Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1764
Revised: June 3, 2024
Accepted: July 5, 2024
Published online: August 15, 2024
Processing time: 146 Days and 21.7 Hours
Impaired hypoglycaemic counterregulation has emerged as a critical concern for diabetic patients who may be hesitant to medically lower their blood glucose levels due to the fear of potential hypoglycaemic reactions. However, the patho-genesis of hypoglycaemic counterregulation is still unclear. Glucagon-like peptide-1 (GLP-1) and its analogues have been used as adjunctive therapies for type 1 diabetes mellitus (T1DM). The role of GLP-1 in counterregulatory dys-function during hypoglycaemia in patients with T1DM has not been reported.
To explore the impact of intestinal GLP-1 on impaired hypoglycaemic counterregulation in type 1 diabetic mice.
T1DM was induced in C57BL/6J mice using streptozotocin, followed by intraperitoneal insulin injections to create T1DM models with either a single episode of hypoglycaemia or recurrent episodes of hypoglycaemia (DH5). Immunofluorescence, Western blot, and enzyme-linked immunosorbent assay were employed to evaluate the influence of intestinal GLP-1 on the sympathetic-adrenal reflex and glucagon (GCG) secretion. The GLP-1 receptor agonist GLP-1(7-36) or the antagonist exendin (9-39) were infused into the terminal ileum or injected intraperitoneally to further investigate the role of intestinal GLP-1 in hypoglycaemic counterregulation in the model mice.
The expression levels of intestinal GLP-1 and its receptor (GLP-1R) were significantly increased in DH5 mice. Consecutive instances of excess of intestinal GLP-1 weakens the sympathetic-adrenal reflex, leading to dysfunction of adrenal counterregulation during hypoglycaemia. DH5 mice showed increased pancreatic δ-cell mass, cAMP levels in δ cells, and plasma somatostatin concentrations, while cAMP levels in pancreatic α cells and plasma GCG levels decreased. Furthermore, GLP-1R expression in islet cells and plasma active GLP-1 levels were significantly increased in the DH5 group. Further experiments involving terminal ileal infusion and intraperitoneal injection in the model mice demonstrated that intestinal GLP-1 during recurrent hypoglycaemia hindered the secretion of the counterregulatory hormone GCG via the endocrine pathway.
Excessive intestinal GLP-1 is strongly associated with impaired counterregulatory responses to hypoglycaemia, leading to reduced appetite and compromised secretion of adrenaline, noradrenaline, and GCG during hypo-glycaemia.
Core Tip: Glucagon-like peptide-1 (GLP-1) is an incretin hormone primarily produced by specific enteroendocrine L-cells in the intestines. The role of GLP-1 in impaired hypoglycaemic counterregulation in type 1 diabetes mellitus (T1DM) has not been reported. In this study, a model of recurrent hypoglycaemia in T1DM mice was established, and it was found that excessive intestinal GLP-1 is strongly associated with impaired counterregulatory responses to hypoglycaemia, leading to reduced appetite and compromised secretion of adrenaline, noradrenaline, and glucagon during hypoglycaemia.
- Citation: Jin FX, Wang Y, Li MN, Li RJ, Guo JT. Intestinal glucagon-like peptide-1: A new player associated with impaired counterregulatory responses to hypoglycaemia in type 1 diabetic mice. World J Diabetes 2024; 15(8): 1764-1777
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1764.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1764
Patients with type 1 diabetes mellitus (T1DM) are prone to iatrogenic hypoglycaemia due to the administration of insulin or sulfonylureas, which occur an average of 1-2 times per week. Notably, approximately 40% of T1DM patients experience severe hypoglycaemia[1-3]. Severe hypoglycaemia events can manifest as symptoms such as confusion, coma, seizures, and, in extreme cases, death[4]. Studies indicate that hypoglycaemia contributes to up to 10% of fatalities in individuals living with diabetes[5].
When hypoglycaemia occurs in healthy individuals, the body perceives a decrease in blood glucose levels and initiates counterregulatory responses to restore glucose levels. Insulin secretion decreases when glucose levels decrease to 4.4-4.7 mmol/L, while glucagon (GCG) and adrenaline secretion begin to increase when glucose levels reach 3.6-3.9 mmol/L. At approximately 3.2 mmol/L, symptoms such as palpitations, tremors, anxiety, sweating, and hunger manifest due to autonomic nerve excitement, prompting the body to take corrective measures for hypoglycaemia. These symptoms of autonomic hypoglycaemia are commonly referred to as hypoglycaemia warnings. When glucose drops to 2.8 mmol/L, neuroglycopenic symptoms, such as cognitive impairment, epilepsy, or coma, appear[6].
Recurrent hypoglycaemia[7], defined as multiple episodes within a day or consecutive episodes across multiple days, especially if each episode lasts for more than 30 min, can lead to impaired hypoglycaemic counterregulation. This impairment includes decreased GCG secretion and compromised sympathetic nerve excitation, ultimately affecting the body's ability to regulate hypoglycaemia[6]. Impaired hypoglycaemic counterregulation has emerged as a critical concern for diabetic patients who may be hesitant to medically lower their blood glucose levels due to the fear of potential hypoglycaemic reactions. Clinical studies have demonstrated that hypoglycaemic warning symptoms can be reversed after 2 wk of hypoglycaemia prevention in T1DM patients. After preventing hypoglycaemia for 3 mo, neuroendocrine counterregulation, such as GCG and adrenaline secretion, normalizes[8]. However, the pathogenesis of impaired hypoglycaemic counterregulation is still unclear, making it challenging to develop effective prevention and treatment strategies for hypoglycaemia.
GCG-like peptide-1 (GLP-1) is an incretin hormone primarily produced by specific enteroendocrine L-cells in the terminal ileum and colon through the preproglucagon (PPG) gene[9,10]. When GLP-1 in the intestines binds to its receptor (GLP-1R), it can activate brain neurons related to autonomic nerves, stimulate adrenal medulla secretion, and elevate blood catecholamine levels[11,12]. Additionally, GLP-1 can enhance insulin release and suppress GCG secretion by interacting with the GLP-1R on pancreatic islet cells[8,13]. Moreover, by influencing the GLP-1R on vagal afferent nerve fibers and afferent neurons in the gastrointestinal tract, GLP-1 can delay gastric emptying, reduce intestinal peristalsis and gastric acid secretion, and stimulate a satiation signal, thereby leading to weight loss and an improvement in body mass index[8,14,15]. Therefore, GLP-1 and its analogues are commonly used in the clinical treatment of type 2 diabetes mellitus and as adjuvant therapies for T1DM. Given that GLP-1 can induce hypoglycaemia and satiety, what role does GLP-1 play in counterregulation dysfunction and even loss of hunger awareness during hypoglycaemia in patients with T1DM? There are currently no reports available on this topic. Therefore, this study aimed to establish a recurrent hypoglycaemia model in T1DM mice to investigate changes in GLP-1 expression in the intestinal tract, explore the mechanisms underlying hypoglycaemic counterregulatory dysfunction and awareness impairment, and further provide experimental evidence for the prevention and treatment of hypoglycaemia in T1DM patients.
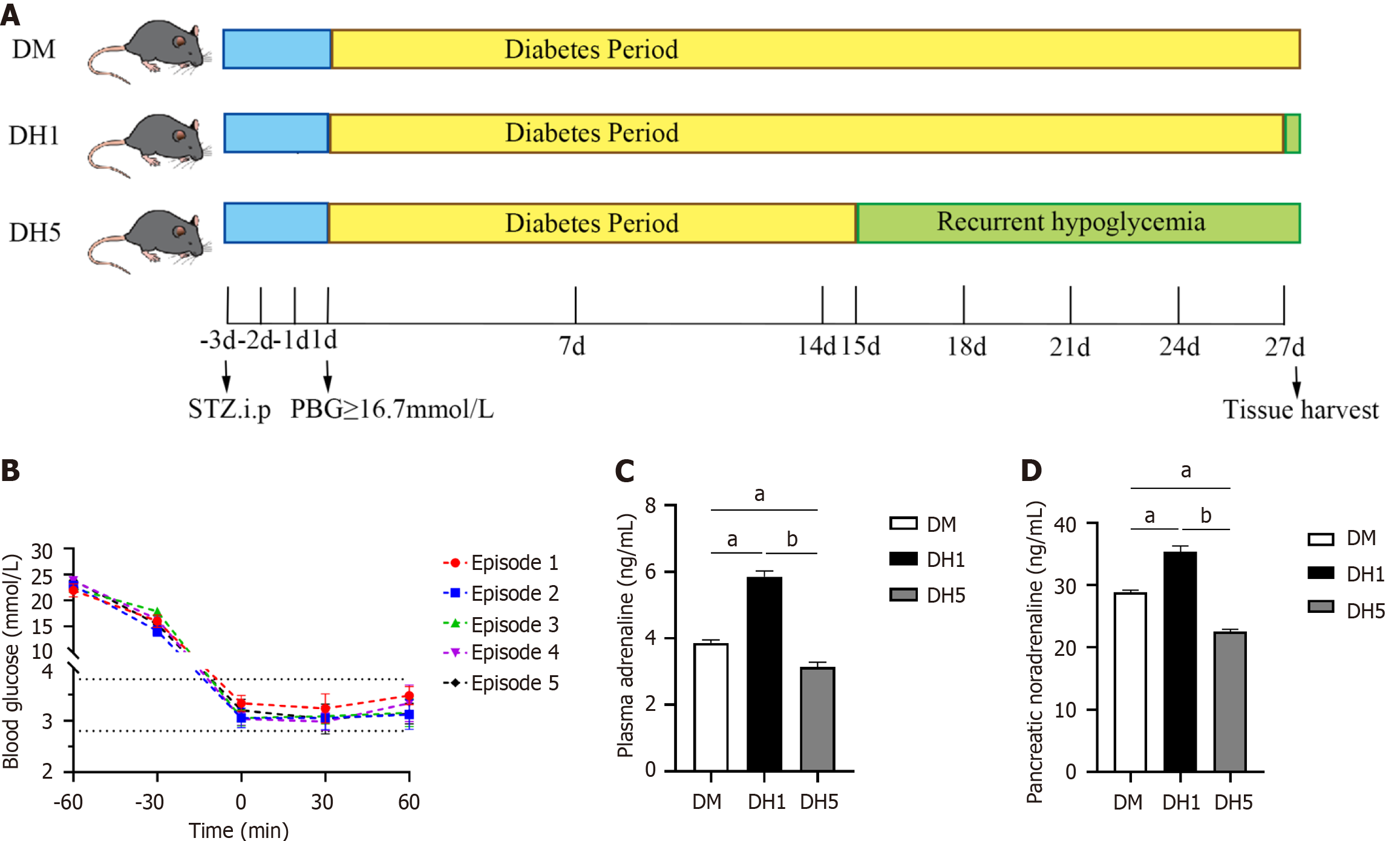
Male C57BL/6J mice aged 10 wk and weighing 22.5 ± 0.5 g were obtained from the Experimental Animal Center at Shandong Second Medical University (No. 2019SDL029; Shandong Province, China). The mice were allowed to acclimate and fed at room temperature (approximately 25 °C) for 1 wk prior to the study. To induce T1DM, all mice were fasted for 6 h, followed by intraperitoneal administration of 220 mg/kg streptozotocin (STZ) dissolved in 0.01 mol/L citrate buffer (pH 4.2). The successful establishment of the T1DM model was confirmed on the third day post-STZ injection when postprandial blood glucose levels in the tail vein blood of mice reached ≥ 16.7 mmol/L, indicating the onset of diabetes. A model of recurrent hypoglycaemia in T1DM mice was created using a standardized protocol with modifications[13-15]. T1DM mice were injected with short-acting insulin after a 6-h fast, resulting in blood glucose levels decreasing to less than 3.9 mmol/L (approximately 3.3 ± 0.5 mmol/L) for more than 60 min, representing a single episode of hypo-glycaemia. Beginning on day 15 of diabetes, hypoglycaemia was induced once every 3 d for a total of 5 consecutive episodes to establish a recurrent hypoglycaemic model in T1DM mice (DH5 group). On the 27th day of diabetes, coinciding with the final hypoglycaemia episode in the DH5 group, a second group of T1DM mice underwent the same procedure to induce hypoglycaemia once, creating the T1DM one hypoglycaemia model (DH1 group).
Throughout the modeling process, the activity status of the model mice was closely monitored, and the plasma adrenaline and pancreatic noradrenaline levels were measured using enzyme-linked immunosorbent assay (ELISA) to evaluate the hypoglycaemic counterregulatory response. Pentobarbital sodium was administered intraperitoneally, followed by the collection of retroocular plasma, ileum, colon, and pancreas samples from the mice. Some tissue samples were fixed in 4% paraformaldehyde and processed into paraffin sections, while others were stored in a freezer at -80 °C.
On the 25th day after diabetes induction, following the 4th hypoglycaemic episode, the mice in the DH1 and DH5 groups were subcutaneously administered 0.05 mg/kg buprenorphine for preoperative analgesia. Anaesthesia was induced with 3% isoflurane and maintained at 2%. The abdomen and neck were prepared by shaving and disinfecting with iodine. A midline incision was made in the abdomen to remove the ileum. A 25 G needle was used to puncture the end of the ileum 5 cm from the ileocecal valve, and Micro-Renathane Tubing (Braintree Scientific, United States) was inserted into the puncture hole. Purse-string sutures were placed around the catheter. A small incision was made on the back of the neck, and the catheter was subcutaneously introduced, fixed in the interscapular area, and sutured. Subcutaneous administration of 0.05 mg/kg/d of buprenorphine was provided for analgesia on the day of surgery and for 2 d postoperatively. Exendin (9-39) amide (Abcam, United Kingdom) or GLP-1(7–36) amide (AnaSpec, United States) was freshly prepared in sterile saline. They were infused at a dose of 100 pmol/kg in 1 mL at a rate of 0.5 mL/min through an external catheter and administered 10 min before the conclusion of the final hypoglycaemic session.
Mice in the DH5 group were injected intraperitoneally with either 5 μg of exendin (9-39) or 5 μg of GLP-1(7-36) 20 min before the conclusion of the final hypoglycaemic session. Plasma samples were then collected and prepared for subsequent experiments.
Adrenaline ELISA kit (Sin-troch, China), GLP-1-Active Form Assay Kit (Immuno-Biological Laboratories, Japan), GCG-ELISA Kit (Cloud-Clone, China), and SST-ELISA Kit (Cloud-Clone, China) were used to analyze the levels of adrenaline, active GLP-1, GCG, and somatostatin (SST) in the plasma of the mice in each group, respectively. A noradrenaline ELISA kit (Sin-troch, China) was used to assess the level of noradrenaline in pancreatic tissue homogenates of the model mice. ELISA was conducted following the instructions of the respective kits.
Intestinal paraffin sections (5 μm thick) were deparaffinized in water, microwaved for repair, and blocked with 10% goat serum (Solarbio, China) at 37 °C for 30 min, after which mouse anti-GLP-1 antibody (Abcam, United Kingdom, 1:200) was added at 4 °C overnight. Subsequently, the sections were incubated in the dark with a fluorescently labeled secondary antibody at 37 °C for 50 min and mounted with DAPI (Solarbio, China) sealing reagent.
Five fluorescently stained sections were extracted from mouse tissue (0.2 mm between sections), and ten randomly selected fields of view from each section were observed under a 200 × fluorescence microscope. The integrated optical density (IOD) values in each image were subsequently calculated using Image-Pro Plus 6.0.
Pancreatic paraffin sections (5 μm thick) were deparaffinized in water, microwaved for repair, and blocked with 3% hydrogen peroxide and 10% goat serum at 37 °C for 30 min. Subsequently, rabbit anti-cAMP antibody (Abcam, United Kingdom, 1:100) was added, and the sections were incubated overnight at 4 °C. The sections were incubated with a biotin-labeled goat anti-rabbit secondary antibody (ZSGB-BIO, China) at 37 °C for 50 min, followed by incubation with horseradish peroxidase-labeled streptavidin for 30 min. Tyramide working solution (APExBIO, United States, 1:800 dilution, containing 0.3% hydrogen peroxide) was then added, and the sections were incubated at room temperature for 10 min. After washing with PBS, the sections were blocked with serum for 50 min and incubated with mouse anti-GCG (BOSTER, United States, 1:200) or mouse anti-SST (Santa Cruz Biotechnology, United States, 1:200) antibody at 37 °C for 1.5 h, followed by incubation with a fluorescently labeled secondary antibody at 37 °C for 50 min. Finally, the sections were mounted with DAPI sealing reagent.
The statistical analysis was in line with the immunofluorescence staining process.
Frozen intestinal tissues were homogenized and ultrasonically lysed to extract proteins, which were quantified using the bicinchoninic acid method. Each sample contained 30 μg of protein, which was transferred to a PVDF membrane using a wet method and then blocked with 5% skim milk for 2 h at room temperature on a shaker. Mouse anti-GLP-1 (1:2000), mouse anti-GLP-1R (DSHB, United States, 1:1000), or mouse anti-β-actin (Proteintech, United States, 1:10000) antibody was individually added and incubated overnight at 4 °C with shaking. The next day, the membranes were incubated with a secondary antibody (Proteintech, United States, 1:5000) for 2 h at room temperature and then visualized using enhanced chemiluminescence reagent. Data analysis was conducted using Image-Pro Plus software, and the relative expression of the target protein was calculated as the ratio of the optical density of the target protein to that of β-actin.
Statistical analyses were conducted using SPSS 25.0 software, while GraphPad Prism 9.0 software was utilized for creating visual representations. Normally distributed measurement data are expressed as the mean ± SE. Comparisons between two groups were assessed using the t test, while comparisons among multiple groups were analyzed using one-way ANOVA. P < 0.05 was considered to indicate statistical significance.
The blood glucose and body weight of model mice meet the modeling criteria: The flow diagram of the modeling process is presented in Figure 1A. Prior to STZ injection, there were no significant differences in blood glucose levels among the groups (P > 0.05). On the first day of diabetes, blood glucose levels significantly increased and exceeded 16.7 mmol/L (P < 0.01). By the seventh day of diabetes, a significant decrease in body weight was observed in each group (P < 0.01). These elevated blood glucose levels and decreased body weight align with the criteria for diabetes modeling, as shown in Table 1. On the 15th day of diabetes, fasted diabetic mice experienced hypoglycaemia, with 1 episode every 3 d, for a total of 5 episodes. The fluctuations in blood sugar levels are illustrated in Figure 1B. Following insulin injection during these episodes, blood glucose levels in the DH5 and DH1 groups decreased to 3.3 ± 0.5 mmol/L within 60 min. Since fasting blood glucose levels < 3.9 mmol/L can induce hypoglycaemia syndrome in diabetic patients, the blood glucose levels during repeated hypoglycaemic episodes in this study met the criteria for postdiabetes hypoglycaemia modeling.
Activity state, plasma adrenaline, and pancreatic noradrenaline levels in DH5 model mice reflect the development of impaired hypoglycaemic counterregulation: This study revealed activity changes in DH5 model mice during episodes of hypoglycaemia, as indicated by increased activity and foraging behaviors during the first two episodes, which gradually decreased during subsequent episodes. This decrease in activity is consistent with the decrease in hypoglycaemic warning symptoms resulting from impaired counterregulation.
To further clarify whether activity changes in DH5 mice are linked to impaired hypoglycaemic counterregulation, sympathetic response indices such as plasma adrenaline and pancreatic noradrenaline were measured using ELISA. The results indicated that plasma adrenaline and pancreatic noradrenaline levels were significantly lower in the DH5 group than in the diabetic mice (DM) and DH1 groups (P < 0.01; Figure 1C and D), suggesting that DH5 group mice exhibit impaired hypoglycaemic counterregulation.
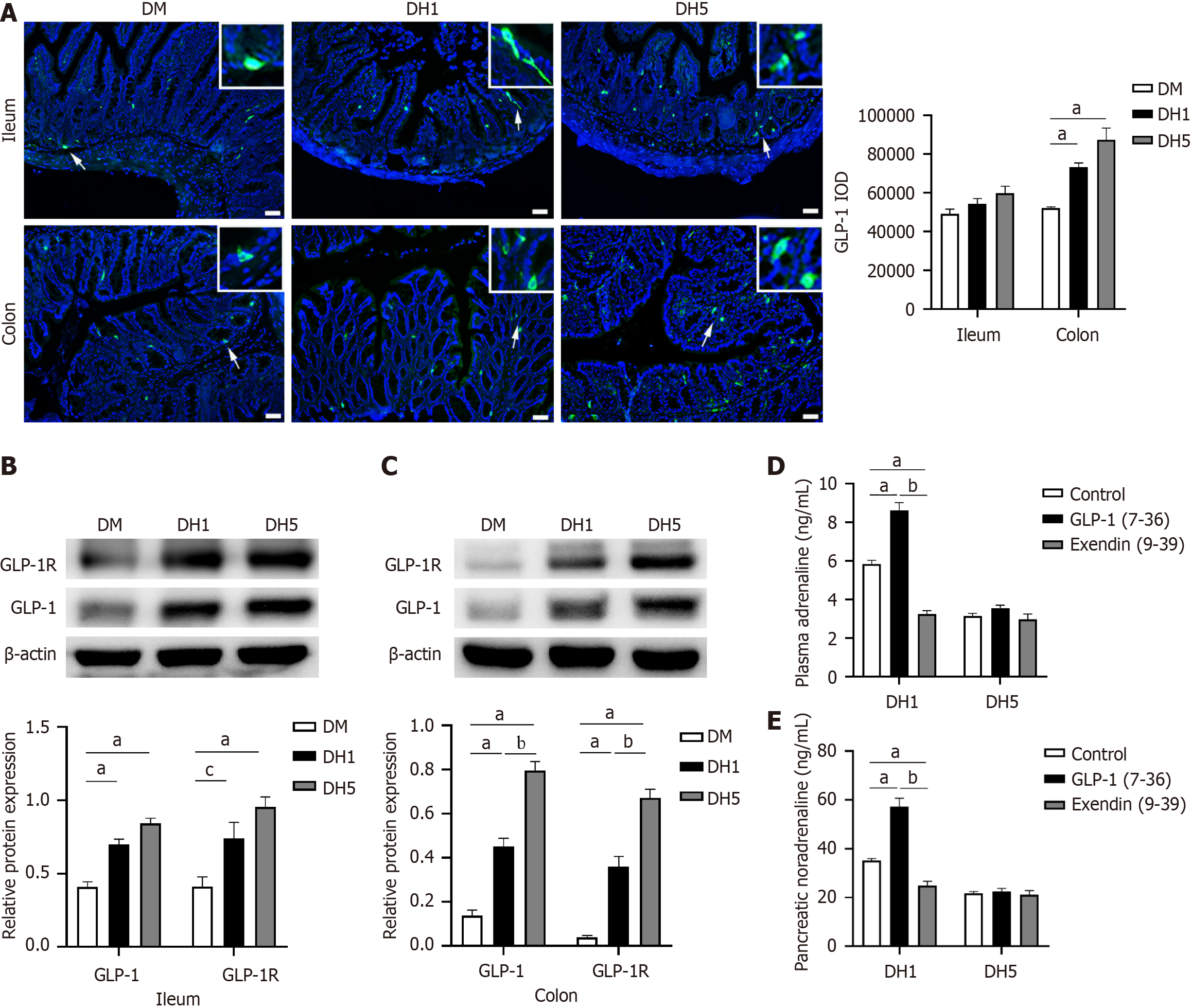
GLP-1 is predominantly present in L cells in the distal small intestine and colon. This study aimed to assess changes in GLP-1 expression in the distal ileum and colon using immunofluorescence staining and Western blot analysis. Active GLP-1-positive cells were primarily located in the glandular epithelium, with occasional presence in the covering epithelium. The fluorescence images revealed a greater number of GLP-1-positive cells in the DH1 and DH5 groups than in the DM group. The GLP-1 IOD in the colon was significantly greater in the DH1 and DH5 groups than in the DM group (P < 0.01; Figure 2A). Since the ileum and colon are relatively long, further verification is needed to determine the total amount of GLP-1 reflected by the GLP-1 IOD in these sections. Therefore, Western blot experiments were conducted, and the results indicated that GLP-1 levels tended to increase sequentially in the DM, DH1, and DH5 groups, with a significant increase in the colon (P < 0.01; Figure 2B and C, Supplementary material).
Given that intestinal GLP-1 primarily acts through neighboring GLP-1Rs, this study aimed to investigate changes in intestinal GLP-1R expression through Western blot analysis. The results indicated a sequential increase in GLP-1R levels in the DM, DH1, and DH5 groups, with a significant increase observed in the colon of the DH5 group (P < 0.01; Figure 2B and C). These results suggest heightened levels of intestinal GLP-1 and GLP-1R expression in the DH1 and DH5 groups, indicating an enhanced paracrine effect, especially in the DH5 group.
Adrenaline and noradrenaline serve as vital markers of the body's stress response. To investigate the role of intestinal GLP-1 in the stress response through paracrine effects, the levels of plasma adrenaline and pancreatic noradrenaline were measured following infusions of the GLP-1R agonist GLP-1(7-36) or the antagonist exendin (9-39) into the terminal ileum. GLP-1(7-36) increased plasma adrenaline and pancreatic noradrenaline levels in DH1 mice (P < 0.01), while exendin (9-39) decreased adrenaline and pancreatic noradrenaline levels (P < 0.01). In contrast, neither GLP-1(7-36) nor exendin (9-39) affected plasma adrenaline or pancreatic noradrenaline levels in DH5 mice (P > 0.05; Figure 2D and E). These results indicate that heightened intestinal GLP-1 expression in DH1 mice can enhance sympathetic nerve excitation and adrenaline secretion, whereas excessive intestinal GLP-1 expression in DH5 mice hinders the activation of the sympathetic response following repeated hypoglycaemia. Excessive intestinal GLP-1 expression in DH5 mice appears to be linked to dysfunction in the secretion of hypoglycaemic counterregulatory hormones such as adrenaline and noradrenaline.
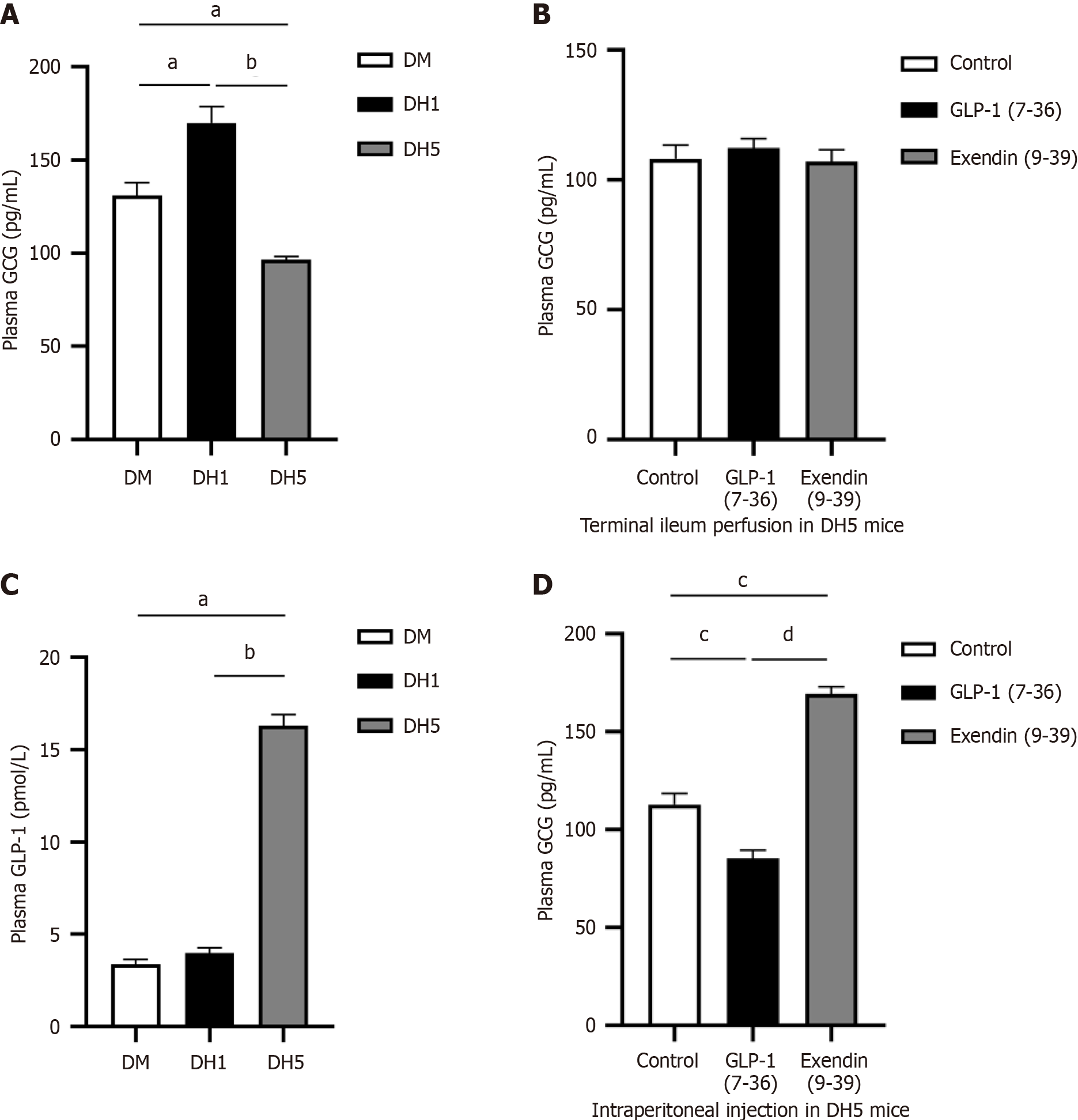
Intestinal GLP-1 participates in secretion of the counterregulatory hormone glucagon during hypoglycaemia through its endocrine effect: To further confirm impaired hypoglycaemic counterregulation in DH5 mice, the plasma level of GCG, another indicator of hypoglycaemic counterregulation, was measured using ELISA. The results showed a significant increase in plasma GCG levels in the DH1 group and a significant decrease in the DH5 group (P < 0.01; Figure 3A), supporting the conclusion of impaired hypoglycaemic counterregulation in DH5 mice.
This study investigated the relationship between plasma GCG levels and intestinal GLP-1 secretion in DH5 mice. ELISA tests conducted after infusion of GLP-1(7-36) or exendin (9-39) revealed no significant changes in plasma GCG levels in the DH5 group (P > 0.05; Figure 3B), suggesting that the reduction in plasma GCG levels in the DH5 mice was not due to the paracrine effect of intestinal GLP-1. However, higher plasma GLP-1 levels were detected in the DH5 group than in the DM and DH1 groups (P < 0.01; Figure 3C), indicating an enhanced endocrine effect of GLP-1 in the DH5 group. Further experiments using intraperitoneal injections demonstrated that GLP-1 has an effect on GCG secretion in DH5 mice through endocrine pathways. Following the injection of GLP-1(7-36), there was a significant decrease in plasma GCG levels, whereas injection of exendin (9-39) led to a significant increase (P < 0.01; Figure 3D).
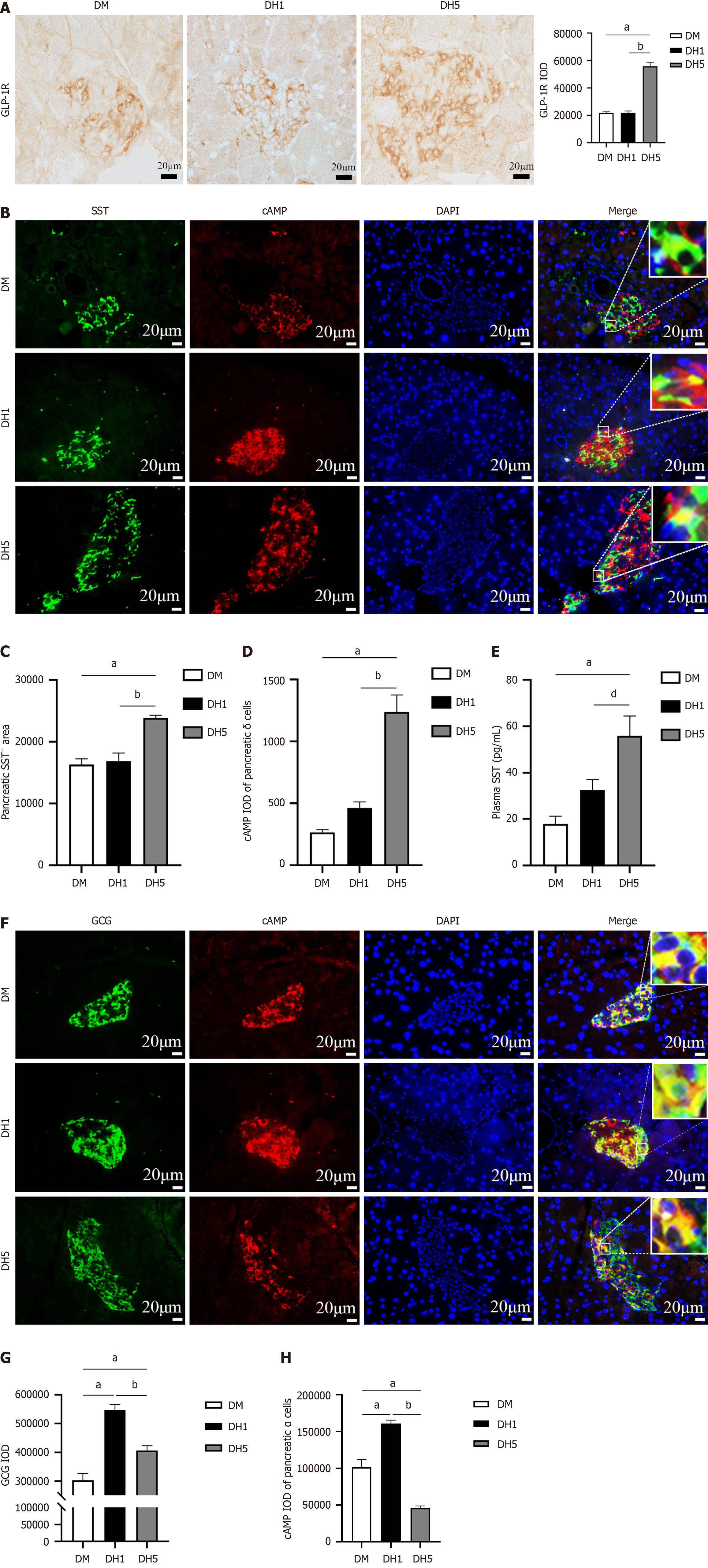
Elevated plasma GLP-1 levels enhance function of pancreatic δ cells through endocrine pathways, leading to suppression of pancreatic α cell secretion: To investigate the endocrine impact of intestinal GLP-1 on the pancreas, the expression of pancreatic GLP-1R was assessed using immunohistochemistry. The DH5 group exhibited significantly greater GLP-1R expression than the DM and DH1 groups (P < 0.01; Figure 4A). Given the scarcity of pancreatic β cells in T1DM mice, GLP-1R is predominantly expressed on pancreatic δ cells. To demonstrate the influence of GLP-1 on δ cells through its receptor, the area of SST-positive cells was measured. Surprisingly, the area of SST+ cells in the pancreas was significantly greater in the DH5 group than in the DM and DH1 groups (P < 0.01; Figure 4B and C). The signaling molecule cAMP plays a crucial role in the secretion of pancreatic SST. To investigate changes in SST secretion in pancreatic δ cells, the cAMP IOD values of these cells were assessed using immunofluorescence. The results revealed a significantly greater cAMP IOD in the pancreatic δ cells in the DH5 group than in the DM and DH1 groups (P < 0.01; Figure 4D). SST secreted by pancreatic δ cells can enter the bloodstream. To further evaluate SST secretion from pancreatic δ cells, the concentration of SST in plasma was measured using ELISA, and the results were consistent with the cAMP findings (Figure 4E). Taken together, these results indicate that the elevated levels of intestinal GLP-1 in DH5 mice enhance SST secretion in pancreatic δ cells through endocrine mechanisms.
SST can inhibit GCG secretion from pancreatic α cells through paracrine effects. Next, the synthesis and secretion of GCG by pancreatic α cells were assessed using immunofluorescence staining and ELISA. The results of immunofluorescence staining revealed a significant difference in the pancreatic GCG IOD between the DH1 and DH5 groups and the DM group. Specifically, the DH5 group exhibited lower GCG levels than the DH1 group (P < 0.01; Figure 4F and G), suggesting a reduction in GCG synthesis in pancreatic α cells of the DH5 mice. Additionally, the cAMP IOD in pancreatic α cells was greatest in the DH1 group but significantly lower in the DH5 group than in the DM and DH1 groups (P < 0.01; Figure 4H). cAMP is a crucial indicator of GCG secretion, suggesting reduced GCG secretion from pancreatic α cells in DH5 mice. Furthermore, plasma GCG levels were significantly decreased in the DH5 group (Figure 3A). These results showed that GCG synthesis and secretion by pancreatic α cells were increased in the DH1 group but decreased in the DH5 group. In summary, the elevated levels of intestinal GLP-1 in the DH5 group may suppress GCG secretion from pancreatic α cells via SST released by pancreatic δ cells through endocrine pathways, leading to impaired hypoglycaemic counterregulatory responses.
Numerous studies have demonstrated that diabetic mice induced by a high dose of STZ injection are commonly utilized as models for T1DM[16,17]. While most research on recurrent hypoglycaemia typically focuses on nondiabetic animals, there have also been reports of recurrent hypoglycaemia in T1DM rats[18-20]. Sankar et al[7] investigated different animal species, onset methods, and nervous system changes during hypoglycaemia, highlighting that recurrent insulin-induced hypoglycaemia in rodents (rats and mice) is the optimal approach for establishing a model of recurrent hypoglycaemia with impaired hypoglycaemic counterregulation. They observed that episodes of hypoglycaemia lasting 30 min or longer, totaling 90 min, can notably alleviate autonomic symptoms, leading to impaired hypoglycaemic counterregulation[7]. In this study, based on previous modeling experiences, male C57BL/6J mice were chosen as model organisms. Blood glucose levels on the third day after intraperitoneal STZ injection increased by ≥ 16.7 mmol/L, remaining consistently above this threshold thereafter, accompanied by a significant reduction in the weight of the mice. These findings strongly indicate the successful establishment of diabetic models.
In this experiment, rapid-acting insulin was administered intraperitoneally to lower blood glucose levels to 3.3 ± 0.5 mmol/L for 60 min, meeting the criteria for diabetic hypoglycaemia with fasting blood glucose levels ≤ 3.9 mmol/L. Previous studies have indicated that experiencing 2-3 episodes of hypoglycaemia per week can result in impaired hypoglycaemic counterregulation[6,18-20]. In this study, a hypoglycaemic frequency of 1 episode every 3 d was utilized for modeling, with the number of hypoglycaemic episodes increasing to 5 instead of the typical 3 reported in previous literature[20]. This adjustment aimed to further solidify and enhance the impaired hypoglycaemic counterregulation, creating a more robust model.
Observations following 3 episodes of hypoglycaemia revealed that DH5 mice exhibited reduced activity, excitement, and foraging behavior during hypoglycaemia. To investigate the potential link between changes in mouse activity in the DH5 group and impaired hypoglycaemic counterregulation, ELISA analyses were performed, which revealed lower levels of plasma adrenaline and pancreatic noradrenaline in the DH5 group than in the DM and DH1 groups. Given that adrenaline plays a significant role in increasing blood glucose levels and that sympathetic axons can stimulate GCG secretion by contacting pancreatic α cells[21,22], the decreased levels of plasma adrenaline and pancreatic noradrenaline in DH5 mice suggest the development of impaired hypoglycaemic counterregulation.
Research has shown that the primary source of endogenous GLP-1 is predominantly L-cells in the terminal ileum and colon, particularly in the terminal colon. GLP-1 secretion is typically low in the fasting and interprandial states but increases rapidly during meal intake, depending on the meal size, and is closely linked to gastric emptying[15,23]. In this study, immunofluorescence and Western blot analyses revealed an increase in intestinal GLP-1 levels in T1DM mice following a single hypoglycaemic episode in the absence of food intake, with a further significant increase observed after multiple hypoglycaemic events. The question arises as to why intestinal GLP-1 levels rise in a fasting state.
Several studies have demonstrated a correlation between hypoglycaemia and oxidative stress[24]. Hypoglycaemia has been found to increase oxidative stress markers in nondiabetic individuals, individuals with diabetes, individuals who have undergone bariatric surgery, and in experimental cell cultures[24-27]. Furthermore, existing knowledge from both experimental and clinical studies indicates that GLP-1 or GLP-1 analogues play a significant role in antioxidant activity, offering therapeutic potential against micro- and macrovascular diabetic complications[28,29]. In this study, we investigated whether the elevated intestinal GLP-1 levels observed in DH1 and DH5 mice are linked to hypoglycaemic oxidative stress or whether they represent the body's protective response to such stress. Further research is needed to address this question.
However, recent research has shown that GLP-1 can serve as a crucial neuromodulator in the body's response to stress. Activation of intestinal GLP-1R leads to c-Fos expression in neurons located in autonomic control regions in the rat brain and adrenal medulla, which in turn promotes adrenal medullary secretion and increases the circulating levels of catecholamines[11,30,31]. The nucleus tractus solitarius (NTS) is identified as a crucial region involved in processing autonomic stress responses in both acute and chronic situations[32]. Through various techniques such as optogenetics and in vivo ganglion imaging, Erika found that the NTS plays a crucial role in processing visceral afferent information and transmitting it to spinal cord nuclei[12]. PPG neurons in the NTS receive monosynaptic input from vagal sensory neurons in the nodose ganglion and act directly on the sympathetic nuclei of the spinal cord to regulate adrenal secretion activity[33-36]. Studies have indicated the presence of GLP-1Rs in vagal afferent fibers and vagal neurons in the lamina propria and submucosa of the gastrointestinal tract[15]. Chemogenetics-mediated activation of GLP-1Rs in vagal afferent fibers can trigger c-Fos expression in NTS neurons, while blocking sympathetic nerve activity can diminish or eliminate the GLP-1R stress response[11,37]. Therefore, Diz-Chaves et al[38] proposed the concept of an intestinal GLP-1-neural reflex pathway, suggesting that intestinal GLP-1 can act on GLP-1R to activate the autonomic nerve and upload signals to the NTS PPG neurons, which in turn relay the signals to the sympathetic nerve, contributing to stress responses. Thus, intestinal GLP-1 has emerged as a critical neuromodulator that enhances the body's stress responses[38].
Hypoglycaemia, a detrimental condition of the body, can induce stress by activating the autonomic sympathetic nervous system[38]. In this study, after inducing stress from a single hypoglycaemic episode, the DH1 group showed higher levels of intestinal GLP-1 expression than the DM group, as evidenced by immunofluorescence and Western blot analysis. Moreover, Western blot analysis also demonstrated a noticeable upwards trend in GLP-1R expression in the intestine. Additionally, ELISA results indicated a significant increase in plasma adrenaline and pancreatic noradrenaline levels in DH1 mice, which exhibited hyperactive and agitated behaviors. These findings imply that increased intestinal GLP-1 levels resulting from hypoglycaemia can initiate an intestinal GLP-1-neural reflex, triggering stress responses and activating the autonomic sympathetic nervous system. To validate the involvement of intestinal GLP-1 in the stress response to hypoglycaemia-induced sympathetic activation, the GLP-1R agonist GLP-1(7-36) or the antagonist exendin (9-39) was infused into the terminal ileum. The findings indicated that GLP-1(7-36) increased plasma adrenaline levels in DH1 mice, while exendin (9-39) had an inhibitory effect, further supporting the role of intestinal GLP-1 in stress responses to hypoglycaemia-induced sympathetic stimulation.
In addition, this study revealed that under conditions of recurrent hypoglycaemia, the expression of intestinal GLP-1 and GLP-1R in DH5 mice was greater than that in the other groups. Interestingly, the plasma adrenaline levels in the DH5 mice were significantly lower, and the mice did not exhibit typical excitement or foraging behavior during hypoglycaemia. These results indicate that intestinal GLP-1 levels continue to rise under recurrent hypoglycaemic stress, while sympathetic excitatory responses are significantly reduced. The discrepancy between single and recurrent hy-poglycaemia may be partially explained by the impact of excessive stress on the adrenal gland, resulting in its hypertrophy and decreased sensitivity. Previous studies have shown that recurrent hypoglycaemic stress can induce adrenal gland hypertrophy and reduce sensitivity[39]. Ma et al[40] showed that a single hypoglycaemic stress can irritate the sympathoadrenal reflex, but recurrent stimulation can decrease adrenaline secretion and increase neuropeptide Y (NPY) secretion from chromaffin cells in the adrenal glands. Additionally, inhibition of NPY or NPY Y1 receptor signaling in adrenal chromaffin cells, either transgenically or pharmacologically, prevented the attenuated adrenaline release induced by recurrent hypoglycaemia[40]. Therefore, it is speculated that excessive intestinal GLP-1 due to recurrent hypoglycaemia may overly stimulate the sympathetic-adrenal reflex, leading to decreased plasma adrenaline levels in DH5 mice. Further experiments using ileum infusions of GLP-1(7-36) or exendin (9-39) in DH5 mice did not reveal a significant impact on adrenaline secretion, further confirming the above speculation. In summary, excessive intestinal GLP-1 due to recurrent hypoglycaemia can lead to overactivation of the sympathoadrenal reflex, resulting in impaired hypoglycaemic sympathetic-adrenal counterregulation.
The major hormones involved in hypoglycaemic counterregulation include adrenaline and GCG. In this study, the plasma GCG levels of the DH5 mice were significantly lower than those of the DH1 mice. To investigate the reason behind this decrease, this study measured plasma adrenaline and pancreatic noradrenaline levels in DH5 mice following terminal ileum infusion of GLP-1(7-36) or exendin (9-39). There were no significant changes in plasma epinephrine or pancreatic norepinephrine levels. Therefore, changes in GCG levels in DH5 mice experiencing recurrent hypoglycaemia are not related to the paracrine effect of intestinal GLP-1, indicating that alterations in GCG levels in DH5 mice are not due to the paracrine effect of intestinal GLP-1.
Previous research has demonstrated that peripheral dipeptidyl peptidase-4 (DPP-IV) degrades intestinal GLP-1, and the remaining undegraded GLP-1 enters the liver through the portal vein, with only approximately 10%-15% entering the systemic bloodstream in the active form to act on pancreatic islets[9,10]. In this study, the DH5 mice exhibited a significant increase in plasma levels of active GLP-1, which was more than 4 times greater than that in the DH1 group, indicating that intestinal GLP-1 has enhanced endocrine effects on DH5 mice. Furthermore, intraperitoneal injection of GLP-1(7-36) or exendin (9-39) in DH5 mice resulted in a decrease in plasma GCG levels following GLP-1(7-36) injection and an increase after exendin (9-39) injection. These findings suggest that GLP-1 can potentially suppress GCG secretion through endocrine pathways in DH5 mice.
To explore the underlying mechanism of GCG reduction in DH5 mice, this study focused on endocrine cells in the pancreas. Given the scarcity of pancreatic β cells in patients with T1DM, more attention has been given to the effect of GLP-1 on pancreatic α and δ cells. Previous research has shown that GLP-1R expression in δ cells is significantly greater than that in α cells, with only approximately 1% of α cells expressing GLP-1R[41]. The increased expression of GLP-1R in the pancreas of the DH5 mice in this experiment indicated an enhanced effect of GLP-1 on pancreatic δ cells. Studies by Ørgaard et al[42] using pancreatic perfusion demonstrated that GLP-1 can boost SST secretion while inhibiting GCG secretion through GLP-1R[42,43]. In contrast, blocking SST receptor 2, which is primarily found in α cells, can abolish the paracrine effect of GLP-1 on GCG secretion. Therefore, it is hypothesized that the suppressive effect of recurrent hypoglycaemia on GCG secretion might be due to the endocrine activity of GLP-1, which promotes SST release from pancreatic δ cells, ultimately leading to the suppression of GCG secretion from pancreatic α cells.
In this study, it was unexpectedly discovered for the first time that the pancreatic δ-cell mass in DH5 mice was significantly greater than that in DM and DH1 mice, indicating that the increase in pancreatic δ cells is additional new evidence of impaired hypoglycaemic counterregulation. However, an increase in pancreatic δ cells may not necessarily improve function. As cAMP is the main signaling molecule that plays a dominant role in SST secretion, this study revealed that the cAMP levels in the pancreatic δ cells of DH5 mice were significantly elevated, accompanied by a noticeable increase in plasma SST levels, indicating a significant increase in SST secretion. Moreover, in the present study, the cAMP levels in pancreatic α cells were reduced in DH5 mice, indicating that GCG secretion in DH5 mice decreased. Overall, the increase in pancreatic δ cells in DH5 mice, under the influence of the oversecretion of GLP-1, enhances SST secretion, which in turn inhibits adjacent pancreatic α cells and decreases their GCG secretion. Ultimately, excessive intestinal GLP-1 induced by recurrent hypoglycaemia may impair the secretion of the counterregulatory hormone GCG during hypoglycaemia.
The NTS can receive signals from the visceral vagus nerve and plays a crucial role in regulating food intake[44]. Rats treated with intravenous GLP-1 demonstrate a reduction in food intake, which is reversed after transection of the subphrenic vagus nerve[45]. Acting as a prandial satiation signal to suppress food intake, intestinal GLP-1 reduces meal size and increases intermeal intervals[15,46]. In addition, recent studies using optogenetic and chemogenetic approaches in transgenic mice have shown that intestinal GLP-1 causes gastric distention and reduces appetite[47]. Intravital measurements of calcium transients have confirmed that ileal myenteric neurons expressing GLP-1 receptors are robustly responsive to GLP-1[47]. By the selective ablation of ileal myenteric neurons, the effects of intestinal GLP-1 can be eliminated, while chemogenetic stimulation of these neurons is sufficient to recapitulate the gastric anorectic effects of GLP-1[47]. Therefore, it is speculated that the decrease in foraging behavior during hypoglycaemia observed in the DH5 mice in this study is related to satiety induced by excessive intestinal GLP-1 and intestinal GLP-1R. The decreased appetite during hypoglycaemia in DH5 mice may be closely related to the activation of the central response through the neural pathway of intestinal GLP-1 and GLP-1 receptors to suppress food intake.
Currently, decreased awareness of hunger during hypoglycaemia in patients is generally believed to be linked to alterations in energy sources within brain neurons. As glucose levels in the brain repeatedly decrease, there is a shift in the energy supply from glucose to lactate or ketones, resulting in reduced motivation to take the initiative to eat[48]. This study, along with related research on the connection between GLP-1 and appetite, provides new experimental evidence on the excessive increase in intestinal GLP-1 and expands on the theoretical understanding of the lack of hunger awareness during hypoglycaemia.
In conclusion, the increased expression of intestinal GLP-1 in the DH5 model of T1DM mice is a crucial factor con-tributing to impaired counterregulatory responses to hypoglycaemia. The administration of GLP-1 or its agonists in T1DM treatment should be carefully monitored to avoid adverse effects. Proper dosing is crucial for effective T1DM therapy, as excessive amounts may worsen the impaired counterregulatory response to hypoglycaemia, leading to decreased appetite and compromised secretion of adrenaline, noradrenaline, and GCG during hypoglycaemia.
The authors thank the staff in Shandong Second Medical University for their assistance in providing animals, sample preparation, and equipment. The authors also thank Dr. Qiang Wang for statistical review of the statistical methods and techniques mentioned in this study.
| 1. | Ratzki-Leewing A, Harris SB, Mequanint S, Reichert SM, Belle Brown J, Black JE, Ryan BL. Real-world crude incidence of hypoglycemia in adults with diabetes: Results of the InHypo-DM Study, Canada. BMJ Open Diabetes Res Care. 2018;6:e000503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Pinés Corrales PJ, Arias Lozano C, Jiménez Martínez C, López Jiménez LM, Sirvent Segovia AE, García Blasco L, Botella Romero F. Prevalence of severe hypoglycemia in a cohort of patients with type 1 diabetes. Endocrinol Diabetes Nutr (Engl Ed). 2021;68:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Lin YK, Agni A, Chuisano S, Fetters MD, Funnell M, Pop-Busui R, DeJonckheere MJ. Patient-Reported Usefulness and Challenges in Using Hypoglycemia-Informing Features of Continuous Glucose Monitors to Manage Hypoglycemia. Sci Diabetes Self Manag Care. 2023;49:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Hölzen L, Schultes B, Meyhöfer SM, Meyhöfer S. Hypoglycemia Unawareness-A Review on Pathophysiology and Clinical Implications. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Amiel SA. The consequences of hypoglycaemia. Diabetologia. 2021;64:963-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Muneer M. Hypoglycaemia. Adv Exp Med Biol. 2021;1307:43-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Sankar A, Khodai T, McNeilly AD, McCrimmon RJ, Luckman SM. Experimental Models of Impaired Hypoglycaemia-Associated Counter-Regulation. Trends Endocrinol Metab. 2020;31:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Davis EM, Sandoval DA. Glucagon-Like Peptide-1: Actions and Influence on Pancreatic Hormone Function. Compr Physiol. 2020;10:577-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Guglielmi V, Sbraccia P. GLP-1 receptor independent pathways: emerging beneficial effects of GLP-1 breakdown products. Eat Weight Disord. 2017;22:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Wang K, Zhang Z, Hang J, Liu J, Guo F, Ding Y, Li M, Nie Q, Lin J, Zhuo Y, Sun L, Luo X, Zhong Q, Ye C, Yun C, Zhang Y, Wang J, Bao R, Pang Y, Wang G, Gonzalez FJ, Lei X, Qiao J, Jiang C. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Science. 2023;381:eadd5787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 13. | Lafferty RA, O'Harte FPM, Irwin N, Gault VA, Flatt PR. Proglucagon-Derived Peptides as Therapeutics. Front Endocrinol (Lausanne). 2021;12:689678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Moore PW, Malone K, VanValkenburg D, Rando LL, Williams BC, Matejowsky HG, Ahmadzadeh S, Shekoohi S, Cornett EM, Kaye AD. GLP-1 Agonists for Weight Loss: Pharmacology and Clinical Implications. Adv Ther. 2023;40:723-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 15. | Krieger JP. Intestinal glucagon-like peptide-1 effects on food intake: Physiological relevance and emerging mechanisms. Peptides. 2020;131:170342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Pan H, Ding Y, Yan N, Nie Y, Li M, Tong L. Trehalose prevents sciatic nerve damage to and apoptosis of Schwann cells of streptozotocin-induced diabetic C57BL/6J mice. Biomed Pharmacother. 2018;105:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Cao M, Peng Y, Lu Y, Zou Z, Chen J, Bottino R, Knoll M, Zhang H, Lin S, Pu Z, Sun L, Fang Z, Qiu C, Dai Y, Cai Z, Mou L. Controls of Hyperglycemia Improves Dysregulated Microbiota in Diabetic Mice. Transplantation. 2021;105:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes. 2006;55:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes. 2008;57:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | McNeilly AD, Gallagher JR, Dinkova-Kostova AT, Hayes JD, Sharkey J, Ashford ML, McCrimmon RJ. Nrf2-Mediated Neuroprotection Against Recurrent Hypoglycemia Is Insufficient to Prevent Cognitive Impairment in a Rodent Model of Type 1 Diabetes. Diabetes. 2016;65:3151-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Krivova YS, Proshchina AE, Otlyga DA, Leonova OG, Saveliev SV. Prenatal development of sympathetic innervation of the human pancreas. Ann Anat. 2022;240:151880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Tang SC, Peng SJ, Chien HJ. Imaging of the islet neural network. Diabetes Obes Metab. 2014;16 Suppl 1:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Maselli DB, Camilleri M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv Exp Med Biol. 2021;1307:171-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 24. | Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J Diabetes Res. 2020;2020:7489795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 25. | Kahal H, Halama A, Aburima A, Bhagwat AM, Butler AE, Graumann J, Suhre K, Sathyapalan T, Atkin SL. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci Rep. 2020;10:4750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 27. | Lupoli R, Calcaterra I, Annunziata G, Tenore G, Rainone C, Schiavo L, Capaldo B, Di Minno MND. Post-Bariatric Hypoglycemia Is Associated with Endothelial Dysfunction and Increased Oxidative Stress. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Park B, Bakbak E, Teoh H, Krishnaraj A, Dennis F, Quan A, Rotstein OD, Butler J, Hess DA, Verma S. GLP-1 receptor agonists and atherosclerosis protection: the vascular endothelium takes center stage. Am J Physiol Heart Circ Physiol. 2024;326:H1159-H1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 29. | Luna-Marco C, de Marañon AM, Hermo-Argibay A, Rodriguez-Hernandez Y, Hermenejildo J, Fernandez-Reyes M, Apostolova N, Vila J, Sola E, Morillas C, Rovira-Llopis S, Rocha M, Victor VM. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023;66:102849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Parker JA, McCullough KA, Field BC, Minnion JS, Martin NM, Ghatei MA, Bloom SR. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes (Lond). 2013;37:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Smits MM, Tonneijck L, Muskiet MH, Hoekstra T, Kramer MH, Diamant M, van Raalte DH. Heart rate acceleration with GLP-1 receptor agonists in type 2 diabetes patients: an acute and 12-week randomised, double-blind, placebo-controlled trial. Eur J Endocrinol. 2017;176:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2503] [Cited by in RCA: 2221] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 33. | Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes. 2010;59:1890-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, Madisen L, Zeng H, Krasnow MA, Knight ZA. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell. 2019;179:1129-1143.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 314] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 35. | Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol. 2009;587:5613-5631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Llewellyn-Smith IJ, Marina N, Manton RN, Reimann F, Gribble FM, Trapp S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience. 2015;284:872-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1373] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 38. | Diz-Chaves Y, Herrera-Pérez S, González-Matías LC, Lamas JA, Mallo F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, Vigo E, Brubaker PL, González-Matías LC, Mallo F. Effects of prolonged exendin-4 administration on hypothalamic-pituitary-adrenal axis activity and water balance. Am J Physiol Endocrinol Metab. 2013;304:E1105-E1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Ma Y, Wang Q, Joe D, Wang M, Whim MD. Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity. J Clin Invest. 2018;128:3866-3871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 42. | Ørgaard A, Holst JJ. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017;60:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Farhat R, Aiken J, D'Souza NC, Appadurai D, Hull G, Simonson E, Liggins RT, Riddell MC, Chan O. ZT-01: A novel somatostatin receptor 2 antagonist for restoring the glucagon response to hypoglycaemia in type 1 diabetes. Diabetes Obes Metab. 2022;24:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016;310:R885-R895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 45. | Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, Afaghani MH, Lee SJ, Scott K, Maske C, Langhans W, Krause E, de Kloet A, Gribble FM, Reimann F, Rinaman L, de Lartigue G, Trapp S. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3:258-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 46. | Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 47. | Zhang T, Perkins MH, Chang H, Han W, de Araujo IE. An inter-organ neural circuit for appetite suppression. Cell. 2022;185:2478-2494.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 48. | Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev. 2019;40:768-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |