Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.102196
Revised: February 9, 2025
Accepted: March 18, 2025
Published online: May 15, 2025
Processing time: 195 Days and 7.1 Hours
Injury to the glomerular filtration barrier causes diabetic kidney disease (DKD), and glomerular endothelial-mesenchymal transition damages the filtration barrier of glomerular endothelial cells. Shenfushu granules (SFSGs) can treat chronic renal failure; however, their role and mechanism in DKD remain unclear.
To investigate the role of SFSGs in delaying DKD progression and their under
The microalbumin content in the urine and the blood glucose, creatinine, and blood urea nitrogen levels in the serum were measured. The expression and distribution of α-smooth muscle actin (α-SMA), heparan sulfate (HS) and cluster of differentiation (CD) 31 were observed through immunofluorescence or immunohistochemistry. Western blotting was conducted to measure the expression of CD31, α-SMA, PIK3R1, protein kinase B (AKT), phospho-PIK3R1, phospho-AKT, and heparanase-1. Network pharmacology was conducted to screen and identify the core components and targets of SFSGs. Molecular docking and dynamic si
Compared with those in the model group, the 24-hour microalbuminuria (188.2 ± 20.1 and 140.4 ± 24.7 vs 323.2 ± 44.4), serum creatinine (79.4 ± 2.6 and 68.7 ± 6.0 vs 110.2 ± 4.8), blood urea nitrogen (14.4 ± 1.1 and 13.1 ± 0.5 vs 19.5 ± 1.1), and renal index (20.3 ± 1.0 and 19.6 ± 0.8 vs 25.3 ± 1.7) were significantly lower in the SFSGs (2.08 and 4.16 g/kg/day extract)-treated DKD mice. SFSGs inhibited the down
Collectively, these results suggest that SFSGs can significantly delay DKD progression and inhibit injury to the glycocalyx and the endothelial-mesenchymal transition of glomerular endothelial cells. This mechanism is related to PIK3R1/AKT/heparanase-1 signaling pathway regulation.
Core Tip: Shenfushu granules (SFSGs) can delay the progression of diabetic kidney disease (DKD) by reducing the levels of serum creatinine, urea nitrogen, and urinary microalbumin in DKD mice and reducing damage to glomeruli. SFSGs reduce glomerular endothelial-mesenchymal transition, which is characterized by filtration barrier damage. SFSGs affect the endothelial-mesenchymal transition mediated by the PIK3R1/protein kinase B/heparanase-1 signaling pathway. The main active ingredient of SFSGs strongly binds with PIK3R1.
- Citation: Yang XD, Ma SJ, Xiang DX, Yang YY. Shenfushu granules attenuate diabetic kidney disease by inhibiting PIK3R1/protein kinase B/heparanase-mediated endothelial-mesenchymal transition. World J Diabetes 2025; 16(5): 102196
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/102196.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.102196
Diabetic kidney disease (DKD) is one of the most pervasive and grave microvascular complications associated with diabetes and is currently the primary cause of end-stage renal disease[1]. The incidence of DKD is increasing in tandem with the increasing prevalence of diabetes. In advanced DKD, the current armamentarium lacks efficacious pharmaceutical interventions for arresting or reversing disease progression, underscoring the importance of measures to mitigate the advancement of nephropathy. The glomerular filtration barrier, a pivotal component of renal function, comprises the innermost layer of fenestrated endothelial cells, glomerular basement membranes, and podocytes[2]. Impairment of any of these constituents causes an increase in proteinuria[3]. Notably, glomerular endothelial cells (GEnCs) serve as integral components of the filtration barrier and are involved in crucial physiological processes, including hemostasis, an
An important factor in glomerular filtration barrier degradation is the endothelial-mesenchymal transition (EndMT) in GEnCs[5]. Glomerular EndMT occurs when the inherent connections between vascular monolayer endothelial cells are disrupted, leading to the loss of the original phenotype: Downregulation of cluster of differentiation (CD) 31 expression and acquisition of a mesenchymal cell phenotype characterized by an increase in α-smooth muscle actin (α-SMA)[6,7]. Under hyperglycemic conditions, GEnCs undergo EndMT, which compromises the glomerular filtration barrier and accelerates the progression of DKD. The glycocalyx is an important component of the filtration membrane of GEnCs, and heparanase-mediated degradation and remodeling of the glycocalyx component of heparan sulfate (HS) in GEnCs are key mechanisms in EndMT and the onset of DKD[7]. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway regulates heparanase expression[8,9]; therefore, modulating PI3K/heparanase-mediated EndMT may provide an effective avenue for mitigating the progression of DKD.
Shenfushu granules (SFSGs) are composed of six traditional Chinese medicinal ingredients: Ostrea rivularis Gould, Glycyrrhiza uralensis Fisch, Rheum tanguticum Maxim ex Balf, Salvia miltiorrhiza Bunge, Smilax glabra Roxb, and Citrus × aurantium (preparation approval number: Xiang Z20210657). Yang[10] reported that the main components of SFSGs in
Valsartan was purchased from the Hunan Qianjin Xiangjiang Pharmaceutical Co. Ltd. Mouse microalbumin, BUN assay kits, and serum creatinine were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China). Masson and hematoxylin and eosin (HE) staining kits were obtained from the Beijing Solarbio Technology Co., Ltd. (Beijing, China). The radioimmunoprecipitation assay (RIPA) buffer was acquired from the Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). The PI3 kinase p85α antibody was purchased from Beyotime. The phosphor-p85α antibody was acquired from Affinity. CD31 and anti-α-SMA antibodies were obtained from the Hunan Aifang Biotechnology Co., Ltd. (Hunan Province, China). Hpa-1 antibody was obtained from ABclonal (Wuhan, Hubei Province, China). Monoclonal anti-heparin sulfate (JM403 epitope) was acquired via AMSBIO (Cam
In brief, 781 g of Rheum tanguticum Maxim ex Balf, 625 g of Salvia miltiorrhiza Bunge, 469 g of Smilax glabra Roxb, 156 g of Ostrea rivularis Gould, 312 g of Citrus × aurantium, and 625 g of Glycyrrhiza uralensis Fisch were weighed and soaked in water for 10 hours. Later, the mixture was decocted twice for 1.5 hours each, and the filtrates were pooled, concentrated to an extract with a relative density of 1.15-1.30 (60 °C) at lower pressure, and later spray dried to obtain the SFSGs extract.
The Second Xiangya Hospital of Central South University’s Animal Ethics and Welfare Committee approved the animal experimental protocol (No. 2021558), which was carried out in compliance with the guidelines for the care and use of laboratory animals. We bought 48 male C57BL/6J mice from SiLai KeJingDa (Changsha, Hunan Province, China), weighing between 18 and 22 g. For one week, the mice had unrestricted access to food and water in an adapted feeding environment at 25 ± 2 °C with 40%-60% humidity. The mice were randomly assigned to two groups: Normal (n = 8) and model (n = 40). After fasting overnight, the mice in the model group were given a continuous intraperitoneal injection of STZ (60 mg/kg, citric acid-sodium citrate buffer, potential of hydrogen = 4.0, 0.1 mol/L) for five days, whereas those in the control group received an injection of the same volume of sodium citrate buffer containing 0.1 mol/L citric acid. After two days, the blood glucose level was determined, and a blood glucose concentration ≥ 16.7 mmol/L was considered to indicate successful establishment of the diabetic mouse model. After one week, eight mice per group were randomly assigned to the model control group; the low-, medium-, and high-dose SFSGs groups; and the positive control drug group (valsartan). The mice in the SFSGs group were intragastrically administered 1.04, 2.08, or 4.16 g/kg/day SFSGs extract [0.5% sodium carboxymethylcellulose sodium (CMCNa) suspension]. The dose for the mice was calculated based on the maximum dose for humans of 16 g extract/day, and the dose for the mice was 2.08 g/kg/day according to the body surface area, which was the medium dose. The mice in the positive control group received valsartan (40 mg/kg/day), with an administration volume of 0.20 mL. The mice in the normal and model control groups were administered 0.5% CMCNa solution (0.2 mL). Urine was collected from a mouse metabolic cage after 12 weeks of treatment, and blood glucose levels and body weights were recorded. After sodium pentobarbital (50 mg/kg) was administered to the animals to induce anesthesia, blood samples were taken. Kidney tissues were collected after the mice died.
The obtained blood samples were stored at 37 °C for 30 minutes to allow natural coagulation. The blood was sub
The 24-hour microalbumin content in the urine was measured using a microalbumin assay kit in accordance with the manufacturer’s instructions after the 24-hour urine of the mice in each group was collected in a metabolic box.
The kidney tissues were embedded in paraffin and sectioned into 5-μm thick slices. Thereafter, the sections were dewaxed with xylene at room temperature and rehydrated with an ethanol gradient (100%, 95%, 80%, and 50%). As directed by the manufacturer, HE, periodic acid-Schiff (PAS), and Masson staining kits were utilized. Images were examined under an Olympus microscope.
Sections were dewaxed and hydrated again and then placed in antigen retrieval solution at 80 °C for 20 minutes. The sections were blocked for one hour at room temperature with 5% bovine serum albumin (BSA) blocking solution. The sections were then incubated with HS (1:200) and α-SMA (1:250) overnight at 4 °C and then with secondary antibody (1:2000) for another hour. The sections were treated with CD31 primary antibody overnight, followed by an additional 1-hour incubation with rabbit secondary antibody (1:2000) at 37 °C in the dark. 4’,6-diamidino-2-phenylindole (DAPI) was then added to counterstain the nuclei for 10 minutes. Antofluorescence quenching sealing medium was added for sealing. Finally, an Olympus microscope was used to view and take pictures of the sections.
After dewaxing and hydration, the sections were added to the antigen retrieval solution at 80 °C for 20 minutes. The sections were blocked for 1 hour at room temperature with a 5% BSA blocking solution. The sections were then treated with the primary antibody CD31 (1:250) at 4 °C overnight, followed by an additional 1 hour at 37 °C for horseradish peroxidase-conjugated secondary antibody incubation. Later, the 3,3’-diamonobenzidine developing solution was added, followed by counterstaining with hematoxylin dye. After sealing, the sections were observed and photographed under a microscope.
After the mice were sacrificed, a 2% lanthanum nitrate-glutaraldehyde stationary liquid solution was injected into the aorta at 2.5 mL/minute. Thereafter, the renal cortical tissues were collected and fixed overnight with a 2% lanthanum nitrate-glutaraldehyde stationary liquid solution. Then, the kidney tissues were washed with 0.1 mol/L phosphate-buffered saline (PBS) for 15 minutes, which was repeated three times, followed by fixation with 1% osmic acid stationary liquid for 12 hours. Thereafter, the kidney tissues were soaked in 50%, 70%, 90%, and 90% acetone; 90% ethanol; and 100% acetone for 20 minutes for dehydration, followed by embedding with an acetone embedding agent. Later, the kidney tissues were cut into 50 nm-60 nm sections using an ultrathin microtome, and 3% uranyl acetate-lead citrate stain was applied to the sections. Ultimately, a transmission electron microscope was used to examine and obtain images of the sections.
The semiquantitative glomerulosclerosis index was evaluated on a scale ranging from 0-4 points, with 0 points indicating normal; 1, 2, 3 and 4 points suggesting involvement of 1%-25%, 26%-50%, 51%-75%, and 76%-100% of the glomeruli, respectively.
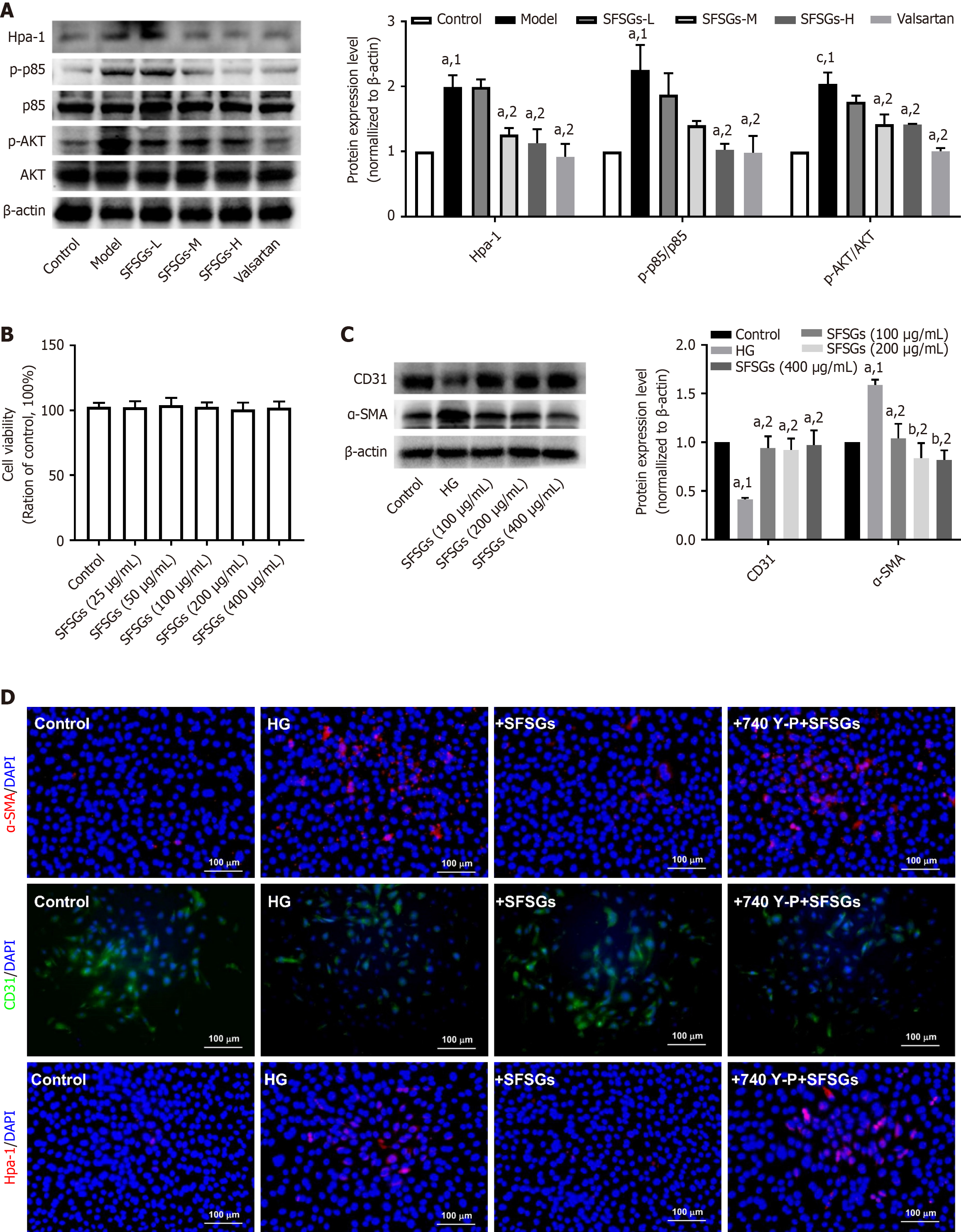
Using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the impact of SFSGs extract on HRGECs viability was assessed. HRGECs were inoculated into 96-well plates and separated into SFSGs extract and control groups. The cells in the SFSGs group were exposed to different concentrations of SFSGs extract (25, 50, 100, 200, and 400 μg/mL) for 24 hours. In contrast, the same volume of PBS was applied to the cells in the control group. Each well was then filled with MTT reagent (0.5 mg/mL), and the mixture was incubated for 2 hours. The formazan was dissolved in dimethyl sulfoxide (DMSO) after the supernatant was removed, and a microplate reader was used to measure the optical density.
HRGECs were divided into four groups: The control, high glucose (HG) group, HG + SFSGs, and HG + SFSGs + 740Y-P groups. In brief, the cells in the HG + SFSGs + 740Y-P group were pretreated with 10 nM 740Y-P and SFSGs extract (200 μg/mL) for 12 hours. The cells in the HG + SFSGs group were pretreated with SFSGs extract (200 μg/mL) for 12 hours. The cells in the HG, HG + SFSGs, and HG + SFSGs + 740Y-P groups were treated with 35 mmol/L HG for 24 hours. Following treatment with an equivalent volume of DMSO solution (0.1%), the cells in the control group were fixed for 15 minutes with 4% paraformaldehyde. Following 10 minutes of treatment with PBS with Tween 20, the cells were blocked with 4% BSA for 1 hour and incubated with primary antibodies against Hpa-1, CD31, and α-SMA overnight. After another 1-hour incubation with a fluorescent secondary antibodies, the nuclei were stained with DAPI. Images were taken via a fluorescence microscope.
Appropriate amounts of kidney tissue or treated cells were collected, and an appropriate amount of RIPA protein lysate was added. The bicinchoninic acid assay technique was used to measure the protein concentration after the supernatant was collected. Thereafter, a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel was prepared, and 35 μg of protein sample was added to each gel well. After electrophoresis for protein separation, a polyvinylidene fluoride membrane was used to receive the separated proteins. Then, the nonspecific antigens were blocked with 5% skim milk powder for 1 hour, and β-actin (1:500), Hpa-1 (1:500), PIK3R1 (1:500), phospho-PIK3R1 (1:500), CD31 (1:250), and α-SMA (1:500) were added to the membranes, which were incubated at 4 °C overnight. The membranes were washed three times for 5 minutes each, after which they were washed with tris buffered saline with Tween 20 (TBST). After that, the membranes were incubated at 37 °C for 1 hour, after which the secondary antibody (dilution: 1:5000) was added. After washing with TBST three times (5 minutes each), an enhanced chemiluminescence solution was added for imaging. The gray values of the protein bands were examined using the Image J program. Relative protein expression = (target protein/β-actin).
The chemical components of SFSGs were obtained from the traditional Chinese medicine systems pharmacology (TCMSP) database (http://tcmspw.com/tcmsp.php) and the Batman database (http://bionet.ncpsb.org/batman-tcm/). Components with an oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 were screened on the basis of the TCMSP database. For compounds without OB and DL data, the SwissADME database (http://www.swissadme.ch/) was used to calculate the absorption, distribution, metabolism, and excretion values. The compounds with “high” gas
The drug and disease targets were imported into the Venny2.1 online drawing tool platform to draw a Venn diagram. The intersections of these genes were used to obtain common drug-disease targets. To create the “SFSGs-component-DKD-target” network diagram, 134 putative active components and common drug-disease targets were subsequently entered into Cytoscape. Isolated components that did not intersect with targets were then removed. The Network Analyzer tool in Cytoscape 3.9.1 software was used to perform topological analysis of the primary active components of the SFSGs to identify the core components.
The STRING database (https://string-db.org/) was used to construct a protein-protein interaction (PPI) network of common drug-disease targets. The minimum interaction threshold was 0.9, and “Homo sapiens” was selected as the protein species. An PPI network diagram was created. Cystoscape 3.9.2 was subsequently used to import the PPI network relationship data. Using the network analyzer program, topological analysis was carried out, and a network diagram of core targets was created.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the common targets were performed via the DAVID database. A platform for the micro-bioinformatics network was used to visualize the results.
The small-molecule structure data format file was obtained from the PubChem database and converted into a protein data bank file using PyMOL. In addition, the protein structure was obtained from the protein data bank database. Molecular docking was performed using AutoDock Vina. The molecular docking data were visualized via discovery studio visualizer, and molecular dynamics simulations were carried out with AMBER 18 software.
Statistical analysis was performed via GraphPad software, and the data are expressed as the means ± SE. A Q-Q plot was used to assess the normality of the data, whereas Levene’s test was employed to evaluate the homogeneity of variance. If the data satisfied the normal distribution and homogeneity of variance criteria, multiple groups were compared using one-way analysis of variance, followed by Tukey’s test for post hoc tests. If the data did not satisfy normality and homogeneity of variance, the Kruskal-Wallis test was adopted, and Dunnett’s test was used for post hoc comparisons. P < 0.05 was considered statistically significant.
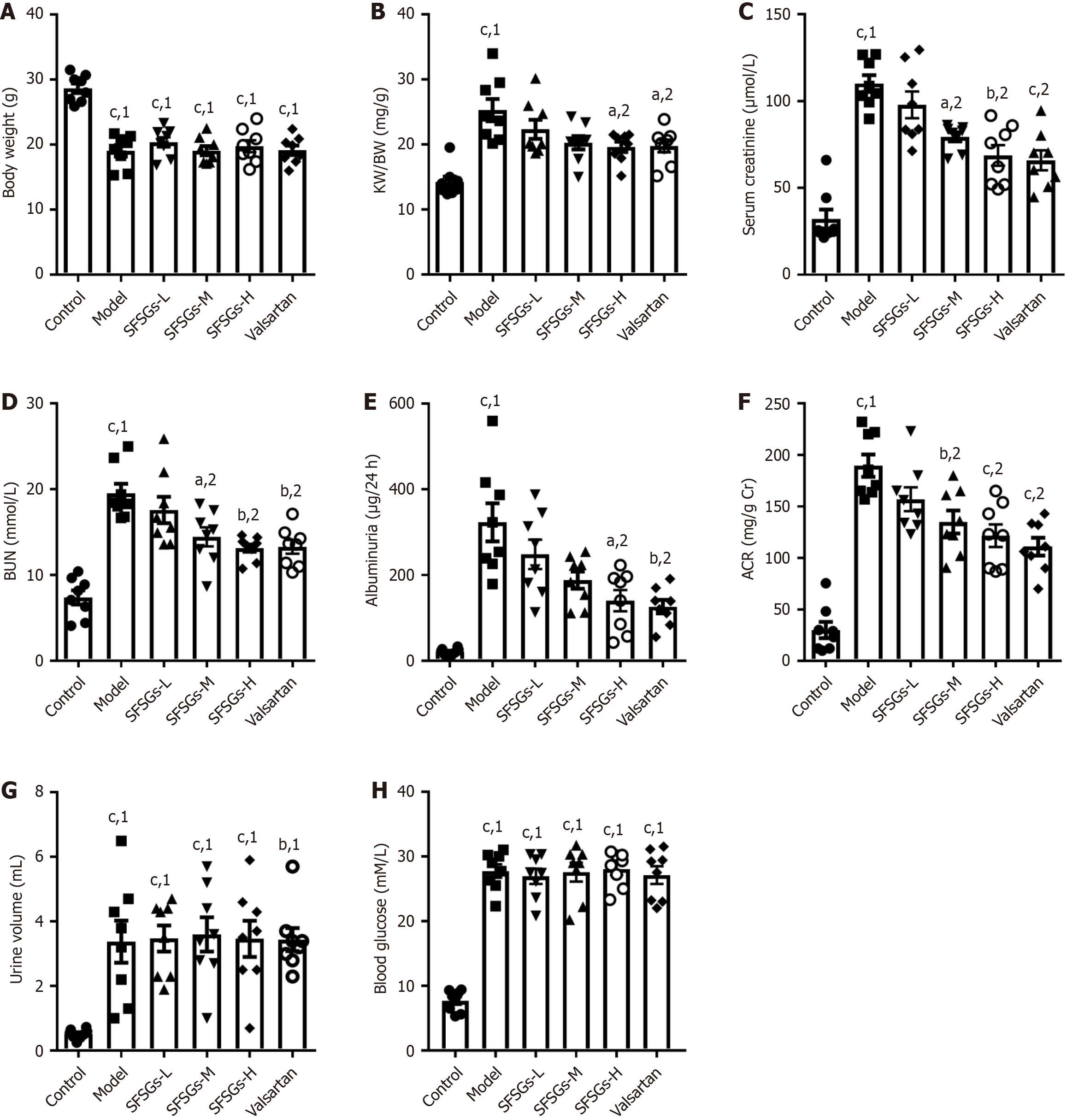
The body weights of the mice in the model group were much lower than those of the mice in the control group, and SFSGs did not improve body weight reduction (Figure 1A). The kidney index, BUN, serum creatinine, albumin/urine creatinine ratio (ACR), 24-hour urinary protein, blood glucose, and urine volume were significantly greater in the model group than in the control group. The kidney index, serum creatinine, BUN, urinary protein, and ACR levels in the SFSGs treatment group were markedly lower than those in the model group (Figure 1B-F), whereas SFSGs had no effect on the increase in blood glucose or urine volume (Figure 1G and H). These results indicate that SFSGs can delay the progression of DKD.
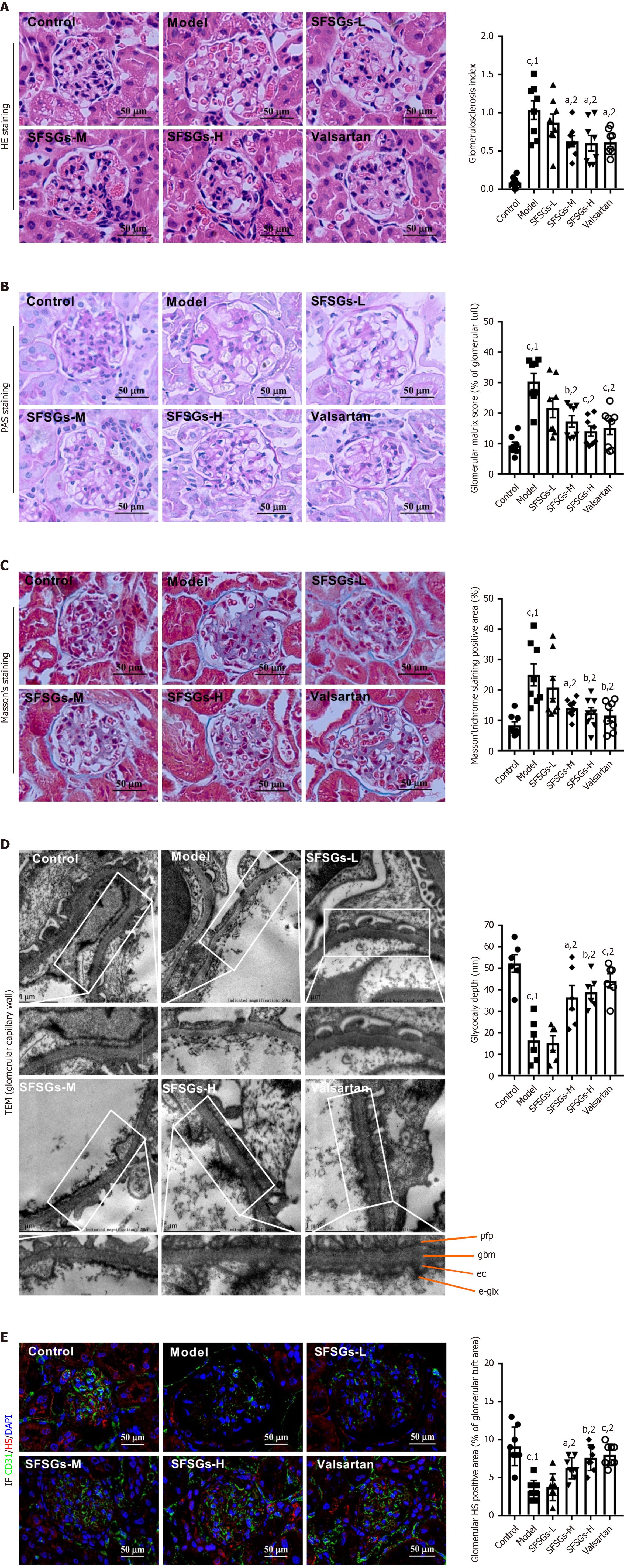
The glomerular damage index in the model group was much greater than that in the control group, as demonstrated by HE staining, and medium to high doses of SFSGs alleviated the increase in the glomerular injury index (Figure 2A). PAS staining revealed that SFSGs reduced the mesangial matrix in the glomeruli of DKD model mice. (Figure 2B). Masson’s trichrome staining also revealed that SFSGs significantly reduced collagen deposition in the glomerular area, thereby improving the occurrence of renal fibrosis (Figure 2C). Transmission electron microscopy revealed that the glycocalyx thickness of endothelial cells in the model group was significantly lower than that in the control group, whereas SFSGs treatment inhibited the reduction in glycocalyx thickness (Figure 2D). Immunofluorescence also revealed a significant reduction in HS in the model group, which was reversed by SFSGs treatment (Figure 2E). These findings suggested that SFSGs reduced the kidney damage caused by HG.
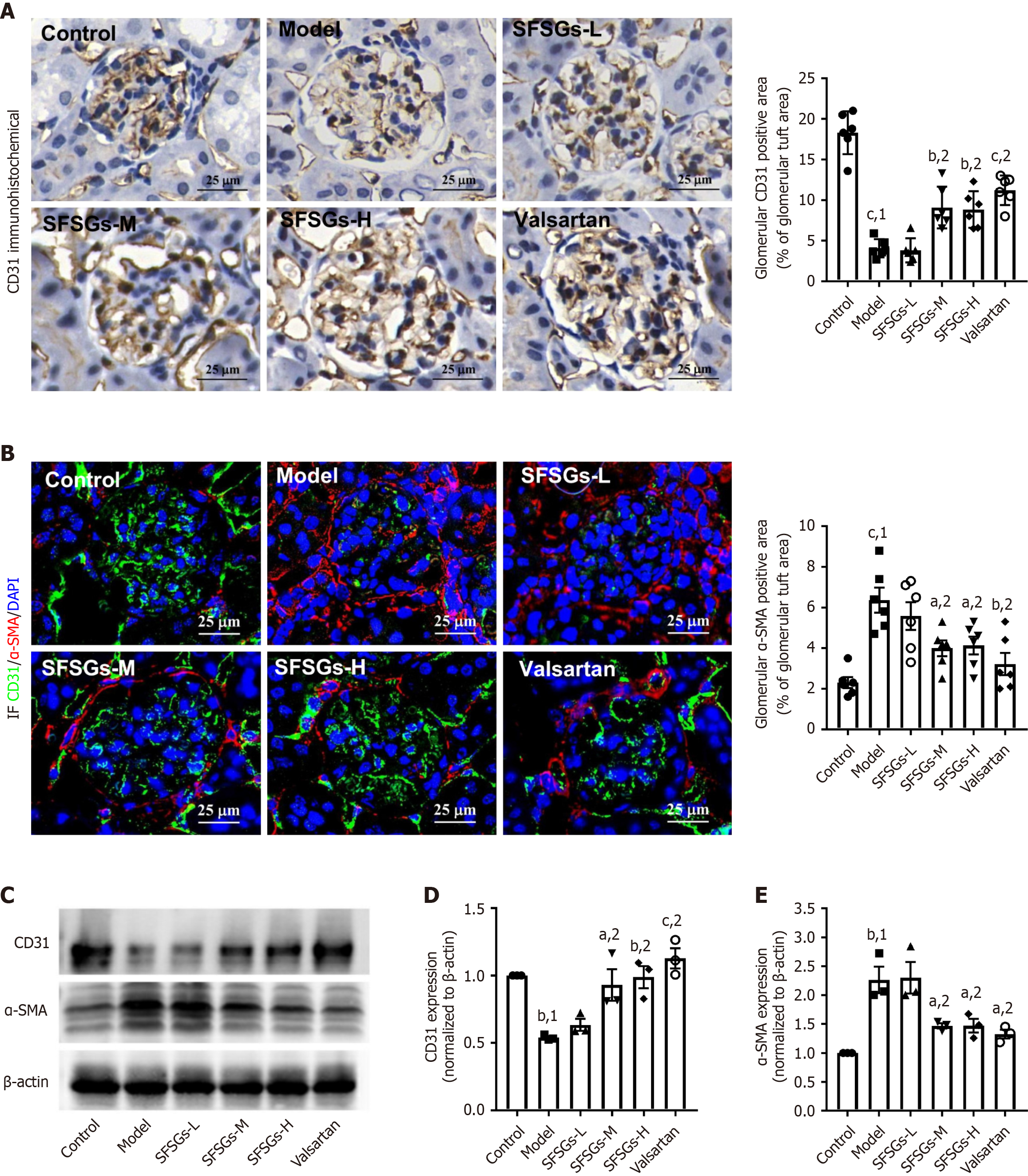
CD31 and α-SMA are markers of endothelial cells and mesenchymal cells, respectively. The immunohistochemistry results revealed a significant reduction in the expression level of CD31 in the glomeruli of the model group compared with those of the control group, whereas SFSGs treatment increased the expression level of CD31 (Figure 3A). Immunofluorescence staining revealed that SFSGs treatment markedly inhibited the increase in α-SMA levels in the renal tissue of DKD model mice (Figure 3B), as confirmed by Western blotting (Figure 3C-E). These results suggest that SFSGs can inhibit EndMT in the glomeruli of mice with DKD.
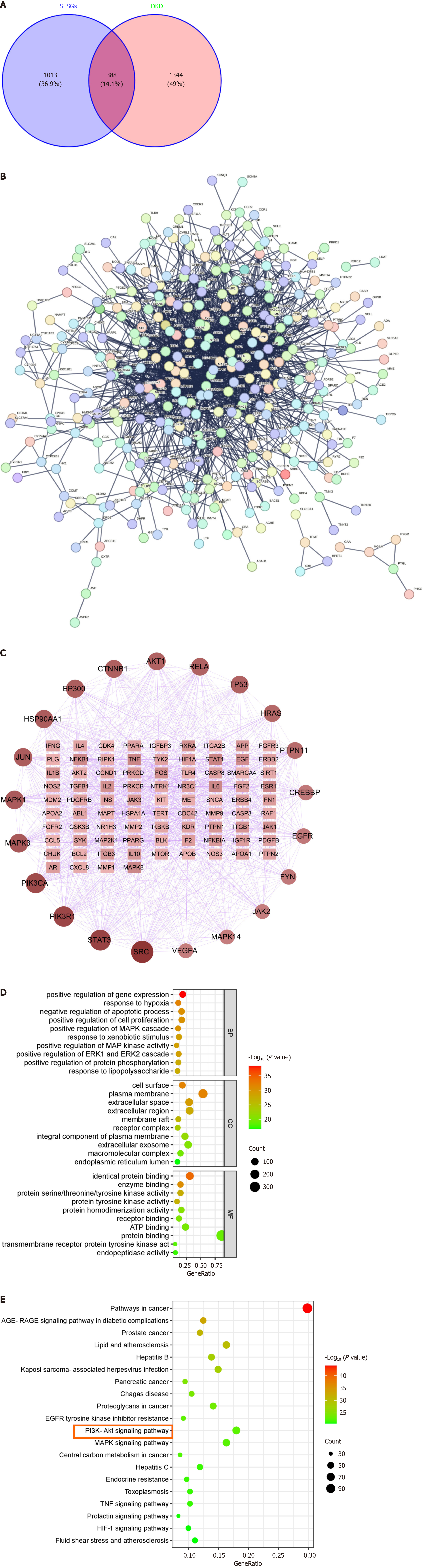
Network pharmacology was used to study the mechanism of SFSGs in treating DKD by screening 134 potential active ingredients (Supplementary Table 1) and their corresponding 1401 potential targets (Supplementary Table 2). A total of 1732 DKD targets were identified (Supplementary Table 3). The overlap between potential active ingredients and disease-related targets yielded 388 drug-disease targets (Figure 4A) (Supplementary Table 4). A PPI network of common targets was constructed (Figure 4B), and topological analysis of the core targets was conducted (Figure 4C). The node size and color vary according to the node degree, with a larger and darker node having a higher node rank. On the basis of the degree values, the top five core targets identified were SRC, STAT3, PIK3R1, PIK3CA, and MAPK3. GO enrichment and KEGG analyses were also performed on the drug-disease targets. The results of the GO analysis suggested that the targets of SFSGs were associated with biological processes, including the negative control of the apoptotic process, the response to hypoxia, and the positive regulation of gene expression. The cellular components included mainly the cell surface, plasma membrane, and extracellular space, and the molecular functions included mainly identical protein binding and enzyme binding (Figure 4D). KEGG pathway analysis also suggested that the PI3K-AKT signaling pathway might be involved in the mechanism by which SFSGs delay DKD (Figure 4E).
The expression of Hpa-1, phospho-AKT, and phospho-PIK3R1 (p-p85) was significantly increased in the renal tissue of the model group, whereas SFSGs treatment significantly inhibited the elevated expression of these proteins (Figure 5A). Cell experiments also revealed that 25-400 μg/mL SFSGs extract had no effect on the viability of HRGECs (Figure 5B). SFSGs extract at concentrations of 100-400 μg/mL significantly inhibited HG-induced EndMT in HRGECs (Figure 5C). Preincubation with the PIK3R1 agonist 740Y-P reversed the effect of SFSGs extract on EndMT (Figure 5D). These results indicate that SFSGs inhibit diabetic glomerular EndMT through the PIK3R1/AKT pathway.
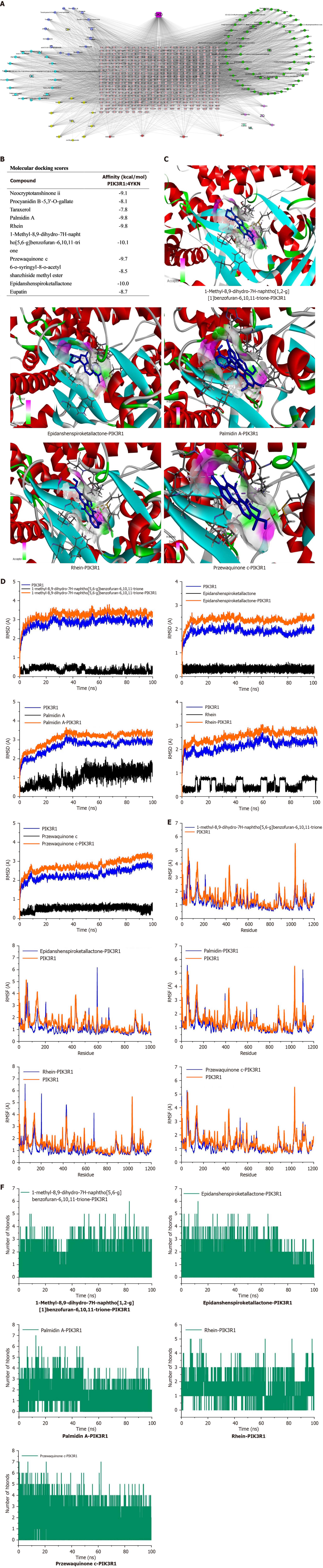
We constructed a drug-target-disease-target network diagram (Figure 6A) that included 507 nodes and 5142 edges. Topological analysis of this network identified the core compounds (Supplementary Table 5). According to the degree value, the top ten ranked compounds were neocryptotanshinone II, procyanidin B-5,3'-O-gallate, taraxerol, palmidin A, rhein, 1-methyl-8,9-dihydro-7H-naphtho[5,6-g]benzofuran-6,10,11-trione, przewaquinone C, 6-O-syringyl-8-O-acetyl shanzhiside methyl ester, epidanshenspiroketallactone, and eupatin. These ten compounds docked with PIK3R1. These findings demonstrated that the binding energies of these ten compounds to PIK3R1 were less than -7 kcal/mol, indicating strong interactions between the compounds and the target (Figure 6B). Docking visualization is shown in Figure 6C. Molecular dynamics simulations were conducted on the top five compound-target pairs, which were selected on the basis of their binding energy. The root mean square deviation results indicated stable molecular motion during the dynamic simulation, with no changes in protein or compound conformation, and good stability of the complexes (Figure 6D). The findings of the root mean square fluctuations revealed that the five compounds reduced the flexibility of the target protein at amino acid residues 200-400 (Figure 6E). During the dynamics simulation, przewaquinone C, 1-methyl-8,9-dihydro-7H-naphtho[5,6-g]benzofuran-6,10,11-trione, and epidanshenspiroketallactone maintained approximately three hydrogen bonds with PIK3R1, whereas the other compounds remained between one and two (Figure 6F). These results indicate that PIK3R1 can bind effectively to the core compounds.
DKD formation and occurrence are complicated processes, and persistent hyperglycemia leads to the generation of toxic mediators. Hyperglycemia and/or toxic mediators can activate different signaling pathways, which can damage kidney cells, ultimately resulting in modifications to the anatomy and function of the kidneys. In renal fibrosis, EndMT plays a role in myofibroblast accumulation and fibroblast activation. In a rodent model of unilateral ureteral blockage-induced experimental kidney fibrosis, myofibroblasts produced by EndMT accounted for approximately 10% of all myofibroblasts[13]. In addition, during the EndMT of GEnCs, fibrosis is enhanced, and the monolayer filtration barrier function is damaged[14]. The inhibition of EndMT maintains the function of the endothelial barrier and delays the progression of nephropathy[15]. SFSGs dramatically slowed the progression of DKD (Table 1), prevented renal EndMT from occurring, and blocked the downregulation of CD31 and the overexpression of α-SMA in GEnCs.
| Item | Control | Model vs control | SFSGs vs model |
| Physiological attributes | |||
| Body weight | Normal | Decrease | - |
| Src | Normal | Increase | Decrease |
| BUN | Normal | Increase | Decrease |
| Albuminuria | Normal | Increase | Decrease |
| ACR | Normal | Increase | Decrease |
| Urine volume | Normal | Increase | - |
| Blood glucose | Normal | Increase | - |
| Pathological attributes | |||
| Glomerulosclerosis | Normal | Increase | Decrease |
| Glomerular fibrosis | Normal | Increase | Decrease |
| Glycocaly depth | Normal | Decrease | Increase |
The glycocalyx, together with the fenestrated pores, constitutes a filtration barrier with a molecular size and charge-specific selectivity[16]. When DKD first begins, the glycocalyx is damaged, which manifests as the degradation of gly
Patients with kidney disease benefit from angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, sodium-glucose cotransporter 2 inhibitors, nonsteroidal corticosteroid receptor antagonists, and glucagon-like peptide-1 receptor agonists[18]. However, current treatments cannot effectively prevent the progression of some patients with DKD. The pathogenesis of DKD is complex and results from the combined action of multiple pathogenic factors and signaling pathways[2]. Therefore, a combination of treatment options, including traditional medicines based on multiple components, is needed. Traditional herbal medicines in SFSGs, such as Rheum tanguticum Maxim ex Balf[19], Glycyrrhiza uralensis Fisch[20], and Salvia miltiorrhiza Bunge[21], have been proposed as a means of delaying the progression of renal disease. In this work, we employed network pharmacology to examine the connections between DKD targets and the active ingredients in SFSGs. Topological analysis revealed that SRC, STAT3, PIK3R1, PIK3CA and MAPK3 were the top five core proteins targeted by SFSGs. In addition, SRC family kinases, STAT3, and MAPK3 are associated with the deve
Basic cellular processes such as transcription, proliferation, translation, growth, and survival are regulated by the PI3K-AKT signaling pathway[26]. PI3K is composed of regulatory and catalytic subunits and is a macrolipase that phos
Our work has several limitations. In this study, we used an STZ-induced type 1 diabetic nephropathy model to evaluate the efficacy of SFSGs and obtained useful results, which provide an experimental basis for the clinical use of SFSGs. Since the progression of DKD commonly combines with other risk factors, such as hypertension and hyperlipidemia, a single animal model cannot summarize all the important functional, structural, and pathological features of the kidneys under the complex etiology of DKD. The use of multiple models for further confirmation of effectiveness in subsequent studies may offer additional reference points for the translation of new drugs. Currently, in clinical practice, SFSGs are often combined with therapeutic agents for DKD, such as hypoglycemic, antihypertensive, lipid-lowering, or diuretic drugs. In the future, rigorous randomized controlled trials should be conducted to facilitate further evaluation of the safety, advantages and disadvantages of SFSGs vs conventional DKD therapies, assessment of the interactions of SFSGs with combined medications, and determination of their optimal dosing regimen. Although acute toxicity tests have shown that the maximum tolerated dose of SFSGs in mice is greater than 214.4 g/kg dry extract, which is 136 times greater than the clinical adult dose, long-term toxicity tests have shown that the maximum tolerated dose of SFSGs in rats is greater than 136 g/kg dry extract, which is 136 times greater than the clinical adult dose (unpublished data).
Collectively, the findings of this study suggest that SFSGs delay the development of DKD and suppress EndMT in GEnCs, which is associated with the PIK3R1-AKT signaling pathway. This work offers a pharmacological explanation for the advantages of SFSGs in the therapeutic management of DKD.
| 1. | Hu Y, Shi R, Mo R, Hu F. Nomogram for the prediction of diabetic nephropathy risk among patients with type 2 diabetes mellitus based on a questionnaire and biochemical indicators: a retrospective study. Aging (Albany NY). 2020;12:10317-10336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Hu S, Hang X, Wei Y, Wang H, Zhang L, Zhao L. Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review. Cell Commun Signal. 2024;22:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 3. | Daehn IS, Duffield JS. The glomerular filtration barrier: a structural target for novel kidney therapies. Nat Rev Drug Discov. 2021;20:770-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 4. | Finch NC, Neal CR, Welsh GI, Foster RR, Satchell SC. The unique structural and functional characteristics of glomerular endothelial cell fenestrations and their potential as a therapeutic target in kidney disease. Am J Physiol Renal Physiol. 2023;325:F465-F478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Zhang J, Chen S, Xiang H, Xiao J, Zhao S, Shu Z, Chai Y, Ouyang J, Liu H, Wang X, Quan Q, Fan J, Gao P, Chen AF, Lu H. S1PR2/Wnt3a/RhoA/ROCK1/β-catenin signaling pathway promotes diabetic nephropathy by inducting endothelial mesenchymal transition and impairing endothelial barrier function. Life Sci. 2023;328:121853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 7. | Sol M, Kamps JAAM, van den Born J, van den Heuvel MC, van der Vlag J, Krenning G, Hillebrands JL. Glomerular Endothelial Cells as Instigators of Glomerular Sclerotic Diseases. Front Pharmacol. 2020;11:573557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Masola V, Gambaro G, Tibaldi E, Onisto M, Abaterusso C, Lupo A. Regulation of heparanase by albumin and advanced glycation end products in proximal tubular cells. Biochim Biophys Acta. 2011;1813:1475-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Yang QF. The study on the content determint, fingerprint and dissolution of ShenFuShu Granules (Degree). M.Sc. Thesis, Central South University. 2011. Available from: https://kns.cnki.net/kcms2/article/abstract?v=jNHD1hIvxn1m2tCJZAICqE0J2SzmsWqBqRxaaNf2Ze3LeDAhuijqnu1qMQVcP594QNgqhxG1Ufd3i8rwQWpHtA7VImKt-eHh8LNcB7C0MVOaR9PfeUZgv1HHIXZBVzPbMPk_D-BX3rGm81t0YNyq85euJ2K-3lf5VE5QNsmTr63s88gNojaOILB6vyFlPDA-&uniplatform=NZKPT&language=CHS. |
| 11. | Zhao X, Xiang D, Jiang YS, Yang YY, Xiang DX. [Mechanism of action of Shenfushu granules in treatment of chronic renal failure]. Hunan Zhongyi Zazhi. 2023;39:149-156. [DOI] [Full Text] |
| 12. | Zhong Y, Deng XY, Zhao CC, Yan M, Zhang BK. [Analysis on appllication of Shenfushu granules in the second xiangya hospital of central south university during 2012-2016]. Zhongguo Yiyuan Yongyao Pingjia Yu Fenxi. 2017;17:300-303. [DOI] [Full Text] |
| 13. | LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1026] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 14. | Lovisa S, Fletcher-Sananikone E, Sugimoto H, Hensel J, Lahiri S, Hertig A, Taduri G, Lawson E, Dewar R, Revuelta I, Kato N, Wu CJ, Bassett RL Jr, Putluri N, Zeisberg M, Zeisberg EM, LeBleu VS, Kalluri R. Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Ma TK, Xu L, Lu LX, Cao X, Li X, Li LL, Wang X, Fan QL. Ursolic Acid Treatment Alleviates Diabetic Kidney Injury By Regulating The ARAP1/AT1R Signaling Pathway. Diabetes Metab Syndr Obes. 2019;12:2597-2608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Yang YY, Chen Z, Yang XD, Deng RR, Shi LX, Yao LY, Xiang DX. Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp Ther Med. 2021;22:1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Chang K, Xie Q, Niu J, Gu Y, Zhao Z, Li F, Qin Q, Liu X. Heparanase promotes endothelial-to-mesenchymal transition in diabetic glomerular endothelial cells through mediating ERK signaling. Cell Death Discov. 2022;8:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Pethő ÁG, Tapolyai M, Csongrádi É, Orosz P. Management of chronic kidney disease: The current novel and forgotten therapies. J Clin Transl Endocrinol. 2024;36:100354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Wang H, Song H, Yue J, Li J, Hou YB, Deng JL. Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane Database Syst Rev. 2012;2012:CD008000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yokozawa T, Liu ZW, Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 2000;6:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Shen Z, Cui T, Liu Y, Wu S, Han C, Li J. Astragalus membranaceus and Salvia miltiorrhiza ameliorate diabetic kidney disease via the "gut-kidney axis". Phytomedicine. 2023;121:155129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 22. | Dorotea D, Jiang S, Pak ES, Son JB, Choi HG, Ahn SM, Ha H. Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via inhibition of Fyn kinase-mediated endoplasmic reticulum stress. Exp Mol Med. 2022;54:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 23. | Tang P, Xu Y, Zhang J, Nan J, Zhong R, Luo J, Xu D, Shi S, Zhang L. miR-223-3p mediates the diabetic kidney disease progression by targeting IL6ST/STAT3 pathway. Biochem Biophys Res Commun. 2023;648:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Ma L, Wu F, Shao Q, Chen G, Xu L, Lu F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des Devel Ther. 2021;15:3207-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Qian X, He L, Hao M, Li Y, Li X, Liu Y, Jiang H, Xu L, Li C, Wu W, Du L, Yin X, Lu Q. YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol. 2021;58:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Margaria JP, Campa CC, De Santis MC, Hirsch E, Franco I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cell Signal. 2020;66:109468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Liu Q, Zhang Z, Yang Y, Zhou Y, Yan H, Wang X, Li X, Zhao J, Hu J, Yang S, Tian Y, Yao Y, Qiu Z, Song Y, Yang Y. The regulatory role of PI3K in ageing-related diseases. Ageing Res Rev. 2023;88:101963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Khanra R, Bhattacharjee N, Dua TK, Nandy A, Saha A, Kalita J, Manna P, Dewanjee S. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed Pharmacother. 2017;94:726-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Xiong D, Hu W, Han X, Cai Y. Rhein Inhibited Ferroptosis and EMT to Attenuate Diabetic Nephropathy by Regulating the Rac1/NOX1/β-Catenin Axis. Front Biosci (Landmark Ed). 2023;28:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |