Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.99108
Revised: December 13, 2024
Accepted: January 6, 2025
Published online: March 15, 2025
Processing time: 191 Days and 5.8 Hours
Identification of myocardial injury has traditionally relied on high-sensitivity troponin T (hs-TnT) levels exceeding the 99th percentile threshold. However, patients with detectable hs-TnT levels below this threshold represent a heterogeneous group with an inadequately characterized risk profile.
To investigate the association between hs-TnT levels below the 99th percentile and the presence of diabetic kidney disease (DKD) in patients with diabetes mellitus.
This study analyzed data from the National Health and Nutrition Examination Survey obtained between 1999 and 2004, focusing on adults with type 2 diabetes mellitus. Serum hs-TnT concentrations were evaluated. DKD was defined as im
The study included 2505 patients with a mean age of 55.02 (standard error: 0.72) years, of whom 44.87% were females. Among the participants, 909 (32.34%) were diagnosed with DKD. Multivariable logistic regression ana
Our study findings suggest that in individuals with type 2 diabetes, detectable hs-TnT levels below the 99th percentile are associated with DKD.
Core Tip: High-sensitivity troponin T levels below the 99th percentile show a significant linear association with diabetic kidney disease in type 2 diabetes mellitus patients. This association, derived from National Health and Nutrition Exa
- Citation: Luo XY, Huang LH, Kang KP. Association between high-sensitivity troponin T levels below the ninety-ninth percentile and diabetic kidney disease: A cross-sectional study. World J Diabetes 2025; 16(3): 99108
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/99108.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.99108
Diabetes mellitus presents a global health crisis, affecting an estimated 463 million individuals worldwide in 2019, with projections reaching 700 million cases by 2045[1]. Type 2 diabetes mellitus (T2DM) accounts for most of these cases and continues to rise at an alarming rate. Among its complications, diabetic kidney disease (DKD) is a leading cause of morbidity and mortality, contributing significantly to the burden of end-stage renal disease[2]. Despite advancements in diabetes management, including sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists, the risk of developing DKD remains substantial in patients with T2DM[3]. This highlights an urgent need for novel biomarkers and mechanisms to understand better and manage the onset and progression of DKD.
High-sensitivity troponin T (hs-TnT) has recently gained attention as a biomarker traditionally used to diagnose myocardial injury, with elevated levels above the 99th percentile indicating myocardial injury[4]. However, patients with hs-TnT levels below this threshold represent a heterogeneous group with an inadequately defined risk profile and are often overlooked clinically. Emerging evidence suggests that even sub-threshold hs-TnT levels are associated with a higher burden of cardiovascular risk factors, greater cardiac pathology, and poorer prognosis[5,6]. This raises the possibility that low hs-TnT levels might indicate subclinical organ damage, including kidney damage in patients with diabetes. Evidence has demonstrated a potential relationship between hs-TnT levels and renal outcomes in diabetic populations[7]. Recent studies further highlight the predictive value of hs-TnT assays for peripheral microangiopathy in T2DM[8], and increased risks of renal and cardiovascular events in patients with DKD[9].
The pathophysiology of DKD is complex, involving a cascade of events triggered by chronic hyperglycaemia, including oxidative stress, inflammation, and endothelial dysfunction, which collectively contribute to progressive kidney function decline[10]. These mechanisms might plausibly lead to elevated hs-TnT levels, even below the myocardial injury th
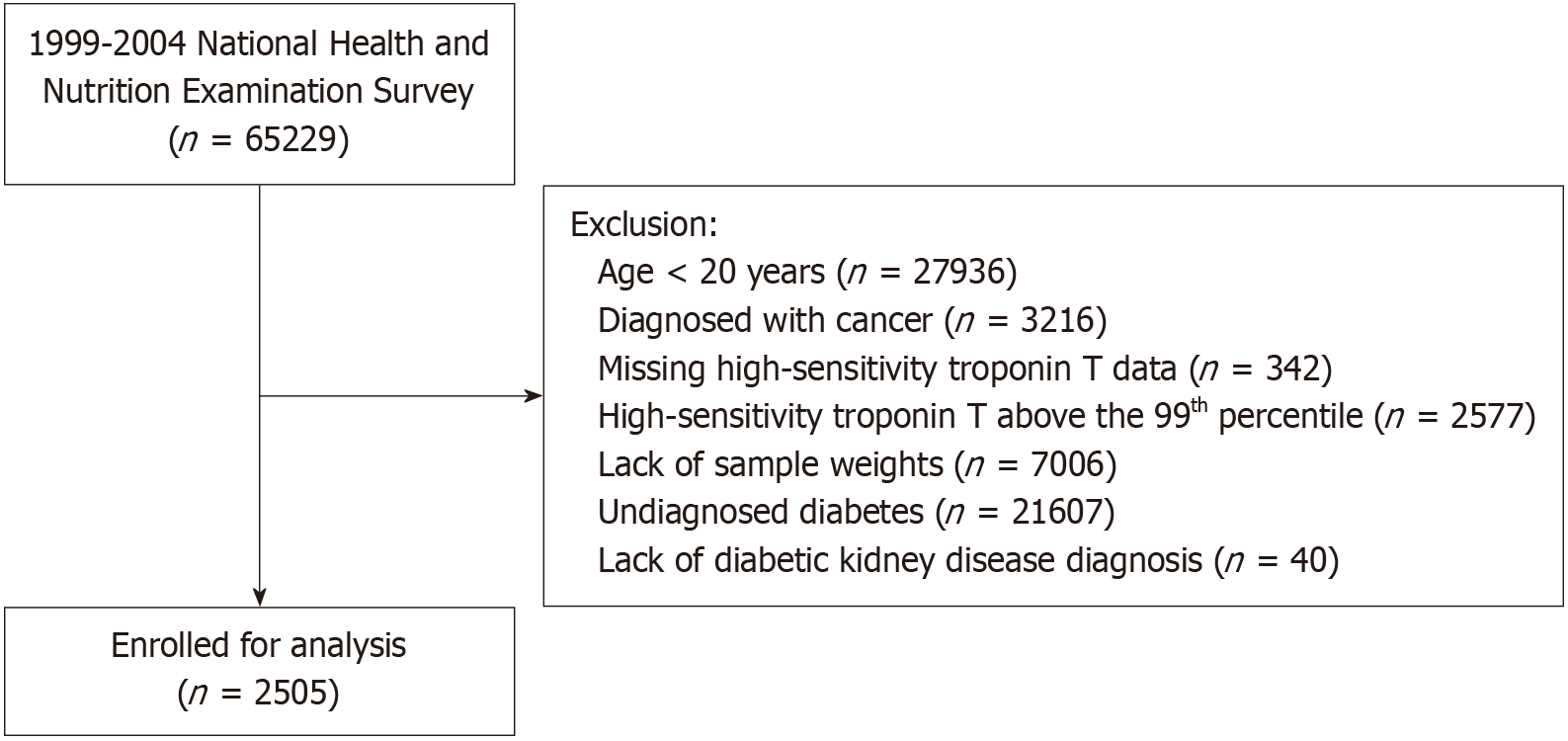
The NHANES program was designed to represent the United States population through a complex, stratified, multistage probability cluster sampling method, selecting participants from the noninstitutionalized civilian population. This study analyzed NHANES data obtained from 1999 to 2004, focusing on participants aged ≥ 20 years. The exclusion criteria included individuals with undiagnosed diabetes, baseline cancer, missing sample weights, or incomplete hs-TnT data. Additionally, participants with hs-TnT values exceeding the sex-specific 99th percentile of the upper reference limit were excluded. A final cohort of 2505 participants was included, with the detailed inclusion and exclusion process shown in Figure 1. The NHANES protocol and the measurement of hs-TnT in stored samples were approved by the Ethics Review Board of the National Centre for Health Statistics. Written informed consent was obtained from all participants.
hs-TnT levels were measured using Elecsys reagents on the Roche Cobas e601 platform, which received Food and Drug Administration clearance on January 18, 2016. The lower limit of detection (LoD) for this fifth-generation assay is 3 ng/L. The sex-specific 99th percentile upper reference limits for hs-TnT, as defined by the International Federation of Clinical Chemistry and Laboratory Medicine[12], are 14 ng/L for females and 22 ng/L for males when using the Roche hs-TnT assay[12].
Diabetes mellitus was defined based on one or more of the following criteria: (1) A prior diagnosis by healthcare professionals; (2) Fasting plasma glucose levels of ≥ 7.0 mmol/L; (3) Glycosylated haemoglobin (HbA1c) levels of ≥ 6.5%; and (4) The current use of diabetes medications. Albuminuria was evaluated using the urine albumin-to-creatinine ratio, while estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula. DKD in patients with T2DM was diagnosed if albumin-to-creatinine ratio was ≥ 30 mg/g and/or eGFR was < 60 mL/minute/1.73 m²[13].
Covariates included age (years), sex (male or female), race/ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, other Hispanic, or other races including multi-racial), educational level (< 9 years, 9-13 years, or ≥ 13 years), household poverty income ratio (PIR), body mass index (BMI), smoking status (never smoker, current smoker, or former smoker), alcohol intake (never, moderate, or heavy), use of diabetes medications (none, other medications, insulin, or oral medications), and diabetes duration (< 10 years or ≥ 10 years). Hypertension was defined as systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure of ≥ 90 mmHg, self-reported hypertension, physician-diagnosed hypertension, or the use of antihypertensive medication. Self-reported cardiovascular disease (CVD) included congestive heart failure, angina, myocardial infarction, or coronary heart disease. Hyperlipidaemia was defined as triglyceride levels of ≥ 150 mg/dL (1.7 mmol/L), total cholesterol levels of ≥ 200 mg/dL (5.18 mmol/L), low-density lipoprotein cholesterol levels of ≥ 130 mg/dL (3.37 mmol/L), or high-density lipoprotein cholesterol levels of < 40 mg/dL (1.04 mmol/L) for men, and < 50 mg/dL (1.30 mmol/L) for women. Healthy Eating Index (HEI) scores were computed based on the HEI-2015 guidelines. Physical activity was defined as engaging in moderate- or vigorous-intensity sports, fitness programs, or recreational activities for more than 10 minutes per week. Participants were classified as inactive if they engaged in such activities for ≤ 10 minutes per week.
To account for the complex sampling design of NHANES, our analyses incorporated sample weights, clustering, and stratification to ensure valid and representative results. Weighted means were calculated for continuous variables, while weighted percentages were used for categorical variables. Statistical comparisons were conducted using analysis of variance or the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. The hs-TnT levels were categorized into tertiles: Tertile 1 (< 5.93 ng/L), tertile 2 (5.94-9.79 ng/L), and tertile 3 (9.80-21.88 ng/L). Weighted multivariable logistic regression analyses were used to assess the correlation between hs-TnT levels and DKD across three models: Model I: Adjusted for age and sex; Model II: Included additional adjustments for race/ethnicity, education level, PIR, BMI, smoking status, eGFR, and comorbidities, including hypertension, CVD, and hyperlipidaemia, as well as physical activity and alcohol intake; Model III: Further as adjusted for HbA1c, HEI, diabetes duration, and diabetes medication use. To investigate the potential nonlinear relationship between hs-TnT and DKD, a restricted cubic spline analysis with three knots was performed. Multiple imputation was used to address missing values in covariates.
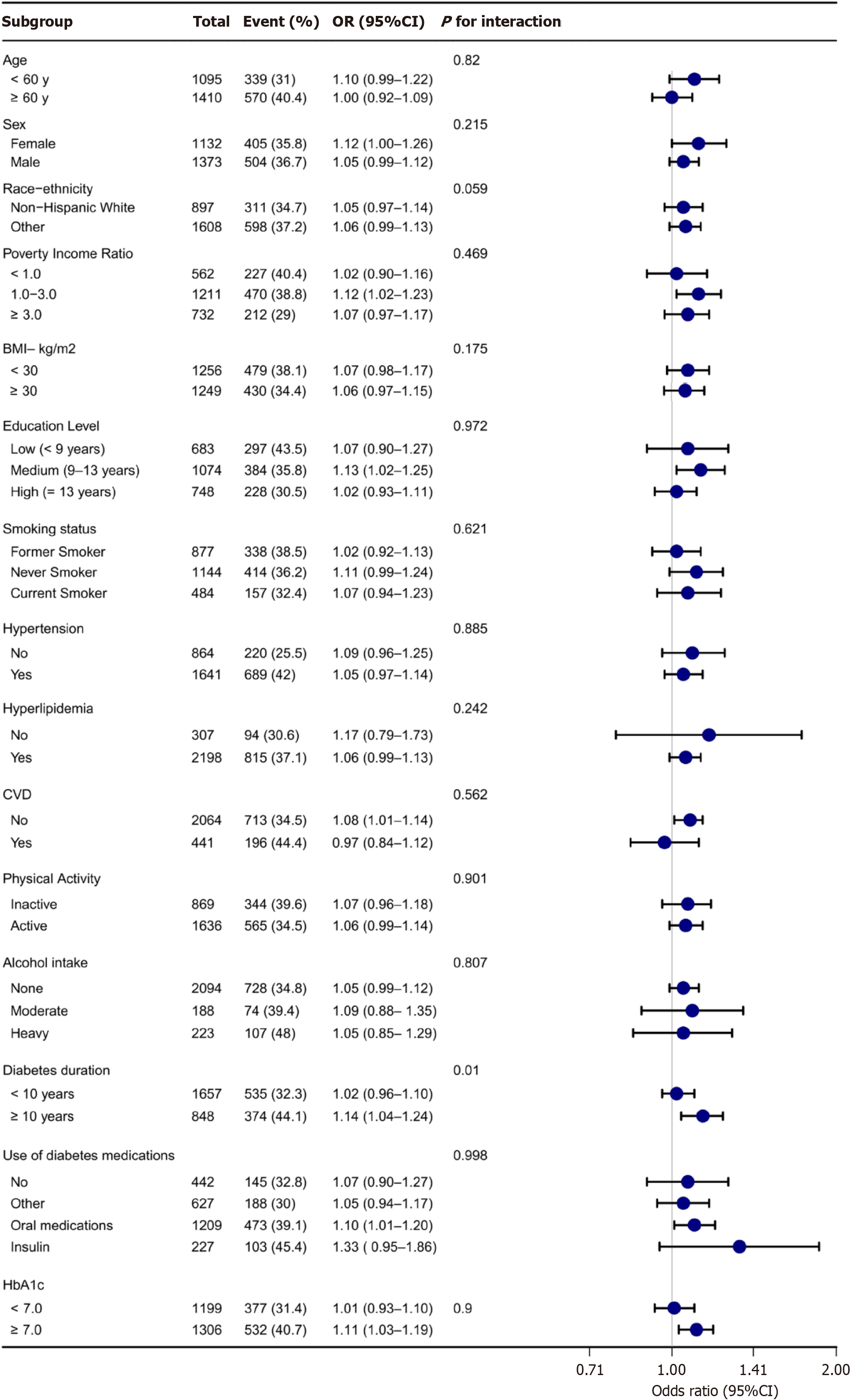
Stratified analyses were performed based on age (< 60 years or ≥ 60 years), sex (female or male), race (White or non-White), PIR (< 1.0, 1.0-3.0, or ≥ 3.0), BMI (< 30 kg/m² or ≥ 30.0 kg/m²), education level (< 9 years, 9-13 years, ≥ 13 years), smoking status (never smoker, former smoker, or current smoker), physical activity (inactive or active), alcohol intake (none, moderate, or heavy), diabetes duration (< 10 years or ≥ 10 years), use of diabetes medications (none, other, or insulin/oral medications), HbA1c (< 7.0% or ≥ 7.0%), and the presence of comorbid conditions including hypertension (yes or no), CVD (yes or no), and hyperlipidaemia (yes or no). Interaction terms between hs-TnT and these stratified variables were evaluated, and their significance was assessed using P values. The results of these analyses were visually presented in a forest plot. To assess the robustness of the findings, sensitivity analyses were conducted. First, compa
Among the final cohort of 2505 individuals with diabetes, the mean age was 55.02 (standard error: 0.72) years , and 44.87% of the participants were females. Baseline characteristics of the study population, stratified by hs-TnT tertiles, are detailed in Table 1. Participants with higher hs-TnT levels were more likely to be older, male, non-Hispanic white, and former smokers. They also exhibited higher rates of hypertension, CVD, and DKD. Additionally, these individuals tended to have a longer duration of diabetes, were more likely to use insulin, had more severe proteinuria, and demonstrated lower eGFRs.
| Variables | Total (n = 2505) | High-sensitivity troponin T (ng/L) | P value | ||
| < 5.93 | 5.94-9.79 | 9.80-21.88 | |||
| Tertile 1 (n = 839) | Tertile 2 (n = 831) | Tertile 3 (n = 835) | |||
| Age, years | 55.02 (0.72) | 47.64 (1.00) | 56.69 (1.09) | 62.99 (0.93) | < 0.001 |
| Sex | < 0.001 | ||||
| Female | 1132 (44.87) | 569 (63.57) | 359 (38.07) | 204 (27.68) | - |
| Male | 1373 (55.13) | 270 (36.43) | 472 (61.93) | 631 (72.32) | - |
| Race-ethnicity | 0.010 | ||||
| Mexican American | 798 (8.10) | 313 (11.07) | 277 (6.98) | 208 (5.43) | - |
| Non-Hispanic black | 537 (14.49) | 178 (16.02) | 159 (12.53) | 200 (14.71) | - |
| Non-Hispanic white | 897 (60.58) | 234 (52.97) | 286 (60.22) | 377 (71.23) | - |
| Other Hispanic | 158 (8.53) | 67 (9.92) | 64 (9.97) | 27 (4.95) | - |
| Other race | 115 (8.31) | 47 (10.02) | 45 (10.30) | 23 (3.68) | - |
| Poverty income ratio | 2.62 (0.08) | 2.63 (0.15) | 2.72 (0.12) | 2.48 (0.11) | 0.220 |
| BMI, kg/m2 | 32.62 (0.50) | 33.05 (0.61) | 32.69 (0.90) | 31.96 (0.52) | 0.306 |
| Education level | 0.108 | ||||
| Low (< 9 years) | 683 (11.73) | 216 (11.02) | 232 (10.82) | 235 (13.74) | - |
| Medium (9-13 years) | 1074 (47.90) | 362 (46.04) | 334 (44.80) | 378 (54.02) | - |
| High (≥ 13 years) | 748 (40.37) | 261 (42.94) | 265 (44.38) | 222 (32.25) | - |
| Smoking status | 0.030 | ||||
| Never smoker | 1144 (45.76) | 446 (51.51) | 360 (41.46) | 338 (43.06) | - |
| Former smoker | 877 (32.26) | 214 (25.21) | 306 (34.64) | 357 (38.95) | - |
| Current smoker | 484 (21.98) | 179 (23.28) | 165 (23.90) | 140 (17.99) | - |
| Comorbidity | |||||
| Hyperlipidemia | 0.948 | ||||
| No | 307 (10.33) | 92 (10.00) | 97 (10.78) | 118 (10.24) | - |
| Yes | 2198 (89.67) | 747 (90.00) | 734 (89.22) | 717 (89.76) | - |
| Hypertension | 0.001 | ||||
| No | 864 (39.05) | 375 (48.26) | 286 (40.55) | 203 (24.92) | - |
| Yes | 1641 (60.95) | 464 (51.74) | 545 (59.45) | 632 (75.08) | - |
| CVD | < 0.001 | ||||
| No | 2064 (83.38) | 767 (90.46) | 702 (87.41) | 595 (69.16) | - |
| Yes | 441 (16.62) | 72 (9.54) | 129 (12.59) | 240 (30.84) | - |
| Alcohol intake | 0.510 | ||||
| None | 2094 (79.83) | 703 (79.11) | 683 (77.18) | 708 (83.89) | - |
| Moderate | 188 (10.27) | 56 (9.83) | 75 (12.65) | 57 (8.10) | - |
| Heavy | 223 (9.90) | 80 (11.07) | 73 (10.17) | 70 (8.00) | - |
| Physical activity | 0.060 | ||||
| Inactive | 869 (28.65) | 285 (26.14) | 285 (25.77) | 299 (35.41) | - |
| Active | 1636 (71.35) | 554 (73.86) | 546 (74.23) | 536 (64.59) | - |
| Diabetes duration | < 0.001 | ||||
| < 10 years | 1657 (69.37) | 653 (80.08) | 548 (70.91) | 456 (53.18) | - |
| ≥ 10 years | 848 (30.63) | 186 (19.92) | 283 (29.09) | 379 (46.82) | - |
| Use of diabetes medications | 0.001 | ||||
| No | 442 (18.47) | 200 (24.69) | 154 (18.38) | 88 (10.23) | - |
| Other1 | 627 (26.07) | 196 (26.25) | 224 (27.90) | 207 (23.69) | - |
| Insulin | 227 (9.70) | 62 (7.64) | 71 (7.50) | 94 (15.03) | - |
| Oral medications | 1209 (45.76) | 381 (41.42) | 382 (46.21) | 446 (51.05) | - |
| ACR category | 0.004 | ||||
| A1 (< 30 mg/g) | 1758 (73.89) | 617 (77.88) | 616 (75.15) | 525 (67.04) | - |
| A2 (30-300 mg/g) | 600 (21.64) | 209 (20.30) | 174 (21.96) | 217 (23.07) | - |
| A3 (≥ 300 mg/g) | 147 (4.47) | 13 (1.82) | 41 (2.89) | 93 (9.89) | - |
| Diabetic kidney disease | < 0.001 | ||||
| No | 1596 (67.66) | 612 (77.51) | 556 (68.63) | 428 (53.28) | - |
| Yes | 909 (32.34) | 227 (22.49) | 275 (31.37) | 407 (46.72) | - |
| HbA1c (%) | 7.56 (0.09) | 7.55 (0.18) | 7.55 (0.11) | 7.59 (0.14) | 0.977 |
| Healthy eating index | 51.34 (0.71) | 50.36 (1.20) | 52.11 (1.07) | 51.77 (0.93) | 0.409 |
| eGFR (mL/minute/1.73 m2) | 89.94 (0.79) | 100.45 (1.33) | 88.53 (1.48) | 77.47 (1.38) | < 0.001 |
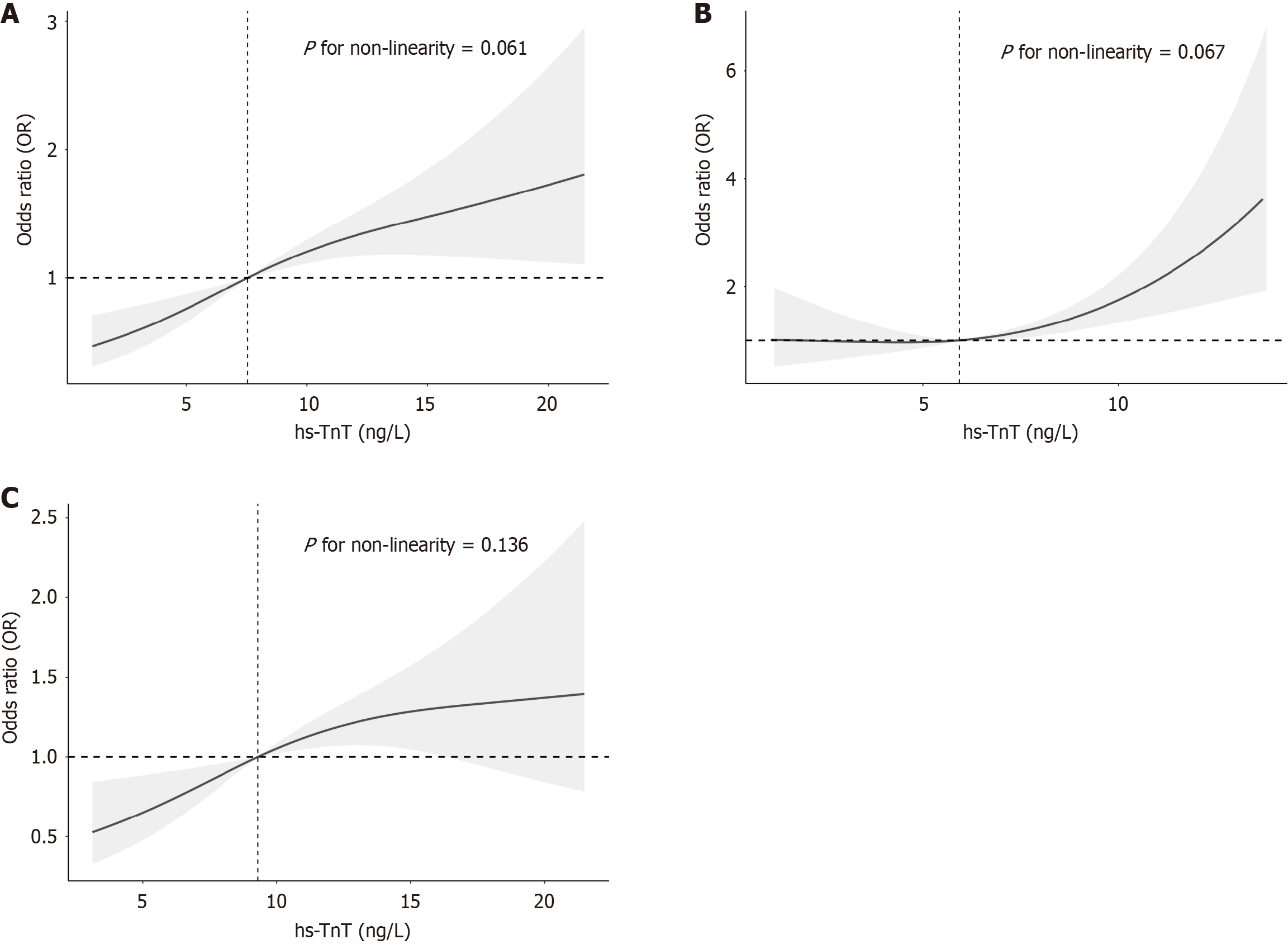
In Model III of the weighted multivariate logistic regression analysis, a linear relationship between hs-TnT levels and DKD was observed. Compared to tertile 1 (< 5.93 ng/L), the odds ratios for tertile 2 (5.94-9.79 ng/L) and tertile 3 (9.80-21.88 ng/L) were 1.25 [95% confidence interval (CI): 0.77-2.02] and 2.07 (95%CI: 1.13-3.80), respectively, with a significant trend (P for trend = 0.022). When hs-TnT was analyzed as a continuous variable, the odds ratio was 1.07 (95%CI: 1.01-1.13). Smoothed curve fitting further supported a linear association between hs-TnT and DKD across the total population (P for nonlinearity = 0.061), as well as among males (P for nonlinearity = 0.136) and females (P for nonlinearity = 0.067) (Figure 2 and Table 2).
| Variable | DKD/total | Non-adjusted model | P value | Model I | P value | Model II | P value | Model III | P value |
| n | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||
| Continuous variable | |||||||||
| hs-troponin T (ng/L) | 909/2505 | 1.11 (1.06-1.16) | < 0.001 | 1.11 (1.05-1.16) | < 0.001 | 1.08 (1.02-1.15) | 0.017 | 1.07 (1.01-1.13) | 0.030 |
| Binary variable | |||||||||
| Tertile 1 (< 5.93) | 227/839 | Reference | - | Reference | - | Reference | - | Refrence | - |
| Tertile 2 (5.94-9.79) | 275/831 | 1.58 (1.00-2.48) | 0.048 | 1.51 (0.91-2.50) | 0.110 | 1.32 (0.82-2.13) | 0.239 | 1.25 (0.77-2.02) | 0.350 |
| Tertile 3 (9.80-21.88) | 407/835 | 3.02 (1.87-4.88) | < 0.001 | 2.80 (1.62-4.83) | < 0.001 | 2.30 (1.20-4.41) | 0.015 | 2.07 (1.13-3.80) | 0.022 |
| P for trend | - | - | < 0.001 | - | < 0.001 | - | 0.015 | - | 0.022 |
Stratified analysis across various demographic and clinical factors, including age, sex, race, PIR, BMI, education level, smoking status, physical activity, alcohol intake, diabetes duration, use of diabetes medications, HbA1c, hypertension, CVD, and hyperlipidaemia, did not reveal significant modifications to the linear relationship between hs-TnT levels and DKD (Figure 3). However, within the subgroup of individuals with a diabetes duration of ≥ 10 years, a higher risk of DKD was associated with increasing hs-TnT levels. No significant interactions were observed within the other stratified variables (Figure 3). Comparisons between the complete dataset and the dataset with imputed covariates showed consistent findings (Supplementary Table 1). Similarly, excluding data points where hs-TnT was < LoD yielded com
In this large, cross-sectional study using nationally representative NHANES data, a linear association was observed between hs-TnT levels below the 99th percentile and an increased risk of DKD among United States adults with T2DM. This relationship persisted in the overall population and was consistent when analyzed separately in males and females. Additionally, the findings were robust across multiple stratified and sensitivity analyses. Our results build on previous research investigating the association between troponin levels and kidney outcomes in individuals with diabetes. For example, a secondary analysis of the ADVANCE trial demonstrated that elevated troponin levels were associated with an increased risk of new or worsening kidney disease in individuals with diabetes and CVD[8]. Similarly, Bidadkosh et al[9] reported a significant association between elevated hs-TnT levels and renal events in patients with type 2 diabetes and nephropathy. Findings from the Edinburgh type 2 diabetes study further supported this association, linking hs-TnT levels to incident kidney disease[14]. Moreover, Anderson et al[15] identified worse kidney function associated with hs-TnT even among individuals without diabetes. Our study contributes to this growing body of evidence by highlighting a linear association between hs-TnT levels below the 99th percentile and DKD in a large, nationally representative sample of United States adults with diabetes. These results suggest that even slight elevations in hs-TnT, potentially indicative of subclinical myocardial injury, are associated with renal dysfunction in this high-risk population.
The mechanisms underlying the observed association between hs-TnT and DKD are complex and multifactorial, likely involving direct and indirect pathways that link cardiac injury to renal dysfunction. One potential explanation is the concept of heart-kidney crosstalk, supported by animal studies demonstrating that myocardial damage in mild renal impairment could exacerbate glomerular sclerosis and proteinuria[16]. These findings suggest that cardiac injury might directly contribute to kidney damage through haemodynamic alterations, neurohumoral activation, or other mediators. Alternatively, elevated hs-TnT levels might reflect systemic processes driving the progression of cardiac and renal diseases. Neurohormonal activation, chronic inflammation, and oxidative stress, which are prevalent pathogenic me
Although the precise mechanisms remain to be fully elucidated, our findings underscore a complex interplay between subclinical cardiac injury and kidney dysfunction in individuals with diabetes. Further research integrating mechanistic studies, longitudinal designs, and multimodal evaluations of cardiovascular and renal health is crucial to unravel these pathophysiological connections. Such efforts might facilitate the development of novel therapeutic strategies addressing cardiac and renal risks in this high-risk population. Our findings highlight the prognostic value of hs-TnT in individuals with diabetes, even at levels below the 99th percentile typically used for diagnosing acute myocardial infarction. This indicates its potential as a marker of subclinical CVD and its associated renal risks. Incorporating hs-TnT into risk stratification models could help identify patients with diabetes who might benefit from more intensive cardiorenal protective interventions. From a public health standpoint, our results highlight the substantial burden of subclinical cardiovascular injury and DKD among adults with diabetes in the United States. Given the high prevalence of diabetes and its associated morbidity, mortality, and healthcare costs, there is an urgent need for early detection and intervention strategies to address these interconnected complications. Population-level strategies focusing on improving glycaemic control, optimising cardiac health, and preventing or delaying DKD onset are crucial.
The strengths of our study include its large, nationally representative sample, comprehensive assessment of potential confounders, and the robustness of findings across numerous sensitivity analyses. However, several limitations should be acknowledged. The cross-sectional design limits the ability to infer temporality and causality. Additionally, our analysis was based on a single hs-TnT measurement, which might not reflect long-term or dynamic changes in subclinical cardiac injury. Although extensive adjustments were made, the possibility of residual confounding cannot be excluded. Future research should focus on the longitudinal trajectories of hs-TnT and kidney function among individuals with diabetes, as well as the underlying mechanisms linking these variables. Furthermore, studies should assess whether hs-TnT could enhance clinical prediction models to improve DKD risk stratification and inform therapeutic strategies. Ultimately, randomized controlled trials will be necessary to determine whether interventions targeting hs-TnT levels could lead to improved renal outcomes in at-risk diabetic populations.
In conclusion, our cross-sectional analysis of adults with diabetes in the United States demonstrates a robust linear association between hs-TnT levels below the 99th percentile and DKD. These findings suggest that subclinical myocardial injury might contribute to DKD pathogenesis, highlighting the close relationship between cardiac and kidney health in individuals with diabetes. Assessing hs-TnT as a novel cardiorenal risk marker and exploring its potential as a thera
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5938] [Article Influence: 989.7] [Reference Citation Analysis (8)] |
| 2. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 581] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 3. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1923] [Article Influence: 320.5] [Reference Citation Analysis (0)] |
| 4. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 2495] [Article Influence: 356.4] [Reference Citation Analysis (1)] |
| 5. | Parikh RH, Seliger SL, de Lemos J, Nambi V, Christenson R, Ayers C, Sun W, Gottdiener JS, Kuller LH, Ballantyne C, deFilippi CR. Prognostic Significance of High-Sensitivity Cardiac Troponin T Concentrations between the Limit of Blank and Limit of Detection in Community-Dwelling Adults: A Metaanalysis. Clin Chem. 2015;61:1524-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Michel L, Jehn S, Dykun I, Anker MS, Ferdinandy P, Dobrev D, Rassaf T, Mahabadi AA, Totzeck M. Detectable troponin below the 99(th) percentile predicts survival in patients undergoing coronary angiography. Int J Cardiol Heart Vasc. 2024;52:101419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Roointan A, Shafieizadegan S, Ghaeidamini M, Gheisari Y, Hudkins KL, Gholaminejad A. The potential of cardiac biomarkers, NT-ProBNP and troponin T, in predicting the progression of nephropathy in diabetic patients: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2023;204:110900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 8. | Welsh P, Woodward M, Hillis GS, Li Q, Marre M, Williams B, Poulter N, Ryan L, Harrap S, Patel A, Chalmers J, Sattar N. Do cardiac biomarkers NT-proBNP and hsTnT predict microvascular events in patients with type 2 diabetes? Results from the ADVANCE trial. Diabetes Care. 2014;37:2202-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Bidadkosh A, Lambooy SPH, Heerspink HJ, Pena MJ, Henning RH, Buikema H, Deelman LE. Predictive Properties of Biomarkers GDF-15, NTproBNP, and hs-TnT for Morbidity and Mortality in Patients With Type 2 Diabetes With Nephropathy. Diabetes Care. 2017;40:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63:S63-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 895] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 12. | The International Federation of Clinical Chemistry and Laboratory Medicine. HighSensitivity Cardiac Troponin I and T Assay Analytical Characteristics Designated by Manufacturer IFCC Committee on Clinical Applications of Cardiac Bio-markers (C-CB) v092021. 2022. Available from: https://ifcc.web.insd.dk/media/479205/high-sensitivity-cardiac-troponin-i-and-t-assay-analytical-characteristics-designated-by-manufacturer-v092021-3.pdf. |
| 13. | de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 758] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 14. | Jenks SJ, Conway BR, McLachlan S, Teoh WL, Williamson RM, Webb DJ, Welsh P, Sattar N, Strachan MWJ, Price JF. Cardiovascular disease biomarkers are associated with declining renal function in type 2 diabetes. Diabetologia. 2017;60:1400-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Anderson AH, Xie D, Wang X, Baudier RL, Orlandi P, Appel LJ, Dember LM, He J, Kusek JW, Lash JP, Navaneethan SD, Ojo A, Rahman M, Roy J, Scialla JJ, Sondheimer JH, Steigerwalt SP, Wilson FP, Wolf M, Feldman HI; CRIC Study Investigators. Novel Risk Factors for Progression of Diabetic and Nondiabetic CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2021;77:56-73.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | van Dokkum RP, Eijkelkamp WB, Kluppel AC, Henning RH, van Goor H, Citgez M, Windt WA, van Veldhuisen DJ, de Graeff PA, de Zeeuw D. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J Am Soc Nephrol. 2004;15:3103-3110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Xiao Y, Wu QQ, Duan MX, Liu C, Yuan Y, Yang Z, Liao HH, Fan D, Tang QZ. TAX1BP1 overexpression attenuates cardiac dysfunction and remodeling in STZ-induced diabetic cardiomyopathy in mice by regulating autophagy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1728-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR, Zhang X, Wu QQ, Liao HH, Ni J, Tang QZ. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Grauslund J, Nybo M, Green A, Sjølie AK. N-terminal pro brain natriuretic peptide reflects long-term complications in type 1 diabetes. Scand J Clin Lab Invest. 2010;70:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Hellemons ME, Lambers Heerspink HJ, Gansevoort RT, de Zeeuw D, Bakker SJ. High-sensitivity troponin T predicts worsening of albuminuria in hypertension; results of a nested case-control study with confirmation in diabetes. J Hypertens. 2013;31:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771-2782b. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |