Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1726
Revised: June 7, 2024
Accepted: July 10, 2024
Published online: August 15, 2024
Processing time: 123 Days and 1.1 Hours
The prevalence of pelvic organ prolapse (POP) increases with age and parity. Specifically, the prevalence of POP among women aged 20 to 39 is 9.7%, while it rises to 49% among women over 80 years old. Additionally, as the number of deliveries increases, the prevalence of POP also rises accordingly, with a rate of 12.8% for women with one delivery history, 18.7% for those with two deliveries, and 24.6% for women with three or more deliveries. It causes immense suffering for pregnant women.
To evaluate the relationship between the levator ani muscle’s hiatus (LH) area and POP in patients with gestational diabetes mellitus (GDM) using perineal ultra-sound.
The study cohort comprised 104 patients aged 29.8 ± 3.7 years who sought medical care at our institution between January 2021 and June 2023. All were singleton pregnancies consisting of 75 primiparas and 29 multiparas, with an average parity of 1.7 ± 0.5. According to the POP diagnostic criteria, the 104 subjects were divided into two groups with 52 members each: POP group (patients with GDM combined with POP) and non-POP group (patients with GDM without POP). Perineal ultrasound was used to measure differences in the anteroposterior diameter, transverse diameter, and LH area. Receiver operating characteristic curves were drawn to determine the optimal cutoff values for the LH anteroposterior diameter, transverse diameter, and area for diagnosing POP.
Statistically significant increase in the LH area, anteroposterior diameter, and lateral diameter were observed in the POP group compared with the non-POP group (P < 0.05). Both groups exhibited markedly elevated incidence rates of macrosomia and stress urinary incontinence. For the POP group, the area under the curve (AUC) for the LH area was 0.906 with a 95% confidence interval (CI): 0.824-0.988. The optimal cutoff was 13.54cm², demonstrating a sensitivity of 83.2% and a specificity of 64.4%. The AUC for the anteroposterior diameter reached 0.836 with a 95%CI: 0.729-0.943. The optimal cutoff was 5.53 cm with a sensitivity of 64.2% and a specificity of 73.4%. For the lateral diameter, its AUC was 0.568 with a 95%CI: 0.407-0.729. The optimal cutoff was 4.67 cm, displaying a sensitivity of 65.9% and a specificity of 69.3%. Logistic regression analysis unveiled that age, body weight, number of childbirths, total number of pregnancies, and gestational weight gain constituted the independent risk factors for the cooccurrence of GDM and POP.
Three-dimensional perineal ultrasonography of LH size and shape changes can effectively diagnose POP. Age, weight, number of births, number of pregnancies, and weight gain during pregnancy are independent risk factors affecting the cooccurrence of GDM and POP. GDM can increase the LH area in patients, and an enlarged LH leads to an increased incidence of POP.
Core Tip: This study aimed to evaluate the relationship between the levator ani muscle’s hiatus (LH) area and pelvic organ prolapse (POP) in patients with gestational diabetes mellitus using perineal ultrasound. Conclusion: Three-dimensional perineal ultrasonography of LH size and shape changes can effectively diagnose POP.
- Citation: Wang QH, Liu LH, Ying H, Chen MX, Zhou CJ, Li H. Clinically significant changes in anal sphincter hiatal area in patients with gestational diabetes mellitus and pelvic organ prolapse. World J Diabetes 2024; 15(8): 1726-1733
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1726.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1726
The levator ani muscle is the most robust layer of the female pelvic floor and plays the most important supportive role[1]. Each side of the levator ani muscle is composed of three parts: Pubococcygeus, iliococcygeus, and ischiococcygeus. The fibers of the pubococcygeus on both sides extend posteriorly, downward, and inward to form an elliptical gap known as the “levator ani muscle’s hiatus” (LH). The LH is the herniation pathway for organ prolapse, with the urethra, vagina, and rectum sequentially passing through it from front to back. To a certain extent, the size of the LH can reflect the support provided by the pelvic floor structure to the pelvic organs[2,3].
Gestational diabetes mellitus (GDM) is a disease unique to female pregnancy and its incidence shows an annually increasing trend[4,5]. GDM increases the incidence of complications in mothers and fetuses and the risk of pelvic floor functional disorders in patients. It is one of the high-risk factors affecting female pelvic floor function[6,7]. Inadequate glycemic control escalates the likelihood of substantial weight gain among expectant mothers and the probability of giving birth to infants with a birth weight above average for their gestational age, thereby imposing pronounced stress on the integrity of the pelvic floor structure. Furthermore, suboptimal glycemic management may result in significant diminishments in the levator ani muscle's thickness, constrictions in ligamentous width, and a diminution in collagen concentration within both the pelvic floor and the rectus abdominis muscular fibers, resulting in varying degrees of muscle weakness[8,9]. The incidence of pelvic organ prolapse (POP) is steadily rising, making it a significant health burden for women worldwide[10]. In patients with POP, the pelvic support structures are damaged and their supporting strength is weakened, leading to the displacement of the uterus, bladder, vagina, and surrounding tissues from their normal anatomical positions and causing damage to the functional state of these pelvic organs. This condition poses a considerable threat to the female reproductive system and affects the patients’ psychological health and quality of life. In this study, a comparative analysis was conducted to investigate the correlation between the LH area and POP in individuals diagnosed with GDM. By utilizing transperineal ultrasound as the primary diagnostic tool, 52 patients with GDM and concurrent POP were compared with another group of 52 patients diagnosed with GDM but without POP. The findings are presented below.
This study was approved by the Ethics Committee of People's Hospital Affiliated to Shandong First Medical University, who agreed to waive the informed consent. A total of 104 patients who sought medical care at our institution between January 2021 and June 2023 were meticulously chosen as the study cohort. Their ages spanned 21-42 years with a mean age of 29.8 ± 3.7 years. All the participants experienced singleton pregnancies. Among them, 75 were primiparous and 29 had previously given birth, resulting in an average parity of 1.7 ± 0.5. According to the diagnostic criteria for POP, the participants were categorically divided into two distinct groups with 52 individuals each: POP group and non-POP group. The inclusion criteria were as follows: (1) Met the diagnostic criteria for GDM; (2) Met the diagnostic criteria for POP according to “Practical Pelvic Floor Ultrasonography”; (3) No history of POP before pregnancy; (4) No history of chronic cough or urinary incontinence before pregnancy; and (5) Was informed and voluntarily joined the study. An oral glucose tolerance test was conducted between 23 and 25 weeks of pregnancy and involved the intake of 250-300 mL of sugar water containing 75 g of glucose powder. The normal blood glucose levels at fasting and 1 and 2 hours post consumption were < 5.1, 10.0, and 8.5 mmol/L, respectively. A diagnosis of GDM was made if any blood glucose value reached or exceeded these standards. The exclusion criteria were as follows: (1) Not meeting the diagnostic criteria for GDM; (2) Preexisting diabetes before pregnancy; and (3) History of chronic cough or urinary incontinence before pregnancy. No statistically significant distinctions in the demographic characteristics were found between the two cohorts of patients (P > 0.05), affirming their comparability. For detailed data, please refer to Tables 1 and 2.
| Group | Height (cm) | Age (years) | Weight (kg) | Primiparas (cases) | Multiparas (cases) | Pregnant once (cases) | Pregnant ≥ 2 times (cases) | Weight gain during pregnancy (kg) |
| POP group (n = 52) | 163.74 ± 6.11 | 28.28 ± 3.15 | 59.88 ± 8.41 | 39 | 13 | 35 | 17 | 20.05 ± 2.78 |
| Non-POP group (n = 52) | 165.39 ± 6.53 | 28.36 ± 2.64 | 58.44 ± 9.59 | 36 | 16 | 37 | 15 | 19.98 ± 2.59 |
| Group | Number | LH area (cm²) | LH anteroposterior diameter (cm) | LH lateral diameter (cm) |
| POP group | 52 | 14.94 ± 1.58 | 6.69 ± 0.72 | 4.18 ± 0.56 |
| Non-POP group | 52 | 13.65 ± 0.97 | 5.15 ± 0.45 | 4.04 ± 0.17 |
Detection method: Following enrollment into the study, the patients in the POP group underwent POP Quantification (POP-Q) staging[11]. All patients were subjected to fasting ultrasonography. The GE Voluson E8 color Doppler ultrasound diagnostic system with a RAB4-8-D three-dimensional (3D) volume probe was utilized at a frequency of 2-8 MHz. Prior to the examination, the patients were instructed to empty their rectum and bladder (with appropriate bladder filling, residual urine < 50 mL) and then assisted into the lithotomy position. A disposable condom was used to cover the probe, which was then placed at the perineum for two-dimensional ultrasound imaging. This imaging captured the mid-sagittal plane of the patient’s pelvic floor, measuring the position and movement of pelvic organs, checking for funnel formation at the internal urethral orifice, and assessing the openness of the posterior angle of the bladder and urethra. Afterward, the 3D imaging acquisition mode was activated to capture standard 3D volume images for the LH. After image collection, a four-dimensional volume offline analysis software was used for postprocessing and data analysis. All ultrasound examinations were conducted by the same physician with extensive clinical experience in the relevant department.
Observational indicators: The anteroposterior and transverse dimensions of the LH in the patients were quantified by utilizing the two-point distance method, and the LH area was determined by employing the area tracing method.
POP assessment: According to the POP-Q staging criteria[12], prolapse was categorized into stage 0, I-II (mild prolapse), and III-IV (severe prolapse). The stage of prolapse for each parturient was recorded based on the organ with the maximum prolapse.
All data were subjected to rigorous statistical analysis using SPSS software version 26.0. Quantitative data were presented as mean ± SD. Prior to intergroup comparisons, assessments for normality and homogeneity of variances were executed. For group comparisons, independent samples t-tests were employed, and statistical significance was denoted by P < 0.05. Receiver operating characteristic (ROC) curves were meticulously constructed to delineate the incidence rates of POP within both cohorts and scrutinize its correlation with the anteroposterior and lateral dimensions of the LH. The area under the curve (AUC) was also computed to quantify these associations.
Comparison was performed between the two study groups. The following notable findings were observed in the POP group: 47 cases of stress urinary incontinence, 36 cases of bladder prolapse, 12 cases of uterine prolapse, 4 cases of rectal prolapse, 23 cases of macrosomia, and 35 cases of diminished pelvic floor muscle strength. Meanwhile, the non-POP group exhibited 45 instances of stress urinary incontinence, 27 cases of macrosomia, and 35 cases of reduced pelvic floor muscle strength. The LH area, anteroposterior diameter, and lateral diameter in the POP group were significantly enlarged compared with those in the non-POP group (P < 0.05). In particular, the AUC for the LH area within the POP group was 0.906 with a 95%CI: 0.824-0.988. The optimal cutoff was 13.54 cm², showcasing a sensitivity of 83.2% and a specificity of 64.4%. The AUC for the anteroposterior diameter of the LH reached 0.836 with a 95%CI: 0.729-0.943. The optimal cutoff was 5.53 cm, displaying a sensitivity of 64.2% and a specificity of 73.4%. For the lateral diameter of the LH, the AUC was 0.568 with a 95%CI: 0.407-0.729. The optimal cutoff was at 4.67 cm, exhibiting a sensitivity of 65.9% and a specificity of 69.3%. During examinations, attention should be paid to discerning the occurrence of POP. Logistic regression analysis indicated that age, weight, number of births, number of pregnancies, and weight gain during pregnancy are independent risk factors for the cooccurrence of GDM and POP. The incidence rates of macrosomia and stress urinary incontinence were relatively high in both groups, indicating that GDM can increase the LH area. An enlarged LH increases the incidence of POP and the risk of pelvic organ dysfunction and fetal complications.
In the non-POP group, the LH appeared diamond-shaped with a compact and orderly internal structure. In the POP group, the LH morphology was primarily diamond shape, “V”-shape, or “U”-shape with a loosely arranged and disordered internal structure. The anteroposterior diameter, transverse diameter, and area of the LH in the POP group were significantly larger than those in the control group.
The LH in the POP group had an area of 14.94 ± 1.58 cm², anteroposterior diameter of 6.69 ± 0.72 cm, and lateral diameter of 4.18 ± 0.56 cm, all of which were significantly greater than those in the non-POP group (13.65 ± 0.97, 5.15 ± 0.45, and 4.04 ± 0.17 cm, respectively; P < 0.05) as indicated in Table 2.
Among the 52 patients in the POP group, 35 (34.61%) were in POP-Q stage I, 8 (15.38%) in stage II, 7 (13.46%) in stage III, and 2 (3.84%) in stage IV. The LH area, anteroposterior diameter, and lateral diameter for each stage are presented in Table 3.
| POP-Q stage | Number | LH area (cm²) | LH anteroposterior diameter (cm) | LH lateral diameter (cm) |
| I | 35 | 14.58 ± 0.54 | 5.86 ± 0.41 | 3.57 ± 0.55 |
| II | 8 | 15.73 ± 0.50 | 6.86 ± 0.62 | 4.28 ± 0.23 |
| III | 7 | 16.23 ± 0.29 | 7.23 ± 0.18 | 4.75 ± 0.11 |
| IV | 0 | 0 | 0 | 0 |
In the POP group of 52 patients, 23 (44.23%) had macrosomia and 47 (90.38%) showed stress urinary incontinence. In the non-POP group of 52 patients, 27 (52.93%) had macrosomia and 45 (86.54%) showed stress urinary incontinence. However, the disparity was not significant (P > 0.05) as illustrated in Table 4.
| Group | Number | Macrosomia | Stress urinary incontinence | Pelvic floor muscle weakness |
| POP group | 52 | 23 (44.23) | 47 (90.38) | 35 (67.31) |
| Non-POP Group | 52 | 27 (52.93) | 45 (86.54) | 26 (50.00) |
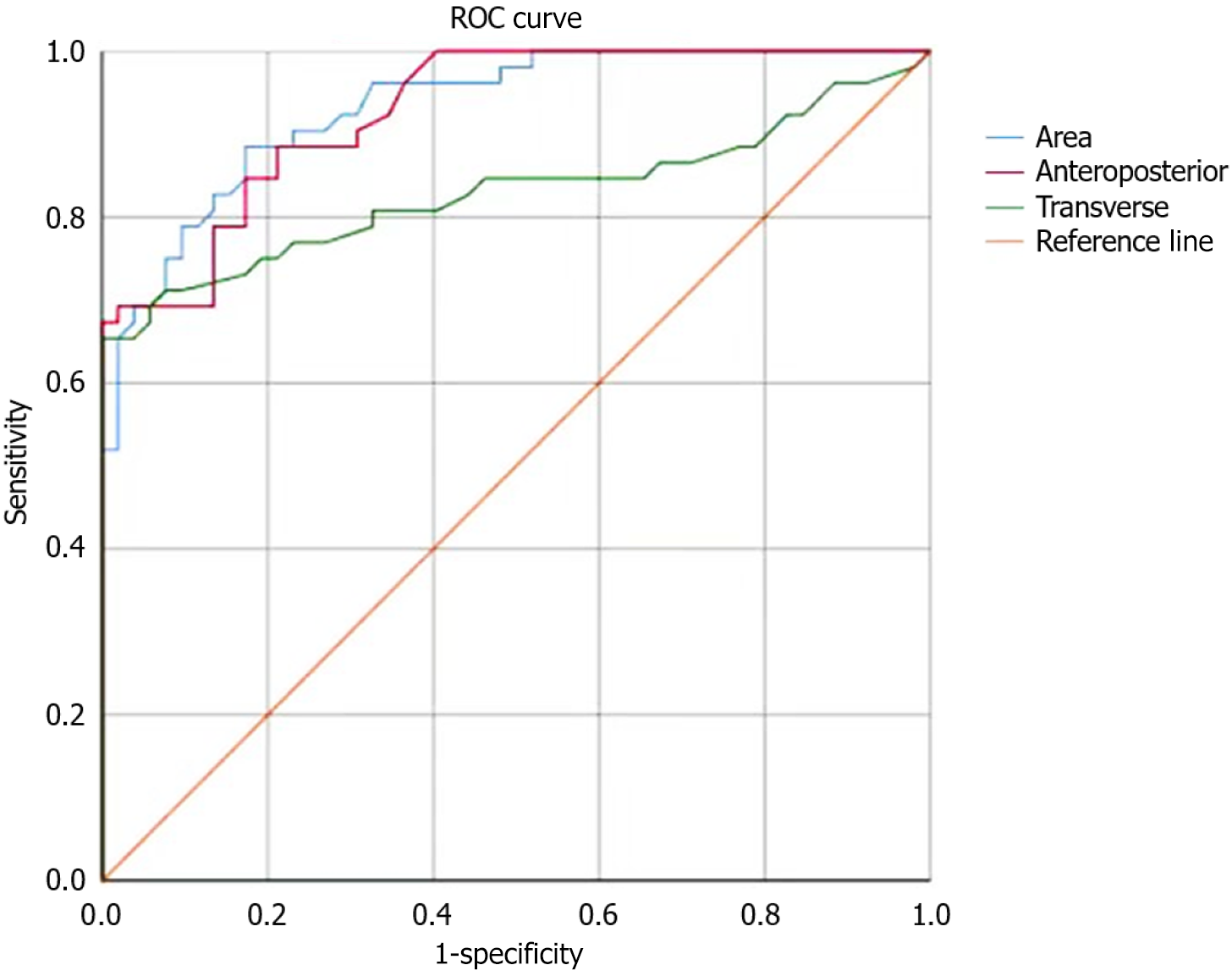
In the POP group, the LH area yielded an AUC of 0.906 with a 95%CI: 0.824-0.988. The optimal cutoff was 13.54 cm², with an associated sensitivity of 83.2% and specificity of 64.4%. For the anteroposterior diameter of the LH in the same group, the AUC was 0.836 with a 95%CI: 0.729-0.943. The optimal cutoff was 5.53 cm, demonstrating a sensitivity of 64.2% and a specificity of 73.4%. For the lateral diameter of the LH within the POP group, the AUC was 0.568 with a 95%CI: 0.407-0.729. The optimal cutoff was 4.67 cm, with a sensitivity of 65.9% and a specificity of 69.3%. These significant findings are visually represented in Figure 1.
Logistic regression analysis revealed that age, weight, number of births, number of pregnancies, and weight gain during pregnancy are independent risk factors influencing the cooccurrence of GDM and POP as shown in Table 5.
| Factor | β | SE | Wald statistic | 95%CI |
| Age | 3.005 | 0.570 | 4.698 | 1.542-14.399 |
| Weight | 2.819 | 0.799 | 6.112 | 1.778-40.774 |
| Parity (number of births) | 1.052 | 0.501 | 3.301 | 1.737-4.721 |
| Gravidity (number of pregnancies) | 2.318 | 0.452 | 3.686 | 2.467-3.741 |
| Gestational weight gain | 1.401 | 0.672 | 3.500 | 3.418-3.895 |
GDM is a distinct condition among pregnant women, occurring exclusively during pregnancy. Its incidence has gradually increased in recent years. According to literature, the incidence of GDM in the Asian population is approximately 11.5%[13]. Poor glycemic control during pregnancy heightens the risk of complications for the mother and fetus and the likelihood of macrosomia. The majority of women with GDM experience weight gain. Gomes et al[14] showed that maternal overweight or obesity can lead to an increased risk of the same condition in children. Skrypnik et al[15] argued that persistent hyperglycemia in utero raises the risk of macrosomia, resulting in severe strain on the maternal pelvic floor. Pinheiro et al[16] found that women with GDM experience varying degrees of muscle weakness in the pelvic floor and rectus abdominis during pregnancy, along with a reduction in type I/II collagen in fast and slow muscle fibers. Marini et al[17] noted a significant increase in the incidence of urinary incontinence 2 years post-cesarean section in women with GDM; this phenomenon can be attributed to long-term hyperglycemia damaging the extracellular matrix and striated muscles of the urethra. A retrospective study by Sangsawang[18] suggested that the most common type of urinary incontinence during pregnancy is stress urinary incontinence. Physiological changes during pregnancy, such as the increased weight of the uterus and fetus, exert pressure on the pelvic floor muscles. GDM exacerbates this risk by increasing the weight of the uterus and fetus, thus raising the incidence of POP in women.
POP has a high incidence rate. In our study involving 104 patients, the POP group had significantly larger LH area and anteroposterior and transverse diameters than the non-POP group. In addition, the number of women with stress urinary incontinence in both groups was higher than the general incidence rate[19]. The levator ani muscle, a major muscle of the pelvic floor, attaches bilaterally to the inner side of the pelvic wall, is symmetrically arranged, and forms a downward funnel shape. It is divided into two parts based on the arrangement of its muscle fibers: Iliococcygeus and pubococcygeus. Both are integral to the levator ani and play a supportive role in maintaining the normal position of pelvic organs. The LH, formed by the bilateral levator ani and the pubic bone in front, is the largest portal in the peritoneum and a primary pathway for the descent of pelvic organs. The integrity of the levator ani and the morphology of the LH reflect the position and structural changes of the pelvic organs. An increased LH size is a significant factor in the increased risk of POP. This study compared the size of the LH area in women with GDM with or without POP using perineal ultrasonography. The findings revealed that the anteroposterior diameter, transverse diameter, and area of LH were significantly enlarged in the POP group (P < 0.05). A possible reason is the prolonged high glucose state during pregnancy that affects the uterine environment and increases the fetal weight, consequently stretching the pelvic floor muscles and enlarging the LH. As a result, the incidence of POP increases.
This study established cutoff values for the LH area and anteroposterior and lateral diameters for the early screening and treatment of POP in GDM using ROC curve analysis. In the POP group, the LH area demonstrated an AUC of 0.906 with a 95%CI: 0.824-0.988. The optimal cutoff was 13.54 cm², showcasing a sensitivity of 83.2% and a specificity of 64.4%. For the anteroposterior diameter, the AUC was 0.836 with a 95%CI: 0.729-0.943. The optimal cutoff was 5.53 cm, revealing a sensitivity of 64.2% and a specificity of 73.4%. For the lateral diameter, the calculated AUC was 0.568 with a 95%CI: 0.407-0.729. The optimal cutoff was 4.67 cm, displaying a sensitivity of 65.9% and a specificity of 69.3%. Dietz[20] suggested that an LH area exceeding 25 cm² serves as a diagnostic criterion for abnormal expansion, which is contradictory with our study findings. This discrepancy may be attributed to potential variations in race and geographical region. Logistic regression analysis showed that age, weight, number of births, number of pregnancies, and weight gained during pregnancy are independent risk factors for the cooccurrence of GDM and POP.
Women diagnosed with GDM face an elevated probability of experiencing diminished pelvic floor muscle strength, POP, postpartum stress urinary incontinence, and macrosomia subsequent to childbirth. GDM stands as a substantial risk factor for the development of postpartum pelvic floor dysfunction.
| 1. | Eickmeyer SM. Anatomy and Physiology of the Pelvic Floor. Phys Med Rehabil Clin N Am. 2017;28:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (68)] |
| 2. | Siahkal SF, Iravani M, Mohaghegh Z, Sharifipour F, Zahedian M, Nasab MB. Investigating the association of the dimensions of genital hiatus and levator hiatus with pelvic organ prolapse: a systematic review. Int Urogynecol J. 2021;32:2095-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (50)] |
| 3. | Schulten SFM, Claas-Quax MJ, Weemhoff M, van Eijndhoven HW, van Leijsen SA, Vergeldt TF, IntHout J, Kluivers KB. Risk factors for primary pelvic organ prolapse and prolapse recurrence: an updated systematic review and meta-analysis. Am J Obstet Gynecol. 2022;227:192-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (67)] |
| 4. | Prudencio CB, Rudge MVC, Pinheiro FA, Sartorão Filho CI, Nunes SK, Pedroni CR, Junginger B, Barbosa AMP. Negative impact of gestational diabetes mellitus on progress of pelvic floor muscle electromyography activity: Cohort study. PLoS One. 2019;14:e0223261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (68)] |
| 5. | Phelan S, Kanaya AM, Ma Y, Vittinghoff E, Barrett-Connor E, Wing R, Kusek JW, Orchard TJ, Crandall JP, Montez MG, Brown JS; Diabetes Prevention Program Research Group. Long-term prevalence and predictors of urinary incontinence among women in the Diabetes Prevention Program Outcomes Study. Int J Urol. 2015;22:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (73)] |
| 6. | Figueiredo VB, Nascimento SL, Martínez RFL, Lima CTS, Ferreira CHJ, Driusso P. Effects of individual pelvic floor muscle training vs individual training progressing to group training vs group training alone in women with stress urinary incontinence: A randomized clinical trial. Neurourol Urodyn. 2020;39:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Nazzal Z, Khatib B, Al-Quqa B, Abu-Taha L, Jaradat A. The prevalence and risk factors of urinary incontinence amongst Palestinian women with type 2 diabetes mellitus: A cross-sectional study. Arab J Urol. 2020;18:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Sartorão Filho CI, Pinheiro FA, Prudencio CB, Nunes SK, Takano L, Enriquez EMA, Orlandi MIG, Junginger B, Hallur RLS, Rudge MVC, Barbosa AMP. Impact of gestational diabetes on pelvic floor: A prospective cohort study with three-dimensional ultrasound during two-time points in pregnancy. Neurourol Urodyn. 2020;39:2329-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Rudge MVC, Souza FP, Abbade JF, Hallur RLS, Marcondes JPC, Piculo F, Marini G, Vesentini G, Thabane L, Witkin SS, Calderon IMP, Barbosa AMP; Diamater Study Group, Rudge MV, Barbosa AMP, Calderon IMP, Souza FP, Abbade JF, Hallur LSR, Piculo F, Marini G, Vesentini G, Thabane L, Palma MS, Graeff CFO, Arni RK, Herculano RD, Salvadori DF, Mateus S, Dal Pai Silva M, Magalhães CG, Costa RA, Lima SAM, Felisbino SL, Barbosa W, Atallah A, Girão MJB, Di Bella Z, Uchoa SM, Payão S, Hijas A, Berghman B, De Bie R, Sobrevia L, Junginger B, Alves FCB, Rossignoli PS, Prudencio CB, Orlandi MIG, Gonçalves MI, Nunes SK, Catinelli BB, Quiroz S, Sarmento BV, Pinheiro FA, Sartorão CI, Lucas RR, Reyes DRA, Quiroz SBCV, Enriquez EMA, Oliveira RG, Floriano JF, Marcondes JPC, Barneze S, Dangió TD, Pascon T, Rossignoli P, Freitas JV, Takano L, Reis F, Caldeirão TD, Fernandes JN, Carr AM, Gaitero MVC, Corrente JE, Nunes HRC, Candido AF, Costa SMB, Dangió TD, Pascon T, Melo JVF, Takano L, Reis FVDS, Caldeirão TD, Carr AM, Garcia GA, Rabadan GB, Bassin HCM, Suyama KS, Damasceno LN, Takemoto MLS, Menezes MD, Bussaneli DG, Nogueira VKC, Lima PR, Lourenço IO, Marostica de Sá J, Megid RA, Caruso IP, Rasmussen LT, Prata GM, Piculo F, Vesentini G, Arantes MA, Ferraz GAR, Camargo LP, Kron MR, Corrente JE, Nunes HRC. Study protocol to investigate biomolecular muscle profile as predictors of long-term urinary incontinence in women with gestational diabetes mellitus. BMC Pregnancy Childbirth. 2020;20:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Souza HDC, Pires LMT, Vieira GC, Castro EAB, Moura EA, Engelmann J, Fonseca DS. Prevalence of pelvic floor disorders and the associated quality of life among institutionalized and noninstitutionalized elderly women: A cross-sectional study. Curr Urol. 2023;17:184-187. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Mendes LC, Bezerra LRPS, Bilhar APM, Neto JAV, Vasconcelos CTM, Saboia DM, Karbage SAL. Symptomatic and anatomic improvement of pelvic organ prolapse in vaginal pessary users. Int Urogynecol J. 2021;32:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Madhu C, Swift S, Moloney-Geany S, Drake MJ. How to use the Pelvic Organ Prolapse Quantification (POP-Q) system? Neurourol Urodyn. 2018;37:S39-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, Wan Sulaiman WA, Suppiah S, Mohamed MH, Veettil SK. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 14. | Gomes D, Le L, Perschbacher S, Haas NA, Netz H, Hasbargen U, Delius M, Lange K, Nennstiel U, Roscher AA, Mansmann U, Ensenauer R. Predicting the earliest deviation in weight gain in the course towards manifest overweight in offspring exposed to obesity in pregnancy: a longitudinal cohort study. BMC Med. 2022;20:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Skrypnik D, Bogdański P, Zawiejska A, Wender-Ożegowska E. Role of gestational weight gain, gestational diabetes, breastfeeding, and hypertension in mother-to-child obesity transmission. Pol Arch Intern Med. 2019;129:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Pinheiro FA, Sartorão Filho CI, Prudencio CB, Nunes SK, Pascon T, Hallur RLS, Takano L, Enriquez EMA, Catinelli BB, Carr AM, Junginger B, Rudge MVC, Barbosa AMP; Diamater Study Group. Pelvic floor muscle dysfunction at 3D transperineal ultrasound in maternal exposure to gestational diabetes mellitus: A prospective cohort study during pregnancy. Neurourol Urodyn. 2022;41:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Marini G, Rinaldi Jde C, Damasceno DC, Felisbino SL, Rudge MV. [Changes in the extracellular matrix due to diabetes and their impact on urinary continence]. Rev Bras Ginecol Obstet. 2014;36:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sangsawang B. Risk factors for the development of stress urinary incontinence during pregnancy in primigravidae: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2014;178:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Dietz HP. Ultrasound in the assessment of pelvic organ prolapse. Best Pract Res Clin Obstet Gynaecol. 2019;54:12-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |