Published online Apr 15, 2025. doi: 10.4239/wjd.v16.i4.93630
Revised: September 16, 2024
Accepted: January 20, 2025
Published online: April 15, 2025
Processing time: 362 Days and 22.2 Hours
At present, the incidence of diabetic nephropathy is increasing year by year, and there are many studies on the pathogenesis of diabetic nephropathy, but it is still not completely clear. The final pathological result of diabetic nephropathy is mainly glomerular cell fibrosis, and the roles of micro-RNA (miRNA)-29 and DNA methyl transferase (DNMTs) in cell fibrosis have been confirmed in other studies, but there is a lack of relevant research in the kidney at present.
To study the potential involvement of miRNA-29a-3p in fibrosis related to dia
The expression of miR-29a-3p, DNMT3A/3B, fibrosis-related molecules, Wnt3a, β-catenin, Janus kinase 2, and signal transducer and activator of transcription 3 was assessed in SV40MES13 cells and diabetic mice using quantitative real-time PCR and western blotting. Furthermore, the expression changes of fibrosis-related molecules were further analyzed using immunofluorescence and immunohistochemical blotting. The renal pathological changes of DKD in each group were also studied using hematoxylin-eosin and periodate-Schiff reaction staining.
In both the in vivo and in vitro experiments, it was observed that high glucose induction significantly decreased miR-29a-3p expression. As a result of this downregulation, DKD-related fibrosis was found to be promoted, as confirmed by elevated expression levels of α-smooth muscle actin, collagen type I, and fibronectin. MiR-29a-3p targets the 3’ non-coding regions of DNMT3A and DNMT3B and inhibits their expression. Inhibition of DNMT3A and DNMT3B can reverse the effect of miR-29a-3p downregulation on DKD-related fibrosis.
MiR-29a-3p can regulate Wnt/β-catenin and Janus kinase/signal transducer and activator of transcription signal pathways by regulating and inhibiting the expression of DNMT3A/3B and thus participate in the inhibition of DKD-related fibrosis.
Core Tip: This study was the first to verify that MiR-29a-3p can regulate Wnt/β-catenin and Janus kinase/signal transducer and activator of transcription signal pathways by regulating and inhibiting the expression of DNMT3A/3B and thus participate in the inhibition of diabetic kidney disease-related fibrosis by in vitro and in vivo experiments. These findings indicated that targeting miR-29a-3p and DNMT3A/3B may hold promise for diabetic kidney disease prevention and treatment.
- Citation: Yang Y, Chen Y, Tang JY, Chen J, Li GQ, Feng B, Mu J. MiR-29a-3p inhibits fibrosis of diabetic kidney disease in diabetic mice via downregulation of DNA methyl transferase 3A and 3B. World J Diabetes 2025; 16(4): 93630
- URL: https://www.wjgnet.com/1948-9358/full/v16/i4/93630.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i4.93630
Diabetic kidney disease (DKD), being one of the severe microvascular complications of diabetes, poses a significant peril to human health, and it is accountable for causing end-stage renal disease[1]. Research evidence indicates that epigenetic modifications, including DNA promoter region methylation, non-coding RNA, and histone regulation, affect factors related to the pathogenesis of DKD. These modifications play a critical role in DKD pathogenesis and ultimately result in vascular complications by regulating downstream target genes and activating various gene signal transduction pathways[2-4]. In various epigenetic modifications, abnormal DNA methylation is considered to be the main regulator affecting the transcriptional activity of DKD-related target genes and presents a metabolic memory mechanism[5,6].
The DNA methyl transferase (DNMT) family catalyzes this process. There are three types of DNMTs present in mammals, including DNMT1, DNMT3A, and DNMT3B. Previous research suggests that DNMT1 aids in preserving DNA methylation patterns, whereas DNMT3A and DNMT3B facilitate methylation at previously unmethylated CpG sites[7]. Elevated levels of DNMT3A/3B have been identified in patients with DKD and appear to be associated with renal fibrosis[8-10]. However, the mechanism of the rise of DNMT3A/3B under high glucose (HG) remains unclear.
MicroRNA (miRNA), a small piece of RNA produced during the synthesis of endogenous nucleotides, has the po
According to previous studies, miR-29a is associated with DNMT3A/3B, so we predict that miR-29a may be involved in the etiology of DKD. In this study, we utilized SV40MES13 cells and diabetic (db/db) mice as in vitro and in vivo models, respectively. We aimed to investigate the regulatory effect of miR-29a on DNMT3A/3B and renal fibrosis as well as its potential pathways by monitoring the expression of DNMT3A/3B and fibrosis-related indexes during the dynamic changes of miR-29a. This study was the first to explore the role of miR-29a-3p in the occurrence and development of DKD. The findings may offer insight for further investigation and examination of clinical approaches for swift in
Mouse glomerular mesangial cells (SV40MES13) were purchased from Wuhan Procell Life Science and Technology Co., Ltd, while RNAiso Plus and reverse transcription reagents were obtained from Chengdu Weike Biotechnology Co., Ltd. Fluorescence quantitative PCR reagents, DNA extraction kits, fast bisulfite conversion kits, and methylation analysis kits were purchased from QIAGEN Taiwan Co., Ltd. MiR-29a-3p mimics, mimics negative control (NC), inhibitor-miR-29a-3p, inhibitor NC, agomiR-29a-3p, and agomir NC were synthesized by Sangon Biotech (Shanghai) Co., Ltd. Refer to Table 1 for detailed information on the antibodies used in the study.
| Molecular | Molecular weight | Source | Reactive species | Company |
| DNMT3A (ab188470) | 102 kDa | Rabbit | Human/mouse/rat | Abcam |
| DNMT3B (L2011188) | 28 kDa | Mouse | Mouse | US Biological |
| α-SMA (ab205719) | 42 kDa | Mouse | Mouse/rat/human/African green monkey | Abcam |
| Collagen I (ab270993) | 139 Da | Rabbit | Mouse/rat | Abcam |
| Fibronectin (ab268020) | 262 kDa | Rabbit | Human/mouse/rat | Abcam |
| Wnt3a (ab219412) | 39 kDa | Rabbit | Human/mouse/rat | Abcam |
| β-catenin (D10A8) | 92 kDa | Rabbit | Human/mouse/rat/monkey | Cell signaling technology |
| JAK2 (D2E12) | 125 kDa | Rabbit | Human/mouse/rat/monkey | Cell signaling technology |
| STAT3 (124H6) | 79 kDa/86 kDa | Mouse | Human/mouse/rat/monkey | Cell signaling technology |
| Tubulin (ab7291) | 55 kDa | Mouse | Mouse/rat/human | Abcam |
| GAPDH (ab8245S) | 37 kDa | Mouse | Mouse/rat/human | Abcam |
Cell culture and transfection: SV40MES13 cells were cultured in HyClone medium containing 5% fetal bovine serum, 1% penicillin-streptomycin, and 1% glutamine. The cells were seeded in culture plates and incubated in either 5.5 mmol/L (normal glucose group) or 30 mmol/L (HG group) glucose medium. In the normal glucose group, the cells were trans
Experimental animals and specific grouping: Twenty 6-week-old male mice were purchased from SPF Biotechnology Co., Ltd, Beijing. The animals were housed under SPF conditions with free access to food and water. The experimental groups included the db/m group, db/db (CON) group, db/db (antagomir NC) group, and db/db (antagomiR-29a-3p) (CON, antagomir NC, and antagomiR-29a-3p) group. These mice received a dosage of 80 mg/kg and one injection per week for three consecutive weeks. The mice were weighed weekly. After 4 weeks, the mice were euthanized by cervical dislocation, and blood was collected by removing the eyeballs. Kidney tissues were then isolated and divided into two halves, with one half stored in liquid nitrogen in a freezing tube. Animal experiments were conducted in accordance with the eighth edition of the “Guidelines for the Care and Use of Experimental Animals” (2011). All procedures were carried out in accordance with the relevant laws and institutional guidelines and have been approved by the appropriate institutional committee, No. LL-202125.
Real-time quantitative PCR: Total RNA was extracted from the transfected cells 24 h post-transfection, using the stem-loop method for reverse transcription. U6 was used as an internal reference, with real-time quantitative PCR (qRT-PCR) performed using a fluorescent quantitative PCR kit. Refer to Table 2 for the primer sequences used in the study.
| Primer | Sequence (5’ to 3’) |
| MiR-29a-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACCG |
| MiR-29a-3p-F | CGCGTAGCACCATCTGAAAT |
| MiR-29a-3p-R | AGTGCAGGGTCCGAGGTATT |
| U6-F | AGAGAAGATTAGCATGGCCCCTG |
| U6-R | AGTGCAGGGTCCGAGGTATT |
Western blotting: Total protein was extracted from mouse cells and kidney tissues using the tissue protein extraction reagent method. Protein concentration was measured using the NanoDrop method. The proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane by electro transfer. After blocking with 5% bovine albumin, the membrane was incubated overnight at 4 °C with primary antibodies (dilution ratio of 1:1000). After washing with tris buffered saline with tween-20, the corresponding horseradish peroxidase-labeled secondary antibodies were added and incubated for 30 min, followed by exposure, development, and fixation. The grayscale values of the bands were analyzed using ImageJ-win64 software.
Serum biochemistry analysis: Mouse blood was placed in an evacuated heparinized tube on ice for 30 min. The serum in the supernatant was collected through centrifugation at 4000 r/min for 10 min and stored in a refrigerator at -80 °C for further detection. The final samples were sent to Wuhan Service Biotech Co., Ltd. and the corresponding operations were carried out in strict accordance with the operating rules. The serum indexes creatinine and blood urea nitrogen were detected using the detection data of an automatic biochemical analyzer (Johnson VITROS5600).
Immunofluorescence analysis: The cells were treated with 100 μL of permeabilization buffer, incubated for 20 min, followed by the addition of 5% bovine serum albumin and further incubation for 60 min. The cells were then incubated overnight with primary antibody (dilution 1:200) in a humidification chamber at 4 °C. After washing with PBS, the appropriate fluorescent secondary antibody was added and incubated for 60 min. The nuclei were stained with Hoechst 33258 dye. After washing with PBS, the liquid was aspirated and the samples mounted using an anti-fading mounting medium. The samples were then examined under a microscope, and the images captured using a fluorescence mi
Hematoxylin-eosin staining: The fixed kidney tissue was embedded in paraffin and sliced using a microtome. The sections were then deparaffinized and rehydrated, followed by staining with hematoxylin for 3-5 min and eosin for 5 min. The samples were dehydrated and mounted and then observed and imaged under a microscope.
Periodic acid-Schiff staining: The tissue sections were deparaffinized and rehydrated, then stained with periodic acid-Schiff (PAS) staining solution B for 10-15 min, followed by staining with PAS staining solution A for 25-30 min, and then with PAS staining solution C for 30 s. After bluing with ammonia water, the samples were dehydrated, mounted, and then observed and imaged under a microscope.
Immunohistochemistry: After deparaffinization and rehydration, the tissue sections were subjected to antigen retrieval by placing them in citrate buffer (potential of hydrogen: 6.0) and heating in a microwave oven (medium heat for 8 min until boiling, then turned off for 8 min, followed by 7 min at low-medium heat, and naturally cooled to room tem
Data analysis was performed using GraphPad Prism8 software. All data were expressed as mean ± SD. Intergroup comparisons were performed using the unpaired t-test, and correlation analysis was performed using the χ2 test. Statistical significance was indicated as P < 0.05, P < 0.01, and P < 0.001.
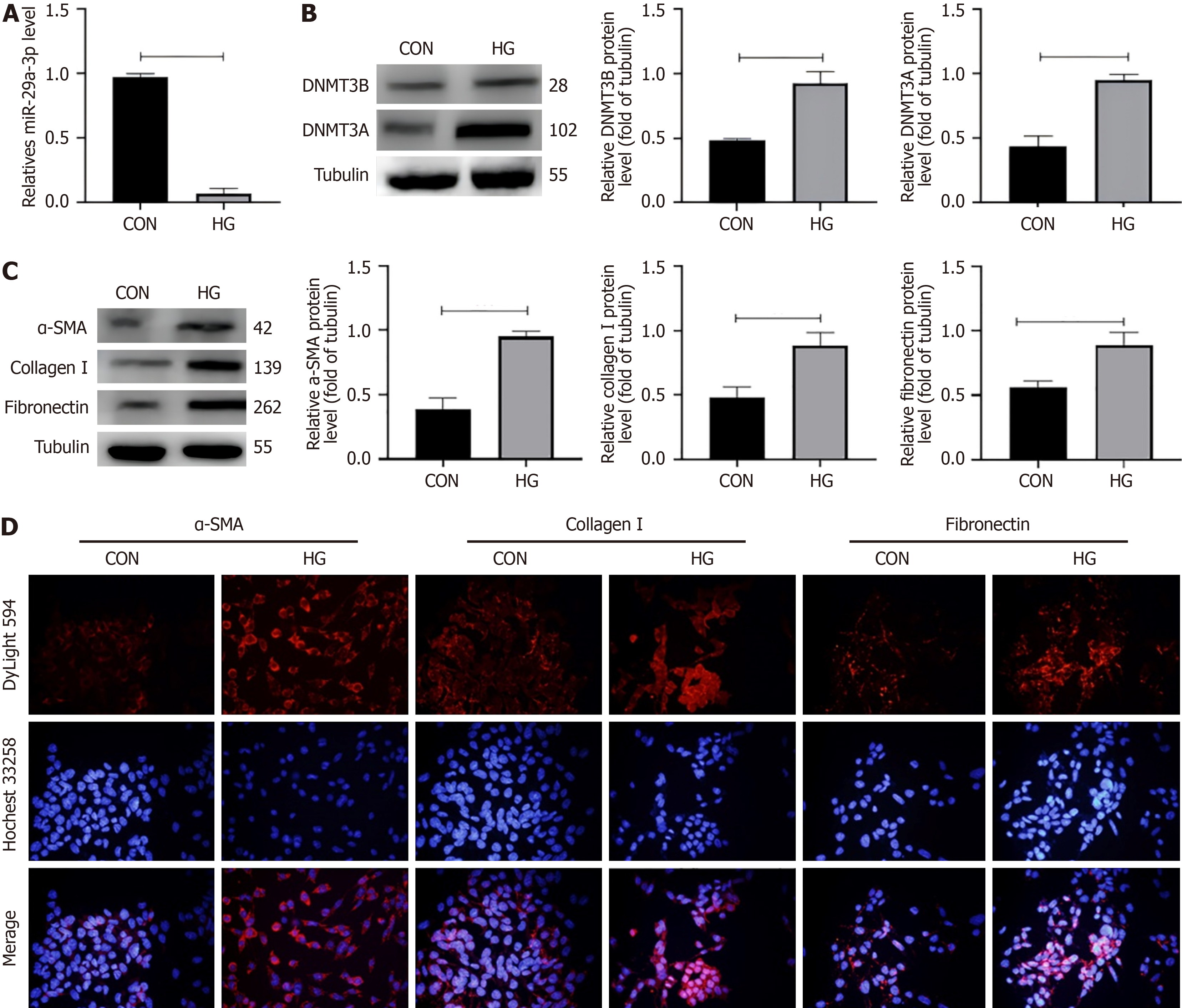
The expression level of miRNA has recently become a potential biomarker of various pathological conditions, while DNMT3A/3B and fibrosis-related molecules have become the key molecules in the occurrence and development of DKD fibrosis. In order to evaluate the role of miR-29a-3p in the pathogenesis of DKD, SV40MES13 cells were cultured with HG to establish an in vitro DKD cell model. qRT-PCR analysis was used to detect the expression of miR-29a-3p, DNMT3A/3B and fibrosis-related molecules (Figure 1).
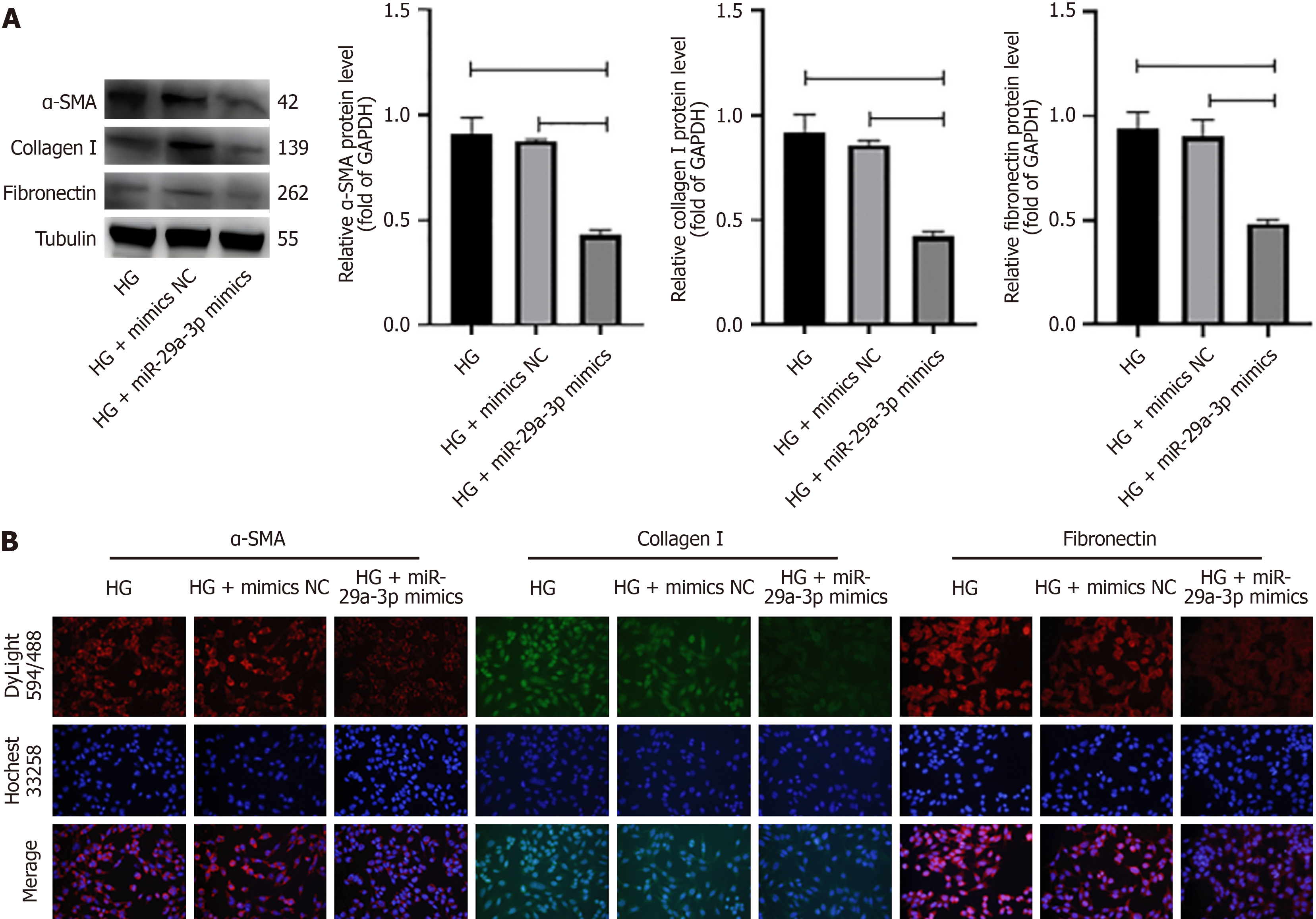
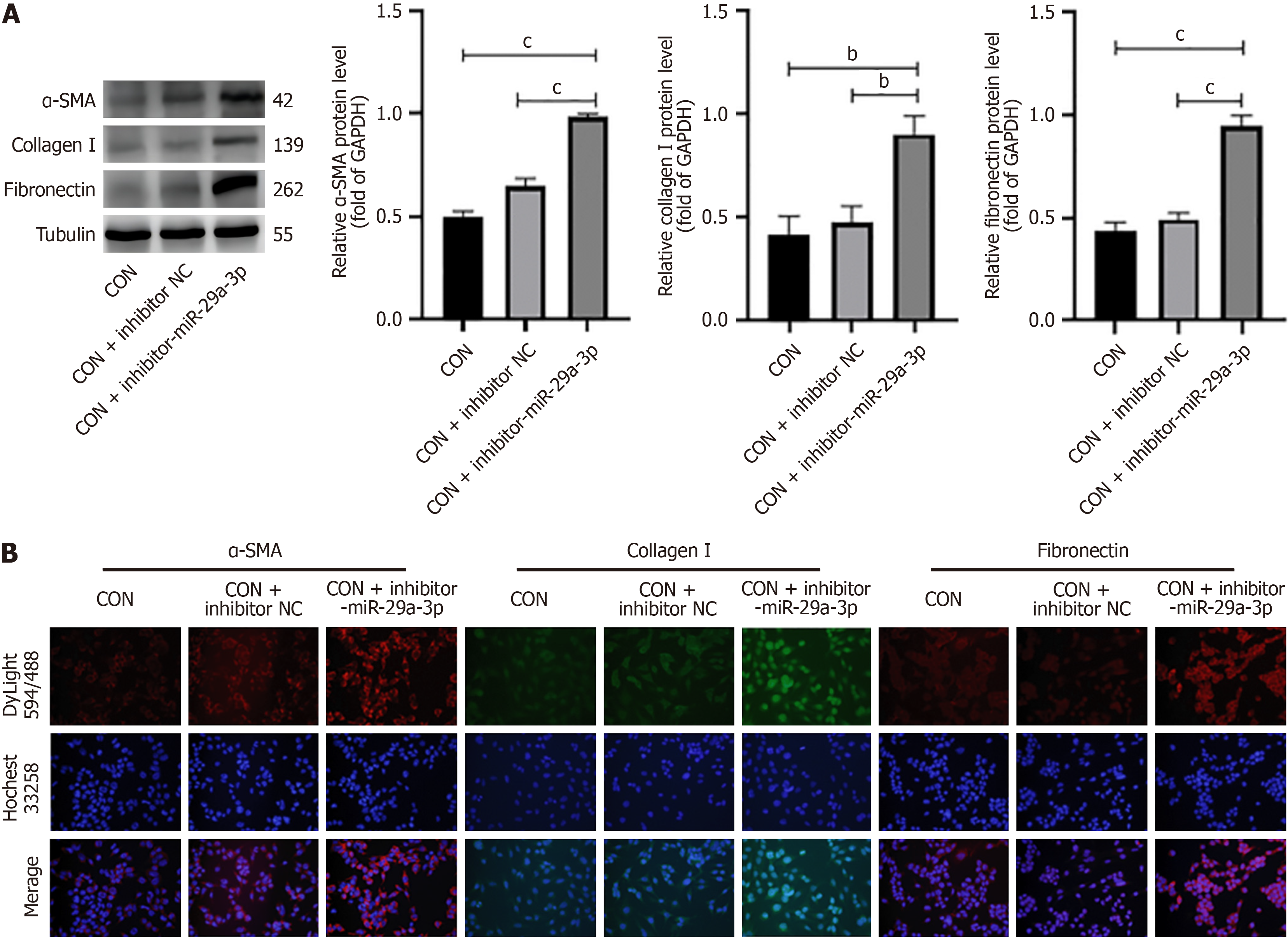
DKD is characterized by glomerulosclerosis or fibrous changes. It is known that miRNA is involved in the process of fibrosis. In order to study the function of miR-29a-3p in DKD, miR-29a-3p inhibitors and miR-29a-3p mimics were added to SV40MES13 cells cultured under different glucose conditions, and the expression of fibrosis molecules was measured by western blotting and immunofluorescence. First of all, we determined the relationship between miR-29a-3p and fibrosis-related molecules and determined that miR-29a was involved in the fibrosis process. In the normal glucose group, the expression levels of alpha smooth muscle actin (α-SMA), collagen I, and fibronectin (cP < 0.001) were significantly increased after transfection with inhibitor-miR-29a-3p (Figure 2). The results showed that the degree of cell fibrosis was enhanced after inhibition of miR-29a-3p. In the HG group, the expression of α-SMA, type I collagen, and fibronectin decreased after adding miR-29a-3p mimic (cP < 0.001, Figure 3). MiR-29a-3p mimics improved the progression of fibrosis. The overexpression of miR-29a in the HG group can improve the expression of fibrosis-related markers.
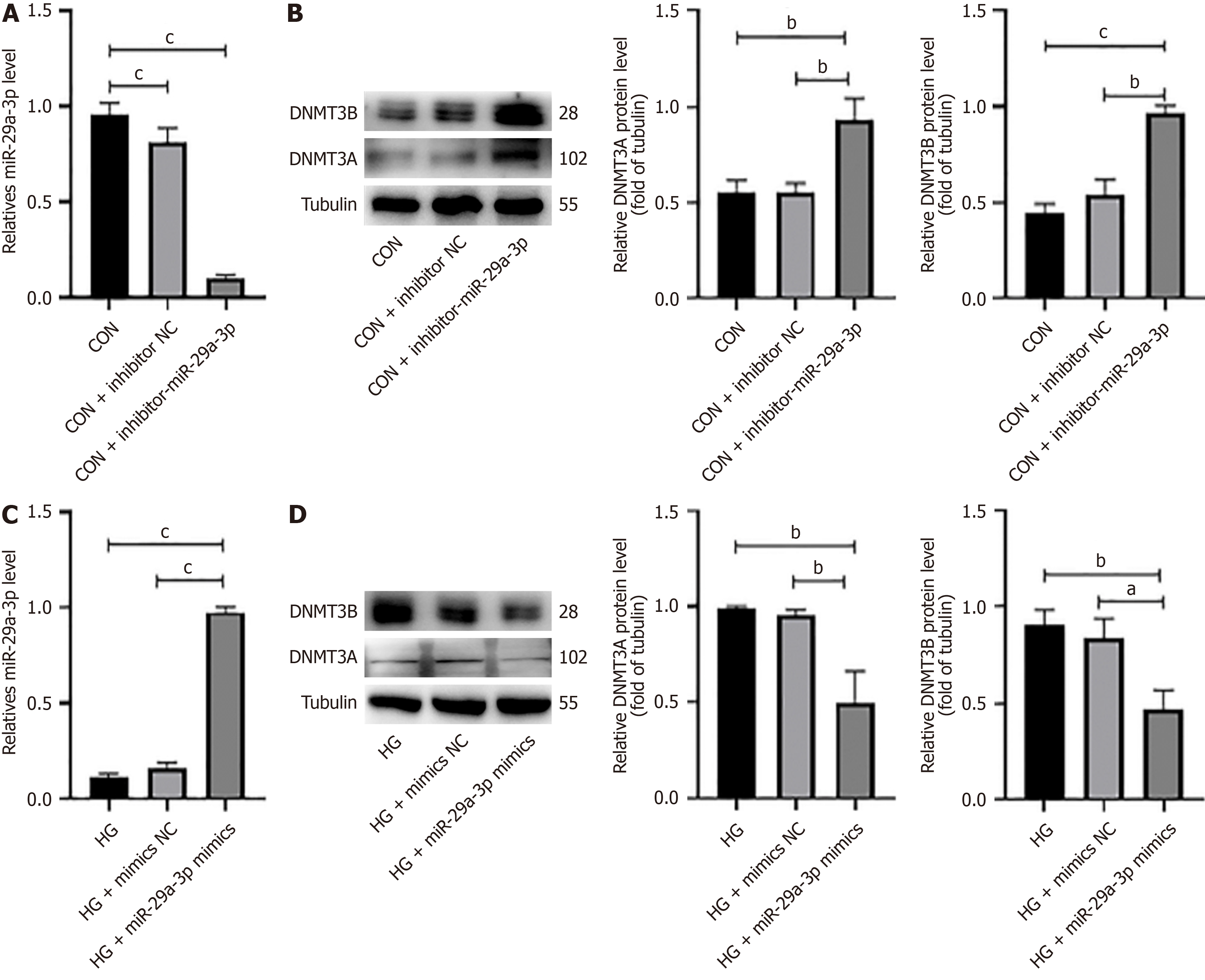
After transfection with inhibitor-miR-29a-3p, qRT-PCR was utilized to measure the expression of several factors. The expression of miR-29a-3p in the normal glucose group was significantly decreased (cP < 0.001), while the expression of DNMT3A and DNMT3B (cP < 0.001) was significantly upregulated (Figure 4A and B). Similarly, we found that after the HG group was transfected with miR-29a-3p mimics, the expression of miR-29a-3p was significantly increased (cP < 0.001) (Figure 4C), and the expression of DNMT3A/3B was significantly downregulated (bP < 0.01) (Figure 4D). It suggested that miR-29a-3p may negatively regulate the expression of DNMT3A/3B protein in SV40MES13 cells.
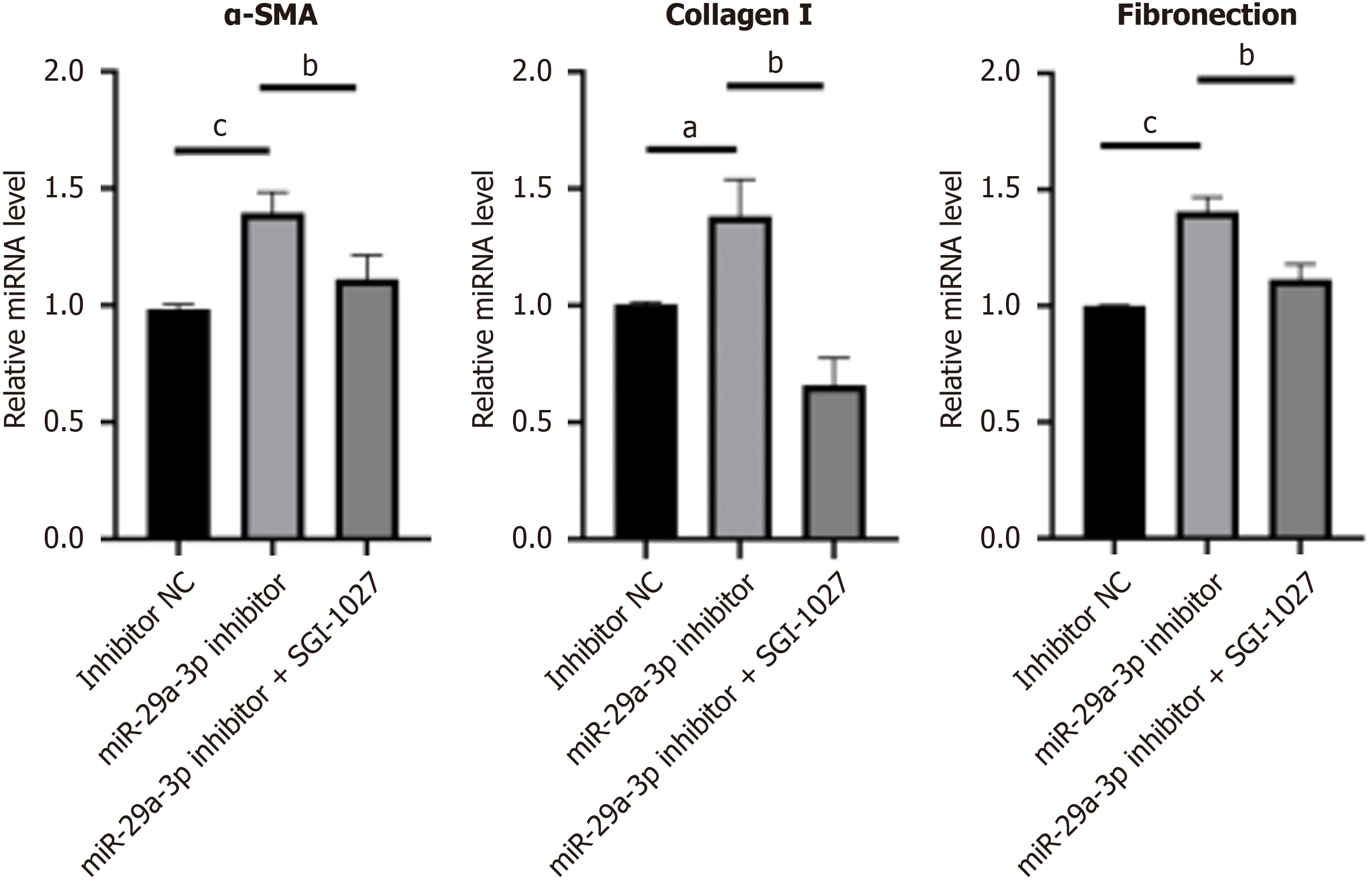
In order to further study whether miR-29a-3p regulates DKD by targeting DNMT3A and DNMT3B, SV40MES13 cells were treated with SGI-1027 after transfection with miR-29a-3p inhibitor to inhibit the expression of DNMT3A and DNMT3B. The results of qRT-PCR (Figure 5) showed that SGI-1027 treatment could significantly reduce the promoting effect of miR-29a-3p inhibitors on the expression of fibrosis-related molecules and enhance the effect of miR-29a-3p inhibitors. These results further suggest that miR-29a-3p can directly downregulate DNMT3A and DNMT3B to inhibit DKD.
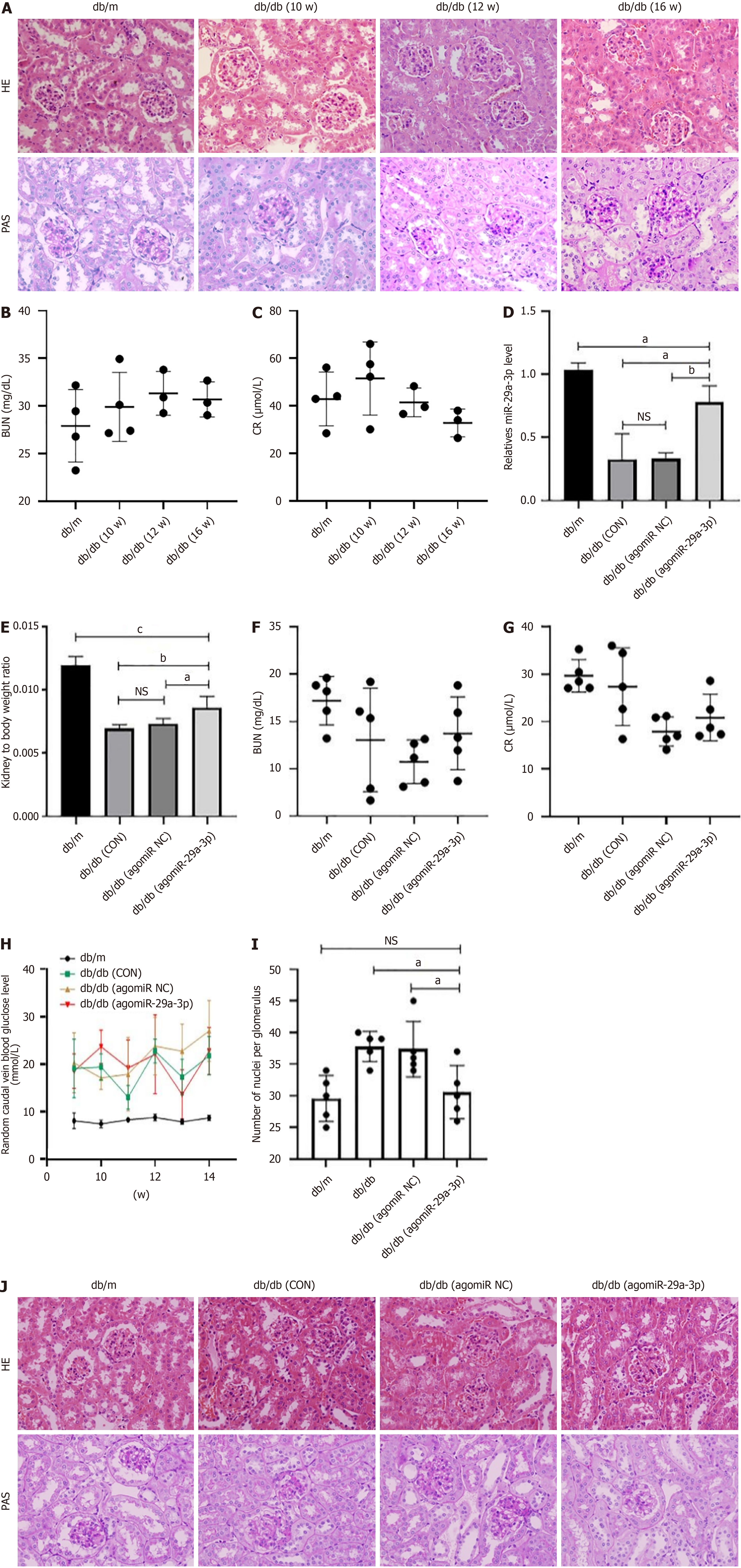
The above in vitro experimental results demonstrated that miR-29a-3p negatively regulated the expression of DNMT3A/3B in SV40MES13 cells, with high expression effectively alleviating cell fibrosis. To further elucidate the role of miR-29a-3p in animals, db/db mice were used as a model of DKD, with db/m mice as normal controls. At the 10th week, we found that the diabetic mice exhibited renal pathological changes such as glomerular hypertrophy, mesangial matrix proliferation, and mesangial area widening. With increasing age, these renal pathological changes worsened, with pathological changes such as glomerulosclerosis appearing by the 16th week (Figure 6A). We also measured serum creatinine (normal range: 10.81-34.74 mg/dL) and blood urea nitrogen (normal range: 10.91-85.09 μ/L), which were shown to be outside the normal range (Figure 6B and C). Therefore, we were able to determine that the db/db mice had already developed kidney disease at the 10th week and were in the early stage of DKD. These results provided a time basis for subsequent experiments.
In our previous study, we intervened in the 10th week by injecting agomiR-29a-3p into db/db mice. Four weeks later, we showed that the kidney index of the agomiR-29a-3p group mice increased significantly, indicating that kidney atrophy had reduced after treatment (Figure 6D). Serum biochemical tests showed that the levels of serum creatinine and blood urea nitrogen in all groups of the db/db mice were within the normal range, indicating that renal function in all groups of mice was in the compensatory period (Figure 6E and F). The blood glucose levels of db/m mice were normal, while db/db mice showed a significant increase, although the difference between the groups was not statistically significant (Figure 6G).
Hematoxylin and eosin staining and PAS staining showed that after treatment with agomiR-29a-3p, pathological changes such as glomerular hypertrophy and mesangial area widening in db/db mice had improved significantly, with no obvious glomerulosclerosis being observed. In contrast, the mice who had not received agomiR-29a-3p treatment developed glomerulosclerosis (Figure 6H-J). Taken together, these results indicate that overexpression of miR-29a-3p delays the kidney structural changes associated with DKD, although whether the signaling pathway was inactivated by DKD methyltransferase requires validation in future experiments.
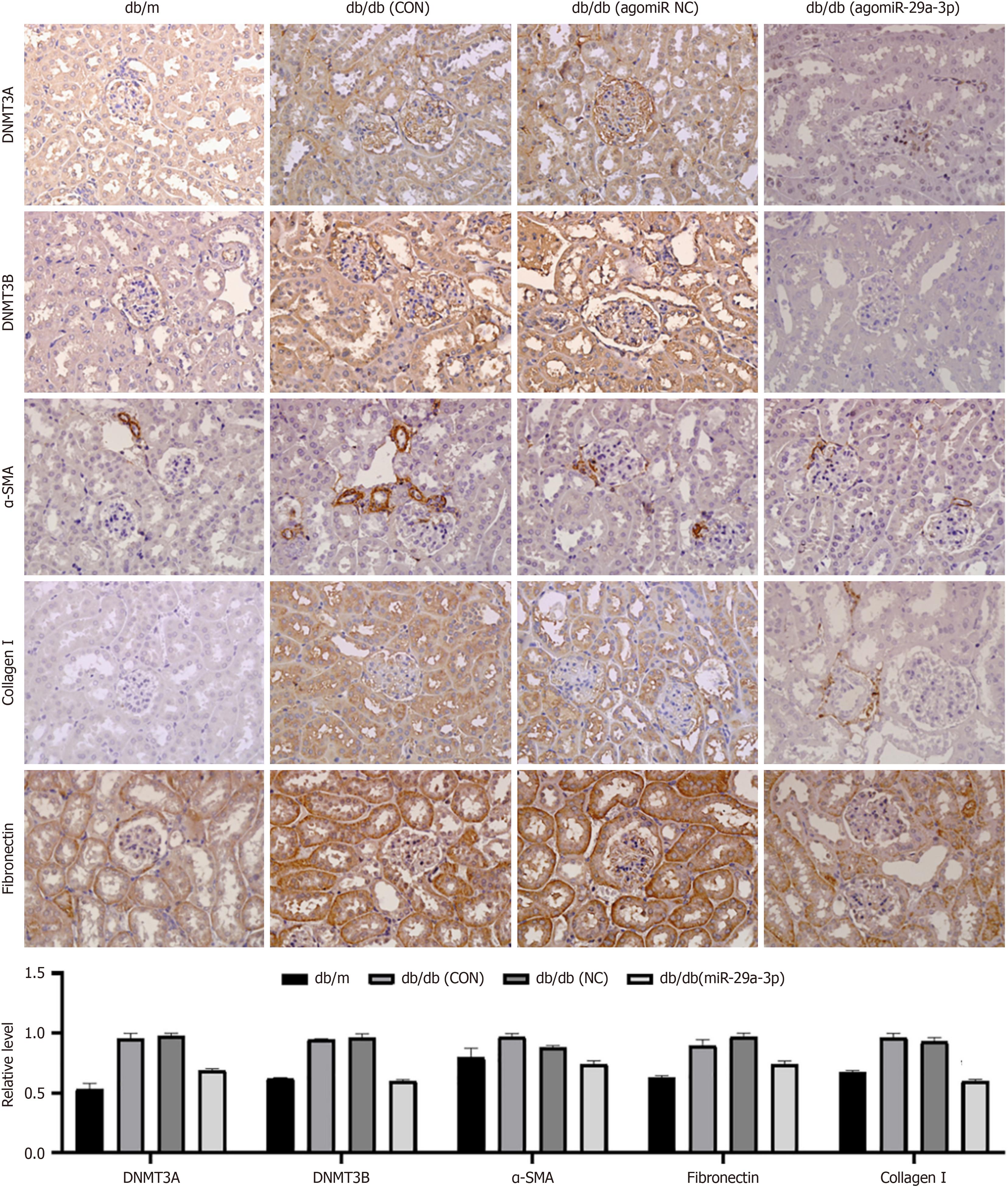
In the in vitro experiments, overexpression of miR-29a reduced the expression of DNMT3A/3B and fibrosis-related molecules. Therefore, we speculated whether this was also the case in an in vivo environment. We showed that treatment with agomiR-29a-3p significantly decreased the expression of DNMT3A/3B and fibrosis-related molecules (α-SMA, collagen type I, and fibronectin) (Figure 7). These results indicated that high expression of miR-29a-3p reduced the expression of renal DNA methyltransferases in DKD mice, delaying the progression of renal fibrosis. This finding was consistent with the results of the in vitro experiments.
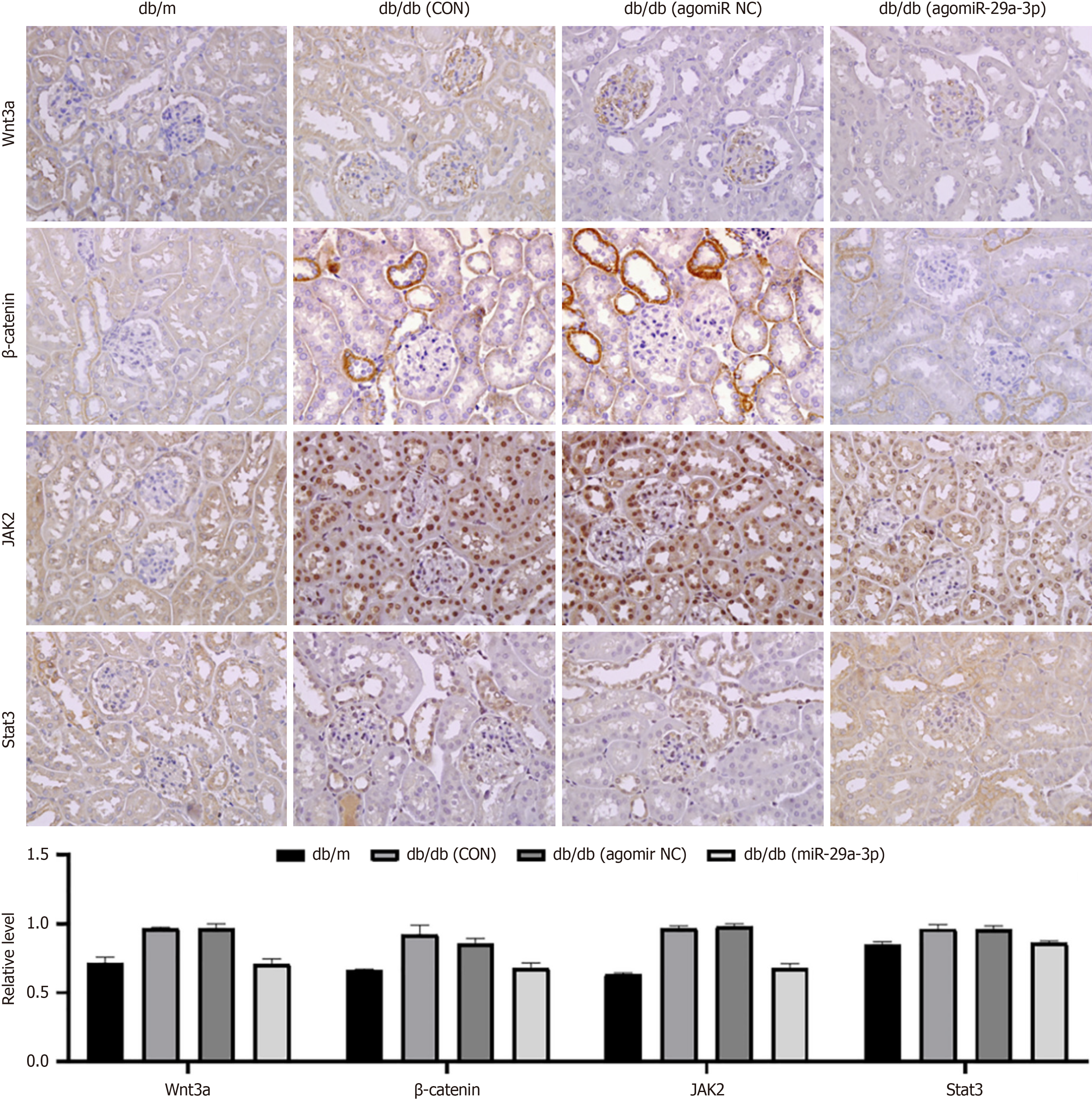
In order to determine potential signaling pathways that may be regulated, we examined the expression of Wnt3a, β-catenin, Janus kinase (JAK) 2, and signal transducer and activator of transcription (STAT) 3 in the Wnt/β-catenin and JAK/STAT signaling pathways. The results showed that the expression of these signaling molecules in db/db mice was significantly higher than that measured in the normal control group. After treatment with agomiR-29a-3p, their ex
MiRNA is considered to be the key regulatory layer of basic biological processes that regulate differentiation, proliferation, embryogenesis, development, and apoptosis by binding to the 3′ untranslated region (UTR) site of its target gene. Several miRNAs are reportedly involved in different tissue fibrosis processes. For instance, miR-21 can induce podocyte differentiation and mesangial cell activation, thereby enhancing renal fibrosis[16]. Meanwhile, miR-325-3p restricts excessive cell proliferation, inflammatory infiltration, and fibrosis in vivo, thus hindering the progression of DKD[17]. MiR-542-3p may have a role in renal fibrosis by inhibiting the expression of AGO1[18].
Additional recent evidence supports the regulatory role of the miR29 gene cluster in tissue fibrosis[9,19-22]. MiR-29a, a highly conserved member of the miR-29 family, is known for its anti-fibrosis properties and has been studied for its mechanism in myocardial, lung, liver, and skin fibrosis[23-26]. However, its role and mechanism in DKD remain unclear. Shi et al[27] observed a reduction in miR-29a-3p expression in early and late diabetic mouse models, whereas no alteration was observed in miR-29b or miR-29c levels. Additionally, Ebadi et al[28] discovered that captopril and spironolactone reduced transforming growth factor β and microalbuminuria levels while enhancing the downregulated expression of miR-29a-3p in db/db mice.
Similarly, we observed a significant decrease in the expression of miR-29a-3p in SV40MES13 cells following stimulation by HG and a significant increase in the expression of fibrosis factors (α-SMA, type I collagen, and fibronectin) (Figure 1). However, the addition of miR-29a-3p mimics resulted in a notable decrease in the expression of fibrosis factors compared with the CON group (Figure 2). To confirm that abnormal expression of fibrosis factors was not caused by pathways mediated by HG, we also conducted verification in the normal glucose group. After inhibiting miR-29a-3p expression, we observed synchronous negative changes in fibrosis factors (Figure 3). This demonstrated that miR-29a-3p is an effective anti-fibrosis molecule as it negatively regulates the expression of fibrosis-related factors, preventing the occurrence and development of fibrosis.
The present study provided additional support to the aforementioned perspective through in vivo experiments. Our findings revealed a notable decrease in the level of fibrosis factor expression within the renal tissue of db/db mice following antagomiR-29a-3p treatment compared with the CON group. DNA methylation is a well-researched epigenetic marker. Several pieces of evidence strongly suggest a close link between miRNA and DNA methylation, particularly in fibrotic diseases[29].
For instance, Fabbri et al[30] uncovered that the regulation of DNMT3A and DNMT3B by miR-29 mediated the reversal of abnormal methylation in lung cancer. The expression of DNMT3A and DNMT3B, two key ab initio DNMTs, is often upregulated in patients with idiopathic pulmonary fibrosis[30]. Wang et al[31] discovered that inhibiting miR-29c en
We conducted additional verification, finding that HG stimulation notably increased the expression of DNMT3A/3B in mesangial cells. Additionally, the expression of fibrosis factors changed synchronously with it. Subsequently, after DNMT3A/3B inhibition, fibrosis factors decreased synchronously, suggesting that it also facilitates cell fibrosis development. Then we used online tools (such as TargetScan, miRDB, etc.) to predict the target genes of miR-29a-3p and found that the 3’-UTR of DNMT3A (862-868 bp, 1305-1311 bp, 5559-5565 bp) and the 3’-UTR of DNMT3B (1202-1909 bp) contained miR-29a-3p (UGGUGCU)-capable sites. It is said that DNMT3A/3B is a possible target of miR-29a-3p, which has also been confirmed by other scholars[15,33-35], but the interaction between them in diabetic nephropathy is not clear.
We further investigated the regulatory relationship between miR-29a-3p and DNMT3A/3B in SV40MES13 cells by inhibiting or overexpressing the former. Our findings revealed that suppressing miR-29a-3p expression resulted in upregulated DNMT3A/3B expression and aggravated cell fibrosis in the normal glucose group. Conversely, overexpressing miR-29a-3p in the HG group led to downregulated DNMT3A/3B expression and decreased cell fibrosis (Figure 4). MiR-29a-3p is proposed to contribute to cellular fibrosis by negatively regulating DNMTs. Furthermore, the expression of fibrosis molecules was observed to decrease upon addition of a DNMT3A/DNMT3B inhibitor to the HG + inhibition miR-29a-3p group, corroborating DNMT3A/DNMT3B as a direct target of miR-29a-3p (Figure 5).
We performed additional in vivo verification by inducing expression of antagomiR-29a-3p in mice through injections. The results demonstrated a significant decrease in the levels of DNMT3A/3B in the kidneys of the antagomiR-29a-3p group compared with the CON group. Additionally, the pathological changes were mild, and no instances of glomerulosclerosis were observed. In contrast, the CON group of db/db mice exhibited more severe renal pathological damage and glomerulosclerosis (Figures 6 and 7). It is postulated that elevated levels of miR-29a-3p in DKD may impede the progression of kidney pathology by suppressing the expression of DNMT3A/3B. Here, we have identified DNMT3A and DNMT3B as the direct targets of miR-29a-3p. The effect of miR-29a-3p on fibrosis factors can be significantly reversed through the use of DNMT inhibitors. Genomic stability is predominantly regulated by genetic and epigenetic mecha
Recent findings indicated that miR-29a-3p induces colon cancer cell apoptosis via the Wnt/β-catenin signaling pathway, inhibits the proliferation of parathyroid cells, and facilitates migration and invasion of ameloblastoma by targeting CTNNBIP1[36-38]. The Wnt/β-catenin signaling pathway is significant in the development of diabetic neph
Additionally, several studies suggest that the JAK/STAT pathway may contribute to the development of DKD through its regulation of cell proliferation, inflammation, and fibrosis[42,43]. For instance, an increase in SOCS1 expression can effectively inhibit the JAK/STAT pathway, resulting in reduced levels of serum creatinine, proteinuria, and renal damage[44,45]. Accordingly, it is speculated that post-inhibition of DNMT3A/3B expression, miR-29a-3p may impede DKD-related chronic inflammation and renal fibrosis by suppressing the Wnt/β-catenin and JKA/STAT signal pathways (Figure 8).
We identified miR-29a-3p as an inhibitor of renal fibrosis in DKD, achieved by its downregulation of DNMT3A/3B. We speculated that its downstream regulatory mechanism might involve feedback from Wnt/β-catenin and JKA/STAT signaling pathways. Notably, DNMT3A and DNMT3B inhibition can counteract the effect of miR-29a-3p downregulation in DKD-related fibrosis. These findings indicate that targeting miR-29a-3p and DNMT3A/3B may hold promise for DKD prevention and treatment.
Thanks to Professor Xu for providing the experimental platform, and we are grateful to the participants and the re
| 1. | De Marinis Y, Cai M, Bompada P, Atac D, Kotova O, Johansson ME, Garcia-Vaz E, Gomez MF, Laakso M, Groop L. Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int. 2016;89:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Lu Z, Liu N, Wang F. Epigenetic Regulations in Diabetic Nephropathy. J Diabetes Res. 2017;2017:7805058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Zheng J, Cheng J, Zhang Q, Xiao X. Novel insights into DNA methylation and its critical implications in diabetic vascular complications. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Stenvinkel P, Ekström TJ. Does the uremic milieu affect the epigenotype? J Ren Nutr. 2009;19:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Bansal A, Pinney SE. DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes. 2017;18:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Wing MR, Devaney JM, Joffe MM, Xie D, Feldman HI, Dominic EA, Guzman NJ, Ramezani A, Susztak K, Herman JG, Cope L, Harmon B, Kwabi-Addo B, Gordish-Dressman H, Go AS, He J, Lash JP, Kusek JW, Raj DS; Chronic Renal Insufficiency Cohort (CRIC) Study. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrol Dial Transplant. 2014;29:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Kumari N, Karmakar A, Ganesan SK. Targeting epigenetic modifications as a potential therapeutic option for diabetic retinopathy. J Cell Physiol. 2020;235:1933-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Zhang HP, Wang YH, Cao CJ, Yang XM, Ma SC, Han XB, Yang XL, Yang AN, Tian J, Xu H, Zhang MH, Jiang YD. A regulatory circuit involving miR-143 and DNMT3a mediates vascular smooth muscle cell proliferation induced by homocysteine. Mol Med Rep. 2016;13:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Gondaliya P, Dasare A, Srivastava A, Kalia K. miR29b regulates aberrant methylation in In-Vitro diabetic nephropathy model of renal proximal tubular cells. PLoS One. 2018;13:e0208044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Li A, Zhang W, Huang Z, Wang J, Yi B. High glucose-induced cytoplasmic translocation of Dnmt3a contributes to CTGF hypo-methylation in mesangial cells. Biosci Rep. 2016;36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, Mikhail A, Hitchcock CL, Wright VP, Nana-Sinkam SP, Piper MG, Marsh CB. Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 410] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 13. | Moraes LN, Fernandez GJ, Vechetti-Júnior IJ, Freire PP, Souza RWA, Villacis RAR, Rogatto SR, Reis PP, Dal-Pai-Silva M, Carvalho RF. Integration of miRNA and mRNA expression profiles reveals microRNA-regulated networks during muscle wasting in cardiac cachexia. Sci Rep. 2017;7:6998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Zamani M, Sadeghizadeh M, Behmanesh M, Najafi F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine. 2015;22:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Qin RH, Tao H, Ni SH, Shi P, Dai C, Shi KH. microRNA-29a inhibits cardiac fibrosis in Sprague-Dawley rats by downregulating the expression of DNMT3A. Anatol J Cardiol. 2018;20:198-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Cui J, Liu N, Chang Z, Gao Y, Bao M, Xie Y, Xu W, Liu X, Jiang S, Liu Y, Shi R, Xie W, Jia X, Shi J, Ren C, Gong K, Zhang C, Bade R, Shao G, Ji X. Exosomal MicroRNA-126 from RIPC Serum Is Involved in Hypoxia Tolerance in SH-SY5Y Cells by Downregulating DNMT3B. Mol Ther Nucleic Acids. 2020;20:649-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Wang X, Gao Y, Tian N, Zou D, Shi Y, Zhang N. Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice. Drug Des Devel Ther. 2018;12:2431-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Sun J, Wang J, Lu W, Xie L, Lv J, Li H, Yang S. MiR-325-3p inhibits renal inflammation and fibrosis by targeting CCL19 in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2020;47:1850-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Li J, Bao H, Zhang K, Yang X, Liu X, Li P, Li Q, Chen W. MiR-542-3p drives renal fibrosis by targeting AGO1 in vivo and in vitro. Life Sci. 2020;255:117845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Chung AC, Lan HY. MicroRNAs in renal fibrosis. Front Physiol. 2015;6:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Alizadeh M, Safarzadeh A, Beyranvand F, Ahmadpour F, Hajiasgharzadeh K, Baghbanzadeh A, Baradaran B. The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J Cell Physiol. 2019;234:19280-19297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Sun CM, Zhang WY, Wang SY, Qian G, Pei DL, Zhang GM. Erratum to: "Fer exacerbates renal fibrosis and can be targeted by miR-29c-3p". Open Med (Wars). 2023;18:20230755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Hsu CH, Liu IF, Kuo HF, Li CY, Lian WS, Chang CY, Chen YH, Liu WL, Lu CY, Liu YR, Lin TC, Lee TY, Huang CY, Hsieh CC, Liu PL. miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Lin L, Qu W, Li Y, Zhu H, Jiang W. MiR-29a-3p/NID1 axis regulates pulmonary fibrosis induced by TGF-β1. Panminerva Med. 2023;65:126-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Fu J, Wu B, Zhong S, Deng W, Lin F. miR-29a-3p suppresses hepatic fibrosis pathogenesis by modulating hepatic stellate cell proliferation via targeting PIK3R3 gene expression. Biochem Biophys Res Commun. 2020;529:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Shi S, Song L, Yu H, Feng S, He J, Liu Y, He Y. Knockdown of LncRNA-H19 Ameliorates Kidney Fibrosis in Diabetic Mice by Suppressing miR-29a-Mediated EndMT. Front Pharmacol. 2020;11:586895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Ebadi Z, Moradi N, Kazemi Fard T, Balochnejadmojarrad T, Chamani E, Fadaei R, Fallah S. Captopril and Spironolactone Can Attenuate Diabetic Nephropathy in Wistar Rats by Targeting microRNA-192 and microRNA-29a/b/c. DNA Cell Biol. 2019;38:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1819] [Cited by in RCA: 2028] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 30. | Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805-15810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1300] [Cited by in RCA: 1279] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 31. | Wang JY, Cheng H, Zhang HY, Ye YQ, Feng Q, Chen ZM, Zheng YL, Wu ZG, Wang B, Yao J. Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett. 2019;24:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Wu F, Yang Q, Mi Y, Wang F, Cai K, Zhang Y, Wang Y, Wang X, Gui Y, Li Q. miR-29b-3p Inhibitor Alleviates Hypomethylation-Related Aberrations Through a Feedback Loop Between miR-29b-3p and DNA Methylation in Cardiomyocytes. Front Cell Dev Biol. 2022;10:788799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Kogure T, Kondo Y, Kakazu E, Ninomiya M, Kimura O, Shimosegawa T. Involvement of miRNA-29a in epigenetic regulation of transforming growth factor-β-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Hepatol Res. 2014;44:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Song G, Tian L, Cheng Y, Liu J, Wang K, Li S, Li T. Antitumor activity of sevoflurane in HCC cell line is mediated by miR-29a-induced suppression of Dnmt3a. J Cell Biochem. 2019;120:18152-18161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Hu W, Dooley J, Chung SS, Chandramohan D, Cimmino L, Mukherjee S, Mason CE, de Strooper B, Liston A, Park CY. miR-29a maintains mouse hematopoietic stem cell self-renewal by regulating Dnmt3a. Blood. 2015;125:2206-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Liu S, Liu D, Liu J, Liu J, Zhong M. miR-29a-3p promotes migration and invasion in ameloblastoma via Wnt/β-catenin signaling by targeting catenin beta interacting protein 1. Head Neck. 2021;43:3911-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Han X, Zheng J, Wang Y, Gao Z. miRNA-29a inhibits colon cancer growth by regulation of the PTEN/Akt/GSK3β and Wnt/β-catenin signaling pathways. Oncol Lett. 2018;16:2638-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Wu Q, Fan W, Zhong X, Zhang L, Niu J, Gu Y. Klotho/FGF23 and Wnt in SHPT associated with CKD via regulating miR-29a. Am J Transl Res. 2022;14:876-887. [PubMed] |
| 39. | Wang S, Nie P, Lu X, Li C, Dong X, Yang F, Luo P, Li B. Nrf2 participates in the anti-apoptotic role of zinc in Type 2 diabetic nephropathy through Wnt/β-catenin signaling pathway. J Nutr Biochem. 2020;84:108451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Hu H, Wan Q, Li T, Qi D, Dong X, Xu Y, Chen H, Liu H, Huang H, Wei C, Zhou W, Jiang S, Mo Z, Liao F, Xu Q, He Y. Circulating MiR-29a, Possible Use as a Biomarker for Monitoring IgA Nephropathy. Iran J Kidney Dis. 2020;14:107-118. [PubMed] |
| 41. | Hsu YC, Chang PJ, Ho C, Huang YT, Shih YH, Wang CJ, Lin CL. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling. Sci Rep. 2016;6:30575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Hu J, Fan X, Meng X, Wang Y, Liang Q, Luo G. Evidence for the involvement of JAK/STAT/SOCS pathway in the mechanism of Tangshen formula-treated diabetic nephropathy. Planta Med. 2014;80:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Moreno JA, Gomez-Guerrero C, Mas S, Sanz AB, Lorenzo O, Ruiz-Ortega M, Opazo L, Mezzano S, Egido J. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs. 2018;27:917-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 44. | Opazo-Ríos L, Sanchez Matus Y, Rodrigues-Díez RR, Carpio D, Droguett A, Egido J, Gomez-Guerrero C, Mezzano S. Anti-inflammatory, antioxidant and renoprotective effects of SOCS1 mimetic peptide in the BTBR ob/ob mouse model of type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J, Mezzano S, Ortiz A, Egido J, Gomez-Guerrero C. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol. 2010;21:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |