Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.101840
Revised: February 14, 2025
Accepted: March 6, 2025
Published online: May 15, 2025
Processing time: 209 Days and 3 Hours
Some non-insulin-based insulin resistance (IR) indices have been found to be associated with metabolic syndrome (MetS); however, few cohort studies have compared the capacities of these indices for predicting incident MetS in young adults.
To investigate the associations of various non-insulin-based IR (NI-IR) indices with new-onset MetS in young military personnel.
A total of 2890 armed forces personnel in Taiwan who were aged 18-39 years and did not have MetS at baseline were followed to monitor the incidence of new-onset MetS from 2014 to the end of 2020. Six NI-IR indices, including the metabolic score for IR (METS-IR), triglyceride (TG)-to-high-density li
During a median follow-up of 5.8 years, there were 673 patients with new-onset MetS (23%). All six of the NI-IR indices were significantly and positively associated with incident MetS. In the entire cohort, the greatest AUROC was found for the METS-IR [0.782; 95% confidence interval (CI): 0.762-0.801; all P values compared to the other NI-IR indices < 0.05], followed by the TG/HDL-C ratio (0.752; 95%CI: 0.731-0.772), ZJU index (0.743; 95%CI: 0.722-0.764), TyG index (0.734; 95%CI: 0.713-0.756), TC/HDL-C ratio (0.731; 95%CI: 0.709-0.752), and then the ALT/AST ratio (0.734; 95%CI: 0.713-0.756).
This study suggests that almost all the NI-IR indices are associated with the development of MetS in military young adults. The METS-IR is the strongest predictor of new-onset MetS before midlife.
Core Tip: This study examined the associations of various non-insulin-based insulin resistance (IR) indices with new-onset metabolic syndrome (MetS) in young military personnel. The greatest area under the receiver operating characteristic curve was found for the metabolic score for IR [METS-IR; 0.782; 95% confidence interval (CI): 0.762-0.801] (all P values compared to the other non-insulin-based IR indices < 0.05), followed by the triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C) ratio (0.752; 95%CI: 0.731-0.772), the Zhejiang University index (0.743; 95%CI: 0.722-0.764), the TG glucose index (0.734; 95%CI: 0.713-0.756), the total cholesterol/HDL-C ratio (0.731; 95%CI: 0.709-0.752), and the alanine transaminase/aspartate transaminase ratio (0.734; 95%CI: 0.713-0.756). In conclusion, the METS-IR is the strongest predictor of new-onset MetS before midlife.
- Citation: Liu WN, Hsu YC, Lin YP, Tsai KZ, Lin YC, Liu PY, Lin GM. Comparisons of various insulin resistance indices for new-onset metabolic syndrome before midlife: The CHIEF cohort study, 2014-2020. World J Diabetes 2025; 16(5): 101840
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/101840.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.101840
Metabolic syndrome (MetS) includes a cluster of interrelated risk factors that increase the likelihood of developing cardiovascular diseases, type 2 diabetes, and other metabolic disorders[1-3]. The core components of MetS include abdominal obesity, hyperglycemia, dyslipidemia, and elevated blood pressure (BP)[4-6]. MetS poses a significant global health challenge, with a prevalence ranging from 20%-25% in adult populations worldwide. In Asia, the prevalence of MetS has increased dramatically over the past two decades, especially in urban areas and developing regions[7]. Recent epidemiological data in Taiwan indicate that approximately 25.5% of adults aged ≥ 20 years meet the criteria for MetS, with concerning trends revealing increasing rates among young adults[8]. This trend is particularly concerning, as MetS in young adults can lead to the earlier onset of cardiovascular complications and diabetes.
Insulin resistance (IR) is a key pathophysiological factor in the development of MetS[5,9]. Although the hyperinsulinemic-euglycemic clamp technique is considered the gold standard for assessing IR, it is time-consuming, expensive, and invasive, making it impractical for routine clinical use[10]. Alternatively, surrogate markers of IR, e.g., the ho
Several cross-sectional studies have reported that the METS-IR, TyG index, and TG/HDL-C ratio are strongly associated with the prevalence of MetS in various populations[18-20]. However, limited data are available on the prospective associations of these indices with new-onset MetS, particularly in young adults. Therefore, this study aimed to evaluate and compare the associations of six NI-IR indices (the METS-IR, TG/HDL-C ratio, TyG index, ZJU index, TC/HDL-C ratio, and ALT/AST ratio) with the development of new-onset MetS in a cohort of young Taiwanese military personnel. The findings of this study may provide valuable insights into the utility of these NI-IR indices for the early identification of individuals who are at high risk for MetS and guide the implementation of targeted preventive strategies in young adults.
The CHIEF study, a cohort study conducted in Taiwan, included 4080 military men and women aged 18 to 50 years at baseline in 2014[21]. The participants did not have diabetes mellitus and were not taking any medications, such as antihypertensive or lipid-lowering agents, at baseline. This study aimed to examine the associations of physical fitness and potential risk factors with metabolic and cardiovascular comorbidities in physically active young adults. At baseline (2014), each participant underwent a comprehensive health evaluation, which included various metrics, such as anthropometrics, hemodynamics, and blood/urine biomarkers. The participants also disclosed their substance use status, categorizing alcohol consumption and smoking tobacco as active habits, previous habits, or something they had never done. Moreover, the participants reported their moderate-intensity physical activity (PA) levels through leisure-time running sessions, which were classified as < 150 minutes/week, 150-299 minutes/week, or ≥ 300 minutes/week over the past 6 months[22]. These data were collected via self-reported responses to a questionnaire that was administered at the Hualien Armed Forces General Hospital in Eastern Taiwan. The cohort study adhered to the ethical guidelines outlined in the Declaration of Helsinki. The study design was evaluated and approved by the Institutional Review Board of the Mennonite Christian Hospital in Hualien, Taiwan (No. 16-05-008). Written informed consent was obtained from all the participants prior to their involvement in the study.
Anthropometric measurements, including waist circumference (WC), height, and weight, were obtained from each participant while standing. Body mass index (BMI) was calculated as the ratio of body weight in kilograms to the square of body height in meters (kg/m2). The resting BP of each participant was measured once on the right arm with an automatic BP device (FT201 Parama-Tech Co., Ltd., Fukuoka, Japan), and it was remeasured if the initial BP was ≥ 130/80 mmHg. The two BP measurements were averaged to obtain the final value[23]. Following a 12-hour overnight fast, blood samples were collected from each participant to measure the serum concentrations of various metabolic biochemical parameters. The metabolic biomarkers included TC, low-density lipoprotein cholesterol (LDL-C), HDL-C, TG, fasting plasma glucose (FPG), and the liver enzymes ALT and AST. An automated analyzer (Olympus AU640, Kobe, Japan) was employed for the analysis of the metabolic biomarkers[24].
In this study, six NI-IR indices were calculated using the following formulas: (1) METS-IR: Ln [(2 × FPG (mg/dL) + TG (mg/dL)] × BMI (kg/m2)/Ln HDL-C (mg/dL)[12]; (2) TG/HDL-C ratio: TG (mg/dL)/HDL-C (mg/dL)[13]; (3) TyG index: Ln [TG (mg/dL) × FPG (mg/dL)/2][14]; (4) ZJU index: BMI (kg/m²) + FPG (mmol/L) + TG (mmol/L) + 3 × ALT (U/L)/AST (U/L) ratio[15]; (5) TC/HDL-C ratio: TC (mg/dL)/HDL-C (mg/dL)[16]; and (6) ALT/AST ratio: ALT (IU/L)/AST (IU/L)[17].
Incident MetS was identified on the basis of each annual health examination (2015-2020) using the International Diabetes Federation criteria, which require the presence of three of the following five features: (1) Abdominal obesity (WC ≥ 90 cm for men and ≥ 80 cm for women); (2) TG ≥ 150 mg/dL; (3) HDL-C < 40 mg/dL in men or < 50 mg/dL in women; (4) systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg, or use of antihypertensive medications; and (5) FPG ≥ 100 mg/dL or previously diagnosed type 2 diabetes with use of antidiabetic medications[5].
The baseline characteristics of the participants who did not have MetS at baseline, who were divided into those who developed incident MetS and those who did not, are presented as the mean ± SD for continuous variables and as n (%) for categorical variables. Continuous variables were compared using analysis of variance (ANOVA), and categorical variables were compared via the χ2 test. Follow-up started in 2014 and continued until the incidence of MetS, loss to follow-up, or the end of the follow-up period on December 31, 2020.
Multivariate Cox proportional hazards regression analysis was performed to determine the associations between the six NI-IR indices (each 1-unit increase) and incident MetS, with initial adjustments for age, sex, alcohol intake status, smoking status, and PA in model 1. The potential covariates were selected due to their crucial role in MetS, according to the findings of a previous study[10]. An additional adjustment for baseline WC, the most critical feature in MetS, in model 2 aimed to verify the independent role of each NI-IR index for new-onset MetS. The area under the receiver operating characteristic curve (AUROC) was utilized to compare the capacities of the NI-IR indices and their related components for predicting new-onset MetS, with comparisons made using the Hanley and McNeil methods[25]. The best cutoff point selected from the AUROC of each NI-IR index was chosen with the aim of maximizing the sum of sensitivity and specificity for MetS. Additionally, the AUROC of each NI-IR index was explored in both men and women. All the statistical analyses were performed utilizing SPSS version 26.0 (IBM Corp., Armonk, NY, United States), and a two-tailed P value < 0.05 was considered statistically significant.
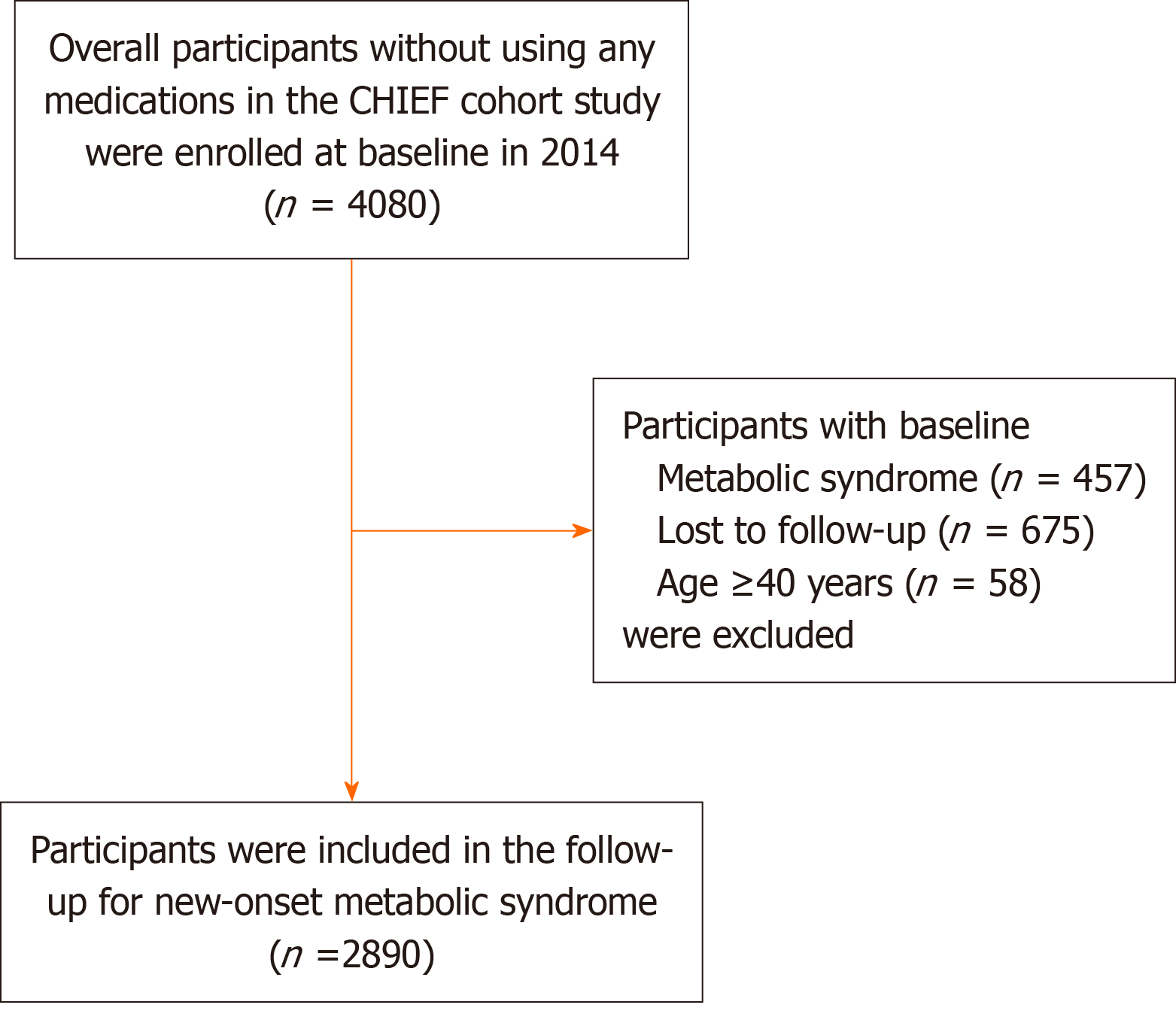
Participants with baseline MetS (n = 457), those who were lost to follow-up (n = 675), and those with a baseline age ≥ 40 years (n = 58) were excluded from the original study population, leaving a final sample of 2890 participants for analysis. Figure 1 shows the flow diagram that was used to select the eligible participants in this study. During a median follow-up of 5.8 years, 673 participants (23%) developed new-onset MetS. Table 1 shows the baseline characteristics of participants stratified by new-onset MetS development. Compared with those who did not develop MetS, those who developed MetS were older, were more likely to be male, and had higher prevalences of current alcohol and tobacco use (all P values < 0.001). Additionally, participants who developed MetS had higher BMI, WC, systolic BP, diastolic BP, FPG, ALT, AST, TC, LDL-C, and TG levels as well as a lower HDL-C level at baseline (all P values < 0.001).
| Without new-onset MetS (n = 2217) | With new-onset MetS (n = 673) | P value | |
| Metabolic index | |||
| METS-IR | 1.94 ± 0.16 | 2.13 ± 0.17 | < 0.001 |
| TyG index | 8.17 ± 0.44 | 8.58 ± 0.51 | < 0.001 |
| ALT/AST | 0.93 ± 0.36 | 1.11 ± 0.42 | < 0.001 |
| TC/HDL-C | 3.38 ± 0.82 | 4.12 ± 0.96 | < 0.001 |
| TG/HDL-C | 1.79 ± 1.15 | 3.01 ± 2.10 | < 0.001 |
| ZJU index | 122.64 ± 10.33 | 131.84 ± 13.57 | < 0.001 |
| Age (years) | 27.80 ± 5.82 | 30.29 ± 5.26 | < 0.001 |
| Male (%) | 1934 (87.2) | 647 (96.1) | < 0.001 |
| Alcohol drinking (%) | 823 (37.1) | 338 (50.2) | < 0.001 |
| Tobacco smoking (%) | 711 (32.4) | 280 (42.2) | < 0.001 |
| Physical activity, min/week (%) | |||
| < 150 | 497 (22.4) | 130 (19.3) | 0.15 |
| 150-299 | 848 (38.2) | 256 (38.0) | |
| ≥ 300 | 872 (39.9) | 287 (42.6) | |
| Systolic BP (mmHg) | 114.52 ± 12.77 | 120.39 ± 12.28 | < 0.001 |
| Diastolic BP (mmHg) | 68.41 ± 9.49 | 72.22 ± 9.58 | < 0.001 |
| BMI (kg/m2) | 23.62 ± 2.83 | 26.40 ± 2.52 | < 0.001 |
| Waist circumference (cm) | 79.73 ± 7.69 | 87.08 ± 6.53 | < 0.001 |
| Blood tests | |||
| TC (mg/dL) | 168.87 ± 30.71 | 182.25 ± 36.18 | < 0.001 |
| LDL-C (mg/dL) | 100.57 ± 27.64 | 114.54 ± 30.58 | < 0.001 |
| HDL-C (mg/dL) | 51.35 ± 10.01 | 45.25 ± 8.46 | < 0.001 |
| TG (mg/dL) | 86.89 ± 45.31 | 130.00 ± 81.42 | < 0.001 |
| FPG (mg/dL) | 91.98 ± 8.86 | 94.67 ± 11.77 | < 0.001 |
| ALT (U/L) | 18.49 ± 12.63 | 27.63 ± 22.13 | < 0.001 |
| AST (U/L) | 18.91 ± 6.94 | 22.55 ± 10.74 | < 0.001 |
Table 2 shows the associations between the NI-IR indices and the risk of incident MetS using multivariable Cox re
| Model 1 | Model 2 | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| METS-IR index | 78.937 | 55.157-112.970 | < 0.001 | 32.996 | 21.706-50.158 | < 0.001 |
| TyG index | 3.671 | 3.231-4.172 | < 0.001 | 2.602 | 2.259-2.997 | < 0.001 |
| ALT/AST | 2.824 | 2.421-3.294 | < 0.001 | 1.495 | 1.238-1.804 | < 0.001 |
| TC/HDL-C | 1.923 | 1.801-2.053 | < 0.001 | 1.606 | 1.489-1.731 | < 0.001 |
| TG/HDL-C | 1.250 | 1.222-1.278 | < 0.001 | 1.187 | 1.157-1.219 | < 0.001 |
| ZJU index | 1.028 | 1.025-1.031 | < 0.001 | 1.023 | 1.019-1.027 | < 0.001 |
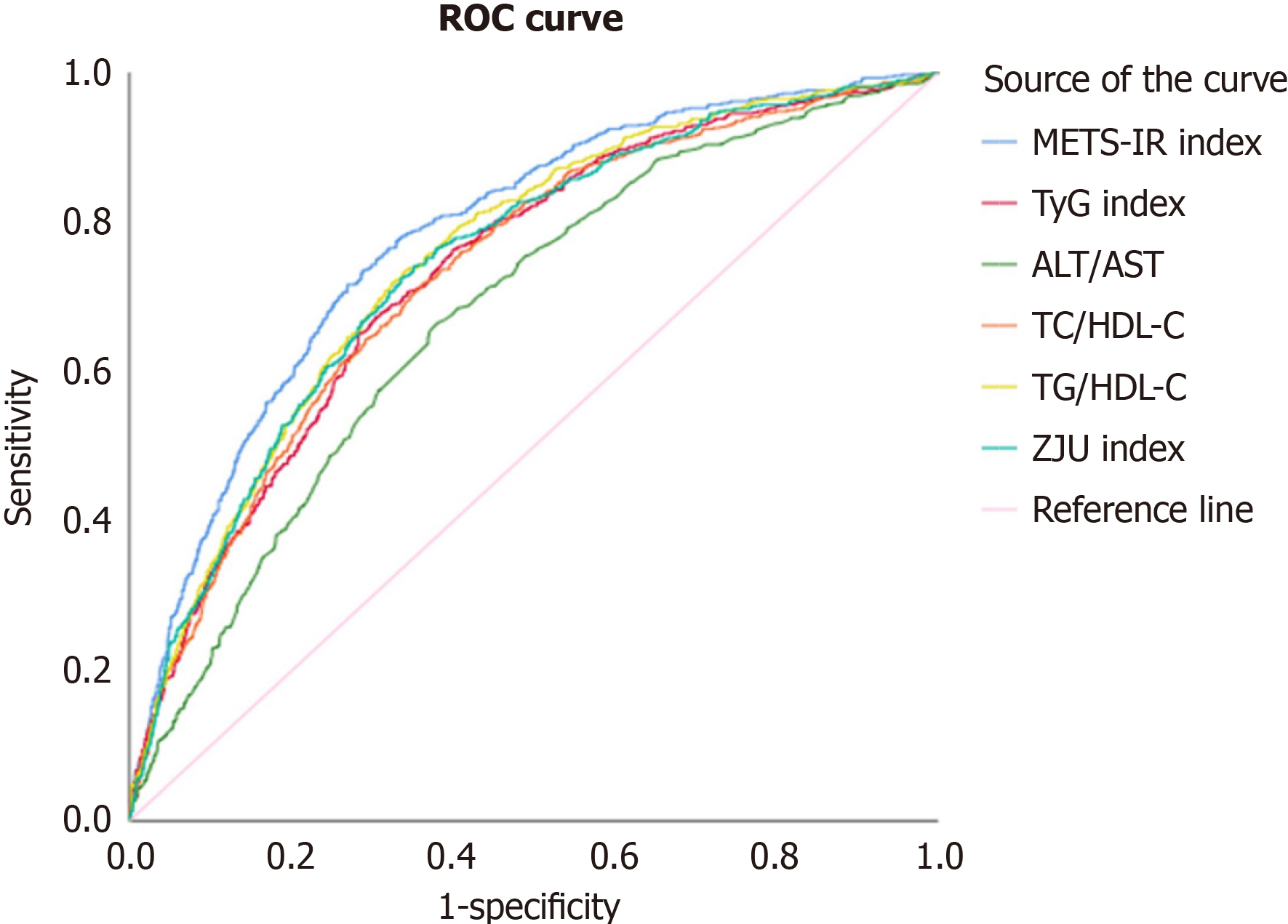
According to the receiver operating characteristic curves shown in Table 3 and Figure 2 for the entire cohort, all six NI-IR indices demonstrated significant capacities for predicting incident MetS (all P values < 0.001; data not shown). The METS-IR revealed the greatest AUROC of 0.782 [95% confidence interval (CI): 0.762-0.801], with all of P values < 0.05 compared with the other indices, followed by the TG/HDL-C ratio (0.752, 95%CI: 0.731-0.772), ZJU index (0.743; 95%CI: 0.722-0.764), TyG index (0.734; 95%CI: 0.713-0.756), TC/HDL-C ratio (0.731; 95%CI: 0.709-0.752), and ALT/AST ratio (0.674; 95%CI: 0.652-0.697). Among the components related to the six NI-IR indices, BMI had the greatest AUROC of 0.771 (95%CI: 0.752-0.790; P = 0.43 compared with METS-IR), followed by TG, with an AUROC of 0.723 (95%CI: 0.701-0.744) in model 2 (Table 3). The cutoff point, sensitivity, and specificity for each index in predicting incident MetS in the entire cohort, the male subcohort, and the female subcohort are shown in Supplementary Tables 1-3. In the sex-specific analyses, the capacities of the six NI-IR indices for predicting incident MetS varied by sex (all P values between men and women were < 0.05), and the results are presented in Table 4. The findings in men were consistent with those in the entire cohort, whereas in women, the greatest AUROC was found for the TC/HDL-C ratio (0.793; 95%CI: 0.684-0.901), followed by the METS-IR (0.764; 95%CI: 0.654-0.874), ZJU index (0.751; 95%CI: 0.658-0.844), TG/HDL-C ratio (0.723; 95%CI: 0.598-0.848), ALT/AST ratio (0.718; 95%CI: 0.614-0.822), and TyG index (0.693; 95%CI: 0.575-0.812), although all the intergroup P values were > 0.05.
| Model 1 | Model 2 | |||||
| AUC | 95%CI | P value | AUC | 95%CI | P value | |
| METS-IR index | 0.781 | 0.761-0.800 | Reference | 0.782 | 0.762-0.801 | Reference |
| TyG index | 0.734 | 0.713-0.755 | 0.001 | 0.734 | 0.713-0.756 | 0.001 |
| ALT/AST | 0.677 | 0.654-0.699 | < 0.001 | 0.674 | 0.652-0.697 | < 0.001 |
| TC/HDL-C | 0.732 | 0.711-0.753 | 0.001 | 0.731 | 0.709-0.752 | < 0.001 |
| TG/HDL-C | 0.751 | 0.731-0.771 | 0.03 | 0.752 | 0.731-0.772 | 0.04 |
| ZJU index | 0.744 | 0.723-0.765 | 0.01 | 0.743 | 0.722-0.764 | 0.008 |
| FPG | 0.608 | 0.584-0.632 | < 0.001 | 0.605 | 0.581-0.630 | < 0.001 |
| TG | 0.721 | 0.700-0.743 | < 0.001 | 0.723 | 0.701-0.744 | < 0.001 |
| BMI | 0.771 | 0.752-0.790 | 0.47 | 0.771 | 0.752-0.790 | 0.43 |
| TC | 0.613 | 0.588-0.638 | < 0.001 | 0.613 | 0.588-0.638 | < 0.001 |
| HDL-C | 0.689 | 0.667-0.712 | < 0.001 | 0.690 | 0.667-0.712 | < 0.001 |
| AST | 0.632 | 0.607-0.656 | < 0.001 | 0.632 | 0.608-0.656 | < 0.001 |
| ALT | 0.693 | 0.671-0.715 | < 0.001 | 0.692 | 0.670-0.714 | < 0.001 |
| Men | Women | P value between men and women | |||||
| AUC | 95%CI | P value | AUC | 95%CI | P value | ||
| METS-IR index | 0.771 | 0.751-0.792 | Reference | 0.764 | 0.654-0.874 | Reference | 0.001 |
| TyG index | 0.726 | 0.704-0.748 | 0.002 | 0.693 | 0.575-0.812 | 0.39 | 0.001 |
| ALT/AST | 0.657 | 0.633-0.681 | < 0.001 | 0.718 | 0.614-0.822 | 0.55 | 0.001 |
| TC/HDL-C | 0.715 | 0.692-0.738 | < 0.001 | 0.793 | 0.684-0.901 | 0.71 | 0.001 |
| TG/HDL-C | 0.741 | 0.719-0.762 | 0.04 | 0.723 | 0.598-0.848 | 0.62 | 0.001 |
| ZJU index | 0.734 | 0.712-0.756 | 0.01 | 0.751 | 0.658-0.844 | 0.85 | 0.001 |
This cohort study investigated the associations of six NI-IR indices (the METS-IR, TG/HDL-C ratio, TyG index, ZJU index, TC/HDL-C ratio, and ALT/AST ratio) with the development of new-onset MetS among young Taiwanese adults. Our findings demonstrated that all NI-IR indices were significantly associated with an increased risk of incident MetS, with adjustments for potential confounders. Additionally, the METS-IR was observed to have the strongest capacity for predicting new-onset MetS among the studied NI-IR indices, particularly in men, whereas the TC/HDL-C ratio was found to have the strongest capacity for predicting new-onset MetS in women.
The METS-IR, which is a composite measure that incorporates BMI, TG, HDL-C, FPG, and SBP, exhibited the greatest capacity for predicting incident MetS in the present study. This finding suggests that the METS-IR may be a more comprehensive marker of IR and MetS risk than other NI-IR indices that include fewer metabolic parameters. The superiority of the METS-IR in predicting incident new-onset MetS, as observed in our study, is further supported by recent findings from a large-scale, cross-sectional study conducted by Cheng et al[26]. The ability of the METS-IR to predict both prediabetes and type 2 diabetes in elderly Chinese individuals highlights its potential for use as a valuable tool for the early identification of those at risk for metabolic disorders across diverse populations. Bello-Chavolla et al[18] also reported that METS-IR outperformed other NI-IR indices in predicting incident hypertension and arterial stiffness. Additionally, Lee et al[16] revealed its utility in predicting advanced liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). The consistency of these findings, along with our results, strengthens the evidence for the superiority of the METS-IR in assessing IR and predicting metabolic risk compared to other NI-IR indices. The physiological basis for the effectiveness of the METS-IR lies in its ability to reflect key pathophysiological mechanisms associated with MetS. Compared with other NI-IR indices, which focus on specific factors, such as lipid metabolism (TG/HDL-C ratio) and glucose homeostasis (TyG index), the METS-IR simultaneously evaluates adiposity (through BMI), lipid metabolism (through TG and HDL-C), and glucose regulation (through FPG). This comprehensive evaluation aligns well with the current understanding of MetS as a multisystem disorder that involves complex interactions between adipose tissue dysfunction, IR, and inflammation.
The TG/HDL-C ratio and the TyG index, which are simple measures based on routine lipid and glucose parameters, demonstrated good capacities for predicting incident MetS in our study. These findings are consistent with those of prior studies that highlighted the potential utility of these indices as surrogate markers of IR and MetS risk. A large-scale cross-sectional study conducted by Liu et al[13] examined the associations between hyperuricemia and three NI-IR indices, i.e., the TG/HDL-C ratio, TyG index, and the METS-IR. The AUROCs of the TG/HDL-C ratio and the TyG index for de
Similarly, a cohort study conducted by Wen et al[14] compared the value of the TyG index with those of other common risk factors in predicting incident prediabetes in Chinese individuals. The predictive capacity of the TyG index (AUROC = 0.60) was superior to that of indices of obesity, lipid profiles, and other NI-IR indices. Although the overall predictive capacity of the TyG index was similar to that of FPG, it tended to be greater in females and obese individuals. Fur
The ZJU index, TC/HDL-C ratio, and ALT/AST ratio were also positively associated with incident MetS. While these indices may not capture the full spectrum of metabolic abnormalities associated with IR and MetS, they focus on specific aspects, e.g., atherogenic lipid metabolism or liver function, which are crucial components of metabolic health. Notably, the ZJU index, which incorporates BMI, FPG, and TG, and the ALT/AST ratio have been shown to be helpful for de
The predictive capacity, sensitivity, and specificity of the NI-IR indices in this study were generally greater than those reported in some prior investigations, which may be due to the younger age and relatively homogeneous nature of this study population as well as the extended follow-up period. Our study suggests that all the NI-IR indices were associated with incident MetS in young adults, and the METS-IR was the strongest predictor of new-onset MetS before midlife. The high predictive capacities along with the suboptimal sensitivity and specificity of these six NI-IR indices highlight their potential utility as screening tools for identifying young individuals who are at high risk for MetS. Early identification of these at-risk individuals is crucial, as it enables timely implementation of lifestyle modifications and preventive measures, which can effectively reduce the risk of developing MetS and related complications later in midlife. The use of NI-IR indices as screening tools in a young population could help streamline the identification process and allocate resources more efficiently, ultimately leading to improved health outcomes and reduced health care costs associated with MetS and related disorders.
Our study has several strengths, including its prospective cohort design, standardized data collection, and comprehensive assessment of multiple NI-IR indices. However, some limitations should be acknowledged. First, since our study focused on young military personnel, who are predominantly male and maintain specific PA requirements, we ac
Our study demonstrates that all the NI-IR indices were significantly associated with new-onset MetS in young adults. Among the NI-IR indices, the METS-IR was observed to have the greatest capacity to predict the development of MetS before midlife, whereas a sex difference was observed, with the TC/HDL-C ratio showing the greatest predictive capacity for MetS among young women. In clinical practice, while the calculation of the METS-IR is more complex than the calculation of simpler indices, such as the TG/HDL-C ratio, all of the components of the METS-IR are usually available in standard health examinations, making the METS-IR a practical and cost-effective screening tool. Additionally, modern electronic health record systems can automate this calculation without adding any clinical burdens. These findings suggest that relevant NI-IR indices may be useful tools for the early identification of specific individuals who are at high risk of new-onset MetS, enabling the implementation of targeted preventive strategies. Further studies are needed to validate these findings in diverse ethnic populations and to evaluate the cost-effectiveness of the use of various NI-IR indices for MetS risk stratification in clinical practice.
| 1. | Ding C, Yang Z, Wang S, Sun F, Zhan S. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: a cohort study. Endocrine. 2018;60:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C; RIVANA Study Investigators. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Hayden MR. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 4. | Demir M, Lang S, Steffen HM. Nonalcoholic fatty liver disease - current status and future directions. J Dig Dis. 2015;16:541-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, Isenovic ER. Link between Metabolic Syndrome and Insulin Resistance. Curr Vasc Pharmacol. 2017;15:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Kumari R, Kumar S, Kant R. An update on metabolic syndrome: Metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab Syndr. 2019;13:2409-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. 2017;17:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 8. | Lin YK, Lin YP, Lee JT, Lin CS, Wu TJ, Tsai KZ, Su FY, Kwon Y, Hoshide S, Lin GM. Sex-specific association of hyperuricemia with cardiometabolic abnormalities in a military cohort: The CHIEF study. Medicine (Baltimore). 2020;99:e19535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int J Sports Med. 2021;42:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 10. | Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, Álvarez-Villalobos NA, González-González JG. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int J Endocrinol. 2020;2020:4678526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 11. | Horáková D, Štěpánek L, Janout V, Janoutová J, Pastucha D, Kollárová H, Petráková A, Štěpánek L, Husár R, Martiník K. Optimal Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Cut-Offs: A Cross-Sectional Study in the Czech Population. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, Sánchez-Lázaro D, Meza-Oviedo D, Vargas-Vázquez A, Campos OA, Sevilla-González MDR, Martagón AJ, Hernández LM, Mehta R, Caballeros-Barragán CR, Aguilar-Salinas CA. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 13. | Liu XZ, Xu X, Zhu JQ, Zhao DB. Association between three non-insulin-based indexes of insulin resistance and hyperuricemia. Clin Rheumatol. 2019;38:3227-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Wen J, Wang A, Liu G, Wang M, Zuo Y, Li W, Zhai Q, Mu Y, Gaisano HY, He Y, Dou J. Elevated triglyceride-glucose (TyG) index predicts incidence of Prediabetes: a prospective cohort study in China. Lipids Health Dis. 2020;19:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, Li Y. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep. 2015;5:16494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Lee JH, Kwon YJ, Park K, Lee HS, Park HK, Han JH, Ahn SB. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Han KY, Gu J, Wang Z, Liu J, Zou S, Yang CX, Liu D, Xu Y. Association Between METS-IR and Prehypertension or Hypertension Among Normoglycemia Subjects in Japan: A Retrospective Study. Front Endocrinol (Lausanne). 2022;13:851338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Martagón AJ, Mehta R, Arellano-Campos O, Gómez-Velasco DV, Almeda-Valdés P, Cruz-Bautista I, Melgarejo-Hernandez MA, Muñoz-Hernandez L, Guillén LE, Garduño-García JJ, Alvirde U, Ono-Yoshikawa Y, Choza-Romero R, Sauque-Reyna L, Garay-Sevilla ME, Malacara-Hernandez JM, Tusié-Luna MT, Gutierrez-Robledo LM, Gómez-Pérez FJ, Rojas R, Aguilar-Salinas CA. Prediction of incident hypertension and arterial stiffness using the non-insulin-based metabolic score for insulin resistance (METS-IR) index. J Clin Hypertens (Greenwich). 2019;21:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 20. | Gasevic D, Frohlich J, Mancini GJ, Lear SA. Clinical usefulness of lipid ratios to identify men and women with metabolic syndrome: a cross-sectional study. Lipids Health Dis. 2014;13:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Huang WC, Tsai KZ, Yang KT, Chen HH, Kwon Y, Lin GM. A comparison of various insulin resistance indices and the possibility of hypertension in military adults: CHIEF study. Diabetol Metab Syndr. 2024;16:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 22. | Tsai KZ, Liu PY, Lin YP, Chu CC, Huang WC, Sui X, Lavie CJ, Lin GM. Do the American guideline-based leisure time physical activity levels for civilians benefit the mental health of military personnel? Front Psychiatry. 2023;14:1255516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Lin GM, Li YH, Lee CJ, Shiang JC, Lin KH, Chen KW, Chen YJ, Wu CF, Lin BS, Yu YS, Lin F, Su FY, Wang CH. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World J Cardiol. 2016;8:464-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Lin YP, Tsai KZ, Chang CY, Su FY, Han CL, Lin GM. Tobacco Smoking and Association between Betel Nut Chewing and Metabolic Abnormalities Among Military Males: The CHIEF Study. Endocr Metab Immune Disord Drug Targets. 2021;21:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5096] [Cited by in RCA: 5068] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 26. | Cheng H, Yu X, Li YT, Jia Z, Wang JJ, Xie YJ, Hernandez J, Wang HHX, Wu HF. Association between METS-IR and Prediabetes or Type 2 Diabetes Mellitus among Elderly Subjects in China: A Large-Scale Population-Based Study. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Fernández-Alonso AM, Chedraui P, Pérez-López FR. Nonalcoholic fatty liver disease risk in polycystic ovary syndrome patients. Gynecol Endocrinol. 2024;40:2359031. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |