Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1962
Revised: July 17, 2024
Accepted: August 12, 2024
Published online: September 15, 2024
Processing time: 83 Days and 3.9 Hours
Diabetes is often associated with gastrointestinal dysfunctions, which can lead to hypoglycemia. Dexmedetomidine (DEX) is a commonly used sedative in perioperative diabetic patients and may affect gastrointestinal function.
To investigate whether sedative doses of DEX alleviate diabetes-caused intestinal dysfunction.
Sedation/anesthesia scores and vital signs of streptozotocin (STZ)-induced diabetic mice under DEX sedation were observed. Diabetic mice were divided into saline and DEX groups. After injecting sedatives intraperitoneally, tight junctions (TJs) and apoptotic levels were evaluated 24 hours later to assess the intestinal barrier function. The role of DEX was validated using Villin-MMP23B flox/flox mice with intestinal epithelial deletion. In vitro, high glucose and hyperosmolarity were used to culture Caco-2 monolayer cells with STZ inter-vention. Immunofluorescence techniques were used to monitor the barrier and mitochondrial functions.
MMP23B protein levels in the intestinal tissue of STZ-induced diabetic mice were significantly higher than those in the intestinal tissue of control mice, with the DEX group displaying decreased MMP23B levels. Diabetes-mediated TJ dis-ruption, increased intestinal mucosal permeability, and systemic inflammation in wild-type mice might be reversed by DEX. In Caco-2 cells, MMP23B was associated with increased reactive oxygen species accumulation, mitochondrial membrane potential depolarization, and TJ disruption.
DEX reduces MMP23B, which may potentially contribute to STZ-induced intestinal barrier dysfunction, affecting TJ modification through mitochondrial dysfunction.
Core Tip: This study investigates the protective role of dexmedetomidine (DEX) in diabetic intestinal injury through the MMP23B pathway. The findings reveal that DEX acts as a sedative and enhances intestinal barrier function by promoting M2 macrophage polarization and reducing mitochondrial dysfunction. Streptozotocin-induced diabetic mice and Caco-2 cell models provide robust evidence of the potential therapeutic benefits of DEX, offering insights into its dual functionality in managing sedation and intestinal healing under diabetic conditions. This research can pave the way for the development of new treatment strategies targeting intestinal complications in diabetic patients.
- Citation: Lu M, Guo XW, Zhang FF, Wu DH, Xie D, Luo FQ. Dexmedetomidine ameliorates diabetic intestinal injury by promoting the polarization of M2 macrophages through the MMP23B pathway. World J Diabetes 2024; 15(9): 1962-1978
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1962.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1962
Type 2 diabetes mellitus (T2DM) is a multifactorial disease characterized by tissue-specific dysregulation, and a complex interplay of genetic and environmental factors contribute to its pathogenesis[1-3]. Although the endocrine aspects of T2DM have been extensively studied, its intestinal component remains poorly explored. The gut, often overlooked, plays a pivotal role in glucose homeostasis and may be a key intervention area.
Intestinal macrophages are key players involved in the homeostasis of the gastrointestinal tract, performing several functions including supporting intestinal neurons, facilitating gastrointestinal motility, participating in oral tolerance to food antigens, and contributing to pathogen clearance. However, intestinal macrophages are also implicated in T2DM pathogenesis. Emerging evidence suggests that alterations in the functional profile of intestinal macrophages can incite metabolic inflammation, thereby accelerating obesity and T2DM progression[4-6].
T2DM can lead to gastrointestinal motility disorders, such as decreased gastric motility. However, the specific mechanisms linking T2DM to these gastrointestinal changes are not fully elucidated. Anesthetics, including dex-medetomidine (DEX), influence various physiological processes, but their impact on intestinal macrophages in diabetic contexts remains poorly understood.
DEX, a selective alpha-2 adrenergic receptor agonist, is widely used for its sedative, analgesic, and anxiolytic properties. In the context of infection and other pathological conditions, DEX enhances intestinal microcirculation, alleviates postoperative pain, and decreases opioid requirements[7-12]. Given its potential influence on inflammatory and immune responses, DEX may modulate the activity of intestinal macrophages, affecting T2DM-associated gastrointestinal dysfunctions.
In the present study, we aimed to delineate the intricate relationship between macrophages and DEX in the context of T2DM. By establishing a streptozotocin (STZ)-induced diabetic mouse model, we investigated the role of DEX in diabetes-induced gastrointestinal injury through comprehensive in vivo and in vitro experiments. Understanding the interaction between DEX and intestinal macrophages can unveil novel mechanisms underlying diabetes-related gastrointestinal complications and identify targets for therapeutic intervention. Our findings provide a fresh perspective on the intricate dynamics of diabetes-related intestinal dysfunction and may pave the way for the development of innovative treatment strategies that leverage the pharmacological potential of DEX to modulate macrophage activity and ameliorate gastrointestinal disturbances in diabetic patients.
Differential gene analysis was performed on data sourced from the Gene Expression Omnibus database GSE63992. Volcano plots and heatmaps were generated, and R language packages were used for data processing[7,8].
A Gene Ontology/Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed on the data sourced from the Gene Expression Omnibus database GSE63992. R language packages were applied for data processing, and the corresponding bubble, circular, and bar charts, which also facilitated the analysis of gene overlaps within the identified Gene Ontology terms, were obtained. Furthermore, the involvement of differentially expressed genes (DEGs) in specific pathways was analyzed using DAVID[9,10].
Gene networks were visualized using Cytoscape 3.6.1, and further analyzed within the STRING database to investigate biological processes.
Villin-CreERT, MMP23B flox/flox, and wild-type (WT) C57BL/6 male mice aged 6-8 weeks were provided by the Xinhua Hospital affiliated with the Shanghai Jiao Tong University School of Medicine. All animals were handled according to the 3R principle and in compliance with animal welfare standards.
The mice were divided into the following groups: Control, diabetes (DB), DB + MMP23B knockout (KO), DEX + DB, and DEX. The DB and DEX + DB mice were injected with STZ (40 mg/kg, intraperitoneal) daily for 5 days[11,12].
We used clodronate liposomes (Cls), which are taken up by macrophages through endocytosis; the release of clodronate by lysosomes induces apoptosis and depletes macrophages. Mice were randomly assigned to one of the following four groups: Sham + phosphate buffered saline (PBS) (Sham; n = 4), DB (n = 4), MMP23B KO + DB + Cls (MMDC; n = 4), and MMP23B KO + PBS (MMP; n = 4). Cls or control (PBS) were intraperitoneally administered at a 24-hour interval before the DB procedure[13,14].
We obtained Caco-2 cells from the Cell Bank of the Chinese Academy of Sciences, and four groups were randomly assigned: PBS (n = 4), MMP23B (n = 4), MMP23B + GM6001 (n = 4).
Immunofluorescence staining was performed on frozen and paraffin tissue sections. Cells and tissues were fixed on glass slides or centrifuge tubes using 4% paraformaldehyde to preserve their morphological structure and protein distribution. Samples were treated with 0.1% Triton X-100 to increase antibody entry and binding. Nonspecific binding sites were blocked by adding an appropriate concentration of bovine serum albumin. Specific antibodies targeting the proteins of interest [CD 86 (AiFang, AF16377, 1:1000, rabbit polyclonal IgG), ZO-1 (Santa Cruz, sc-33725, 1:100, rat monoclonal IgG), and occludin (Santa Cruz, sc-133256, 1:100, mouse monoclonal IgG)] were added, and samples were incubated overnight at 4 °C. Secondary antibodies labeled with specific dyes [FITC-labeled donkey anti-rabbit IgG (Servicebio, GB22403, 1:200) and Cy3-labeled goat anti-mouse IgG (Servicebio, GB21303, 1:200)] were used for fluorescent signal detection. Samples were washed several times with a buffer solution to remove unbound secondary antibodies and other nonspecifically binding molecules. Slides were placed under a fluorescence microscope, and fluorescent signals were observed, detected, and recorded after illuminating with a specific wavelength. All images were captured using an Olympus fluorescence microscope (Olympus, BX53)[15,16].
Samples (tissue extracts) were collected and diluted or processed as needed. The target molecule was dissolved in a buffer solution and added to the wells of an enzyme-linked immunosorbent assay plate. The solid phase was allowed to interact with the target molecule, and the plate was incubated at room temperature or under refrigerated conditions. This step allows the target molecule to adsorb onto the solid phase[17].
The mitochondrial membrane potential (ΔΨm) was determined according to the manufacturer’s instructions. After washing the cells, a JC-1 working solution was labeled for 20 minutes (37 °C). The dual fluorescence imaging of JC-1 was completed using a RuoChuang camera[18]. Using the JC-1 monomer level to assess mitochondrial health is of great significance as it can reflect changes in ΔΨm. ΔΨm is an important indicator of mitochondrial function, and its decrease is usually associated with early apoptosis events. As a fluorescent probe, JC-1 can exhibit different fluorescent properties under different ΔΨm states. When ΔΨm is high, JC-1 is in a polymeric form inside the mitochondria, emitting red fluorescence; however, when ΔΨm decreases, JC-1 exists in a monomeric form in the cytoplasm, emitting green fluorescence. This change in fluorescence color provides an intuitive and sensitive method for detecting ΔΨm.
In research on diabetic intestinal injury, a specific detection method using JC-1 can help reveal changes in mito-chondrial function under diabetic conditions. Diabetes may lead to mitochondrial dysfunction, including decreased ΔΨm, thereby affecting the energy metabolism of intestinal cells and cell apoptosis. Through JC-1 detection, researchers can observe changes in ΔΨm of intestinal cells in a diabetic mouse model and further explore the impact of diabetes on mitochondrial function in intestinal cells.
Furthermore, the versatility of the JC-1 staining method makes it suitable for various cell types, including muscle cells and neurons, and as an indicator of ΔΨm in intact tissues and isolated mitochondria. The high affinity of JC-1 staining reagents for mitochondria and its application in flow cytometry analysis makes it an indispensable tool for studying mitochondrial health and apoptosis.
Statistical analysis was conducted using GraphPad Prism 9.0. The normality of the data was assessed using the Kolmogorov-Smirnov test. Subsequently, Tukey’s post hoc test was used for multiple group comparisons conducted through a one-way analysis of variance. Quantitative data are presented as mean ± SD. P value of < 0.05 were considered statistically significant.
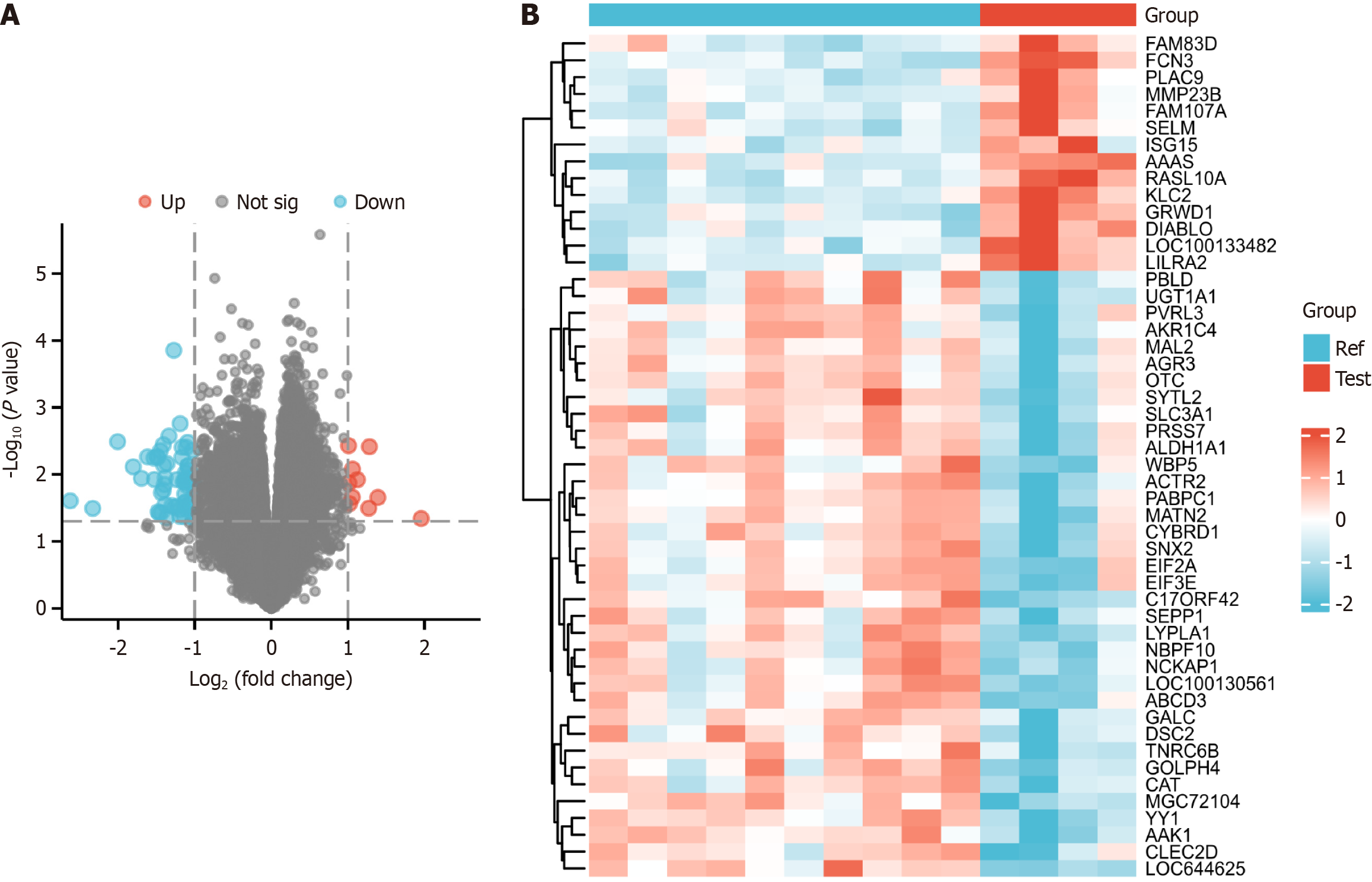
The heatmap of gene differences and volcano plots are presented in the figures. In total, 34687 genes were identified based on the following criteria: |LogFC| > 2 and P value < 0.05 (3 genes); |LogFC| > 1 and P value < 0.05 (75 genes); and |LogFC| > 0.58 and P value < 0.05 (514 genes). Among identified genes, FAM83D, FCN3, PLAC9, MMP23B, FAM107A, SELM, ISG15, AAAS, RASL10A, and KLC2 were upregulated in samples from diabetic individuals (Figure 1).
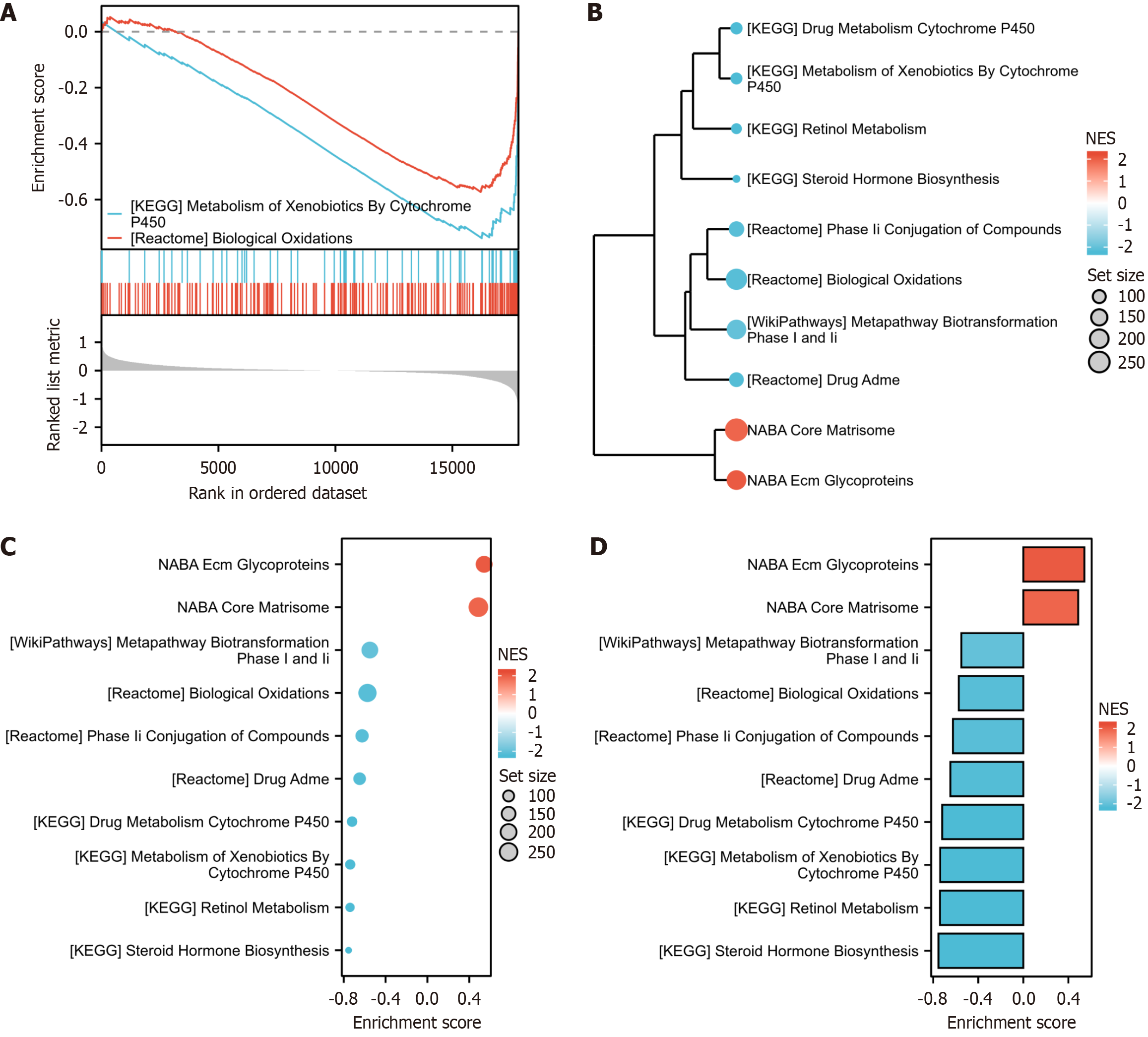
The enriched genes demonstrated significant involvement in diverse biological processes, including the KEGG metabolism of xenobiotics by cytochrome P450, reactome biological oxidations, KEGG drug metabolism cytochrome P450, NABA ECM glycoproteins, KEGG retinol metabolism, NABA core matrisome, reactome drug ADME, KEGG steroid hormone biosynthesis, reactome phase II conjugation of compounds, and WP metapathway biotransformation phase I and II (Figure 2).
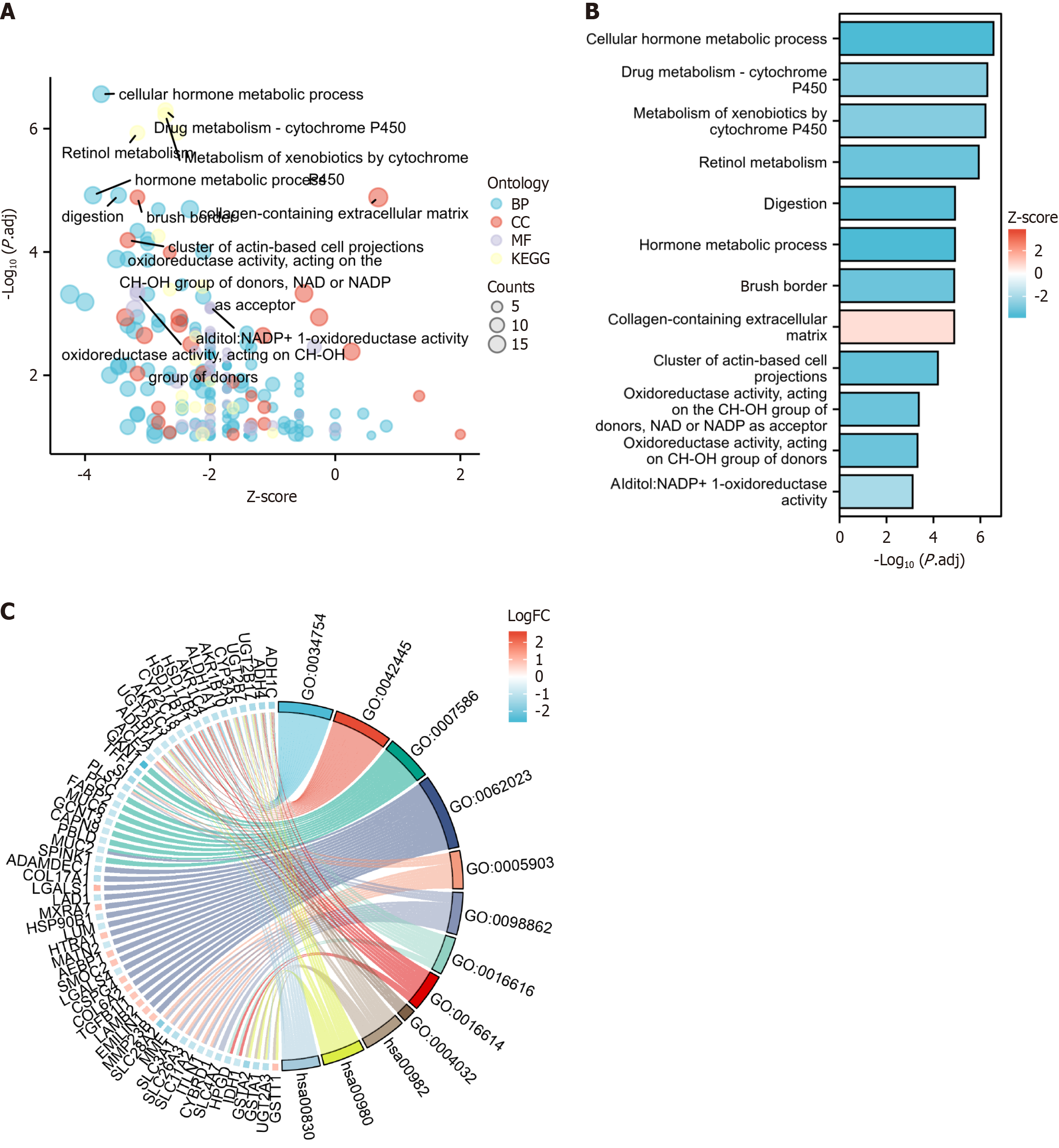
Figure 3A displays the expression of multiple inflammatory factors in the control and experimental groups. These factors are primarily linked to cellular hormone metabolic processes, hormone metabolism, digestion, responses to toxic substances, retinol metabolism, collagen-containing extracellular matrix, brush border, and cluster of actin-based cell projections space. They are also enriched in various KEGG pathways (Figure 3).
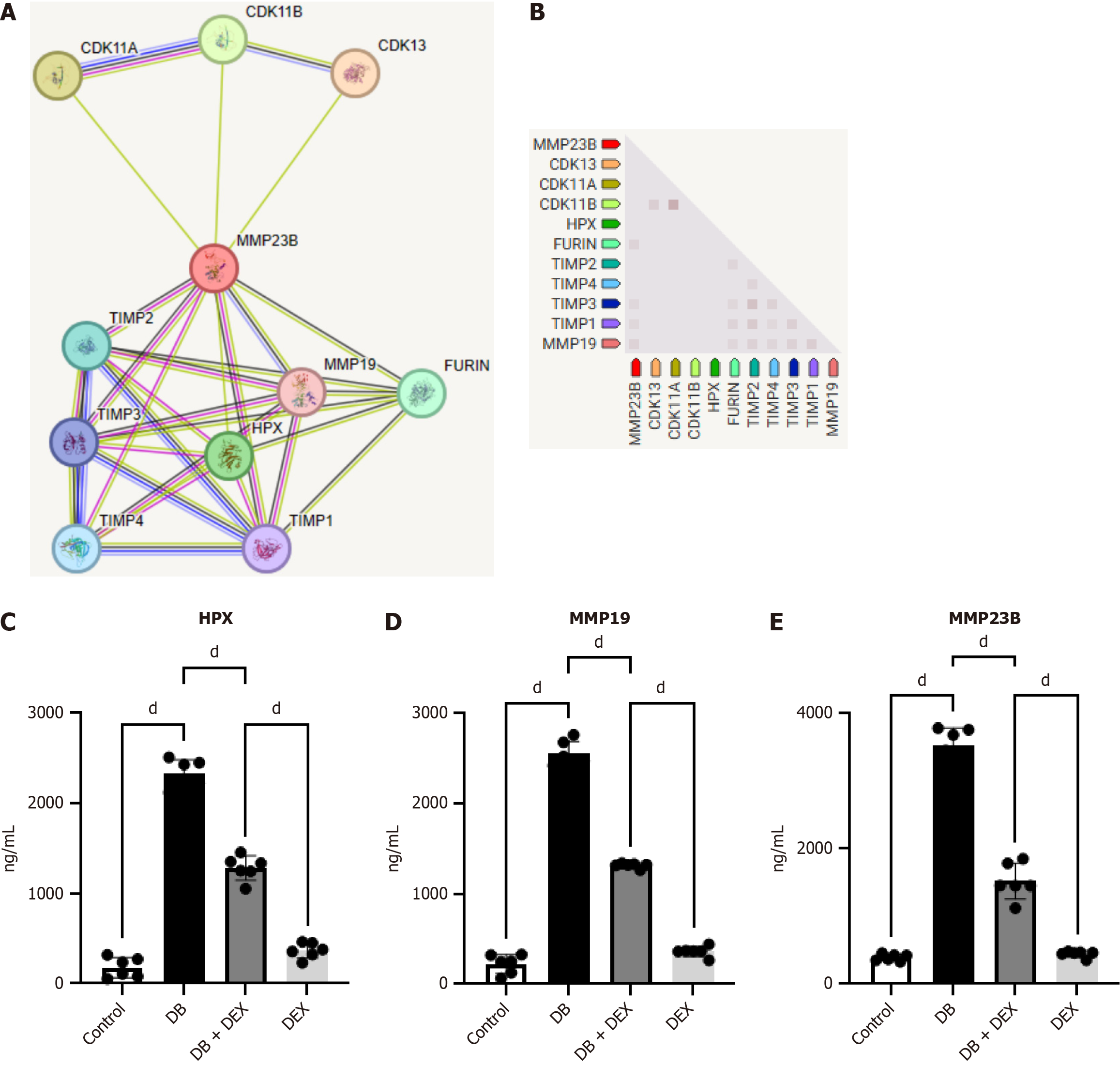
The integrated analysis of LogFC and KEGG pathways highlighted the consistent involvement of MMP23B in these biological processes. Subsequently, a protein-protein interaction network comprising 11 DEGs was established using the STRING database and illustrated through the utilization of the Cytoscape tool (Figure 4A and B). In the diabetic nephropathy (DN) group, MMP23B, MMP19, and HPX were upregulated. Interestingly, DEX treatment reduced the expression levels of inflammatory markers in contrast to the DB group (Figure 4C-E).
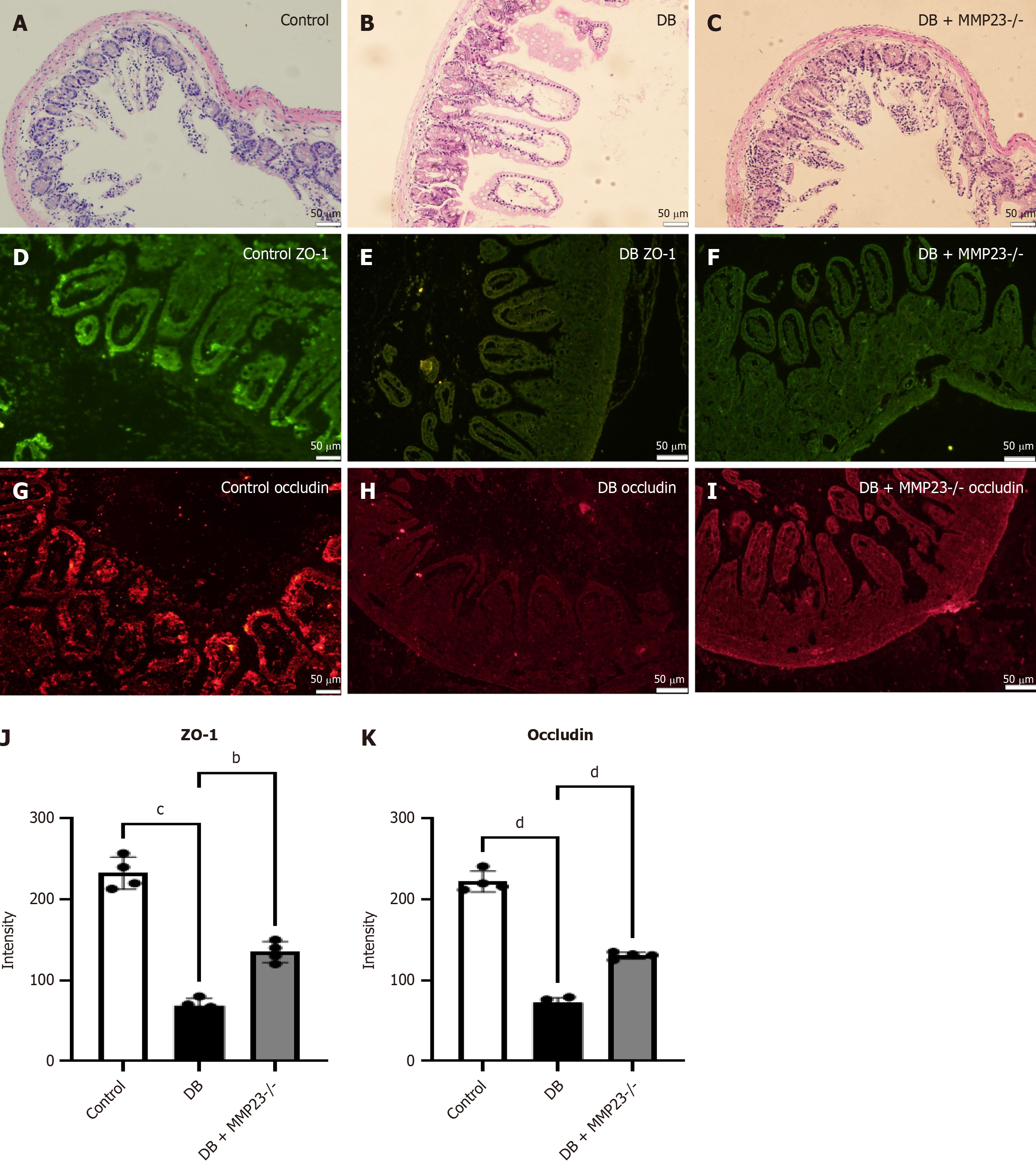
We used MMP23B KO mice to understand the role of MMP23B in intestinal injury. Hematoxylin and eosin staining indicated less severe injury in MMP23B KO mice than in WT ones (Figure 5A-C). Immunofluorescence analysis indicated that the intestinal injury marker was markedly reduced after 24 hours of treatment (Figure 5D-I). Furthermore, protein expression levels of occludin and ZO-1 were markedly increased in the MMP23B KO group compared with the WT group, as observed by immunofluorescence (Figure 5J and K).
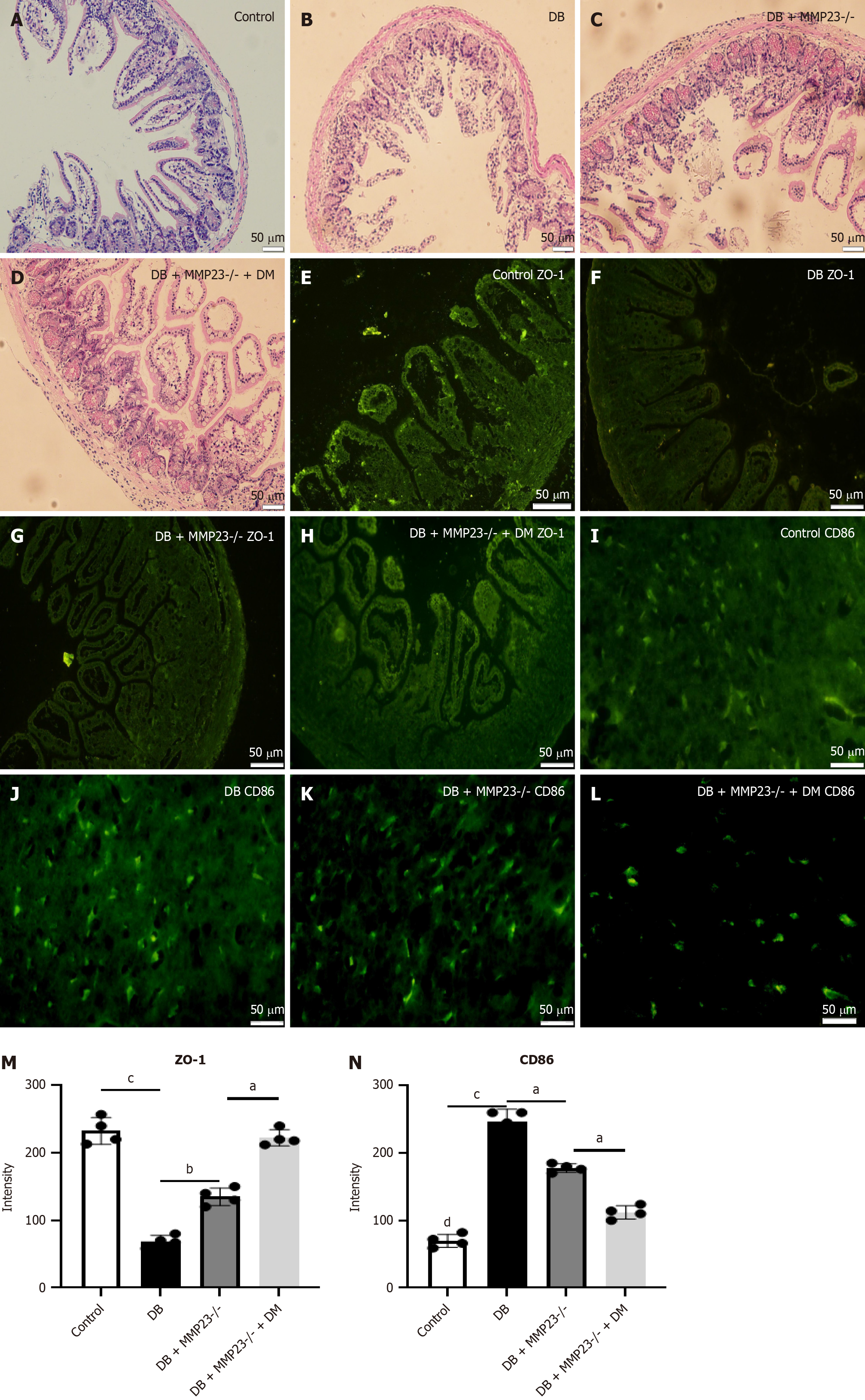
To elucidate the role of macrophages in intestinal injury, we depleted macrophages using Cls in mice. Hematoxylin and eosin staining revealed mild injury in mice treated with Cls (Figure 6A-D). Immunofluorescence analysis demonstrated a significant induction in ZO-1 and reduction in CD86 levels after 24 hours of Cl treatment (Figure 6E-L). In addition, immunofluorescence analysis revealed a significant increase in the protein expression of CD86 and ZO-1 in MMP23B KO mice (Figure 6M and N).
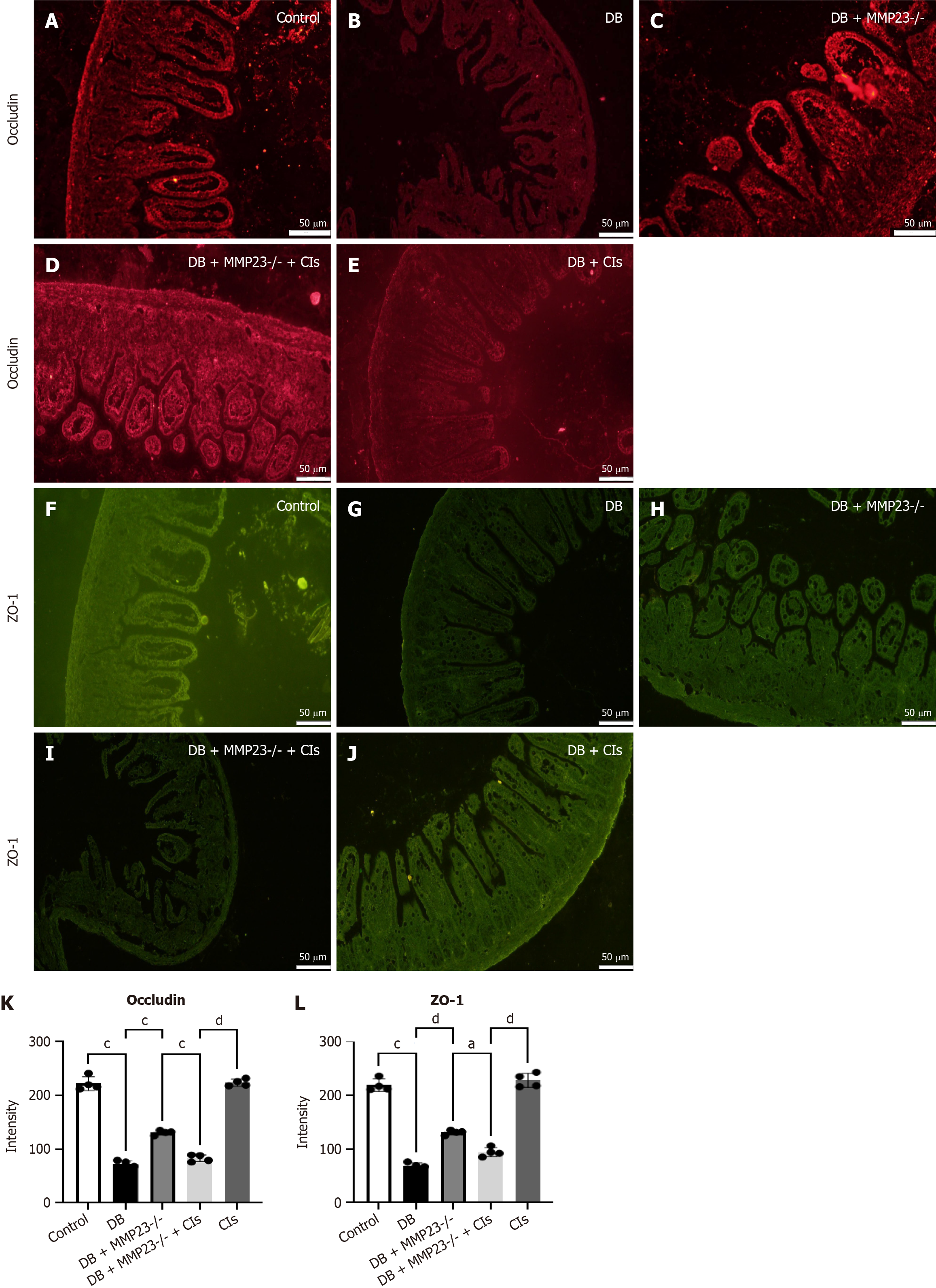
Immunofluorescence analysis demonstrated significantly induced ZO-1 and occludin levels after 24 hours of Cl treatment (Figure 7A-J). Immunofluorescence analysis also revealed a significant decrease in the protein expression of occludin and ZO-1 in MMP23B KO mice (Figure 7K and L).
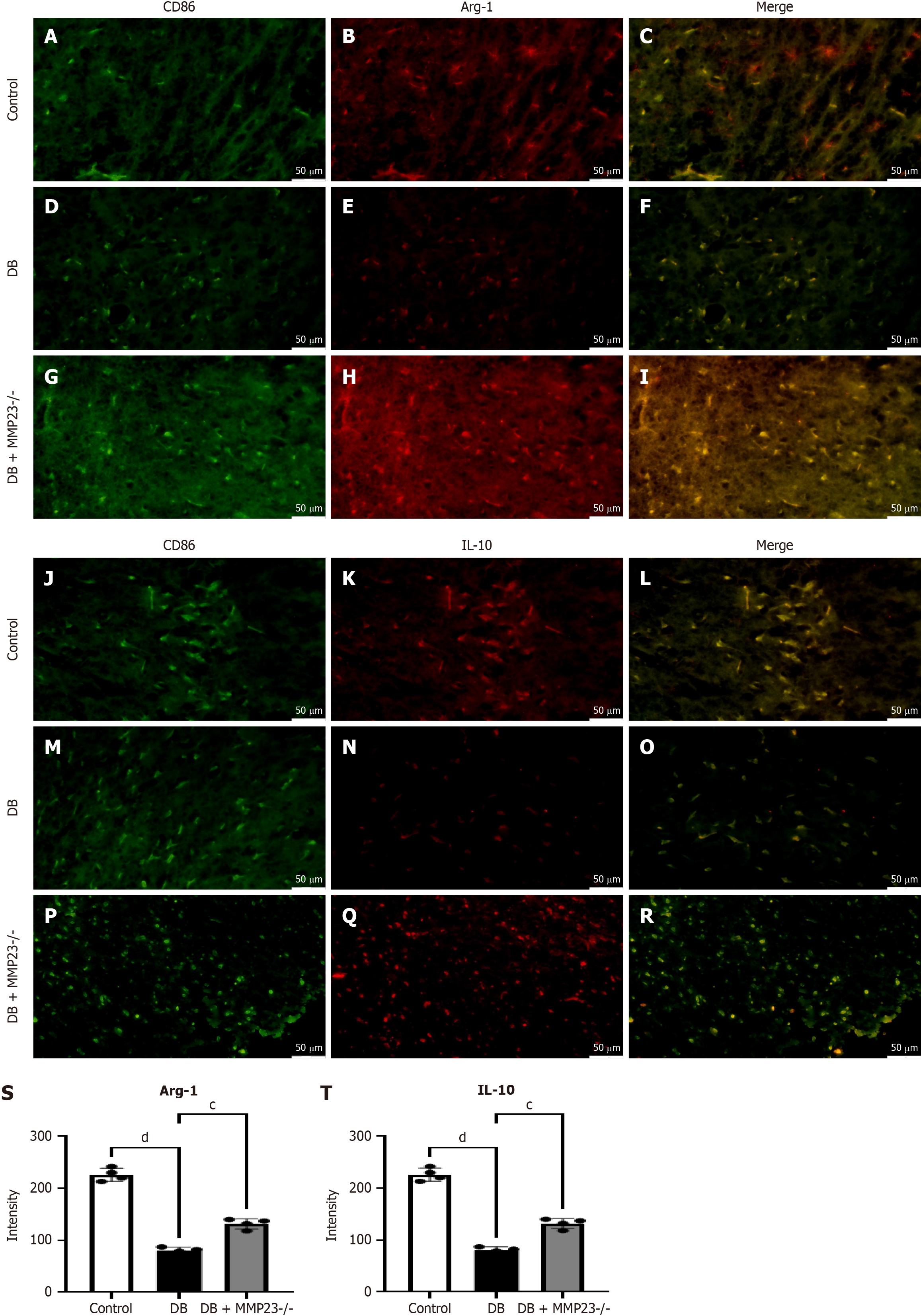
In vivo experiments demonstrated that MMP23B KO resulted in M2 polarization. Following DB induction with STZ, the fluorescence intensity of arginase-1 (Arg-1) (Figure 8A-I) and interleukin (IL)-10 (Figure 8J-R) significantly decreased. Compared with the DB pretreatment levels, the fluorescence intensity levels were induced in MMP23B KO mice (Figure 8S and T).
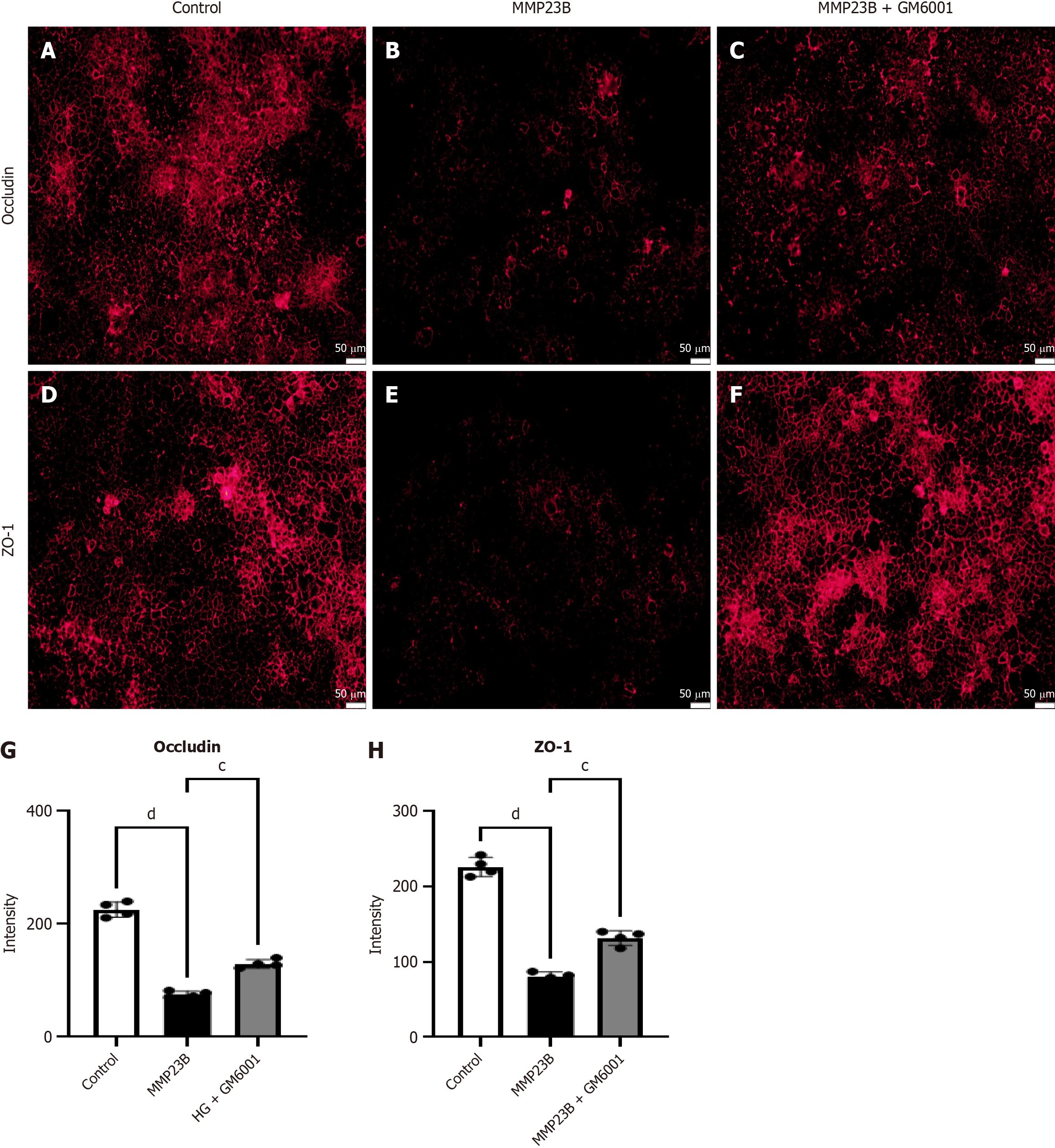
Fluorescence intensity levels of occludin (Figure 9A-C) and ZO-1 (Figure 9D-F) significantly decreased after MMP23B treatment compared with the baseline levels. Treating the MMP23B group with GM6001 improved the fluorescence intensity levels of occludin (Figure 9G) and ZO-1 (Figure 9H).
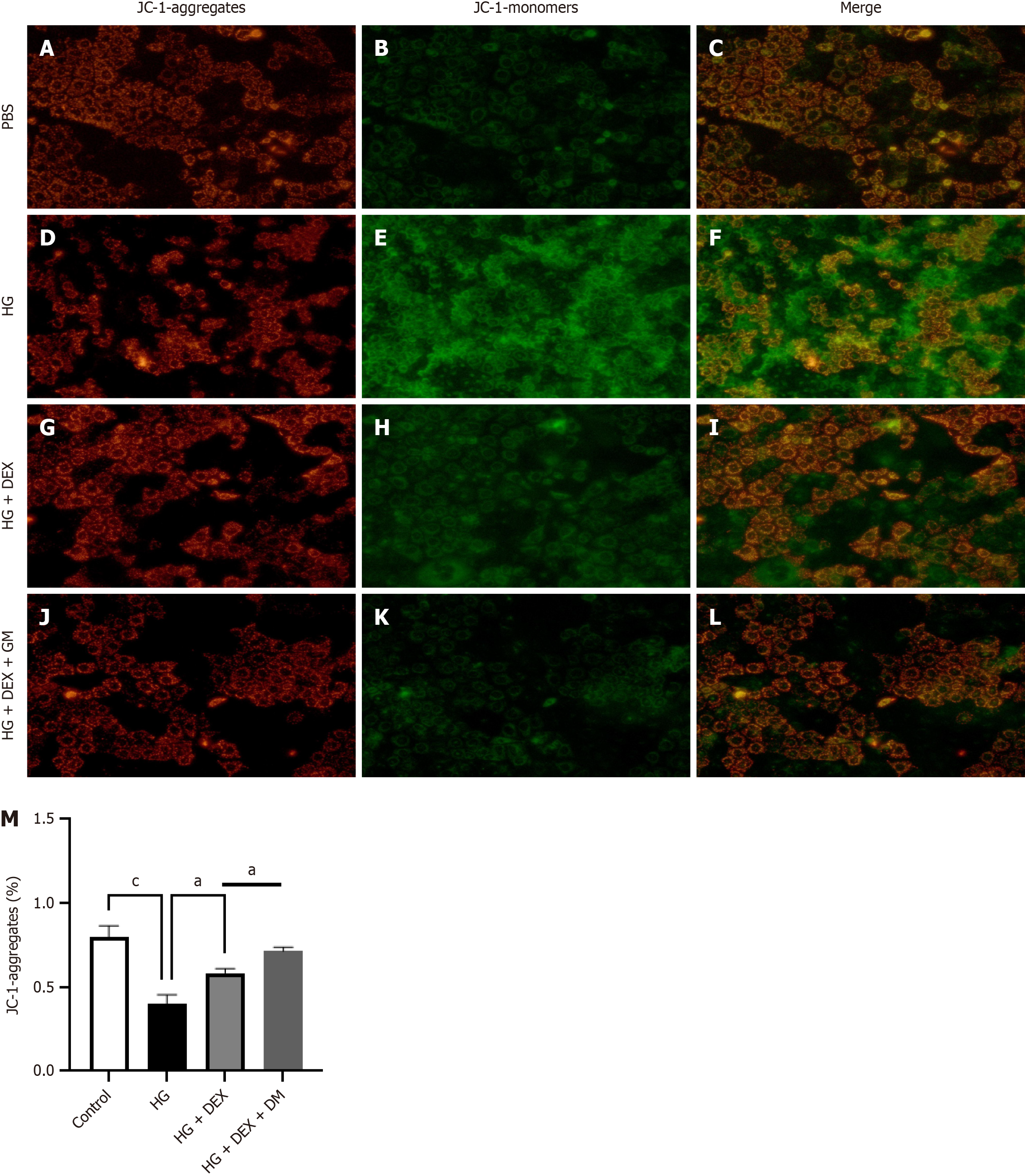
We investigated the effects of DEX on MMP23B-induced mitochondrial dysfunction in Caco-2 cells. Our results revealed that compared with a high glucose environment alone, treating cells with DEX decreases JC-1 monomer levels and increases ΔΨm, indicating that DEX can reverse the mitochondrial damage induced by high glucose (Figure 10).
Our study reveals a novel role of DEX in modulating intestinal function, particularly in the context of diabetes. DEX significantly influences intestinal motility and immune responses by interacting with the MMP23B pathway[19,20]. This finding is pivotal as it suggests that DEX counteracts diabetes-related intestinal tissue damage by regulating the activity of M1 macrophages and expression of MMP23B, a key inflammatory factor.
In the diabetic state, the intestinal microenvironment is altered, leading to inflammation and subsequent damage. This state is characterized by the release of cytokines, which can cause tissue damage when dysregulated[21,22]. Our bioinformatics analysis revealed that in addition to MMP19 and MMP8, MMP23B was upregulated in the diabetic group, indicating its potential role in the pathogenesis of diabetes-related intestinal complications.
Furthermore, our mechanistic experiments revealed that DEX decreased the expression levels of these inflammatory factors, suggesting its protective effect on intestinal tissues. MMP23B, with its capacity to degrade extracellular matrix proteins, is implicated in various biological processes, including central nervous system development and inflammatory responses. Its modulation by DEX may be central to mitigating diabetes-induced intestinal disruptions. Elucidating the effect of DEX on MMP23B can provide a new perspective on the management of diabetes-related gastrointestinal complications and open avenues for future research into targeted therapies that leverage this pathway for intestinal protection and repair.
These cytokines are crucial in regulating immune responses and the inflammatory process, but their excessive or improper release may damage intestinal tissues. Through bioinformatics analysis, we identified three genes with Log|FC| > 2 and P value < 0.05 in the DB group, among which the expression of the inflammatory factor MMP23B was elevated. Further gene enrichment analysis revealed cellular metabolism and extracellular matrix glycoprotein secretion. In the DN group, the expression of MMP23B, MMP19, and MMP8 was elevated. DEX decreased the expression levels of inflammatory factors compared with those in the DB group.
MMP23B is involved in central nervous system development, inflammatory responses, and invasion and metastasis. It has a typical metalloproteinase structure, including a signal peptide sequence, a protease active site domain, and a cysteine/cysteine repeat sequence. The zinc ions in the MMP23B active domain can participate in the degradation of extracellular matrix proteins, playing a role in reshaping the extracellular matrix and regulating cellular behavior[23,24]. However, the impact of MMP23B on intestinal macrophages remains unclear.
M1 macrophages, also known as classically activated macrophages, promote inflammation and host defense in response to infection or injury[25,26]. In diabetes-related intestinal tissue, M1 macrophages can contribute to the immune response and tissue damage. During diabetes, the intestinal tissue can be exposed to bacterial pathogens or their components due to increased intestinal permeability. This exposure triggers an inflammatory response, leading to M1 macrophage activation in the intestinal tissue. These cytokines contribute to recruiting and activating other immune cells, enhancing the inflammatory response in diabetes-related intestinal tissues[27,28]. M1 macrophages have enhanced phagocytic capacity and microbial killing abilities. They can engulf and eliminate pathogens, thereby helping in controlling infection in individuals with diabetes. However, excessive M1 macrophage activation can lead to tissue damage due to the release of cytotoxic molecules. Suppression of IL-10 promotes inflammatory reactions and impairs M1 macrophage polarization by targeting MMP23B, which is critical in macrophage polarization[29-31].
Our study demonstrated that MMP23B is responsible for macrophage polarization, highlighting its unique role in M1 macrophage polarization and potential contribution to the development of diabetes-related intestinal damage in mice. Hematoxylin and eosin staining indicated less severe injury in MMP23B KO mice. Immunofluorescence analysis indicated that the intestinal injury marker was markedly reduced after 24 hours of treatment. Furthermore, the protein expression of occludin and ZO-1 was markedly increased after MMP23B KO, as observed via immunofluorescence analysis.
While the role of MMP23B in the intestine remains unclear, our study revealed that knocking out MMP23B downregulates the intestinal tight junction (TJ) proteins ZO-1 and occludin and upregulates proinflammatory factors. These results provide a deeper understanding of MMP23B. Previous studies have reported that IL-1β stimulates periodontal inflammation during RR, inducing IL-10 and tumor necrosis factor-α synthesis and secretion. However, the initial source of IL-10 and the M2 subtype that possesses anti-inflammatory properties remain unclear. ZO-1, the earliest discovered epithelial TJ protein, is crucial in cell function and permeability[32-36]. Accordingly, blocking or removing ZO-1 in cells did not prevent the assembly of TJs or hinder the development of barrier functions.
We depleted macrophages using Cls in mice to elucidate the role of macrophages in intestinal injury[37,38]. Hematoxylin and eosin staining revealed mild injury in mice treated with Cls. Immunofluorescence analysis demonstrated a significant reduction in ZO-1 and CD86 levels after 24 hours of Cl treatment and a significant increase in the protein expression of CD86 and ZO-1 after MMP23 knockout. In vivo experiments revealed that knocking out MMP23B led to M2 polarization.
Upon STZ induction through DB treatment, fluorescence intensity levels of Arg-1 and IL-10 were notably reduced. Compared with the pretreatment levels, the fluorescence intensity levels displayed a decrease after MMP23B KO. Mitochondria have various critical functions in cells and are closely related to cell damage. During energy production, mitochondria produce some byproducts, such as reactive oxygen species. When reactive oxygen species are produced excessively, they may lead to oxidative stress, thereby damaging mitochondria and other organelles and further exacerbating cell damage[39]. The findings of the present study indicated that treatment with DEX, as opposed to treatment with MMP23B alone, significantly reduced mitochondrial dysfunction, as evidenced by decreased levels of JC-1 monomers and an improved ΔΨm.
Although animal models have provided valuable insights, the complexity of the human diabetic intestinal damage has not been fully understood. Careful consideration and rigorous validation are required to translate our findings from mouse experiments into clinical applications. Moreover, further investigation into the exact role of MMP23B in diabetes-related inflammation is needed.
In conclusion, our study indicated that DEX reduces MMP23B, which is a potential contributor to intestinal barrier dysfunction that may induce diabetes, affecting TJ changes through mitochondrial dysfunction.
| 1. | Elashi AA, Toor SM, Umlai UI, Al-Sarraj YA, Taheri S, Suhre K, Abou-Samra AB, Albagha OME. Correction: Genome-wide association study and trans-ethnic meta-analysis identify novel susceptibility loci for type 2 diabetes mellitus. BMC Med Genomics. 2024;17:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Merlo EM, Tutino R, Myles LAM, Lia MC, Minasi D. Alexithymia, intolerance to uncertainty and mental health difficulties in adolescents with Type 1 diabetes mellitus. Ital J Pediatr. 2024;50:99. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Zeng X, Huang S, Ye X, Song S, He J, Hu L, Deng S, Liu F. Impact of HbA1c control and type 2 diabetes mellitus exposure on the oral microbiome profile in the elderly population. J Oral Microbiol. 2024;16:2345942. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Ahn JH, Lopez L, Tibbs T, Yu YR, Tighe R, Denson L, Arthur J. Zinc restriction by intestinal E. coli - produced Yersiniabactin activates HIF-1α in macrophages to promote CD-associated fibrosis. Inflamm Bowel Dis. 2024;30:S62-S62. [DOI] [Full Text] |
| 5. | Zhang S, Zhang C, Yan H, Yang L, Shi N, Liu C, Chen Y. Sacral Nerve Stimulation Alleviates Intestinal Inflammation Through Regulating the Autophagy of Macrophages and Activating the Inflammasome Mediated by a Cholinergic Antiinflammatory Pathway in Colitis Rats. Neuromodulation. 2024;27:302-311. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Zhu L, Dou Z, Wu W, Hou Q, Wang S, Yuan Z, Li B, Liu J. Ghrelin/GHSR Axis Induced M2 Macrophage and Alleviated Intestinal Barrier Dysfunction in a Sepsis Rat Model by Inactivating E2F1/NF-κB Signaling. Can J Gastroenterol Hepatol. 2023;2023:1629777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Deng S, Li W, Li Z, Wang P, Ma Q. Bright luminescent Zn(2)GeO(4):Mn NP/MXene hydrogel-based ECL biosensor for glioblastoma diagnosis. Talanta. 2024;276:126214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 8. | Liu C, Xu R. Dexmedetomidine protects H9C2 rat cardiomyocytes against hypoxia/reoxygenation injury by regulating the long non-coding RNA colon cancer-associated transcript 1/microRNA-8063/Wnt/β-catenin axis. Bioengineered. 2022;13:13300-13311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Pluskiewicz W, Werner A, Bach M, Adamczyk P, Drozdzowska B. Fracture risk prediction in postmenopausal women from GO Study: the comparison between FRAX, Garvan, and POL-RISK algorithms. Arch Osteoporos. 2024;19:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Rocca MA, Valsasina P, Romanò F, Tedone N, Amato MP, Brichetto G, Boccia VD, Chataway J, Chiaravalloti ND, Cutter G, Dalgas U, DeLuca J, Farrell RA, Feys P, Freeman J, Inglese M, Meza C, Motl RW, Salter A, Sandroff BM, Feinstein A, Filippi M; Cogex Research Team. Cognitive rehabilitation effects on grey matter volume and Go-NoGo activity in progressive multiple sclerosis: results from the CogEx trial. J Neurol Neurosurg Psychiatry. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Chen S, Li Y, Song W, Cheng Y, Gao Y, Xie L, Huang M, Yan X. Insulin eye drops improve corneal wound healing in STZ-induced diabetic mice by regulating corneal inflammation and neuropeptide release. BMC Ophthalmol. 2024;24:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Tabaa MME, Tabaa MME, Rashad E, Elballal MS, Elazazy O. Harmine alleviated STZ-induced rat diabetic nephropathy: A potential role via regulating AMPK/Nrf2 pathway and deactivating ataxia-telangiectasia mutated (ATM) signaling. Int Immunopharmacol. 2024;132:111954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 13. | Culemann S, Knab K, Euler M, Wegner A, Garibagaoglu H, Ackermann J, Fischer K, Kienhöfer D, Crainiciuc G, Hahn J, Grüneboom A, Nimmerjahn F, Uderhardt S, Hidalgo A, Schett G, Hoffmann MH, Krönke G. Addendum: Stunning of neutrophils accounts for the anti-inflammatory effects of clodronate liposomes. J Exp Med. 2023;220. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Mochalova EN, Egorova EA, Komarova KS, Shipunova VO, Khabibullina NF, Nikitin PI, Nikitin MP. Comparative Study of Nanoparticle Blood Circulation after Forced Clearance of Own Erythrocytes (Mononuclear Phagocyte System-Cytoblockade) or Administration of Cytotoxic Doxorubicin- or Clodronate-Loaded Liposomes. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Fang M, Yin W, Qiu C, Song T, Lin B, Wang Y, Xiong H, Wu S. Stromal B Lymphocytes Affecting Prognosis in Triple-Negative Breast Cancer by Opal/TSA Multiplexed Immunofluorescence. Int J Womens Health. 2024;16:755-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Tomimatsu K, Fujii T, Bise R, Hosoda K, Taniguchi Y, Ochiai H, Ohishi H, Ando K, Minami R, Tanaka K, Tachibana T, Mori S, Harada A, Maehara K, Nagasaki M, Uchida S, Kimura H, Narita M, Ohkawa Y. Precise immunofluorescence canceling for highly multiplexed imaging to capture specific cell states. Nat Commun. 2024;15:3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 17. | Roelofs L, Frössling J, Rosander A, Bjerketorp J, Belaghi RA, Hansson I, Frosth S. Digital dermatitis in Swedish dairy herds assessed by ELISA targeting Treponema phagedenis in bulk tank milk. BMC Vet Res. 2024;20:168. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Małota K, Student S, Świątek P. Low mitochondrial activity within developing earthworm male germ-line cysts revealed by JC-1. Mitochondrion. 2019;44:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Ekkapat G, Kampitak W, Theerasuwipakorn N, Kittipongpattana J, Engsusophon P, Phannajit J, Chokengarmwong N. A Comparison of Efficacy between Low-dose Dexmedetomidine and Propofol for Prophylaxis of Postoperative Delirium in Elderly Patients Undergoing Hip Fracture Surgery: A Randomized Controlled Trial. Indian J Crit Care Med. 2024;28:467-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 20. | Sun Y, Darmani NA. A Comparative Study of the Antiemetic Effects of α(2)-Adrenergic Receptor Agonists Clonidine and Dexmedetomidine against Diverse Emetogens in the Least Shrew (Cryptotis parva) Model of Emesis. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Frühbeck G, Gómez-Ambrosi J, Ramírez B, Becerril S, Rodríguez A, Mentxaka A, Valentí V, Moncada R, Reina G, Baixauli J, Casado M, Silva C, Escalada J, Catalán V. Decreased expression of the NLRP6 inflammasome is associated with increased intestinal permeability and inflammation in obesity with type 2 diabetes. Cell Mol Life Sci. 2024;81:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Liu X, Fang H, Pan L, Zhang P, Lin H, Gao H, Ye C, Mao D, Luo Y. S-amlodipine induces liver inflammation and dysfunction through the alteration of intestinal microbiome in a rat model. Gut Microbes. 2024;16:2316923. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Allione A, Pardini B, Viberti C, Giribaldi G, Turini S, Di Gaetano C, Guarrera S, Cordero F, Oderda M, Allasia M, Gontero P, Sacerdote C, Vineis P, Matullo G. MMP23B expression and protein levels in blood and urine are associated with bladder cancer. Carcinogenesis. 2018;39:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Pardini B, Allione A, Viberti C, Giribaldi G, Turini S, Gaetano CD, Guarrera S, Cordero F, Oderda M, Allasia M, Gontero P, Sacerdote C, Naccarati A, Vineis P, Matullo G. Abstract 1258: MMP23B expression and protein levels in blood and urine are associated with bladder cancer risk. Cancer Res. 2018;78:1258. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Choi JY, Byeon HW, Park SO, Uyangaa E, Kim K, Eo SK. Inhibition of NADPH oxidase 2 enhances resistance to viral neuroinflammation by facilitating M1-polarization of macrophages at the extraneural tissues. J Neuroinflammation. 2024;21:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Li Y, Guo X, Zhan P, Huang S, Chen J, Zhou Y, Jiang W, Chen L, Lin Z. TRPV1 Regulates Proinflammatory Properties of M1 Macrophages in Periodontitis Via NRF2. Inflammation. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Gonciarz W, Piątczak E, Chmiela M. The influence of Salvia cadmica Boiss. extracts on the M1/M2 polarization of macrophages primed with Helicobacter pylori lipopolysaccharide in conjunction with NF-kappa B activation, production of cytokines, phagocytic activity and total DNA methylation. J Ethnopharmacol. 2023;310:116386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Mathiesen CBK, Rudjord-Levann AM, Gad M, Larsen J, Sellebjerg F, Pedersen AE. Cladribine inhibits secretion of pro-inflammatory cytokines and phagocytosis in human monocyte-derived M1 macrophages in-vitro. Int Immunopharmacol. 2021;91:107270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Honjoh K, Nakajima H, Hirai T, Watanabe S, Matsumine A. Relationship of Inflammatory Cytokines From M1-Type Microglia/Macrophages at the Injured Site and Lumbar Enlargement With Neuropathic Pain After Spinal Cord Injury in the CCL21 Knockout (plt) Mouse. Front Cell Neurosci. 2019;13:525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Inoue Y, Kamiya T, Hara H. Increased expression of ELOVL7 contributes to production of inflammatory cytokines in THP-1 cell-derived M1-like macrophages. J Clin Biochem Nutr. 2023;72:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Jędrzejewski T, Pawlikowska M, Sobocińska J, Wrotek S. Protein-Bound Polysaccharides from Coriolus Versicolor Fungus Disrupt the Crosstalk Between Breast Cancer Cells and Macrophages through Inhibition of Angiogenic Cytokines Production and Shifting Tumour-Associated Macrophages from the M2 to M1 Subtype. Cell Physiol Biochem. 2020;54:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Georgiadis A, Tschernutter M, Bainbridge JWB, Balaggan KS, Mowat F, West EL, Munro PMG, Thrasher AJ, Matter K, Balda MS, Ali RR. Correction: The Tight Junction Associated Signalling Proteins ZO-1 and ZONAB Regulate Retinal Pigment Epithelium Homeostasis in Mice. PLoS One. 2023;18:e0295782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Schmidt A, Finegan T, Häring M, Kong D, Fletcher AG, Alam Z, Grosshans J, Wolf F, Peifer M. Polychaetoid/ZO-1 strengthens cell junctions under tension while localizing differently than core adherens junction proteins. Mol Biol Cell. 2023;34:ar81. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 34. | Wang K, Hongyan Q, Lassegue B, Eaton DCC, Song C, Mu J, Griendling KK, Hernandes MS. Abstract 12294: Poldip2 Mediates Brain Vascular Permeability by Regulating ZO-1 Phosphorylation and Localization at the Interendothelial Border. Circulation. 2023;148. [DOI] [Full Text] |
| 35. | Wu H, Wu L, Yu W, Gu C, Li Y, Chen K, Zhang L, Qian F. Veronica linariifolia subsp. dilatata ameliorates LPS-induced acute lung injury by attenuating endothelial cell barrier dysfunction via EGFR/Akt/ZO-1 pathway. J Ethnopharmacol. 2024;321:117545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Zhuang X, Martin TA, Ruge F, Zeng JJ, Li XA, Khan E, Dou Q, Davies E, Jiang WG. Expression of Claudin-9 (CLDN9) in Breast Cancer, the Clinical Significance in Connection with Its Subcoat Anchorage Proteins ZO-1 and ZO-3 and Impact on Drug Resistance. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Li Y, Wu L, Yong Y, Niu X, Gao Y, Zhou Q, Xie H, Liu X, Li Y, Yu Z, Abd El-Aty AM, Ju X. Enhancing gut barrier integrity: Upregulation of tight junction proteins by chitosan oligosaccharide through the ERK1/2 signaling pathway. Nutrition. 2024;124:112428. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Tu J, Jiang Y, Tu L, Chen Y, Pan L, Fan X, Tian J, Li J, Wang X, Fu H, Xu B, Feng D. Da-Cheng-Qi decoction improves severe acute pancreatitis capillary leakage syndrome by regulating tight junction-associated proteins. Front Pharmacol. 2024;15:1138251. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Masuda D, Nakanishi I, Ohkubo K, Ito H, Matsumoto KI, Ichikawa H, Chatatikun M, Klangbud WK, Kotepui M, Imai M, Kawakami F, Kubo M, Matsui H, Tangpong J, Ichikawa T, Ozawa T, Yen HC, St Clair DK, Indo HP, Majima HJ. Mitochondria Play Essential Roles in Intracellular Protection against Oxidative Stress-Which Molecules among the ROS Generated in the Mitochondria Can Escape the Mitochondria and Contribute to Signal Activation in Cytosol? Biomolecules. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |