Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.98423
Revised: October 22, 2024
Accepted: December 5, 2024
Published online: February 15, 2025
Processing time: 187 Days and 5.6 Hours
The increasing number of type 2 diabetes mellitus (T2DM) patients leads to higher rates of morbidity and mortality related to lung cancer.
To investigate the utility of the proliferating cell nuclear antigen Ki-67 in patients with lung adenocarcinoma in situ (AIS) complicated by T2DM.
One hundred patients with AIS and T2DM (group A), 100 patients with AIS alone (group B), and 60 patients with benign lung lesions (group C) admitted to the Department of Thoracic Surgery and Endocrinology of the First Affiliated Hospital of Soochow University from November 2021 to December 2022 were enrolled. Ki-67 expression was compared among the groups.
Group A had significantly higher levels of fasting plasma glucose (FPG), total cholesterol (TC), total triglyceride, low-density lipoprotein cholesterol, glyco
Ki-67 expression was higher in patients with AIS complicated by T2DM than in patients with AIS alone. Therefore, detecting the Ki-67 level might assist in the diagnosis of AIS in patients with T2DM.
Core Tip: Type 2 diabetes mellitus (T2DM) is an independent risk factor for lung cancer and increases mortality risk; Ki-67, a proliferation marker, is highly expressed in lung cancer, especially with T2DM; elevated Ki-67 levels in adenocarcinoma in situ (AIS) patients with T2DM suggest its utility as a screening marker. Hyperglycemia and insulin resistance in T2DM may drive cancer progression via the Ras/MAPK and IGF-1 signaling pathways. Regular monitoring of Ki-67, glycosylated hemoglobin, and lipid levels is essential for early detection and better prognosis in patients with AIS and T2DM.
- Citation: Chen K, Wang G, Hu JC, Zhou YY, Ma HT. Clinical significance of Ki-67 in patients with lung adenocarcinoma in situ complicated by type 2 diabetes. World J Diabetes 2025; 16(2): 98423
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/98423.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.98423
Lung cancer is the most prevalent tumor and the primary cause of cancer-related deaths in China, causing 720000 deaths in 2020 as reported by the 2020 GLOBOCAN database[1]. Notably, individuals with diabetes have an increased likelihood of developing malignancies[2]. Lung adenocarcinoma is the most commonly identified histological subtype of non-small-cell lung cancer (NSCLC). The International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) introduced a new classification system for lung adenocarcinoma in 2011. This system classified lung adenocarcinoma as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma, or invasive adenocarcinoma[3].
However, the presence of underlying metabolic conditions such as type 2 diabetes mellitus (T2DM) presents additional diagnostic and treatment challenges. In 2023, the International Diabetes Federation documented 537 million cases of T2DM worldwide. The number is expected to increase to 1.3 billion by 2050[4]. China accounts for a quarter of all patients with diabetes globally, which is the highest prevalence of diabetes in the world. The number of adults with diabetes in China has reached 114 million, accounting for one-tenth of the global adult population[5]. Individuals with T2DM have a 20%-25% higher risk of various malignancies, including lung cancer, than individuals without diabetes[6]. The rising prevalence of T2DM and lung cancer contributes to an increasing burden of both diseases. Epidemiological data suggest that individuals with T2DM have a higher risk of various cancers, including lung cancer, which complicates the clinical course and prognosis[7]. A notable concern is the complexity of diagnosing AIS in patients with diabetes, as diabetes can mask early cancer symptoms or exacerbate cancer progression. Studies suggest that the coexistence of diabetes comp
Uncontrolled cell proliferation is recognized as the principal characteristic of malignancy. With the exception of the G0 phase, the nuclear protein Ki-67 is present throughout the active phases of the cell cycle[8]. According to previous research[9], high Ki-67 expression negatively affects disease-free survival, relapse-free survival, and overall survival in patients with NSCLC. Hence, Ki-67 expression might represent a valuable biomarker for forecasting the prognosis of patients with lung cancer. As a nuclear proliferation antigen, Ki-67 has broad prospects in lung cancer research[10]. However, there are no studies focusing on Ki-67 expression in patients with AIS and T2DM. Therefore, this study explored the clinical significance of Ki-67 in patients with AIS complicated by T2DM, shedding light on its potential utility in improving diagnostic accuracy and prognosis in this high-risk population.
This study employed a case-control design. In total, 160 patients with AIS complicated by T2DM presented to the Department of Thoracic Surgery and Endocrinology of the First Affiliated Hospital of Soochow University between November 2023 and December 2024. Group A comprised all patients with AIS complicated by T2DM (80 men and 80 women; age: 49-81 years). Meanwhile, two hundred patients diagnosed with AIS alone who were admitted to the Department of Thoracic Surgery at the same time comprised Group B (100 men and 100 women; age: 46-85 years). Group C served as a control group and comprised 60 patients with benign pulmonary lesions, including 15 patients with pulmonary cyst, 10 patients with bronchiectasis, 10 patients with pulmonary fibrosis, 5 patients with pulmonary pseudotumor, 10 patients with hamartoma, and 10 patients with tuberculous nodules (30 men and 30 women, age: 44-79 years). The control group was matched to the AIS group by age and sex to ensure comparability. The sample size was calculated using power analysis to detect significant differences in Ki-67 expression with 80% power at a 5% significance level. AIS and benign lesions were confirmed by postoperative pathology according to the TNM staging criteria from the IASLC, ATS, and ERS[3].
The study population consisted of patients with a pre-existing diagnosis of T2DM who subsequently developed AIS. Patients were diagnosed with T2DM according to the diagnostic criteria for diabetes outlined by the World Health Organization in 1999[11], and the diagnosis of AIS was based on the IASLC/ATS/ERS classification. The inclusion criteria for this study were as follows: (1) Confirmed diagnosis of AIS according to established clinical and pathological guidelines; (2) Pre-existing diagnosis of T2DM; and (3) Availability of paraffin-embedded tumor tissues for immunohistochemical analysis. The exclusion criteria were as follows: (1) New diagnosis of T2DM after a diagnosis of AIS; (2) Presence of other malignancies; (3) presence of severe concurrent diseases such as uncontrolled cardiovascular or renal conditions; (4) Recent infection; and (5) insufficient tumor tissue samples for analysis. The study was approved by the institutional review board of The First Affiliated Hospital of Soochow University (No: 2022-459). All processes were conducted in compliance with applicable rules and legislation. Each individual granted written informed consent to participate in this study.
Following a 12-hour fasting period, a 15-mL venous blood sample was collected in the morning from each participant. Of this, 10 mL was allocated for biochemical analysis using a Hitachi 7600 automated biochemical analyzer (Hitachi, Tokyo, Japan). The glycosylated hemoglobin (HbA1c) concentration was measured by high-performance liquid chromatography with a G8 HbA1c analyzer (Tosoh, Tokyo, Japan). Additionally, paraffin-embedded tumor tissue samples were acquired from each participant, from which 4-mm sections were prepared.
Paraffin-embedded tumor tissues were cut into 4-mm-thick sections and subjected to immunohistochemical staining for Ki-67. The primary antibody used was anti-Ki-67 (clone MIB-1, Dako, Agilent Technologies, Santa Clara, CA, United States) at a 1: 200 dilution. A biotinylated secondary antibody and the avidin-biotin complex method were applied, followed by 3,3′-diaminobenzidine substrate development. The stained sections were then counterstained with hematoxylin. Positive Ki-67 expression was quantified by counting the number of positively stained nuclei in 10 high-power fields.
Biochemical parameters: Fasting plasma glucose (FPG), total cholesterol (TC), and triglyceride (TG) levels were evaluated by enzymatic methods. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were assessed by a precipitation method.
Ki-67 expression: No obvious staining or < 10% positive staining indicated negative expression. Weak positive expression was indicated by 10%-25% positive cells. Moderately positive expression was indicated by 25%-50% positive cells, whereas strongly positive expression was indicated by > 50% positive cells.
Statistical analysis was conducted using GraphPad Prism 8.0 software (GraphPad, San Diego, CA, United States). The measurement data were presented as the mean ± SD. A t-test was utilized to compare the groups. The statistical data was presented as percentages. The relationship between Ki-67 and other clinical indicators was evaluated using Pearson’s correlation analysis. Logistic regression modeling was conducted to identify risk factors for AIS with coexisting T2DM, with a significance level set at P < 0.05.
The distribution of gender and age did not differ among the three groups (P > 0.05, Table 1). According to one-way analysis of variance, TC, TG, LDL-C, FPG, HbA1c, and insulin levels were all higher in group A than in group B (P < 0.01), and TC, TG, HDL, FPG, HbA1c, and insulin levels were all higher in group A than in group C (P < 0.01).
| Group | Number of patients (male/female) | Age (years) | BMI (kg/m2) | Smoking (n) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) |
| A | 160 (80/80) | 60.07 ± 10.47 | 25.1 ± 2.9 | 48 | 5.01 ± 1.11 | 1.64 ± 0.97 | 1.09 ± 0.46 |
| B | 200 (100/100) | 61.40 ± 9.97 | 25.0 ± 3.1 | 60 | 4.69 ± 0.75b | 1.27 ± 0.60b | 1.18 ± 0.27 |
| C | 60 (30/30) | 59.99 ± 10.32 | 25.2 ± 3.0 | 14 | 4.76 ± 1.12b | 1.29 ± 0.60b | 1.24 ± 0.28b |
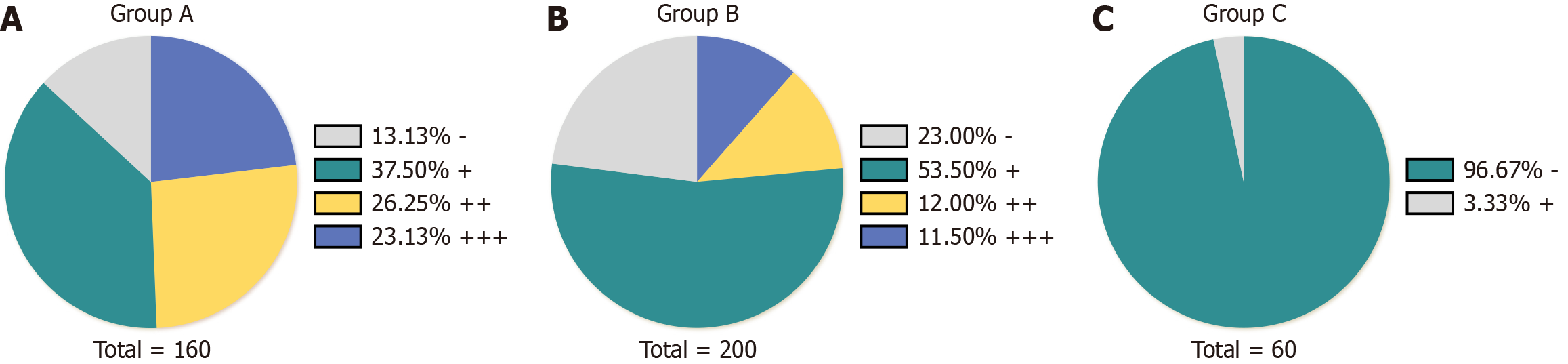
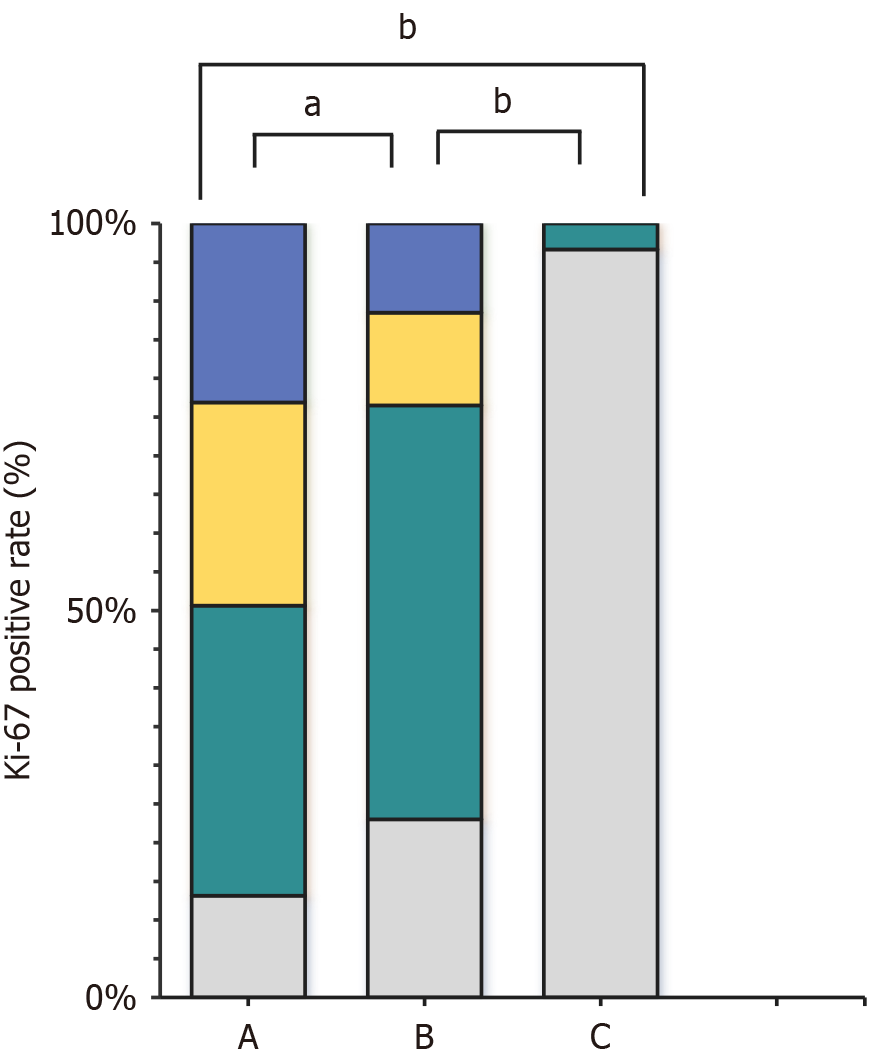
As presented in Table 2, Figure 1 and 2, the Ki-67 positivity rate was highest in group A (86.87%), and the weakly, moderately, and strongly positive rates were 37.50, 26.25, and 23.13%, respectively. The Ki-67 positivity rate in group B was 77%, including weakly, moderately, and strongly positive rates of 53.5, 12, and 11.5%, respectively. The total Ki-67 positivity rate in group C was 3.33%, and all such samples were weakly positive. The weakly positive rate was highest in group B, followed by groups A (P < 0.01) and C (P < 0.01). The total positivity and moderately and strongly positive rates were highest in group A, followed by groups B (P < 0.01) and C (P < 0.01).
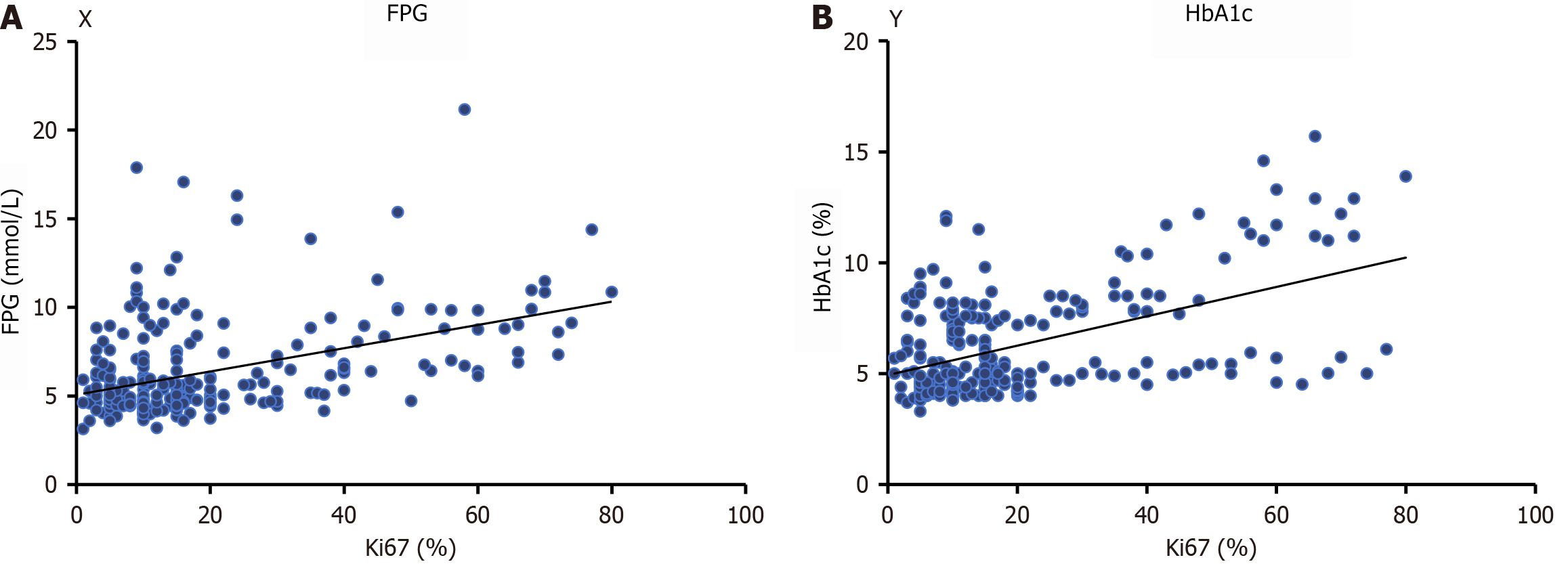
Ki-67 was utilized as the dependent variable in Pearson’s correlation analysis. The independent variables included sex, age, FPG, HbA1c, Insulin, TC, TG, LDL-C, and HDL-C. Ki-67 was positively correlated with FPG (r = 0.192, F = 61.40, P < 0.01) and HbA1c levels (r = 0.251, F = 86.58, P < 0.01, Figure 3).
Logistic regression analysis was conducted on group A to assess binary data, with the presence of T2DM as the dependent variable. Independent variables included Ki-67, FPG, HbA1c, Insulin, TG, TC, LDL-C, and HDL-C. The results indicated that Ki-67 (OR = 0.870, 95%CI: 0.796-0.950, P = 0.002), FPG (OR = 1.923, 95%CI: 1.216-3.043, P = 0.005), HbA1c (OR = 11.563, 95%CI: 2.165-12.016, P < 0.01), Insulin (OR = 0.882, 95%CI: 0.788-0.986, P = 0.027), HDL (OR = 0.043, 95%CI: 0.004-0.409, P = 0.006), and TC (OR = 3.116, 95%CI: 1.135-8.556, P = 0.027) were independent variables for patients with AIS complicated by T2DM (Table 3).
| Independent variables | β | Standard error | Wald | OR (95%CI) | P value |
| Ki-67 | −0.140 | 0.045 | 9.697 | 0.870 (0.796–0.950) | 0.002 |
| FPG | 0.654 | 0.234 | 7.810 | 1.923 (1.216–3.043) | 0.005 |
| HbA1c | 1.452 | 0.690 | 15.036 | 11.563 (2.165-12.016) | < 0.01 |
| Insulin | −0.126 | 0.057 | 4.880 | 0.882 (0.788–0.986) | 0.027 |
| HDL-C | −3.151 | 1.151 | 7.495 | 0.043 (0.004–0.409) | 0.006 |
| TC | 1.137 | 0.515 | 4.864 | 3.116 (1.135–8.556) | 0.027 |
| TG | 0.054 | 0.405 | 0.018 | 1.056 (0.477–2.336) | 0.893 |
| LDL-C | 0.377 | 0.381 | 0.978 | 1.458 (0.691–3.077) | 0.323 |
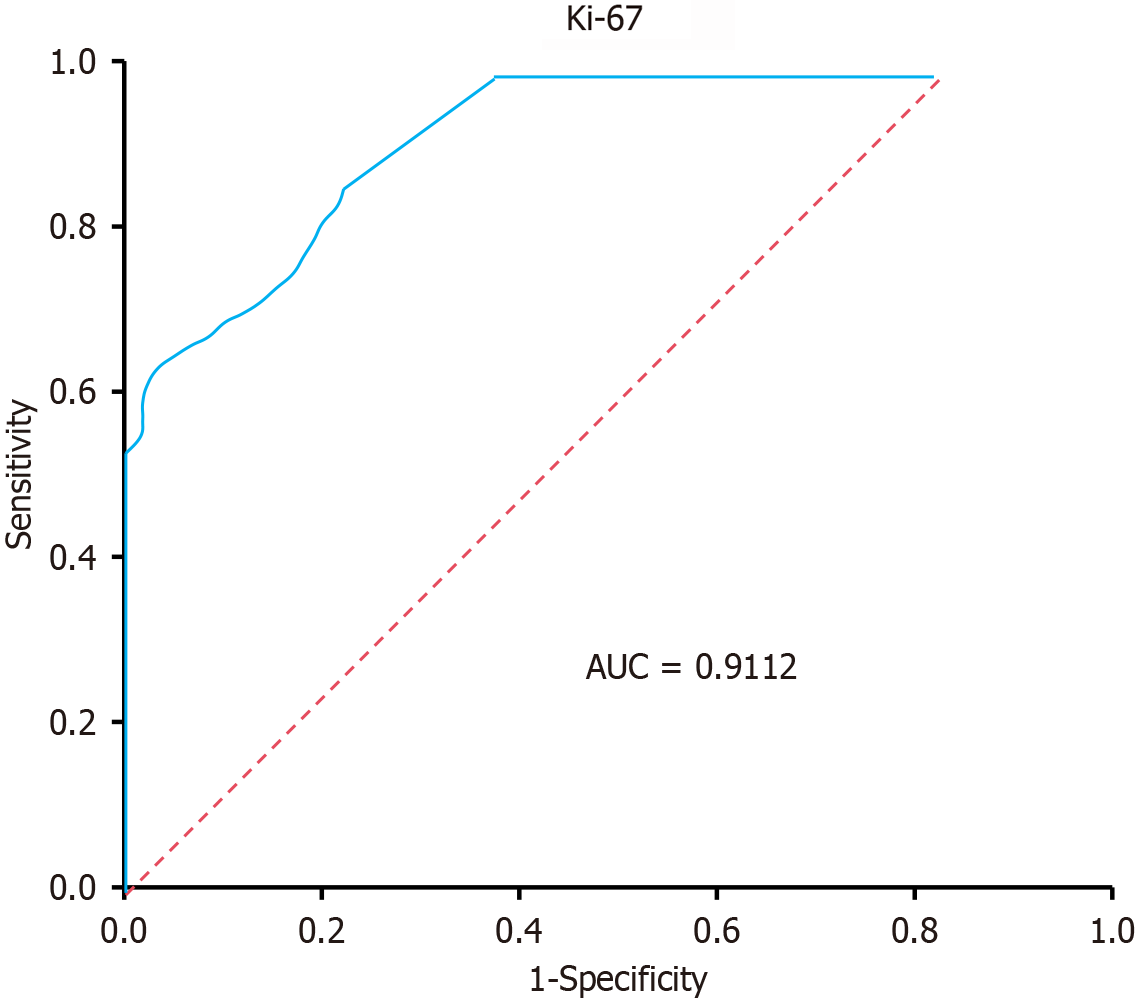
To further evaluate the performance of the logistic regression model, we generated a receiver operating characteristic (ROC) curve. The area under the ROC curve was 0.9112, indicating good predictive power. The sensitivity and specificity of the model for predicting AIS in patients with T2DM were 85.0% and 73.4%, respectively. The ROC curve is displayed in Figure 4.
Studies have increasingly recognized the connection between lung cancer and T2DM as two prevalent chronic non-communicable diseases[12]. A meta-analysis revealed that T2DM was an independent risk factor for developing lung cancer and was also associated with the likelihood of death from lung cancer[13]. A study by Kim et al[14] found that the mortality rate of lung cancer was significantly higher in patients with T2DM. In addition, Karlin et al[15] revealed that lung cancer was the predominant malignant tumor in people with T2DM, and timely detection and treatment of lung cancer might greatly enhance patient prognosis. More measures should be investigated for early lung cancer screening in patients with T2DM.
Ki-67, a marker of cellular proliferation, is highly expressed during the active stages of the cell cycle, specifically in G1, S, G2, and mitotic phases[16]. A high rate of cell proliferation is a characteristic feature of cancer. Poorer prognoses have been observed in patients diagnosed with multiple myeloma, prostate cancer, and breast cancer who exhibit increased Ki-67 expression[9]. A previous study[16] revealed that Ki-67 is highly expressed in lung cancer, and its expression can gradually increase with disease progression. The differentiation and infiltration of cancer cells in patients might enhance the expression of Ki-67[17]. A previously published meta-analysis[18] indicates that the Ki-67 Labeling index holds prognostic value in NSCLC patients, with a high index associated with a poorer prognosis.
Ki-67 expression, as determined through immunohistochemistry using solid tumor tissues, provided valuable insights into tumor proliferation in patients with AIS complicated by T2DM in this study. Although serum Ki-67 measurements are available in many laboratories, this study focused on tissue-based assessment, which is widely used in clinical oncology for prognostic evaluation. In this study, Ki-67 was positive in patients with AIS irrespective of the presence of T2DM according to our findings. Glycans on the cell membrane can undergo structural changes and move to different locations throughout the body during the progression of lung cancer[19]. This might account for the elevated expression and presence of Ki-67 in patients with AIS. Importantly, patients with both AIS and T2DM had higher Ki-67 levels (total positivity, highly positive, and moderately positive rates) than those with benign pulmonary lesions or AIS alone. This might be attributable to the persistent and long-term hyperglycemia experienced by patients with diabetes. This was evidenced by data[20] indicating that FPG and HbA1c levels were positively associated with the expression of Ki-67. Ki-67 could not be detected in the G0 and early G1 phases of the cell cycle, but its expression appeared in the middle G1 phase and gradually increased with the progression of the cell cycle to the S and G2 phases, reaching its peak in the mitotic phase. Subsequently, the antigenic activity of Ki-67 rapidly declines[21]. More than 80% of patients with T2DM exhibit insulin resistance and hyperinsulinemia[22]. Insulin can promote cell division, growth, and proliferation by activating the Ras/MAPK signal pathway, thereby shortening the cell cycle and triggering tumor formation. Hyperglycemia can supply the energy substrates necessary for tumor proliferation[23]. These substrates disrupt the typical composition of the capillary basement membrane, leading to thickening, reduced permeability, mitochondrial malfunction, and respiratory issues. Excessive anaerobic end-product generation with a sluggish metabolic rate can lead to aberrant cell division and cancer development. Oxidative stress triggered by hyperglycemia can harm vascular endothelial cells and stimulate their growth, leading to mitotic activation[24].
The findings in this study indicated that Ki-67, HbA1c, FPG, Insulin, TC, and HDL-C were independent risk factors for patients with AIS complicated by T2DM. A previous study by Yan et al[25] revealed that patients with T2DM exhibited a higher rate of Ki-67 expression, more advanced clinical stage, and higher histological grades. Disordered glucose metabolism could exacerbate cancer progression. Some studies[26,27] reported that T2DM, which is marked by insulin resistance, leads to prolonged elevation of insulin levels in the body, which boosts the expression of growth hormone receptors and enhances the generation of IGF-1 receptor. This in turn triggers the activation of the IGF-1 receptor signaling pathway, which is recognized as a stimulator of tumor advancement. EGF pathways are also associated with cancer metabolism. Inhibiting EGF pathways could enhance the anti-cancer impact in NSCLC by reactivating oxidative phosphorylation[28]. These results could partially explain why patients with AIS complicated by T2DM had a significantly worse prognosis.
The multiple linear regression analysis results showed that Ki-67 independently affected FPG and HbA1c levels in patients with AIS and T2DM. The biochemical tests also revealed that TC and TG levels were substantially higher in patients with AIS complicated by T2DM than in those with benign pulmonary lesions. Several meta-analyses[29-32] revealed that high lipid levels were strongly linked to a higher risk of T2DM, and they could be used alongside other biomarkers to forecast the onset of AIS. This finding indicated that serum Ki-67 could be helpful for the early screening of AIS in patients with T2DM. Therefore, thoracic surgeons and endocrinologists should pay special attention to the postoperative results of these patients and emphasize the necessity of regular follow-up. A proactive monitoring and management strategy is essential to improve patient outcomes and postoperative quality of life, enabling more personalized patient care and treatment plans.
Based on our current knowledge, this study represents the initial investigation of the involvement of Ki-67 in patients with AIS complicated by T2DM. A number of constraints merit scrutiny. First, this study only measured Ki-67 once, which might not accurately represent long-term Ki-67 Levels. In addition, some random measurement errors were unavoidable. Additional research employing a variety of metrics is required to validate our findings.
A variety of potential confounding factors can influence Ki-67 expression. These include smoking, which is known to affect cell proliferation and tumor growth, obesity, which is associated with systemic inflammation and altered metabolic pathways, and other comorbidities such as cardiovascular disease. It is critical to acknowledge potential confounding factors that can influence Ki-67 expression. Although we controlled for major variables such as age and sex, future studies should aim to assess the impact of these additional factors on Ki-67 expression in patients with AIS and T2DM to ensure a clearer interpretation of the biomarker’s role. In addition, we will focus on conducting longitudinal assessments of Ki-67 expression to determine its stability and variability over time in patients with AIS and T2DM. These studies will provide a better understanding of changes in Ki-67 expression during disease progression and utility of these changes for predicting tumor recurrence or response to treatment. Longitudinal data will further strengthen the clinical utility of Ki-67 as a prognostic marker in these patient populations.
In conclusion, when compared with established lung cancer biomarkers such as carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA 21-1), Ki-67 offers additional prognostic value by directly reflecting the proliferative activity of tumor cells. Although CEA and CYFRA 21-1 are widely used in clinical settings, the ability of Ki-67 to predict tumor aggressiveness and recurrence provides a complementary tool for the early diagnosis and monitoring of patients with AIS complicated by T2DM. This highlights the potential for integrating Ki-67 into routine screening protocols alongside these established markers.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64037] [Article Influence: 16009.3] [Reference Citation Analysis (174)] |
| 2. | Briana DD, Malamitsi-Puchner A. Galectin-3: An Early Marker of Gestational Diabetes, Subclinical Atherosclerosis, and Tumor Progression. Angiology. 2020;71:474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Eguchi T, Kadota K, Park BJ, Travis WD, Jones DR, Adusumilli PS. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg. 2014;26:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 428] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 5. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1682] [Article Influence: 841.0] [Reference Citation Analysis (18)] |
| 6. | Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson AM, Ljung R, Wild SH, Kerssens JJ, Harding JL, Magliano DJ, Gudbjörnsdottir S; Diabetes and Cancer Research Consortium. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59:980-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Zhu B, Qu S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front Endocrinol (Lausanne). 2022;13:800995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016;13:496-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Yan J, Wang H, Zhou H, He H, Qiu L, Wang Z. Correlation between expression of Ki-67 and MSCT signs in different types of lung adenocarcinoma. Medicine (Baltimore). 2020;99:e18678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, Goeppert B, Thomas M, Herth FJ, Schirmacher P, Schnabel PA, Hoffmann H, Dienemann H, Muley T, Weichert W. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer. 2014;111:1222-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Schneider H, Shaw J, Zimmet P. Guidelines for the detection of diabetes mellitus--diagnostic criteria and rationale for screening. Clin Biochem Rev. 2003;24:77-80. [PubMed] |
| 12. | Yang J, Yang C, Shen H, Wu W, Tian Z, Xu Q, Cao C, Ye S, Ban L, Tong X, Mei J. Discovery and validation of PZP as a novel serum biomarker for screening lung adenocarcinoma in type 2 diabetes mellitus patients. Cancer Cell Int. 2021;21:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Leiter A, Charokopos A, Bailey S, Gallagher EJ, Hirsch FR, LeRoith D, Wisnivesky JP. Assessing the association of diabetes with lung cancer risk. Transl Lung Cancer Res. 2021;10:4200-4208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kim NE, Kang EH, Ha E, Lee JY, Lee JH. Association of type 2 diabetes mellitus with lung cancer in patients with chronic obstructive pulmonary disease. Front Med (Lausanne). 2023;10:1118863. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Karlin NJ, Amin SB, Buras MR, Kosiorek HE, Verona PM, Cook CB. Patient outcomes from lung cancer and diabetes mellitus: a matched case-control study. Future Sci OA. 2018;4:FSO248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. 2018;127:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 572] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 17. | Davey MG, Hynes SO, Kerin MJ, Miller N, Lowery AJ. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 18. | Wen S, Zhou W, Li CM, Hu J, Hu XM, Chen P, Shao GL, Guo WH. Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: a meta-analysis of published studies involving 32 studies. BMC Cancer. 2015;15:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Čaval T, Alisson-Silva F, Schwarz F. Roles of glycosylation at the cancer cell surface: opportunities for large scale glycoproteomics. Theranostics. 2023;13:2605-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 20. | Fujita Y, Kozawa J, Iwahashi H, Yoneda S, Uno S, Eguchi H, Nagano H, Imagawa A, Shimomura I. Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. J Diabetes Investig. 2018;9:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Remnant L, Kochanova NY, Reid C, Cisneros-Soberanis F, Earnshaw WC. The intrinsically disorderly story of Ki-67. Open Biol. 2021;11:210120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1352] [Article Influence: 270.4] [Reference Citation Analysis (0)] |
| 23. | Leitner BP, Siebel S, Akingbesote ND, Zhang X, Perry RJ. Insulin and cancer: a tangled web. Biochem J. 2022;479:583-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 237] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 25. | Yan X, Gao Z, Zhou Y, Gao F, Li Q. Expressions of IGF-1R and Ki-67 in breast cancer patients with diabetes mellitus and an analysis of biological characteristics. Pak J Med Sci. 2022;38:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Sharma R, Kopchick JJ, Puri V, Sharma VM. Effect of growth hormone on insulin signaling. Mol Cell Endocrinol. 2020;518:111038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Batista TM, Haider N, Kahn CR. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia. 2021;64:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 28. | Kasprzak A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Bhowmik B, Siddiquee T, Mujumder A, Afsana F, Ahmed T, Mdala IA, do V Moreira NC, Khan AKA, Hussain A, Holmboe-Ottesen G, Omsland TK. Serum Lipid Profile and Its Association with Diabetes and Prediabetes in a Rural Bangladeshi Population. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Fu L, Zhang G, Qian S, Zhang Q, Tan M. Associations between dietary fiber intake and cardiovascular risk factors: An umbrella review of meta-analyses of randomized controlled trials. Front Nutr. 2022;9:972399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Deng H, Hu P, Li H, Zhou H, Wu X, Yuan M, Duan X, Lao M, Wu C, Zheng M, Lao XQ, Zhao W, Liu X. Novel lipid indicators and the risk of type 2 diabetes mellitus among Chinese hypertensive patients: findings from the Guangzhou Heart Study. Cardiovasc Diabetol. 2022;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Peng J, Zhao F, Yang X, Pan X, Xin J, Wu M, Peng YG. Association between dyslipidemia and risk of type 2 diabetes mellitus in middle-aged and older Chinese adults: a secondary analysis of a nationwide cohort. BMJ Open. 2021;11:e042821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |