Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.103602
Revised: February 9, 2025
Accepted: March 24, 2025
Published online: May 15, 2025
Processing time: 140 Days and 4.8 Hours
There are conflicting results on the potential correlation between folic acid and gestational diabetes mellitus (GDM), and the correlation between genetic factors related to folic acid metabolism pathways and GDM remains to be revealed.
To examine the association between single-nucleotide polymorphisms (SNPs) of enzyme genes in the folate me
A nested case-control study was conducted with GDM cases (n = 412) and healthy controls (n = 412). DNA was extracted blood samples and SNPs were genotyped using Agena Bioscience’s MassARRAY gene mass spec
The variation allele frequency of melatonin receptor 1B (MTNR1B) rs10830963 was higher in the GDM group than in controls (P < 0.05). MTNR1B rs10830963 mutant G was associated with risk for GDM [adjusted odds ratio (aOR): 1.43; 95% confidence interval (95%CI): 1.13-1.80] in the additive model. MTNR1B rs10830963 GG + GC was significantly associated with the risk for GDM (aOR: 1.65; 95%CI: 1.23-2.22) in the dominant model. The two-locus model of MTNR1B rs10830963 and CHEMERIN rs4721 was the best model (P < 0.05) for gene-gene interactions in the GMDR results. The high-risk rs10830963 × rs4721 type of interaction was a risk factor for GDM (aOR: 2.09; 95%CI: 1.49-2.93).
This study does not find an association between SNPs of folate metabolic enzymes and risk for GDM. The G mutant allele of MTNR1B rs10830963 is identified as a risk factor for GDM in the additive model, and there may be gene-gene interactions between MTNR1B rs10830963 and CHEMERIN rs4721. It is conducive to studying the causes of GDM and provides a new perspective for the precise prevention of this disease.
Core Tip: Single-nucleotide polymorphisms of folate metabolic enzymes are not correlated with gestational diabetes mellitus (GDM). The G mutant allele of melatonin receptor 1B (MTNR1B) rs10830963 is a risk factor for GDM. Interactions between the MTNR1B rs10830963 and CHEMERIN rs4721 gene variants are associated with an increased risk for GDM.
- Citation: Zheng TT, Liu JH, Huang WT, Hong B, Wang D, Liu CY, Zhang J, Li SS, Wu SW, Wang Q, Chen L, Jin L. Single-nucleotide polymorphisms in genes involved in folate metabolism or selected other metabolites and risk for gestational diabetes mellitus. World J Diabetes 2025; 16(5): 103602
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/103602.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.103602
Gestational diabetes mellitus (GDM) is one of the most common metabolic complications during pregnancy. It has near- and long-term complications that can seriously harm the health of pregnant women and their offspring. In 2019, its prevalence was 12.8% worldwide[1]. In China, its incidence was 14.8% between 2013 and 2017[2]. Its incidence has increased over the past three decades, and its pathogenesis is unclear. It is currently thought that the causes of GDM are complex and related to environmental, nutritional, and genetic factors[3].
Studies in the 1990s showed that folic acid supplementation during pregnancy can prevent birth defects, such as neural tube defects[4] and congenital heart disease[5]. The United States began to implement a policy of compulsory folic acid fortification in food in 1998[6], and now more than 90 countries have adopted similar policies in staple foods. Since 2009, the Chinese government has been providing 400 µg daily folic acid tablets for 6 months to women planning pregnancy to prevent fetal neural tube defects. However, over the past decade, several studies have shown that taking folic acid or plasma folate is associated with risk for GDM; in particular, studies in Chinese populations have reached consistent conclusions regarding this[7-9]. Indeed, it has been found that synthetic folic acid in supplements that cannot be fully metabolically utilized in the body induces an inflammatory response[10] and oxidative stress that damages islet cells, leading to the pathogenesis of GDM[11].
Genes that encode the folate metabolic enzymes include RFC-1, MTHFR, MTRR, DHFR, MTR, SHMT, GCP II,
The frequency of the MTHFR C677T gene variant shows spatial and racial differences[15]. The variation rate of MTHFR in Chinese populations is higher than that in the European and American population[16,17]. A recent meta-analysis[18] reported that the frequency of the T allele of the MTHFR C677T gene variant differs by race, being less frequent in Caucasian populations than in Asian ones. In addition, the incidence of GDM is much higher in China than that in Western countries[2,19-21], which highlights the probable importance of different genetic backgrounds. Some gene polymorphisms, such as rs1801282 (PPARG), are significantly associated with GDM risk in Asian but not in Caucasian populations[22].
The most important genes involved in the pathogenesis of GDM identified in the previous studies include PPARG, ADIPOQ, MTNR1B[23-25], CDKAL1[26], HHEX[27], and CHEMERIN[28]. However, to the best of our knowledge, few studies have investigated the gene-gene or gene-environment interactions[29].
Within this context, we propose that the reason for the increased prevalence of GDM in Chinese women may be related to the genetic polymorphisms of enzymes in the folate metabolic pathway and other interactions among genetic and environmental factors. Here, we focused on pregnant women in Beijing. In the analysis of the association between SNPs of genes related to folate-metabolizing enzymes and the risk for GDM, we simultaneously considered the interactions among genes, as well as the impact of gene-environment interactions on the onset of GDM. Such information can comprehensively explain the potential mechanisms underlying the genetic and environmental factors leading to GDM and provide a new perspective for the precise prevention of GDM.
This study used a prospective cohort in Beijing that was constructed at Haidian District Maternal and Child Health Hospital and the Sixth Medical Center of the PLA General Hospital in Beijing.
In all, 2203 participants were recruited from the prospective cohort from August 2019 to July 2021. The inclusion criteria were as follows: Gestational weeks less than 13 at enrollment, spontaneous conception, singleton pregnancy, maternal age between 20 and 45 years, and living in Beijing for 1 year or more. The exclusion criteria were as follows: Suffering from serious chronic or infectious disease (e.g., 1/2 type diabetes mellitus, cancer, chronic cardiovascular and cerebrovascular diseases, chronic renal failure, or HIV infection), missing information on GDM diagnosis, questionnaire was not finished at enrollment, or blood samples not collected at enrollment.
Using these criteria, 826 study subjects were initially included in the study, with 413 patients in the case group, whose discharge diagnosis from pregnancy was clearly GDM. The control group included 413 pregnant women who delivered in the two hospitals during the same period but were not diagnosed with GDM during the gestational period. Due to the failure of one genotyping experiment in each of the case and control groups, 824 study subjects were selected in all.
The study was approved by the Biomedical Ethics Committee of Peking University (No. IRB00001052-18104). Pregnant women were fully informed of the purpose and content of the study and voluntarily signed written informed consent before the beginning of the study. The study was conducted in accordance with the Declaration of Helsinki.
At 4-13 gestational weeks, pregnant women completed the baseline questionnaire online on WeChat using a specialized program, providing maternal sociodemographic data, personal and family medical history, exercise habits, dietary habits, folic acid supplementation, and so forth.
GDM was diagnosed using the standards of the International Association of Diabetes and Pregnancy Study Group. Pregnant women who were not diagnosed with diabetes pre-pregnancy were given a 75 g oral glucose tolerance test after 12 h fasting at 24-28 weeks of gestation, and diagnosis of GDM was made if any of the following criteria were met: Fasting glucose ≥ 5.1 mmol/L, 1 h glucose ≥ 10.0 mmol/L, or 2 h glucose ≥ 8.5 mmol/L.
Fasting anticoagulant venous blood samples (2 mL) were collected at enrollment with a vacutainer. After centrifugation at 3000 rpm for 5 min, the samples were divided into plasma and blood cells in 2 mL sterilized freezing tubes. The blood samples were transported cold to a biological sample bank on the day of collection and frozen in a -80 °C freezer.
DNA was extracted from blood cells that were from fasting blood samples in the first trimester of pregnancy, using the Blood DNA Purification kit (DP348-03, Tiangen), according to the manufacturer’s protocol. The purity and concentration of the extracted DNA were tested using a Nanodrop microspectrophotometer from Thermo, United States. DNA samples with an OD260/OD280 ratio between 1.7-2.0 and a concentration of more than 20 ng/µL were used for genotyping.
The gene loci were screened using the Human Genome Project database, specifically the 1000 Genomes Browser (https://www.ncbi.nlm.nih.gov/variation/tools/tools1000genomes/) based on an extensive review of the literature. Previous studies have suggested that SNPs in genes involved in folate metabolism may affect the function of this metabolic pathway. In all, 10 SNPs in 8 genes associated with folate metabolism enzymes (RFC-1 rs1051266; MTHFR rs1801131, rs1801133, rs2274976; MTRR rs1801394; DHFR rs408626; MTR rs1805087; SHMT1 rs1979277; GCP II rs202676; and MTHFD1 rs2236225) were identified; these had a minor allele frequency > 5% in Han Chinese Beijing in the human genome sequence version 8 (Genome Reference Consortium Human Genome Build 38, GRCh38).
SNPs of genes related to GDM were chosen based on specific criteria. First, the gene must play a role in aspects of the development of GDM, such as insulin secretion, insulin antagonism, glucose and lipid metabolism, and/or inflammatory response. Additionally, the gene’s locus must include landmark SNPs of GDM-related gene that has been confirmed by meta-analyses. Finally, the association between variants of the gene or within the locus and risk for GDM must have been reported in high-quality, original studies. In the end, eight SNPs in six GDM-related genes (ADIPOQ rs2241766, rs266729; MTNR1B rs10830963, rs1387153; CDKAL1 rs7756992; PPARG rs1801282; CHEMERIN rs4721; and HHEX rs5015480) were screened. Detailed information is given in Supplementary Table 1. The SNPs were genotyped by Agena Bioscience’s MassARRAY gene mass spectrometry system. All SNPs were checked, and their consistency with Hardy-Weinberg equilibrium was assessed.
For categorical variables, chi-square tests were conducted for comparisons of demographics and other characteristics between the case and control groups for categorical variables. Likewise, Hardy-Weinberg equilibrium tests and comparison of the allele frequency of SNPs were conducted between the case and control groups. Unconditional logistic regression models were used to estimate the association between SNP genotypes and GDM in the additive, dominant, and recessive models, respectively. Confounders, including age of delivery, pre-pregnancy body mass index (BMI), ethnic group, education level, per capita annual income of the family, primipara, GDM history, family history of diabetes, physical activity, staple food habits, and folic acid supplementation, were adjusted in the logistic regression models. Generalized multi-factor dimensionality reduction (MDR) was used to analyze gene-gene and gene-environment interactions using the software generalized MDR (GMDR), version 0.9. The associations between the SNPs of folate metabolic enzymes and GDM were estimated using an unconditional logistic regression model.
Two-sided values of P < 0.05 were considered statistically significant. The P values were corrected using the Benjamini-Hochberg (B-H) method due to multiple comparisons.
The differences in age of delivery, pre-pregnancy BMI, annual per capita household income, parity and history of GDM, and medians of fasting blood glucose in the first trimester were statistically significant between the case and control groups; the rest were not (Table 1).
| Characteristics | GDM1 | Control1 | χ2 | P value |
| (n = 412) | (n = 412) | |||
| Delivery age (years) | 15.388 | < 0.001 | ||
| 20-30 | 97 (23.6) | 136 (33.0) | ||
| 30-35 | 193 (47.0) | 197 (47.8) | ||
| 35-44 | 121 (29.4) | 79 (19.2) | ||
| Pre-pregnancy BMI (kg/m2) | 51.509 | < 0.001 | ||
| < 18.5 | 32 (8.3) | 55 (13.3) | ||
| 18.5-24.0 | 225 (63.9) | 306 (85.7) | ||
| 24.0-28.0 | 95 (27.0) | 43 (12.0) | ||
| ≥ 28.0 | 32 (8.3) | 8 (1.9) | ||
| Ethnicity | ||||
| Han | 352 (91.4) | 385 (93.4) | 1.164 | 0.281 |
| Other | 33 (8.6) | 27 (6.6) | ||
| Education level | 2.89 | 0.089 | ||
| College school and below | 102 (26.5) | 88 (21.4) | ||
| Bachelor degree and above | 283 (73.5) | 324 (78.6) | ||
| Per capita annual household income (RMB) | 7.26 | 0.027 | ||
| < 50000 | 74 (21.8) | 58 (16.2) | ||
| 50000-100000 | 151 (44.5) | 146 (40.9) | ||
| ≥ 100000 | 114 (33.6) | 153 (42.9) | ||
| Primiparity | 15.812 | < 0.001 | ||
| Yes | 210 (55.6) | 284 (69.3) | ||
| No | 168 (44.4) | 126 (30.7) | ||
| History of GDM | 23.798 | < 0.001 | ||
| Yes | 33 (8.0) | 4 (1.0) | ||
| No | 379 (92.0) | 408 (99.0) | ||
| Family history of diabetes | 3.195 | 0.074 | ||
| Yes | 59 (15.2) | 45 (10.9) | ||
| No | 329 (84.8) | 366 (89.1) | ||
| Amount of exercise | 2.614 | 0.271 | ||
| No | 174 (46.6) | 167 (40.9) | ||
| Some | 144 (38.6) | 176 (43.1) | ||
| A large amount | 55 (14.7) | 65 (15.9) | ||
| Staple food habit (days a week) | 1.152 | 0.562 | ||
| 7 | 164 (44.1) | 177 (43.5) | ||
| 3-6 | 186 (50.0) | 198 (48.6) | ||
| 0-2 | 22 (5.9) | 32 (7.9) | ||
| Periconceptional folic acid supplementation (day) | 3.06 | 0.216 | ||
| ≥ 45 | 85 (22.9) | 83 (20.4) | ||
| 15-44 | 51 (13.7) | 74 (18.2) | ||
| < 15 | 235 (63.3) | 250 (61.4) | ||
| Periconceptional multiple micronutrients containing folic acid supplementation (day) | 0.381 | 0.827 | ||
| ≥ 45 | 102 (27.5) | 104 (25.6) | ||
| 15-44 | 78 (21.0) | 87 (21.4) | ||
| < 15 | 191 (51.5) | 216 (53.1) | ||
| Overview of folic acid supplementation (day) | 0.406 | 0.816 | ||
| ≥ 45 | 165 (44.5) | 172 (42.3) | ||
| 15-44 | 89 (24.0) | 100 (24.6) | ||
| < 15 | 117 (31.5) | 135 (33.2) |
The differences in the allele frequencies of 18 SNPs in 14 genes are shown in Table 2. The SNPs rs10830963 and rs1387153 in the GDM-related MTNR1B gene had higher frequencies of mutant alleles G and T in the GDM group than in controls (P < 0.05). Only the differences in the former were statistically significant (Padjusted < 0.05) after correction using the B-H method.
| Gene | SNP | Allele | GDM | Control | χ2 | P value | Padjusted1 |
| RFC-1 | rs1051266 | T | 361 (44.0) | 362 (44.1) | 0.002 | 0.960 | 0.963 |
| C | 459 (56.0) | 458 (55.9) | |||||
| MTHFR | rs1801131 | G | 116 (14.1) | 129 (15.7) | 0.810 | 0.368 | 0.694 |
| T | 708 (85.9) | 695 (84.3) | |||||
| rs1801133 | G | 364 (44.3) | 378 (46.0) | 0.481 | 0.488 | 0.694 | |
| A | 458 (55.7) | 444 (54.0) | |||||
| rs2274976 | T | 46 (5.6) | 54 (6.6) | 0.661 | 0.416 | 0.694 | |
| C | 776 (94.4) | 770 (93.4) | |||||
| MTRR | rs1801394 | G | 217 (26.4) | 202 (24.8) | 0.581 | 0.446 | 0.694 |
| A | 605 (73.6) | 614 (75.2) | |||||
| DHFR | rs408626 | T | 321 (39.1) | 320 (38.8) | 0.008 | 0.928 | 0.963 |
| C | 501 (60.9) | 504 (61.2) | |||||
| MTR | rs1805087 | G | 69 (8.4) | 85 (10.3) | 1.791 | 0.181 | 0.542 |
| A | 753 (91.6) | 739 (89.7) | |||||
| SHMT1 | rs1979277 | A | 45 (5.5) | 60 (7.3) | 2.289 | 0.130 | 0.535 |
| G | 779 (94.5) | 764 (92.7) | |||||
| GCP II | rs202676 | G | 282 (34.4) | 253 (30.9) | 2.333 | 0.127 | 0.535 |
| A | 538 (65.6) | 567 (69.1) | |||||
| MTHFD1 | rs2236225 | A | 193 (23.5) | 196 (23.8) | 0.014 | 0.905 | 0.963 |
| G | 627 (76.5) | 628 (76.2) | |||||
| ADIPOQ | rs2241766 | G | 207 (25.2) | 220 (26.7) | 0.453 | 0.501 | 0.694 |
| T | 613 (74.8) | 604 (73.3) | |||||
| rs266729 | G | 213 (26.2) | 212 (25.9) | 0.030 | 0.862 | 0.963 | |
| C | 599 (73.8) | 608 (74.1) | |||||
| MTNR1B | rs10830963 | G | 389 (47.6) | 317 (38.6) | 13.517 | < 0.001 | 0.004 |
| C | 429 (52.4) | 505 (61.4) | |||||
| rs1387153 | T | 368 (44.9) | 322 (39.2) | 5.484 | 0.019 | 0.173 | |
| C | 452 (55.1) | 500 (60.8) | |||||
| CDKAL1 | rs7756992 | A | 395 (48.3) | 396 (48.2) | 0.002 | 0.963 | 0.963 |
| G | 423 (51.7) | 426 (51.8) | |||||
| PPARG | rs1801282 | G | 53 (6.5) | 65 (7.9) | 1.253 | 0.263 | 0.676 |
| C | 767 (93.5) | 759 (92.1) | |||||
| Chemerin | rs4721 | G | 367 (44.8) | 387 (47.1) | 0.893 | 0.345 | 0.694 |
| T | 453 (55.2) | 435 (52.9) | |||||
| HHEX | rs5015480 | C | 156 (18.9) | 133 (16.2) | 2.087 | 0.149 | 0.535 |
| T | 668 (81.1) | 687 (83.8) |
Under the additive model, the mutant alleles G of rs10830963 and T of rs1387153 in MTNR1B were both identified as risk factors for GDM, showing statistically significance (P < 0.05) in the univariate logistic model. After B-H adjustment, only the former remained significantly correlated with GDM [odds ratio (OR) = 1.46, 95% confidence interval (95%CI): 1.19-1.78, Padjusted = 0.004). This association persisted even after adding multivariate adjustments, with each additional G allele increasing the risk for GDM by 1.43 times [adjusted OR (aOR) = 1.43, 95%CI: 1.13-1.80, Padjusted = 0.046). The detailed results are given in Supplementary Table 2.
In the dominant model, in rs10830963 and rs1387153 of the MTNR1B gene, the mutant homozygous + heterozygous GG + GC and TT + TC relative to wild homozygous CC were risk factors for GDM and were found to be statistically significant (P < 0.05) in univariate logistic regression. After adjustment for B-H, only the former remained associated with GDM (aOR = 1.65, 95%CI: 1.23-2.22, Padjusted = 0.018). After multivariate adjustment was added, it remained; however, after B-H adjustment the association was no longer significant. The detailed result is given in Supplementary Table 3.
Under the recessive model, the homozygous GG variant of rs10830963 in the MTNR1B gene was identified as a risk factor for GDM compared to the combined heterozygous and wild-type GC and CC variants (P < 0.05). However, this association lost its significance after B-H adjustment. In the multivariate model, no association between the gene loci and the risk for GDM remained statistically significant. Detailed results are given in Supplementary Table 4.
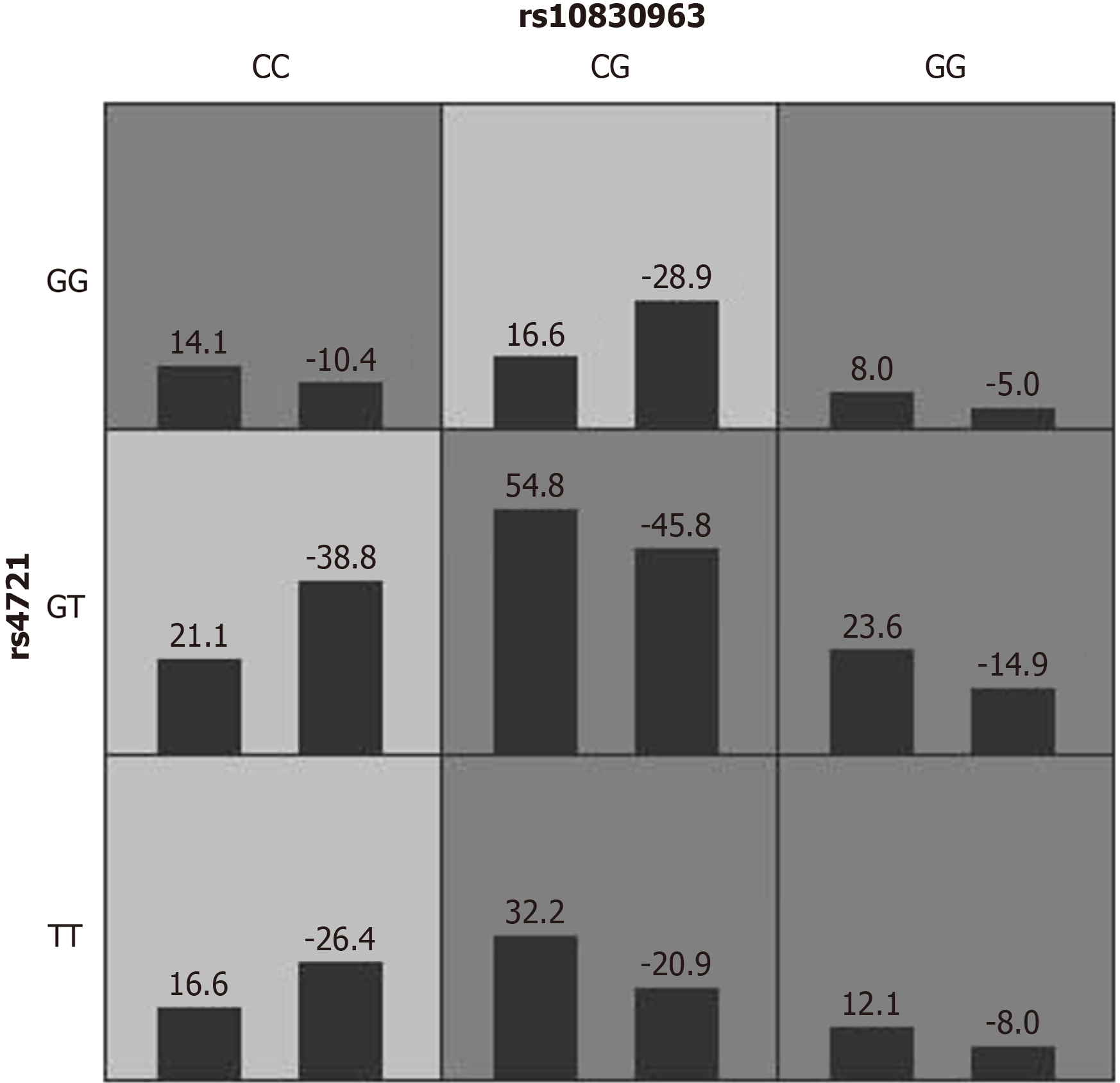
In total, 18 SNP loci of 796 women without missing loci were included to explore the interaction models from one to five sites, and the results are shown in Table 3. The one-site model for rs10830963 was not statistically significant, but the two-site interaction model for rs10830963 with rs4721 was statistically significant (P < 0.05), which could meet 10-fold cross-verification consistency with high prediction accuracy (59.96%). Thus, the two-site model of MTNR1B rs10830963 and CHEMERIN rs4721 was selected as the best.
| Number of sites | The best model | Tr.BA1 | Te.BA2 | P value | CVC3 |
| 1 | rs10830963 | 0.5599 | 0.5599 | 0.055 | 10/10 |
| 2 | rs10830963 rs4721 | 0.5999 | 0.5996 | 0.001 | 10/10 |
| 3 | rs10830963 rs4721 rs1801131 | 0.6219 | 0.5490 | 0.172 | 5/10 |
| 4 | rs10830963 rs4721 rs1801131 rs5015480 | 0.6587 | 0.5420 | 0.623 | 4/10 |
| 5 | rs10830963 rs4721 rs1801131 rs5015480 rs7756992 | 0.7229 | 0.4798 | 0.828 | 2/10 |
The risk pattern of the two-site model of rs10830963 × rs4721 is shown in Figure 1. Univariate and multivariate logistic regressions were used for validation, where the multivariate model was adjusted for 11 variables (delivery age, pre-pregnancy BMI, maternal ethnicity, education, per capita annual household income, primipara, GDM history, family history of diabetes, amount of exercise, staple food habits, and folic acid supplementation). Univariate logistic regression showed that the high-risk type of the rs10830963 × rs4721 interaction was an important risk factor for developing GDM in pregnant women. After adjusting for covariates, the interaction between high-risk type and GDM remained significant in the multivariate logistic regression (aOR = 2.09, 95%CI: 1.49-2.93, P < 0.001); that is, the risk for GDM in the high-risk type was 2.09 times higher than that in the low-risk type.
The 10 SNPs related to the folate metabolism enzymes of 796 people without missing loci were used to explore the interaction model from one site to five sites, and the results are shown in Supplementary Table 5. None of the best models of the interaction at two to five sites were statistically significant. This indicates that no gene-gene interactions involving these SNPs were found on GDM susceptibility.
In all, 18 SNPs of 796 individuals were without missing loci, and the above 11 categorical variables were used to explore the gene-environment interactions for one to five factors. The results are shown in Table 4. The best interaction models for one, two, three, and four factors were statistically significant (P < 0.05). The cross-validation consistencies (CVCs) for the best models for two, three, and four factors, were 9/10, 6/10, and 5/10 respectively, which indicate that the interaction model was insufficient and inferior to the univariate model of the pre-pregnancy BMI. With the exception of the three-factor model, the prediction accuracies of all other models were less than that of the single-factor pre-pregnancy BMI model. Therefore, it was not meaningful to examine the gene-environment interactions between these 18 SNPs and the 11 categorical variables.
The results regarding associations between the SNPs in genes involved in folate metabolism and the risk for GDM are shown in Table 5. None of the associations were statistically significant after adjusting for genetic and non-genetic factors.
| The best model | Tr.BA1 | Te.BA2 | P value | CVC3 |
| Pre-pregnancy BMI | 0.6244 | 0.6242 | 0.001 | 10/10 |
| Pre-pregnancy BMI rs10830963 | 0.6418 | 0.6235 | 0.001 | 9/10 |
| Pre-pregnancy BMI rs10830963 per capita annual household income | 0.6671 | 0.6331 | 0.001 | 6/10 |
| Pre-pregnancy BMI rs10830963 per capita annual household income rs4721 | 0.7071 | 0.5777 | 0.001 | 5/10 |
| Pre-pregnancy BMI delivery age per capita annual household income rs1801133 rs1387153 | 0.7756 | 0.5554 | 0.055 | 6/10 |
After adjusting for GDM-related SNPs, confounding factors, and gene-gene interactions, no significant association between these SNPs and GDM was observed. After adjusting for multivariate factors, the MTNR1B rs10830963 variant was significantly associated with GDM under the additive model. The high-risk rs10830963 × rs4721 interaction was a significant risk factor for GDM, being 2.09 times higher than that of the low-risk type.
Multiple studies have reported that periconception folic acid supplementation can increase or reduce the risk for GDM. Thus, the folate metabolism pathway has reasonably been assumed to have a certain correlation with GDM, and some genetic polymorphism studies have been carried out accordingly. Liu et al[30] recruited 366 Chinese women with singleton pregnancies to be followed up prospectively from early pregnancy and determined their folate concentration in red blood cells and rs1801133 polymorphism in early pregnancy. The results indicated that pregnant women who were homozygous for the mutant T gene had the highest red cell folate level, and pregnant women with high concentrations were at higher risk for GDM than those with low concentrations. However, this association was no longer significant after adjusting for the rs1801133 polymorphism. Jankovic-Karasoulos et al[31] typed several SNPs associated with folate metabolism enzymes, including MTHFR, MTHFD1, MTR, and MTRR. They measured circulating folate, vitamin B12, and Hcy in 3196 primiparas in Europe and Australia and observed significant effects of MTHFD1 rs2236225 and MTHFR rs1801133 on Hcy and serum folate, but no direct correlation between the above SNPs and GDM was found.
In the present study, we not only collected sociodemographic variables but also investigated eating habits, exercise habits, and folic acid use of pregnant women, adjusting for eight known GDM-related SNPs and their interactions; thus, our study provides more convincing evidence on this issue. After adjusting for confounding factors and the genetic background of GDM, we failed to detect an influence of multiple folate metabolic enzymes, including MTHFR, on GDM risk. From this, we know that SNPs have little effect on GDM, which is induced by multiple genes and environmental factors. Steegers-Theunissen et al[32] found that pregnant women who had continuous supplementation of folic acid had higher levels of IGF2 methylation than those who did not supplement after delivery. This suggests that folic acid can directly affect the host’s epigenetic modifications through abnormal DNA methylation. Such modifications can have adverse effects on pancreatic islet cell functions and can participate in the occurrence and development of GDM. It is necessary to conduct further studies to identify GDM pathogenesis.
A global multicenter GWAS study of 5485 GDM women and 347856 non-GDM women confirmed that the G mutant allele of MTNR1B rs10830963 was a risk factor for GDM[33]. In an Asian population, the results of another GWAS study[34] showed that allelic variation in the MTNR1B rs10830962 SNP variation allele was significantly associated with GDM. A study in Chinese women also found that a mutant allele of rs10830962 mutant allele was found to be a risk factor for GDM when using additive model[35]. Rs10830962 and rs10830963 are in complete linkage disequilibrium, meaning that the genetic variants at these two loci are highly correlated. Our results are consistent with these above findings.
A meta-analysis of multiple GWAS studies confirmed that multiple SNPs of MTNR1B are highly correlated with fasting blood glucose levels in the non-diabetic population[36]. A large-scale GWAS involving both European Union and non-European Union populations[37] found that genes associated with glycemic traits in non- pregnant individuals are also crucial during pregnancy. The genes may cause functional changes in islet β cells, which reduce insulin release after glucose ingestion[37]. Melatonin is a hormone that is primarily secreted by the pineal gland, whose function is to adjust the circadian rhythms of multiple organs, including the pancreas[38]. Tuomi et al[39] reported that melatonin treatment inhibits insulin secretion, with at-risk carriers exhibiting higher glucose levels. That study confirmed that the G allele of MTNR1B rs10830963 increased the expression of MTNR1B mRNA, which made islet β cells more sensitive to the inhibitory effects of melatonin. This common variant may lead to delayed secretion of melatonin and a prolonged duration of elevated melatonin levels during dim-light cycles, further affecting circadian rhythms, and increasing fasting blood glucose levels, and increasing insulin resistance[40]. The above findings form a theoretical basis for the significant correlation that we observed between the MTNR1B rs10830963 variant allele and GDM in our sample population.
GMDR, also known as score-based multi-factor dimensionality reduction (MDR), is a non-parameter, high-order method of interaction analysis without a genetic mode that was developed on the basis of MDR. It can reduce multidimensional variables to two levels and then evaluate the explanatory power of this multidimensional variable combination for diseases with good interaction and validation ability through cross validation and replacement testing[41]. The rationale includes scoring statistics and cross-validation. The statistical ideas are as follows. First, some data randomly selected from all of the data are calculated using maximum likelihood estimation. High-risk and low-risk models are divided according to the positive and negative values of the score statistic. The models are tested using the remaining data. This is repeated to avoid the impact of the opportunistic division of data for the results[42]. In this study the total sample was divided into 10 samples, 9 of which were used as model training samples each time, with the remaining one serving as a test sample. After 10-fold cross-validation, the model with the highest prediction accuracy was selected as the best model for the number of n loci. The number of times that a model is selected during this process is called the CVC. The average prediction accuracy from 10 tests was taken as an unbiased estimate for the model-related prediction accuracy, namely, a test of the sample accuracy. Across the best models for all numbers of loci, the model with the highest CVC and the highest accuracy was chosen as the final model for interaction, and P < 0.05 was considered to indicate statistical significance.
The GMDR analysis of 18 SNPs showed that pregnant women with a high risk for the rs10830963 × rs4721 interaction were at significantly higher risk for developing GDM than low-risk women. To the best of our knowledge, this is the first study to observe an interaction between the MTNR1B gene and the CHEMERIN gene. As noted, the mutant G allele of MTNR1B rs10830963 had an effect in inhibiting insulin secretion, and the mutant G allele of CHEMERIN rs4721 may cause abnormal Chemerin secretion and thus reduce serum TG levels. In the multivariate logistic model, the association strength of rs10830963 with GDM and that of rs4721 were both less than that of the rs10830963 rs4721 interaction model, which suggests that MTNR1B and CHEMERIN may have synergistic effects in GDM-related glucose and lipid metabolism. A study found that essential fatty acids play crucial roles in diabetes due to their involvement in various metabolic pathways and their influence on inflammation, lipid metabolism, insulin resistance, glucose homeostasis, and insulin signaling[43]. Leptin is known to be involved in GDM, researchers have not yet fully grasped the ways in which it influences the condition[44]. The level of free fatty acids in blood is high, the leptin production is further exacerbated, which was attributed to the difficulty of the adipose tissue to use energy properly. Furthermore, in patients with insulin resistance the levels of leptin have been shown to rise up regardless of the body fat of the patient[45,46]. These findings are consistent with the rs10830963 × rs4721 interaction having synergistic effects in GDM. However, the mechanisms underlying these effects remain unclear and require further investigation through animal studies and large-scale population research. This finding is helpful for early screening and precise intervention of GDM in pregnant women to improve prognosis.
This study had the following central strengths. First, the correlation between the folate metabolism pathway and GDM at present mainly focuses on a few SNP sites, such as MTHFR rs1801131 and rs1801133. This study included 8 signature SNPs related to GDM and 10 signature SNPs related to 8 metabolic enzymes of folate metabolism to allow us to comprehensively explore the correlations among multiple enzyme genes on the folate metabolism pathway and GDM. Second, conventional gene polymorphism studies usually only adjust basic characteristics such as age, BMI, birth, and are less likely to effectively evaluate exercise habits, dietary habits, folic acid supplementation, and other factors affecting the onset of GDM. We examined the relationship between SNPs of folate metabolism genes and GDM more powerfully, taking full consideration of such confounding factors based on a detailed questionnaire as well as the known genetic background. Finally, the GMDR method was used to explore gene-gene and gene-environment interactions. This is likewise the first time that an interaction between the two sites of rs10830963 and rs4721 was found. Upon testing, the rs10830963 × rs4721 two-locus model was found to have high prediction accuracy and CVC. This finding provides a new perspective for the molecular biological mechanism of GDM.
This study also had some limitations. First, the baseline survey time was weeks 4 to 13 of early pregnancy, and the survey content extended from 3 months before pregnancy to early pregnancy. GDM is diagnosed at 24 to 28 weeks of gestation, during which the lifestyle habits and folic acid administration may have changed. However, we did not investigate the lifestyle habits of pregnant women from early to second-trimester pregnancy, so the data were not fully representative. Second, we did not detect important intermediates or cofactors in folate metabolism pathways such as THF, Hcy, VB6, and VB12, so we could not explore the potential correlations between folate metabolism enzymes and GDM from the factor level to provide stronger evidence. This is worth further research in the future.
Interactions between the MTNR1B rs10830963 and CHEMERIN rs4721 SNPs play a significant role in the development of GDM, whereas SNPs involved in folate metabolism do not show such a correlation. In addition, no significant gene-gene or gene-environment interactions involving the studied SNPs and GDM were identified. These findings are valuable for early screening and precise intervention for GDM in pregnant women, which could ultimately improve maternal and child health.
The authors deeply thank all clinical staff members and patients who participated in this study.
| 1. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1409] [Article Influence: 469.7] [Reference Citation Analysis (1)] |
| 2. | Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. 2019;10:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 3. | Mirghani Dirar A, Doupis J. Gestational diabetes from A to Z. World J Diabetes. 2017;8:489-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (6)] |
| 4. | Folic Acid Supplementation to Prevent Neural Tube Defects: A Limited Systematic Review Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2023 Aug- . [PubMed] |
| 5. | Czeizel AE, Dudás I, Vereczkey A, Bánhidy F. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients. 2013;5:4760-4775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Federal Register. Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid; Correction. [cited 06 December 2024]. Available from: https://www.federalregister.gov/documents/1996/08/05/96-19803/food-standards-amendment-of-standards-of-identity-for-enriched-grain-products-to-require-addition-of. |
| 7. | Cheng G, Sha T, Gao X, He Q, Wu X, Tian Q, Yang F, Tang C, Wu X, Xie Q, Yan Y. The Associations between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus, and Adverse Birth Outcomes based on a Birth Cohort. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Li N, Jiang J, Guo L. Effects of maternal folate and vitamin B12 on gestational diabetes mellitus: a dose-response meta-analysis of observational studies. Eur J Clin Nutr. 2022;76:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Li Q, Zhang Y, Huang L, Zhong C, Chen R, Zhou X, Chen X, Li X, Cui W, Xiong T, Gao Q, Xu S, Wu Y, Wang X, Zhang G, Zhang X, Lin L, Gao D, Xiao M, Xiong G, Yang H, Yang N, Yang X, Hao L, Jin Z, Yang N. High-Dose Folic Acid Supplement Use From Prepregnancy Through Midpregnancy Is Associated With Increased Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care. 2019;42:e113-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Bonamichi BDSF, Lee J. Unusual Suspects in the Development of Obesity-Induced Inflammation and Insulin Resistance: NK cells, iNKT cells, and ILCs. Diabetes Metab J. 2017;41:229-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Sowton AP, Padmanabhan N, Tunster SJ, McNally BD, Murgia A, Yusuf A, Griffin JL, Murray AJ, Watson ED. Mtrr hypomorphic mutation alters liver morphology, metabolism and fuel storage in mice. Mol Genet Metab Rep. 2020;23:100580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Matsuo K, Suzuki R, Hamajima N, Ogura M, Kagami Y, Taji H, Kondoh E, Maeda S, Asakura S, Kaba S, Nakamura S, Seto M, Morishima Y, Tajima K. Association between polymorphisms of folate- and methionine-metabolizing enzymes and susceptibility to malignant lymphoma. Blood. 2001;97:3205-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Zheng W, Zhang Y, Zhang P, Chen T, Yan X, Li L, Shao L, Song Z, Han W, Wang J, Huang J, Ma K, Yang R, Ma Y, Xu L, Zhang K, Yuan X, Li G. Gestational diabetes mellitus is associated with distinct folate-related metabolites in early and mid-pregnancy: A prospective cohort study. Diabetes Metab Res Rev. 2024;40:e3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 15. | Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151:862-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 691] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 16. | Xu X, Li J, Sheng W, Liu L. Meta-analysis of genetic studies from journals published in China of ischemic stroke in the Han Chinese population. Cerebrovasc Dis. 2008;26:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Yang B, Fan S, Zhi X, Xia R, Wang Y, Zheng Q, Sun G. Geographical and ethnic distribution of MTHFR gene polymorphisms and their associations with diseases among Chinese population. Clin Genet. 2017;92:243-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zhu X, Hong X, Chen L, Xuan Y, Huang K, Wang B. Association of methylenetetrahydrofolate reductase C677T and A1298C polymorphisms with genetic susceptibility to polycystic ovary syndrome: A PRISMA-compliant meta-analysis. Gene. 2019;719:144079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, Jacqueminet S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60:636-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Chamberlain C, Joshy G, Li H, Oats J, Eades S, Banks E. The prevalence of gestational diabetes mellitus among Aboriginal and Torres Strait Islander women in Australia: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2015;31:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 22. | Wu L, Cui L, Tam WH, Ma RC, Wang CC. Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci Rep. 2016;6:30539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Dias S, Pheiffer C, Abrahams Y, Rheeder P, Adam S. Molecular Biomarkers for Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Zhang C, Bao W, Rong Y, Yang H, Bowers K, Yeung E, Kiely M. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum Reprod Update. 2013;19:376-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Lambrinoudaki I, Vlachou SA, Creatsas G. Genetics in gestational diabetes mellitus: association with incidence, severity, pregnancy outcome and response to treatment. Curr Diabetes Rev. 2010;6:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wang X, Ding Y, Zhang X, Rao J, Yu H, Pan H. The association between HHEX single-nucleotide polymorphism rs5015480 and gestational diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2020;99:e19478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Wang D, Wang H, Li M, Zhao R. Chemerin levels and its genetic variants are associated with Gestational diabetes mellitus: A hospital-based study in a Chinese cohort. Gene. 2022;807:145888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ren Q, Guo M, Yang F, Han T, Du W, Zhao F, Li J, Li W, Feng Y, Wang S, Zhang Y, Wu W. Association of CPT1A gene polymorphism with the risk of gestational diabetes mellitus: a case-control study. J Assist Reprod Genet. 2021;38:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Mora-Ortiz M, Rivas-García L. Gestational Diabetes Mellitus: Unveiling Maternal Health Dynamics from Pregnancy Through Postpartum Perspectives. Open Res Eur. 2024;4:164. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, Wu LP, Xia LY. Associations Between Gestational Diabetes Mellitus Risk and Folate Status in Early Pregnancy and MTHFR C677T Polymorphisms in Chinese Women. Diabetes Metab Syndr Obes. 2020;13:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Jankovic-Karasoulos T, Furness DL, Leemaqz SY, Dekker GA, Grzeskowiak LE, Grieger JA, Andraweera PH, McCullough D, McAninch D, McCowan LM, Bianco-Miotto T, Roberts CT. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern Child Nutr. 2021;17:e13064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Pervjakova N, Moen GH, Borges MC, Ferreira T, Cook JP, Allard C, Beaumont RN, Canouil M, Hatem G, Heiskala A, Joensuu A, Karhunen V, Kwak SH, Lin FTJ, Liu J, Rifas-Shiman S, Tam CH, Tam WH, Thorleifsson G, Andrew T, Auvinen J, Bhowmik B, Bonnefond A, Delahaye F, Demirkan A, Froguel P, Haller-Kikkatalo K, Hardardottir H, Hummel S, Hussain A, Kajantie E, Keikkala E, Khamis A, Lahti J, Lekva T, Mustaniemi S, Sommer C, Tagoma A, Tzala E, Uibo R, Vääräsmäki M, Villa PM, Birkeland KI, Bouchard L, Duijn CM, Finer S, Groop L, Hämäläinen E, Hayes GM, Hitman GA, Jang HC, Järvelin MR, Jenum AK, Laivuori H, Ma RC, Melander O, Oken E, Park KS, Perron P, Prasad RB, Qvigstad E, Sebert S, Stefansson K, Steinthorsdottir V, Tuomi T, Hivert MF, Franks PW, McCarthy MI, Lindgren CM, Freathy RM, Lawlor DA, Morris AP, Mägi R. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum Mol Genet. 2022;31:3377-3391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 34. | Kwak SH, Kim SH, Cho YM, Go MJ, Cho YS, Choi SH, Moon MK, Jung HS, Shin HD, Kang HM, Cho NH, Lee IK, Kim SY, Han BG, Jang HC, Park KS. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61:531-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Xie K, Chen T, Zhang Y, Wen J, Cui X, You L, Zhu L, Xu B, Ji C, Guo X. Association of rs10830962 polymorphism with gestational diabetes mellitus risk in a Chinese population. Sci Rep. 2019;9:5357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Kaaja R, Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diabet Stud. 2008;5:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Hayes MG, Urbanek M, Hivert MF, Armstrong LL, Morrison J, Guo C, Lowe LP, Scheftner DA, Pluzhnikov A, Levine DM, McHugh CP, Ackerman CM, Bouchard L, Brisson D, Layden BT, Mirel D, Doheny KF, Leya MV, Lown-Hecht RN, Dyer AR, Metzger BE, Reddy TE, Cox NJ, Lowe WL Jr; HAPO Study Cooperative Research Group. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62:3282-3291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1256] [Cited by in RCA: 1150] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 39. | Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen AK, Martikainen S, Räikkönen K, Forsén T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab. 2016;23:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 40. | Zhu H, Zhao ZJ, Liu HY, Cai J, Lu QK, Ji LD, Xu J. The melatonin receptor 1B gene links circadian rhythms and type 2 diabetes mellitus: an evolutionary story. Ann Med. 2023;55:1262-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 41. | Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, Li MD. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007;80:1125-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 454] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 42. | Chen Q, Tang X, Hu YH. [Detecting interaction for quantitative trait by generalized multifactor dimensionality reduction]. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:938-941. [PubMed] |
| 43. | Liang H, Mu HB, Zhang FH, Li WQ, Li GC, Li WD, Liang M, He ZL. Causal relationship between linoleic acid and type 2 diabetes and glycemic traits: a bidirectional Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1277153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 44. | Roca-Rodríguez MDM, Ramos-García P, López-Tinoco C, Aguilar-Diosdado M. Significance of Umbilical Cord Leptin Profile during Pregnancy in Gestational Diabetes Mellitus-A Systematic Review and Meta-Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Liu Y, Li DY, Bolatai A, Wu N. Progress in Research on Biomarkers of Gestational Diabetes Mellitus and Preeclampsia. Diabetes Metab Syndr Obes. 2023;16:3807-3815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Zhang R, Noronha JC, Khan TA, McGlynn N, Back S, Grant SM, Kendall CWC, Sievenpiper JL. The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (2)] |