Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.98085
Revised: October 6, 2024
Accepted: November 21, 2024
Published online: February 15, 2025
Processing time: 195 Days and 18.9 Hours
The association between body mass index (BMI) and bone mineral density (BMD) has shown inconsistent results, varying by sex and skeletal site. Despite normal or elevated bone mass, individuals with type 2 diabetes have an increased risk of hip and vertebral fractures.
To assess lumbar spine trabecular volumetric BMD (vBMD) across different BMI categories in individuals with and without diabetes.
This cross-sectional study included 966 men over 50 years old and 1001 postmenopausal women from the Pinggu Metabolic Disease Study. The vBMD of lumbar vertebrae 2 through 4 was measured using quantitative computed tomography. Total adipose tissue, subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and lumbar skeletal muscle area were also quantified.
In men with obesity (P = 0.038) and overweight (P = 0.032), vBMD was signi
The relationship between BMI and trabecular vBMD differs in individuals with and without diabetes. Overweight and obese men with diabetes exhibit higher vBMD.
Core Tip: The association between body mass index (BMI) and bone mineral density (BMD) has shown contradictory findings, which depends on sex and skeletal sites. By using data from Pinggu Metabolic Disease Study, we examined the association between BMI, body composition, and lumbar spine trabecular volumetric BMD (vBMD) in patients with and without diabetes. We found that men with obesity and overweight aged over 50 years with diabetes had favorable trabecular vBMD than participants without diabetes. The trabecular vBMD had different trend patterns with the increase of BMI in participants with and without diabetes. There was a saturation effect value between BMI (22.33 kg/m2) and vBMD in men without diabetes.
- Citation: Lv F, Cai XL, Zhang XY, Zhou XH, Han XY, Li YF, Ji LN. Association between body mass index and lumbar spine volumetric bone mineral density in diabetic and non-diabetic patients. World J Diabetes 2025; 16(2): 98085
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/98085.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.98085
Osteoporosis, a systemic skeletal disorder, is characterized by low bone mass and deteriorated bone microarchitecture, resulting in increased fragility and fracture risk. In China, the condition affects 32.1% of postmenopausal women and 6.9% of men over 50 years old, according to the China Osteoporosis Prevalence Study[1]. It significantly burdens healthcare systems and diminishes quality of life.
Obesity has traditionally been seen as protective against fractures[2]. However, studies on the relationship between body mass index (BMI) and bone mineral density (BMD) have produced inconsistent findings, influenced by factors such as sex and skeletal site[3-5]. Moreover, fat distribution may differentially impact bone health. Higher trunk fat mass assessed by dual-energy X-ray absorptiometry (DXA) and visceral adipose tissue (VAT) assessed by quantitative computed tomography (QCT) correlated with lower trabecular bone volume, reduced stiffness, decreased bone formation, and increased cortical porosity, as revealed by micro-computed tomography (CT) of transiliac biopsies[6]. Type 2 diabetes (T2D) is a growing global health concern. T2D patients are at increased risk for hip and vertebral fractures despite having normal or higher bone mass[7]. However, there is limited research on how BMI and body composition affect bone mass and microstructure in people with and without diabetes. Understanding the complex relationships among obesity, diabetes, and osteoporosis is vital for developing effective healthcare strategies[8].
DXA remains the gold standard for diagnosing osteoporosis and predicting fracture risk[9]. Most studies examining the impact of BMI on bone focus on lumbar spine and hip areal BMD[8]. However, DXA results can be skewed by factors such as osteophytes, aortic calcifications, and surrounding soft tissues like fat[10]. To address these limitations, QCT offers an alternative, measuring trabecular volumetric BMD (vBMD) alongside abdominal fat, providing a more accurate assessment of fracture risk[11].
This study examined the relationship between BMI, body composition, and lumbar spine trabecular vBMD in individuals with and without diabetes, using data from the Pinggu Metabolic Disease Study (PMDS). Additionally, it explored whether a saturation effect exists between BMI and vBMD in postmenopausal women and men over 50 years old.
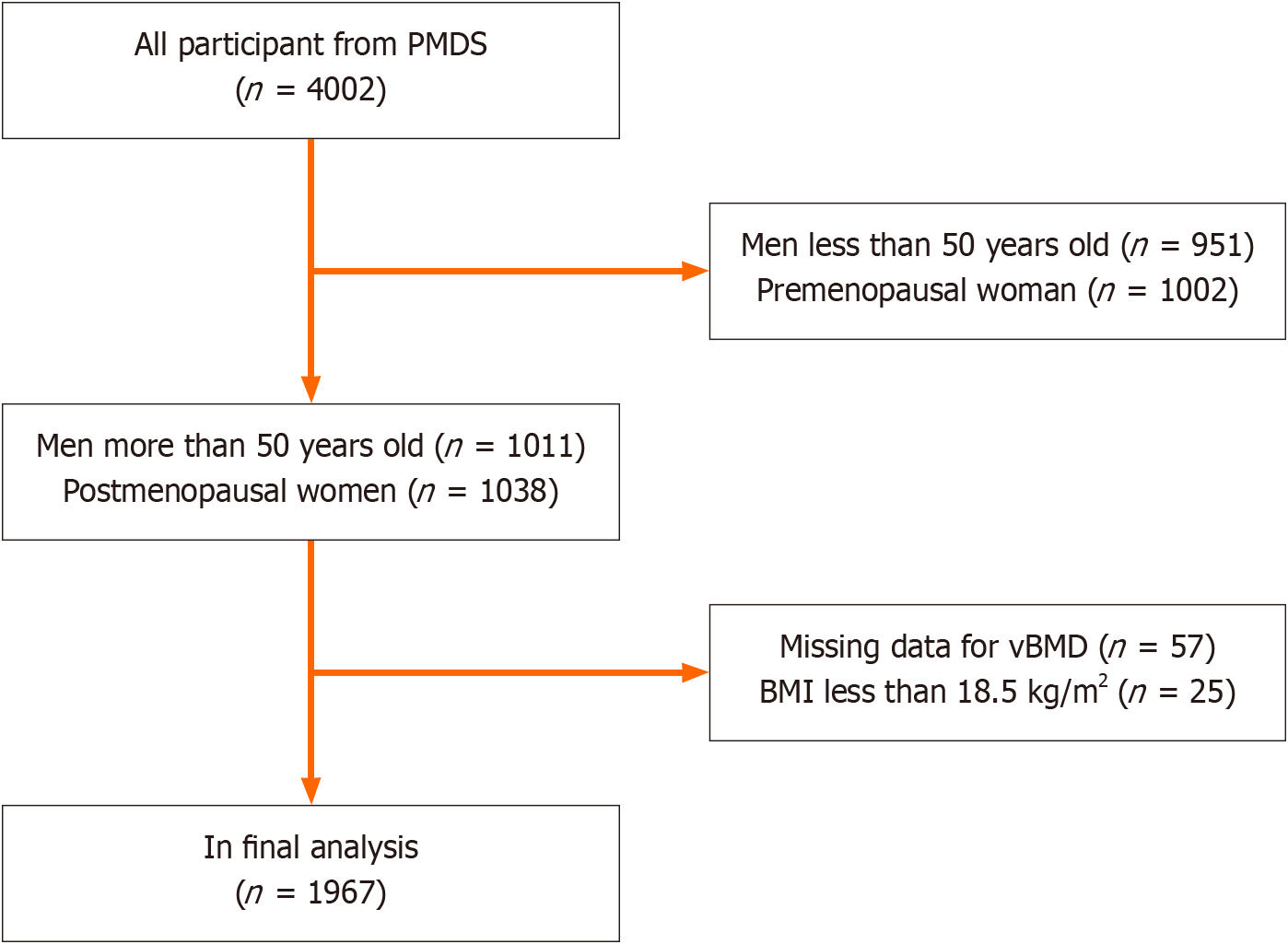
This was a subgroup study of the PMDS, which was carried out in the Pinggu district of Beijing between September 2013 and July 2014. The study involved 4002 participants aged 26 to 76 years. Comprehensive details regarding participant enrollment and study methodology have been previously published[12]. For this analysis, 951 men under 50 years, 1002 premenopausal women, and 57 subjects lacking vBMD data were excluded. Participants identified as underweight (BMI < 18.5 kg/m²) were also omitted due to the known negative correlation between underweight status and BMD. This underweight subgroup was minimal, comprising only 25 individuals. Consequently, data from 1967 participants were included for further analysis (Figure 1).
Ethical approval for the PMDS was received from the ethics committees of Peking University Medical Center and the University of Michigan. Additionally, the specific study received ethics approval from the Peking University People’s Hospital ethics committee (Approval No. 2021PHB441-001).
All participants engaged in face-to-face interviews and completed standardized questionnaires, as described previously[12]. Heights were measured without shoes to the nearest 0.1 cm, and weights were recorded in light clothing to the nearest 0.1 kg. The BMI was calculated using the formula: Weight (kg)/[height (m)]2. Waist circumference (WC) was measured at the midpoint between the lower rib cage and the iliac crest with a flexible non-elastic tape, while hip circumference (HC) was measured at the midpoint between the iliac crest and the most lateral points of the greater trochanter, also to the nearest 0.1 cm. The waist-to-hip ratio (WHR) was calculated by dividing WC by HC. Participants were categorized into BMI groups as defined by the Working Group on Obesity in China[13], including obesity (≥ 28.0 kg/m2), overweight (24.0-27.9 kg/m2), and normal weight (18.5-23.9 kg/m2).
Fasting blood samples were obtained following an 8- to 12-hour interval. Renal and liver functions were evaluated by measuring serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lipid profiles, including total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol using a Coulter UniCel DxC 800 automated biochemical analyzer (Beckman, United States).
Participants without a diabetes diagnosis underwent an oral glucose tolerance test (OGTT) to evaluate their glucose tolerance. For those diagnosed with diabetes, fasting plasma glucose (FPG) was determined by the hexokinase method, and serum insulin levels were measured using radioimmunoassay. Hemoglobin A1c (HbA1c) was quantified through cation-exchange high-pressure liquid chromatography, with serum insulin also assessed by radioimmunoassay (China Institute of Atomic Energy, Beijing, China). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR = fasting insulin (FINS) (mU/mL) × fasting glucose (mmol/L)/22.5.
Participants were classified into glucose tolerance categories based on OGTT results in accordance with the 1999 World Health Organization criteria[14]. Participants with diagnosed diabetes were based on self-reported history and/or current use of hypoglycemic medications.
Participants underwent abdominal CT scans with a GE 64-slice CT scanner (LightSpeed VCT, GE, United States). Lumbar spine trabecular vBMD for the L2-L4 region was assessed via asynchronous calibration using QCT Pro analysis software (Mindways, Austin, TX, United States). According to the American College of Radiology criteria, participants were classified into osteoporosis (vBMD < 80 mg/cm3) or low bone mass (vBMD 80 to 120 mg/cm3)[15].
Abdominal fat was measured at the L4-L5 intervertebral disc space, while skeletal muscle area (SMA) was assessed at the L3 intervertebral disc space. Total adipose tissue (TAT), VAT, and SMA (cm2) were quantified semi-automatically using the Tissue Composition Module of the analysis software (Mindways, Austin, TX, United States). Subcutaneous adipose tissue (SAT) was determined by subtracting VAT from TAT.
Baseline characteristics are expressed as the mean ± SD or as frequencies and percentages where appropriate. Group differences were evaluated using Student’s t-test, Mann-Whitney U test, or Kruskal-Wallis H test for continuous variables, while categorical variables were analyzed by the χ2 test or Fisher’s exact test. Pearson correlation coefficients were calculated to assess the relationships between vBMD and variables including BMI, WC, VAT, SAT, and SMA, with stratification by glucose status and sex. Multivariate linear regression models were applied to examine the relationships between BMI, WC, VAT, SAT, SMA, and vBMD, adjusting for confounding factors. Nonlinear associations between BMI and vBMD were explored through fitting smooth curves and threshold effect analyses. Two piecewise linear regression models were implemented to identify the optimal inflection point on the curve.
Statistical analyses were performed using SPSS software (version 23.0, SPSS Inc., Chicago, IL, United States) and EmpowerStats (version 4.1, X&Y Solutions, Inc., Boston, MA, United States), with a significance threshold set at P ≤ 0.05.
This study comprised 1967 participants, including 966 men over 50 years and 1001 postmenopausal women, with an average age of 59.4 ± 6.7 years. Of these, 427 had diabetes, categorized as follows: 162 were obese, 184 were overweight, and 81 were of normal weight. Table 1 details the characteristics of participants stratified by BMI and glucose status. In the obesity group, diabetic participants had significantly higher WC and WHR, and elevated levels of ALT, FPG, FINS, and HOMA-IR compared to non-diabetics (P < 0.05). In the overweight group, those with diabetes also presented greater WC, HC, WHR, ALT, AST, FPG, FINS, HbA1C, HOMA-IR, and TG, along with lower levels of HDL-C compared to their non-diabetic counterparts (P < 0.05). In the normal weight group, diabetic participants exhibited higher WHR, FPG, HbA1C, HOMA-IR, and TG, as well as lower diastolic blood pressure than non-diabetics (P < 0.05).
| Variable | Total | Obesity | Overweight | Normal weight | |||
| n = 1967 | Diabetes, n = 162 | Non-diabetes, n = 370 | Diabetes, n = 184 | Non-diabetes, n = 683 | Diabetes, n = 81 | Non-diabetes, n = 487 | |
| Age (years) | 59.4 ± 6.7 | 59.5 ± 6.1 | 58.8 ± 6.2 | 59.8 ± 6.9 | 59.2 ± 6.8 | 61.1 ± 6.8 | 59.8 ± 7.20 |
| Men (%) | 49.1 | 35.8 | 37.3 | 53.8 | 50.7 | 54.3 | 57.7 |
| Annual household income (%) | |||||||
| < ¥50000 | 60.3 | 53.7 | 57.0 | 48.9 | 60.8 | 65.4 | 67.8 |
| ¥50000-99000 | 30.1 | 32.1 | 33.6 | 36.9 | 30.0 | 22.2 | 25.9 |
| ≥ ¥100000 | 9.5 | 14.2 | 9.5 | 14.2 | 9.2 | 12.4 | 6.4 |
| Education (%) | |||||||
| Elementary school or lower | 33.6 | 39.5 | 33.2 | 29.3 | 29.9 | 45.7 | 36.8 |
| Middle school | 60.0 | 51.9 | 60.9 | 59.7 | 62.4 | 54.3 | 59.8 |
| College or higher | 6.4 | 8.7 | 5.7 | 10.8 | 7.7 | 0 | 3.5 |
| BMI (kg/m2) | 26.09 ± 3.47 | 30.53 ± 2.25 | 30.40 ± 2.22 | 25.97 ± 1.15 | 26.01 ± 1.14 | 22.35 ± 1.21 | 22.12 ± 1.34 |
| WC (cm) | 88.0 ± 9.6 | 99.0 ± 6.9 | 97.6 ± 6.9a | 89.8 ± 6.1 | 87.5 ± 5.9b | 80.5 ± 5.5 | 80.0 ± 5.7 |
| HC (cm) | 98.4 ± 6.9 | 104.9 ± 5.9 | 105.2 ± 6.2 | 97.3 ± 4.7 | 98.3 ± 4.7b | 92.3 ± 5.04 | 92.7 ± 4.7 |
| WHR | 0.89 ± 0.07 | 0.94 ± 0.06 | 0.93 ± 0.06a | 0.92 ± 0.06 | 0.89 ± 0.06b | 0.87 ± 0.06 | 0.84 ± 0.06b |
| ALT (U/L) | 22.5 ± 12.2 | 28.8 ± 20.6 | 23.9 ± 10.8b | 24.1 ± 16.7 | 22.2 ± 11.0a | 19.3 ± 8.9 | 19.6 ± 7.8 |
| AST (U/L) | 23.3 ± 8.9 | 28.5 ± 14.4 | 23.5 ± 8.7 | 23.2 ± 13.8 | 23.0 ± 7.2a | 21.1 ± 8.0 | 23.2 ± 6.0b |
| Cr (μmol/L) | 63.4 ± 33.6 | 61.6 ± 17.3 | 62.0 ± 14.2 | 68.4 ± 56.9 | 62.1 ± 14.5 | 76.6 ± 103.7 | 62.8 ± 31.5 |
| FPG (mmol/L) | 6.38 ± 1.81 | 8.59 ± 2.28 | 5.80 ± 0.54b | 8.44 ± 2.23 | 5.76 ± 0.58b | 9.54 ± 3.81 | 5.64 ± 0.55b |
| FINS (uIU/mL) | 9.25 ± 6.88 | 15.55 ± 10.09 | 12.02 ± 6.19b | 11.19 ± 10.16 | 8.57 ± 4.11b | 7.42 ± 11.53 | 5.60 ± 3.00 |
| HbA1c (%) | 6.03 ± 1.04 | 7.31 ± 1.36 | 5.80 ± 0.47 | 7.12 ± 1.34 | 5.69 ± 0.40b | 7.30 ± 1.80 | 5.62 ± 0.70b |
| HOMA-IR | 2.74 ± 2.69 | 6.07 ± 4.63 | 3.14 ± 1.82b | 4.22 ± 4.10 | 2.22 ± 1.16b | 3.04 ± 4.94 | 1.42 ± 1.84b |
| SBP (mmHg) | 133 ± 18 | 137 ± 17 | 136 ± 18 | 137 ± 19 | 132 ± 16 | 136 ± 21 | 130 ± 18 |
| DBP (mmHg) | 79 ± 11 | 81 ± 11 | 82 ± 12 | 79 ± 10 | 79 ± 10 | 75 ± 11 | 76 ± 11a |
| Cholesterol (mmol/L) | 5.09 ± 1.41 | 5.27 ± 1.31 | 5.15 ± 1.09 | 5.15 ± 1.12 | 5.03 ± 0.94 | 5.14 ± 1.21 | 5.04 ± 0.92 |
| Triglyceride (mmol/L) | 1.59 ± 1.41 | 2.26 ± 1.49 | 1.87 ± 1.51b | 2.00 ± 2.01 | 1.51 ± 1.13b | 1.41 ± 1.24 | 1.13 ± 1.21a |
| LDL-C (mmol/L) | 2.98 ± 0.84 | 3.06 ± 0.91 | 3.07 ± 0.91 | 2.97 ± 0.93 | 2.99 ± 0.78 | 2.89 ± 0.93 | 2.91 ± 0.77 |
| HDL-C (mmol/L) | 1.18 ± 0.31 | 1.06 ± 0.18 | 1.09 ± 0.24 | 1.11 ± 0.29 | 1.17 ± 0.27b | 1.30 ± 0.44 | 1.33 ± 0.36 |
| vBMD | 103.0 ± 30.8 | 105.9 ± 33.2 | 102.1 ± 29.4 | 104.0 ± 32.9 | 103.9 ± 30.6 | 101.6 ± 30.4 | 100.8 ± 30.9 |
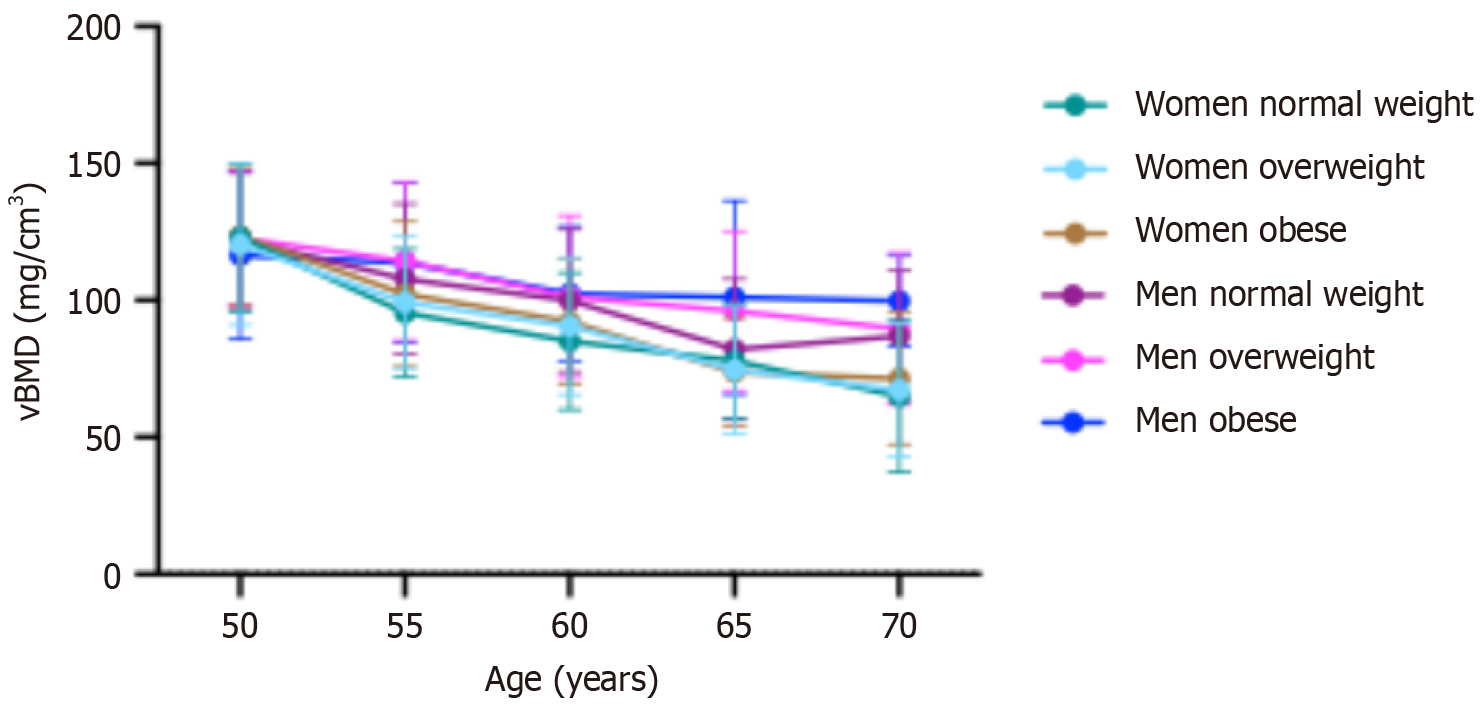
Figure 2 shows the age-dependent mean vBMD stratified by sex and BMI. In the general population, mean vBMD decreased with age, with a more pronounced decline in women than in men. Among men, those with obesity experienced a slower decline in vBMD compared to men without obesity. In women, the prevalence of osteoporosis increased from 5.2% in those under 55 years to 77.9% in those aged 70 years and older. For men, the prevalence was 4.0% between ages 50 and 55, increasing to 36.6% in those aged 70 years and older.
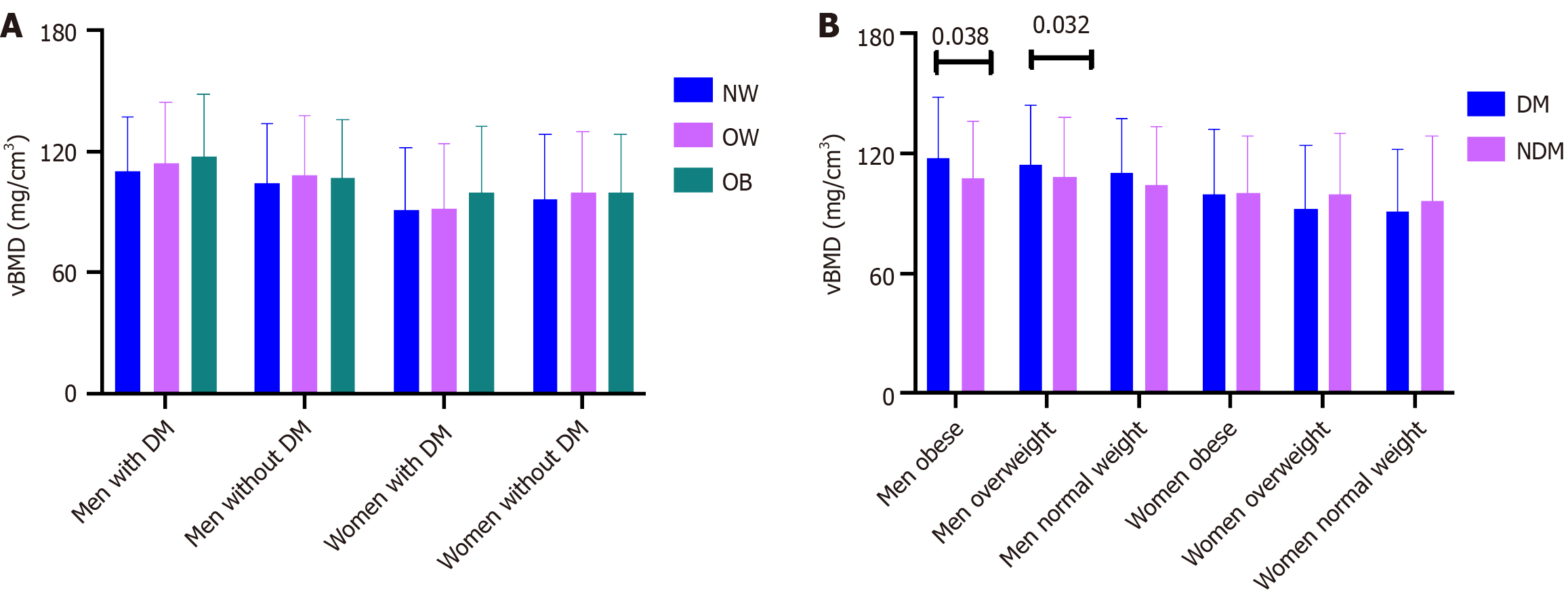
We evaluated the influence of BMI on vBMD by classifying participants into three categories: Normal weight, overweight, and obese. Analysis revealed no significant differences in vBMD among these groups when stratified by sex and glucose metabolism (Figure 3A).
To investigate the impact of diabetes on vBMD, the participants were further divided into two categories: Those with diabetes and those without. In the obese and overweight groups, the mean vBMD was significantly higher in individuals with diabetes compared to their non-diabetic counterparts (obese: 117.47 ± 30.80 mg/cm³ vs 107.28 ± 28.50 mg/cm³, P = 0.038; overweight: 114.40 ± 29.97 mg/cm³ vs 108.23 ± 29.83 mg/cm³, P = 0.032) among men aged over 50 years. In contrast, no significant differences in vBMD were found between diabetic and non-diabetic participants among normal-weight men and postmenopausal women (Figure 3B).
We conducted correlation analyses to explore variables associated with vBMD. In men with diabetes, age showed a negative correlation with vBMD (r = -0.380, P < 0.001). However, no significant associations were observed between vBMD and BMI, WC, WHR, VAT, SAT, SMA, FPG, FINS, or HbA1c. In contrast, in men without diabetes, vBMD was negatively correlated with age (r = -0.385, P < 0.001) and positively correlated with FPG (r = 0.091, P = 0.012), BMI (r = 0.093, P = 0.010), and SMA (r = 0.098, P = 0.009). In women with diabetes, vBMD was negatively associated with both age (r = -0.602, P < 0.001) and VAT (r = -0.138, P = 0.049). Among women without diabetes, vBMD had negative associations with age (r = -0.612, P < 0.001), HbA1c (r = -0.112, P = 0.002), and VAT (r = -0.144, P < 0.001), while it positively correlated with SMA (r = 0.108, P = 0.004).
Additionally, we conducted multiple regression analyses to determine the most significant independent predictors of vBMD. In men, the independent predictors identified were age (β = -0.387, P < 0.001), BMI (β = 0.130, P = 0.004), and VAT (β = -0.145, P = 0.001), accounting for an R² value of 17.9% (P < 0.001). In women, significant predictors included age (β = -0.594, P < 0.001), BMI (β = 0.157, P = 0.004), VAT (β = -0.112, P = 0.001), and SAT (β = -0.068, P = 0.035), resulting in an R² value of 39.4% (P < 0.001).
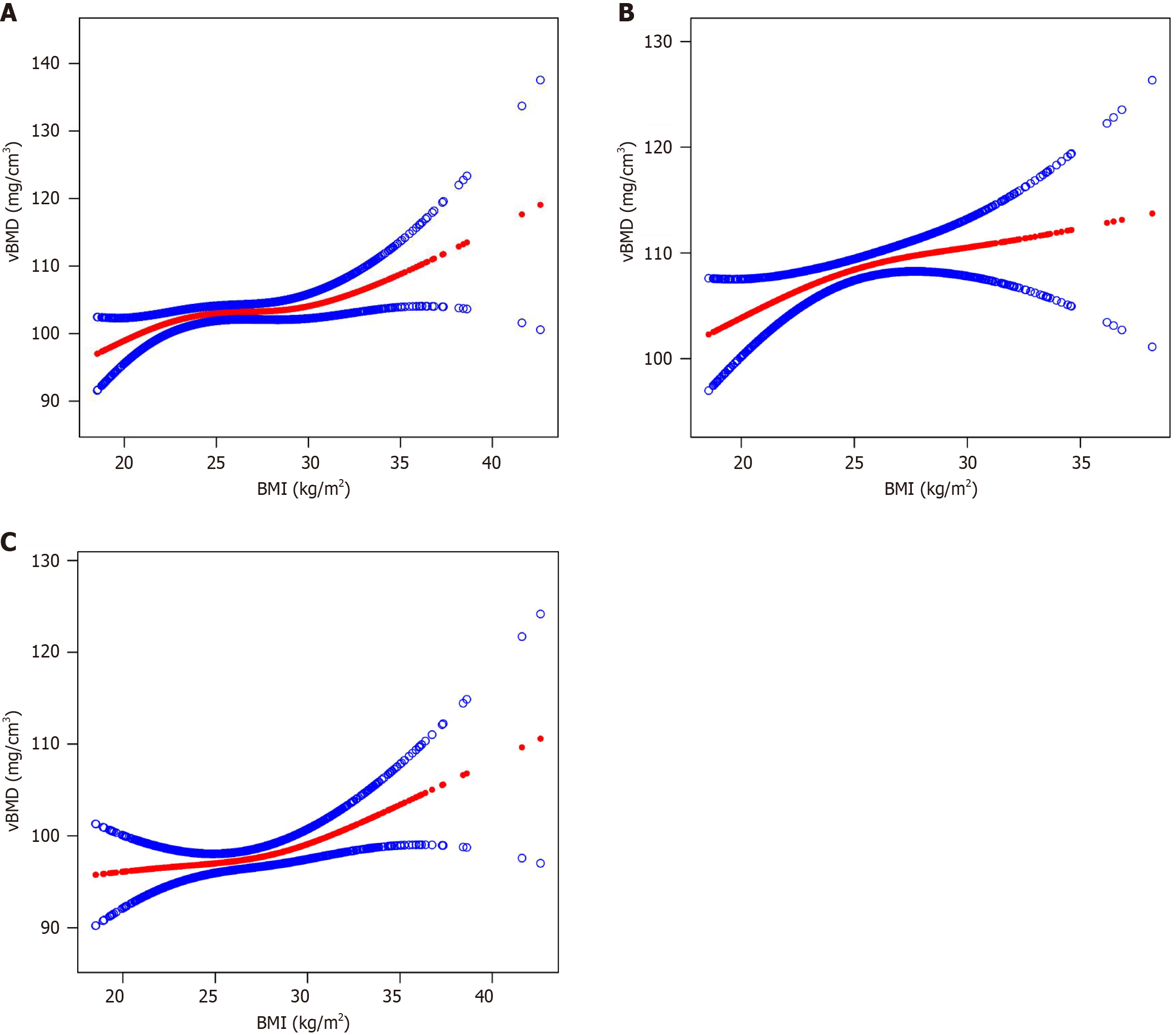
Figure 4 presents smooth curve fits that illustrate the nonlinear relationship between BMI and vBMD. The saturation effect analysis indicated that this relationship was not significant in patients with diabetes or in women without diabetes, as determined by likelihood ratio tests greater than 0.05 after adjusting for age and sex. In men without diabetes, a saturation effect was observed at a BMI of 22.33 kg/m2 (Figure 4 and Table 2). Beyond this point, further increases in BMI did not correspond to significant changes in vBMD.
| Diabetes | Non-diabetes | Total | ||||
| β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | |
| vBMD | ||||||
| Model 1 | ||||||
| One line effect | 0.54 (-0.20, 1.29) | 0.155 | 0.40 (0.01, 0.78) | 0.043 | 0.43 (0.09, 0.77) | 0.014 |
| Model 2 | ||||||
| BMI turning point (K), kg/m2 | 29.24 | 21.48 | 21.42 | |||
| < K, effect 1 | 0.28 (-0.81, 1.38) | 0.612 | 3.84 (0.41, 7.26) | 0.028 | 3.70 (0.33, 7.08) | 0.032 |
| > K, effect 2 | 1.12 (-0.83, 3.06) | 0.261 | 0.21 (-0.22, 0.64) | 0.339 | 0.28 (-0.09, 0.66) | 0.139 |
| Effect 2 - 1 | 0.83 (-1.77, 3.43) | 0.532 | -3.63 (-7.22, -0.04) | 0.048 | -3.42 (-6.93, 0.09) | 0.056 |
| LRT test | 0.529 | 0.047 | 0.056 | |||
| Men | ||||||
| Model 1 | ||||||
| One line effect | 0.33 (-0.86, 1.52) | 0.589 | 0.53 (-0.08, 1.15) | 0.043 | 0.49 (-0.06, 1.04) | 0.080 |
| Model 2 | ||||||
| BMI turning point (K), kg/m2 | 26.45 | 22.33 | 21.65 | |||
| < K, effect 1 | 1.07 (-1.26, 3.39) | 0.370 | 3.51 (0.56, 6.46) | 0.020 | 3.93 (0.14, 7.72) | 0.042 |
| > K, effect 2 | -0.33 (-2.48, 1.82) | 0.763 | 0.05 (-0.73, 0.82) | 0.907 | 0.19 (-0.45, 0.83) | 0.567 |
| Effect 2 - 1 | -1.40 (-5.18, 2.38) | 0.470 | -3.46 (-6.82, -0.11) | 0.043 | -3.74 (-7.82, 0.34) | 0.072 |
| LRT test | 0.465 | 0.043 | 0.072 | |||
| Women | ||||||
| Model 1 | ||||||
| One line effect | 0.7 (-0.2, 1.7) | 0.133 | 0.4 (-0.1, 0.8) | 0.143 | 0.49 (-0.06, 1.04) | 0.080 |
| Model 2 | ||||||
| BMI turning point (K), kg/m2 | 27.3 | 28.7 | 29 | |||
| < K, effect 1 | -0.2 (-2.4, 2.0) | 0.844 | -0.1 (-0.8, 0.6) | 0.760 | -0.1 (-0.7, 0.6) | 0.879 |
| > K, effect 2 | 1.4 (-0.3, 3.1) | 0.108 | 1.3 (0.1, 2.5) | 0.035 | 1.4 (0.4, 2.5) | 0.009 |
| Effect 2 - 1 | 1.6 (-1.8, 5.0) | 0.347 | 1.4 (-0.2, 3.0) | 0.096 | 1.5 (0, 3.0) | 0.049 |
| LRT test | 0.342 | 0.095 | 0.049 | |||
In this cross-sectional study, we found that men over 50 years with obesity or overweight and diabetes had higher lumbar spine trabecular vBMD than their non-diabetic counterparts. The relationship between BMI and vBMD differed between participants with and without diabetes, with a BMI saturation point of 22.33 kg/m2 observed in non-diabetic men.
The associations between BMI, bone mass, microarchitecture, and fracture risk are complex[16-20]. Some studies suggest that obesity is linked to favorable bone characteristics[16,17]. For instance, postmenopausal French women with obesity showed higher volumetric density, lower trabecular separation, more trabeculae, and a larger cortical area at the distal tibia and radius[16]. Similarly, postmenopausal Chinese women with obesity had higher BMD and improved cortical and trabecular parameters compared to normal-weight women[17]. However, results have been inconsistent. Shen et al[18] found that higher BMI was associated with greater trabecular and cortical vBMD, cross-sectional area, and integral volume at the proximal hip in men with obesity, though this relationship did not extend to other bone parameters. A Mendelian randomization study revealed a positive causal relationship between BMI and BMD at the lumbar spine and heel, but no significant effect on BMD at the femoral neck or forearm[5]. These variations may be due to differences in study designs, populations, measurement methods, or skeletal sites analyzed. Fracture risk also varies by skeletal site in individuals with obesity or overweight. The risk of fractures at non-vertebral sites, such as the proximal humerus, upper leg, and ankle, is higher compared to those with normal weight[19], while the risk at vertebral sites and the proximal femur is lower[20]. In this study, lumbar spine trabecular vBMD did not differ significantly across BMI categories. However, future research should assess BMD at additional skeletal sites and incorporate more detailed bone microarchitecture parameters to clarify these relationships.
Higher BMD in individuals with obesity is often attributed to increased mechanical loading on bones, but findings are inconsistent. Some studies report that higher BMI is linked to greater bone mineral content at the femoral neck and lumbar spine, but not at the radius[21,22]. Others have found increased bone mass at the lumbar spine, tibia, and radius in women with obesity, but not in men[23,24]. In addition to mechanical factors, obesity influences bone metabolism through hormones such as leptin and adiponectin[25,26]. Certain fat deposits, particularly visceral fat, have been associated with lower bone mass and detrimental bone microarchitecture, as shown in CT scans. Our study similarly found a negative association between VAT and vBMD in both men and women[6,27]. Obesity also leads to a disproportionate increase in fat mass relative to lean mass, which may explain why bone mass gains in individuals with obesity do not fully correspond to their excess body weight. Relatively lower lean mass in these individuals may partially account for these imbalanced bone mass changes.
Bone mass and microarchitecture in patients with T2D remain complex, with studies reporting conflicting results. Some show no differences in bone structure, while others indicate either worsening or improvement in bone microarchitecture among diabetics[28-32]. The Framingham Study, for instance, found that diabetic patients had lower cortical vBMD, greater cortical porosity, and reduced cross-sectional area at the tibia, but no changes at the radius[29]. Interestingly, trabecular bone indices were similar or more favorable in individuals with T2D compared to non-diabetics, regardless of sex or obesity status[30]. Nilsson et al[28] reported that elderly diabetic women had higher trabecular bone volume fraction, greater distal cortical vBMD, and larger cortical area at the radius and ultradistal tibia compared to non-diabetics. They also noted lower cortical porosity in diabetics at the distal radius, but not at the tibia or ultradistal radius[28]. Similarly, Rasmussen et al[30] observed higher cortical and trabecular vBMD, increased cortical area, and reduced trabecular separation in diabetic patients at both the tibia and radius. In contrast, Vigevano et al[31] found that men with both diabetes and obesity had greater trabecular separation at the tibia and radius than their non-diabetic counterparts, but observed no differences in cortical bone microarchitecture[28]. Burghardt et al[32] further reported that postmenopausal women with T2D exhibited higher trabecular vBMD and thickness, alongside increased cortical porosity at the radius[32]. In our study, no significant differences in trabecular vBMD were observed between diabetic and non-diabetic participants. However, many of the diabetic patients had been newly diagnosed at the time of the QCT assessments, and the relatively short duration of diabetes may have obscured potential differences in trabecular vBMD between the groups.
Postmenopausal women and men over 50 years are at a heightened risk of fractures with increasing age[1]. Furthermore, the incidence of clinical fractures in the past five years is significantly higher among individuals with obesity and overweight[1]. Therefore, it is essential to balance BMI and bone mass in this population. However, previous studies have failed to determine the optimal BMI that enhances bone mass while reduces the risk of obesity-related complications. Recent findings suggested that overweight men show improved trabecular and cortical microarchitecture in the inferomedial and superolateral regions of the femoral neck compared to controls, indicating better resistance to femoral fractures[33]. An analysis of data from the 2017-2020 National Health and Nutrition Examination Survey revealed that adults over 50 years maintain an optimal balance between BMI and femoral neck BMD at approximately 24.3 kg/m²[34]. Another study identified BMI saturation values of 26.13 kg/m2 for the total femur and 26.82 kg/m2 for the total spine among United States adults over 50[35]. In contrast, some research has found no saturation effects between BMI and BMD[17]. In our study, we found a saturation effect between BMI and BMD in men without diabetes, but not in participants with diabetes or in women without diabetes. Notably, the BMI saturation value that we identified was lower than those reported in previous studies. Additional research is needed to investigate saturation effects across different skeletal sites and various bone microarchitecture parameters. Moreover, saturation values should be examined concerning sex, age, and race for a more comprehensive understanding.
Our study has several limitations. First, the cross-sectional design precludes the establishment of a causal relationship between obesity and bone mass. Second, although bone quality encompasses bone mass, microarchitecture, and tissue material properties, we focused solely on lumbar spine trabecular vBMD. This limitation restricts our ability to assess how BMI impacts other skeletal sites, highlighting the need for future studies to explore a wider array of bone microarchitecture parameters. Third, the varying stages of obesity (Class I, Class II, and Class III) may differently affect bone mass. However, since only 24 participants in our study had a BMI greater than 35 kg/m2, we could not adequately evaluate the influence of these stages on bone mass. Lastly, factors such as metabolic-associated fatty liver disease, smoking, alcohol consumption, lifestyle choices, and hypoglycemic medications may also affect bone mass. Unfortunately, we did not consider these confounding variables in our analysis of vBMD.
Men over 50 years with obesity or overweight and diabetes demonstrated higher trabecular vBMD than those without diabetes. The association between trabecular vBMD and BMI revealed distinct patterns based on diabetes status. Understanding how obesity and diabetes affect bone microarchitecture is essential for identifying and managing patients at high risk for fractures.
We thank all staff involved in the Pinggu Metabolic Disease Study.
| 1. | Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, Cui L, Zhao N, Lin Q, Chen L, Lin H, Jin X, Dong Z, Ren Z, Hou Z, Zhang Y, Zhong J, Cai S, Liu Y, Meng R, Deng Y, Ding X, Ma J, Xie Z, Shen L, Wu W, Zhang M, Ying Q, Zeng Y, Dong J, Cummings SR, Li Z, Xia W. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw Open. 2021;4:e2121106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 2. | Khosla S, Atkinson EJ, Riggs BL, Melton LJ 3rd. Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Greco EA, Fornari R, Rossi F, Santiemma V, Prossomariti G, Annoscia C, Aversa A, Brama M, Marini M, Donini LM, Spera G, Lenzi A, Lubrano C, Migliaccio S. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010;64:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Jankowska EA, Rogucka E, Medraś M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia. 2001;33:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Song J, Zhang R, Lv L, Liang J, Wang W, Liu R, Dang X. The Relationship Between Body Mass Index and Bone Mineral Density: A Mendelian Randomization Study. Calcif Tissue Int. 2020;107:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Müller R, Zhao B, Guo X, Lang T, Saeed I, Liu XS, Guo XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98:2562-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Picke AK, Campbell G, Napoli N, Hofbauer LC, Rauner M. Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr Connect. 2019;8:R55-R70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Walsh JS, Vilaca T. Obesity, Type 2 Diabetes and Bone in Adults. Calcif Tissue Int. 2017;100:528-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Tanner SB. Dual-energy X-ray absorptiometry in clinical practice: new guidelines and concerns. Curr Opin Rheumatol. 2011;23:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kim MW, Lee DH, Huh JW, Bai JW. The impact of obesity on the accuracy of DXA BMD for DXA-equivalent BMD estimation. BMC Musculoskelet Disord. 2022;23:1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Cataño Jimenez S, Saldarriaga S, Chaput CD, Giambini H. Dual-energy estimates of volumetric bone mineral densities in the lumbar spine using quantitative computed tomography better correlate with fracture properties when compared to single-energy BMD outcomes. Bone. 2020;130:115100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hu P, Li Y, Zhou X, Zhang X, Zhang F, Ji L. Association between physical activity and abnormal glucose metabolism-A population-based cross-sectional study in China. J Diabetes Complications. 2018;32:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chen C, Lu FC; Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17 Suppl:1-36. [PubMed] |
| 14. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 15. | Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res. 2013;28:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Qi W, Jiang Y, Liu W, Chi Y, Jiajue R, Pang Q, Wang O, Li M, Xing X, Yu W, Xia W. Bone Microarchitecture in Obese Postmenopausal Chinese Women: The Chinese Vertebral Osteoporosis Study (ChiVOS). Front Endocrinol (Lausanne). 2022;13:891413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Shen J, Nielson CM, Marshall LM, Lee DC, Keaveny TM, Orwoll ES; Osteoporotic Fractures in Men MrOS Research Group. The Association Between BMI and QCT-Derived Proximal Hip Structure and Strength in Older Men: A Cross-Sectional Study. J Bone Miner Res. 2015;30:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Compston J. Obesity and fractures in postmenopausal women. Curr Opin Rheumatol. 2015;27:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Travison TG, Araujo AB, Esche GR, McKinlay JB. The relationship between body composition and bone mineral content: threshold effects in a racially and ethnically diverse group of men. Osteoporos Int. 2008;19:29-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 23. | Rexhepi S, Bahtiri E, Rexhepi M, Sahatciu-Meka V, Rexhepi B. Association of Body Weight and Body Mass Index with Bone Mineral Density in Women and Men from Kosovo. Mater Sociomed. 2015;27:259-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Viljakainen HT, Valta H, Lipsanen-Nyman M, Saukkonen T, Kajantie E, Andersson S, Mäkitie O. Bone Characteristics and Their Determinants in Adolescents and Young Adults with Early-Onset Severe Obesity. Calcif Tissue Int. 2015;97:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015;101:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res. 2017;32:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, Boyd SK, McLean RR, Broe KE, Kiel DP, Bouxsein ML. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-pQCT Study. J Bone Miner Res. 2018;33:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 30. | Rasmussen NH, Dal J, Kvist AV, van den Bergh JP, Jensen MH, Vestergaard P. Bone parameters in T1D and T2D assessed by DXA and HR-pQCT - A cross-sectional study: The DIAFALL study. Bone. 2023;172:116753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Vigevano F, Gregori G, Colleluori G, Chen R, Autemrongsawat V, Napoli N, Qualls C, Villareal DT, Armamento-Villareal R. In Men With Obesity, T2DM Is Associated With Poor Trabecular Microarchitecture and Bone Strength and Low Bone Turnover. J Clin Endocrinol Metab. 2021;106:1362-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:5045-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 33. | Jadzic J, Andjelic U, Milovanovic P, Zivkovic V, Nikolic S, Djonic D, Djuric M. Improved femoral micro-architecture in adult male individuals with overweight: fracture resistance due to regional specificities. Int J Obes (Lond). 2024;48:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Pu J. The Saturation Effect of Obesity on Bone Mineral Density for Older People: The NHANES 2017-2020. Front Endocrinol (Lausanne). 2022;13:883862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Ma M, Feng Z, Liu X, Jia G, Geng B, Xia Y. The Saturation Effect of Body Mass Index on Bone Mineral Density for People Over 50 Years Old: A Cross-Sectional Study of the US Population. Front Nutr. 2021;8:763677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |