INTRODUCTION
Diabetic foot ulcer (DFU) is an ulceration occurring in patients with established or newly diagnosed diabetes mellitus, usually associated with lower limb neuropathy and peripheral arterial disease[1]. DFUs are reported to affect about 18.6 million people worldwide yearly[2]. The healing of diabetic wounds is partly influenced by the degree of tissue ischemia, which can potentially delay the healing process[3]. The pathogenesis of DFU is driven by complex factors, and thus its healing mechanisms are not well understood unclear. Diabetic foot can lead to infections and even amputations, causing excruciating pain, mental stress and substantial financial burden on patients, making a major threat to global public health. Therefore, it is imperative to explore new methods for treating DFU.
Dl-3-n-butylphthalide (NBP) was an artificial racemic mixture used for the treatment of ischemic stroke in China. It was initially isolated from the seeds of Apium graveolens Linn[4]. It has been shown to have multi-target effects, including inhibiting platelet activation[5,6], protecting the blood-brain barrier[7], suppressing inflammation and oxidative stress[8], and improving adenosine triphosphate metabolism[9]. Studies have shown that it can promote angiogenesis[10-12], and has the potential to treat diabetes and its complications. The effects of NBP on diabetes-related diseases such as diabetic cognitive deficits[13,14], diabetic cataracts[15], diabetic nephropathy[16], diabetic peripheral neuropathy[17], diabetic vascular complications[18], and diabetes-induced brain ischemia[19] have been documented. Findings from such studies indicate that NBP can protect against diabetes-related diseases. Although numerous studies have aimed to develop the treatments for diabetic wound, the translation of results from preclinical studies into clinical use is limited. For instance, the use of nanoparticles to treat diabetic wound is hindered by environment and efficiency of synthesis, as well as the toxicity of such nanoparticles[20]. Several wound dressings have also been proposed, however, the need to change wound dressings regularly in clinical settings, coupled with the high demand for human and financial resources make such wound dressings less desirable. Studies have demonstrated that NBP, a mature medication, can effectively treat other diabetic complications. In this study, we aimed to investigate whether NBP can alleviate the DFU symptoms and reveal the associated mechanisms.
Network pharmacology is a methodology that integrates systems biology, network analysis, connectivity, redundancy, and pleiotropy. This approach provides a comprehensive framework for drug discovery, allowing researchers to determine clinical efficacy of drugs and understand their side effects and toxicity[21]. The organ-centric approach in medicine and the “one disease-one, target-one drug” dogma are significant limitations to the rapid drug development. Network pharmacology have revolutionized how we define, diagnose, and treat diseases[22]. It has enhanced the identification of multiple drug targets within complex biological networks and allowed researchers to explore drug interactions within these networks and elucidate their mechanisms of action at a systems level.
This study utilized the network pharmacology method to construct a protein-protein interaction (PPI) network comprising shared targets of NBP and DFU. In addition, hub genes within the PPI network were identified and the core targets were investigated to explore the target and target pathways of the active ingredients of NBP, employing network pharmacology, gene ontology (GO), and Kyoto Encyclopedia of genes and genomes (KEGG) biological pathway functional enrichment analyses. Finally, molecular docking analysis and experimental validation were performed to validate the predictions.
MATERIALS AND METHODS
Construction of PPI network
The intersection targets between NBP and DFU were explored on the STRING database (https://string-db.org/ver.11.0) to determine the PPI relationships based on a combined score > 0.4. “CytoHubba” was used to calculate the weight of each gene and explore the hub genes of the PPI network, and “MCODE” was used to identify the representative modules using Cytoscape software 3.8.1 (https://cytoscape.org).
Biology of functional analysis
The enrichment of the identified targets was determined through GO and KEGG enrichment analyses using the R “clusterProfiler,” “enrichplot,” and “ggplot2” packages.
Molecular docking
The core protein structure of the PPI network was downloaded from the Protein Data Bank database (http://www.rcsb.org/). The molecular structure of NBP was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The macromolecular protein was processed using PyMOL 2.4.0, which involved the removal of water molecules and ligands, followed by molecular docking analysis using AutoDock Tools 1.5.6. Results visualization and graphical analysis were performed using PyMOL 2.4.0.
Animal experiment and treatment
The four-week-old healthy male BALb/c mice (20 ± 2 g weight) were purchased from the Animal Experimental Center of Xuzhou Medical University. The Experimental Animal Administrative Committee of Xuzhou Medical University approved the animal experimental protocol. BALB/c mice were allowed to acclimatize for one week, then fasted (with water) for 12 hours. Eight mice were randomly selected as the control group. The remaining mice were intraperitoneally injected with 1% streptozotocin (Sigma, Saint Louis, MO, United states) solution at 100 mg/kg daily for three consecutive days. Two weeks later, fasting blood glucose levels were measured used venous blood for two consecutive days. Mice with blood glucose levels ≥ 16.7 mmol/L, polydipsia, polyphagia, and polyuria were considered to have established diabetes. The dorsal hair was shaved, a 10 mm full-thickness skin excision wound was created under sterile conditions to establish a DFU ischemic model. The wound site was cleaned with 70% ethanol before excision, and carefully excised with sterile surgical scissors. Twenty successfully modeled mice were randomly divided into DFU model group and the NBP treatment group, with 10 mice in each group. The mice were randomized using a computer-generated random number list to avoid bias. The NBP group was administered with a daily topical application of 10 μm/L NBP (MCE, Shanghai, China) solution to the dorsal wound, while the DFU and control groups received an equal volume of phosphate buffered saline (PBS). On days 1, 3, 7, and 14, the photographs of the foot wounds were taken, and the wound area was measured. The healing rate was calculated using the formula: Healing rate = (healed area/original wound area) × 100%.
Histological analysis
The obtained wound margin tissues were fixed, dehydrated, embedded in paraffin, and sectioned at 6 μm thickness. They were then subjected to Masson’s trichrome and hematoxylin-eosin (HE) and then observed under a light microscope (20 ×) and images were taken for further analysis.
Immunofluorescence staining
The tissues sections were permeabilized with 0.3% Triton X-100 for 10 minutes and blocked with 5% bovine serum albumin for 1 hour. This was followed by incubation with primary antibodies against Ki67 and CD31 overnight at 4 °C. The sections were washed and incubated with fluorophore-conjugated secondary antibodies for 1 hour at room temperature in the dark. Cell nuclei were counterstained with DAPI for 10 minutes. Finally, the sections were mounted with antifade medium and analyzed using a fluorescence microscope to assess Ki67 and CD31 expression.
Experimental cells
Human umbilical vein endothelial cells (HUVEC), human keratinocyte cells (HaCaT), and human dermal fibroblasts (HDF) were cultured in our laboratory in complete high-glucose Dulbecco’s modified eagle medium (DMEM, Gibco, Grand Island, NE, United States) supplemented with 10% foetal bovine serum (Gibco, Grand Island, NE, United States) at 37 °C in a humidified incubator with 5% CO2. Subculturing was performed every 2 to 3 days.
Experimental grouping and treatment
In vitro experiments were performed using HUVEC, HaCaT, and HDF to simulate the pro-angiogenic effects of NBP. The cells were divided into groups as follows: Control, 40 mmol/L glucose (high glucose, HG), and 40 mmol/L glucose + 15 μM NBP (HG + NBP). Those in the control group was cultured in standard complete medium, while cells in the HG group were cultured in medium containing 40 mmol/L glucose, and the HG + NBP group was cultured in medium containing 40 mmol/L glucose and 15 μM NBP.
Cell counting kit 8 assay
Cells were seeded in 96-well plates at a density of 3 × 103 cells per well. After 8 hours, cells adhered to the wells and were treated with the drugs, with six replicates per group for 48 hours. This was followed by incubation with the Cell Counting Kit 8 reagent (New Saimei Biotechnology Co., Ltd, Suzhou, China). Briefly, 10 μL of Cell Counting Kit 8 reagent was added to each well and incubated for 2 hours at 37 °C. The optical density was measured at 450 nm using a microplate reader, and the optical density values were used to determine the cell proliferation for each group.
EdU assay
The EdU cell proliferation assay kit was purchased from Ruibo Biotechnology Co., Ltd (Guangzhou, China). Cells were seeded in 96-well plates at a density of 4 × 103 cells per well and allowed to adhere for 8 hours. They were treated according to the groups, with six replicates per group for 48 hours, 100 μL of EdU medium was added to each well and incubated for 2 hours at 37 °C. The EdU medium was prepared fresh and protected from light to avoid degradation of the EdU reagent. Cells were washed 1-3 times with PBS, and the fixed with paraformaldehyde for 30 minutes at room temperature. They were then treated with glycine for 5 minutes on a decolorization shaker, and then washed with PBS. This was followed by permeabilization with 0.5% Triton X-100 for 10 minutes at room temperature and staining with Apollo reaction solution for 30 minutes at room temperature in the dark. The reaction solution was discarded, and cells were washed with PBS, counterstained with Hoechst 33342 for 30 minutes at room temperature in the dark, washed with PBS 1-3 times, and observed under an inverted fluorescence microscope. ImageJ software was used to quantify EdU-positive cells in each group.
Wound healing assay
Cells were seeded in 6-well plates at a density of 1 × 106 cells per well and cultured until they reached a 90% confluence. A scratch wound was made on the center of each well with a 100 μL pipette tip, followed by two washes with PBS. Cells were then treated with the respective drugs and imaged at 0 and 24 hours using an inverted phase-contrast microscope to measure the migration distance.
Transwell assay
The Transwell assay was performed to evaluate cell migration. Complete DMEM with 10% foetal bovine serum was added to the lower chamber of a 24-well Transwell chamber (LABSELECT, Beijing, China). Cells were seeded in the upper chamber at a density of 1 × 104 cells per well in serum-free DMEM and treated according to the designated groups. After 24 hours of incubation at 37 °C, cells were washed three times with PBS, fixed with paraformaldehyde for 20 minutes, and stained with crystal violet for 20 minutes, followed by another three-times washes with PBS. Cells on the upper surface of the chamber membrane were removed with a cotton swab. The cells were dried and those on the lower surface were observed under a microscope. Five fields of view per well were photographed, and the average number of migrated cells was quantified using ImageJ software. Cell counts were determined by averaging cell numbers across five fields of view. Cell migration was quantified by comparing the total number of cells in the lower chamber to the control group.
Annexin V-FITC/PI assay
Annexin V-FITC/PI double staining apoptosis detection kit was purchased from Kgi Biological Co. (Jiangsu, China). Briefly, the cells were seeded in 6-well plates at a density of 1 × 104 cells per well and allowed to adhere for 8 hours followed by incubation with the drugs for 48 hours. The supernatant from each group was collected and transferred into flow cytometry tubes, the adherent cells were harvested with EDTA-free trypsin and combined with the corresponding supernatant. Cells were centrifuged at 2000 rpm for 5 minutes, washed twice with PBS, and resuspended. For accurate cell counting, cells were resuspended in 1 mL PBS to a concentration of approximately 1× 106 cells/mL before staining. Binding buffer (500 μL), FITC (5 μL), and PI (5 μL) were added to each tube, mixed gently, and incubated for 5-15 minutes at 4 °C in the dark. They were finally analyzed by flow cytometry.
TUNEL assay
Cells were seeded in 6-well plates at a density of 1 × 104 cells per well. After 8 hours of adherence, they were treated with the drugs according to the designated groups, with six replicates per group 48 hours. This was followed by washing three times with PBS and fixed with paraformaldehyde for 30 minutes at room temperature, and then washed with PBS three times. Cells were permeabilized with 0.5% Triton X-100 for 10 minutes at 4 °C, washed three times with PBS, and incubated with 50 μL of TUNEL reaction mixture (TUNEL apoptosis assay kit, Proteintech, Wuhan, China) per well at 37 °C for 60 minutes in the dark. Cells were washed three times with PBS and stained with DAPI (50 μL per well) for 3 minutes at room temperature. Samples were observed under a fluorescence microscope, and ImageJ software was used to quantify TUNEL-positive cells in each group.
Quantitative realtime polymerase chain reaction
HUVEC cells were seeded in culture dishes and allowed to adhere for 8 hours. They were then treated with the drugs for 48 hours and washed three times with PBS. Total intracellular RNA was extracted using Trizol and reverse transcribed to synthesize cDNA. Quantitative real-time polymerase chain reaction (qPCR) was performed using the SYBR Green Mix kit of TaKaRa Company (Dalian, China). The expression of target genes was calculated by the 2-ΔΔCt method.
Western blot assays
Cells were seeded in culture dishes and allowed to adhere for 8 hours and then treated in line with the designated groups for 48 hours. They were washed three times with PBS, and 300 μL of cell lysis buffer (radio immunoprecipitation assay lysis buffer with phenylmethanesulfonyl fluoride at a ratio of 100:1) was added into each dish. Cells were lysed on ice, and proteins were collected. Protein concentration was determined using the bicinchoninic acid method and the proteins were separated by sodium-dodecyl sulfate gel electrophoresis and transferred to nitrocellulose membranes, which were incubated with primary antibodies overnight at 4 °C on a horizontal shaker. The primary antibodies used were rabbit anti-human B cell leukemia/lymphoma 2 (BCL2, Proteintech, Wuhan, China, 1:2000), rabbit anti-human BCL2 associated X (Proteintech, Wuhan, China, 1:2000), rabbit anti-human advanced glycosylation end-product specific receptor (Proteintech, Wuhan, China, 1:3000), and mouse anti-human β-actin (Proteintech, Wuhan, China, 1:5000). The membranes were washed with Tris-buffered saline with Tween (3 times for 5 minutes each), and then incubated with secondary antibodies (1:5000) for 2 hours at room temperature in the dark. Membranes were washed with Tris-buffered saline with Tween (3 times for 5 minutes each) and incubated with enhanced chemiluminescence substrate for chemiluminescent detection. Protein bands were photographed and analyzed using ImageJ software.
Detection of reactive oxygen species, malondialdehyde, and superoxide dismutase levels in HUVEC cells
HUVEC cells were seeded in culture dishes to allow adherence to the plate for 8 hours. They were then treated with the drug for 48 hours, washed twice with serum-free medium and then incubated with dichlorodihydrofluorescein diacetate (50 μmol/L) at 37 °C for 20 minutes. During the incubation, the dishes were gently shaken every 5 minutes to ensure even distribution and complete penetration of the dye. Subsequently, cells were washed three times with PBS to remove unbound dye. Cellular fluorescence intensity was then quantified using flow cytometry. To further evaluate intracellular oxidative stress levels, malondialdehyde and superoxide dismutase levels were determined using commercially available assay kits following the manufacturer’s instructions.
Statistical analysis
Data were analyzed using GraphPad Prism 9.0 software. Results are presented as mean ± SD, from at least three experimental repeats. Differences among multiple groups were analyzed using analysis of variance, and differences between two groups were compared using the t-test. A P value of < 0.05 was considered statistically significant.
RESULTS
Identifying NBP targets in DFU
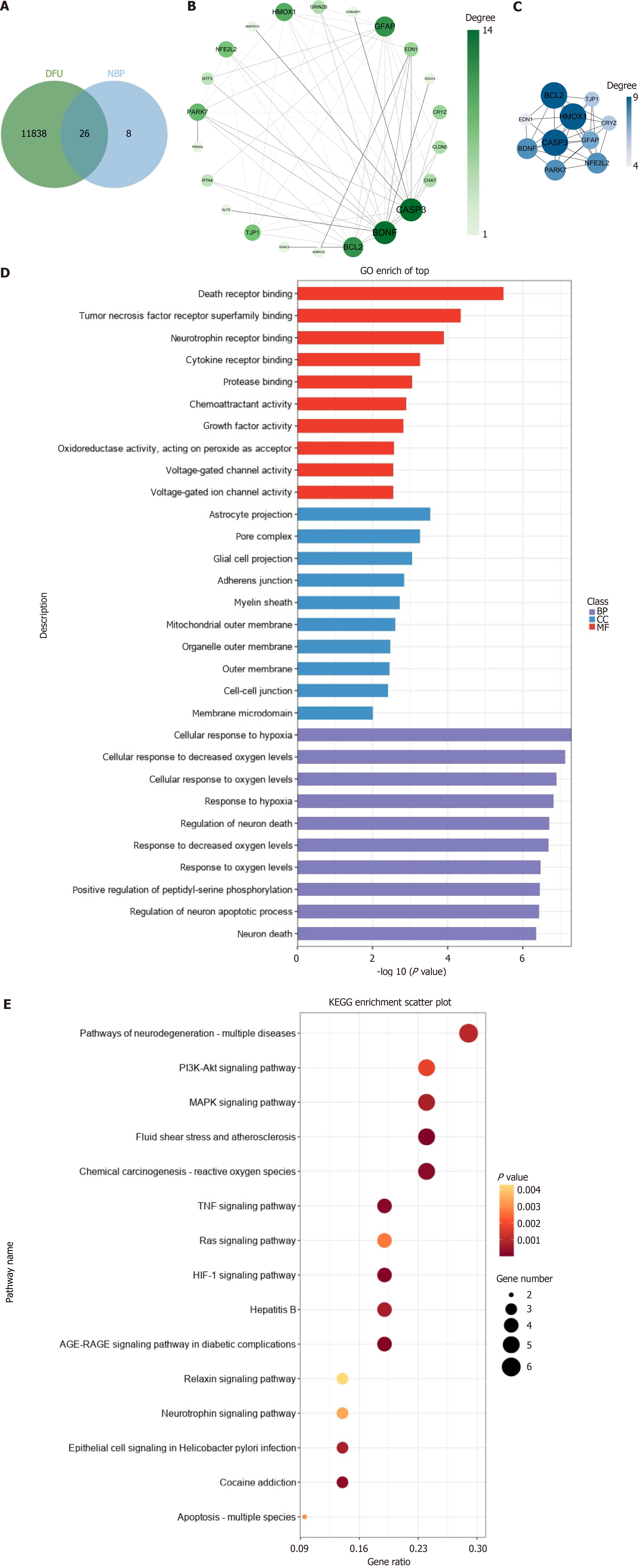
A total of 277 pharmacological targets of NBP were predicted using the Coremine database, and the repetitive genes were deleted using the UniProt database. An overlap of 11864 DFU targets with 34 NBP pharmacological targets revealed 26 intersection genes of NBP against DFU (Figure 1A). The PPI network of the 26 intersection targets was analyzed using STRING (Figure 1B), and cytoHubba was used to identify ten core gene targets, namely, heme oxygenase 1 (HMOX1), caspase 3 (CASP3), BCL2, brain derived neurotrophic factor (BDNF), nuclear factor erythroid 2 (NFE2) L2, glial fibrillary acidic protein, parkinsonism associated deglycase 7, crystallin zeta, tight junction protein 1, endothelin 1 (Figure 1C). GO analyses of the 26 genes showed that NBP was enriched in cellular response to hypoxia, regulation of neuron death, tumor necrosis factor receptor superfamily binding, death receptor binding, among others (Figure 1D). Additionally, 48 KEGG pathways were significantly enriched (P adjusted < 0.05), including the hypoxia inducible factor 1 (HIF-1) signaling pathway, tumor necrosis factor signaling pathway, advanced glycation end products-receptor of advanced glycation end products (AGE-RAGE) signaling pathway in diabetic complications, and pathways of neurodegeneration-multiple diseases (Figure 1E).
Figure 1 Pharmacological targets of Dl-3-n-butylphthalide in diabetic foot ulcers.
A: Twenty-six intersection genes of Dl-3-n-butylphthalide against diabetic foot ulcers; B and C: Protein-protein interaction network of the 19 melatonin targets; D and E: Gene ontology and Kyoto Encyclopedia of genes and genomes enrichment analysis of the 26 Dl-3-n-butylphthalide targets. DFU: Diabetic foot ulcers; NBP: Dl-3-n-butylphthalide; GO: Gene ontology; KEGG: Kyoto Encyclopedia of genes and genomes.
The potential NBP alleviated DFU-like phenotypes in mice
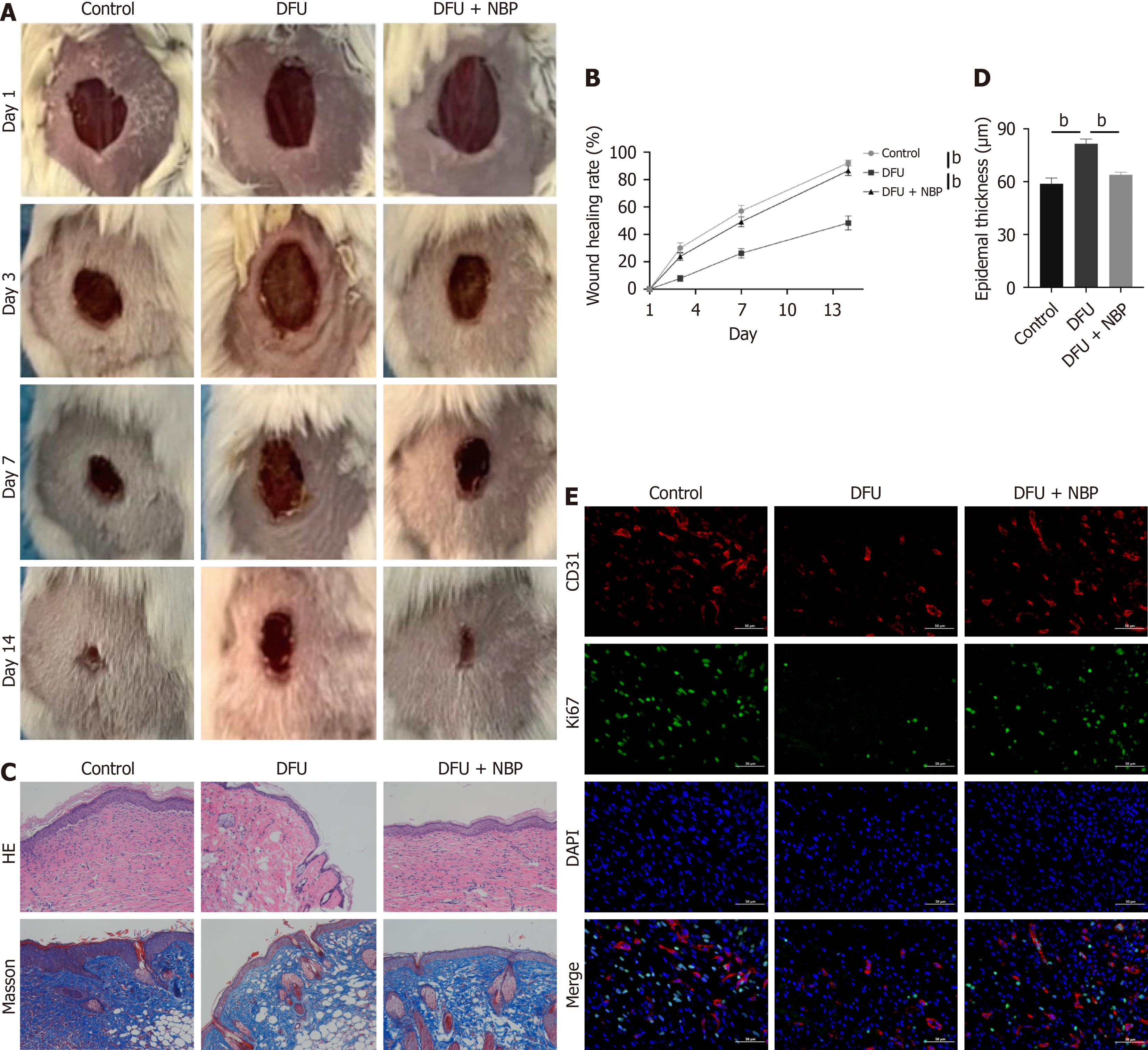
To explore the efficacy of NBP in treating DFU, a streptozotocin-induced mouse DFU mode was constructed to evaluate the therapeutic effect of butylphthalide and sodium chloride injection. The healing of the back wounds in mice was assessed through Masson’s trichrome, HE staining, and immunofluorescence staining. It was observed that NBP administration promoted wound healing in mice (Figure 2A and B). The HE and Masson’s trichrome staining results showed that the NBP group significantly increased neovascularization and fibroblast proliferation relative to the model group (Figure 2C). Epidermal thickening is a pathological change usually observed in abnormal scarring, such as keloids[23]. In this study, the epidermis in the NBP treatment group was thinner than that in the DFU group (Figure 2D), suggesting that NBP could reshape the surface structure of diabetic wounds. Ki67/CD31 double-staining is often used to quantify angiogenesis. Immunofluorescence results demonstrated higher expressions of Ki67 and CD31 in the NBP group than in the model group (Figure 2E), indicating that NBP enhanced the formation of new blood vessel formation. In conclusion, NBP could treat DFU by promoting angiogenesis.
Figure 2 Wound healing of different time points in diabetic foot ulcers mice.
A: Images of mice wounds at different time points; B: The healing rate of foot wounds at different time points in mice; C: The wound tissues of mice were stained with hematoxylin-eosin and Masson 14 days after surgery; D: The epidermal thickness of mice 7 days after surgery; E: Immunofluorescence staining showed capillary density analysis of wound tissues in mice. Data expressed as individual values with mean ± SE. bP < 0.01. DFU: Diabetic foot ulcers; NBP: Dl-3-n-butylphthalide; HE: Hematoxylin-eosin.
NBP increases angiogenesis and reepithelialization by promoting the proliferation and migration of HUVEC and HDF under HG
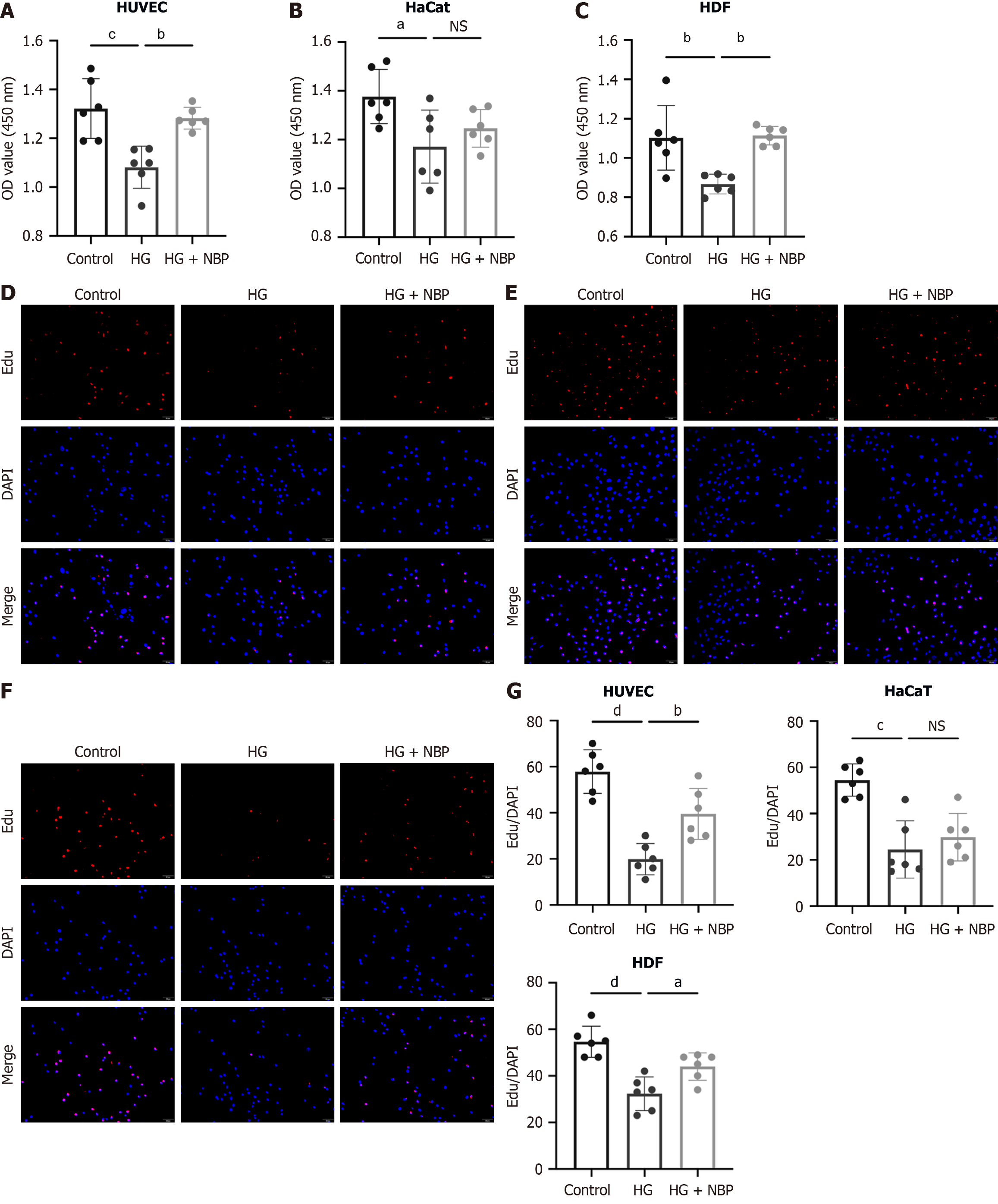
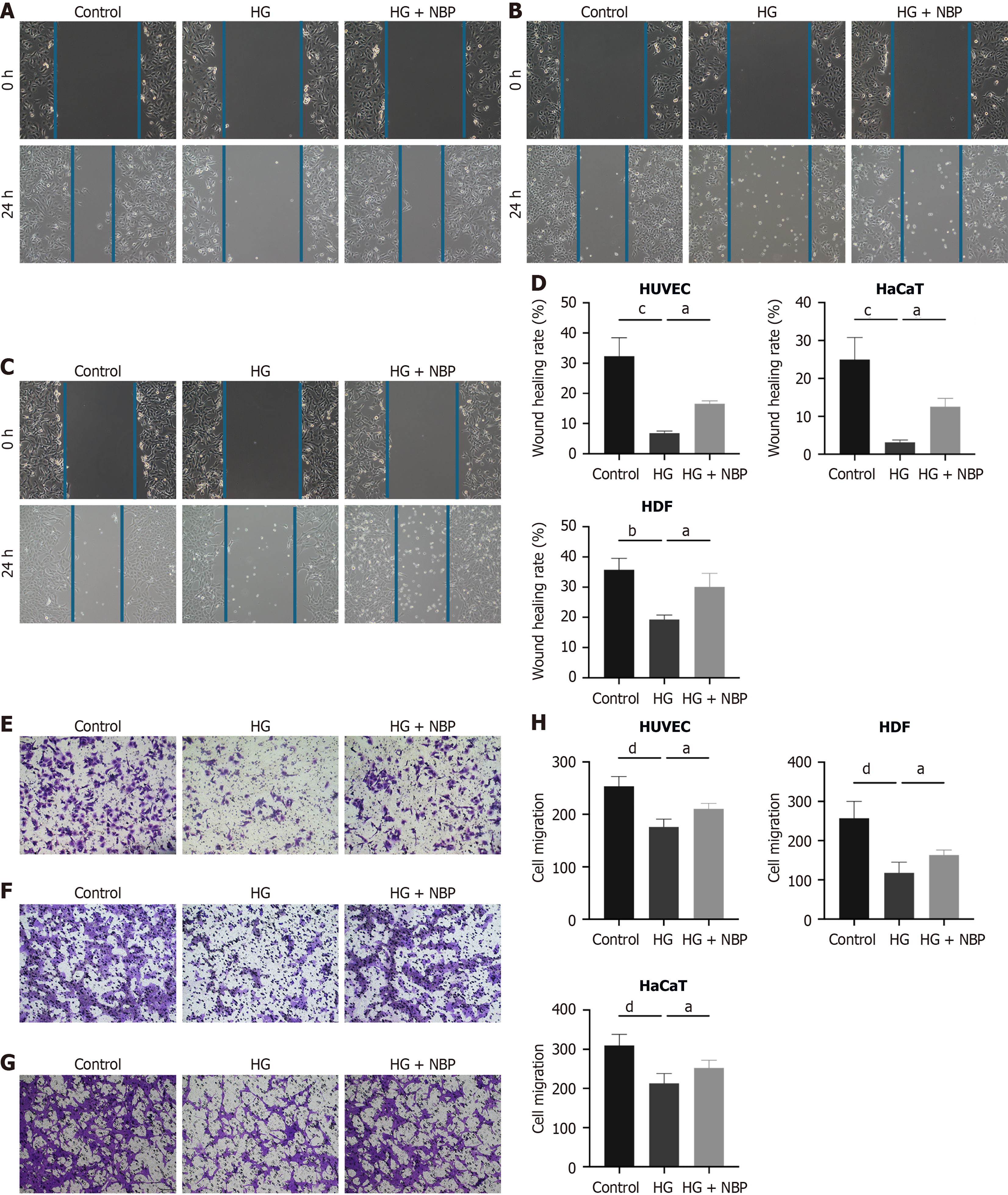
Vascular endothelial cells and epithelial cells are the primary repair cells in the wound healing process. In this study, we explored the effects of NBP on angiogenesis and reepithelialization in HG-treated HUVEC, HDF, and HaCaT cells. As shown in Figure 3, NBP treatment significantly enhanced the proliferation of HUVEC and HDF cells under HG conditions. However, it did not significantly affect the proliferation of HaCaT. The ability of endothelial and epithelial cells to migrate is crucial for wound healing. Impaired migration decreases the healing rates and chronic wounds. The results showed that in all three cell types, the area of scratch wound healing was significantly greater in the NBP + HG group than in the HG group (Figure 4A-D). Moreover, the number of cells migrating through the Transwell membrane was significantly higher in the NBP + HG group compared to the HG group in the three cell types (Figure 4E-H). These data suggested that NBP treatment effectively restored the migration ability of HUVEC, HaCaT, and HDF cells in an HG environment (Figure 4E-H), providing evidence that NBP is a promising drug for vascular and epidermal regeneration in DFU.
Figure 3 Dl-3-n-butylphthalide promotes cellular proliferation under high glucose conditions.
A-C: Cell Counting Kit 8 assay was used to assess the effect of Dl-3-n-butylphthalide on cell proliferation; D: Edu assay showed the cell proliferation of human umbilical vein endothelial cells; E: Edu assay showed the cell proliferation of human keratinocytes cells; F: Edu assay showed the cell proliferation of human dermal fibroblasts; G: Quantification of Edu corporation assay in human umbilical vein endothelial cells, human keratinocytes cells, and human dermal fibroblasts. Data expressed as individual values with mean ± SE. dP < 0.0001, cP < 0.001, bP < 0.01, aP < 0.05, nsP > 0.05. HUVEC: Human umbilical vein endothelial cells; HaCaT: Human keratinocyte cells; HDF: Human dermal fibroblasts; HG: High glucose; NBP: Dl-3-n-butylphthalide.
Figure 4 Dl-3-n-butylphthalide promotes cellular migration under high glucose conditions.
A: The scratch assay showed the cell migration of human umbilical vein endothelial cells; B: Cell migration of human keratinocytes cells; C: Cell migration of human dermal fibroblasts; D: Quantitative analysis of migration cells in a scratch assay; E: Transwell migration experiment detected the migration abilities of Human umbilical vein endothelial cells; F: Transwell migration experiment of human keratinocytes cells; G: Transwell migration experiment of human dermal fibroblasts; H: Quantification of the number of cells that migrated in Transwell assays. Data expressed as individual values with mean ± SE. dP < 0.0001, cP < 0.001, bP < 0.01, aP < 0.05. HG: High glucose; NBP: Dl-3-n-butylphthalide; HUVEC: Human umbilical vein endothelial cells; HaCaT: Human keratinocyte cells; HDF: Human dermal fibroblasts.
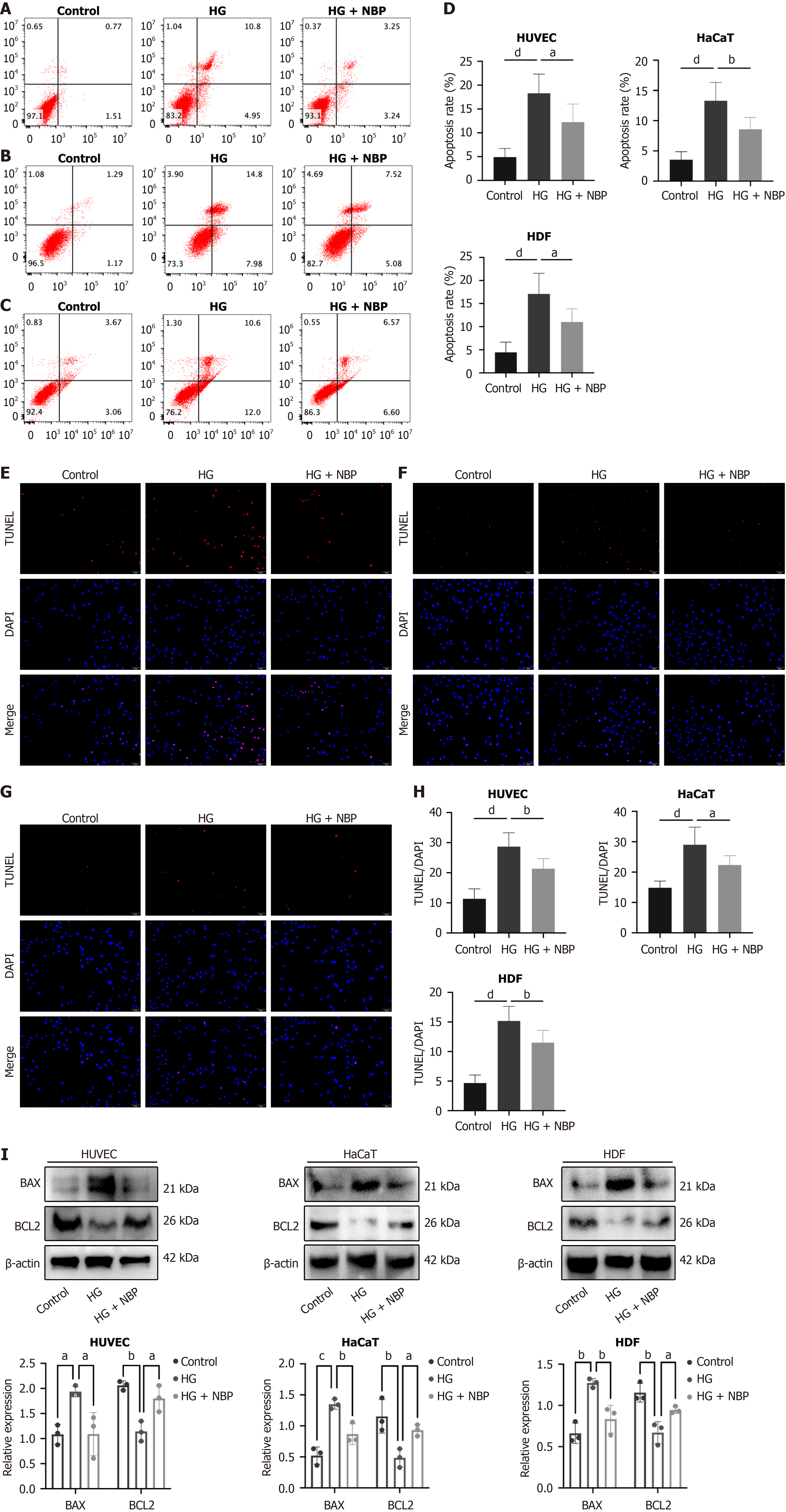
NBP reduced the apoptosis of HUVEC, HaCaT, and HDF cells under HG
HG conditions induced apoptosis to varying degree in different cell types within the wound tissues, which impaired wound healing. The results showed that, compared to the HG group, the apoptosis rate in the NBP + HG group was markedly reduced in HUVEC, HaCaT, and HDF cells (Figure 5A-H). The Western blot results showed that in the NBP + HG group, BCL2 associated X protein expression was decreased, and BCL2 protein expression was increased compared to the HG group (Figure 5I). These suggested that NBP inhibited apoptosis in HUVEC, HaCaT, and HDF cells under HG conditions.
Figure 5 Dl-3-n-butylphthalide reduces cell apoptosis under high glucose conditions.
A: Flow cytometry detection of cell apoptosis of Human umbilical vein endothelial cells; B: Flow cytometry detection of human keratinocytes cells; C: Flow cytometry detection of human dermal fibroblasts; D: Statistical results of apoptotic cells in flow cytometry; E: TUNEL staining was detected the cell apoptosis of human umbilical vein endothelial cells; F: TUNEL staining of human keratinocytes cells; G: TUNEL staining of human dermal fibroblasts; H: Statistical analysis of the percentage of apoptotic cells according to the result of the TUNEL assay; I: Apoptosis-related protein expression was examined by Western blot analysis. Data expressed as individual values with mean ± SD. dP < 0.0001, cP < 0.001, bP < 0.01, aP < 0.05. HG: High glucose; NBP: Dl-3-n-butylphthalide; HUVEC: Human umbilical vein endothelial cells; HaCaT: Human keratinocyte cells; HDF: Human dermal fibroblasts.
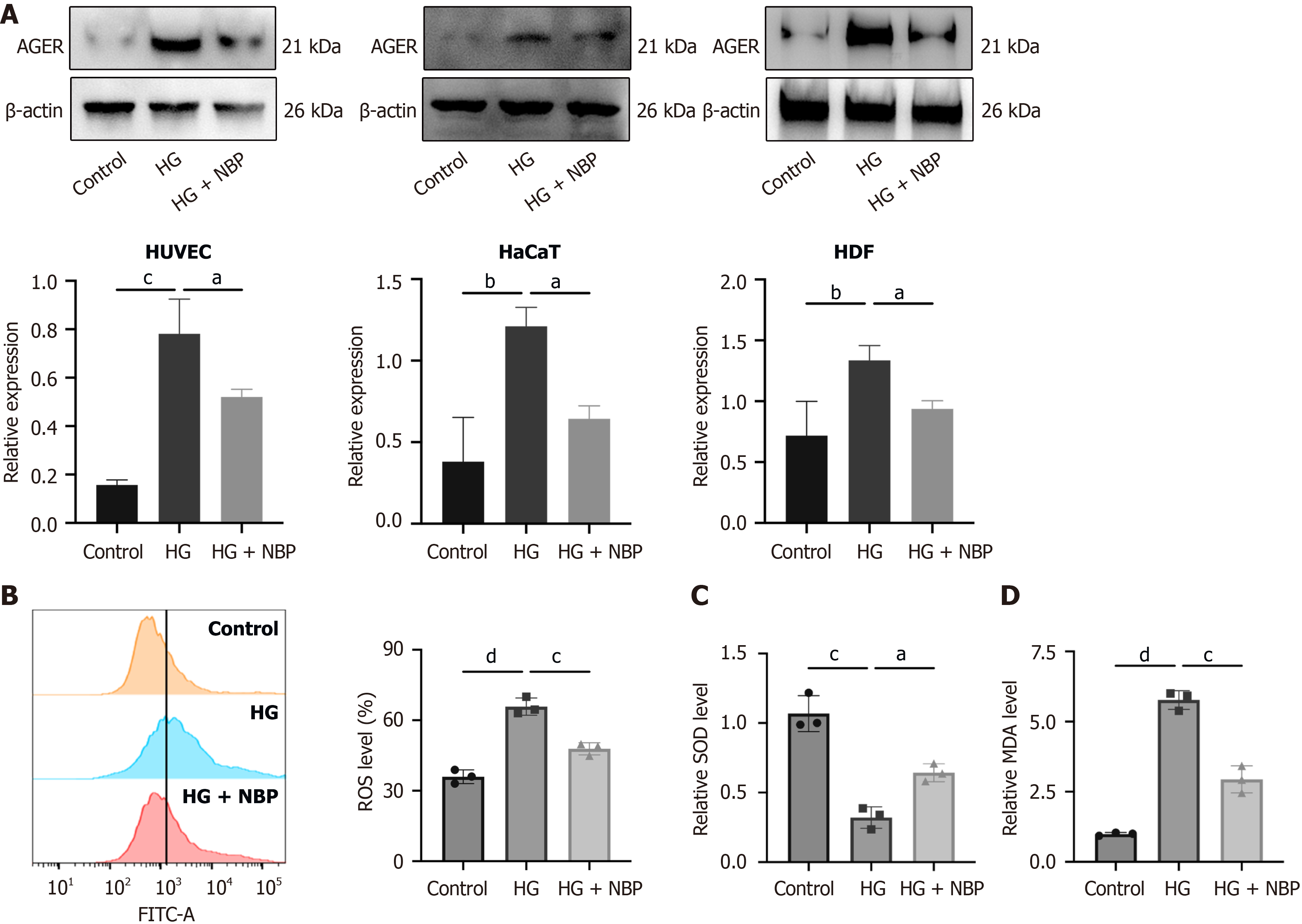
NBP exerts biological effects by modulating the AGE-RAGE signaling pathway
Figure 1 reveals that the NBP-associated target genes in DFU treatment were mainly enriched in the AGE-RAGE signaling pathway. The AGE-RAGE pathway regulates inflammatory signaling pathway in various diseases, including diabetes, cardiovascular diseases, and neurological disorders. Western blot indicated that advanced glycosylation end-product specific receptor protein expression was lower in the NBP + HG group than in the HG group in the three cell types, suggesting that NBP activated the AGE-RAGE signaling pathway and modulated the biological functions of HUVEC, HaCaT, and HDF cells, to treat DFU (Figure 6A). Figure 6B-D indicates that, compared with the HG group, the NBP treated group showed decreased expression of reactive oxygen species and malondialdehyde under HG conditions and increased superoxide dismutase expression in HUVEC, suggesting that NBP suppressed the oxidative stress.
Figure 6 Dl-3-n-butylphthalide exerts anti-apoptotic and anti-oxidative stress effects by modulating the advanced glycation end products-receptor of advanced glycation end products signaling pathway under high glucose conditions.
A: Western blot analysis of the protein expression of advanced glycosylation end-product specific receptor; B: Flow cytometry analysis of reactive oxygen species levels; C and D: Kit-based detection of superoxide dismutase and malondialdehyde levels. Data expressed as individual values with mean ± SD. dP < 0.0001, cP < 0.001, bP < 0.01, aP < 0.05. AGER: Advanced glycosylation end-product specific receptor; HG: High glucose; NBP: Dl-3-n-butylphthalide.
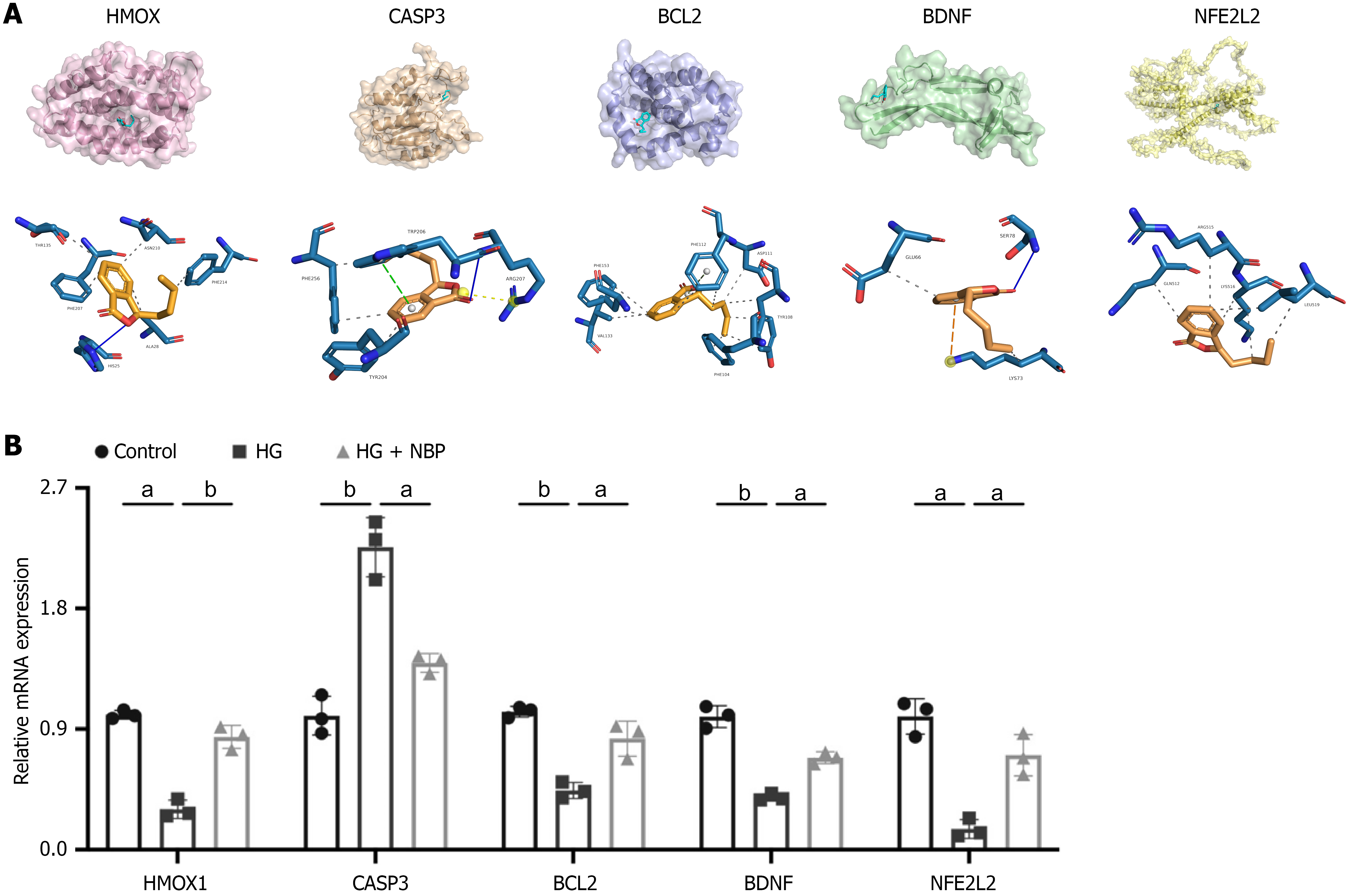
Molecular docking analysis and the effect of NBP on related gene expression in HUVEC
Molecular docking was performed to identify the potential binding between NBP and seven core targets. It was observed that NBP could bind to HMOX1, CASP3, BCL2, BDNF, and NFE2 L2 with docking energy of -6.105 kcal/mol, -5.514 kcal/mol, -6.152 kcal/mol, -4.338 kcal/mol, and -5.023 kcal/mol, respectively. The binding energies for docking interactions were negative, indicating a strong binding affinity between NBP and the core targets. HMOX1 and BDNF interact with NBP via hydrogen bonds and hydrophobic interactions. The CASP3 interacts with NBP via hydrogen bonds, electrostatic and hydrophobic interactions. The interactions between BCL2, NFE2 L2, and NBP are primarily characterized by hydrophobic interactions (Figure 7A). To investigate the anti-apoptotic and anti-oxidative stress effects of NBP on HUVECs, we performed qPCR to assess the expression of relevant genes. As depicted in Figure 7B, mRNA expression of HMOX1, BCL2, BDNF, and NFE2 L2 was upregulated in HUVECs under HG conditions. Conversely, NBP treatment significantly decreased CASP3 mRNA expression.
Figure 7 Molecular docking analysis and mRNA expression analysis of core genes.
A: Molecular docking revealed the binding of Dl-3-n-butylphthalide to its targets. Blue solid line: Hydrogen bond; Gray dashed line: Hydrophobic interaction; Yellow dashed line: Electrostatic interaction; B: The quantitative real-time polymerase chain reaction analysis of the mRNA levels of heme oxygenase 1, caspase 3, B cell leukemia/lymphoma 2, brain derived neurotrophic factor and nuclear factor erythroid 2 L2. Data expressed as individual values with mean ± SD. bP < 0.01, aP < 0.05. HMOX: Heme oxygenase; CASP3: Caspase 3; BCL2: B cell leukemia/lymphoma 2; BDNF: Brain derived neurotrophic factor; NFE2: Nuclear factor erythroid 2; HG: High glucose; NBP: Dl-3-n-butylphthalide.
DISCUSSION
Epidemiological data indicate that DFUs are a highly prevalent and severe complication of diabetes globally. The pathogenesis of DFUs involves a well-established sequence of events, including loss of sensation (neuropathy), ischemia, and minor trauma. Among the estimated 537 million individuals worldwide with diabetes, approximately 19% to 34% may develop DFU during their lifetime[24]. Notably, the available treatments for DFUs, such as wound debridement, glycemic control, and infection management, do not effectively cure the disease. Consequently, patients with DFUs are at an elevated risk of lower limb amputation. This has called for the design of new and effective drugs to promote the healing of chronic, non-healing diabetic wounds. Innovative approaches, including advanced wound dressings[25], negative pressure wound therapy[26], and stem cell therapy[27], have emerged as potential solutions for DFU treatment. Notably, NBP, a drug developed in China for ischemic stroke, has was reported to confer protection against other diabetic complications. However, its efficacy and underlying mechanisms in treating DFUs remain to be fully elucidated. In this study, we explored mechanisms through network pharmacology.
In this study, we identified numerous potential therapeutic targets and signaling pathways mediating the effects of NBP on DFU through network pharmacology. The GO/KEGG enrichment results suggested that NBP targeted cellular response to hypoxia and the HIF-1 signaling pathway. In animal experiment, NBP treatment enhanced neovascularization and fibroblasts proliferation compared with the DFU group, indicating that the NBP treatment promoted wound healing. An important target of HIF-1 is vascular endothelial growth factor (VEGF), which participates in angiogenesis and neovascularization. Studies have shown that the VEGF mRNA level cannot be induced by hypoxia in HIF-1β mutant cells further validating the important role of HIF-1 in the secretion of VEGF[28]. In vitro, we demonstrated that NBP promoted the proliferation and migration of HUVEC and HDF cells under HG. These results revealed that NBP could treat DFU by promoting angiogenesis. The AGE-RAGE axis has been implicated in the regulation of disease onset and progression by modulating apoptosis. Fu et al[29] found that the AGE-RAGE axis can promote apoptosis in renal endothelial cells, contributing to diabetic nephropathy. Moreover, it has been shown that NBP mitigates renal IRI by decreasing apoptosis, oxidative stress, and inflammation[30]. In further in vitro experiments, it was found that NBP significantly reduced apoptosis in a high-glucose environment and facilitated wound healing.
The AGE-RAGE signaling pathway can regulate the cellular biological behavior via various mechanisms such as inflammation, apoptosis, and oxidative stress[31,32]. RAGE is expressed on the surface of many cells, including endothelial cells, monocytes-macrophages, vascular smooth muscle cells, glomerular epithelial cells, astrocytes, and microglial cells[33]. This suggests that these cells are potential sites for the physiological and pathological effects of AGEs and RAGE interactions. Activation of the AGE-RAGE axis in both immune-related and somatic cells transduce signals was found to increase oxidative stress and cell apoptosis, thereby promoting AGE formation and contributing to AGE-related diseases. These biological responses can damage the vascular endothelial cells, renal mesangial, endothelial, and retinal pericytes, inducing diabetic triopathy[34]. Vascular endothelial cells and fibroblasts are the primary repair cells involved in the wound-healing process, and the integrity of their structure and function influences the speed of diabetic wound healing. In this study, we demonstrated that NBP decreased the expression level of the RAGE in cells under HG conditions, suggesting that the effects of NBP were mediated via the AGE-RAGE signaling pathway.
Network pharmacology analysis revealed that the effects of NBP were associated with neurotrophin receptor, neuron death, and neurodegeneration pathways. In chick embryo, a positive reciprocal association was found between nerves and wound repair[35]. Evidence from prior investigations has indicated that cutaneous sensory innervation participates in the diabetic wound healing pathology[36]. The NBP administration restored the function and structure of the brain following intracerebral hemorrhage mainly manifested as alleviation of neuronal apoptosis, suppression of neuroinflammation and oxidative stress, neurovascular remodeling, and eventually improvement of neurological deficits[37]. Although little is known about the role of nerves during skin healing, we postulate that NBP may accelerate skin healing in DFU by inhibiting neuronal apoptosis. However, we used a single database and software and the information available in existing databases is limited and incomplete, and thus, our findings may carry some bias. Future studies are needed to validate our findings.
Molecular docking analysis identified that the direct targets of NBP included NFE2 L2, HMOX1, BDNF, BCL2 and CASP3, which were apoptosis- and oxidative stress-related genes. The NFE2 L2 signaling pathway can alleviate oxidative stress and repair skin damage induced by oxidative stress[38]. NFE2 L2 has been identified as a promising target for the treatment of diabetes and its complications. For instance, NFE2 L2 was shown to treat diabetic nephropathy via suppressing antioxidant effects[39], and ameliorate dyslipidemia in diabetes[40]. The antioxidant enzyme HMOX1 is a downstream protein of NFE2 L2 and a key enzyme in heme degradation, catalyzing the degradation of heme to produce biliverdin and CO, which effectively reduces the inflammatory response and oxidative stress damage to protect cells. Abnormal expression of NFE2 L2 was detected in non-healing diabetic wounds, and overexpression of HMOX1 was reported to enhance wound healing through its anti-inflammatory effects[41]. It was also found to be upregulated during wound healing[42]. The NFE2 L2/HMOX1 signaling pathway function to maintain the redox balance by regulating the production of oxidative and antioxidant substances, reducing oxidative stress response, and inhibiting cell apoptosis[43]. Currently, researchers are investigating the role of cell apoptosis in wound healing. It has been shown that apoptosis is involved in and regulates multiple stages of wound healing. Overexpression of BCL2 protein can reduce the production of oxygen free radicals, thereby inhibiting cell apoptosis and promoting wound healing[44]. BCL2-modified adipose-derived stem cells were reported to delay adipose-derived stem cells apoptosis and thus promote wound healing in diabetic mice[45]. CASP3 is a widely studied apoptotic regulatory factor. Its overexpression can limit the release of cyt-c and the activation of downstream CASP3 protease, to improve cell survival or death[46]. BDNF is a neurotrophic factor that acts as a neurotransmitter modulator that maintains neuronal survival and plasticity[47]. It is involved in oxidative stress and inflammatory injury associated with diabetes, protecting cells by inhibiting apoptosis [48]. We plan to strengthen future studies by investigating the affinity between NBP and its five core targets, adding depth and credibility. The qPCR results demonstrated that HMOX-1, BCL2, BDNF, NFE2 L2 mRNA levels in HUVEC under HG environment were increased, while CASP-3 mRNA expression was decreased following NBP treatment. Analysis of literature showed that these target proteins contribute to the effects of NBP in treating DFU.
The binding modes of NBP with HMOX1, CASP3, BCL2, BDNF, and NFE2 L2 are primarily driven by hydrogen bonding and hydrophobic interactions, which affects their enzymatic activity and transcriptional function. These interactions enhance the NBP’s antioxidant, anti-apoptotic, and reparative effects. Notably, NBP exerts therapeutic effects by activating related transcription factors, regulating oxidative stress and apoptosis signaling pathways, and possibly indirectly affecting the gene transcription of HMOX1, BDNF, NFE2 L2, CASP3, and BCL2 and increasing their mRNA levels. This multi-pathway mechanism of action provides a novel idea for developing individualized treatments. Further experimental studies are warranted to further explore the specific mechanisms by which NBP regulates the expression of these target genes and the potential of this action in clinical applications.
The safety of NBP as a drug for the treatment of cerebral ischemia has been documented in several studies, and the efficacy of NBP in the treatment of other diseases such as vascular dementia[49], Parkinson’s disease[50] was reported. Notably, this drug was found to induce few adverse reactions. It has also been reported to treat post-stroke cognitive impairment, acute ischemic stroke and other diseases, without increasing the risk of adverse reactions when applied in combination with other drugs[51-53]. No adverse effects of NBP in the treatment of diabetes-related complications have not been reported so far, and further clinical trials are needed to determine whether NBP induces adverse effects when combined with multiple medications in diabetic patients. To date, NBP has been shown to treat diabetic peripheral neuropathy, suggesting that it may improve diabetic wound healing via neuroprotective mechanisms. The combination of NBP with other drugs may yield better therapeutic effects. A study reported that NBP combined with α-lipoic acid exerted neuroprotective effects[54], suggesting that this combination strategy may be suitable for the treatment of diabetic wounds.
Polymorphisms in the BDNF gene may affect the efficacy of this drug in the treatment of DFU, with studies indicating the polymorphisms in BDNF are associated with type 2 diabetes and hyperglycemia[55]. Chronic hyperglycemia affects wound healing and neuroprotection. Patients with poorly controlled diabetes show poor response to NBP, and the drug may be more effective in settings where infection is well controlled. Environmental factors, including smoking, pollution, and poor nutrition, can increase oxidative stress and inflammation, potentially interfering with the therapeutic effects of NBP, which aims to mitigate these factors. Investigating the interplay between genetic and environmental factors and NBP response may provide valuable insights into treatment efficacy and facilitate the development of more personalized treatment strategies for DFU. Further research in this area could significantly improve the management of diabetes complications.
To explore the efficacy and application of NBP in DFUs models, high-quality clinical trials are needed. Currently, there are no specific treatments for DFU. As innovative therapeutic options for DFU are developed, NBP is expected to serve as an adjunct to DFU treatment or in combination therapy with other therapeutic options (e.g., antibiotics, anti-inflammatory drugs, etc.). Notably, the DFU treatment is expensive, the use of NBP can reduce long-term hospitalization and save on surgical costs. Widespread NBP application can positively impact global health by addressing needs in developed countries and improving outcomes for diabetic patients in developing countries through accessible and effective treatments.