Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1916
Revised: June 4, 2024
Accepted: July 2, 2024
Published online: September 15, 2024
Processing time: 174 Days and 4 Hours
Diabetic nephropathy (DN) is the most frequent chronic microvascular conse
To explore the protective effect of Cor against podocyte injury in DN mice and the underlying mechanisms.
Streptozotocin and a high-fat diet were combined to generate DN mice models, which were then divided into either a Cor group or a DN group (n = 8 in each group). Mice in the Cor group were intraperitoneally injected with Cor (30 mg/kg/d) for 12 wk, and mice in the DN group were treated with saline. Bio
Compared with the control group, the DN mice models had increased fasting blood glucose, glycosylated hemoglobin, triglycerides, and total cholesterol, decreased nephrin and podocin expression, increased apoptosis rate, elevated inflammatory cytokines, and enhanced oxidative stress. All of the conditions mentioned above were alleviated after intervention with Cor. In addition, Cor therapy improved SIRT1 and AMPK expression (P < 0.001), inhibited reactive oxygen species and oxidative stress, and elevated autophagy in HG-induced podocytes (P < 0.01).
Cor alleviates podocyte injury by regulating autophagy via the SIRT1-AMPK pathway, thereby exerting its protective impact on renal function in DN mice.
Core Tip: This study explored the protective effects of Corilagin (Cor) against podocyte injury in diabetic nephropathy (DN). Cor treatment markedly attenuated fasting blood glucose, glycosylated hemoglobin, triglycerides, and total cholesterol, increased nephrin and podocin expression, reduced apoptosis rate, and inhibited inflammatory cytokines and oxidative stress. In addition, Cor contributed to improving SIRT1 and AMPK expression, inhibited reactive oxygen species and oxidative stress, and elevated autophagy in high-glucose-treated podocytes. Thus, Cor might be a potential therapeutic agent for the treatment and clinical management of DN.
- Citation: Lou Y, Luan YT, Rong WQ, Gai Y. Corilagin alleviates podocyte injury in diabetic nephropathy by regulating autophagy via the SIRT1-AMPK pathway. World J Diabetes 2024; 15(9): 1916-1931
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1916.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1916
Diabetic nephropathy (DN) is a major cause of end-stage renal disease (ESRD) and is a common complication of diabetes. It is primarily caused by persistently high blood sugar levels and the resulting chronic inflammatory response[1,2]. Approximately 35%-40% of diabetic patients eventually develop DN[3], leading to a significant number of diabetic deaths and posing a severe menace to the quality of life in diabetics[4]. Prolonged diabetes circumstances can impair numerous physical organs, resulting in incapacitating and sometimes fatal consequences such as cardiovascular disease, neu
Autophagy is a highly selective cellular mechanism that destroys and recycles misfolded or malfunctioning proteins and damaged organelles via the lysosome pathway to preserve cellular homeostasis[7]. Autophagy is modulated by dietary intake and intracellular stress, both of which are altered in diabetic patients, and alterations in the SIRT1, AMPK, and mammalian target of rapamycin (mTOR) autophagy pathways can aggravate organelle disorders and lead to DN[8]. Under diabetes circumstances, high glucose (HG) and nutritional imbalances enhance intracellular stress and prevent autophagy by suppressing SIRT1 and AMPK and stimulating mTOR, resulting in the onset and development of DN[7]. These findings suggest that hyperglycemia-induced changes in autophagic activity cause diabetes-related podocyte injury. Therefore, activating autophagy could be a potential therapeutic target to treat and prevent podocyte injury in DN.
SIRT1 is an element of the Sir2-like protein family[9]. SIRT1 has a role in various physiological processes, including metabolism, cell proliferation, mitochondrial homeostasis, autophagy, and apoptosis[9-11]. As an autophagy positive regulator, SIRT1 enhances autophagy by deacetylating autophagy-related proteins such as Beclin-1, ATG5, ATG7, LC3B, and p62 once activated[12,13]. Moreover, SIRT1 can interact with the AMPK and mTOR pathways, regulating the metabolism of energy and pro-survival processes such as autophagy[7]. SIRT1 expression was low in injured podocytes during DN[14]. Furthermore, SIRT1 knockdown in podocytes caused severe podocyte destruction and elevated proteinuria, but SIRT1 overexpression dramatically reduced kidney damage in diabetic animals[9,14]. Therefore, SIRT1 expression in podocytes might regulate the onset and progression of podocyte injury in DN.
Corilagin (Cor) is a tannin-family ellagitannin found in various medicinal plants[15]. Previous studies discovered Cor has multiple biological and pharmacological effects, including hepatoprotective, anti-inflammatory, antibacterial, antioxidant, anti-hypertensive, anti-diabetic, and anti-tumor capabilities[16]. It can trigger apoptosis and limit cell proliferation, slowing the growth of many cancer cells[15]. Cor has been shown to have hepatoprotective properties in the treatment of a variety of liver diseases, including hepatocellular cancer, drug-induced liver damage, and liver fibrosis[17]. Cor has been found in recent studies to reduce schistosomiasis-induced hepatic fibrosis by modulating the IL-13, GATA3, and miR21/smad7/ERK signaling pathways[18-21]. However, the protective effect of Cor against podocyte injury in DN and the mechanisms involved in this process remain unknown. Therefore, the current study investigated Cor's protective role against podocyte injury in DN in mice and the potential underlying mechanisms.
The Animal Care and Use Committee of Shanghai Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine approved this entire study. The experimental procedure was carried out in accordance with the ARRIVE guidelines.
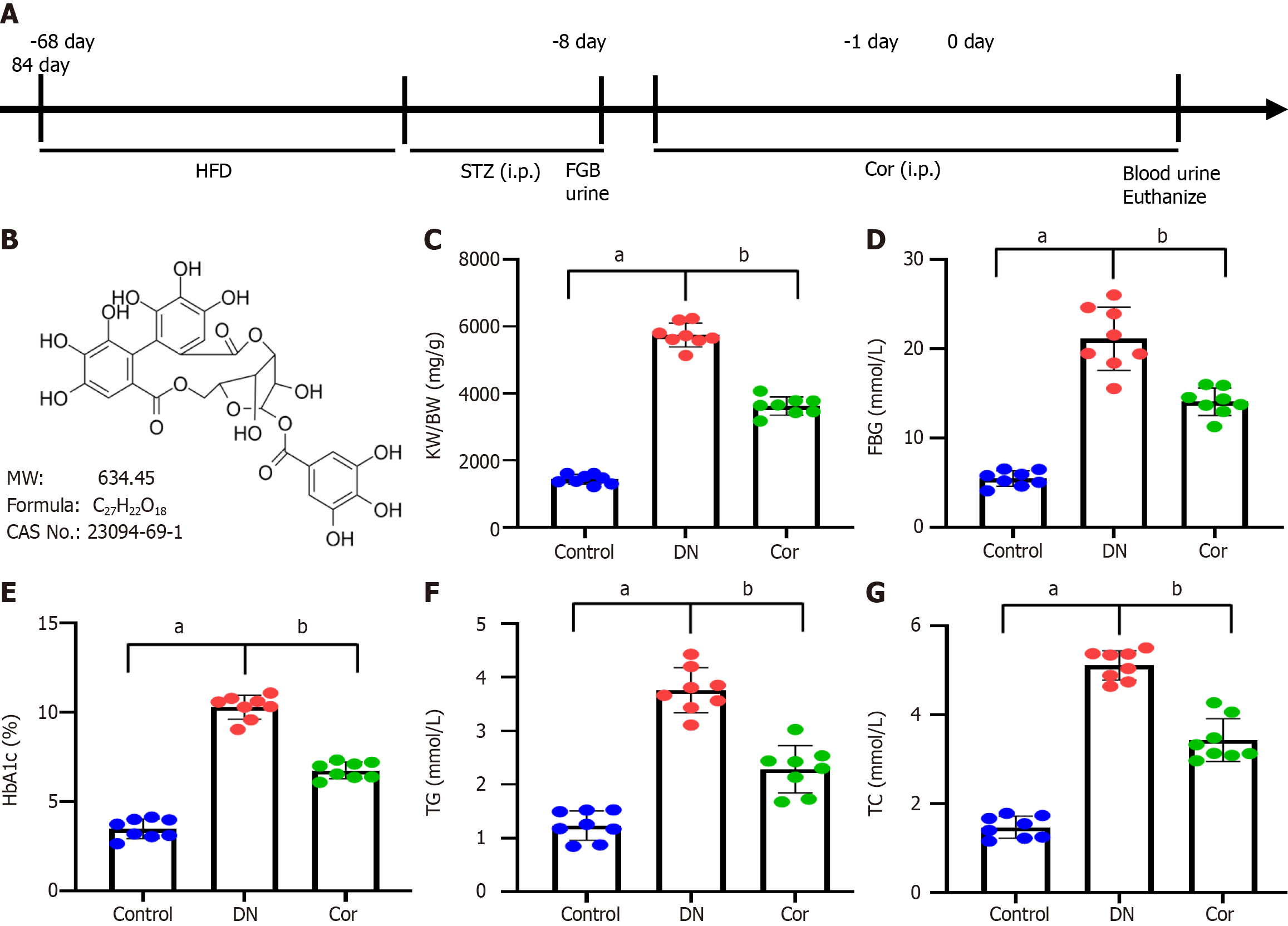
C57BL/6 mice (male, 6-8 weeks, n = 24) were purchased from Shanghai Slake Experimental Animal Company. The mice were maintained in a specific pathogen-free laboratory animal room with standard light/dark cycles and 60% humidity for 1 wk. Mice were fed a high-fat diet (HFD) for 2 mo, followed by intraperitoneal injections of streptozotocin (STZ; 50 mg/kg, S0130, Sigma, United States) for seven continuous days. Normal control mice were intraperitoneally injected with an equal amount of sodium citrate buffer. Fasting blood glucose (FBG) levels were assessed using a glucose meter 24 h after STZ injections, and urine samples were collected in a metabolic cage to determine the 24-h urinary albumin excretion rate (24-h UAER). DN mice were characterized by FBG > 16.9 mmol/L and increased 24-h UAER (Figure 1A).
DN mice were divided into two groups, with eight mice per group: (1) DN group: Mice were intraperitoneally injected with an equal amount of saline; and (2) Cor group: Mice were intraperitoneally injected with Cor (30 mg/kg/d, HY-N0462, MedChemExpress, Figure 1B). After 12 wk of continuous treatment with Cor, blood was collected via the femoral artery to determine FBG and glycosylated hemoglobin (HbA1c), and urine samples were collected in the metabolic cage for 24 h to determine 24-hUAER.
Mice were placed in a metabolic cage, urine was collected for 24 h, and urine volume was recorded. The urine was centrifuged, and the supernatant was separated and packaged before being placed in a -80 °C refrigerator. Fasting mice were anaesthetized by inhaling 3% isoflurane; blood was taken from the femoral artery to separate serum and stored at -80 °C. The mice were euthanized for cervical dislocation, and the abdominal cavity was quickly opened. The kidneys were cut off, and the renal capsule was peeled off. The kidneys were divided into two parts: One was fixed in 4% paraformaldehyde for histological examination, and the other part was frozen in a -80 ℃ freezer for mRNA and protein expression detection.
After 12 wk of continuous intervention, the mice's body weight (BW) was measured. After cutting off the kidney, the kidney weight (KW) was measured, and the renal hypertrophy index (KW/BW) was calculated based on the ratio of KW (mg) to BW (g).
Before and 12 wk after treatment, 5 mL of blood was drawn via the femoral artery from each mouse. FBG was measured with a glucose meter (Roche, Germany). HbA1c was measured by using ELISA (ml401824, Shanghai Enzyme-linked Biotechnology Co., Ltd., China). An automatic biochemical analyzer was used to measure the concentrations of triglyceride (TG), total cholesterol (TC), serum creatinine (Scr), and blood urea nitrogen (BUN).
After 12 wk of Cor treatment, mice were transferred into the metabolic cage, and 24-h urine was collected. The total volume of urine was recorded. After centrifugation (3000 rpm, 10 min), the urine supernatant was stored at -80 °C. Urinary albumin was detected using a mouse albumin ELISA Kit (ml063626, Shanghai Enzyme-linked Biotechnology Co., Ltd., China). 24-h UAER was defined as urinary albumin (µg/mL) × urine volume (mL).
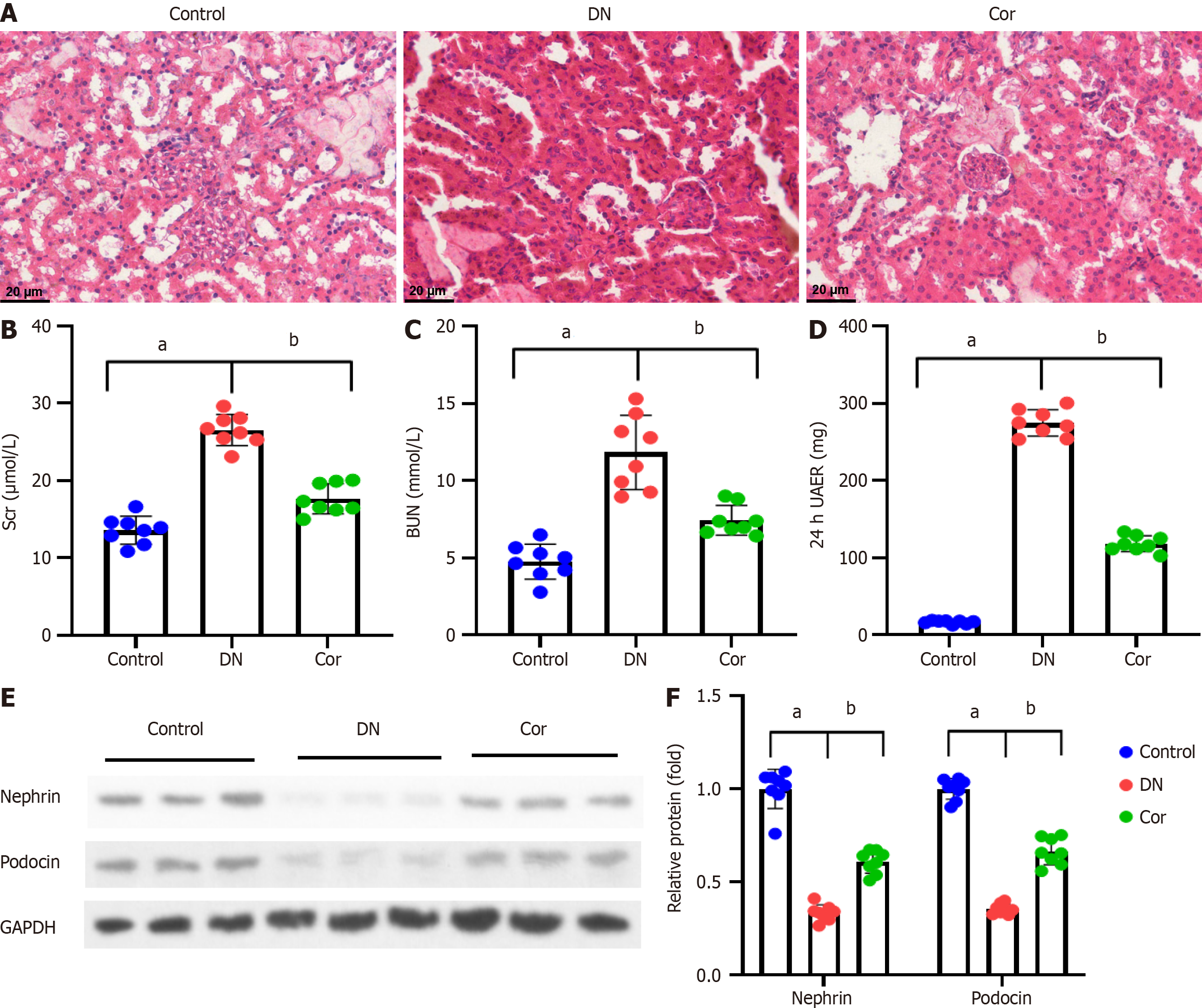
Kidney tissue slices were fixed for 48 h in 4% paraformaldehyde at 4 °C before being embedded in paraffin and cut into 5 μm thick pieces. Dewaxed and hydrated paraffin slices were stained with hematoxylin for 5 min before being rinsed with water. Sections were stained with eosin for 3 min and photographed using an Olympus IX51 microscope (Japan).
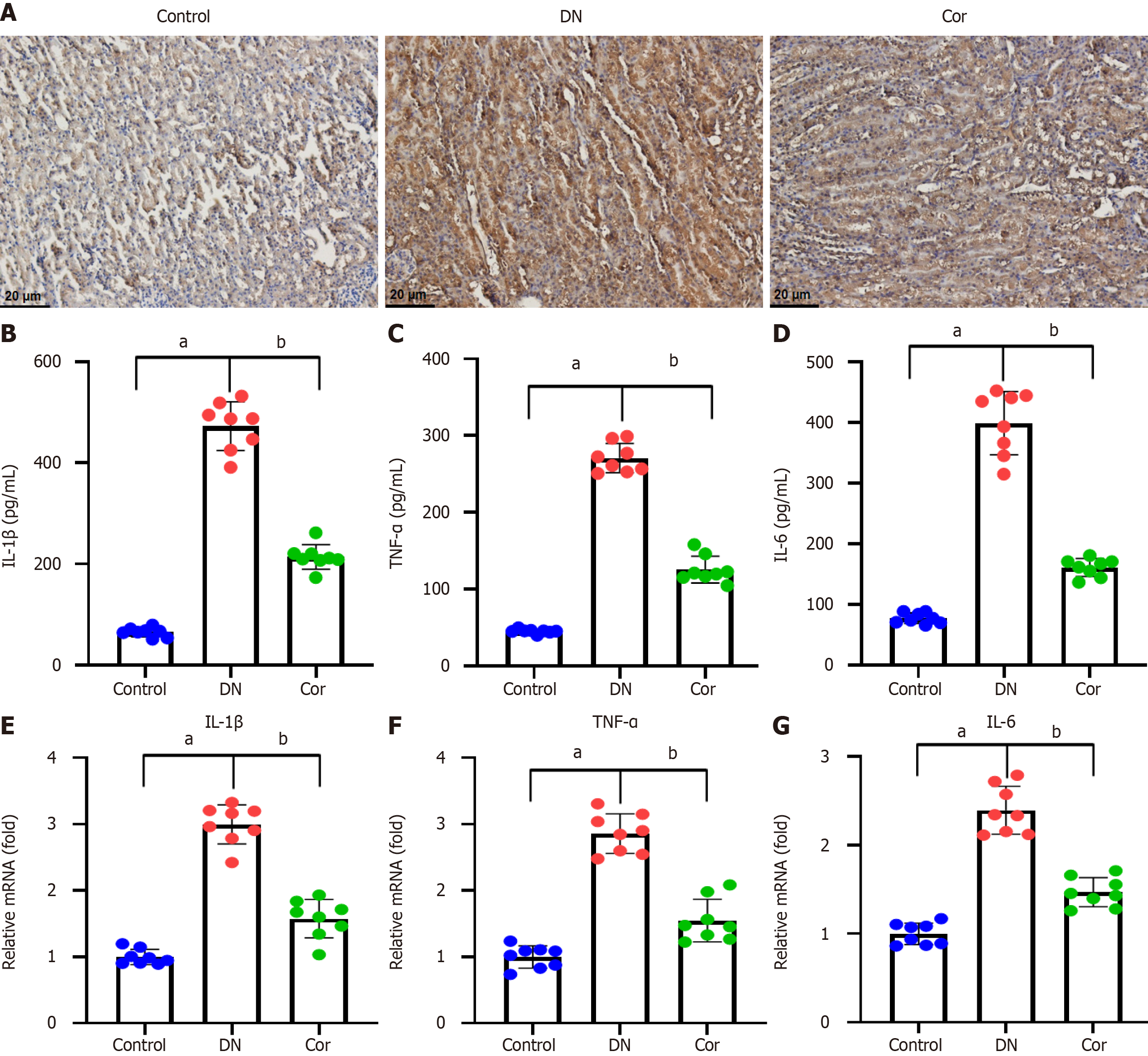
For immunohistochemical staining, histological sections (5-μm thickness) of kidney tissue were deparaffinized with xylene and dehydrated through a graded series of alcohol. Sections were then boiled at 120 °C for 4 min in 0.01 M citrate buffer (pH 6.0) for antigen repair. Sections were treated with 0.3% hydrogen peroxide to block endogenous peroxidase. Sections were treated with IL-1β primary antibody (1:400, sc-52012, Santa Cruz, United States) overnight at 4 °C. Next, samples were exposed to a biotin-labeled secondary antibody for 1 h. Finally, the slides were counterstained with hematoxylin.
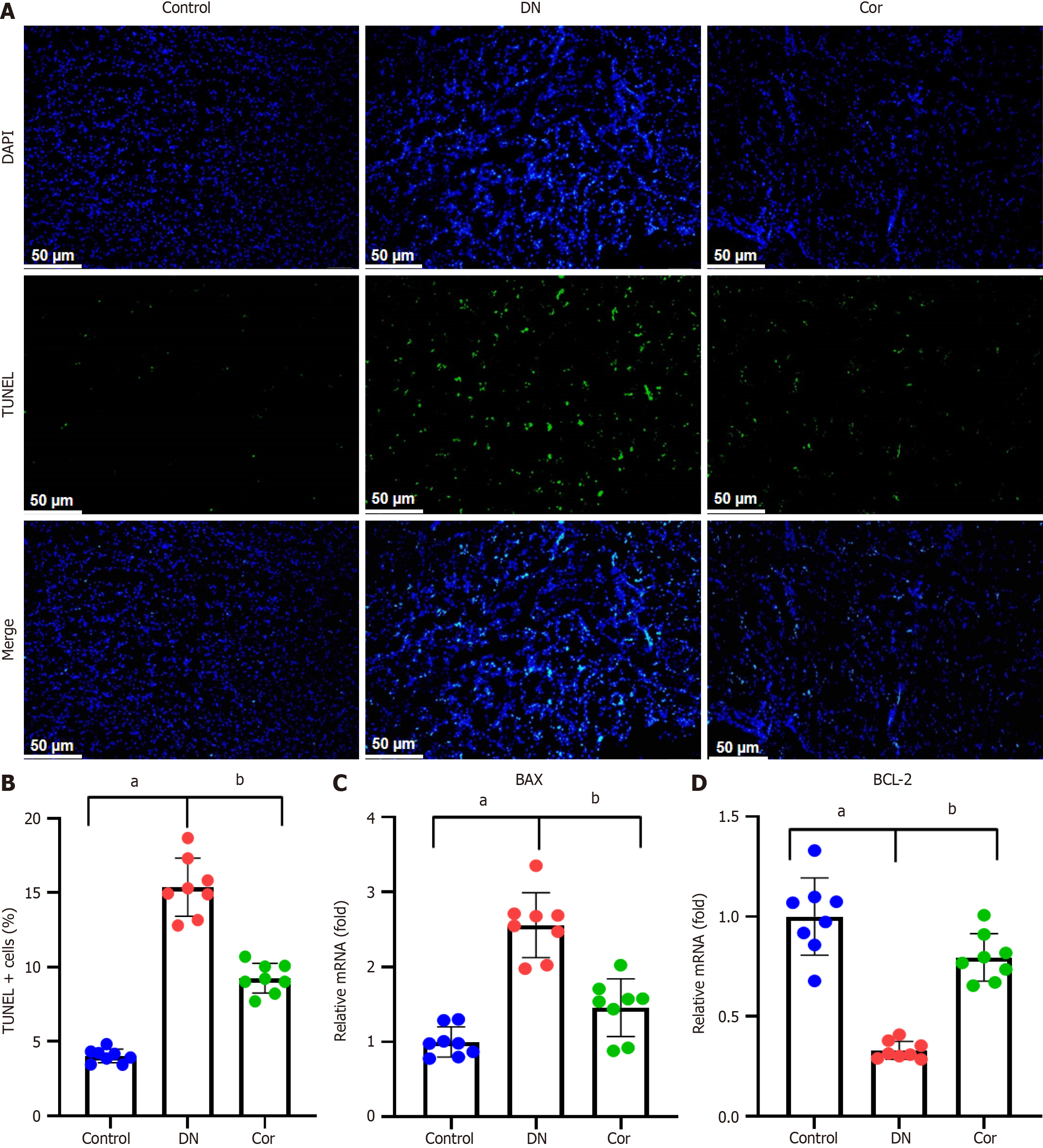
Frozen sections of mouse kidney tissue were stained with a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit (C1086, Beyotime, Shanghai, China) and DAPI (S0063, Beyotime, Shanghai, China) and washed three times in phosphate-buffered saline (PBS) before observation under an inverted fluorescence microscope (IX51, Olympus, Japan). TUNEL-positive cells were counted, and the apoptosis rate was assessed as the percentage of TUNEL-positive cells in the DAPI-positive cells.
The kidney tissue of mice was collected and cut into small pieces to obtain tissue homogenate. The levels of IL-1β (MLB00C), TNF-α (MTA00B), and IL-6 (M6000B) were measured using ELISA kits (R&D Systems) following the manufacturer's instructions.
Mouse MPC5 podocytes were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS). Undifferentiated podocytes were treated with recombinant mouse IFN-γ (10 U/mL) containing 10% FBS and were cultured in a 5% CO2 incubator at 33 °C. After passage, the cells were treated with 10% FBS with no IFN-γ. The culture was induced to differentiate for 2 wk in 5% CO2 incubator at 37 °C. MPC5 cells were pretreated for pathway inhibition experiments with the SIRT1 inhibitor EX-527 (10 μM, HY-15452, MedChemExpress).
MPC5 cells in the logarithmic growth stage were randomly divided into four groups: (1) Control group: Treated with 5 mmol/L glucose; (2) Cor group: Treated with Cor (50 μM); (3) HG group: Treated with 30 mmol/L glucose; and (4) HG + Cor group: Treated with 30 mmol/L glucose and Cor (50 μM). After 48 h of treatment, MPC5 cells were collected for subsequent experimentation.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was employed to analyze cell viability. MPC5 cells were seeded in 96-well plates (1 × 104 cells/well) and incubated with HG and/or Cor for 48 h. MTT solution (1 mg/mL, M1020, Solarbio, Beijing, China) was added into the cell well and incubated for 4 h at 37 °C. Formazan crystal was dissolved with 100 μL/well of DMSO. A microplate reader was used to measure absorbance at 570 nm.
Apoptotic cells were determined using the Annexin V-FITC/propidium iodide (PI) assay kit (CA1020, Solarbio, Beijing, China). Cells were cultured in a 24-well plate (5 × 104 cells/well) and were treated for 48 h. Then, cells were digested by trypsin and incubated at room temperature with Annexin V-FITC and PI for 15 min. Cell apoptosis rate was estimated by flow cytometry.
Reactive oxygen species (ROS) production was investigated by dihydroethidium (DHE) and 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) staining. For in vivo experiments, frozen kidney tissues were incubated with DHE solution (10 μM, S0063, Beyotime, Shanghai, China) at 37 °C for 1 h, followed by DAPI staining. Then, sections were washed with PBS solution and examined under a fluorescence microscope. For in vitro experiments, MPC5 cells were seeded into a glass bottom cell culture dish and then incubated with a DCFH-DA solution (10 μM, S0033S, Beyotime, Shanghai, China) for 1 h at 37 °C, followed by DAPI staining. The cells were washed with PBS and then examined under a fluorescence microscope.
MPC5 cells and kidney tissues were lysed on ice for 30 min with cell lysis solution (ml095543, Shanghai Enzyme-linked Biotechnology Co., Ltd., China). The cell lysate underwent centrifugation at 1600 × g for 10 min, and the supernatant was collected. Protein concentration quantification, malondialdehyde (MDA) (S0131S) content, and superoxide dismutase (SOD) (S0109) and catalase (CAT) (S0051) activities were determined using the oxidative stress detection kit (Beyotime, Shanghai, China).
MPC5 cells were fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.2% Triton X-100 for 15 min. After blocking with 5% BSA (Thermo Fisher Scientific, Inc.) for 1 h at room temperature, the cells were cultured with the primary antibody anti-LC3-II (1:100; ab192890, Abcam) at 4 ˚C overnight. On the second day, the cells were incubated with FITC-conjugated goat anti-rabbit IgG (1:200) for 1 h at room temperature. The nuclei were counterstained with DAPI. Autophagic cells were observed and photographed with a fluorescence microscope.
Total RNA was extracted from cultured podocytes using Trizol reagent (Invitrogen) and reverse transcribed into cDNA with a Prime Script RT Reagent Kit (Takara Biotechnology, Dalian, China). Real-time quantitative PCR (RT-qPCR) assays were performed using the SYBR Green PCR kit (Takara) on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster, CA, United States). The sequences of the primers used are shown in Table 1. The relative mRNA expression was quantified using the 2-ΔΔCT method using GAPDH as the endogenous control.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
| BAX | GCCTCCTCTCCTACTTCGG | AAAAATGCCTTTCCCCTTC |
| BCL-2 | CTCGTCGCTACCGTCGTGACTTCG | CAGATGCCGGTTCAGGTACTCAGTC |
| IL-1β | CAGGATGAGGACATGAGCACC | CTCTGCAGACTCAAACTCCAC |
| TNF-α | ATGAGAAGTTCCCAAATGGCC | TCCACTTGGTGGTTTGCTACG |
| IL-6 | GGAAATCGTGGAAATGAG | GCTTAGGCATAACGCACT |
| GAPDH | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
Total protein in podocytes was extracted using a lysis buffer [0.1% Triton X-100, 50 mmol/L Tris (pH 7.0), 100 Mm NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF] and quantified using the Pierce BCA Protein Assay Kit (NCI3225CH, Thermo Scientific). Total protein (50 µg) was separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk for 1 h at room temperature, and were incubated with the following primary antibodies: Rabbit anti- Nephrin (1:1000, ab216341, Abcam); rabbit anti-Podocin (1:1000, ab181143, Abcam); rabbit anti-SIRT1 (1:1000, ab189494, Abcam); rabbit anti-p-AMPK (Thr172, 1:1000, #5256, Cell Signaling Technology); rabbit anti-t-AMPK (1:1000, #2532, Cell Signaling Technology); rabbit anti-Beclin1 (1:1000, ab210498, Abcam); rabbit anti-p62 (1:2000, ab109012, Abcam); and rabbit anti-GAPDH (1:2000, ab9485, Abcam) antibody at 4 ˚C overnight. Then the membranes were incubated with HRP-conjugated secondary antibody (1:2000, ab6721, Abcam) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence kit (Millipore Co., Billerica, MA, United States), and quantitative analysis was carried out using Image J software.
Data, presented as the means ± SD, were analyzed using GraphPad Prism 9.5. One-way ANOVA was used to analyze the difference among three or more groups, followed by the Bonferroni post hoc test. All experiments were repeated three times. One-sided P < 0.001 was considered statistically significant.
A DN mouse model was established by administering an HFD combined with STZ injections. Then, mice were administrated with Cor for a further 12 wk. The BW and KW were measured to calculate their ratio (KW/BW) to evaluate renal hypertrophy. FBG, HbA1c, TG, and TC of mice were measured and compared with the control group. The KW/BW ratio significantly increased in DN mice (P < 0.001). After 12 wk of treatment with Cor, the Cor group mice showed a significant reduction in KW/BW (P < 0.001) (Figure 1C). Compared with the control group, the FBG, HbA1c, TG, and TC of the DN group mice were significantly increased (P < 0.001). Compared with the DN mouse model group, Cor administration significantly reduced the FBG, HbA1c, TG, and TC (P < 0.001) (Figure 1D-G).
The renal tissues of mice were stained with HE to manifest the tissue morphology. The structure of the glomerulus and renal tubules in the control group mice was relatively straightforward, the epithelial cells were arranged neatly, and the basement membrane was relatively intact. Compared with the control group, the DN group of mice showed glomerular hypertrophy, increased mesangial extracellular matrix, thickened basement membrane, dilation of renal tubular lumen, and infiltration of inflammatory cells around renal tubules and interstitium. Compared with the DN group, the Cor group mice showed reduced renal lesions and improved renal tissue damage to varying degrees (Figure 2A). The Scr, BUN, and 24-h UAER of mice were measured to assess the renal function of mice. Compared with the control group, the levels of Scr, BUN, and 24-h UAER in the DN group mice were significantly increased (P < 0.001). After continuous administration of Cor for 12 wk, the serum Scr, BUN, and 24-h UAER levels were significantly reduced (P < 0.001) (Figure 2B-D). The presence of proteinuria is related to podocyte injury, so Western blotting was carried out to determine the expression of proteins related to podocyte injury. The protein levels of Nephrin and Podocin in kidney tissues of DN mice were decreased (P < 0.001). After Cor treatment, the protein levels of Nephrin and Podocin were enhanced (P < 0.001) (Figure 2E and F).
To evaluate the effect of Cor on renal tissue apoptosis, we performed TUNEL assay. The number of TUNEL + cells in the kidney tissue of DN mice increased significantly (green fluorescence), but this increase was weakened considerably in the mice treated with Cor (Figure 3A and B). RT-qPCR analysis was used to determine the mRNA expression of apoptosis-related genes. Compared with DN mice, Cor treatment significantly reduced the expression of BAX mRNA and increased the expression of BCL-2 mRNA in kidney tissue (Figure 3C and D).
Immunohistochemistry was performed to evaluate the expression of the inflammatory protein IL-1β in the kidney tissue of each mouse. Compared with the control group, there was a significant increase of IL-1β expression around the glomerulus and renal tubules of mice in the DN group, and the IL-1β expression was decreased after Cor treatment (Figure 4A). The ELISA assay was performed to evaluate the inflammatory factor secretion in the kidney tissues. The concentrations of IL-1β, TNF-α, and IL-6 were increased in DN mice compared with the control mice. Cor treatment significantly reduced the concentrations of IL-1β, TNF-α, and IL-6 (Figure 4B-D). In addition, the mRNA expression of the three inflammatory cytokine genes in mouse kidney tissue was measured using RT-qPCR. DN mice showed significantly increased mRNA levels of IL-1β, TNF-α, and IL-6, but Cor treatment markedly attenuated these changes (Figure 4E-G).
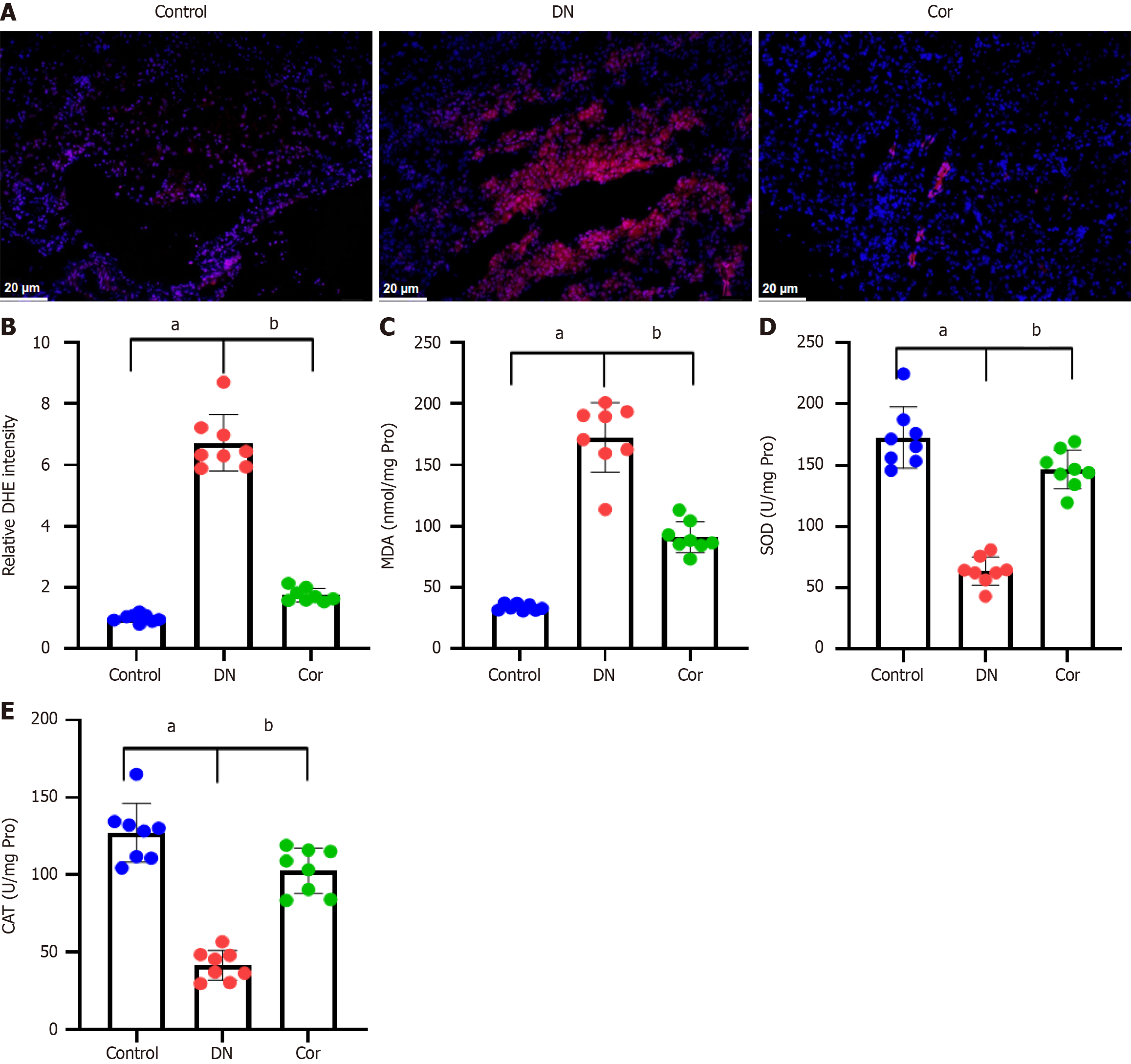
To evaluate the effect of Cor on the production of ROS, we performed DHE staining on renal tissue slices of mice. Compared with the control group, the red fluorescence in renal tissue of DN mice was significantly increased, but this increase was weakened in the mice treated with Cor (Figure 5A). Quantitative analysis showed that Cor significantly reduced the relative fluorescence intensity of DHE in mouse kidney tissue (Figure 5B). Key biomarkers of oxidative stress, including MDA, SOD, and CAT, were measured in renal tissue lysate. Compared with the control group, the content of MDA in the renal tissue of DN mice increased, and the activities of SOD and CAT decreased, while Cor treatment completely reversed these changes (Figure 5C-E).
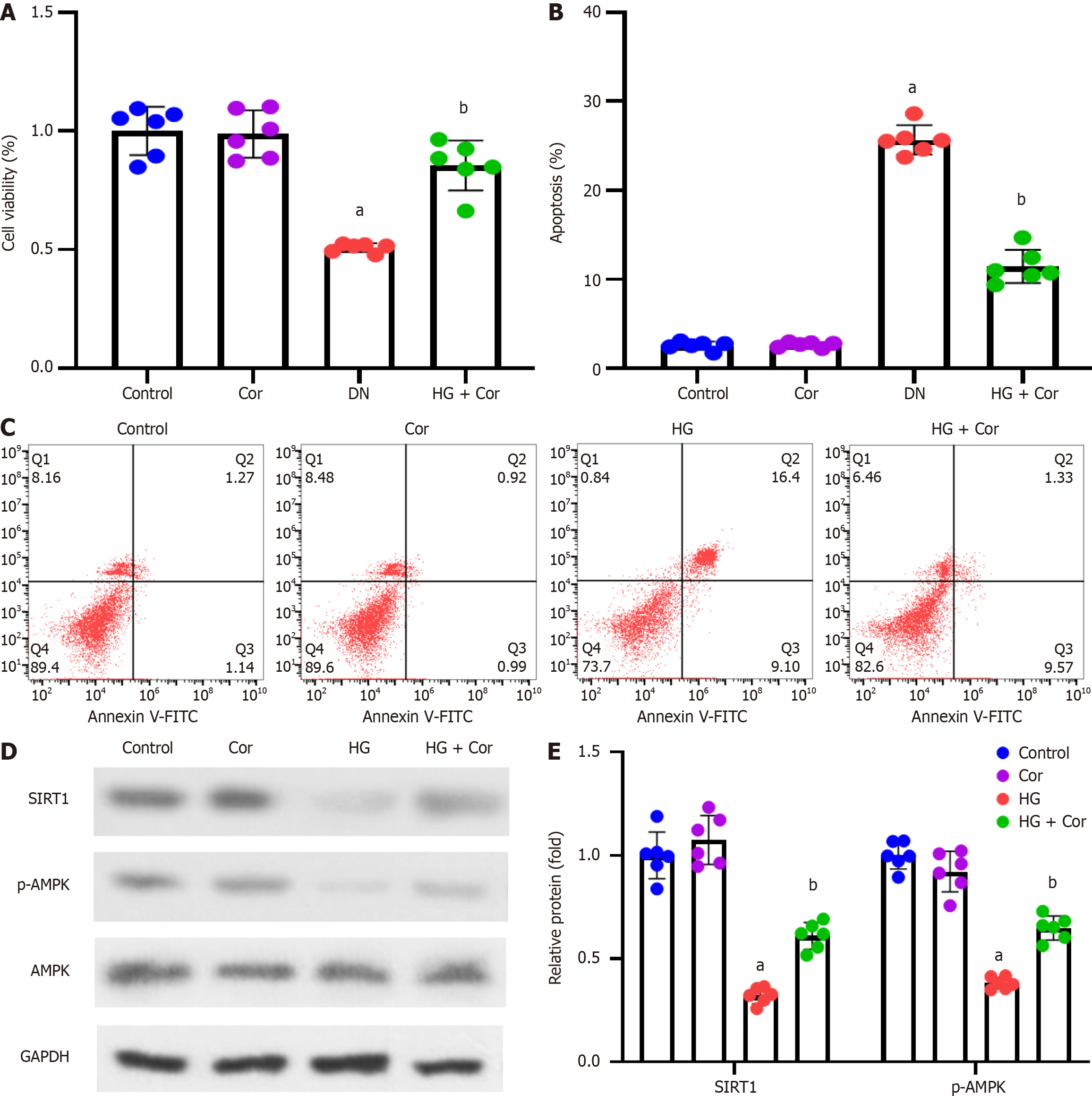
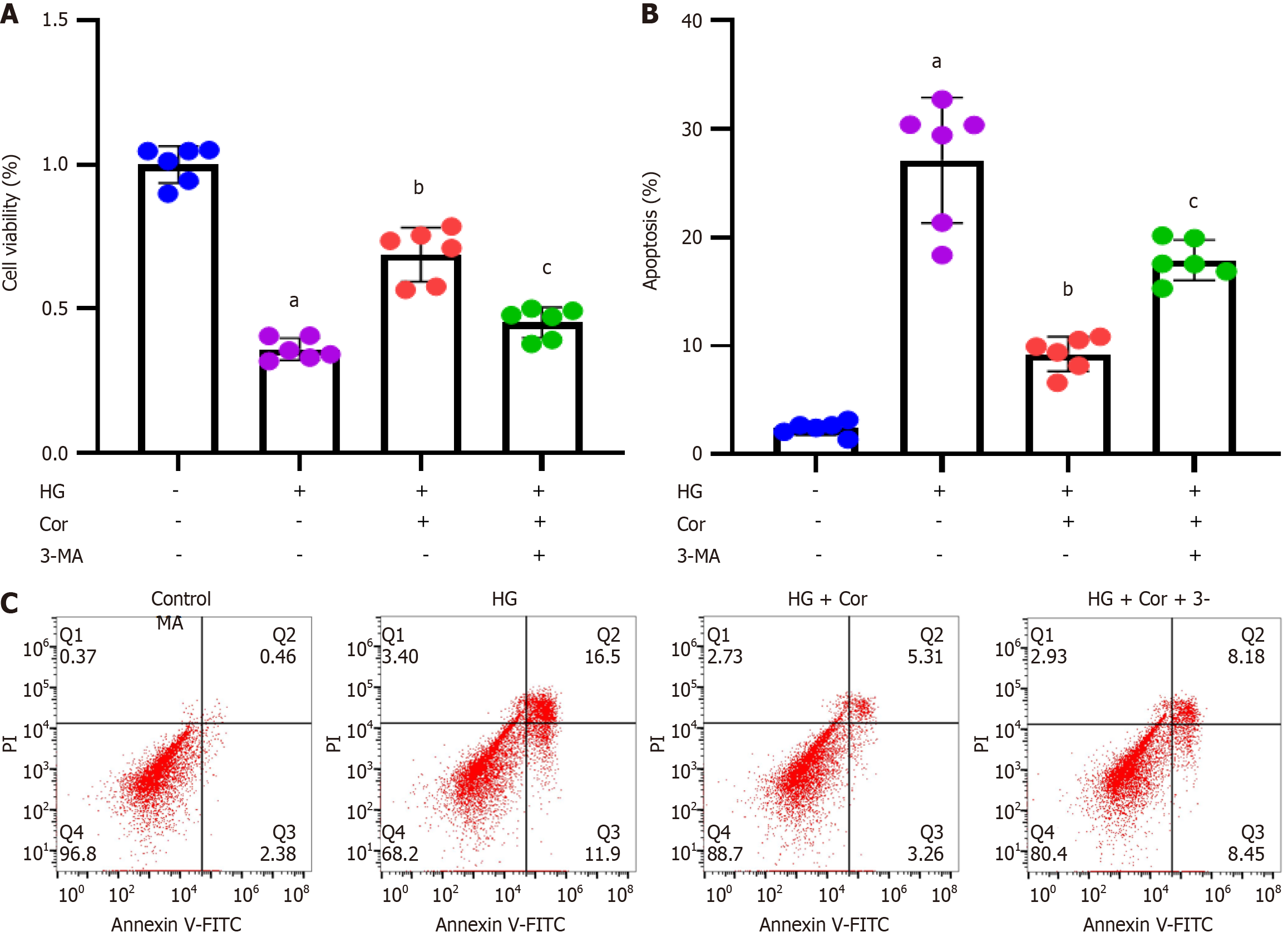
MPC5 podocytes were incubated with HG to establish a cell model of DN, followed by intervention with Cor (50 μM) and HG (D-glucose 30 mmol/L) for a further 48 h. MTT assay was used to detect the viability of cells in different groups. Compared with the control group, the cell viability of the HG group was significantly reduced, while Cor treatment increased cell viability compared to the HG group (P < 0.001) (Figure 6A). The apoptotic rate was determined using double staining with Annexin V-FITC and PI. Cor treatment markedly attenuated HG-induced increase in the apoptotic rate of MPC5 cells (Figure 6B and C). The expression of proteins of related pathways was determined using Western blotting. The protein levels of SIRT1 and p-AMPK in podocytes were decreased by HG treatment. After Cor treatment, the protein levels of SIRT1 and p-AMPK were enhanced (P < 0.001) (Figure 6D and E).
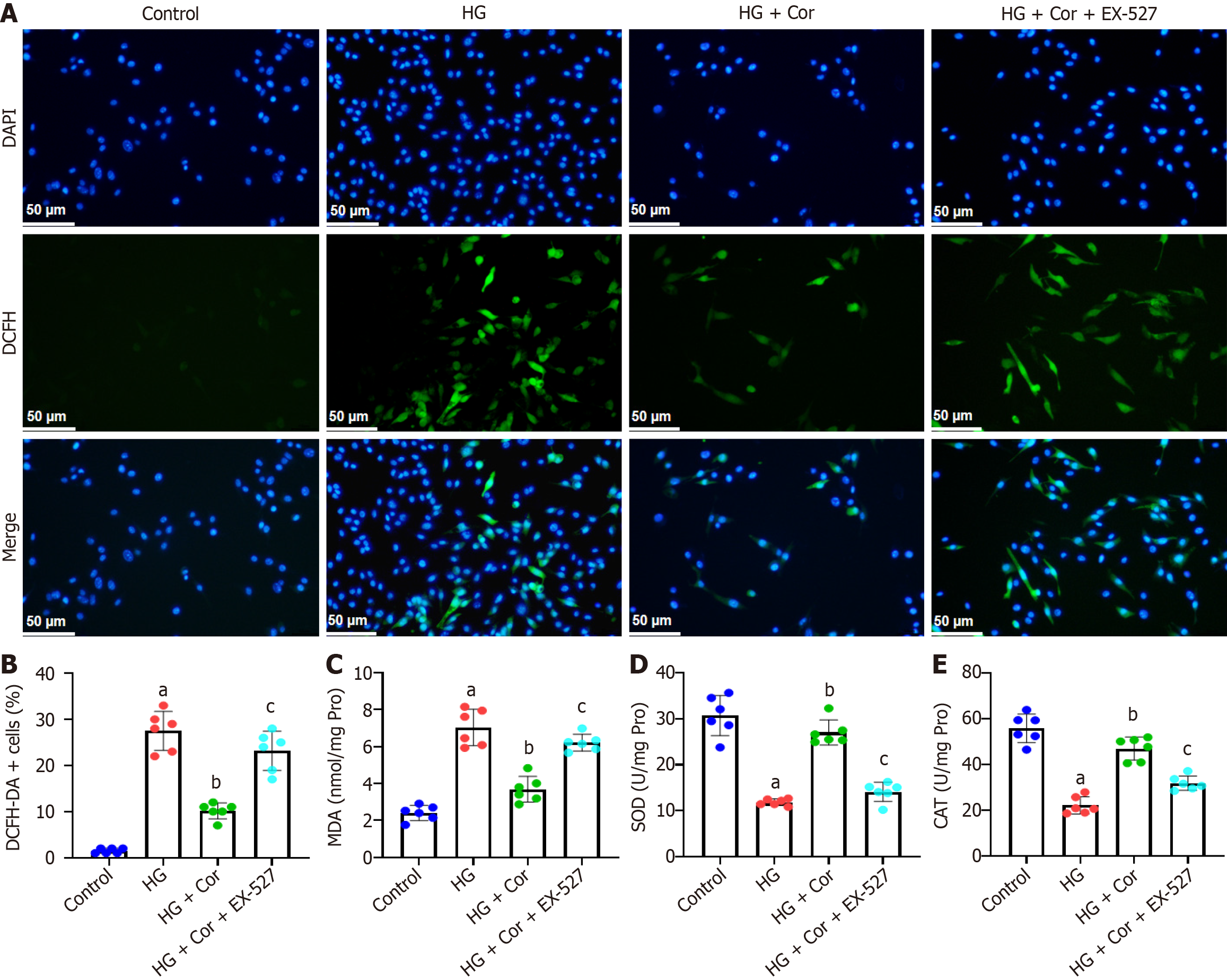
To investigate whether SIRT1 is involved in the protective effects of Cor on podocytes, MPC5 cells were pretreated with a SIRT1 inhibitor (EX-527, 10 μM) for 2 h, and then incubated with Cor (50 μM) and HG (D-glucose 30 mmol/L) for a further 48 h. MPC5 cells were stained with DCFH-DA to evaluate the effect of Cor on the production of ROS. Compared with cells in the HG group, cells in the HG group showed enhanced green fluorescence, which was markedly attenuated by EX-527 pretreatment (Figure 7A). Quantitative analysis showed that Cor significantly reduced the percentage of DCFH-DA-positive cells in HG-induced cells (Figure 7B). To investigate the effect of Cor on indicators of oxidative stress, we measured intracellular MDA levels and SOD and CAT activities. Cor significantly decreased the MDA content and reduced SOD and CAT activities. However, these changes were significantly reversed by pretreatment with EX-527 (P < 0.01) (Figure 7C-E).
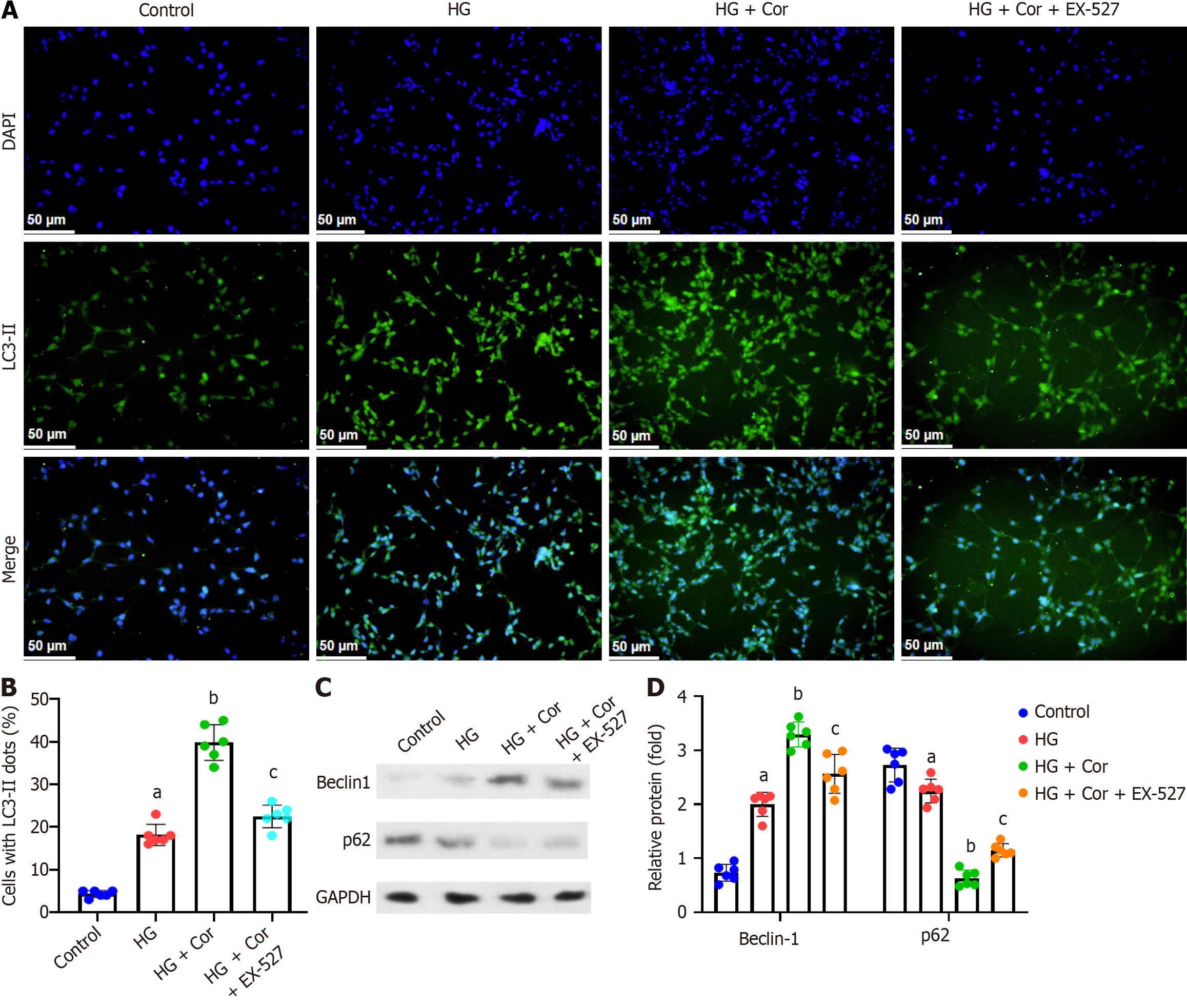
To evaluate whether Cor regulates autophagy in HG-induced podocytes, we monitored the degree of autophagy by staining MPC5 cells with FITC-labeled anti-LC3-II antibody and observed it under a fluorescence microscope. Compared to the control group, the HG group showed increased cellular autophagy in podocytes, and Cor treatment further enhanced podocyte autophagy, manifested as a decrease in green fluorescence intensity (LC3-II spots). The improved cell autophagy by Cor was markedly attenuated by EX-527 pretreatment (Figure 8A). Quantitative analysis showed that Cor significantly increased the percentage of LC3-II spot cells in a SIRT-dependent manner (P < 0.001) (Figure 8B). Then, Western blotting was applied to analyze the expression of the autophagy-related proteins Beclin-1 and p62. Cor increased the protein level of Beclin-1 and decreased the protein level of p62 in podocytes. Cotreatment with EX-527 reduced the expression of Beclin-1 and increased the expression of p62 (P < 0.01) (Figure 8C and D).
To investigate whether Cor inhibits damage to podocytes induced by HG, MPC5 cells were exposed to an autophagy inhibitor (3-MA at 1 mg/mL), Cor (at 50 μM), and HG (30 mmol/L D-glucose) for 48 h. Cell viability in different groups was assessed using MTT assay. Compared to the control group, cell viability significantly decreased in the HG group. However, treatment with Cor led to increased cell viability compared to the HG group. This increase was further reduced by 3-MA treatment (P < 0.001) (Figure 9A). Cell apoptosis was evaluated using Annexin V-FITC/PI double staining and flow cytometry. The results showed that Cor treatment significantly reduced the HG-induced increase in the apoptotic rate of MPC5 cells. Additionally, the autophagy inhibitor 3-MA (1 mg/mL) reversed the increased apoptotic rate (Figure 9B and C).
In the present study, we investigated the potential effects of Cor against podocyte injury in DN. HFD, in combination with STZ injection, was used to generate the DN mouse model. The KW/BW ratio, FBG, HbA1c, TG, and TC were enhanced in DN mice, which were reduced after Cor treatment. Cor treatment potentially: (1) Improved histopathological injury and renal function; (2) Reduced apoptosis in renal tissue; (3) Attenuated inflammatory infiltration in the kidney tissue, and (4) Alleviated oxidative stress in DN mice. In addition, HG-induced podocyte injury was reduced, and SIRT1 and AMPK protein expression was improved by Cor treatment. Cor potentially suppresses cellular ROS production and oxidative stress and promotes HG-induced podocyte autophagy via SIRT1. Furthermore, Cor treatment attenuated HG-induced podocyte injury by improving autophagy. Thus, our results demonstrate that Cor might have a protective role against podocyte injury in DN.
DN represents a significant microvascular complication associated with diabetes. Recent research has indicated a noticeable increase in the prevalence of DN in developed nations[22]. DN frequently progresses to ESRD[2], thus posing severe health risks. DN distinguishes itself from other renal conditions due to its intricate metabolic irregularities, complicating the treatment process. Therefore, the research and development (R&D) of drugs that may effectively pre
Traditional Chinese medicine treatments for DN have demonstrated efficacy in recent years by utilizing active components to prevent the progression of DN. Research findings indicate that curcumin shows potential benefits in mitigating adriamycin-induced nephrotic syndrome in rats through the inhibition of the NF-κB pathway[23]. Similarly, another study revealed that resveratrol could attenuate early podocyte damage in obese rats following an ovariectomy with an HFD by modulating lipid metabolism, enhancing insulin sensitivity, and reducing inflammatory responses[24]. Furthermore, Cor exhibits diverse pharmacological properties such as hepatoprotective, anti-inflammatory, antibacterial, antioxidant, antihypertensive, antidiabetic, and antitumor effects[16]. Our current investigation demonstrated that Cor effectively reduces renal hypertrophy, FBG, HbA1c, TG, TC, and inflammatory cytokines levels while also protecting against podocyte injury in DN. These findings suggest that Cor has a distinct therapeutic potential in the management of DN and could potentially alleviate podocyte injury in DN.
Apoptosis, identified as programmed cell death, serves a significant function in cellular regeneration. However, an overabundance of apoptosis can have deleterious impacts on organ functionality. The occurrence of renal cell apoptosis stands as a crucial element influencing the performance of the kidneys[25]. In this research, the apoptosis rate was increased in the DN group mice. However, Cor treatment remarkably reduced the apoptosis rate in kidney tissue. These results indicate that Cor administration alleviated the apoptosis in the DN mice, which suggests that Cor could serve as a potential therapeutic agent to treat podocyte injury in DN.
The activation of macrophages is a critical factor in the inflammatory injury observed in DN[26]. Recent focus has shifted towards investigating the polarization of M1/M2 macrophages in the progression of DN[27,28]. M1 macrophages are known for their production of significant quantities of pro-inflammatory cytokines like iNOS, TNF-α, MCP-1, and other mediators that enhance inflammation, thereby exacerbating damage in the pathogenesis of DN[29,30]. Conversely, M2 macrophages play a role in suppressing renal inflammation and alleviating injury through the secretion of anti-inflammatory cytokines, such as IL-10 and Arg-1[31,32]. In our study, the pro-inflammatory cytokine secretions were increased in DN group mice. However, Cor treatment significantly decreased the levels of pro-inflammatory cytokines in kidney tissue. These results demonstrate that Cor could serve as an anti-inflammatory agent to treat podocyte injury in DN.
Oxidative stress is recognized as a crucial process in the pathogenesis of renal impairment in diabetes, influenced by metabolic and hemodynamic perturbations[33]. The onset of oxidative stress occurs when there is an imbalance between the production of ROS and the body's intrinsic antioxidant defense system, resulting in the oxidation of bioactive molecules, including carbohydrates, proteins, lipids, and DNA, ultimately leading to cellular injury[34]. A study con
Autophagy plays a significant role in the development of DN[7]. Under normal conditions in podocytes, a high level of autophagic activity helps in breaking down and removing damaged proteins and aging organelles, thus maintaining cell balance[36,37]. Podocyte injury and dysfunction are significant factors in the progression of DN, making them important targets for treatment in DN patients[38]. Therefore, autophagic abnormalities, particularly in podocytes, are believed to be linked to the progression of DN[7,38]. Autophagy alterations, such as increased expression of autophagy-related proteins (LC3-II and Beclin-1) and enhanced production of autophagosomes, have been found in podocytes from STZ-induced DN rats and mouse podocytes grown in HG[39,40]. In the current study, the expression of LC3-II was increased in the HG group than in the control group. However, Cor treatment increased the expression of LC3-II and Beclin-1 and decreased the expression of p62, which activated the autophagy, resulting in podocyte repair in vitro. These findings are consistent with the previously reported studies[41,42].
Many studies have shown that SIRT1 plays a crucial role in the development of DN. Certain synthetic drugs and natural substances can increase SIRT1 expression and activity, thereby preserving renal function[43]. However, there is no literature exploring the effect of Cor on SIRT1 in DN. Under glucose deprivation, AMPK enhances autophagy by stimulating ULK1 through Ser317 and Ser777 phosphorylation. Conversely, mTOR or AKT activity at HG levels decreases ULK1 activation by phosphorylating ULK1 at Ser757, disrupting the connection between ULK1 and AMPK and reducing AMPK downstream signaling for autophagy induction[44-46]. Previous research suggests that restoring autophagy activity by pharmacologically activating AMPK with AICAR or metformin significantly reduces kidney damage in diabetic mice[47,48]. Therefore, AMPK-mediated induction of autophagy plays a crucial role in protecting against DN. In our research, we observed that the protein levels of SIRT1 and p-AMPK in podocytes were reduced by HG treatment, while Cor treatment increased the SIRT1 and p-AMPK protein levels. The results demonstrate that Cor suppresses HG-induced podocyte injury by increasing SIRT1 and AMPK protein expression.
In conclusion, our research indicates that Cor can alleviate the symptoms of renal impairment in DN mice. Cor might have a therapeutic effect by regulating the SIRT1/AMPK signaling pathway, thereby reducing podocyte injury. However, due to the complexity of metabolism in DN mice and its potential influence on multiple pathways, further investigation is needed to identify the specific targets of Cor. Additionally, as Cor is a traditional Chinese medicine, its potential side effects have not been well defined. Therefore, more research is required in this area.
| 1. | Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 674] [Article Influence: 61.3] [Reference Citation Analysis (1)] |
| 2. | Lassén E, Daehn IS. Molecular Mechanisms in Early Diabetic Kidney Disease: Glomerular Endothelial Cell Dysfunction. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, Dong Z. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75:669-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: A new perspective for a new era. Mol Metab. 2019;30:250-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 6. | Yamahara K, Yasuda M, Kume S, Koya D, Maegawa H, Uzu T. The role of autophagy in the pathogenesis of diabetic nephropathy. J Diabetes Res. 2013;2013:193757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15-R30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 8. | Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2886] [Cited by in RCA: 2757] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Liu W, Zhang Z, Hu Y, Zhang X, Sun Y, Lei Q, Sun D, Liu T, Fan Y, Li H, Ding W, Fang J. Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy. Ren Fail. 2021;43:128-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Aventaggiato M, Vernucci E, Barreca F, Russo MA, Tafani M. Sirtuins' control of autophagy and mitophagy in cancer. Pharmacol Ther. 2021;221:107748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374-3379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1182] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 14. | Hong Q, Zhang L, Das B, Li Z, Liu B, Cai G, Chen X, Chuang PY, He JC, Lee K. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 2018;93:1330-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 15. | Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Li X, Deng Y, Zheng Z, Huang W, Chen L, Tong Q, Ming Y. Corilagin, a promising medicinal herbal agent. Biomed Pharmacother. 2018;99:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Yan F, Cheng D, Wang H, Gao M, Zhang J, Cheng H, Wang C, Zhang H, Xiong H. Corilagin Ameliorates Con A-Induced Hepatic Injury by Restricting M1 Macrophage Polarization. Front Immunol. 2021;12:807509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Du P, Ma Q, Zhu ZD, Li G, Wang Y, Li QQ, Chen YF, Shang ZZ, Zhang J, Zhao L. Mechanism of Corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model. Eur J Pharmacol. 2016;793:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Li HR, Li G, Li M, Zhang SL, Wang H, Luo T, Wu F, Dong JH, Guo YJ, Zhao L. Corilagin ameliorates schistosomiasis hepatic fibrosis through regulating IL-13 associated signal pathway in vitro and in vivo. Parasitology. 2016;143:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Li YQ, Chen YF, Dang YP, Wang Y, Shang ZZ, Ma Q, Wang YJ, Zhang J, Luo L, Li QQ, Zhao L. Corilagin Counteracts IL-13Rα1 Signaling Pathway in Macrophages to Mitigate Schistosome Egg-Induced Hepatic Fibrosis. Front Cell Infect Microbiol. 2017;7:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Zhou X, Xiong J, Lu S, Luo L, Chen ZL, Yang F, Jin F, Wang Y, Ma Q, Luo YY, Wang YJ, Zhou JB, Liu P, Zhao L. Inhibitory Effect of Corilagin on miR-21-Regulated Hepatic Fibrosis Signaling Pathway. Am J Chin Med. 2019;47:1541-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Feng Y, Weng H, Ling L, Zeng T, Zhang Y, Chen D, Li H. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int J Biol Macromol. 2019;132:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Fan HY, Wang XK, Li X, Ji K, Du SH, Liu Y, Kong LL, Xu JC, Yang GQ, Chen DQ, Qi D. Curcumin, as a pleiotropic agent, improves doxorubicin-induced nephrotic syndrome in rats. J Ethnopharmacol. 2020;250:112502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Li B, Xiao X, Miao Y, Guo L, Zhen J, Li X, Jiang B, Hu Z. Resveratrol alleviates obesity-associated podocyte injury in ovariectomized obese rats. Exp Ther Med. 2020;19:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhong X, He J, Zhang X, Li C, Tian X, Xia W, Gan H, Xia Y. UCP2 alleviates tubular epithelial cell apoptosis in lipopolysaccharide-induced acute kidney injury by decreasing ROS production. Biomed Pharmacother. 2019;115:108914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Yang X, Mou S. Role of Immune Cells in Diabetic Kidney Disease. Curr Gene Ther. 2017;17:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Yang Y, Zhao Y. Macrophage phenotype and its relationship with renal function in human diabetic nephropathy. PLoS One. 2019;14:e0221991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 819] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 31. | Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda). 2015;30:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 32. | Chen T, Cao Q, Wang Y, Harris DCH. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 2019;95:760-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 33. | Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63:S63-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 861] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 35. | Xue R, Xiao H, Kumar V, Lan X, Malhotra A, Singhal PC, Chen J. The Molecular Mechanism of Renal Tubulointerstitial Inflammation Promoting Diabetic Nephropathy. Int J Nephrol Renovasc Dis. 2023;16:241-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1338] [Cited by in RCA: 1272] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 37. | Tharaux PL, Huber TB. How Is Proteinuric Diabetic Nephropathy Caused by Disturbed Proteostasis and Autophagy in Podocytes? Diabetes. 2016;65:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Liu N, Xu L, Shi Y, Zhuang S. Podocyte Autophagy: A Potential Therapeutic Target to Prevent the Progression of Diabetic Nephropathy. J Diabetes Res. 2017;2017:3560238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Jin Y, Liu S, Ma Q, Xiao D, Chen L. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol. 2017;794:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | Huang SS, Ding DF, Chen S, Dong CL, Ye XL, Yuan YG, Feng YM, You N, Xu JR, Miao H, You Q, Lu X, Lu YB. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci Rep. 2017;7:45692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Wang Y, Li Y, Zhang T, Chi Y, Liu M, Liu Y. Genistein and Myd88 Activate Autophagy in High Glucose-Induced Renal Podocytes In Vitro. Med Sci Monit. 2018;24:4823-4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Wang W, Sun W, Cheng Y, Xu Z, Cai L. Role of sirtuin-1 in diabetic nephropathy. J Mol Med (Berl). 2019;97:291-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 44. | Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4208] [Cited by in RCA: 5535] [Article Influence: 395.4] [Reference Citation Analysis (0)] |
| 45. | Yao F, Zhang M, Chen L. 5'-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B. 2016;6:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Fang Y, Li F, Qi C, Mao X, Wang F, Zhao Z, Chen JK, Zhang Z, Wu H. Metformin effectively treats Tsc1 deletion-caused kidney pathology by upregulating AMPK phosphorylation. Cell Death Discov. 2020;6:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617-F627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 48. | Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |