Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.97910
Revised: August 28, 2024
Accepted: November 13, 2024
Published online: February 15, 2025
Processing time: 200 Days and 22.7 Hours
Tacrolimus (FK506) is a key calcineurin inhibitor used to prevent organ transplant rejection and is effective in improving graft survival. However, it is linked to hyperglycemia and insulin resistance, contributing to new-onset diabetes after transplantation and negatively affecting islet function.
To study the effects of tacrolimus on the insulin signaling pathway of hepatocytes.
HL7702 cells were treated with different concentrations of tacrolimus (0.1 mg/L, 1 mg/L, 5 mg/L) for 24 hours. The proteins involved in insulin signaling were detected by Western blotting.
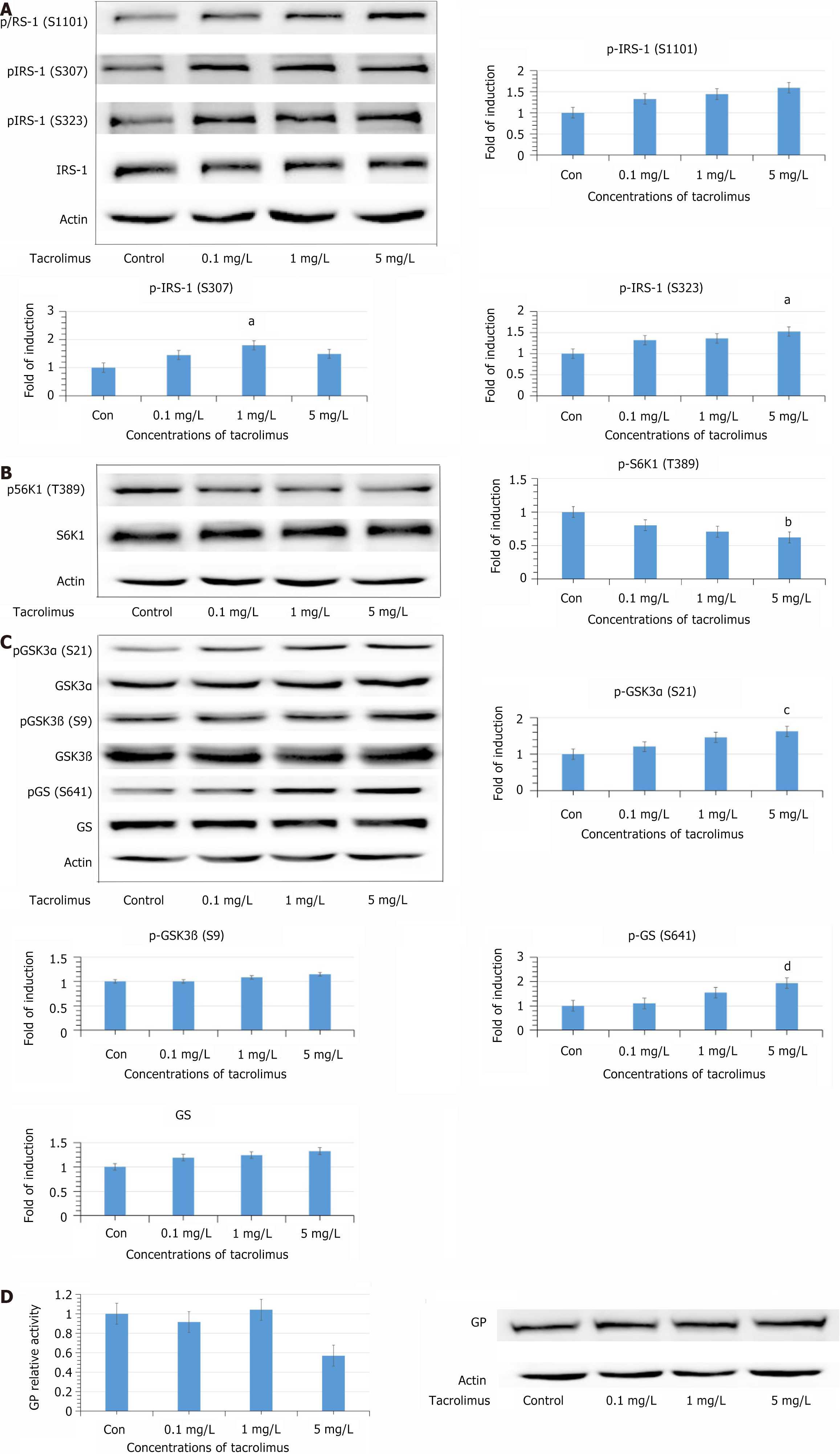
Compared with the control group, phosphorylation of insulin receptor substrate (IRS) 1 at Ser 307 and Ser 323 were increased significantly when the tacrolimus concentration reached 1 and 5 mg/L. Phosphorylation of IRS1 at Ser 1101 was also increased, although not significantly. However, phosphorylation of Ribosomal protein S6 kinase beta-1 at Thr 389 was decreased significantly. The levels of phosphorylated glycogen synthase kinase 3α Ser 21 and Ser 9 were increased. Surprisingly, phosphorylation of glycogen synthase at Ser 641 was increased. There was no significant change in the activity of glycogen phosphorylase.
Tacrolimus has no direct effect on hepatic glucose metabolism, but inhibits IRS1-mediated insulin signaling. This may be one of the underlying mechanisms by which tacrolimus induces insulin resistance.
Core Tip: Tacrolimus, a calcineurin phosphatase inhibitor, prevents transplant rejection but induces insulin resistance and diabetes. This study explores its direct effects on insulin signaling in hepatocytes, revealing mechanisms involving calcineurin inhibition, dyslipidemia, and altered glucose metabolism.
- Citation: Li HY, Wang Y, Ran M, Gao F, Zhu BY, Xiao HY, Xu C. Tacrolimus induces insulin receptor substrate 1 hyperphosphorylation and inhibits mTORc1/S6K1 cascade in HL7702 cells. World J Diabetes 2025; 16(2): 97910
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/97910.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.97910
Tacrolimus (FK506) is a calcineurin phosphatase inhibitor that has been used to prevent acute rejection after solid organ transplantation, improving graft survival[1]. However, tacrolimus has a significant ability to induce hyperglycemia and hyperinsulinemia, which suggests that insulin resistance occurs in patients treated with tacrolimus[2,3]. Moreover, it has been observed that the capacity of islet secretion can be negatively influenced by tacrolimus from an early stage after transplantation[4]. Animal experiments also show that tacrolimus contributes to development of diabetes, and promotes expression of intestinal glucose transporter (GLUT) and strengthens glucose absorption[5,6]. Our previous study has demonstrated that islet cell injury occurred in a tacrolimus-treated rat model, but the injury was reversible. During the period of tacrolimus discontinuation, the islet cell injury was improved[7]. Therefore, it has been confirmed that tacrolimus is associated with the development of new-onset diabetes after transplantation (NODAT). NODAT leads to serious deterioration of graft function, and tacrolimus is more diabetogenic than other calcineurin phosphatase inhibitors[8-10].
Many studies have been performed to discover the underlying mechanisms of tacrolimus-induced diabetes. Calci
The mechanisms of tacrolimus-associated insulin resistance are still controversial. Animal experiments indicated that tacrolimus did not suppress insulin signaling in all tissues of rats[15]. Pereira et al[16], in 2013 discovered that tacrolimus enhanced lipolysis and inhibited lipogenesis, which ultimately led to dyslipidemia[16]. Dyslipidemia plays an important role in the development of insulin resistance. The mechanisms by which dyslipidemia causes insulin resistance have not been completely elucidated, but some important factors have been identified, including oxidative and endoplasmic reticulum stress, proinflammatory cytokines and generation of lipid metabolites[17]. Accordingly, tacrolimus may induce insulin resistance through an indirect pathway. Further study has shown that tacrolimus reduces glucose uptake of adipocytes by increasing GLUT4 endocytosis without inhibiting the insulin signaling pathway[18]. These results provide us with a novel insight in the study of tacrolimus-induced insulin resistance.
Liver is the backbone in substance metabolism. In hepatocytes, glycogen synthase and glycogen phosphorylase are the main enzymes in glucose metabolism. In fasting conditions, glucagon promotes glycogenolysis by activating glycogen phosphorylase and inhibiting glycogen synthase[19]. During the postprandial phase, gastrointestinal nutrients are absorbed into the liver prior to entering the blood circulation, and insulin promotes glycogenesis by reversing the action of glucagon to maintain glucose homeostasis[19]. It has been suggested that insulin receptor substrate (IRS) 2 of hepatocytes is mainly expressed in the periportal area of the liver, which dominates glucose metabolism and is suppressed by high levels of insulin induced by excess free fatty acids (FFAs)[20,21]. Therefore, disorder of insulin signaling in hepatocytes is decisive in development of insulin resistance and there is a need to explore the direct effects of tacrolimus on hepatocytes. Our previous study demonstrated that tacrolimus increased phosphorylation at Thr 308 of Akt in HL7702 cells[22]. Accordingly, the effects of tacrolimus on other important sites in insulin signaling of hepatocytes will be further investigated in this study.
FK506 was purchased from Cell Signaling Technology (3 Trask Lane, Danvers, MA, United States). The primary antibodies were as follows: Anti-phospho-GSK3α (Ser 21) rabbit monoclonal antibody (mAb), anti-GSK3α rabbit mAb, anti-phospho-GSK3β (Ser 9) rabbit mAb, anti-GSK3β rabbit mAb, anti-phospho-GS (Ser 641) rabbit Ab, anti-GS rabbit Ab, anti-phospho-S6K1 (Thr 389) rabbit mAb, anti-phospho-IRS1 (Ser 307) rabbit mAb, anti-phospho-IRS1 (Ser 323) rabbit mAb, anti-phospho-IRS1 (Ser 1101) rabbit Ab, and anti-IRS1 rabbit Ab (Cell Signaling Technology). An anti-S6K1 rabbit antibody was obtained from Millipore. An anti-glycogen phosphorylase (GP) antibody was obtained from Proteintech Group (Chicago, IL, United States). An anti-actin mouse mAb was obtained from Abcam. The secondary antibodies for western blotting were horseradish-peroxidase-linked anti-mouse/rabbit IgG (Cell Signaling Technology). Other chemicals and reagents were purchased from commercial sources.
Human liver cell line HL7702 was obtained from Cell Bank of the Chinese Academy of Science. HL7702 cells were cultivated in RPMI-1640 medium (Gibco) supplemented with 10% newborn bovine serum (Zhejiang Tianhang Biotechnology). The atmosphere was humidified with 5% CO2 at 37 °C. Before the medium was replaced with fresh medium that contained different concentrations of FK506 (0.1, 1 and 5 mg/L, dissolved in DMSO), HL7702 cells were allowed to grow for 24 hours in logarithmic phase. The fresh medium was used to treat HL7702 cells in the control group.
HL7702 cells in each group were lysed by RIPA buffer [1% NP-40, 150 mmol/L NaCl, 0.1% SDS, 0.5% deoxycholate, 50 mmol/L Tris (pH 7.4), protease inhibitors] to extract total protein. The concentration of protein was quantified by BCA assay. The protein was heated for 5 minutes in boiled water and denatured. SDS-PAGE was used to separate the denatured protein. The denatured protein was transferred onto nitrocellulose (NC) membranes. NC membranes were blocked by Tris-Buffered Saline with Tween-20 for 3 hours which contained 5% fat-free milk. NC membranes incubated with the corresponding primary antibodies overnight at 4 °C and secondary antibodies at room temperature for 1 hours. The target bands were visualized by the chemiluminescence imaging system and quantified by ImageJ software.
GP activity was measured according to the kit instruction of manufacturer (GenMed, Plymouth, MN, United States). Total protein was added to a buffer containing substrate and measured once every 60 seconds for 5 minutes at an absorbance of 340 nm. The difference between the immediate absorbance and the absorbance after 5 minutes represented the activity.
The data are shown as the mean ± SD from three independent experiments (n = 3). The significance of the differences between the control and tacrolimus treatment groups was analyzed with SPSS version 17.0 using one-way analysis of variance. P < 0.05 was defined as statistically significant.
HL7702 cells were exposed to tacrolimus at different concentrations for 24 hours, and we detected phosphorylation at the serine sites of IRS1. Phosphorylation of IRS1 (Ser 307 and Ser 323) was significantly increased at 1 and 5 mg/L (P < 0.05; Figure 1A). Phosphorylation of IRS1 at Ser 1101 was also increased, but there was no significant change. The total levels of IRS-1 did not show any significant change.
Our previous study showed that tacrolimus increased phosphorylation at Thr 308 of Akt in HL7702 cells after 24 hours treatment. IRS1 serine residues were mediated by the PI3K–Akt–S6K1 negative feedback loop, so the phosphorylation level of S6K1 (Thr 389) was further detected. However, phosphorylation of S6K1 (Thr 389) was decreased significantly (P < 0.05; Figure 1B).
Phosphorylation of glycogen synthase kinase (GSK)3α (Ser 21) and GSK3β (Ser 9) was increased, which demonstrated that activity of GSK3 was inhibited (Figure 1C). Surprisingly, the phosphorylation of the GSK3 substrate, GS (Ser 641), was not decreased but increased. Moreover, the total levels of GS were increased after tacrolimus exposure. There was no significant change in the activity of GP (Figure 1D).
In this study, tacrolimus decreased the level of p-S6K1 (Thr 389), which indicated that the mTORC/S6K1 pathway was inhibited. Besides, IRS1 was found to be phosphorylated at Ser 323, Ser 307 and Ser 1101. These results demonstrated that the activity of IRS1 was also inhibited. Insulin signaling mediated by IRS1 in hepatocytes is mainly responsible for lipogenesis[21]. Therefore, tacrolimus may inhibit lipid synthesis by disrupting insulin signaling associated with IRS1, and then increasing FFA concentration in the circulation. FFAs have been identified as important signaling molecules in insulin secretion. The experiment proved that FFAs are able to promote insulin secretion stimulated by glucose from pancreatic β-cells[23]. High level of insulin in the fasting phase can inhibit the expression of IRS2 in hepatocytes and induce hyperglycemia.
Our previous study found that tacrolimus increased phosphorylation at Thr 308 of Akt in HL7702 cells, which indicated that the activity of Akt had been promoted. It is well known that the PI3K/Akt pathway is important in substance metabolism. The PI3K/Akt pathway is activated by insulin binding to cell surface receptors, and activated Akt increases expression of GLUT proteins and promotes their translocation[24]. In hepatocytes, glucose transport is equivalent to free diffusion and not regulated by insulin[25]. Our previous study has also shown that insulin cannot mediate GLUT2 expression and translocation in HL7702 cells[26]. Accordingly, the enzymes associated with glucose metabolism are crucial in maintaining blood glucose homeostasis. GS plays a major role in glucose metabolism, and defective glycogen synthesis is an important cause of insulin resistance[27]. A lack of hepatic glycogen synthesis promotes lipid synthesis, and excess lipid impairs insulin signaling in hepatocytes[28]. Therefore, we detected the proteins associated with glycogen synthesis.
GSK3, the main substrate of Akt, is the principal kinase that is responsible for GS (Ser 641) phosphorylation[29]. GSK3 is important in regulating the expression of GS, which can be significantly promoted when the activity of GSK3 is inhibited[30]. In this study, tacrolimus increased phosphorylation of GSK3α at Ser 21 and GSK3β at Ser 9, which suggested that activity of GSK3 was inhibited. However, phosphorylation of GS (Ser 641) was elevated and there was no significant increase in GS expression. An animal study demonstrated that GS activity was strongly related to phosphorylation of Ser 641[31]. Increased phosphorylation at Ser 641 suggested that activity of GS was inhibited. To ascertain the effects of tacrolimus on glycogen synthesis and explore the mechanism by which tacrolimus leads to phosphorylation of GS at Ser 641, GP activity was further measured. GP determines the rate of glycogen degradation, and inhibiting GP activity has been identified as one of therapeutic measures in type 2 diabetes. Phosphorylated GS and phosphorylated GP are both substrates of protein phosphatase (PP)1[32,33]. After 24 h exposure of tacrolimus, the change in GP activity failed to achieve significance and there was no change in its expression. Phosphorylated GP is the active form. Dephosphorylation of GP is a critical action in glycogen synthesis, and this process is catalyzed by PP1[32]. Therefore, this result suggests that tacrolimus does not influence the activity of PP1, and phosphorylation of GS induced by tacrolimus is not achieved by inhibiting PP1.
FFAs have been identified as a critical factor in insulin resistance development. Clinical experiments have shown that insulin resistance can be induced by excess FFAs in the circulation, and endurance training cannot eliminate the negative effect[34]. Moreover, lowering plasma FFAs level can ameliorate insulin resistance and improve glucose tolerance[35]. Pancreatic β-cells are stimulated by FFAs to secrete insulin[36]. In hepatocytes, the insulin signaling pathway mediated by IRS2 is responsible for the regulation of glucose metabolism. The activity of IRS2 is suppressed in hyperinsulinemia[20,21].
Sterol-regulatory-element-binding proteins (SREBPs), which control the expression of lipogenic genes, are important transcription factors in lipogenesis. SREBPs are regulated by mTORC/S6K1 signaling. Inhibiting mTORC/S6K1 signaling can reduce the expression of SREBPs[37]. Triglyceride breakdown is negatively regulated by mTORC/S6K1 signaling. The process of lipolysis is catalyzed by adipose triglyceride lipase (ATGL), and mTORC1 influences the lipolytic process by regulating the expression of ATGL. The expression of ATGL significantly increases when the activity of mTORC1 is inhibited[38].
There were some limitations to this study. Due to the complexity of the regulation of glycogen synthesis, allosteric regulation is also a critical pathway in glycogen synthesis[39]. Therefore, the phosphorylation regulation cannot completely determine glycogen synthesis, and the rate of glycogen synthesis should be detected in future experiments.
This study shows that tacrolimus has no direct effect on hepatic glucose metabolism, but inhibits IRS1-mediated insulin signaling. This effect may result in hepatic lipid metabolism disorder and elevated FFAs in the circulation. This may be one of the underlying mechanisms by which tacrolimus induces insulin resistance in the body.
| 1. | Li CJ, Li L. Tacrolimus in preventing transplant rejection in Chinese patients--optimizing use. Drug Des Devel Ther. 2015;9:473-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Dmitrewski J, Krentz AJ, Mayer AD, Buckels JA, Barnes AD, Smith J, Nattrass M. Metabolic and hormonal effects of tacrolimus (FK506) or cyclosporin immunosuppression following renal transplantation. Diabetes Obes Metab. 2001;3:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Wyzgał J, Paczek L, Sańko-Resmer J, Ciszek M, Nowak M, Rowiński W, Szmidt J, Durlik M. Insulin resistance in kidney allograft recipients treated with calcineurin inhibitors. Ann Transplant. 2007;12:26-29. [PubMed] |
| 4. | van Duijnhoven EM, Christiaans MHL, Boots JMM, Nieman FHM, Wolffenbuttel BHR, van Hooff JP. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol. 2002;13:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Larsen JL, Bennett RG, Burkman T, Ramirez AL, Yamamoto S, Gulizia J, Radio S, Hamel FG. Tacrolimus and sirolimus cause insulin resistance in normal sprague dawley rats. Transplantation. 2006;82:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Li Z, Sun F, Zhang Y, Chen H, He N, Chen H, Song P, Wang Y, Yan S, Zheng S. Tacrolimus Induces Insulin Resistance and Increases the Glucose Absorption in the Jejunum: A Potential Mechanism of the Diabetogenic Effects. PLoS One. 2015;10:e0143405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Xu C, Niu YJ, Liu XJ, Teng YQ, Li CF, Wang HY, Yin JP, Wang LT, Shen ZY. Tacrolimus reversibly reduces insulin secretion, induces insulin resistance, and causes islet cell damage in rats. Int J Clin Pharmacol Ther. 2014;52:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Liu JY, You RX, Guo M, Zeng L, Zhou P, Zhu L, Xu G, Li J, Liu D. Tacrolimus Versus Cyclosporine as Primary Immunosuppressant After Renal Transplantation: A Meta-Analysis and Economics Evaluation. Am J Ther. 2016;23:e810-e824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Song JL, Gao W, Zhong Y, Yan LN, Yang JY, Wen TF, Li B, Wang WT, Wu H, Xu MQ, Chen ZY, Wei YG, Jiang L, Yang J. Minimizing tacrolimus decreases the risk of new-onset diabetes mellitus after liver transplantation. World J Gastroenterol. 2016;22:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Malvezzi P, Rostaing L. The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf. 2015;14:1531-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Oetjen E, Baun D, Beimesche S, Krause D, Cierny I, Blume R, Dickel C, Wehner S, Knepel W. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003;63:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Speckmann T, Sabatini PV, Nian C, Smith RG, Lynn FC. Npas4 Transcription Factor Expression Is Regulated by Calcium Signaling Pathways and Prevents Tacrolimus-induced Cytotoxicity in Pancreatic Beta Cells. J Biol Chem. 2016;291:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Rodríguez-Rodríguez AE, Triñanes J, Porrini E, Velázquez-García S, Fumero C, Vega-Prieto MJ, Díez-Fuentes ML, Luis Lima S, Salido E, Torres A. Glucose homeostasis changes and pancreatic β-cell proliferation after switching to cyclosporin in tacrolimus-induced diabetes mellitus. Nefrologia. 2015;35:264-272. [DOI] [Full Text] |
| 14. | Prokai A, Fekete A, Pasti K, Rusai K, Banki NF, Reusz G, Szabo AJ. The importance of different immunosuppressive regimens in the development of posttransplant diabetes mellitus. Pediatr Diabetes. 2012;13:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Shivaswamy V, Bennett RG, Clure CC, Ottemann B, Davis JS, Larsen JL, Hamel FG. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl Res. 2014;163:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, Eriksson JW. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol Cell Endocrinol. 2013;365:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 18. | Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, Eriksson JW. Cyclosporine A and tacrolimus reduce the amount of GLUT4 at the cell surface in human adipocytes: increased endocytosis as a potential mechanism for the diabetogenic effects of immunosuppressive agents. J Clin Endocrinol Metab. 2014;99:E1885-E1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab. 2014;16 Suppl 1:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, Sasako T, Moroi M, Sugi K, Kakuta S, Iwakura Y, Noda T, Ohnishi S, Nagai R, Tobe K, Terauchi Y, Ueki K, Kadowaki T. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Kubota N, Kubota T, Kajiwara E, Iwamura T, Kumagai H, Watanabe T, Inoue M, Takamoto I, Sasako T, Kumagai K, Kohjima M, Nakamuta M, Moroi M, Sugi K, Noda T, Terauchi Y, Ueki K, Kadowaki T. Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Nat Commun. 2016;7:12977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Yao Y, Wang HY, An J, Wang Y, Zhu BY, Gao F, Xu C. [Effects of tacrolimus on the expression of Akt in HL7702 cells]. Zhonghua Zaihai Jiuyuan Yixue. 2019;3:154-157. [DOI] [Full Text] |
| 23. | Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1239] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 24. | Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism. 2016;65:124-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 25. | Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Liu J, Xu C, Zhang S, Li H, Chen K, Huang P, Guo Z, Xu L. Microcystin-LR disrupts insulin signaling by hyperphosphorylating insulin receptor substrate 1 and glycogen synthase. Environ Toxicol. 2018;33:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Turnbull J, Tiberia E, Pereira S, Zhao X, Pencea N, Wheeler AL, Yu WQ, Ivovic A, Naranian T, Israelian N, Draginov A, Piliguian M, Frankland PW, Wang P, Ackerley CA, Giacca A, Minassian BA. Deficiency of a glycogen synthase-associated protein, Epm2aip1, causes decreased glycogen synthesis and hepatic insulin resistance. J Biol Chem. 2013;288:34627-34637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Irimia JM, Meyer CM, Segvich DM, Surendran S, DePaoli-Roach AA, Morral N, Roach PJ. Lack of liver glycogen causes hepatic insulin resistance and steatosis in mice. J Biol Chem. 2017;292:10455-10464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2:101-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 341] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | MacAulay K, Blair AS, Hajduch E, Terashima T, Baba O, Sutherland C, Hundal HS. Constitutive activation of GSK3 down-regulates glycogen synthase abundance and glycogen deposition in rat skeletal muscle cells. J Biol Chem. 2005;280:9509-9518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Burén J, Lai YC, Lundgren M, Eriksson JW, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys. 2008;474:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Agius L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol Aspects Med. 2015;46:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Somsák L, Czifrák K, Tóth M, Bokor E, Chrysina ED, Alexacou KM, Hayes JM, Tiraidis C, Lazoura E, Leonidas DD, Zographos SE, Oikonomakos NG. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr Med Chem. 2008;15:2933-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Chow LS, Seaquist ER, Eberly LE, Mashek MT, Schimke JM, Nair KS, Mashek DG. Acute free fatty acid elevation eliminates endurance training effect on insulin sensitivity. J Clin Endocrinol Metab. 2012;97:2890-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Haber EP, Procópio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic beta-cell function. Int Rev Cytol. 2006;248:1-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 459] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 38. | Caron A, Richard D, Laplante M. The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr. 2015;35:321-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 39. | Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, Voshol PJ, Jensen J, Sakamoto K. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 2010;12:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |