Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.102277
Revised: December 9, 2024
Accepted: January 3, 2025
Published online: March 15, 2025
Processing time: 98 Days and 3.2 Hours
The duodenum plays a significant role in metabolic regulation, and thickened mucous membranes are associated with insulin resistance. Duodenal mucosal resurfacing (DMR), a new-style endoscopic procedure using hydrothermal energy to ablate this thickened layer, shows promise for enhancing glucose and lipid metabolism in type 2 diabetes (T2D) patients. However, the mechanisms driving these improvements remain largely unexplored.
To investigate the mechanisms by which DMR improves metabolic disorders us
Rats with T2D underwent a revised DMR procedure via a gastric incision using a specialized catheter to abrade the duodenal mucosa. The duodenum was evaluated using histology, immunofluorescence, and western blotting. Serum assays measured glucose, lipid profiles, lipopolysaccharide, and intestinal hormones, while the gut microbiota and metabolomics profiles were analyzed through 16S rRNA gene sequencing and ultra performance liquid chromatography-mass spectrum/mass spectrum, severally.
DMR significantly improved glucose and lipid metabolic disorders in T2D rats. It increased the serum levels of cholecystokinin, gastric inhibitory peptide, and glucagon-like peptide 1, and reduced the length and depth of duodenal villi and crypts. DMR also enhanced the intestinal barrier integrity and reduced lipopolysaccharide translocation. Additionally, DMR modified the gut microbiome and metabolome, particularly affecting the Blautia genus. Correlation analysis revealed significant links between the gut microbiota, metabolites, and T2D phenotypes.
This study illustrates that DMR addresses metabolic dysfunctions in T2D through multifaceted mechanisms, highlighting the potential role of the Blautia genus on T2D pathogenesis and DMR’s therapeutic impact.
Core Tip: Duodenum is a particular metabolic signaling center, and the thickened mucous membranes cause duodenal dysfunction and promote insulin resistance. This study explored the mechanisms by which duodenal mucosal resurfacing (DMR) affects type 2 diabetes (T2D) using a rat model. It highlights the potential role of the Blautia genus in the pathogenesis of T2D and the therapeutic effect of DMR. The results provide a theoretical basis for performing DMR in humans with T2D and identify several areas requiring further research.
- Citation: Nie LJ, Cheng Z, He YX, Yan QH, Sun YH, Yang XY, Tian J, Zhu PF, Yu JY, Zhou HP, Zhou XQ. Role of duodenal mucosal resurfacing in controlling diabetes in rats. World J Diabetes 2025; 16(3): 102277
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/102277.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.102277
Influenced by genetics, environmental factors, stress, diet, lifestyle, and various other elements, the incidence of type 2 diabetes (T2D) is on the rise, with a projected global prevalence expected to reach 12.2% by 2045[1,2]. In individuals with T2D, damage to tissues and organs increases the incidence of complications, leading to increased morbidity and mortality and affecting both longevity and quality of life. Despite the availability of lifestyle interventions and advanced medical treatment for T2D, over half of affected individuals do not attain the treatment goal of achieving a glycemic hemoglobin level ≤ 53 mmol/mol[3]. Furthermore, current T2D treatment requires daily or weekly active interventions owing to the deterioration of β-cell function and the recurrence of hyperglycemia on treatment discontinuation[4]. Therefore, disease-modifying therapeutic interventions that can revolutionize the management of T2D are urgently needed.
The pathogenesis of T2D is intricate and multifaceted, with insulin resistance serving as the central mechanism[5]. Furthermore, the contribution of the stomach and small intestine to T2D pathogenesis is being increasingly recognized, contributing to the emergence of metabolic surgery as a viable option[6,7]. Recent research has underscored the duode
Considering the complexity of T2D pathogenesis, DMR might exert a regulatory effect on metabolic processes via multiple pathways. In this study, we applied the DMR procedure to diabetic rats and conducted an in-depth investigation of the potential underlying mechanisms. In our preliminary experiments, we found that the clinical endoscopic and hydrothermal ablation method used in humans is not suitable for use in diabetic rats owing to the narrow lumen and thin walls of the esophagus and intestine in rats. Based on a previous study[11], we designed a DMR catheter that uses mechanical wear to induce necrosis and detachment of the duodenal mucosa in diabetic rats to simulate hydrothermal ablation used in clinical practice. Our research revealed that mechanical wear, as well as hydrothermal ablation can significantly improve metabolic function in diabetic rats and we conducted a preliminary investigation into the underlying mechanisms.
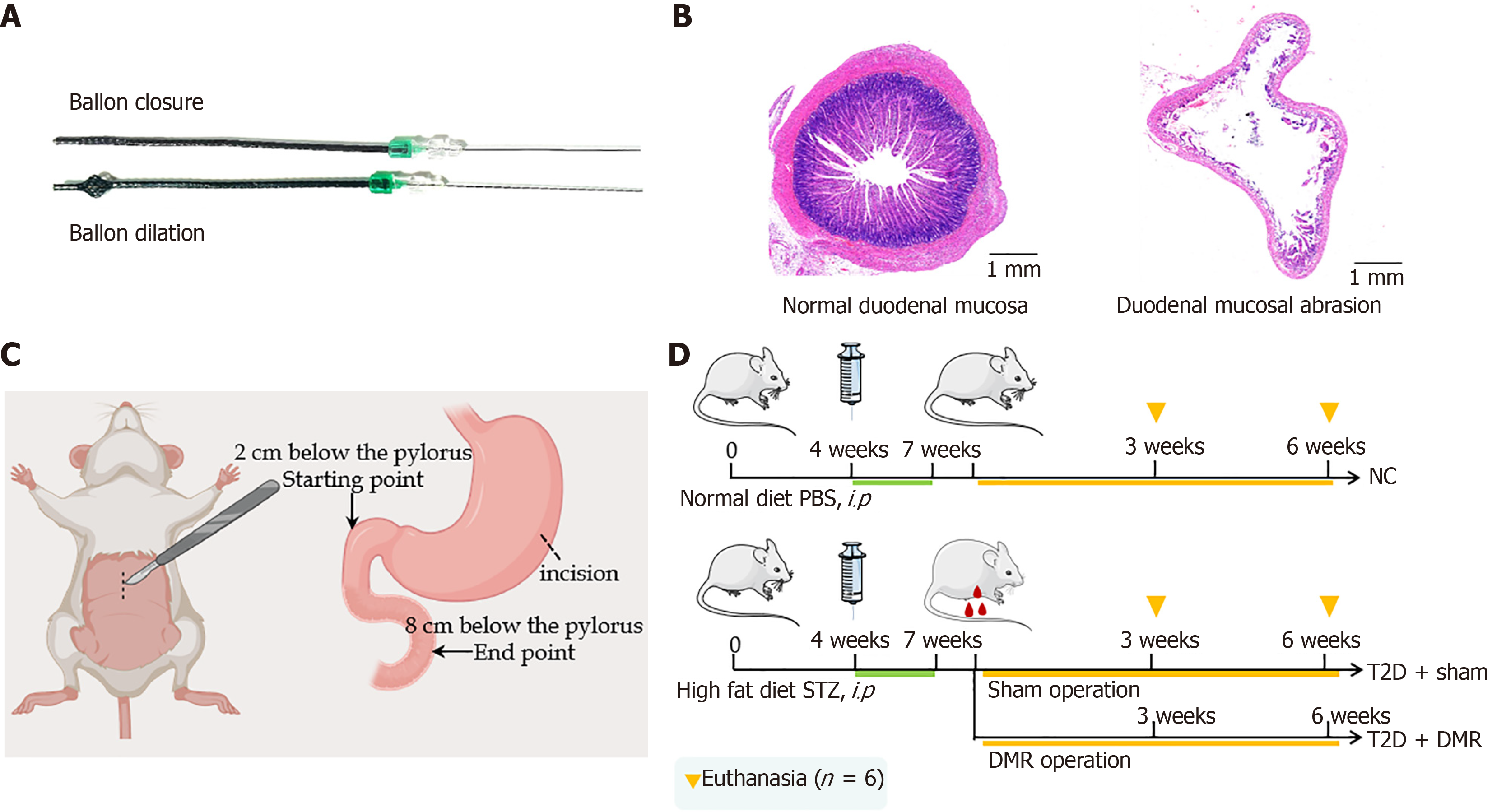
Six weeks old male Sprague-Dawley rats (200 ± 20 g) were purchased from Zhejiang Charies River Animal Technology Co., Ltd. [Jiaxing, China, permission No. SCXK (Zhejiang) 2019-0001]. The rats were housed in groups of three to four per cage under the specific pathogen free conditions (temperature: 22.5 ± 2.0 °C; relative humidity: 60% ± 10%; 12-hour light-dark cycle: 07:00-19:00). The rats were acclimated for 1 week before the experiment and then randomly assigned to normal control (NC) and T2D groups. The rats in the NC group (n = 12) consumed a standard rodent diet. The rats in the T2D group (n = 40) were fed a high-fat diet (HFD) (containing 45% fat, 35% carbohydrate, 20% protein; product code: XTHF45; Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd., Nanjing, Jiangsu Province, China) for 4 weeks to induce insulin resistance. The diabetic state is then induced by intraperitoneal injection of streptozotocin (STZ)
All appropriate measures were taken to minimize pain or discomfort to the animals. The Ethics Committee of the Jiangsu Center for Safety Evaluation of Drugs approved the study (No. IACUC-20220613-01, approval date: June 13, 2022) and all animal experiments were conducted in accordance with the ARRIVE guidelines.
Endoscopic and hydrothermal ablation methods are not suitable for use in rats owing to the narrow lumen and thin walls of the esophagus and duodenum; therefore, we chose gastric incision and physical mechanical wear of the duodenal mucosa. Based on previous studies[11], we designed a DMR catheter with an adjustable polyethylene terephthalate mesh balloon at the distal end, which had a certain friction force (Figure 1A). During the procedure, the balloon size was adjusted according to the size and degree of wear of the duodenal lumen. The abrasion area of the duodenum was approximately 80% without muscle layer damage, as shown in Figure 1B.
Isoflurane (RWD Life Science Co., Ltd., Shenzhen, China) was administered to the rats after a 12-hour fast. The DMR procedure was initiated using a 4-cm mid line incision. Then, a longitudinal incision, approximately 0.5 cm long, was made in the greater curvature of the stomach and the DMR catheter was inserted into the duodenum. Once positioned
Food intake, body weight, and FBG levels were checked weekly during study. All rats were fasted overnight and were euthanized at postoperative week 3 or 6 (six rats per group) (Figure 1D). Blood samples were taken through the abdominal aorta, and the serum was stored at -80 °C after centrifugation (4000 rpm/minute, 15 minutes). Duodenum, liver and colonic contents were collected for further analysis.
The rats were administered a glucose solution (1 g/kg) by oral gavage after overnight fasting. Blood glucose levels were measured using a glucose analyzer [OGM-161, ACON Biotech (Hangzhou) Co., Ltd., Hangzhou, Zhejiang Province, China] at 0, 15, 30, 60, and 120 minutes after glucose administration, and the area under curve of oral glucose tolerance test (OGTT) (AUCOGTT) was computed according to the data.
The insulin (Catalog CSB-E05070r), cholecystokinin (CCK) (Catalog CSB-E08114r), gastric inhibitory peptide (GIP) (Catalog CSB-E17969r), lipopolysaccharide (LPS) (Catalog CSB-E14247r), and glucagon-like peptide 1 (GLP-1) (Catalog CSB-E08117r) enzyme-linked immunosorbent assay kits were bought from Wuhan Huamei Biotech Co., Ltd. (Wuhan, Hubei Province, China). Following the instructions, these kits were used to measure the serum levels of CCK, GIP, LPS, GLP-1, and insulin at room temperature. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using a Dimension EXL200 fully automatic biochemical meter (Siemens AG, Berlin, Germany).
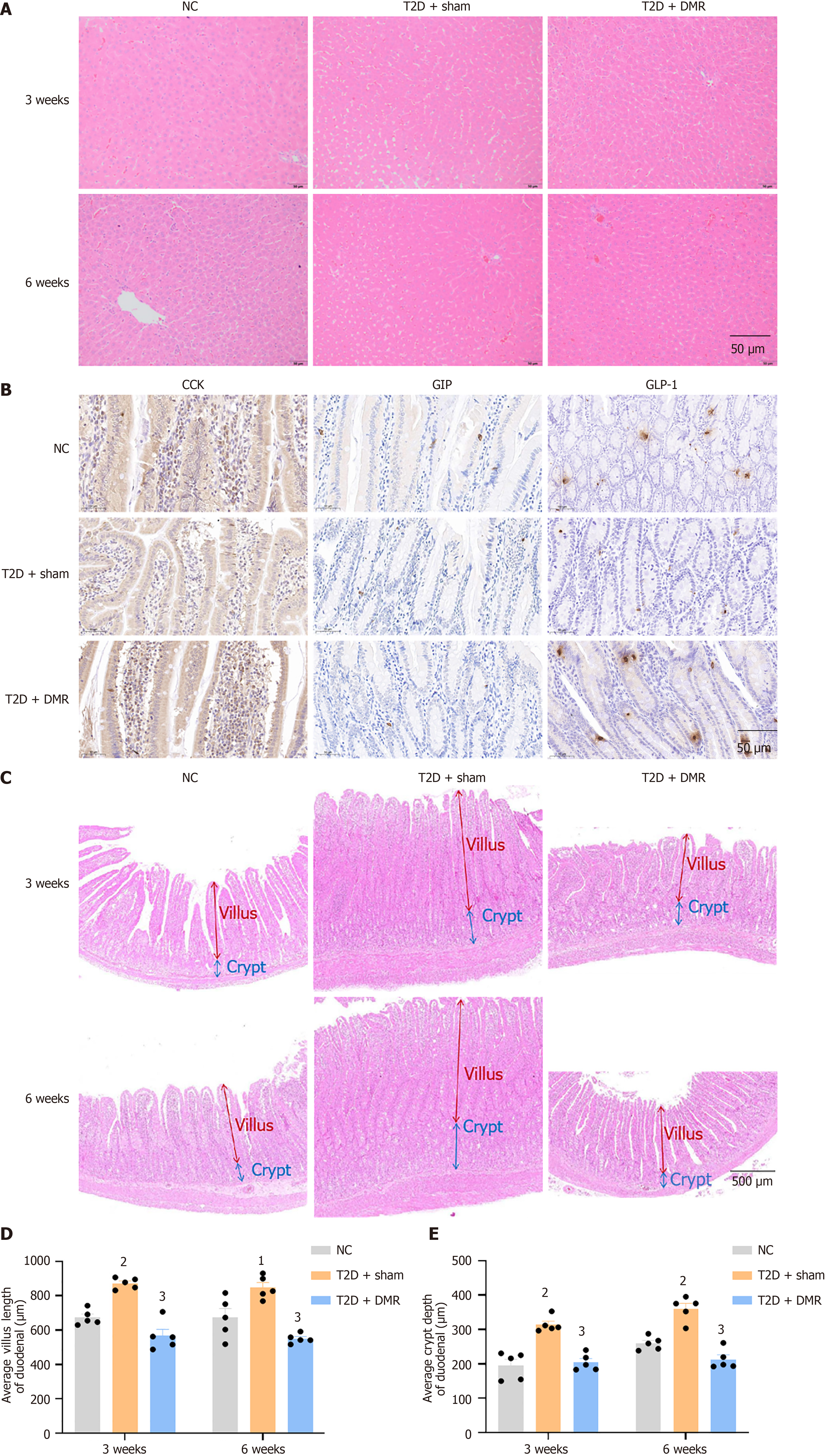
The liver and duodenal segments preserved in formalin were buried with paraffin, and sectioned with 5-μm-thick. Histological changes in the duodenum were assessed using hematoxylin and eosin (HE) staining. Images were collected using the Olympus CX21 optical binocular microscope (Olympus, Tokyo, Japan) equipped with a 5 × or 40 × objective lens. The length of the duodenal villi and depth of the crypts were measured using Aperio ImageScope software. For each rat (n = 5), ten intact villi and crypts were selected for measurement. The length of villi and depth of crypts were measured using a previously reported method and the mean values were calculated for each rat[17]. The villi were measured from the apex to the villus-crypt junction, while the crypts were measured from the base to the crypt-villus junction.
For protein extraction, the duodenal tissue was treated with RIPA buffer containing protease and phosphatase inhibitors. The protein concentration was checked by employing Bradford protein assay kit (Thermo Fisher, Catalog 23227). Then, the protein extract solution was added to the loading buffer (Biorbyt, Catalog orb90545) and boiled for denaturation. The proteins were electrophoretically separated on a BeyoGel plus PAGE prefabricated gel (Beyotime, 4%-15%, Catalog P0519S) and electro-imprinted on a polyvinylidene difluoride membrane (PALL, Catalog 65421). The 5% milk solution was used to block the membrane. The membrane was incubated overnight at 4 °C in a solution of occludin (1:1000, Abcam, Catalog ab216327), zonula occludens-1 (ZO-1) (1:5000, Santa Cruz, Catalog sc-33725) and β-actin (1:5000, Abcam, Catalog ab8226) antibody. The membranes were then incubated with horseradish peroxidase-conjugated secondary anti-rabbit antibodies (1:5000, Biosharp, Catalog BL003A). The blots were visualized using enhanced chemiluminescence reagent (Biosharp, Catalog BL523A). The Image J computer software analysis system was employed to normalize the β-actin signal and conduct semi-quantitative analysis.
For immunofluorescence staining, serial duodenal sections were incubated with antibodies against occludin (1:150; Abcam, Catalog ab216327) and ZO-1 (1:50; Santa Cruz Biotechnology, Catalog sc-33725), followed by the appropriate secondary antibody. The nuclei were counterdyed with 4’,6-diamidino-2-phenylindole (Sigma-Aldrich, Catalog MBD0020) at the end of the immunofluorescence procedure. Immunofluorescence images were obtained and analyzed using Image J software. Likewise, for immunohistochemically stained duodenal slices were incubated with antibodies against CCK (1:200, Absin, Catalog abs138035), GIP (1:100, Abcam, Catalog ab209792), and GLP-1 (1:2000, Starter, Catalog S0B0387).
Colonic content samples were collected from the NC, T2D-sham, and T2D-DMR rats at postoperative week 6 were for 16S rRNA gene sequencing. The extraction, library preparation, sequencing, and analysis were performed by Metabo-Profile Biotechnology Co., Ltd. (Shanghai, China). Targeted metabolomic analyses of the colonic contents were performed using the Q300 metabolite analysis kit (Human Metabolomics Institute, Inc., Shenzhen, Guangdong Province, China) based on a previously published method, with modifications[18]. The specific experimental details were as described previously[19].
Statistical analysis was finished employing statistical product and service solutions 21.0 Version. All quantitative data were shown as mean ± SE. AUCOGTT were computed by trapezoidal integration. Comparisons between more than two groups were performed using one-way analysis of variance (ANOVA). For comparison between two groups, we have used the Student’s t test (two-tailed). The correlations between gut microbiota, metabolites and T2D-related traits were analyzed employing Spearman’s correlation analysis. P < 0.05 was thought to be statistically meaning.
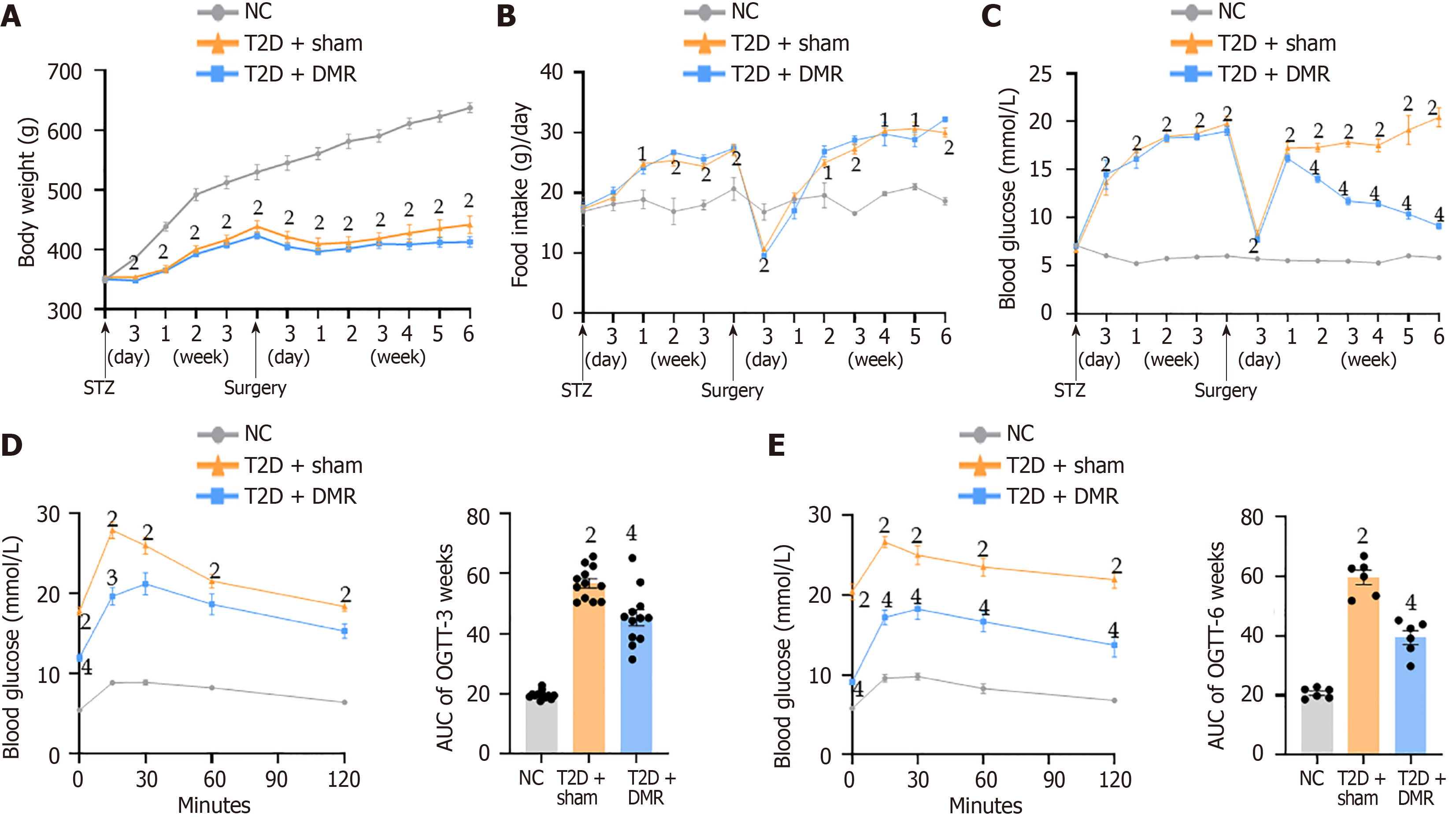
The DMR and sham surgeries were performed on the T2D rats according to the study protocol. The post-surgery body weight and food intake were comparable between the DMR and sham groups (Figure 2A and B). Both the DMR and sham groups exhibited a decline in their FBG levels on day 3 after surgery. Subsequently, the FBG levels in the sham group continued to escalate, whereas those in the DMR group decreased. From postoperative week 2, the FBG levels of T2D rats were significantly decreased by DMR procedure (all P < 0.05; Figure 2C). Furthermore, DMR procedure improved glucose tolerance at postoperative weeks 3 and 6, as evidenced by the lower values of AUCOGTT (all P < 0.05; Figure 2D and E). Additionally, the DMR procedure led to increased serum insulin levels at postoperative week 6 (P < 0.01; Table 1). Collectively, these findings indicate that the DMR procedure significantly ameliorated metabolic disorders in blood glucose in T2D rats.
| Variable | Preoperative | Postoperative week 3 | Postoperative week 6 | ||||||
| NC | T2D + sham | T2D + DMR | NC | T2D + sham | T2D + DMR | NC | T2D + sham | T2D + DMR | |
| Insulin (μIU/mL) | 18.86 ± 1.34 | 13.24 ± 1.082 | 13.78 ± 1.361 | 19.49 ± 1.15 | 14.62 ± 1.501 | 15.79 ± 1.07 | 19.67 ± 1.02 | 12.76 ± 1.312 | 18.07 ± 1.045 |
| HDL-C (mmol/L) | 1.39 ± 0.26 | 0.60 ± 0.132 | 0.59 ± 0.102 | 1.54 ± 0.25 | 0.66 ± 0.132 | 0.88 ± 0.152 | 1.52 ± 0.12 | 0.66 ± 0.162 | 1.03 ± 0.21 |
| LDL-C (mmol/L) | 0.96 ± 0.15 | 1.71 ± 0.231 | 1.67 ± 0.221 | 1.09 ± 0.16 | 1.68 ± 0.26 | 1.37 ± 0.20 | 1.06 ± 0.15 | 1.67 ± 0.231 | 1.10 ± 0.114 |
| TG (mmol/L) | 1.45 ± 0.19 | 2.15 ± 0.231 | 2.23 ± 0.261 | 1.60 ± 0.17 | 2.05 ± 0.21 | 1.87 ± 0.22 | 1.67 ± 0.19 | 2.37 ± 0.231 | 1.78 ± 0.144 |
| TC (mmol/L) | 1.67 ± 0.19 | 2.43 ± 0.211 | 2.45 ± 0.331 | 1.47 ± 0.25 | 2.39 ± 0.261 | 2.12 ± 0.25 | 1.76 ± 0.25 | 2.54 ± 0.141 | 1.74 ± 0.244 |
| FFA (mmol/L) | 0.51 ± 0.13 | 1.41 ± 0.212 | 1.42 ± 0.222 | 0.52 ± 0.06 | 1.30 ± 0.232 | 1.11 ± 0.101 | 0.51 ± 0.08 | 1.39 ± 0.282 | 0.98 ± 0.15 |
| AST (U/L) | 234.00 ± 28.21 | 314.50 ± 20.941 | 316.00 ± 21.491 | 222.17 ± 33.79 | 315.67 ± 28.901 | 304.67 ± 21.67 | 238.5 ± 24.66 | 325.17 ± 26.711 | 262.67 ± 19.49 |
| ALT (U/L) | 89.33 ± 14.19 | 169.17 ± 22.511 | 183.17 ± 25.082 | 88.67 ± 11.95 | 179.83 ± 20.492 | 156.33 ± 15.01 | 90.50 ± 12.86 | 181.33 ± 28.141 | 141.50 ± 21.61 |
| GLP-1 (pg/mL) | 2.37 ± 0.24 | 1.50 ± 0.171 | 1.48 ± 0.251 | 2.67 ± 0.15 | 1.62 ± 0.152 | 1.70 ± 0.222 | 2.63 ± 0.37 | 1.67 ± 0.151 | 2.30 ± 0.234 |
| GIP (pg/mL) | 103.36 ± 14.26 | 39.77 ± 4.553 | 40.90 ± 5.073 | 111.47 ± 17.48 | 40.34 ± 6.182 | 62.90 ± 7.891 | 97.89 ± 14.54 | 38.27 ± 7.262 | 72.74 ± 9.974 |
| CCK (pg/mL) | 26.61 ± 2.86 | 13.61 ± 2.432 | 15.52 ± 2.372 | 28.20 ± 3.21 | 15.02 ± 1.642 | 20.67 ± 1.931 | 28.32 ± 4.75 | 12.85 ± 2.561 | 22.92 ± 2.994 |
After consuming a HFD and STZ intraperitoneal injection, the serum LDL-C, TC, TG, free fatty acids, AST, and ALT levels increased significantly and HDL-C levels decreased (all P < 0.05; Table 1). These metabolic changes improved after the DMR procedure. For instance, the serum LDL-C and TC levels of T2D rats in the DMR group were signally decreased at postoperative week 6 (all P < 0.05; Table 1). Additionally, HE staining revealed that the DMR procedure reduced lipid deposition in the livers of T2D rats (Figure 3A). Collectively, these results highlight the ability of the DMR procedure to improve lipid metabolism in T2D rats.
Intestinal hormones play an important role in metabolic surgery[20]. The serum levels of GLP-1, GIP, and CCK decreased significantly after HFD feeding and STZ intraperitoneal injection. However, DMR procedure significantly increased the levels of these hormones (all P < 0.05; Table 1). Additionally, DMR procedure increased the number of CCK-positive cells and GLP-1-positive cells at postoperative week 6 (Figure 3B).
As shown in Figure 3C-E, histological findings showed increased duodenal villus length and crypt depth in diabetes rats. However, DMR procedure reversed these changes. The duodenum is an important part for the digestion and absorption of nutrients. These results suggest that DMR procedure may lessen nutrient absorption by reducing villus length and crypt depth, thereby improving glucose and lipid parameters.
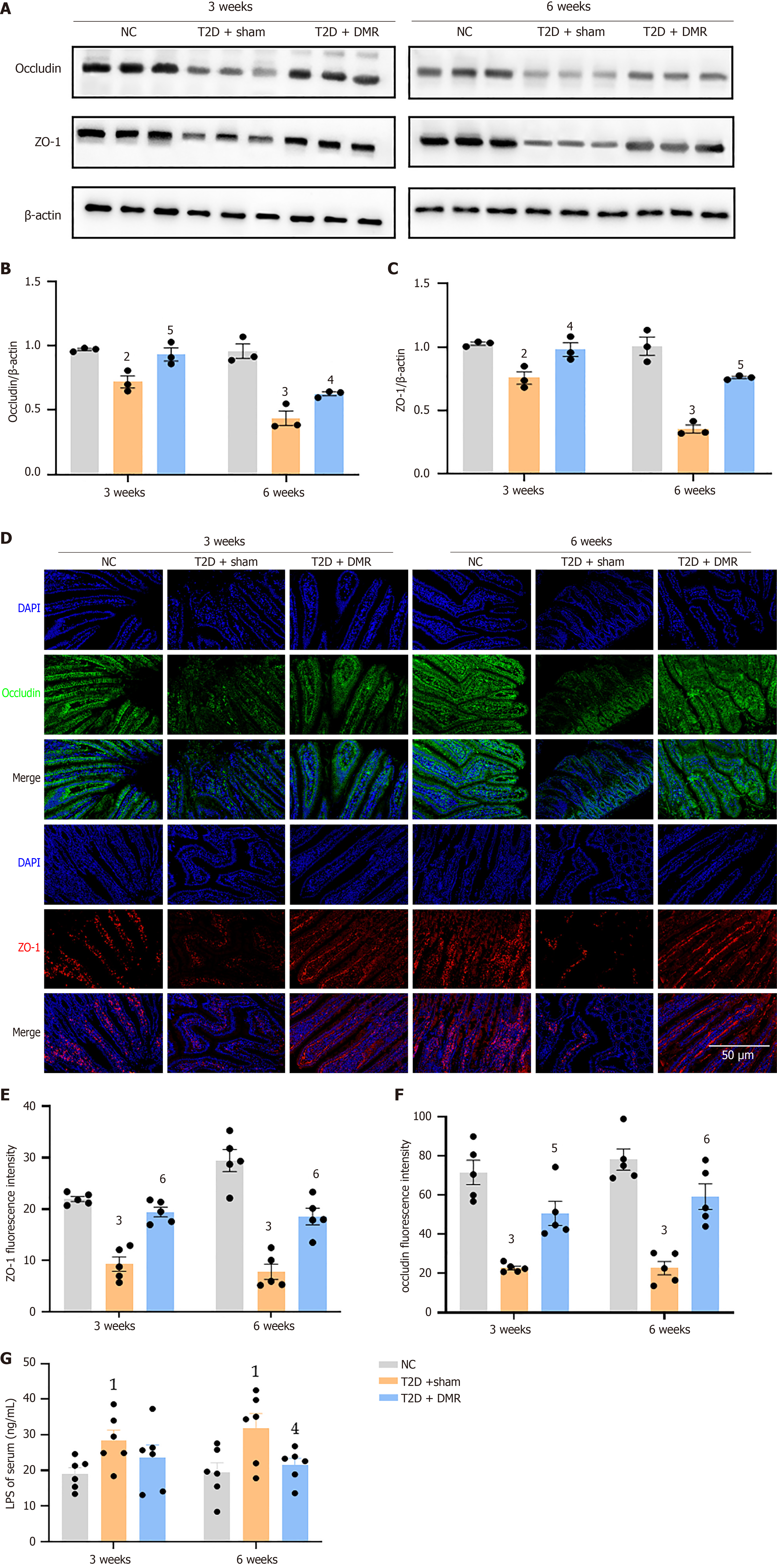
The expression levels of the tight junction proteins in the duodenum were estimated using western blot and immunofluorescence. Western blot indicated that occludin and ZO-1 expression was increased by the DMR procedure (all P < 0.05; Figure 4A-C). Immunofluorescence showed similar results (all P < 0.05; Figure 4D-F). In addition, the serum LPS levels of rats in the T2D-sham group were increased, and the DMR procedure reduced serum LPS levels at postoperative week 6 (P < 0.05; Figure 4G). These results indicate that the DMR procedure significantly enhanced duodenal mucosal barrier.
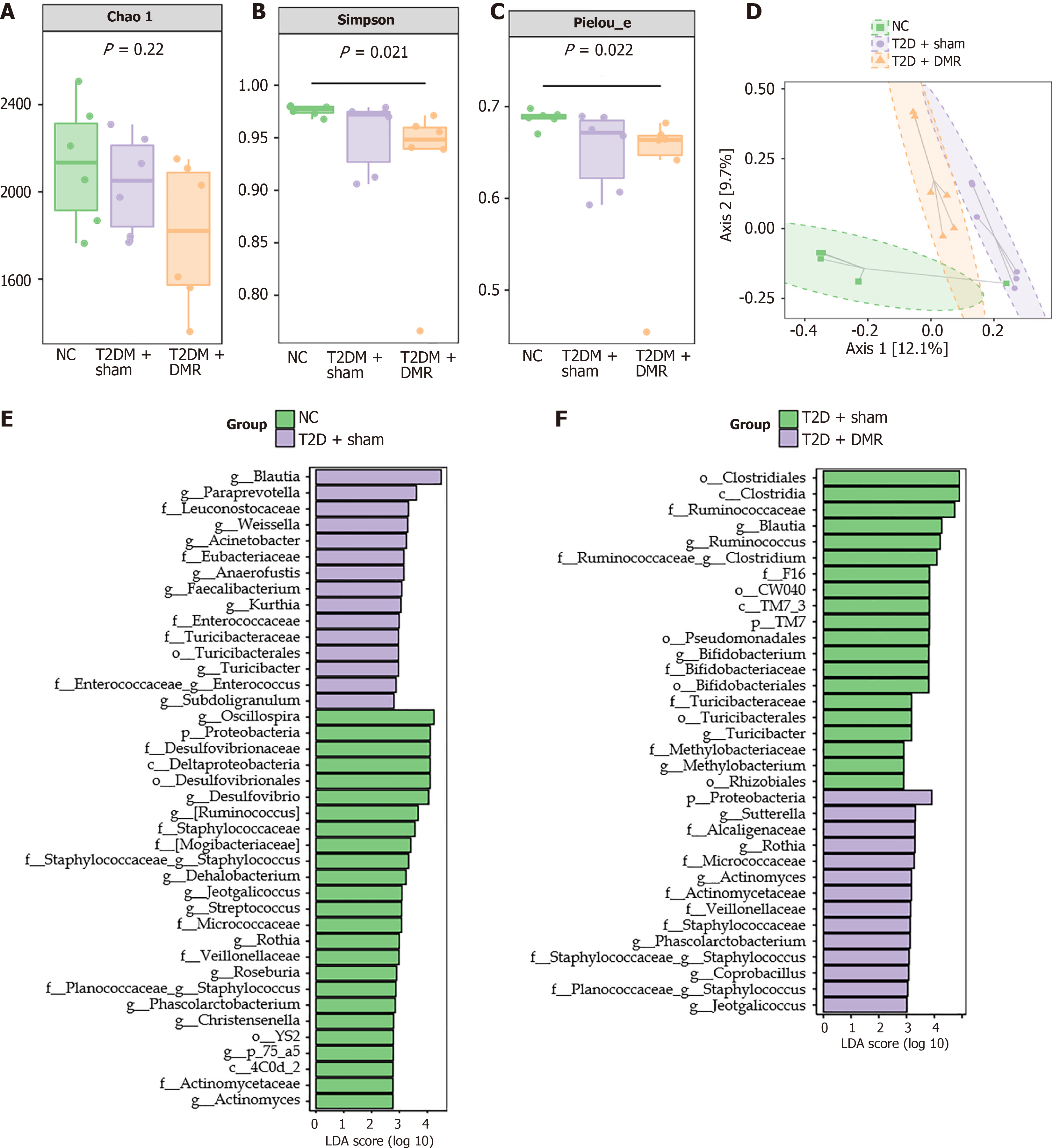
High-throughput sequencing of the 16S rRNA gene was performed to study the effects of DMR on the microbiota profiles of colonic content samples from T2D rats. Specific analysis of α-diversity showed that, there were no marked discrepancies in microbiome richness between groups, but multiformity and evenness differed markedly between groups (Figure 5A-C). Moreover, principal coordinates analysis of β-diversity showed differences in the composition of gut microbiota among the NC, T2D-sham, and T2D-DMR groups (Figure 5D). At the phylum level, Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria were predominant in all groups, with the proportion exceeding 96% (Supplementary Figure 1A). The relative abundance of Firmicutes was increased in the T2D-sham group. However, the relative abundance of Bacteroidetes and Proteobacteria was decreased in the T2D-sham group. At the genus level, the relative abundance of some genera were disparate among the three groups. For example, the relative abundance of Blautia was merely 1.21% in the NC group, which increased to 7.29% in the T2D-sham group, and recovered to 3.84% in the T2D-DMR group (Supplementary Figure 1B).
In addition, linear discriminant analysis effect size analysis revealed that the intestinal microbiota underwent significant changes at different taxonomic levels. The DMR procedure reversed the changes in abundance of Proteobacteria in the colonic contents at the phylum level and changes in the abundance of Blautia, Jeotgaleliccus, Rothia, Turicibacter, Phascolarctobacter, Staphylococcus and Actinomyces at the genus level (Figure 5E and F). Mapping the gene families of these 16S rRNA characteristic sequences to the Kyoto encyclopedia of genes and genomes database to predict related pathways revealed that they were mainly associated with the metabolic pathways of carbohydrates and amino acids (Supplementary Figure 2). In summary, these results indicate that the DMR procedure regulates the gut microbiota in T2D rats.
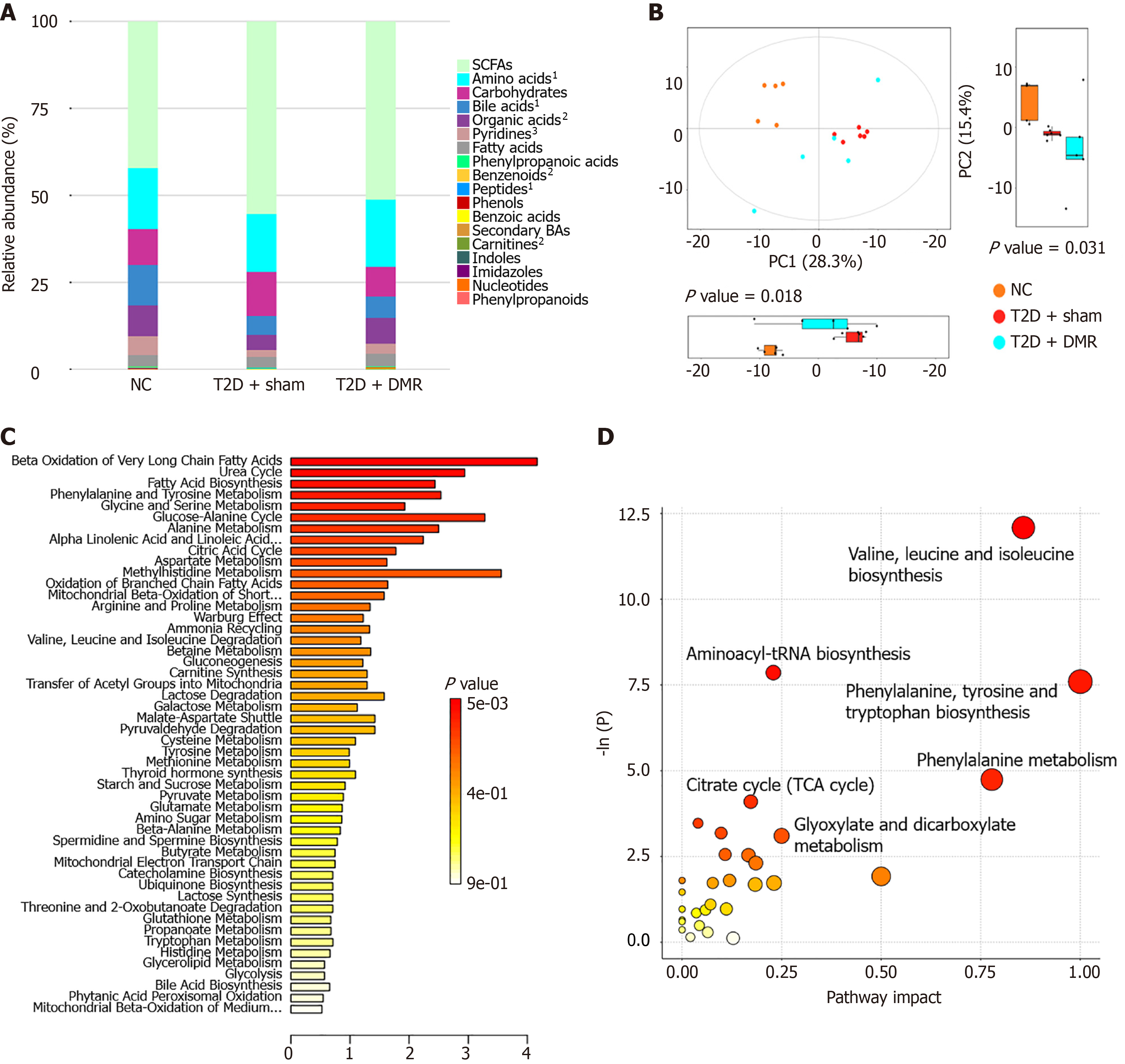
Ultra-high performance liquid chromatography with quadrupole time-of-flight mass spectrometry was used to profile the metabolites across the three groups. The total amounts of amino acids, bile acids, pyridines, benzenoids, peptides and carnitines differed significantly between the T2D-DMR, T2D-sham, and NC groups (Figure 6A). Principal component analysis revealed that each group of samples was distinctly arranged into disparate blocks, indicating that T2D and the DMR procedure generated distinct metabolite profiles (Figure 6B). Candidate biomarkers were confirmed employing ANOVA and the Kruskal-Wallis test. 87 altered metabolites with variable importance in projection > 1 and P < 0.05 were chosen. Compared with NC group, a total of 14 metabolites were increased, and 73 metabolites were reduced in the T2D-sham group. Further analysis revealed that the DMR procedure reversed the changes in 25 metabolites (Supplementary Table 1). Pathway-associated metabolite sets database and the rno databank were used for pathway enrichment analysis of the differential metabolites (Figure 6C and D). The results showed that the metabolism pathway affected by the DMR procedure might be involved with β oxidation and biosynthesis of fatty acids, biosynthesis and metabolism of amino acids, the uric acid cycle, and the glucose-alanine cycle.
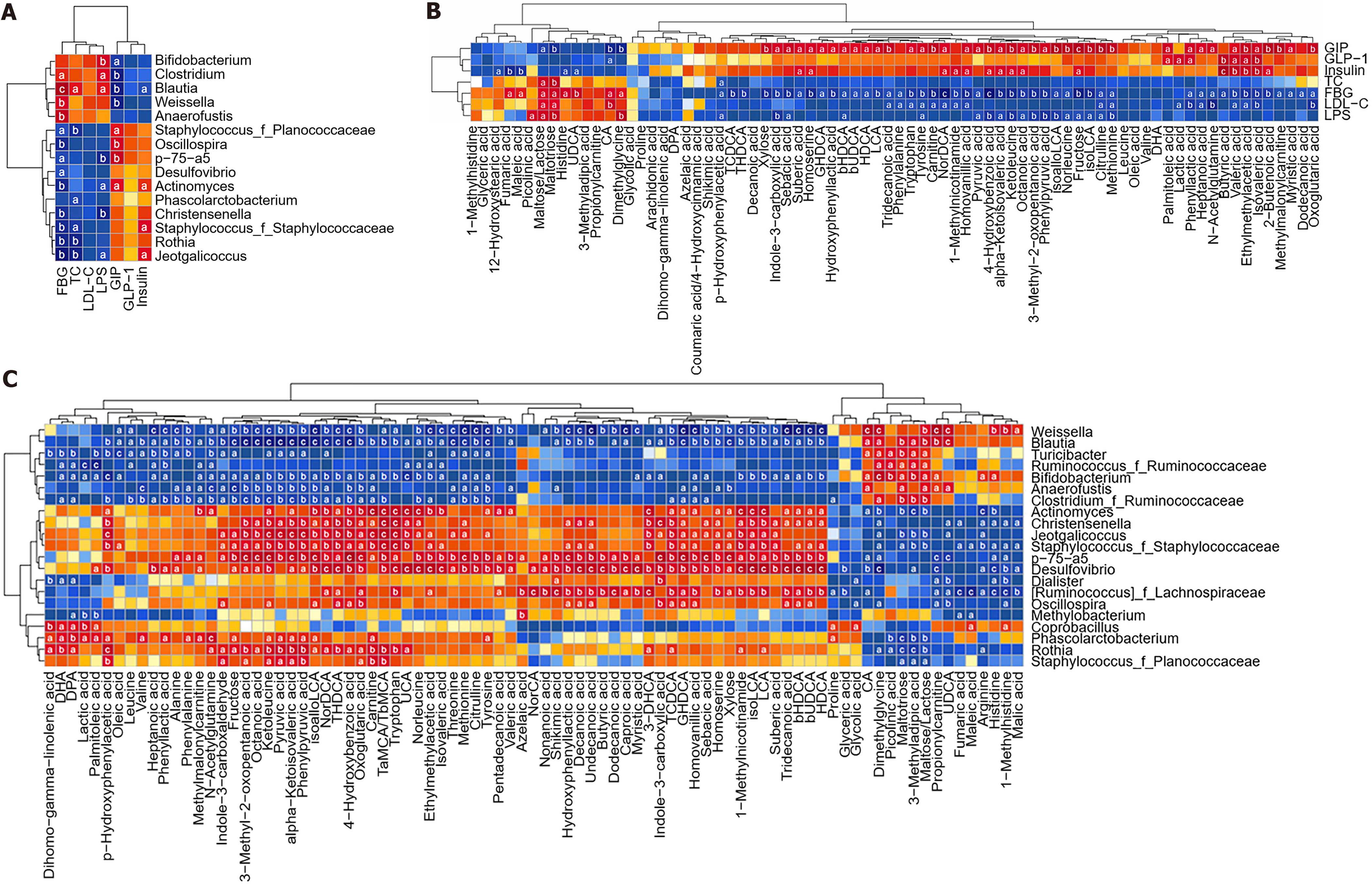
Significant correlations were observed between T2D-related traits, gut microbiota, and metabolites. Specifically, the abundance of the Blautia genus showed positive association with FBG, LPS, and TC levels, while displaying negative correlation with GIP and insulin levels. In contrast, the abundance of the Jeotgalelicus, Rothia, Phascolarctobacter, and Actinomyces genera exhibited negative correlation with FBG, LPS or TC levels, while displaying positive correlation with GIP or insulin levels (Figure 7A). Similarly, the majority of the 25 metabolites influenced by the DMR procedure were correlated with glucose and lipid parameters, as well as LPS and intestinal hormones (Figure 7B). Further analysis revealed that levels of the Blautia and Turiciactor genera were positively correlated with maltose/lactose, maltotriose, and 3-methyladipic acid levels, and negatively correlated with ketoleucane levels (Figure 7C). Conversely, the levels of Phascolarctobacterum, Rothia, Jeotgallicus genera were negatively correlated with maltose/lactose, maltotriose, and 3-methyladipic acid, and positively correlated with ketoleucane levels (Figure 7C).
In this study, we successfully performed DMR surgery in T2D rats. Although our mechanical abrasion method is different from the clinical hydrothermal ablation, both methods significantly improved diabetes control, confirming the importance of the duodenal mucosa in the pathogenesis and treatment of T2D. Our findings strongly suggest that the DMR-induced improvements in glucose and lipid metabolism in T2D rats involved multiple pathways.
Numerous studies have reported that the levels of gastrointestinal hormones such as GLP-1 and CCK increase significantly after metabolic surgery[21]. The upregulation of endogenous GLP-1 signaling is considered the primary pathway for enhancing glucose metabolism after metabolic surgery[22,23]. This study also found that the amount of GLP-1-positive cells in the duodenum increased after DMR. CCK plays a role in satiety stimulation and insulin secretion[24,25], contributing to metabolic control. The DMR procedure shares similarities with duodenal-jejunal bypass sleeve (DJBS) surgery in that it reduces nutrient absorption in the duodenum. Speck et al[26] found an increase in serum GIP levels after DJBS surgery, which partially explains the elevated serum GIP levels following the DMR procedure. However, the effects of endogenous GIP after surgical metabolic interventions are not fully understood. Endogenous GLP and GIP might function synergistically to yield greater metabolic benefits. The DMR procedure had no impact on the expression level of GIP-positive cells in the duodenum. The increase in serum GIP and GLP-1 levels may be related to a rise in the amount of GIP or GLP-1 positive cells in other intestinal segments or the DMR procedure may directly stimulate the secretion of GIP and GLP-1. However, further research is needed to verify these phenomena.
Previous studies have revealed that high-fat and high-sugar diets can induce duodenal mucosal hyperplasia in rodents, triggering insulin resistance signaling and contributing to metabolic diseases[9]. This concept underpins the design of the DMR procedure[8]. Additionally, diabetes can promote mucosal hyperplasia, unrelated to food intake. This mucosal thickening is primarily attributable to the elongation of the intestinal villi and deepening of the crypts[27,28]. This increases the intestinal surface area, enhancing digestion and absorption in rats. The potential mechanism whereby the DMR procedure improves the control of T2D may involve reducing nutrient absorption by modulating villus length and crypt depth.
Dysfunction in the gut barrier, gut microbiota, and metabolic products is closely associated with T2D[29-31]. Numerous studies have provided evidence that the gut microbiota produce a effect in T2D. However, specific taxonomic groups associated with T2D vary considerably between studies[29]. In our research, we primarily observed significant correlations between certain bacteria and T2D-related parameters at the genus level. Among these, Blautia were the most strongly correlated with T2D-related parameters. As a genus within the Lachnospiraceae family, several human and rodent studies have shown an association between Blautia and T2D, although these associations have varied between studies. Some studies have suggested that Blautia, as an anaerobic probiotic, is negatively associated with metabolic disorders[32], and that oral administration of Blautia wexlerae ameliorates obesity and T2D by altering the gut microbiota[33]. Conversely, some clinical studies have shown that there is no correlation between Blautia and T2D[34]. Nevertheless, most clinical and animal studies have reported an increase in the abundance of Blautia in T2D or prediabetic individuals[35-38], with decreases observed after metabolic surgery[39,40]. Anhê et al[40] found that following bariatric surgery gut microbiota, specifically higher Parabacteroides and lower Blautia abundance, improved blood glucose independent of changes in obesity, insulin, or insulin resistance. Consistent with most studies, this study demonstrated an increase in the Blautia genus in T2D rats, which significantly decreased after the DMR procedure. Further investigation revealed that the level of the Blautia genus was strongly correlated with T2D-related traits. Additionally, Leclercq et al[41] found that an increase in Blautia abundance was linked to high intestinal permeability. Blautia may increase the intestinal barrier permeability, promote LPS leakage, trigger insulin resistance, and consequently elevate blood glucose levels. Based on the correlation between Blautia and metabolites, Blautia may participate primarily in amino acid biosynthesis and metabolism. However, the precise role of Blautia after the DMR procedure warrants further investigation. In addition, we only studied the impact of DMR on the gut microbiota in the colon. Future research should include an investigation of changes in the gut microbiota in duodenum.
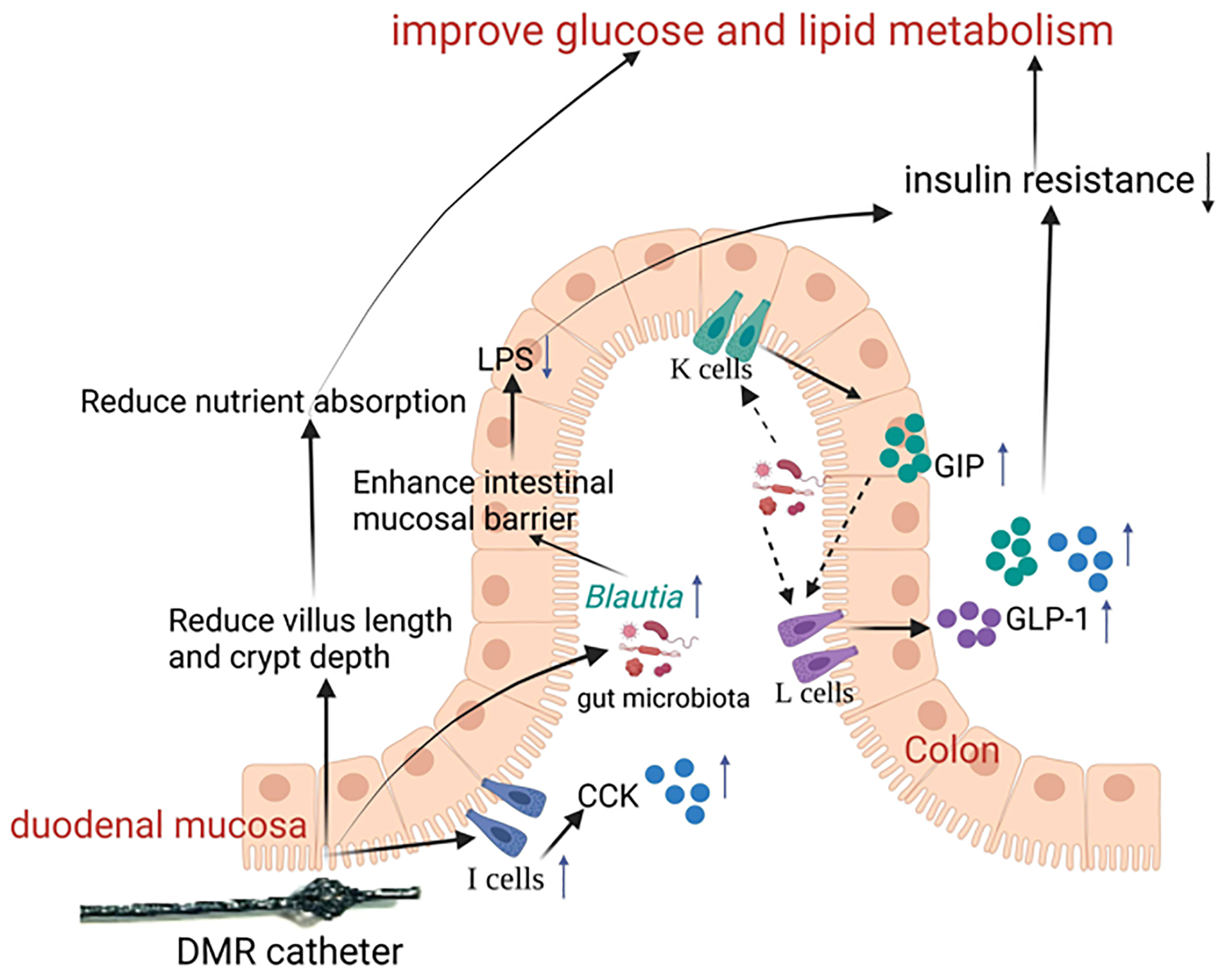
The pathogenesis of T2D is multifaceted, and DMR improves metabolic disorder through multiple interconnected pathways[10]. The changes induced by the DMR procedure interact with each other. This study suggests that the DMR procedure initially reduce villus length and crypt depth, thereby decreasing nutrient absorption. This may lead to improvements in the gut microbiota and gut hormone secretion, and a reduction in LPS production. These alterations in the gut microbiota, and metabolite and gut hormone levels may regulate the proliferation and apoptosis of intestinal epithelial cells, enhance barrier function, reduce LPS leakage, reduce inflammation, and ultimately reduce insulin resistance, thereby enhancing glucose and lipid metabolism (Figure 8).
This study has several limitations. First, we only conducted short-term postoperative observation. In the future, long-term changes in glucose and lipid metabolism and mucosal changes should be investigated. Second, this was a preliminary observational study without in-depth exploration of the mechanisms. In-depth research on the role of Blautia in the pathogenesis and treatment of T2D, as well as the interplay between the microbiota, villi, and intestinal hormones is needed. Third, the surgical methods used in the animal experiments differed from those of the DMR procedure used in clinical trials. Clinical DMR is a minimally invasive endoscopic procedure that causes minimal trauma in humans. We performed laparotomy, which causes significant trauma in T2D rats. To eliminate interference from surgery, we used a sham-surgery group as a control. In addition, hot water has been used to damage the mucosal layer in clinical trials, whereas we used mechanical force in this study. Although the methods are different, the common goal was to cause necrosis and shedding of mucosal cells without damaging the muscle layer.
Collectively, our findings strongly indicate that the duodenal mucosa is important in the pathogenesis and treatment of T2D, and that DMR may effectively alleviate insulin resistance and metabolic disorders in T2D rats through a multitude of pathways. These mechanisms may include a reduction of villus length and crypt depth, augmentation of intestinal hormone secretion, reinforcement of intestinal barrier function, reduction in LPS leakage, and modulation of gut microbiota and metabolites. This study serves as a preliminary investigation and further animal experiments are required to corroborate these findings. Nevertheless, our research provides theoretical groundwork for the potential clinical application of the DMR procedure in humans with T2D.
The authors wish to thank Dr. Wang HP for providing technical guidance with the animal surgery, and Mr. Han DZ and Nanjing Kangyou Medical Technology Co., Ltd. for designing the DMR catheters.
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4368] [Article Influence: 624.0] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4698] [Article Influence: 1566.0] [Reference Citation Analysis (36)] |
| 3. | Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | van Baar ACG, Devière J, Hopkins D, Crenier L, Holleman F, Galvão Neto MP, Becerra P, Vignolo P, Rodriguez Grunert L, Mingrone G, Costamagna G, Nieuwdorp M, Guidone C, Haidry RJ, Hayee B, Magee C, Carlos Lopez-Talavera J, White K, Bhambhani V, Cozzi E, Rajagopalan H, J G H M Bergman J. Durable metabolic improvements 2 years after duodenal mucosal resurfacing (DMR) in patients with type 2 diabetes (REVITA-1 Study). Diabetes Res Clin Pract. 2022;184:109194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 496] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 6. | Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, Buob D, Pigeyre M, Mathurin P, Pattou F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893-8; discussion 898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Wu W, Lin L, Lin Z, Yang W, Cai Z, Hong J, Qiu J, Lin C, Lin N, Wang Y. Duodenum Exclusion Alone Is Sufficient to Improve Glucose Metabolism in STZ-Induced Diabetes Rats. Obes Surg. 2018;28:3087-3094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Cherrington AD, Rajagopalan H, Maggs D, Devière J. Hydrothermal Duodenal Mucosal Resurfacing: Role in the Treatment of Metabolic Disease. Gastrointest Endosc Clin N Am. 2017;27:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Pereira JNB, Murata GM, Sato FT, Marosti AR, Carvalho CRO, Curi R. Small intestine remodeling in male Goto-Kakizaki rats. Physiol Rep. 2021;9:e14755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Nie L, Yan Q, Zhang S, Cao Y, Zhou X. Duodenal Mucosa: A New Target for the Treatment of Type 2 Diabetes. Endocr Pract. 2023;29:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Haidry RJ, van Baar AC, Galvao Neto MP, Rajagopalan H, Caplan J, Levin PS, Bergman JJ, Rodriguez L, Deviere J, Thompson CC. Duodenal mucosal resurfacing: proof-of-concept, procedural development, and initial implementation in the clinical setting. Gastrointest Endosc. 2019;90:673-681.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Mingrone G, van Baar AC, Devière J, Hopkins D, Moura E, Cercato C, Rajagopalan H, Lopez-Talavera JC, White K, Bhambhani V, Costamagna G, Haidry R, Grecco E, Galvao Neto M, Aithal G, Repici A, Hayee B, Haji A, Morris AJ, Bisschops R, Chouhan MD, Sakai NS, Bhatt DL, Sanyal AJ, Bergman JJGHM; Investigators of the REVITA-2 Study. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: the randomised, double-blind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut. 2022;71:254-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | de Oliveira GHP, de Moura DTH, Funari MP, McCarty TR, Ribeiro IB, Bernardo WM, Sagae VMT, Freitas JR Jr, Souza GMV, de Moura EGH. Metabolic Effects of Endoscopic Duodenal Mucosal Resurfacing: a Systematic Review and Meta-analysis. Obes Surg. 2021;31:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D, Rodriguez Grunert L, Galvao Neto M, Vignolo P, Hayee B, Mertens A, Bisschops R, Tijssen J, Nieuwdorp M, Guidone C, Costamagna G, Devière J, Bergman JJGHM. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2020;69:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Boye A, Acheampong DO, Gyamerah EO, Asiamah EA, Addo JK, Mensah DA, Brah AS, Ayiku PJ. Glucose lowering and pancreato-protective effects of Abrus Precatorius (L.) leaf extract in normoglycemic and STZ/Nicotinamide - Induced diabetic rats. J Ethnopharmacol. 2020;258:112918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Sun K, Ding M, Fu C, Li P, Li T, Fang L, Xu J, Zhao Y. Effects of dietary wild bitter melon (Momordica charantia var. abbreviate Ser.) extract on glucose and lipid metabolism in HFD/STZ-induced type 2 diabetic rats. J Ethnopharmacol. 2023;306:116154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Atiq A, Shal B, Naveed M, Khan A, Ali J, Zeeshan S, Al-Sharari SD, Kim YS, Khan S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur J Pharmacol. 2019;843:292-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Kuang J, Wang J, Li Y, Li M, Zhao M, Ge K, Zheng D, Cheung KCP, Liao B, Wang S, Chen T, Zhang Y, Wang C, Ji G, Chen P, Zhou H, Xie C, Zhao A, Jia W, Zheng X, Jia W. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023;35:1752-1766.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 19. | Shen S, Huang D, Qian S, Ye X, Zhuang Q, Wan X, Dong Z. Hyodeoxycholic acid attenuates cholesterol gallstone formation via modulation of bile acid metabolism and gut microbiota. Eur J Pharmacol. 2023;955:175891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 21. | Svane MS, Bojsen-Møller KN, Martinussen C, Dirksen C, Madsen JL, Reitelseder S, Holm L, Rehfeld JF, Kristiansen VB, van Hall G, Holst JJ, Madsbad S. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology. 2019;156:1627-1641.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Shah A, Prasad M, Mark V, Holst JJ, Laferrère B. Glucagon-like peptide-1 effect on β-cell function varies according to diabetes remission status after Roux-en-Y gastric bypass. Diabetes Obes Metab. 2022;24:2081-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, Alikhan M, Clements BA, Abumrad NN, Flynn CR. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology. 2019;156:1041-1051.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Koizumi H, Mohammad S, Ozaki T, Muto K, Matsuba N, Kim J, Pan W, Morioka E, Mochizuki T, Ikeda M. Intracellular interplay between cholecystokinin and leptin signalling for satiety control in rats. Sci Rep. 2020;10:12000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Rushakoff RJ, Goldfine ID, Carter JD, Liddle RA. Physiological concentrations of cholecystokinin stimulate amino acid-induced insulin release in humans. J Clin Endocrinol Metab. 1987;65:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923-E932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Castro VMD, Medeiros KCP, Lemos LIC, Pedrosa LFC, Ladd FVL, Carvalho TG, Araújo Júnior RF, Abreu BJ, Farias NBDS. S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue Cell. 2021;69:101483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K. Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J. 2003;50:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 1065] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 30. | Cao Y, Ren G, Zhang Y, Qin H, An X, Long Y, Chen J, Yang L. A new way for punicalagin to alleviate insulin resistance: regulating gut microbiota and autophagy. Food Nutr Res. 2021;65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 31. | Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, Joosten LAB, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 999] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 32. | Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. 2021;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 806] [Article Influence: 268.7] [Reference Citation Analysis (0)] |
| 33. | Hosomi K, Saito M, Park J, Murakami H, Shibata N, Ando M, Nagatake T, Konishi K, Ohno H, Tanisawa K, Mohsen A, Chen YA, Kawashima H, Natsume-Kitatani Y, Oka Y, Shimizu H, Furuta M, Tojima Y, Sawane K, Saika A, Kondo S, Yonejima Y, Takeyama H, Matsutani A, Mizuguchi K, Miyachi M, Kunisawa J. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat Commun. 2022;13:4477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 203] [Reference Citation Analysis (0)] |
| 34. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2139] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 35. | Kashtanova DA, Tkacheva ON, Doudinskaya EN, Strazhesko ID, Kotovskaya YV, Popenko AS, Tyakht AV, Alexeev DG. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, Kautzky-Willer A, Paulweber B, Hackl E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 37. | Egshatyan L, Kashtanova D, Popenko A, Tkacheva O, Tyakht A, Alexeev D, Karamnova N, Kostryukova E, Babenko V, Vakhitova M, Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2016;5:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Ibrahim KS, Bourwis N, Dolan S, Lang S, Spencer J, Craft JA. Characterisation of gut microbiota of obesity and type 2 diabetes in a rodent model. Biosci Microbiota Food Health. 2021;40:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 40. | Anhê FF, Zlitni S, Zhang SY, Choi BS, Chen CY, Foley KP, Barra NG, Surette MG, Biertho L, Richard D, Tchernof A, Lam TKT, Marette A, Schertzer J. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut. 2023;72:460-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 41. | Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485-E4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (0)] |