Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.98438
Revised: October 19, 2024
Accepted: November 25, 2024
Published online: February 15, 2025
Processing time: 187 Days and 1.5 Hours
Gestational diabetes mellitus (GDM) is a growing public health concern, particularly in regions with diverse ethnic populations. Understanding the incidence and risk factors of GDM is crucial for early prevention and management, es
To investigate the incidence of GDM and identify its associated risk and pro
A multi-center retrospective study was conducted, dividing participants into GDM and non-GDM groups according to standardized diagnostic criteria. Data were collected from 103629 deliveries across 40 hospitals in Guizhou. Various demographic, clinical, and laboratory parameters were analyzed using logistic regression to identify risk and protective factors for GDM.
Among the 103629 deliveries, 18957 cases of GDM were identified, with an incidence of approximately 18.3%. The risk of GDM was higher in the Han ethnic group compared to minority ethnic groups. The Dong ethnic group had the lowest incidence among the minorities. Key risk factors identified included older age (especially > 35 years), higher pre-pregnancy body mass index (BMI), light physical activity, gravidity, family history of diabetes, hemoglobin, aspartate aminotransferase, alanine aminotransferase, and direct bilirubin. Protective factors included higher education level, total protein, and albumin. There were also differences based on blood type, with type A associated with higher risk.
The incidence rate in Guizhou is 18.3%. Older age (especially > 35 years), Han ethnicity, lower education level, higher pre-pregnancy BMI, light physical activity, and higher gravidity are the main risk factors for GDM. Laboratory findings indicate that higher hemoglobin, higher liver function parameters (alanine aminotransferase, aspartate aminotransferase, and direct bilirubin), and lower total protein and albumin are associated with a higher risk of GDM. Blood type A has a higher risk of GDM compared to blood types AB and O.
Core Tip: Guizhou Province, located in southwest China, is a multi-ethnic inhabited area with a unique geographical environment, including plateaus, mountains, and hilly areas. Moreover, medical resources in Guizhou are relatively limited. This study investigates the incidence and related high-risk factors of gestational diabetes mellitus (GDM) in Guizhou, evaluates the prevention and treatment measures for gestational diabetes in Guizhou, and puts forward corresponding preventive measures to guide disease monitoring and early intervention for different high-risk factors, so as to provide a reference for the prevention and treatment of GDM in the region, so as to reduce the incidence of maternal and infant complications.
- Citation: Dong L, Zhong W, Qiao T, Wang Z, Liang Y, Zhao DQ. Investigation and study on the epidemiology of gestational diabetes mellitus in Guizhou. World J Diabetes 2025; 16(2): 98438
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/98438.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.98438
Gestational diabetes mellitus (GDM) is characterized by normal blood glucose levels prior to pregnancy, which shift to abnormal glucose tolerance during the gestational period. This condition represents approximately 80%-90% of all diabetes cases diagnosed in pregnant women. Typically, most women will see a return to normal blood glucose levels after childbirth[1]. The incidence of GDM is increasing worldwide, becoming a significant issue affecting maternal and fetal health. GDM may lead to serious adverse outcomes and severe short-term and long-term complications for both mother and child[2,3]. Guizhou Province, located in southwestern China, is economically underdeveloped, and research on GDM in this region is sparse. This study focuses on exploring the epidemiology and risk factors of GDM in a multi-ethnic region, providing valuable insights for early prevention and management of GDM. At present, the research focus on GDM predominantly centers on major urban centers and developed regions, both domestically and internationally. Relatively speaking, there is limited research on this issue in the Guizhou region. Situated in the southwestern part of China, Guizhou Province comprises nine prefecture-level cities and is characterized by its multi-ethnic population and unique geographical traits, encompassing high plateaus, mountainous terrain, and hilly landscapes. Moreover, the rapid socioeconomic advancement and elevation of living standards in Guizhou have brought about notable shifts in the populace’s lifestyles and dietary patterns. These factors could potentially result in divergence in the incidence and attributes of GDM in Guizhou compared to other areas. Besides, the availability of medical resources in Guizhou is relatively constrained, and the infrastructure is less developed in comparison to more affluent regions. Hence, the implementation of an epidemiological investigation and research on GDM in Guizhou is of paramount importance. In this present study, the incidence rate of gestational diabetes in Guizhou was obtained and compared with the national incidence rate of gestational diabetes, as well as with other regions in China, and the incidence rate of gestational diabetes in various regions of Guizhou was compared to evaluate the effect of prevention and control measures for gestational diabetes in Guizhou, which has guiding significance for diabetes prevention and control and policy formulation in Guizhou. It provides reference and scientific basis for prevention and control policies in other similar areas, and provides ideas for the prevention and control of gestational diabetes nationwide and globally. Moreover, the study will validate the recognized risk factors of GDM, explore potential influencing factors, and provide new clues and research directions for further exploration of the causes and mechanisms of GDM. Based on this research, high-risk groups can be identified, and personalized intervention measures can be provided to reduce the incidence of maternal and neonatal complications[4-7].
Although GDM is a common and prevalent disease, there is limited research on this disease in the Guizhou region. This study is innovative in providing the overall incidence rate of GDM in Guizhou and its various regions, comparing it with the national and domestic rates, and evaluating the prevalence in high-altitude areas. Additionally, the unique multi-ethnic settlement characteristics of Guizhou will be explored to investigate potential differences in GDM prevalence among different ethnic groups, providing a basis for further in-depth research.
Study objects: Part I study objects: A cross-sectional stratified sampling survey was conducted using the medical record information system. The total number of deliveries and GDM patients in 40 cooperative hospitals (tertiary and secondary) in Guizhou Province from January 1, 2021 to December 31, 2021 were reviewed. A total of 103629 deliveries were collected, including 18957 GDM patients.
Part II study objects: Clinical data of pregnant women who delivered in representative hospitals in Guizhou Province during the study period was retrospectively collected using the Wenjuanxing platform. Participants were screened according to strict inclusion and exclusion criteria. A total of 2838 pregnant women were selected as the GDM group, and 2800 healthy pregnant women were selected as the non-GDM group.
Inclusion criteria for the GDM group: (1) Abnormal results in the oral glucose tolerance test (OGTT) at 24-28 weeks of gestation; (2) No diagnosis of diabetes or impaired glucose tolerance before pregnancy; (3) Singleton, naturally conceived; and (4) Good clinical compliance.
Exclusion criteria for the GDM group: (1) Presence of mental disorders; and (2) Incomplete clinical data.
Inclusion criteria for the non-GDM group: (1) Normal OGTT results; (2) No history of major organ structural or functional abnormalities; (3) Good clinical compliance; and (4) Standardized birth inspection.
Exclusion criteria for the non-GDM group: (1) Presence of mental disorders; (2) Unable or refusing to cooperate with investigators; (3) Diagnosed with diabetes mellitus, chronic hypertension, rheumatism, or polycystic ovary syndrome; and (4) Incomplete clinical data.
Sample size calculation for part I: The sample size for part I was calculated based on the incidence of GDM in Guizhou Province. According to the descriptive epidemiological study, a cross-sectional stratified sampling survey was designed. According to the reference, the incidence of GDM in China is approximately 17.5%[8]. With = 0.05, = 1.96, = 0.175, and a tolerance error of 10%, the sample size was calculated using equation
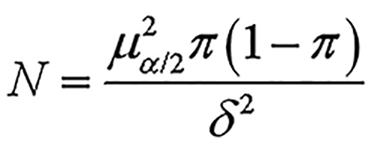
to be 18120. Considering a dropout rate of 10%, the final sample size was determined to be 20140. With = 0.05, = 1.96, = 0.175, = 0.1. Finally, during the investigation, 103629 total deliveries were obtained, meeting the sample size requirements for part I.
Sample size calculation for part II: The sample size for part II was designed as a case-control study. Binary logistic regression was used to screen for risk factors. According to the events per variable (EPV) method, an EPV of 10 could ensure the stability of statistical results[9]. Considering 20 variables [e.g., age, body mass index (BMI), education level, occupation, gravidity, parity, ethnicity, family history, blood type, hemoglobin, and 10 unknown variables] and an incidence of GDM of approximately 17.5%, the sample size was calculated using equation to be 1142 cases. A total of 2838 GDM cases and 2800 non-GDM cases were collected, meeting the sample size requirements for Part II.
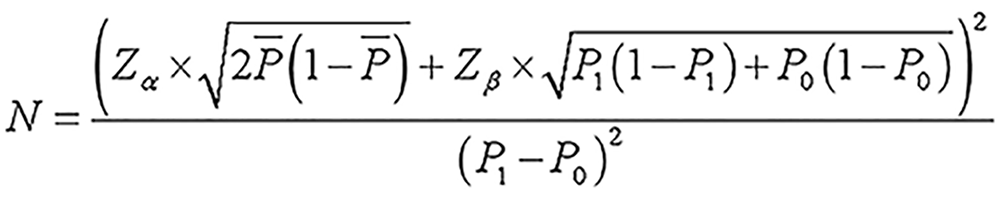
Sample allocation including: (1) Geographical areas were assigned according to different regions of Guizhou to ensure the geographical representation of the samples; (2) Selected hospitals in different areas included tertiary and secondary hospitals with large local delivery volumes to reflect the stratified sampling study and compare disease distribution under different medical levels and resources; (3) Considering the feasibility of the investigation, communication with medical institutions in each area was conducted to ensure smooth implementation of the research based on the principle of voluntary cooperation; (4) Selected hospitals served different population groups with varying ages, socioeconomic status, and ethnic backgrounds, helping to improve the universality and external validity of the study findings; and (5) Selected hospitals had good medical record systems and data collection capabilities, providing high-quality and reliable clinical data to improve the accuracy and validity of the epidemiological studies.
GDM diagnosis criteria: The unified diagnostic criteria from the ninth edition of the textbook “Obstetrics and Gynecology” (People’s Medical Publishing House) were used. All subjects underwent an OGTT between 24 and 28 weeks of gestation. GDM was diagnosed when one or more abnormal glucose tolerance test results were observed (fasting blood glucose ≥ 5.1 mmol/L; 1-hour ≥ 10.0 mmol/L; 2-hour ≥ 8.5 mmol/L).
OGTT method: The 75 g OGTT method was employed: Patients fasted for 8-10 hours before examination and were given a normal diet for 3 consecutive days prior to the examination, ensuring a daily carbohydrate intake greater than or equal to 150 g. During the examination, patients remained sedentary and refrained from smoking. A 300-mL glucose solution (based on 75 g powder) was orally ingested within 5 minutes, and venous blood samples were collected before drinking, as well as 1 hour and 2 hours after drinking.
Observation index: Medical records were reviewed, and the Wenjuanxing was utilized to complete the registration, primarily encompassing: (1) General information about pregnant women: Maternal age, ethnicity, education level, occupation, pre-pregnancy body mass index (pre-pregnancy BMI), gravidity, parity, etc; (2) Family history and past history: Family history of diabetes, hypertension, previous history of GDM, and endocrine diseases in immediate family members; (3) Laboratory examination: Blood routine tests (white blood cell, platelet, hemoglobin), biochemical tests (albumin, total protein, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, alkaline phosphatase, creatinine, urea nitrogen, uric acid, etc.), blood type, etc. Remarks: Relevant laboratory test results were collected within 24 hours post-admission, with the first test result selected if multiple measurements were taken during this period. In cases of missing test results, outpatient prenatal examination results from the day of admission were substituted; and (4) Mode of delivery, pregnancy complications, complications, adverse fetal outcomes, neonatal out
Quality control of data collection and processing: Data collection was carried out by trained healthcare personnel following standardized procedures to ensure consistency across all 40 hospitals. Additionally, each hospital had designated personnel responsible for a secondary review of the collected data to ensure accuracy and completeness.
During data entry, a double-entry system was implemented to minimize input errors. Prior to analysis, data cleaning procedures were conducted to identify and exclude outliers and missing values. A random sampling of the data was re-validated to ensure data integrity.
Data processing steps included data cleaning, classification, archiving, and analysis. During data cleaning, the completeness and accuracy of the data were verified, abnormal data were eliminated, and the collected data were classified and archived. Statistical product and service solutions 27.0 statistical software was employed to process the study indicators. Initially, the one-sample K-S goodness-of-fit method was used. mean ± SD was adopted for the statistical description of normally distributed data. The two independent sample t-test was used to compare means between the two groups, while the median (P25, P75) was used to describe the median of non-normally distributed data. The rank-sum test was used for group comparisons, with the statistic expressed as Z. Enumeration data were described by (%), and the χ2 test was used for statistical analysis. Disordered multi-category qualitative data were compared between groups with Bonferroni based on the χ2 test. After identifying statistically significant factors for GDM incidence through single factor difference analysis and considering potential confounding effects among these factors, binary multivariate logistic regression analysis was further performed to calculate the odds ratio (OR) value, estimating the strength of association between each factor and GDM while controlling for confounding bias. A P value of < 0.05 was considered statistically significant. The Hosmer-Lemeshow test was used to assess model goodness of fit, and receiver operating characteristic (ROC) curve analysis was employed to evaluate risk prediction efficiency.
GDM morbidity in Guizhou: A total of 103629 deliveries across 40 hospitals were collected, among which 18957 cases were GDM, resulting in an incidence of approximately 18.3% in Guizhou Province.
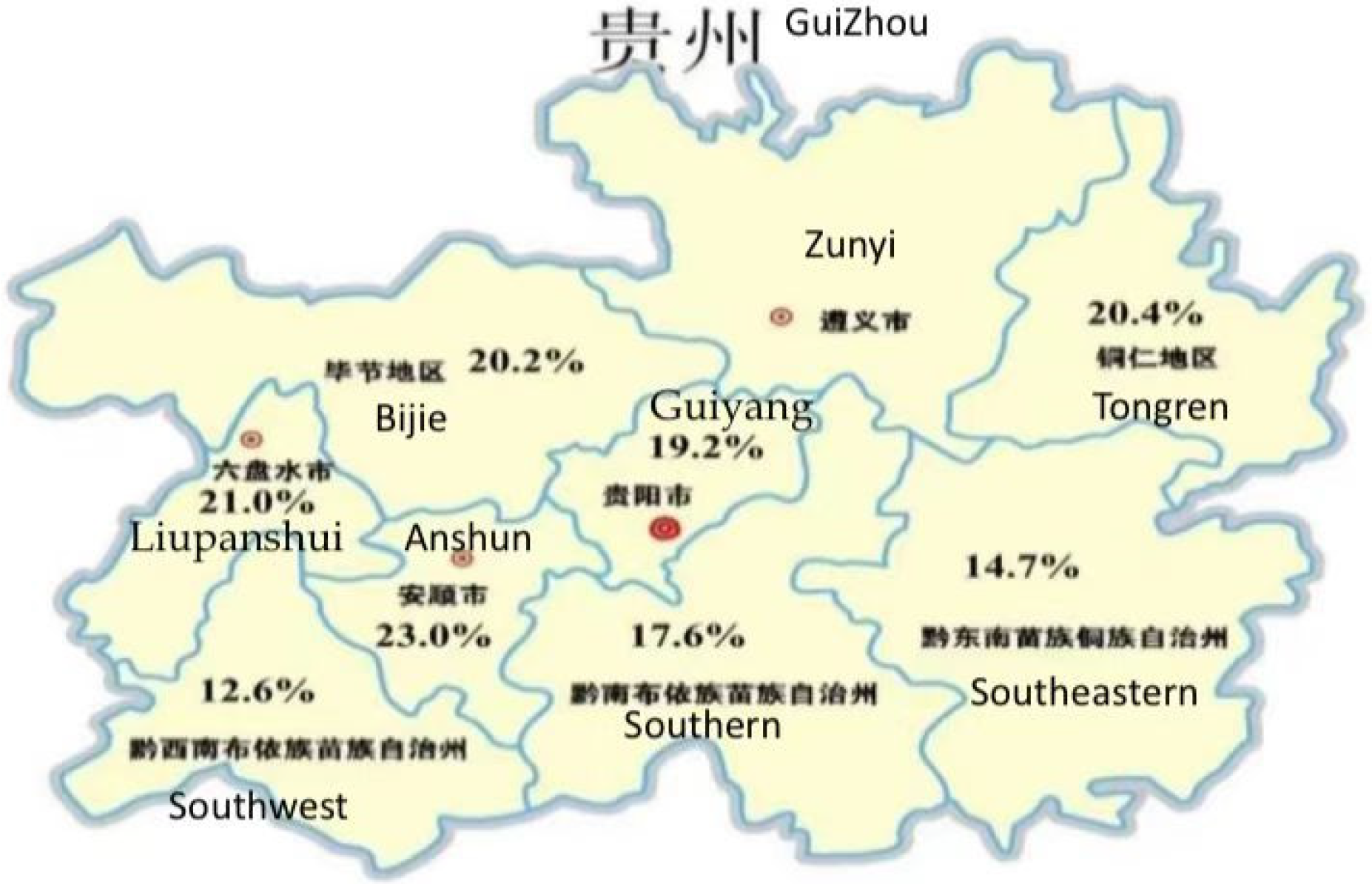
GDM morbidity in different regions of Guizhou: In order to ensure the representative of the regional hospital, adopt the sampling survey, each region choose hospital inclusion criteria for: tertiary hospital (delivery more than 2000), secondary hospital (delivery more than 1000) as a representative of regional typical hospital, Zunyi area in the sample survey into the sample size is less, consider the possible results bias, so not included in the criteria. The results showed that the incidence of GDM in Guiyang was 19.2%, Tongren 20.4%, Bijie 20.2%, Anshun 23.0%, Liupanshui 21.0%, Southwest Guizhou 12.6%, Southeastern Guizhou 14.7%, and Southern Guizhou 17.6% (Figure 1).
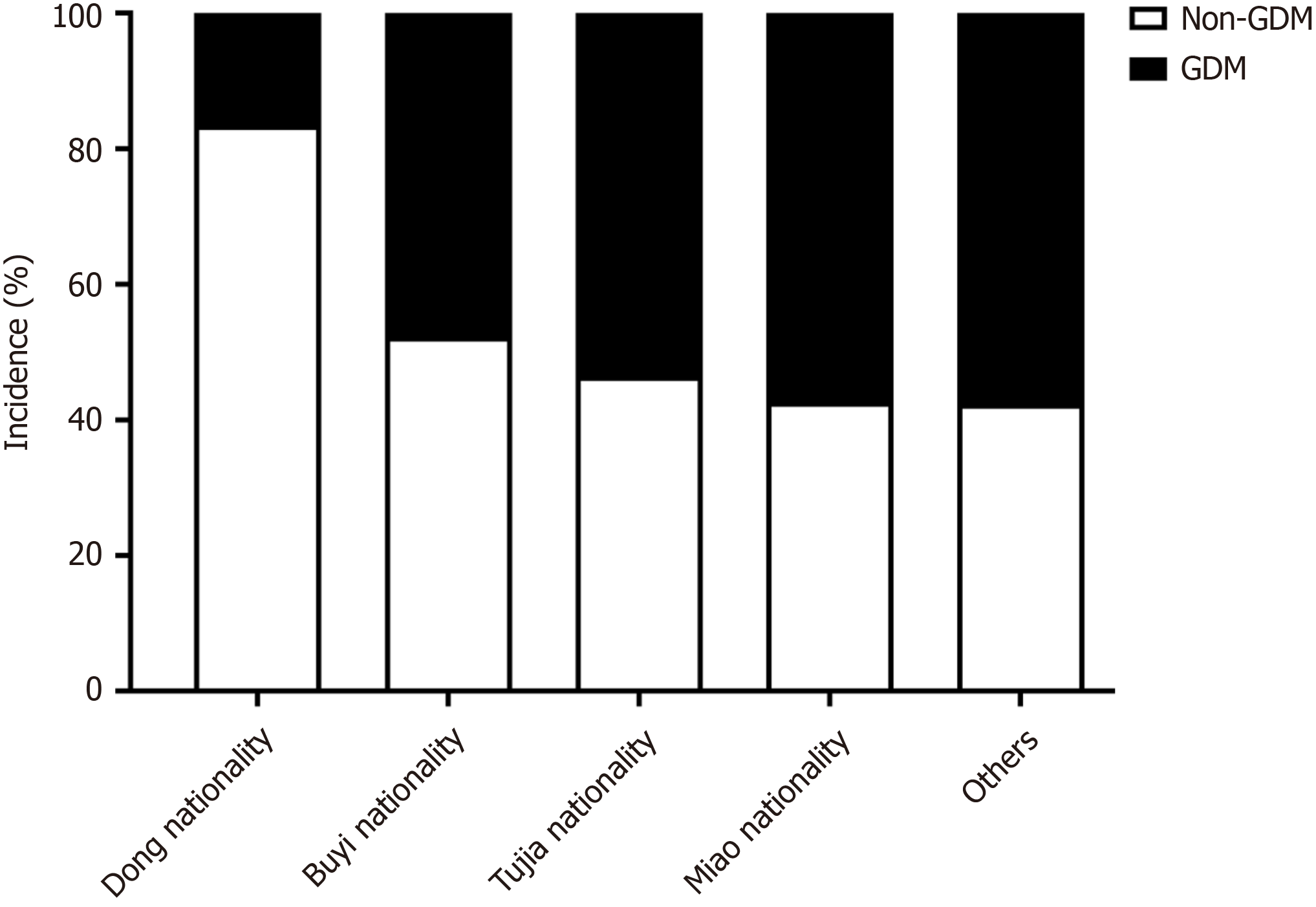
Incidence of GDM in different ethnic minorities in Guizhou: The Dong ethnic group had a significantly lower GDM incidence compared to other ethnic groups [χ² = 84.714; degrees of freedom (df) = 4; P < 0.001] (see Table 1 for detailed rates), highlighting potential cultural or lifestyle differences (Figure 2). The observed differences in GDM incidence across ethnic groups and regions may be attributed to a variety of factors, including dietary habits, cultural practices, so
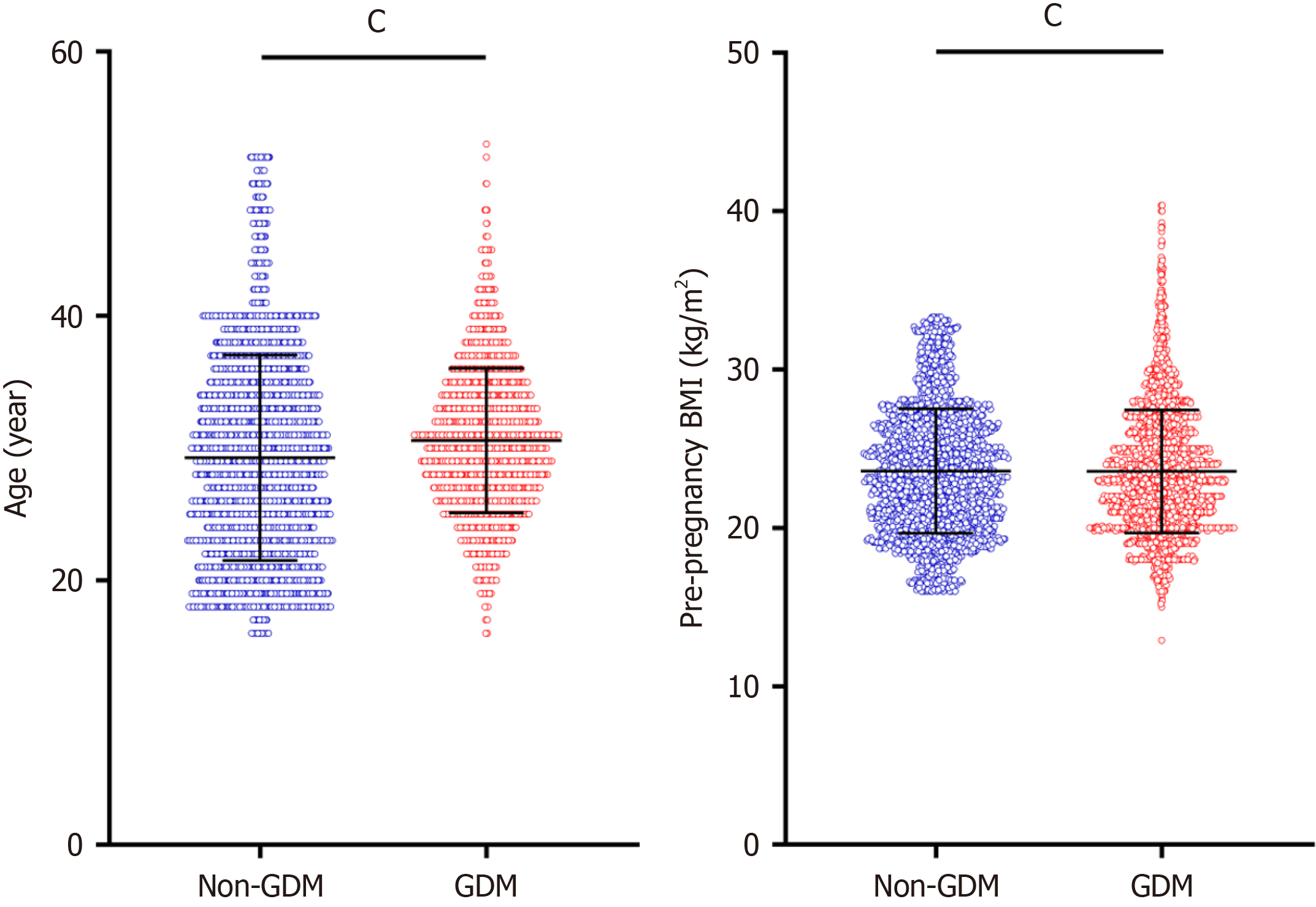
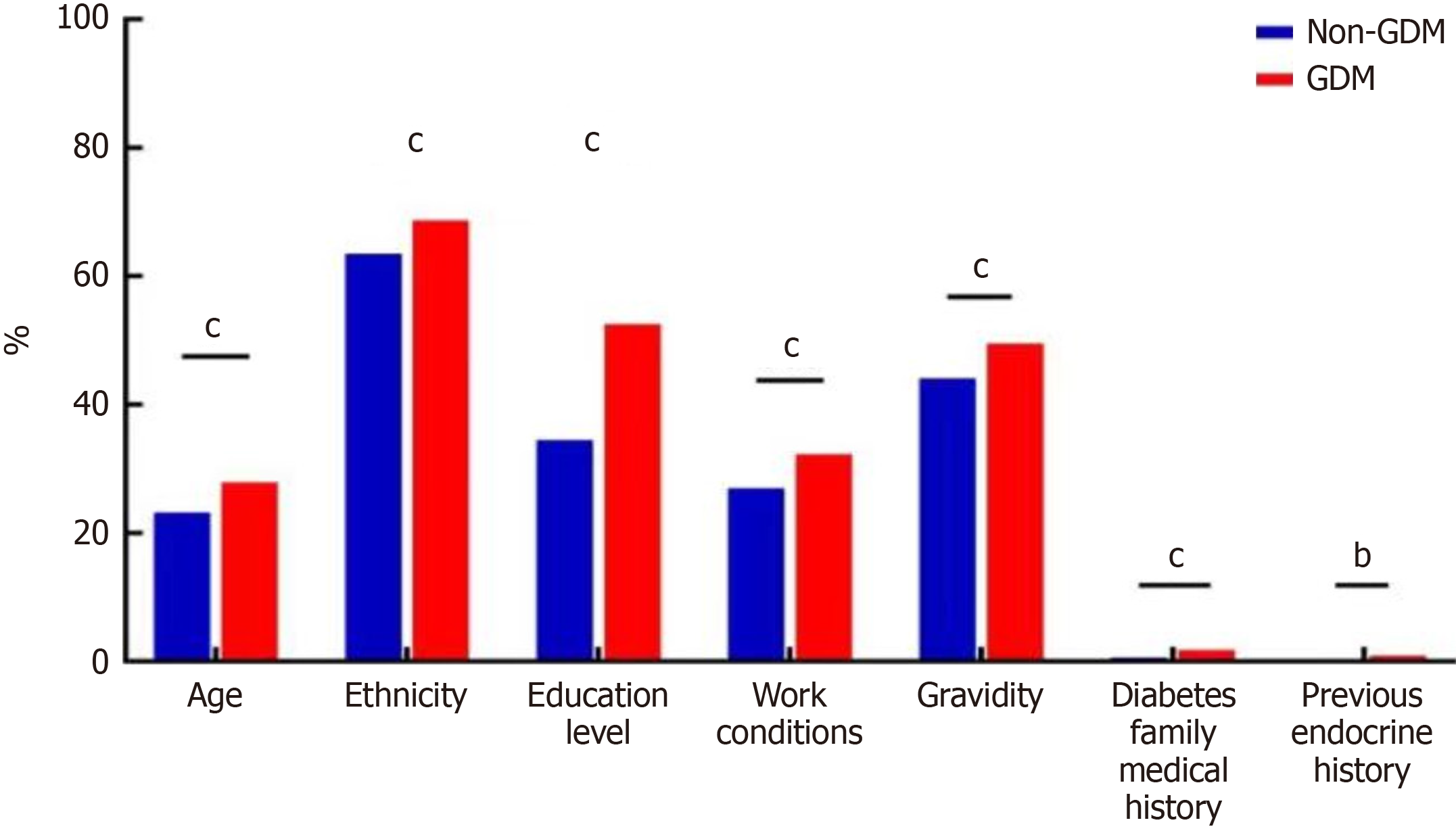
Single factor difference analysis of general data between GDM group and non-GDM group: Significant differences were observed between the two groups in terms of age, pre-pregnancy BMI, nationality, education level, occupation, gravidity, family history of diabetes, and previous history of endocrine diseases (P < 0.001). However, no significant differences were found in parity, previous history of GDM, or family history of hypertension between the groups (P > 0.05). See Table 2, Figure 3 and 4.
| Normal information | Non-GDM group (n = 2800) | GDM group (n = 2838) | χ²/t | P value |
| Age, year, mean ± SD | 28.28 ± 7.76 | 30.57 ± 5.47 | -7.212 | < 0.001 |
| Age segment, year | 16.069 | < 0.001 | ||
| < 35 | 2150 (76.8) | 2047 (72.1) | ||
| ≥ 35 | 650 (23.2) | 791 (27.9) | ||
| Pre-pregnancy BMI (kg/m²), mean ± SD | 23.59 ± 3.93 | 25.57 ± 3.88 | -19.036 | < 0.001 |
| Ethnicity | 17.081 | < 0.001 | ||
| Han | 1777 (63.5) | 1951 (68.7) | ||
| Minority | 1023 (36.5) | 887 (31.3) | ||
| Education level | 210.721 | < 0.001 | ||
| Junior-senior high school | 966 (34.5) | 1489 (52.5) | ||
| Technical secondary school and junior college | 1231 (44.0) | 786 (27.7) | ||
| Bachelor degree or above | 603 (21.5) | 563 (19.8) | ||
| Occupation1 | 19.567 | < 0.001 | ||
| None | 2045 (73.0) | 1920 (67.7) | ||
| Yes | 755 (27.0) | 918 (32.3) | ||
| Gravidity, times | 20.180 | < 0.001 | ||
| 1 | 702 (25.1) | 691 (24.4) | ||
| 2 | 864 (30.8) | 742 (26.1) | ||
| ≥ 3 | 1234 (44.1) | 1405 (49.5) | ||
| Parity | 2.674 | 0.263 | ||
| 0 | 443 (15.8) | 491 (17.2) | ||
| 1 | 1245 (44.4) | 1217 (42.8) | ||
| ≥ 2 | 1112 (39.8) | 1130 (40.0) | ||
| Family history of diabetes | 17.263 | < 0.001 | ||
| None | 2784 (99.4) | 2788 (98.2) | ||
| Yes | 16 (0.6) | 50 (1.8) | ||
| Past GDM medical history | 1.240 | 0.266 | ||
| None | 2785 (99.4) | 2816 (99.2) | ||
| Yes | 15 (0.6) | 22 (0.8) | ||
| Family history of hypertension | 1.787 | 0.181 | ||
| None | 2787 (99.5) | 2817 (99.2) | ||
| Yes | 13 (0.5) | 21 (0.8) | ||
| History of previous endocrine disorders | 7.360 | 0.007 | ||
| None | 2791 (99.7) | 2813 (99.1) | ||
| Yes | 9 (0.3) | 25 (0.9) |
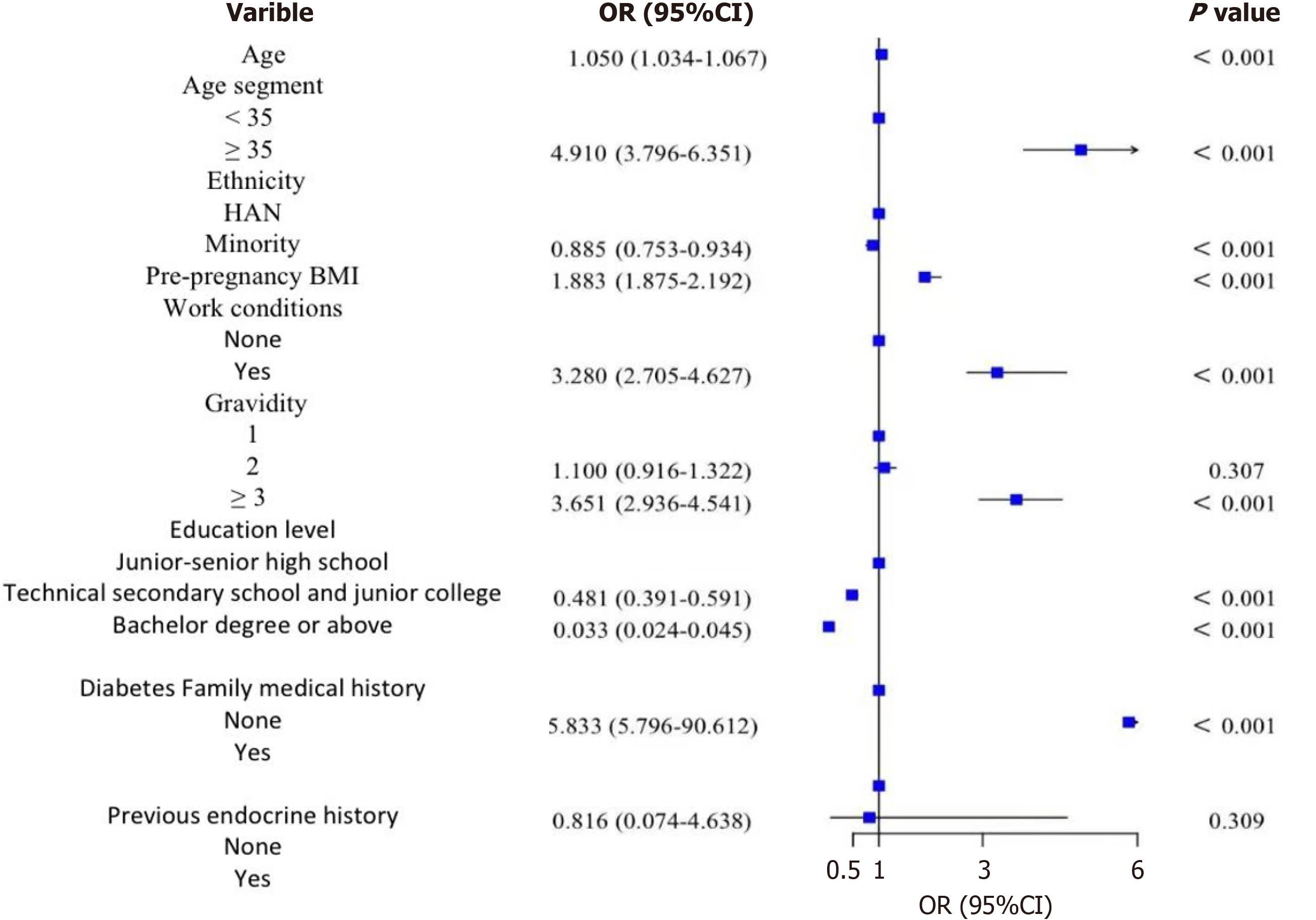
Binary logistic regression model analysis of general data: The variables with statistical significance between groups were analyzed using a binary logistic regression model. Age was found to be statistically significant for the incidence of GDM (B = 0.049; OR = 1.050; P < 0.001), indicating that the older the age, the higher the risk of GDM. For every one-year increase in maternal age, the risk of GDM increased by 0.05 times. The age segment was also statistically significant for GDM (P < 0.001), with the risk of GDM being higher in the population aged ≥ 35 years compared to those aged < 35 years (B = 1.591; OR = 4.910; P < 0.001). The risk of GDM in ethnic minorities was lower than that in the Han nationalities (B = -0.251; OR = 0.885; P < 0.001). Pre-pregnancy BMI was statistically significant for GDM (B = 0.124; OR = 1.883; P < 0.001), with a higher pre-pregnancy BMI associated with a higher risk of GDM. For every 1 kg/m2 increase in pre-pregnancy BMI, the risk of GDM increased by 0.883 times. The working population had a statistically significant effect on the occurrence of GDM (B = 3.342; OR = 3.280; P < 0.001), with people who were employed or had light working status having a higher risk of GDM. The number of pregnancies was statistically significant for the incidence of GDM (P < 0.001). Although there was no significant difference in the risk of GDM between two pregnancies and one pregnancy, the risk of GDM in the population with more than three pregnancies was higher than that in the population with one pregnancy (B = 1.295; OR = 3.651; P < 0.001). The risk of GDM in the group with junior college education was lower than that in the group with junior high school education (B = -0.733; OR = 0.481; P < 0.001), while the risk of GDM in the group with a bachelor’s degree or above was lower than that in the group with junior high school education (B = -3.423; OR = 0.033; P < 0.001), indicating that higher education levels were associated with a lower risk of GDM. Family history of diabetes was statistically significant for the occurrence of GDM (P < 0.001), with people having a family history of diabetes exhibiting a higher risk of GDM (B = 3.132; OR = 5.833; P < 0.001). The analysis is shown in Table 3 and Figure 5.
| Impact factors | Grouping | B | Standard error | Wald | OR | 95%CI | P value |
| Age | 0.049 | 0.008 | 36.97 | 1.050 | 1.034-1.067 | < 0.001 | |
| Age segment | Less than 35 years old | ||||||
| ≥ 35 | 1.591 | 0.131 | 146.888 | 4.910 | 3.796-6.351 | < 0.001 | |
| Ethnicity | Han | ||||||
| Minority | -0.251 | 0.153 | 2.708 | 0.885 | 0.753-0.934 | < 0.001 | |
| Pre-pregnancy BMI | 0.124 | 0.005 | 684.835 | 1.883 | 1.875-2.192 | < 0.001 | |
| Work conditions | None | ||||||
| Yes | 3.342 | 0.159 | 21.387 | 3.280 | 2.705-4.627 | < 0.001 | |
| Gravidity | 1 | 164.439 | < 0.001 | ||||
| 2 | 0.096 | 0.094 | 1.042 | 1.100 | 0.916-1.322 | 0.307 | |
| ≥ 3 | 1.295 | 0.111 | 135.59 | 3.651 | 2.936-4.541 | < 0.001 | |
| Education level | Junior-senior high school | 439.713 | < 0.001 | ||||
| Technical secondary school and junior college | -0.733 | 0.105 | 48.348 | 0.481 | 0.391-0.591 | < 0.001 | |
| Bachelor degree or above | -3.423 | 0.163 | 438.66 | 0.033 | 0.024-0.045 | < 0.001 | |
| Diabetes family medical history | None | ||||||
| Yes | 3.132 | 0.701 | 19.938 | 5.833 | 5.796-90.612 | < 0.001 | |
| Previous endocrine history | None | ||||||
| Yes | 1.661 | 1.002 | 0.536 | 0.816 | 0.074-4.638 | 0.309 |
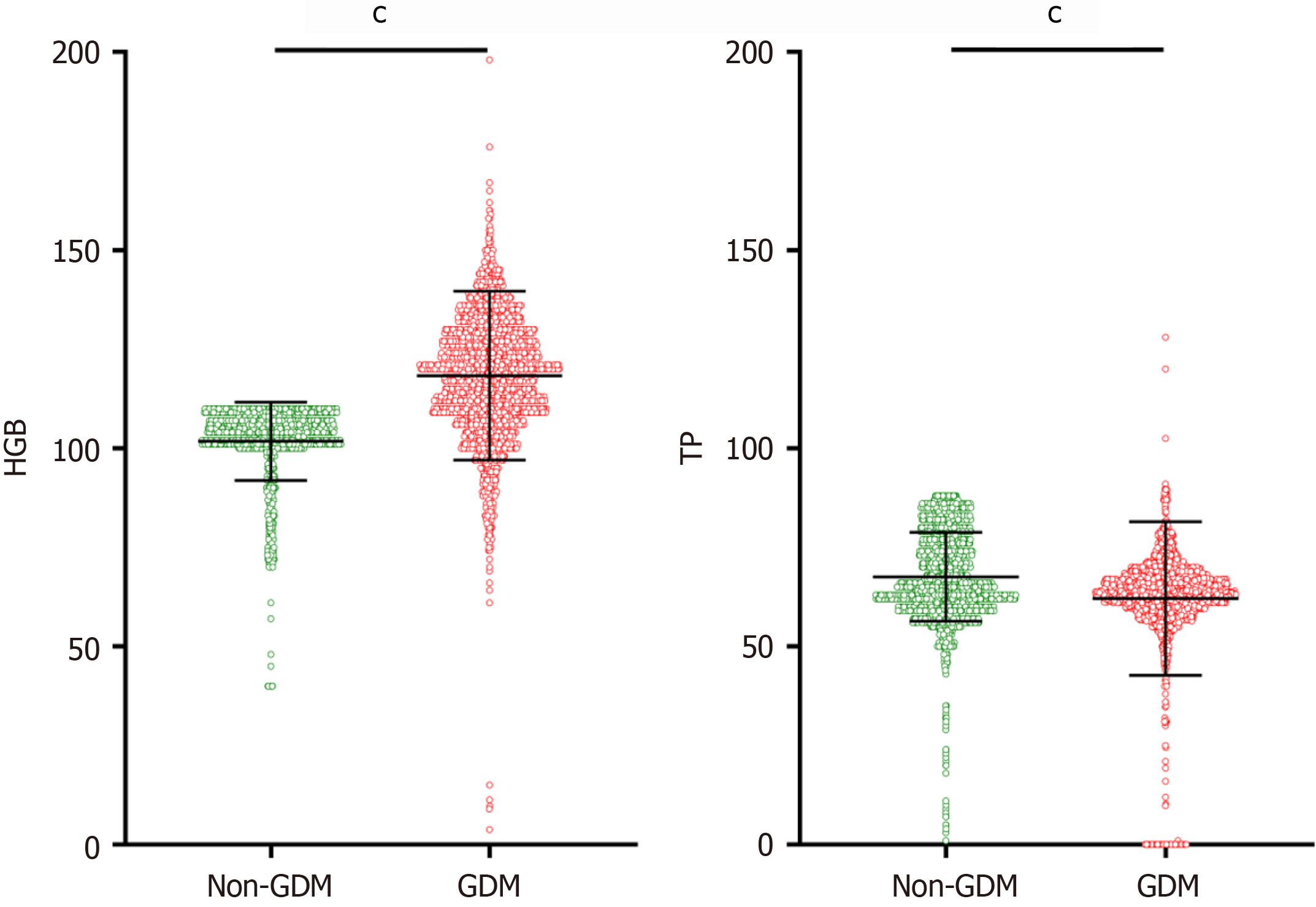
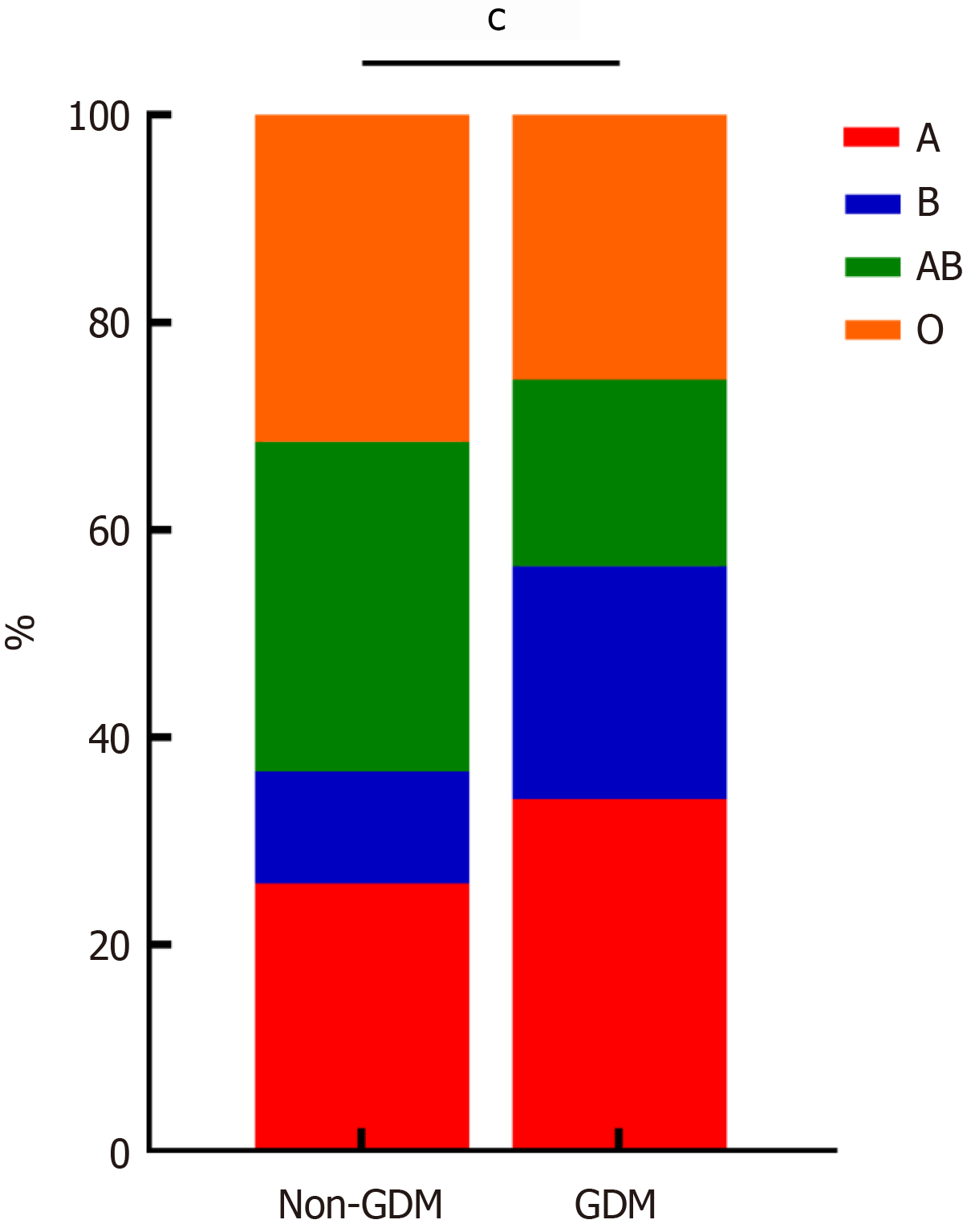
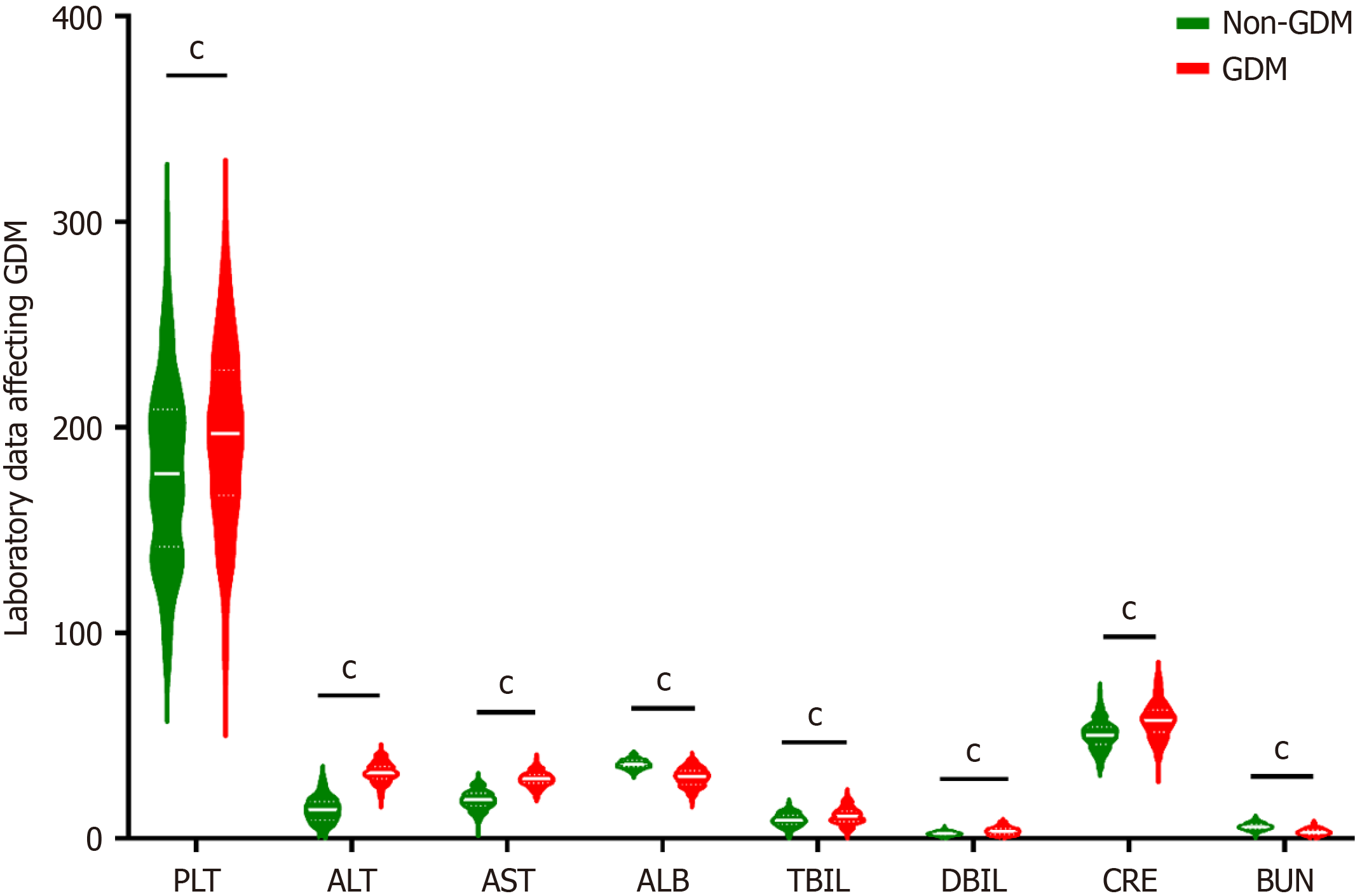
Single factor difference analysis of laboratory data between the GDM group and non-GDM group: The laboratory examination data for the two groups were compared, including blood routine (platelet, hemoglobin), biochemical examination (albumin, total protein, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, creatinine, blood urea nitrogen, etc.), and blood type. The differences were statistically significant (P < 0.001), while there was no statistically significant difference in white blood cell, alkaline phosphatase, or uric acid (P > 0.05), as shown in Table 4, Figure 6, Figure 7, and Figure 8.
| Normal information | Non-GDM group (n = 2800) | GDM group (n = 2838) | χ²/Z/t | P value |
| HGB | 101.75 ± 0.187 | 118.31 ± 0.400 | 44.472 | < 0.001 |
| WBC | 9.0 (6.0, 12.0) | 8.8 (7.0, 10.8) | -1.512 | 0.130 |
| PLT | 179.0 (137.0, 224.0) | 194.0 (153.0, 235.0) | -9.706 | < 0.001 |
| ALT | 13.0 (9.8, 22.9) | 32.0 (27.0, 36.0) | -48.016 | < 0.001 |
| AST | 18.1 (15.0, 24.7) | 29.0 (25.0, 32.0) | -42.249 | < 0.001 |
| TP | 67.56 ± 0.212 | 62.08 ± 0.363 | -12.468 | < 0.001 |
| ALB | 36.0 (33.7, 38.0) | 30.0 (27.0, 35.0) | -18.978 | < 0.001 |
| TBIL | 9.0 (6.4, 12.7) | 10.7 (6.9, 14.2) | -8.577 | < 0.001 |
| DBIL | 2.4 (1.8, 3.7) | 3.5 (1.7, 5.2) | -9.686 | < 0.001 |
| AKP | 157.0 (123.0, 178.0) | 158.0 (117.1, 198.0) | -4.772 | 0.152 |
| CRE | 50.0 (44.0, 58.0) | 58.0 (51.0, 68.0) | -26.454 | < 0.001 |
| BUN | 5.80 (4.2, 7.5) | 3.20 (2.6, 4.1) | -40.192 | < 0.001 |
| UA | 308.5 (259.1, 360.0) | 312.1 (261.0, 368.0) | -7.159 | 0.212 |
| Blood type | 272.546 | < 0.001 | ||
| Type A | 724 (25.9) | 966 (34.0) | ||
| Type B | 303 (10.8) | 638 (22.5) | ||
| Type AB | 890 (31.8) | 510 (18.0) | ||
| Type O | 883 (31.5) | 724 (25.5) |
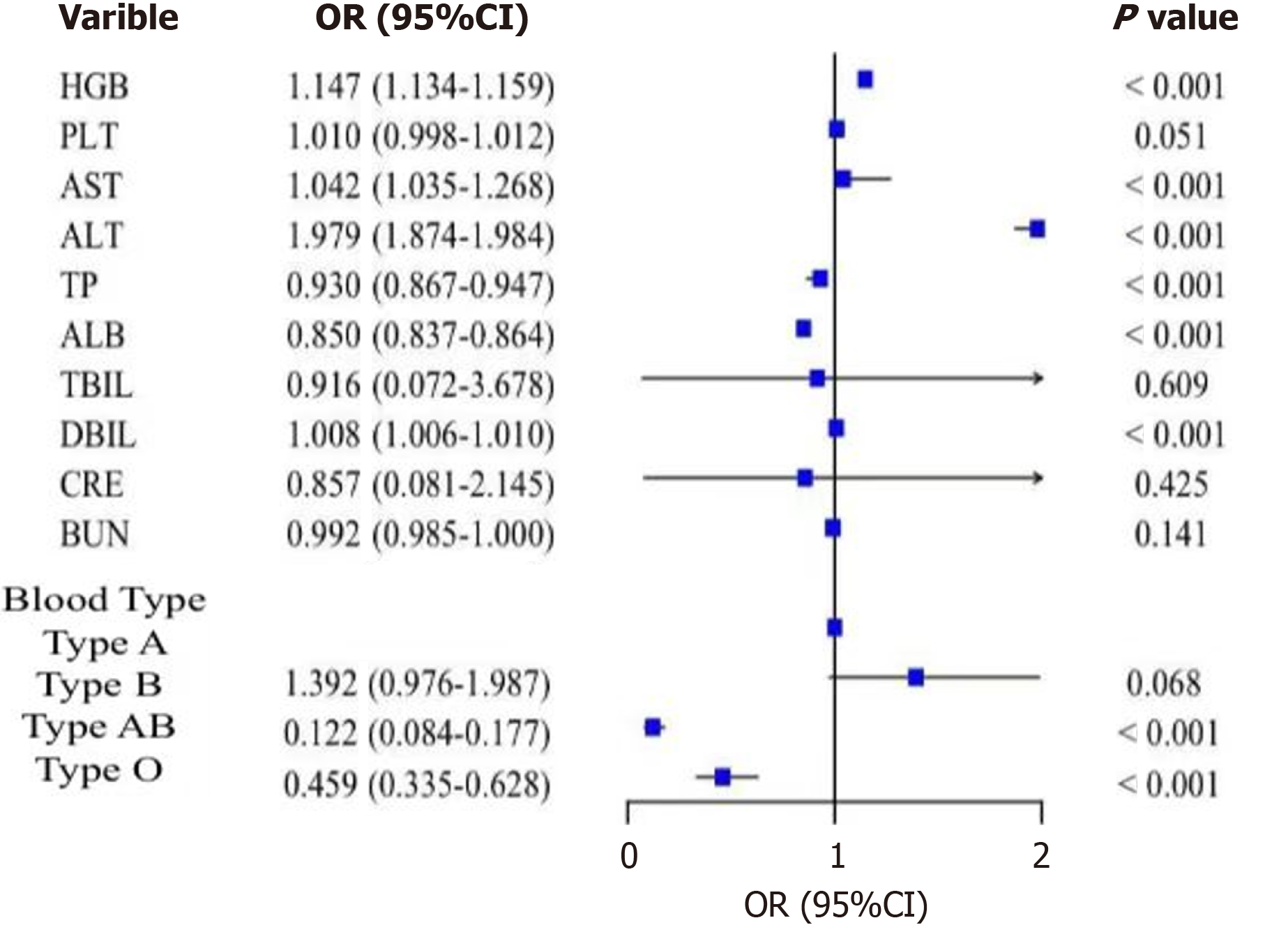
Binary logistic regression model analysis of laboratory data: Binary logistic regression analysis of laboratory data with statistical significance in the single-factor difference analysis showed that hemoglobin had a statistically significant effect on the occurrence of GDM (B = 0.137; OR = 1.147; P < 0.001). Higher hemoglobin levels were associated with a higher risk of GDM. Aspartate aminotransferase (B = 0.121; OR = 1.042; P < 0.001), alanine aminotransferase (B = 0.021; OR = 1.979; P < 0.001), and direct bilirubin (B = 0.008; OR = 1.008; P < 0.001) were also positively associated with GDM risk. Total protein (B = -0.266; OR = 0.930; P < 0.001) and albumin (B = -0.162; OR = 0.850; P < 0.001) were negatively associated with GDM risk, indicating that lower total protein and albumin levels were associated with a higher risk of GDM. Blood type differences were statistically significant for the occurrence of GDM (P < 0.001). The risk of GDM in blood group AB was lower than that in blood group A (B = -2.103; OR = 0.122; P < 0.001), and the risk of GDM in blood group O was lower than that in blood group A (B = -0.779; OR = 0.459; P < 0.001). The study showed that blood group A had a higher risk of GDM compared to blood groups AB and O. As shown in Table 5 and Figure 9.
| Impact factors | B | Standard error | Wald | OR | 95%CI | P value |
| HGB | 0.137 | 0.006 | 598.921 | 1.147 | 1.134-1.159 | < 0.001 |
| PLT | 0.010 | 0.001 | 71.200 | 1.010 | 0.998-1.012 | 0.051 |
| AST | 0.121 | 0.0035 | 68.145 | 1.042 | 1.035-1.268 | < 0.001 |
| ALT | 0.021 | 0.003 | 59.191 | 1.979 | 1.874-1.984 | < 0.001 |
| TP | -0.266 | 0.015 | 316.813 | 0.930 | 0.867-0.947 | < 0.001 |
| ALB | -0.162 | 0.008 | 393.139 | 0.850 | 0.837-0.864 | < 0.001 |
| TBIL | 0.635 | 1.012 | 43.623 | 0.916 | 0.072-3.678 | 0.609 |
| DBIL | 0.008 | 0.001 | 53.904 | 1.008 | 1.006-1.010 | < 0.001 |
| CRE | 0.635 | 1.712 | 69.623 | 0.857 | 0.081-2.145 | 0.425 |
| BUN | -0.008 | 0.004 | 4.159 | 0.992 | 0.985-1.000 | 0.141 |
| Blood Type | ||||||
| Type A | 146.589 | < 0.001 | ||||
| Type B | 0.331 | 0.181 | 3.336 | 1.392 | 0.976-1.987 | 0.068 |
| Type AB | -2.103 | 0.191 | 121.663 | 0.122 | 0.084-0.177 | < 0.001 |
| Type O | -0.779 | 0.160 | 23.646 | 0.459 | 0.335-0.628 | < 0.001 |
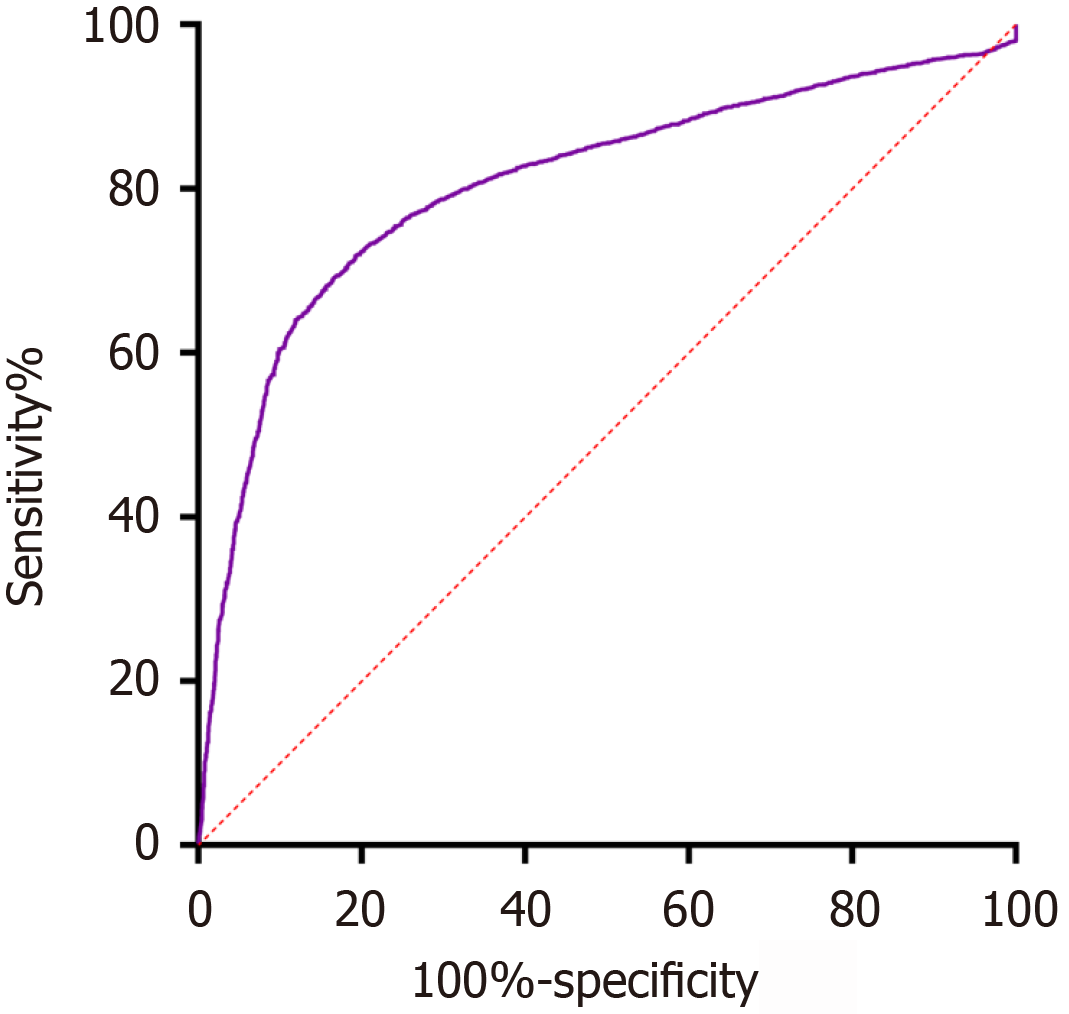
Evaluation of the ROC curve for prediction efficiency of the model: The Hosmer-Lemeshow goodness of fit test results of the logistic regression equation model showed P = 0.204 > 0.05 (χ2 = 5.940; df = 8), indicating a good goodness of fit effect of the equation model. The area under the ROC curve was 0.805 [95% confidence interval (CI): 0.794-0.817], indicating good model discrimination, with a specificity of 0.807, a sensitivity of 0.719. The maximum Youden index of 0.526 was obtained at the optimal cut-off point on the ROC curve. This index represents the point at which the model achieves the best balance between sensitivity and specificity. A Youden index of 0.526 indicates that the model has good discriminatory power, optimizing both the true positive rate (sensitivity) and the true negative rate (specificity) for identifying GDM cases (Figure 10).
Comparison between the incidence of GDM in Guizhou and the national incidence of GDM: This study found that the incidence of GDM in Guizhou Province is 18.3%, slightly higher than the national average of 17.5%. Given the limited healthcare resources in Guizhou, some patients may have insufficient awareness of prenatal check-ups, potentially underestimating the actual GDM incidence. Increasing awareness and improving the capabilities of local hospitals are essential strategies to reduce the GDM incidence and ensure maternal and child safety. The regional and ethnic differences in GDM incidence observed in this study may be influenced by several factors. For example, the higher incidence in Han populations could be linked to dietary patterns rich in starches and fats, while ethnic minorities such as the Dong, who traditionally consume a more balanced diet with lower fat intake, showed lower incidence rates. Additionally, lifestyle factors such as physical activity levels, socioeconomic disparities, and access to prenatal care services likely contribute to these variations. Future research should investigate these factors in more detail to provide a more comprehensive understanding of the mechanisms behind the ethnic and regional differences in GDM risk[10].
Occupation as a risk factor: Our findings indicate that light physical activity are associated with a higher risk of GDM, possibly due to lower levels of physical activity and longer sedentary periods. Future interventions should focus on promoting physical activity and reducing sedentary behavior, particularly in workplace settings. These findings highlight the importance of incorporating occupational factors into GDM prevention strategies.
Comparison of the incidence of GDM between Guizhou and other regions in China: Compared to other regions, such as Chongqing (26.2%) and Beijing (20%), Guizhou’s GDM incidence is lower but higher than that of coastal regions like Hebei and Shenzhen. The unique geographic, dietary, and environmental conditions in Guizhou, along with unequal access to medical resources, likely contribute to this higher incidence. Further research should focus on the influence of altitude, dietary habits, and healthcare access in this region[11-16].
Comparison of GDM incidence among different ethnic groups in Guizhou: Significant regional variations in GDM incidence were observed within Guizhou, with Anshun having the highest rate (23%) and Qianxinan the lowest (12.6%). This disparity may be related to local dietary habits and differences in medical care. Further studies should explore these regional differences to develop targeted prevention strategies, especially in high-incidence areas like Anshun.
Comparison of the risk of GDM between Han and ethnic minorities in Guizhou: The study found that GDM incidence was lower among ethnic minorities, particularly the Dong ethnic group (16.5%), compared to the Han population. Lifestyle, dietary habits, and genetic factors may contribute to these differences. Future studies should further explore the role of ethnicity in GDM incidence to provide more personalized prevention and intervention strategies[17,18].
The relationship between age and GDM: Han women exhibited a higher risk of GDM than ethnic minorities, which may be related to dietary and cultural differences. Future research should examine both genetic and environmental factors to better understand this disparity and inform targeted early interventions. Age is a significant risk factor for GDM, with older women (especially over 35 years) showing a higher risk. Efforts should be made to encourage women to plan pregnancies before the age of 35 to reduce age-related GDM risks.
Relationship between pre-pregnancy BMI and GDM: Higher pre-pregnancy BMI is strongly associated with GDM risk. Weight control through a balanced diet and regular exercise should be emphasized during pregnancy to lower GDM incidence[19].
Relationship between education level and GDM: Lower education levels were associated with higher GDM risk. Raising awareness through prenatal education and promoting healthy lifestyle choices can help reduce GDM incidence in less-educated populations[20,21].
Relationship between work status and GDM: Light physical activity women exhibited a higher GDM risk, possibly due to stress and lack of physical activity. Future studies should explore the impact of work stress and lifestyle factors on GDM development.
The relationship between gravidity and GDM: Multiple pregnancies increase the risk of GDM, particularly in older women. As multiple pregnancies become more common, early detection and intervention will be critical in managing GDM risk[22].
The relationship between blood type and GDM: ABO blood groups are classified based on the presence or absence of A and B antigens on red blood cells. Numerous studies have demonstrated an association between ABO blood groups and certain diseases (e.g., infections, cardiovascular diseases, and nervous system disorders). Previous studies have investigated the relationship between the ABO blood group and GDM, but the results have been inconsistent. Although the precise mechanism linking blood type and gestational diabetes remains unclear, some studies have suggested that blood type may be related to physiological processes such as insulin resistance, insulin secretion, and glucose regulation[23,24]. During pregnancy, a woman's insulin sensitivity may decrease, leading to increased insulin resistance, which can trigger diabetes. However, pregnant women with different blood types may exhibit variations in this physiological process, resulting in different disease risks. In addition to genetic factors, lifestyle and dietary habits may also influence the association between blood type and gestational diabetes. Some studies have shown that factors such as dietary com
The relationship between hemoglobin and GDM: Higher hemoglobin levels were linked to an increased risk of GDM, suggesting that beyond anemia, elevated hemoglobin should be monitored closely during pregnancy[25-27].
The relationship between liver function and GDM: Abnormal liver function was associated with GDM in this study, with elevated liver enzyme levels and reduced total protein and albumin being significant risk factors. Further studies are needed to clarify the relationship between liver dysfunction and GDM[28]. In this study, elevated levels of aspartate aminotransferase, alanine aminotransferase, and direct bilirubin, which reflect increased liver metabolic function, suggest a disorder in liver metabolic function and an increased risk of GDM. Furthermore, decreased levels of total protein and albumin, which reflect liver synthesis function, indicate liver synthesis dysfunction and an increased risk of GDM. Currently, the specific relationship between abnormal liver function and GDM remains unclear, and further studies are needed to explore its underlying mechanisms.
The findings of this study have important clinical and public health implications. GDM not only increases the risk of adverse maternal outcomes, such as preeclampsia, cesarean delivery, and future type 2 diabetes, but also poses significant risks for neonatal health, including macrosomia, preterm birth, and long-term metabolic disorders in the offspring. Early identification and management of high-risk groups, particularly older women, those with higher pre-pregnancy BMI, and women of Han ethnicity, are critical for improving maternal and neonatal outcomes.
From a public health perspective, the study highlights the need for targeted interventions and preventive measures, especially in high-risk populations identified in this research. Public health campaigns should focus on raising awareness about the importance of prenatal care, promoting healthy lifestyles, and providing access to early screening and management of GDM. These measures could significantly reduce the incidence of GDM and its associated complications, improving both maternal and neonatal health outcomes.
As a retrospective study, our research is subject to certain limitations. First, despite efforts to ensure data completeness and accuracy by utilizing hospital electronic medical record systems and double-checking processes, recall bias and incomplete records remain inherent challenges. However, most of the key variables, including OGTT results and laboratory parameters, were directly derived from standardized hospital records, minimizing subjectivity. Second, the retrospective nature of this study limits our ability to establish causal relationships between identified risk factors and GDM. Our findings are correlational and require confirmation through prospective cohort studies to explore causality and underlying mechanisms. Future research could also focus on potential confounders not accounted for in this study, such as environmental and genetic factors.
This study did not collect detailed information on dietary habits, physical activity levels, or socioeconomic factors, which are known to significantly influence the risk of GDM. While we partially addressed socioeconomic factors through education level and light physical activity and non-working occupation, other dimensions such as income, access to healthcare, and lifestyle details were not included in the dataset. We acknowledge that these factors might introduce residual confounding effects. Future research should prioritize the inclusion of such variables through prospective cohort studies or standardized surveys to provide a more comprehensive understanding of the multifactorial nature of GDM risk. Additionally, qualitative research methods could complement quantitative analyses to explore cultural and beha
We identified an intriguing association between blood type A and an increased risk of GDM. However, the precise mechanisms underlying this relationship remain unclear. Previous research suggests potential links between blood types and metabolic processes such as insulin sensitivity, systemic inflammation, and glucose regulation, but these hypotheses require further exploration. Given the retrospective nature of our study, we could not examine potential interactions between blood type and other metabolic factors. The observed association should be interpreted cautiously and validated in future research. Large-scale, multi-center cohort studies and investigations at the genetic and molecular levels are necessary to elucidate the biological mechanisms and assess the generalizability of this finding.
The innovation of this study lies in the epidemiological investigation of GDM in Guizhou Province, revealing the incidence of GDM and its influencing factors in the entire region and each sub-region. The study not only confirms the currently recognized influencing factors of GDM (such as age, education level, pre-pregnancy BMI, light physical activity, gravidity, hemoglobin, and liver function) but also identifies other potential influencing factors (such as blood group differences, higher risk in the Han nationality compared to ethnic minorities, and lower incidence of GDM in the Dong nationality compared to other ethnic minorities). The clinical significance of this study is multifaceted. It provides important guidance for diabetes prevention, control, and policy-making in Guizhou, as well as a reference and scientific basis for prevention and control policies in other similar regions. The study offers ideas for the prevention and treatment of GDM in China and proposes corresponding preventive measures based on different risk factors to guide disease monitoring and early intervention, providing a reference for the prevention and treatment of GDM in this region. Early identification of high-risk factors, early detection of high-risk groups, and personalized intervention measures can reduce the incidence of maternal and infant complications. This study emphasizes the importance of early identification and management of high-risk factors, and all localities should strengthen the education of women of childbearing age to ensure they understand the high-risk factors of GDM. Formulating different countermeasures according to different risk factors has important clinical and social significance in improving the health of pregnant women and fetuses and reducing medical costs. This study will provide new clues and research ideas for further exploring the etiology and pathogenesis of GDM in cohort studies, basic research, and clinical and basic research in the future.
Based on the epidemiological investigation of GDM in Guizhou, this study discusses the incidence of GDM in this area, comparing it with the incidence of GDM in China as a whole, other regions of China, and different areas within Guizhou Province. The results show that the incidence of GDM in Guizhou is at a moderate level in China, however, compared to plain areas, coastal regions, and economically developed areas, the incidence of GDM in Guizhou is higher. The incidence of GDM varies across different regions of Guizhou, with Anshun having a higher incidence and the three minority autonomous regions having a lower incidence. Attention should be paid to the influence of regional factors on GDM, and prevention and control policies from the three minority autonomous regions should be adopted to reduce the incidence of GDM in Guizhou and further decrease the incidence of gestational diabetes throughout the country. This study also demonstrates that the Han nationality is more susceptible to GDM than other ethnic minorities, while the Dong nationality has a lower incidence compared to other ethnic minorities, with the difference being statistically significant. Additionally, single factor analysis and binary logistic regression analysis revealed that older age (especially over 35 years), Han nationality, lower education level, higher pre-pregnancy BMI, employment, and a higher number of pregnancies were the main risk factors for GDM in the general information. Laboratory tests showed that a higher hemoglobin count, elevated levels of alanine aminotransferase, aspartate aminotransferase, and direct bilirubin, as well as lower levels of total protein and albumin, were associated with a higher risk of GDM. Studies have also indicated that blood group A has a higher risk of GDM compared to blood groups AB and O.
| 1. | Zhang Y, Xiao CM, Zhang Y, Chen Q, Zhang XQ, Li XF, Shao RY, Gao YM. Factors Associated with Gestational Diabetes Mellitus: A Meta-Analysis. J Diabetes Res. 2021;2021:6692695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1908] [Cited by in RCA: 1918] [Article Influence: 137.0] [Reference Citation Analysis (1)] |
| 3. | Gong KX, Zhu BB, Tao FB. [Research progress in the relationship between sleep during pregnancy and gestational diabetes mellitus]. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:1162-1166. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, Pineda-Cortel MRB. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 5. | Li J, Song C, Li C, Liu P, Sun Z, Yang X. Increased risk of cardiovascular disease in women with prior gestational diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:324-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Szmuilowicz ED, Josefson JL, Metzger BE. Gestational Diabetes Mellitus. Endocrinol Metab Clin North Am. 2019;48:479-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 7. | Yuen L, Wong VW. Gestational diabetes mellitus: Challenges for different ethnic groups. World J Diabetes. 2015;6:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 8. | Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, Wu HR, Li N, Zhang MH, Liu XH, Zhang H, Wang YH, Niu JM, Gan YJ, Zhong LR, Wang YF, Kapur A. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care. 2013;36:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Norman G, Monteiro S, Salama S. Sample size calculations: should the emperor's clothes be off the peg or made to measure? BMJ. 2012;345:e5278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3698] [Article Influence: 217.5] [Reference Citation Analysis (0)] |
| 11. | Atlaw D, Sahiledengle B, Assefa T, Negash W, Tahir A, Regasa T, Tekalegn Y, Mamo A, Enegeda ZT, Solomon D, Gezahegn H, Bekele K, Zenbaba D, Desta F, Tasew A, Nugusu F, Beressa G, Shiferaw Z, Feleke Z, Regassa Z, Duguma N, Chattu VK. Incidence and risk factors of gestational diabetes mellitus in Goba town, Southeast Ethiopia: a prospective cohort study. BMJ Open. 2022;12:e060694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Huifen Z, Yaping X, Meijing Z, Huibin H, Chunhong L, Fengfeng H, Yaping Z. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J Diabetes Complications. 2022;36:108186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Liu X, Wang S, Wang G. Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-analysis. J Clin Nurs. 2022;31:2665-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 14. | Nazarpour S, Shokati Poursani A, Mousavi M, Ramezani Tehrani F, Behboudi-Gandevani S. Investigation of the relationship between air pollution and gestational diabetes. J Obstet Gynaecol. 2024;44:2362962. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Tian ML, Du LY, Ma GJ, Zhang T, Ma XY, Zhang YK, Tang ZJ. Secular increase in the prevalence of gestational diabetes and its associated adverse pregnancy outcomes from 2014 to 2021 in Hebei province, China. Front Endocrinol (Lausanne). 2022;13:1039051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Cavicchia PP, Liu J, Adams SA, Steck SE, Hussey JR, Daguisé VG, Hebert JR. Proportion of gestational diabetes mellitus attributable to overweight and obesity among non-Hispanic black, non-Hispanic white, and Hispanic women in South Carolina. Matern Child Health J. 2014;18:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Pu J, Zhao B, Wang EJ, Nimbal V, Osmundson S, Kunz L, Popat RA, Chung S, Palaniappan LP. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors. Paediatr Perinat Epidemiol. 2015;29:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Tan J, Xiong Y, Wang X, Wei S, Luo C, Huang S, Yang Y, Chen J, Chen J, Xu M, Wu F. Influencing factors for postpartum depression in women with gestational diabetes mellitus. Front Endocrinol (Lausanne). 2024;15:1423127. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Zhao F, Xiao B. Factors Influencing Adverse Pregnancy Outcomes in Gestational Diabetes Mellitus. Comput Intell Neurosci. 2022;2022:5177428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 397] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 22. | Zhao XH, Zhang ZH. Risk factors for postpartum depression: An evidence-based systematic review of systematic reviews and meta-analyses. Asian J Psychiatr. 2020;53:102353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 23. | Shimodaira M, Yamasaki T, Nakayama T. The association of maternal ABO blood group with gestational diabetes mellitus in Japanese pregnant women. Diabetes Metab Syndr. 2016;10:S102-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kataria Y, Wu Y, Horskjær PH, Mandrup-Poulsen T, Ellervik C. Iron Status and Gestational Diabetes-A Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Tarim E, Kilicdag E, Bagis T, Ergin T. High maternal hemoglobin and ferritin values as risk factors for gestational diabetes. Int J Gynaecol Obstet. 2004;84:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zein S, Rachidi S, Awada S, Osman M, Al-Hajje A, Shami N, Sharara I, Cheikh-Ali K, Salameh P, Hininger-Favier I. High iron level in early pregnancy increased glucose intolerance. J Trace Elem Med Biol. 2015;30:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Zeng Y, He G. Association of blood parameters in early pregnancy with anemia during late pregnancy: a multicenter cohort study in China. J Matern Fetal Neonatal Med. 2024;37:2299110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Zhao M, Yang S, Su X, Hung TC, Liu Y, Zheng W. Hepatitis B Virus Infection and Increased Risk of Gestational Diabetes Regardless of Liver Function Status: A Xiamen Area Population-Based Study. Front Physiol. 2022;13:938149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |