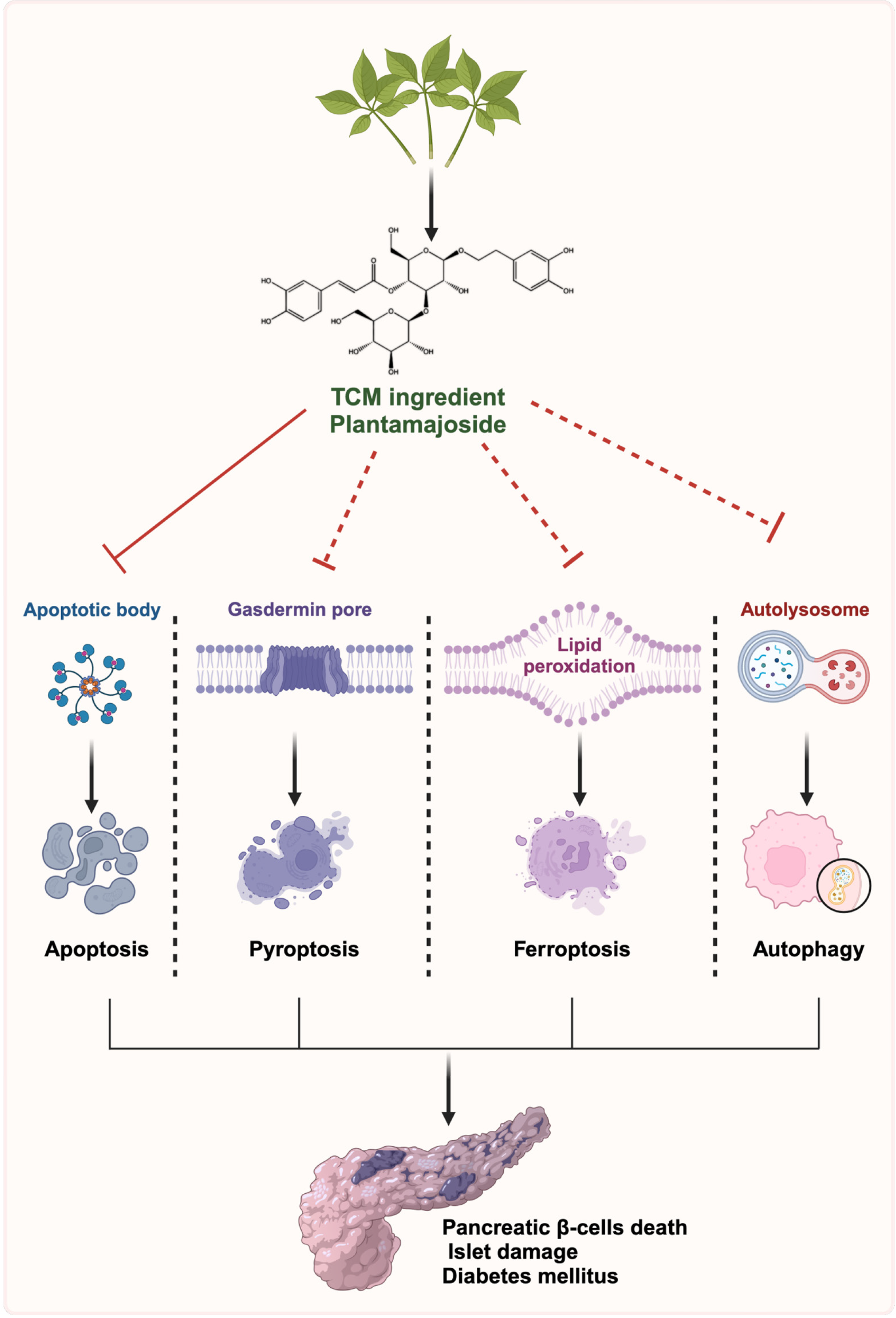
Pancreatic β-cells, located in the islets of the Langerhans, are essential for insulin production and secretion. Defects in the function or number of these cells can ultimately contribute to the progression of T1DM and T2DM[33-35]. A plethora of evidence shows that multiple forms of regulated cell death (RCD) in pancreatic β-cells, such as apoptosis, ferroptosis, pyroptosis and autophagy, are closely involved in the initiation and progression of DM and its complications[36,37] (Figure 1). Inhibition of β-cell death represents a promising therapeutic approach for DM. Thus, elucidating the various models of RCD in β-cells and the specific pathways involved is beneficial for developing effective strategies to enhance the survival and functionality of these cells, improving the outcomes of β-cell-based therapies.
Apoptosis is a form of RCD characterized by cellular shrinkage, plasma membrane blebbing, and nuclear frag
Ferroptosis, a type of RCD driven by iron-dependent lipid peroxidation, exhibits considerable heterogeneity in morphology and underlying biological mechanisms compared with classical forms of RCD[46,47]. The morphological alterations are distinguished by abnormally shrunken mitochondria with diminished cristae and condensed membrane density. Ferroptosis is strongly associated with various pathological conditions such as neurodegenerative disorders, ischemic injury, and tumors[48,49]. Numerous studies have shown that ferroptosis plays significant roles in the pathophysiology and pathogenesis of DM and its complications. For instance, chronic arsenic exposure can induce β-cell ferroptosis and inflammation via the glutathione peroxidase 4/Ager/p65 axis, consequently leading to islet dysfunction and onset of DM[50-52]. Quercetin, a bioactive flavonoid with therapeutic potential for T2DM management, can protect β-cells from ferroptosis by inhibiting iron deposition and reducing lipid peroxidation[53].
Pyroptosis, a caspase-dependent form of pro-inflammatory RCD, is distinguished by multiple features including cytoplasmic swelling, pore membrane formation, chromatin concentration and inflammatory responses[54,55]. This process is predominantly triggered by inflammasomes (especially nucleotide-binding oligomerization domain-like receptor protein 3 [NLRP3] inflammasomes) and executed by gasdermin (GSDM) family members, particularly GSDMD. It plays significant roles in various physiological and pathological processes, including DM and its complications[56-58]. Accumulating evidence has indicated that inhibiting β-cell pyroptosis is becoming a promising therapeutic strategy for DM[59]. For example, several agents such as salidroside (a primary ingredient of the traditional herbal medicine Rhodiola species)[60] and vitamin D[61], can protect β-cells against pyroptosis by suppressing the NLRP3/GSDMD pathway, thereby mitigating progression of DM.
Autophagy is an evolutionarily conserved and lysosome-dependent catabolic process characterized by the lysosomal degradation of cellular components, followed by recycling of proteins and organelles to preserve intracellular homeostasis. This process is initiated by the formation of autophagosomes, which subsequently fuse with lysosomes to facilitate degradation of cellular cargoes[62,63]. Autophagy typically serves as one of the major quality control me
Mounting evidence has demonstrated that other types of RCD, including necroptosis, PANoptosis as well as cuproptosis, play crucial roles in the pathogenesis of numerous diseases, including DM[68-70]. Hyperglycemia has been shown to induce β-cell necroptosis by promoting the formation of necrosome complexes, which consist of receptor-interacting protein kinase 1, receptor-interacting protein kinase 2 and mixed lineage kinase domain-like protein, resulting in cell swelling and membrane disruption[4]. Moreover, impaired copper metabolism has been reported in T2DM, highlighting the potential significance of cuproptosis, a recently identified copper-dependent form of RCD, in the progression of DM[71]. Given the significance of RCD in β-cells in the pathogenesis of DM and its complications, further research is required to elucidate the precise mechanisms underlying these RCD processes, the crosstalk between different types of RCD, and therapeutic potential of targeting RCD in DM.
Although PMS has been demonstrated to possess the capacity to mitigate multiple diseases via different pathways, its potential roles in DM remain poorly understood. In a previous study[72], the researchers revealed that PMS upregulated the expression of DnaJ heat shock protein family (Hsp40) member C1 (DNAJC1), a molecule associated with endoplasmic reticulum stress, thereby enhancing its interaction with the molecular chaperone glucose-regulated protein 78. This interaction subsequently alleviates the unfolded protein response and endoplasmic reticulum stress. As a result, PMS downregulates the expression of proapoptotic proteins B-cell lymphoma 2 (Bcl-2)-associated X protein and cytochrome c, while upregulating the antiapoptotic molecule Bcl-2 to inhibit β-cell apoptosis, ultimately alleviating β-cell damage and ameliorating T2DM progression[72]. Given the novel and protective effects of PMS on β-cells and its modulation of DNAJC1 in these cells, a mechanism that has never been extensively explored, these findings provide innovative mechanistic insights into DM pathogenesis, and highlight PMS as a promising therapeutic candidate for DM. However, the underlying mechanisms through which PMS modulates DNAJC1 remain unclear. Moreover, it remains to be determined whether PMS inhibits β-cell apoptosis through other molecules or pathways. Considering that PMS has been shown to modulate key signaling pathways involved in various types of RCD, such as apoptosis and autophagy[16,73], PMS holds significant potential to protect β-cells from other forms of RCD, including apoptosis, ferroptosis, pyroptosis and autophagy (Figure 1). Therefore, further exploration into whether PMS shields pancreatic β-cells from these types of RCD, coupled with unraveling the underlying molecular mechanisms, will provide a deeper understanding of how PMS may influence pancreatic β-cell survival under stress conditions and facilitate the advancement of more effective therapeutic strategies for DM. Given that glycosides generally exhibit low oral bioavailability, future structural modifications of PMS analogs should focus on improving their pharmacokinetic properties, particularly enhancing oral absorption, to facilitate drug development.
RCD of pancreatic β-cells plays a pivotal role in the onset and progression of DM, as the loss of functional β-cells leads to impaired insulin secretion and disrupted glucose homeostasis. Targeting RCD of β-cells has therefore emerged as a promising therapeutic strategy for DM. PMS, a bioactive compound derived from TCM, is characterized by its versatility, safety and suitability for industrial synthesis, exhibiting diverse pharmacological properties through multiple pathways, such as inhibiting autophagy and apoptosis in different diseases. Although current research on the regulation of RCD such as ferroptosis, PANoptosis, and cuproptosis by PMS in DM is limited, the ongoing investigation is highly likely to uncover additional RCD pathways influenced by PMS. By specifically targeting the RCD of β-cells, PMS presents a potentially novel therapeutic strategy with a multitarget approach for improving DM management. However, there is still a lack of large-scale clinical trials assessing the effects of PMS in humans, and much of the existing preclinical data remains preliminary. While the molecular mechanisms discussed in the aforementioned research appear promising, the translation of PMS from bench to bedside remains a significant challenge, further investigation is warranted to comprehensively elucidate PMS pharmacological effects and evaluate its clinical applications.
We would like to express our sincere appreciation to Xiong ZJ for his expert advice and guidance on this manuscript.
| 1. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3447] [Article Influence: 313.4] [Reference Citation Analysis (16)] |
| 2. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 937] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 3. | IDF Diabetes Atlas. International Diabetes Federation 2021. [cited February 12, 2025]. Available from: https://diabetesatlas.org/atlas/tenth-edition/. |
| 4. | Prasad MK, Mohandas S, Ramkumar KM. Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic β-cells in diabetes. Apoptosis. 2023;28:958-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 5. | Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 541] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 6. | Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8:e2100275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 7. | Michaelidou M, Pappachan JM, Jeeyavudeen MS. Management of diabesity: Current concepts. World J Diabetes. 2023;14:396-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 8. | Cai W, Davis DB. Imaging and therapy of diabetes: State of the art. Adv Drug Deliv Rev. 2019;139:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Tang JL, Liu BY, Ma KW. Traditional Chinese medicine. Lancet. 2008;372:1938-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Guo DA, Lu A, Liu L. Modernization of traditional Chinese medicine. J Ethnopharmacol. 2012;141:547-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, Cai H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 12. | Li S, Wu Z, Le W. Traditional Chinese medicine for dementia. Alzheimers Dement. 2021;17:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Zhou YM, Cao YH, Guo J, Cen LS. Potential prospects of Chinese medicine application in diabetic retinopathy. World J Diabetes. 2024;15:2010-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Liu F, Huang X, He JJ, Song C, Peng L, Chen T, Wu BL. Plantamajoside attenuates inflammatory response in LPS-stimulated human gingival fibroblasts by inhibiting PI3K/AKT signaling pathway. Microb Pathog. 2019;127:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Zeng G, An H, Fang D, Wang W, Han Y, Lian C. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation-induced injury through regulating the akt/Nrf2/HO-1 and NF-κB signaling pathways. J Recept Signal Transduct Res. 2022;42:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Pei S, Yang X, Wang H, Zhang H, Zhou B, Zhang D, Lin D. Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and -2. BMC Cancer. 2015;15:965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Yan D. Plantamajoside exerts antifibrosis effects in the liver by inhibiting hepatic stellate cell activation. Exp Ther Med. 2019;18:2421-2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Xu H, Yu H, Fu J, Zhang ZW, Hu JC, Lu JY, Yang XY, Bu MM, Jiang JD, Wang Y. Metabolites analysis of plantamajoside based on gut microbiota-drug interaction. Phytomedicine. 2023;116:154841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Guo YN, Liu J, Wang LH, Wu HQ, Wang T, Deng B, Wang JY, Lu L, Chen ZX, He JQ, Liang BR, Li H, Huang YS, Yang ZQ, Xian SX, Wang LJ, Ye XH. Plantamajoside attenuates cardiac fibrosis via inhibiting AGEs activated-RAGE/autophagy/EndMT pathway. Phytother Res. 2023;37:834-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Lin S, Lu J, Chen Q, Jiang H, Lou C, Lin C, Wang W, Lin J, Pan X, Xue X. Plantamajoside suppresses the activation of NF-κB and MAPK and ameliorates the development of osteoarthritis. Int Immunopharmacol. 2023;115:109582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Choi SY, Jung SH, Lee HS, Park KW, Yun BS, Lee KW. Glycation inhibitory activity and the identification of an active compound in Plantago asiatica extract. Phytother Res. 2008;22:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Amakura Y, Yoshimura A, Yoshimura M, Yoshida T. Isolation and characterization of phenolic antioxidants from Plantago herb. Molecules. 2012;17:5459-5466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, Li C, Deng G. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Int Immunopharmacol. 2016;35:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Yin W, Xu J, Li C, Dai X, Wu T, Wen J. Plantamajoside inhibits the proliferation and epithelial-to-mesenchymal transition in hepatocellular carcinoma cells via modulating hypoxia-inducible factor-1α-dependent gene expression. Cell Biol Int. 2020;44:1616-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Zuo X, Li L, Sun L. Plantamajoside inhibits hypoxia-induced migration and invasion of human cervical cancer cells through the NF-κB and PI3K/akt pathways. J Recept Signal Transduct Res. 2021;41:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Zhakipbekov K, Turgumbayeva A, Issayeva R, Kipchakbayeva A, Kadyrbayeva G, Tleubayeva M, Akhayeva T, Tastambek K, Sainova G, Serikbayeva E, Tolenova K, Makhatova B, Anarbayeva R, Shimirova Z, Tileuberdi Y. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Li W, Wang L, Wang B, Suo J. Novel inhibition of Staphylococcus aureus sortase A by plantamajoside: implications for controlling multidrug-resistant infections. Appl Environ Microbiol. 2025;91:e0180424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Darwish SF, Elbadry AMM, Elbokhomy AS, Salama GA, Salama RM. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Front Aging. 2023;4:1231706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 29. | Guo X, Chen L, Li J. Plantamajoside Alleviates Substantia Nigra Damage in Parkinson's Disease Mice by Inhibiting HDAC2/MAPK Signaling and Reducing Microglia Polarization. ACS Chem Neurosci. 2023;14:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Živković JČ, Barreira JCM, Šavikin KP, Alimpić AZ, Stojković DS, Dias MI, Santos-Buelga C, Duletić-Laušević SN, Ferreira ICFR. Chemical Profiling and Assessment of Antineurodegenerative and Antioxidant Properties of Veronica teucrium L. and Veronica jacquinii Baumg. Chem Biodivers. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Park BG, Lee HS, Jung SH, Hong CO, Won HJ, Park HY, Ryu YS, Lee SJ, Kim KH, Park KW, Lee KW. A 90 day repeated oral toxicity study on plantamajoside concentrate from Plantago asiatica. Phytother Res. 2007;21:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Ravn HW, Mondolot L, Kelly MT, Lykke AM. Plantamajoside — A current review. Phytochem Lett. 2015;12:42-53. [DOI] [Full Text] |
| 33. | Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 670] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 34. | Jain C, Ansarullah, Bilekova S, Lickert H. Targeting pancreatic β cells for diabetes treatment. Nat Metab. 2022;4:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 514] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 36. | Rojas J, Bermudez V, Palmar J, Martínez MS, Olivar LC, Nava M, Tomey D, Rojas M, Salazar J, Garicano C, Velasco M. Pancreatic Beta Cell Death: Novel Potential Mechanisms in Diabetes Therapy. J Diabetes Res. 2018;2018:9601801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3031] [Cited by in RCA: 3038] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 38. | Wan H, Yan YD, Hu XM, Shang L, Chen YH, Huang YX, Zhang Q, Yan WT, Xiong K. Inhibition of mitochondrial VDAC1 oligomerization alleviates apoptosis and necroptosis of retinal neurons following OGD/R injury. Ann Anat. 2023;247:152049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 1622] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 40. | Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Tang Q, Shi W, Liu M, Tang L, Ren W, Shi S. Mitochondrial protein MPV17 promotes β-cell apoptosis in diabetogenesis. Clin Sci (Lond). 2023;137:1195-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Ryan A, Murphy M, Godson C, Hickey FB. Diabetes mellitus and apoptosis: inflammatory cells. Apoptosis. 2009;14:1435-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1149] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 44. | Das AK, Hossain U, Ghosh S, Biswas S, Mandal M, Mandal B, Brahmachari G, Bagchi A, Sil PC. Amelioration of oxidative stress mediated inflammation and apoptosis in pancreatic islets by Lupeol in STZ-induced hyperglycaemic mice. Life Sci. 2022;305:120769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 45. | Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50 Suppl 1:S44-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1595] [Article Influence: 531.7] [Reference Citation Analysis (0)] |
| 47. | Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25:424-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 340] [Article Influence: 340.0] [Reference Citation Analysis (0)] |
| 48. | Zeng F, Nijiati S, Tang L, Ye J, Zhou Z, Chen X. Ferroptosis Detection: From Approaches to Applications. Angew Chem Int Ed Engl. 2023;62:e202300379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 49. | Chen J, Shi Z, Zhang C, Xiong K, Zhao W, Wang Y. Oroxin A alleviates early brain injury after subarachnoid hemorrhage by regulating ferroptosis and neuroinflammation. J Neuroinflammation. 2024;21:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Reference Citation Analysis (0)] |
| 50. | He J, Li Z, Xia P, Shi A, FuChen X, Zhang J, Yu P. Ferroptosis and ferritinophagy in diabetes complications. Mol Metab. 2022;60:101470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 51. | Liu P, Zhang Z, Cai Y, Li Z, Zhou Q, Chen Q. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 52. | Hong H, Lin X, Xu Y, Tong T, Zhang J, He H, Yang L, Lu Y, Zhou Z. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci Total Environ. 2022;849:157819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 53. | Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 54. | Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P, Wang Z. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310-4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 375] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 55. | Kovacs SB, Miao EA. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 984] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 56. | Zhou J, Qiu J, Song Y, Liang T, Liu S, Ren C, Song X, Cui L, Sun Y. Pyroptosis and degenerative diseases of the elderly. Cell Death Dis. 2023;14:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 57. | Liu P, Zhang Z, Chen H, Chen Q. Pyroptosis: Mechanisms and links with diabetic cardiomyopathy. Ageing Res Rev. 2024;94:102182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 58. | Chen Y, Long T, Chen J, Wei H, Meng J, Kang M, Wang J, Zhang X, Xu Q, Zhang C, Xiong K. WTAP participates in neuronal damage by protein translation of NLRP3 in an m6A-YTHDF1-dependent manner after traumatic brain injury. Int J Surg. 2024;110:5396-5408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 59. | Cao Z, Huang D, Tang C, Lu Y, Huang S, Peng C, Hu X. Pyroptosis in diabetes and diabetic nephropathy. Clin Chim Acta. 2022;531:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 60. | Zhou J, Yan S, Guo X, Gao Y, Chen S, Li X, Zhang Y, Wang Q, Zheng T, Chen L. Salidroside protects pancreatic β-cells against pyroptosis by regulating the NLRP3/GSDMD pathway in diabetic conditions. Int Immunopharmacol. 2023;114:109543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 61. | Wu M, Lu L, Guo K, Lu J, Chen H. Vitamin D protects against high glucose-induced pancreatic β-cell dysfunction via AMPK-NLRP3 inflammasome pathway. Mol Cell Endocrinol. 2022;547:111596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 62. | Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023;14:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 369] [Reference Citation Analysis (0)] |
| 63. | Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 676] [Article Influence: 338.0] [Reference Citation Analysis (0)] |
| 64. | Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 65. | Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K, Cecconi F, Choi AMK, Choi ME, Chu CT, Codogno P, Colombo MI, Cuervo AM, Deretic V, Dikic I, Elazar Z, Eskelinen EL, Fimia GM, Gewirtz DA, Green DR, Hansen M, Jäättelä M, Johansen T, Juhász G, Karantza V, Kraft C, Kroemer G, Ktistakis NT, Kumar S, Lopez-Otin C, Macleod KF, Madeo F, Martinez J, Meléndez A, Mizushima N, Münz C, Penninger JM, Perera RM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Sadoshima J, Santambrogio L, Scorrano L, Simon HU, Simon AK, Simonsen A, Stolz A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Galluzzi L, Pietrocola F. Autophagy in major human diseases. EMBO J. 2021;40:e108863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 979] [Article Influence: 244.8] [Reference Citation Analysis (0)] |
| 66. | Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 663] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 67. | Chen ZF, Li YB, Han JY, Wang J, Yin JJ, Li JB, Tian H. The double-edged effect of autophagy in pancreatic beta cells and diabetes. Autophagy. 2011;7:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Wan H, Yang YD, Zhang Q, Chen YH, Hu XM, Huang YX, Shang L, Xiong K. VDAC1, as a downstream molecule of MLKL, participates in OGD/R-induced necroptosis by inducing mitochondrial damage. Heliyon. 2024;10:e23426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Hu XM, Zheng S, Zhang Q, Wan X, Li J, Mao R, Yang R, Xiong K. PANoptosis signaling enables broad immune response in psoriasis: From pathogenesis to new therapeutic strategies. Comput Struct Biotechnol J. 2024;23:64-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 70. | Wan H, Ban X, He Y, Yang Y, Hu X, Shang L, Wan X, Zhang Q, Xiong K. Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury. Neural Regen Res. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 71. | Qu J, Wang Y, Wang Q. Cuproptosis: potential new direction in diabetes research and treatment. Front Endocrinol (Lausanne). 2024;15:1344729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Wang D, Wang YS, Zhao HM, Lu P, Li M, Li W, Cui HT, Zhang ZY, Lv SQ. Plantamajoside improves type 2 diabetes mellitus pancreatic β-cell damage by inhibiting endoplasmic reticulum stress through Dnajc1 up-regulation. World J Diabetes. 2025;16:99053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Wang Z, Zuo J, Zhang L, Zhang Z, Wei Y. Plantamajoside promotes metformin-induced apoptosis, autophagy and proliferation arrest of liver cancer cells via suppressing Akt/GSK3β signaling. Hum Exp Toxicol. 2022;41:9603271221078868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |