Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.102126
Revised: December 7, 2024
Accepted: January 8, 2025
Published online: March 15, 2025
Processing time: 102 Days and 18.8 Hours
Diabetic wound injury is a significant and common complication in individuals with diabetes. N6-methyladenosine (m6A)-related epigenetic regulation is widely involved in the pathogenesis of diabetes complications. However, the function of m6A methyltransferase Wilms tumor 1-associated protein (WTAP) in diabetic wound healing remains elusive.
To investigate the potential epigenetic regulatory mechanism of WTAP during diabetic wound healing.
Human umbilical vein endothelial cells (HUVECs) were induced with high glu
The expression of several m6A methyltransferases, including METTL3, METTL14, METTL16, KIAA1429, WTAP, and RBM15, were measured. WTAP exhibited the most significant elevation in HG-induced HUVECs compared with the normal control. WTAP depletion notably restored cell viability and enhanced tube formation ability and migration of HUVECs suppressed by HG. The unclosed wound area of mice was smaller in WTAP knockdown-treated mice than in control mice at nine days post-wounding, along with enhanced re-epithelialization rate and collagen deposition. The m6A levels on DNMT1 mRNA in HUVECs were repressed by WTAP knockdown in HUVECs. The mRNA levels and expression of DNMT1 were inhibited by WTAP depletion in HUVECs. Overexpression of DNMT1 in HUVECs notably reversed the effects of WTAP depletion on HG-induced HUVECs.
WTAP expression is elevated in HG-induced HUVECs and epigenetically regulates the m6A modification of DNMT1 to impair diabetic wound healing.
Core Tip: Diabetes mellitus is significantly correlated with non-healing wounds, which cause physiological and psychological distress to patients. N6-methyladenosine (m6A)-associated epigenetic regulation plays a crucial role in diabetes mellitus. However, the function of m6A methyltransferase tumor 1-associated protein (WTAP) in diabetic wound healing remains unclear. Our study demonstrated that WTAP was elevated in high glucose-induced human umbilical vein endothelial cells and epigenetically regulates the m6A modification of DNA methyltransferase 1, repressing diabetic wound healing. This study provides novel insights into the mechanisms by which the m6A regulator WTAP inhibits diabetic wound healing.
- Citation: Xiao RJ, Wang TJ, Wu DY, Yang SF, Gao H, Gan PD, Yi YY, Zhang YL. N6-methyladenosine methyltransferase Wilms tumor 1-associated protein impedes diabetic wound healing through epigenetically activating DNA methyltransferase 1. World J Diabetes 2025; 16(3): 102126
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/102126.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.102126
Diabetes mellitus is a pervasive global chronic condition[1]. According to the World Health Organization's epidemiological survey, the prevalence of diabetes among adults aged 20 to 79 is projected to rise from approximately 7.7% in 2010 to a higher proportion by 2030[2]. Diabetes mellitus leads to various complications[3], among which non-healing wounds represent a chronic complication of diabetes, inflicting both significant physiological and psychological distress on patients and imposing a substantial burden on the economy and society at large[4,5].
Hyperglycemia-induced vascular damage stands out as a significant contributor to severe diabetic complications, such as diabetic foot ulcers[6]. In normal circumstances, wound closure involves four interconnected and synchronized stages: Hemostasis, inflammation, proliferation, and remodeling[7]. However, this process is hindered and prolonged in individuals with diabetes and non-healing wounds, primarily due to the impairment of these four stages[8,9]. Notably, endothelial cell dysfunction and disrupted microcirculation are frequently observed in patients with diabetes, con
N6-methyladenosine (m6A) stands as a widely explored RNA modification, playing a central role in the "epigenetic regulation" of mRNAs. It is ubiquitously present in eukaryotic cells[11]. Alterations in m6A levels are intricately linked to the modulation of RNA metabolism, responses to heat shock stress, as well as the initiation and progression of various diseases[12-14]. The m6A modifications are orchestrated by the m6A writer complex, which is composed of RNA methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), Wilms tumor 1-associated protein (WTAP), and other proteins with methyltransferase capabilities[15]. Previous studies have indicated that WTAP-mediated m6A modification of NLRP3 mRNA influences kidney injury in diabetic nephropathy[16].
DNA methyltransferase 1 (DNMT1) is an enzyme central to DNA methylation, responsible for adding a methyl group to the carbon atom at the cytosine 5 position of the CpG dinucleotide sequence[17,18]. DNMT1 plays a key role in the maintenance of the DNA methylation pattern and plays an important role in the development of several diseases[19,20]. In particular, the role of DNMT1 in diabetes mellitus and its complications has garnered significant attention[21]. It has been shown that DNMT1 expression levels are elevated in patients with diabetes mellitus and correlate with disease severity[22]. In individuals with diabetes mellitus, increased expression of DNMT1 is associated with inflammatory responses, particularly with the expression of interleukin-6, a pro-inflammatory cytokine that activates the expression of DNMT1, which in turn affects the function of renal cells and accelerates the disease progression in diabetes mellitus[23].
In this study, we aimed to determine whether WTAP modulates the pathological process of diabetic wound healing by regulating DNMT1 and explored the potential underlying molecular mechanisms.
All animal experiments were approved by the Animal Care Committee of The Second Affiliated Hospital of Jiangxi Medical College of Nanchang University on March 6, 2024. Eight-week-old male BALB/c mice were intraperitoneally injected with streptozotocin (50 mg/kg) for five days to induce diabetes. Two weeks post-injection, blood glucose levels were measured using a blood glucose monitor. Successful induction of diabetes was confirmed when the blood glucose level exceeded 16.7 mmol/L and was sustained for an additional four weeks before creating full-thickness cutaneous wounds. Prior to surgery, diabetic mice were anesthetized with pentobarbital sodium (Sigma, United States) at a concentration of 1% (50 mg/kg body weight). Following shaving and sterilization, a full-thickness excision wound with a diameter of 20 mm was made on the back of all mice. Subsequently, the mice were randomly divided into two groups: The control group was treated with siNC and siWTAP mimics (2 nmol/20 g body weight). siNC and siWTAP was injected subcutaneously at the edge of wounds on days 0, 3, and 6 post-wounding. Digital photographs were captured on days 0, 6, and 9, and the wound area was quantified using Image J software.
On day nine, full-thickness traumatic tissue samples were obtained from the outer edge of the entire wound. The tissues were divided into two equal portions: One portion was fixed in 4% paraformaldehyde at 4 °C overnight, embedded in paraffin, and processed for histological analysis; the other portion was stored in liquid nitrogen for RNA and protein expression analysis. Tissue sections of 4 μm thickness were affixed to glass slides for histological examination. He
Human umbilical vein endothelial cells (HUVECs) were obtained from the Shanghai Cell Bank of Chinese Science Academy and were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, United States) that was added with 10% fetal bovine serum (FBS; Hyclone, United States), 100 μg/mL of streptomycin, and 100 U/mL of penicillin. The cells were maintained in a 5% CO2 humidified atmosphere at 37 °C. HUVECs were stimulated with 5.5 mmol/L normal glucose (as control) or 30 mmol/L high glucose (HG) for 48 hours. Additionally, 24.5 mmol/L mannitol was added to the normal glucose group to maintain osmolarity.
The siWTAP, DNMT1 overexpression vectors, and their corresponding negative controls (siNC and empty vectors) were procured from Guangzhou Ribobio and GenePharma (Shanghai, China). HUVECs were transfected using Lipofectamine 2000 Reagent (Invitrogen, United States) following the manufacturer's instructions. Briefly, cells were seeded into 6-well plates and grown to 60% confluence. Then, siRNAs (20 nM) and plasmids (1 µg) were mixed with 10 µL transfection reagent and added to the cell culture medium. In vitro assays were conducted 48 hours post-transfection.
For detection of cell proliferation, cell counting kit 8 (CCK-8) and colony formation assay were performed. For the CCK-8 assay, HUVECs were transfected with the indicated siRNAs or overexpression vectors and seeded into 96-well plates at a density of 5000 cells per well. After incubation for 48 hours, CCK-8 reagent (Beyotime, China) was added into each well and incubated for 2 hours; then, absorbance values at 450 nm were measured by a spectrophotometer (Thermo, United States). For the colony formation assay, single cells were suspended in a culture medium at a density of 1000 cells per well. After incubation at 37 °C for 10 days, colonies were stained with crystal violet (Sigma, United States). Images of colonies were taken using a digital camera.
The migration ability of HUVECs from different treated groups, using a 24-well Transwell chamber (8.0 μm pore size, Corning, United States), was evaluated. HUVECs suspended in serum-free DMEM were added to the upper chamber with 16000 cells per well. Subsequently, the lower chamber was added with a complete culture medium containing 10% FBS for 24 hours. Afterward, the HUVECs that migrated to the bottom surface were stained with crystal violet (Solarbio, Beijing, China) and counted under a microscope.
For the tube formation assay, HUVECs from the treated groups were seeded in a 96-well culture plate at 25000 cells per well. The wells were pre-coated with Matrigel matrix (BD Biosciences, CA, United States). After 6 hours of incubation, tube formation was observed under a microscope, and the total length of the endothelial tubes was measured using Image J software.
RNA was isolated from full-thickness traumatic tissue using TRIzol reagent. The subsequent synthesis of cDNA was executed using the Prime-Script® RT reagent Kit (TAKARA, Japan). Quantitative PCR (qPCR) assay was performed using TB Green Premix Ex Taq II kit (TAKARA, Japan) according to the manufacturer’s protocol. The housekeeping gene β-actin was adopted for normalizing the relative expression levels of each RNA sample. The standard 2−ΔΔCt method was employed for data analysis.
For protein extraction, RIPA lysis buffer with proteinase inhibitor (Roche, Switzerland) was employed to extract total proteins. Equal amounts of total protein (40 μg) were separated by 10% to 15% SDS-PAGE, blotted onto a PVDF membrane (Millipore, United States), and hatched with 5% skimmed milk. The blots were then hatched with specific primary antibodies for WTAP (Abcam, United States), DNMT1 (Abcam, United States), and β-actin (Proteintech, China) at 4 °C overnight. Subsequently, the membrane underwent incubation with secondary anti-mouse or anti-rabbit an
A methylated RNA immunoprecipitation (MeRIP) experiment was performed to analyze m6A enrichment on mRNA using the Magna MeRIP m6A Kit (Millipore, United States) in accordance with the manufacturer’s protocol. The precipitants were analyzed using qPCR assay.
Data are presented as the mean ± SD. Group differences were assessed using a one-way analysis of variance followed by Tukey’s post-test or student’s t-test using GraphPad Prism 7.0 software. Statistical significance was considered at P < 0.05.
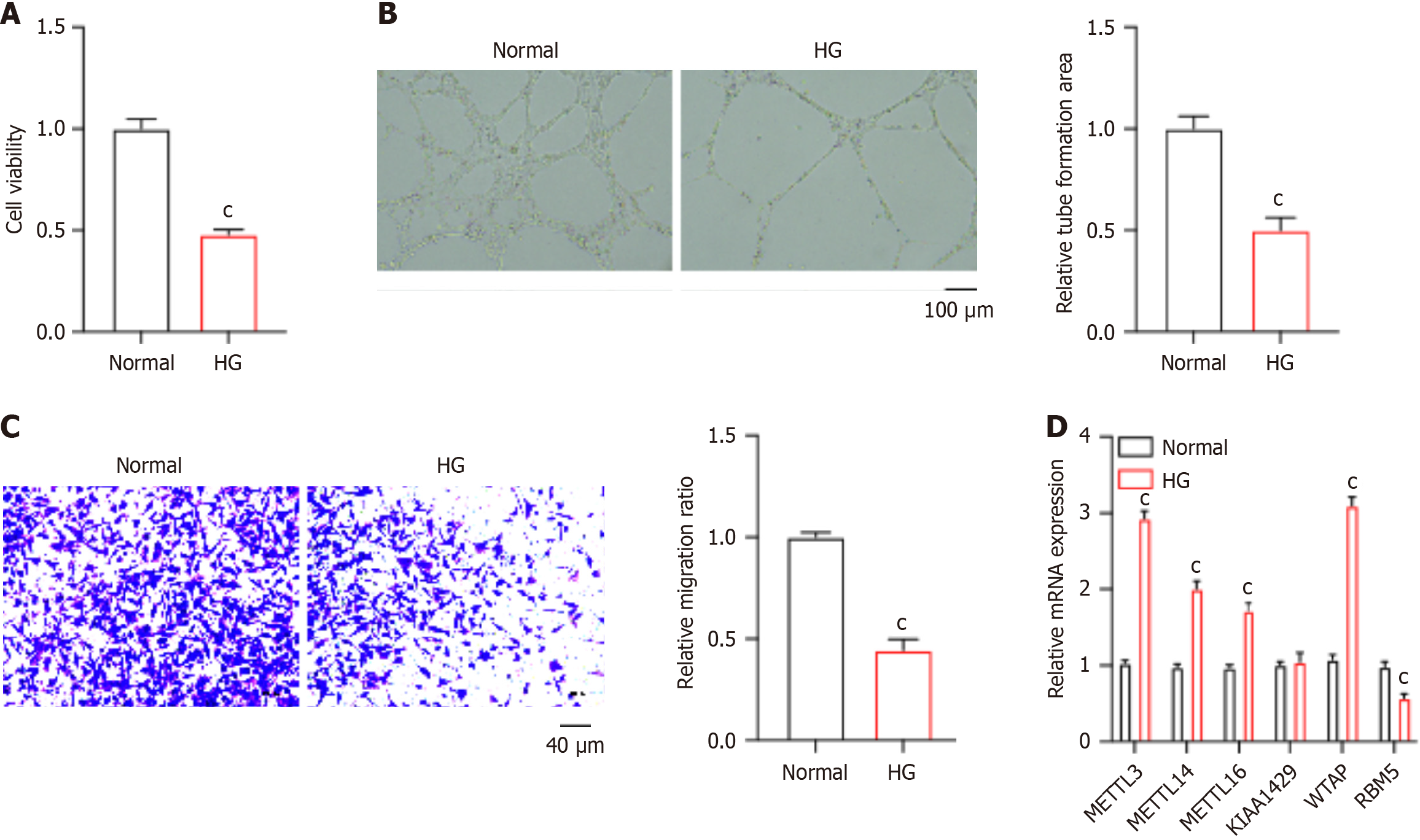
To determine the epigenetic regulators that are closely correlated with diabetic wound healing, we established an in vitro model using HG-stimulated HUVECs. HUVECs were treated with mannitol as a control to exclude the potential side effects caused by osmolarity. We observed that HG-stimulated HUVECs notably repressed the viability of HUVECs (Figure 1A). Results from the tube formation assay and Transwell experiment showed that HG induction led to reduced tube formation (Figure 1B) and migration (Figure 1C) of HUVECs. Notably, we analyzed the expression of several RNA methyltransferases, including the METTL3, METTL14, METTL16, KIAA1429, WTAP, and RBM15, among which WTAP exhibited the most significant elevation in the HG group compared with the normal control (Figure 1D). These data demonstrated that HG-repressed HUVEC function is potentially regulated by m6A modification.
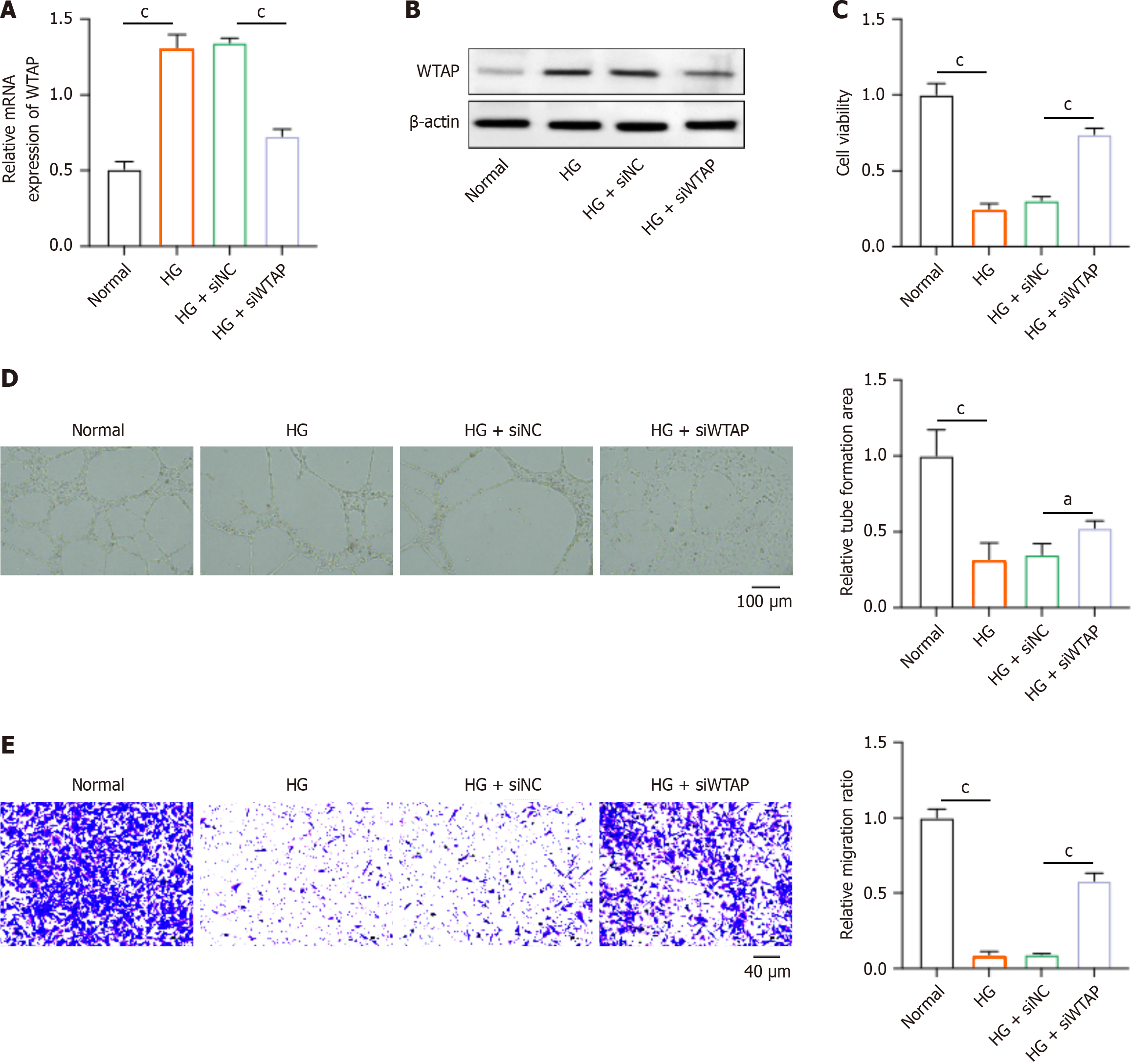
Next, we investigated the effects of WTAP on HUVECs via conducting depletion of WTAP by siRNAs. As shown in Figure 2A and B, transfection of siWTAP could significantly downregulate the RNA and protein levels of WTAP in HUVECs. The depletion of WTAP notably recovered the viability of HUVECs that were suppressed by HG (Figure 2C). Moreover, the siWTAP treatment could promote the tube formation ability (Figure 2D) and migration (Figure 2E) of HG-stimulated HUVECs. These data suggested that the knockdown of WTAP could protect HUVECs from HG-induced dysfunction.
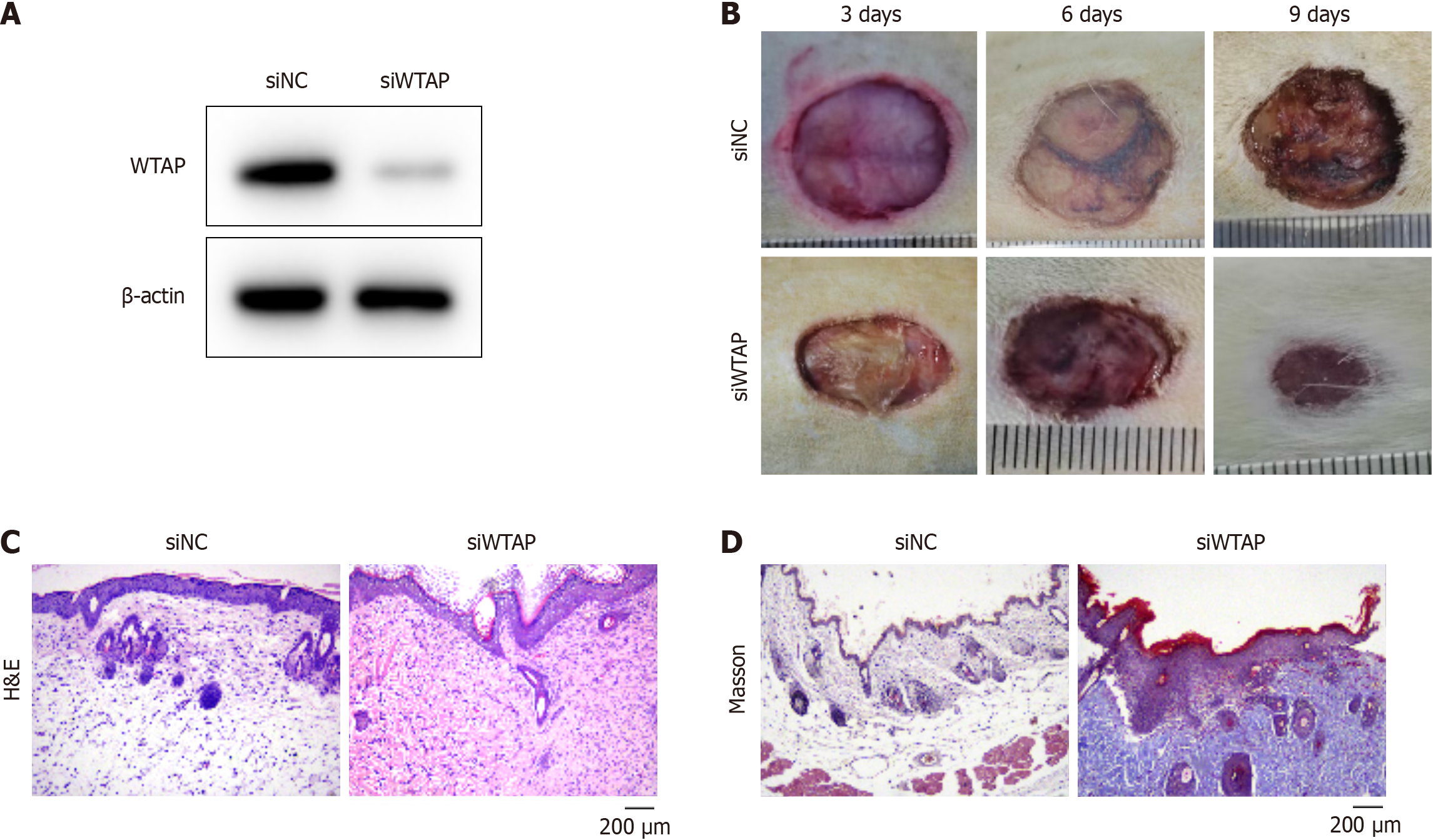
We established an in vivo mouse model to mimic diabetic wound healing by administering siWTAP treatment. The siWTAP treatment notably reduced the expression of WTAP in mouse skin tissue (Figure 3A). The unclosed wound area of mice was smaller in siWTAP-treated mice compared with that of the siNC-treated mice at nine days post-wounding (Figure 3B). Histological staining was conducted to analyze the wound repair effects after treatment at day nine after operation. Results from hematoxylin & eosin staining demonstrated that treatment with siWTAP caused a higher re-epithelialization rate than the siNC treatment group (Figure 3C). Furthermore, the Masson staining results indicated that collagen deposition of mice treated with siWTAP was stronger compared with the control group (Figure 3D). These findings demonstrate that depletion of WTAP accelerates the healing of diabetic wounds.
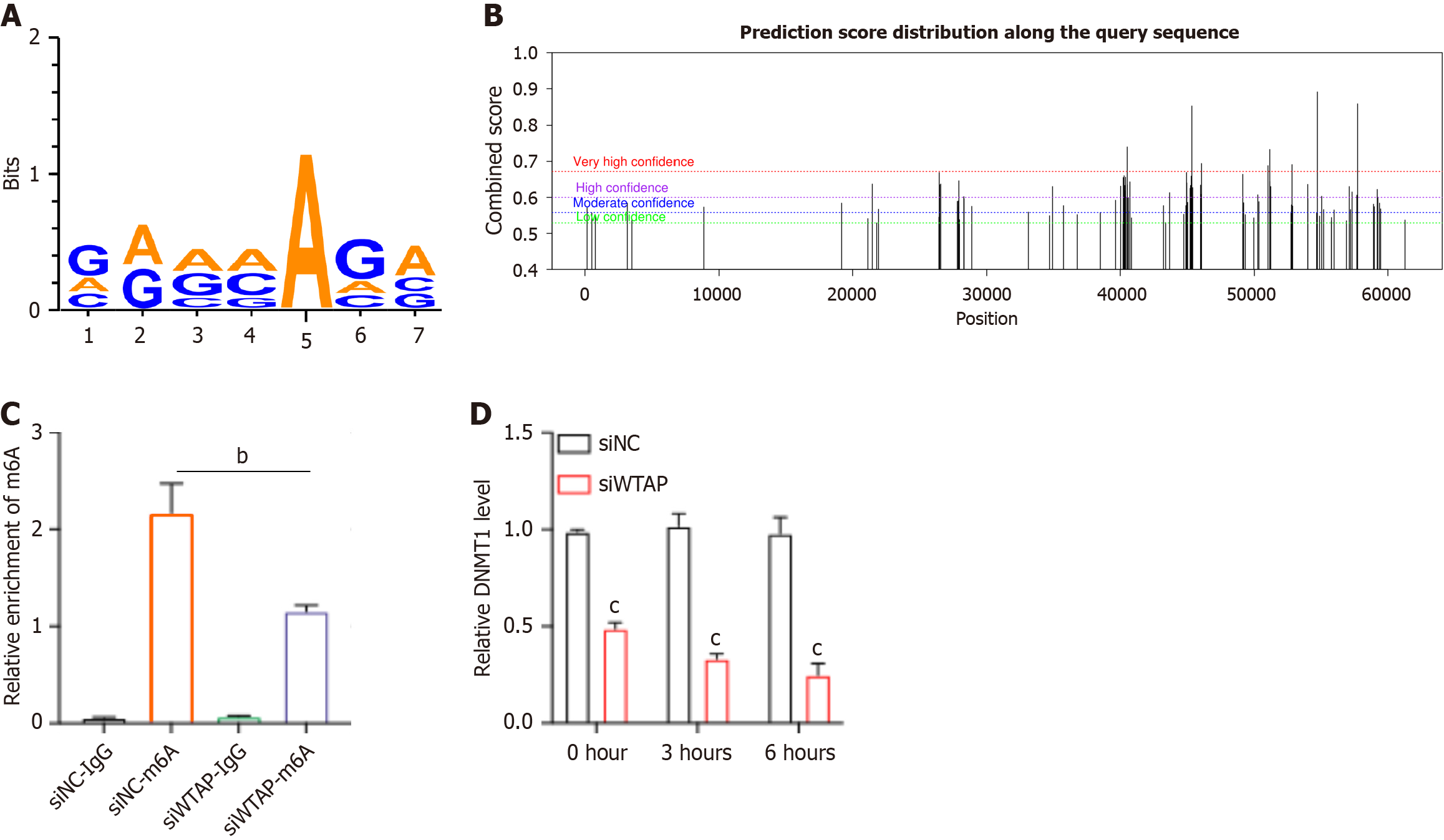
To identify WTAP’s downstream targets, we conducted online analysis using https://rna.sysu.edu.cn/rmbase/ to explore potential mRNAs in HG-induced HUVECs. The m6A modified motif on DNMT1 mRNA associated with WTAP was identified to be GAAAAGA (Figure 4A). Additionally, we utilized a sequence-based m6A modification site predictor SRAMP (http://www.cuilab.cn/sramp) to screen for m6A modified sites on DNMT1 mRNA (Figure 4B). Subsequently, m6A modified content on DNMT1 mRNA was identified through MeRIP-PCR analysis. As shown in Figure 4C, knockdown of WTAP resulted in reduced m6A levels on DNMT1 mRNA. Moreover, the depletion of WTAP significantly downregulated DNMT1 mRNA expression (Figure 4D).
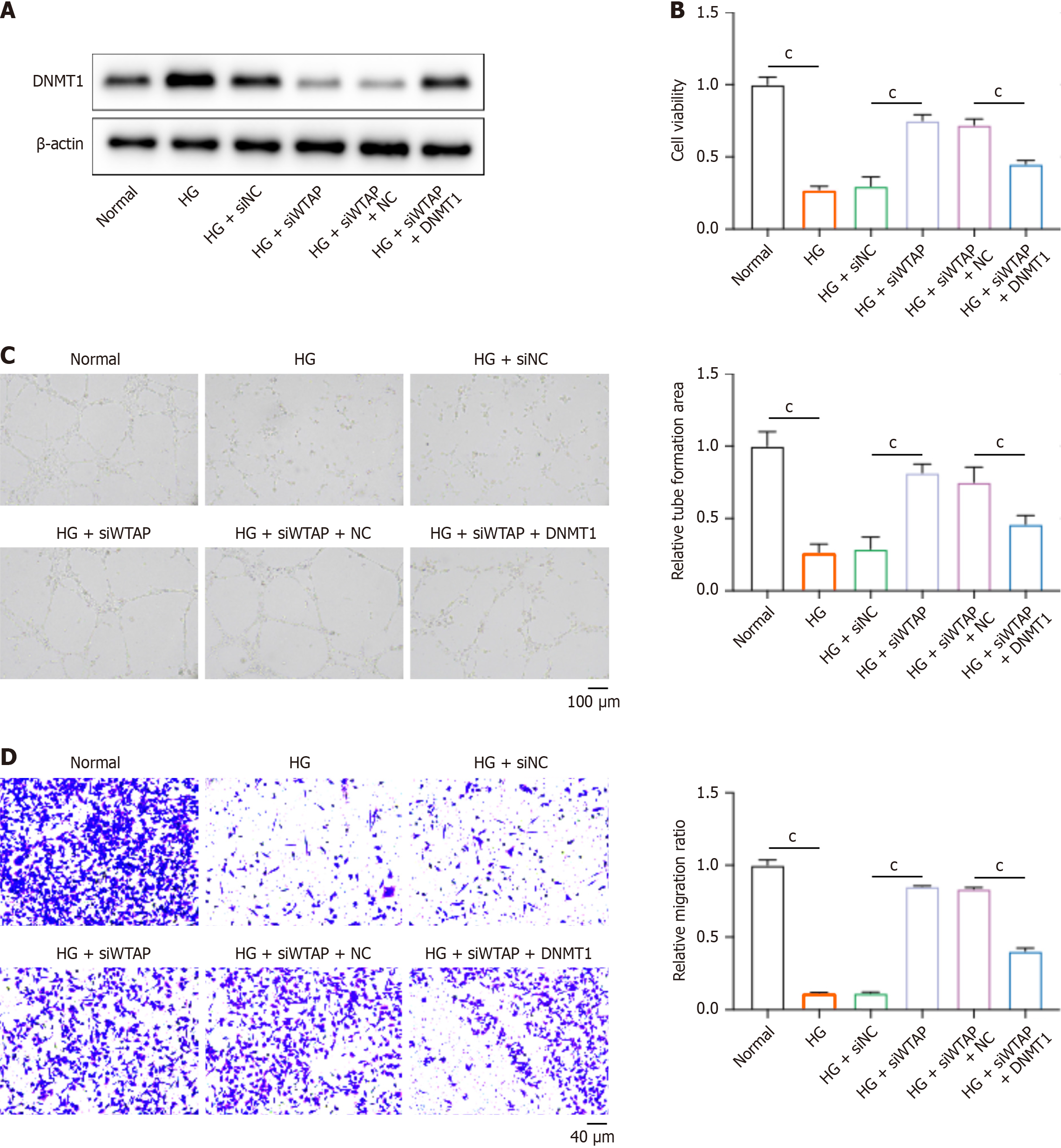
To identify the regulatory axis of WTAP-DNMT1 in HUVECs during diabetic wound healing, we treated HUVECs with siWTAP and DNMT1 overexpression vectors. DNMT1 Level was elevated in HG-induced HUVECs compared with normal cells. WTAP knockdown reduced the expression of DNMT1, whereas DNMT1 overexpression vectors effectively recovered DNMT1 expression in HUVECs (Figure 5A). Furthermore, the knockdown of WTAP in HUVECs restored cell viability (Figure 5B), tube formation (Figure 5C), and migration (Figure 5D) in HG-induced HUVECs, whereas overexpression of DNMT1 abolished these effects of siWTAP.
The complications associated with wound healing, often resulting in foot ulcers and, in severe cases, amputation, are significant contributors to mortality in individuals with diabetes[24-26]. Accordingly, there is a pressing need for novel therapeutics aimed at enhancing wound healing, particularly in patients with diabetes. Wound healing is a dynamic and systematic process that encompasses epithelialization, angiogenesis, granulation tissue formation, and wound contraction and involves multiple cell types, such as keratinocytes, immune cells, vascular endothelial cells, and fibroblasts[7,27,28]. At the inflammation stage, monocytes migrate to the wound area and differentiate into macrophages, aiding in pathogen clearance[7]. During the proliferative phase of wound healing, fibroblasts infiltrate the wound bed, secreting extracellular matrix proteins to form granulation tissue[29]. On the other hand, endothelial progenitor cells play a crucial role in wound neovascularization, and endothelial cells regulate vasoconstriction and vasodilation[28]. In this study, we identified that RNA methyltransferases such as METTL3, METTL14, METTL16, and WTAP were significantly elevated in HG-induced vascular endothelial cells. We further determined that WTAP knockdown alleviated the damage to HUVECs caused by HG, manifested as enhanced proliferation, migration, and tube formation. Consistently, previous studies indicated that knockdown of WTAP attenuates HG-induced cell pyroptosis and NLRP3-related pro-inflammatory cytokines in HK-2 cells and diabetes mice[16].
Accumulating studies have revealed that endothelial dysfunction is indeed a key factor in the development of vascular complications in diabetes, and inflammation is intricately involved, often influenced by epigenetic changes[30]. M6A is a common epigenetic modification found in eukaryotic cells and has been implicated in various cellular processes, including those related to endothelial function and dysfunction[31,32]. For example, the m6A demethylase FTO, has emerged as a crucial regulator in diabetes-associated dysfunction of vascular endothelial cells. Increased FTO activity leads to a reduction in the overall m6A levels in the context of hyperglycemia, and knockdown of FTO in endothelial cells has been shown to mitigate inflammation and impair cell migration and tube formation[32]. Additionally, increased levels of METTL3 have been observed in HG-induced HUVECs, accompanied by elevated m6A methylation levels. Functionally, silencing METTL3 suppresses apoptosis and restores the proliferation of HUVECs exposed to HG conditions[31]. These findings highlight the important roles of epigenetic regulation in diabetic complications. Here, we identified elevated expression of WTAP, another well-known RNA methyltransferase, in HG-induced HUVECs, and identified the potential downstream target. We conducted online analysis and identified potential m6A modified motif on DNMT1 mRNA associated with WTAP. Meanwhile, multiple studies have reported the crucial role of DNMT1 in wound healing. It has been reported that DNMT1 inhibits macrophage motility by repressing cholesterol levels in wound healing[33]. Interestingly, diabetes disrupts wound healing through a mechanism involving DNMT1-mediated dysregulation of hematopoietic stem cell differentiation toward macrophages[34]. Another study reported that transient hyperglycemia increases DNMT1 expression, leading to sustained activation of NF-κB and consequent endothelial dysfunction. Inhibiting DNMT1 has been found to enhance angiogenesis and expedite diabetic wound healing through modulation of the Ang-1/NF-κB signaling pathway[35]. Despite the fact that our findings indicated the critical role of WTAP in regulating endothelial function during diabetic complications, there are certain limits in the current study. Other regulators of RNA methylation, including METTL3 METTL14, METTL16, and RBM5 were also observed to be altered under HG condition. Further experiments, such as gene mutation and knockdown studies, should be performed to identify whether these regulators are involved in the regulation of DNMT1 in diabetic complications. Future studies may involve the exploration of WTAP inhibitors as therapeutic avenues and the interplay of WTAP with other signaling molecules pertinent to diabetic wound healing. Moreover, this potential regulatory axis could be verified in other diabetic complications in subsequent research.
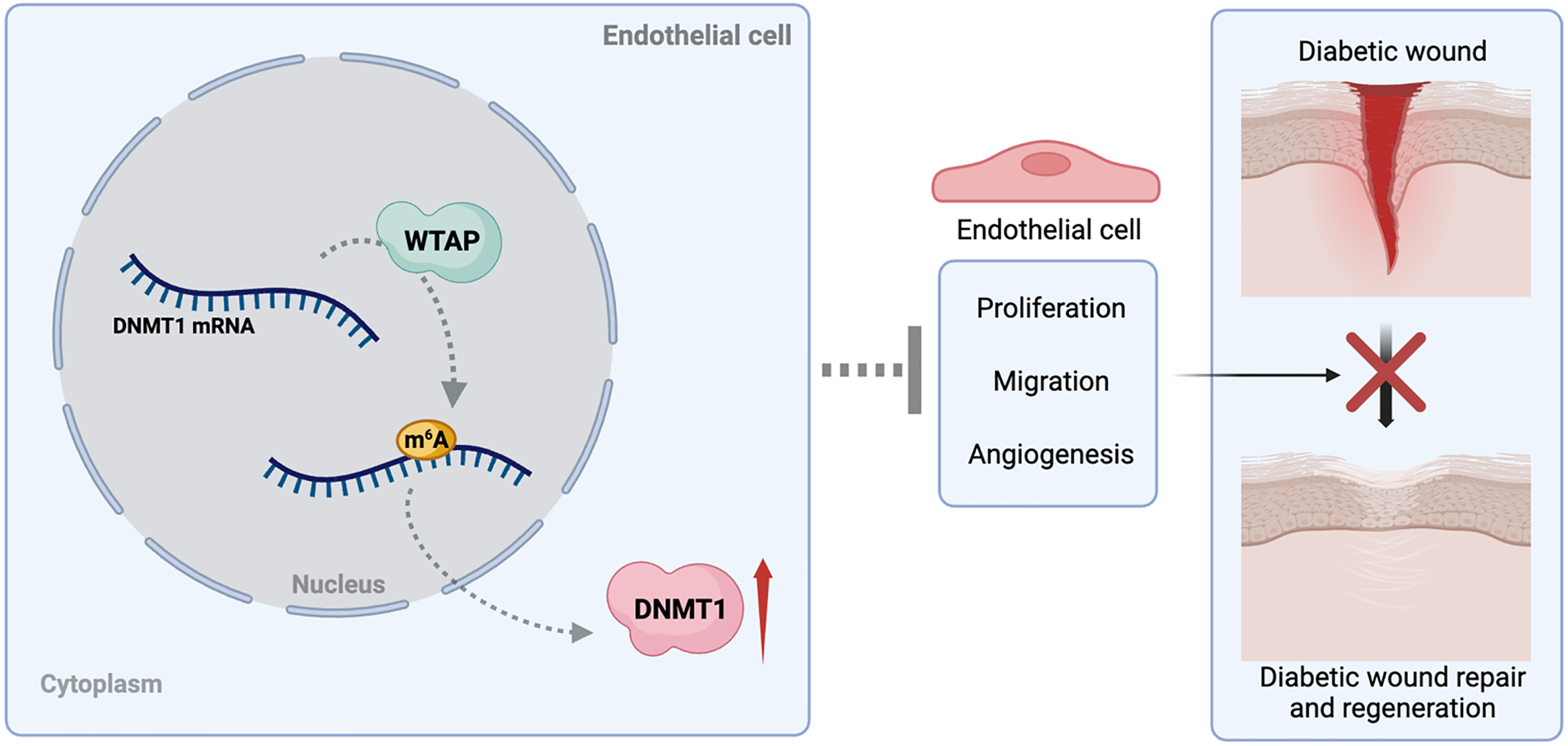
In this study, we analyzed the involvement of epigenetic regulation in diabetic wound healing. We identified that the RNA methyltransferase WTAP is significantly elevated under HG induction. Molecular mechanism analysis identified that WTAP elevated the m6A modification on DNMT1 and subsequently suppressed proliferation, migration, and angiogenesis of HUVECs, consequently inhibiting diabetic wound healing (Figure 6). Our findings provided a novel regulatory mechanism underlying diabetic wound healing and presented WTAP as a promising therapeutic target. Nevertheless, clinical translation will require extensive validation studies.
| 1. | Cheng AYY, Gomes MB, Kalra S, Kengne AP, Mathieu C, Shaw JE. Applying the WHO global targets for diabetes mellitus. Nat Rev Endocrinol. 2023;19:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4362] [Article Influence: 623.1] [Reference Citation Analysis (0)] |
| 3. | Ceriello A, Prattichizzo F. Variability of risk factors and diabetes complications. Cardiovasc Diabetol. 2021;20:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Joslin EP. The Prevention of Diabetes Mellitus. JAMA. 2021;325:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1536] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 6. | Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X, Yao MD, Li XM, Yao J, Zhou RM, Zhang SJ, Jiang Q, Zhao C, Yan B. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019;116:7455-7464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 7. | Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1617] [Article Influence: 269.5] [Reference Citation Analysis (0)] |
| 8. | Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep. 2018;18:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 9. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 10. | Grennan D. Diabetic Foot Ulcers. JAMA. 2019;321:114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Huang W, Chen TQ, Fang K, Zeng ZC, Ye H, Chen YQ. N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. J Hematol Oncol. 2021;14:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 12. | Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2803] [Cited by in RCA: 3071] [Article Influence: 219.4] [Reference Citation Analysis (0)] |
| 13. | Zhang N, Ding C, Zuo Y, Peng Y, Zuo L. N6-methyladenosine and Neurological Diseases. Mol Neurobiol. 2022;59:1925-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 929] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 15. | Huang Q, Mo J, Liao Z, Chen X, Zhang B. The RNA m(6)A writer WTAP in diseases: structure, roles, and mechanisms. Cell Death Dis. 2022;13:852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Lan J, Xu B, Shi X, Pan Q, Tao Q. WTAP-mediated N(6)-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell Mol Biol Lett. 2022;27:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Chen Z, Zhang Y. Role of Mammalian DNA Methyltransferases in Development. Annu Rev Biochem. 2020;89:135-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 18. | Mohan KN. DNMT1: catalytic and non-catalytic roles in different biological processes. Epigenomics. 2022;14:629-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 19. | Ren Y. Regulatory mechanism and biological function of UHRF1-DNMT1-mediated DNA methylation. Funct Integr Genomics. 2022;22:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 20. | Yan L, Geng Q, Cao Z, Liu B, Li L, Lu P, Lin L, Wei L, Tan Y, He X, Li L, Zhao N, Lu C. Insights into DNMT1 and programmed cell death in diseases. Biomed Pharmacother. 2023;168:115753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 21. | Cao K, Lv W, Wang X, Dong S, Liu X, Yang T, Xu J, Zeng M, Zou X, Zhao D, Ma Q, Lin M, Long J, Zang W, Gao F, Feng Z, Liu J. Hypermethylation of Hepatic Mitochondrial ND6 Provokes Systemic Insulin Resistance. Adv Sci (Weinh). 2021;8:2004507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Chen YT, Lin WD, Liao WL, Tsai YC, Liao JW, Tsai FJ. NT5C2 methylation regulatory interplay between DNMT1 and insulin receptor in type 2 diabetes. Sci Rep. 2020;10:16087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Zhao S, Yuan Q, Zhu L, Li F, Wang H, Kong D, Hao J. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death Dis. 2021;12:642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Liu Y, He W, Mu X, Wu X, Deng J, Nie X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front Immunol. 2022;13:918223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 25. | Sharifiaghdam M, Shaabani E, Faridi-Majidi R, De Smedt SC, Braeckmans K, Fraire JC. Macrophages as a therapeutic target to promote diabetic wound healing. Mol Ther. 2022;30:2891-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 26. | Dixon D, Edmonds M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs. 2021;81:29-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Rognoni E, Watt FM. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018;28:709-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019;204:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 29. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3157] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 30. | Yuan J, Liu Y, Zhou L, Xue Y, Lu Z, Gan J. YTHDC2-Mediated circYTHDC2 N6-Methyladenosine Modification Promotes Vascular Smooth Muscle Cells Dysfunction Through Inhibiting Ten-Eleven Translocation 2. Front Cardiovasc Med. 2021;8:686293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Li Z, Meng X, Chen Y, Xu X, Guo J. N(6)-methyladenosine (m(6)A) writer METTL3 accelerates the apoptosis of vascular endothelial cells in high glucose. Heliyon. 2023;9:e13721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Zhou C, She X, Gu C, Hu Y, Ma M, Qiu Q, Sun T, Xu X, Chen H, Zheng Z. FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1. J Clin Invest. 2023;133:e160517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 33. | Zhao C, Yang Q, Tang R, Li W, Wang J, Yang F, Zhao J, Zhu J, Pang W, Li N, Zhang X, Tian XY, Yao W, Zhou J. DNA methyltransferase 1 deficiency improves macrophage motility and wound healing by ameliorating cholesterol accumulation. NPJ Regen Med. 2023;8:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, Fazzio TG, Messina LM. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun. 2018;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 35. | Zhao J, Yang S, Shu B, Chen L, Yang R, Xu Y, Xie J, Liu X, Qi S. Transient High Glucose Causes Persistent Vascular Dysfunction and Delayed Wound Healing by the DNMT1-Mediated Ang-1/NF-κB Pathway. J Invest Dermatol. 2021;141:1573-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |