Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1858
Revised: June 4, 2024
Accepted: July 2, 2024
Published online: September 15, 2024
Processing time: 210 Days and 23.2 Hours
It is widely recognized that chronic hyperglycemia decreases bone quality, although little is known about the impact of the rapid correction of chronic hyperglycemia on the quality of bone remodeling. This spotlight article explores this correlation by focusing on the stages of bone remodeling linked to glucose levels.
Core Tip: The protein osteocalcin involved in bone remodeling is affected by glucose metabolism and variation. Osteoprotegerin and receptor activator of NF-kB ligand also involved in bone modeling are equally sensitive to glucose variation. Bone remodeling is impaired when glucose levels are reduced and only not only when the blood glucose threshold is exceeded.
- Citation: Dardari D, Segurens B. Rapid correction of chronic hyperglycemia and bone remodeling, warning against overdoing. World J Diabetes 2024; 15(9): 1858-1861
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1858.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1858
The protein osteocalcin (OC) involved in bone remodeling is affected by glucose metabolism and variation.
Osteoprotegerin (OPG) and receptor activator of NF-kB ligand also involved in bone modeling are equally sensitive to glucose variation.
Bone remodeling is impaired when glucose levels are reduced and only not only when the blood glucose threshold is exceeded.
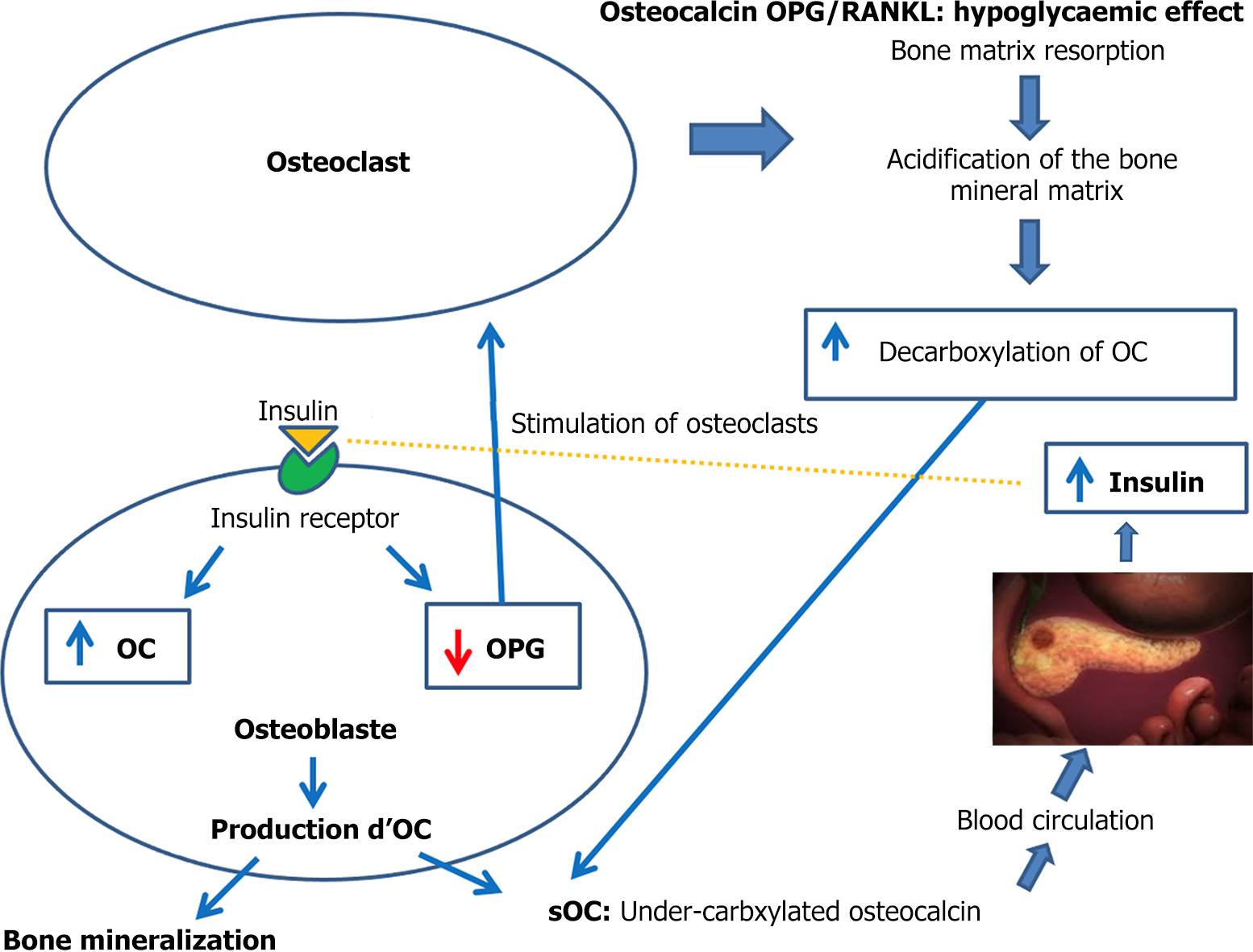
It has been suggested that bone contributes to the overall physiology of the body as an endocrine hormone[1]. To maintain its activity, bone is constantly remodeled by osteoblasts (OB) and osteoclasts[2]. During the various stages of OB differentiation, different biomarkers such as the OC are involved. Secreted by OB, OC is a bone component and biomarker of bone mineralization. Nevertheless, there are several shortcomings regarding the idea of OC as an endocrine hormone[3]. OC increases the insulin sensitivity of the target organs by stimulating glucose uptake by the muscles, increasing adiponectin production by adipose tissue, reducing lipid accumulation and inflammation in the liver, and promoting insulin secretion by the pancreas. The latter action is directly related to glucose metabolism[3]. This demonstrates the link between bone remodeling and glucose metabolism via the impact of glucose metabolism on OC. Patients with hyperglycemia have low levels of OC[4,5]. If hyperglycemia is corrected rapidly, the level of OC will also rise, thus accelerating the bone remodeling process.
Furthermore OPG is a soluble glycoprotein produced mainly by OB, which inhibits osteoclast genesis by preventing the binding of the receptor activator of nuclear factor-κB ligand (RANKL) to its receptor RANK[6]. However, RANKL is an osteoclast differentiation factor produced by OB, which triggers osteoclast genesis by binding to RANK, a membrane receptor expressed by osteoclast precursors[7]. In a study[8], OPG was inhibited by the correction of hyperglycemia, twenty-two patients with newly diagnosed type 1 diabetes were treated with insulin, with a drop in HbA1c from 11.1% (98 mmol/mol) at diagnosis to 6.2% (44 mmol/mol) after 6 months. The plasma OPG level in the patients before treatment was 3.1 ng/L, and after 6 months treatment it was decreased to 2.6 ng/L (P < 0.001) but was still higher than in 28 healthy controls (2.1 ng/L). Elevated levels of OPG have previously been shown in patients with both type 1 and type 2 diabetes and have been associated to hyperglycemia[9,10]. We would therefore conclude that rapidly reducing hyperglycaemia inhibits OPG and increases RANKL.
In a different vein, OB use glucose during differentiation via both oxidative phosphorylation and aerobic glycolysis[11]. However, it is also known that hyperglycemia plays a role in OB differentiation, whereas OB have insulin receptors. The binding of insulin to its OB receptor induces bone formation and the production of OC as well as a decrease in the expression of OPG[11]. It can therefore be concluded that the lack of insulin induced by hyperglycemia reduces the OB differentiation. However, insulin can stimulate the secretion of OC. OB that produce proteins also express an insulin receptor. Following its binding to its receptor, insulin not only induces bone formation but also decreases the expression of OPG, which inhibits the differentiation and activation of osteoclasts, the cells responsible for the resorption of the bone matrix.
Glucose levels, glucose metabolism, and insulin seem play a role in every stage of bone remodeling: They activate OC, reduce OPG, and differentiate OB. While previous studies have focused on the role played by hyperglycemia in the disruption and imbalance of bone remodeling, few have focused on the possible impact of hyperglycemia on bone remodeling, as presented in Figure 1, which illustrates how the correction of hyperglycemia impacts bone remodeling. However, in our focus we have not put the spotlight on the role of RANK due to the lack of studies on the topic.
This spotlight highlights the impact of the rapid correction of hyperglycemia on bone remodeling. In the literature, this impact has rarely been described. It has been suggested that certain profiles of patients living with chronic hyperglycemia should undergo a bone health assessment prior to the correction of chronic hyperglycemia. For example, simple bone densitometry can guide physicians or give clues about the state of the bone structure. This type of low-cost exploration can reduce the risk of bone diseases whose mechanism is linked to the alteration of bone modeling such as osteoporosis or Charcot’s neuroarthropathy[12]. Finally, it is possible that the anti-RANKL therapies used in certain diseases such as osteoporosis could be a means of preventing the perturbation of bone modelling for people living with chronic hyperglycaemia and for whom a rapid reduction in this hyperglycaemia is planned in a short-term perspective.
The authors acknowledge Natacha Vitrat Gip Genopole Genopole Campus 1 20, Rue Henri Desbruères F-91030 Evry, FRANCE.
| 1. | Veldhuis-Vlug AG, Fliers E, Bisschop PH. Bone as a regulator of glucose metabolism. Neth J Med. 2013;71:396-400. [PubMed] |
| 2. | Weivoda MM, Bradley EW. Macrophages and Bone Remodeling. J Bone Miner Res. 2023;38:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2030] [Cited by in RCA: 1804] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 4. | Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008;396:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World J Orthop. 2013;4:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. [Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients]. Zhonghua Yi Xue Za Zhi. 2007;87:1234-1237. [PubMed] |
| 9. | Rasmussen LM, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006;154:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Medeiros C, Wallace JM. High glucose-induced inhibition of osteoblast like MC3T3-E1 differentiation promotes mitochondrial perturbations. PLoS One. 2022;17:e0270001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Dardari D. Trends in the pathophysiology of Charcot neuroarthropathy. Trends Endocrinol Metab. 2023;34:61-62. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |