Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1683
Revised: May 14, 2024
Accepted: June 7, 2024
Published online: August 15, 2024
Processing time: 114 Days and 0 Hours
In this editorial, we discuss the recent article by Zhao et al published in the World Journal of Diabetes, which highlights the importance of recognizing the risk indicators associated with diabetes mellitus (DM). Given the severe implications of healthcare-associated infections (HAIs) in hospitalized individuals- such as heightened mortality rates, prolonged hospitalizations, and increased costs- we focus on elucidating the connection between DM and nosocomial infections. Diabetic patients are susceptible to pathogenic bacterial invasion and subsequent infection, with some already harboring co-infections upon admission. Notably, DM is an important risk factor for nosocomial urinary tract infections and surgical site infections, which may indirectly affect the occurrence of nosocomial blood
Core Tip: Diabetes mellitus (DM) is an important risk factor for nosocomial urinary tract infections and surgical site infections, which may indirectly affect the occurrence of nosocomial bloodstream infections, especially in DM patients with poor glycemic control. Diabetic patients should therefore pay more attention to the prevention of healthcare-associated infections, with a focus on the management of their blood glucose level.
- Citation: Yu XL, Zhou LY, Huang X, Li XY, Pan QQ, Wang MK, Yang JS. Urgent call for attention to diabetes-associated hospital infections. World J Diabetes 2024; 15(8): 1683-1691
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1683.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1683
Originally denoting infections linked to hospitalization (formerly termed nosocomial infections)[1], healthcare-associated infections (HAIs) now encompass infections occurring in hospital environments among patients or medical personnel. This includes infections emerging during hospitalization, as well as those arising within hospitals and after discharge (e.g. 48 hours post-hospitalization, within 30 days after receiving health care, or up to 90 days after undergoing certain surgical procedures), excluding infections existing pre-admission or present upon admission[2]. The prevalence of HAIs ranges from 3%-10%[3], presenting a significant challenge to healthcare workers (HCWs). HAIs can affect the treatment of primary diseases, increasing mortality and disability rates, as well as escalating the economic burden on patients' families and society, thereby straining healthcare systems worldwide, especially in developing countries[4]. HAIs yield worse outcomes than community-acquired infections, attributed to the compromised health status of hospitalized patients and the higher prevalence of multidrug-resistant (MDR) bacteria in hospital settings compared to community settings[5]. Severe HAIs often induce sepsis, estimated at 48.9 million cases globally in 2017 and remaining a leading cause of mortality worldwide[6]. In the recent issue of the World Journal of Diabetes, Zhao et al[7] presented an insightful article. Through an analysis of diabetes mellitus (DM) prevalence and risk factors among the elderly, the authors proposed that heightened attention to the abnormalities of related risk signs can aid in improving their overall health. This prompts consideration of HAI management in hospitalized patients with DM, given their higher risk of infection compared to the general population.
The microenvironment shaped by DM triggers molecular changes in key components of the defense system, including neutrophils, natural killer cells, and macrophages, thereby affecting innate immunity[8]. Concurrently, hyperglycemia disrupts cytokine equilibrium, inhibiting the adaptive immune response against invading pathogens, and further increasing the susceptibility of diabetic patients to microbial infections[8]. Compared to the general population, patients with DM face an elevated risk of infection[9]. The severity of systemic microangiopathy in diabetic patients can lead to tissue damage, creating a favorable milieu for pathogenic bacteria due to the high sugar content, thereby fostering the growth of fungi and bacteria. Consequently, these wounds may serve as sites for secondary infections or facilitate the spread of infection to adjacent soft tissues or deeper into bones[10]. A significant proportion of hospitalized patients with DM present with preexisting infections upon admission, predominantly complex skin and soft tissue lesions. These patients carry common pathogens such as methicillin-resistant Staphylococcus aureus (S. aureus), thereby increasing the likelihood of HAI occurrence, prolonged hospital stays, and increased susceptibility to adverse drug events[9,11]. Genito et al[12] revealed that co-infection with S. aureus and Pseudomonas aeruginosa in diabetic mice enhanced virulence potential. Meta-analyses corroborate the established association between HAIs and DM prevalence[13,14]. Notably, patients with severe DM, particularly those with elevated HbA1c levels, exhibit heightened susceptibility to intensive care unit (ICU) infections compared to non-diabetic patients and those with well-controlled blood glucose (Table 1). During the coronavirus disease 2019 (COVID-19) pandemic, patients with DM experienced a more severe disease course and heightened mortality rates[15]. Poor blood glucose management in patients with diabetes often precipitates recurrent infections, consequently escalating disability rates[10]. Therefore, heightened vigilance is imperative for preventing HAIs in hospitalized diabetic patients.
| Type of HAIs | Primary disease | Results | Research type | Country | Ref. |
| NUTIs | - | The overall incidence of NUTIs in the diabetic group was significantly higher than in the non-diabetic group (13.67% vs 6.40%; P = 0.004) | Cross-sectional study | Pakistan | Ramrakhia et al[19] |
| Undergoing surgery for colorectal cancer | DM with chronic complications is an independent risk factor of NUTIs | Retrospective study | United States | Kang et al[20] | |
| - | No significant association between DM and NUTIs | Retrospective study | France | Girard et al[21] | |
| - | DM was an independent risk factor for CAUTIs in elderly hospitalized patients | Case-control study | China | Shen et al[22] | |
| HCAPs | - | A high mortality rate from HAPs was strongly correlated with DM | Prospective study | Egypt | Yakoub et al[30] |
| - | The incidence of all types of pneumonia analyzed was significantly higher in patients with T2DM than in patients with non-T2DM | Retrospective study | Spain | Lopez-de-Andres et al[31] | |
| Acute cerebral infarction | HCAPs occurred in 80% of all patients with DM, which was significantly higher than that in non-DM patients (72.2%) | Retrospective study | China | Liu et al[32] | |
| Acute cerebral infarction | DM is not a risk factor for non-VAPs | Retrospective study | China | Yang et al[33] | |
| - | DM is not a risk factor for the development of HAPs or an increased mortality factor for HAP-related hospital complications | Meta-analysis | - | Vardakas et al[34] | |
| - | MDR bacteria-induced VAPs were not associated with DM | Meta-analysis | - | Hu et al[35] | |
| - | VAPs after cardiac surgery were not associated with DM | Meta-analysis | - | He et al[36] | |
| SSIs | ACLR | DM may increase the risk of SSIs after ACLR | Meta-analysis | - | Zhao et al[40] |
| HNC tumor resection | The risk of SSIs is more than 3 times higher in diabetic patients than in people without DM | Retrospective study | China | Gan et al[41] | |
| - | DM was an independent risk factor for SSIs for multiple surgical procedure types, and this association was highest for cardiac surgery compared with other types of surgeries | Meta-analysis | - | Martin et al[42] | |
| Noncardiac surgery | Glucose control in the first 24 hours after surgery was poor and the mean serum glucose concentrations of ≥ 150 mg/dL during this time were associated with increased rates of postoperative infectious complications | Meta-analysis | America | King et al[43] | |
| NBSIs | - | A significant increase was noted in NBSIs and mortality in patients with DM, but DM was not an independent risk factor for NBSIs | Prospective observational study | Greece | Tsakiridou et al[37] |
| - | Diabetic patients showed a 1.7-fold probability of developing ICU-acquired NBSIs compared to nondiabetic subjects | Prospective observational study | Greece | Michalia et al[48] | |
| - | CRBSIs in diabetic patients were 4.32 times higher than in non-diabetic patients | Prospective study | China | Jia et al[49] | |
| - | COVID-19 patients with DM were at higher risk of developing NBSIs | Descriptive cross-sectional study | India | Samantaray et al[50] | |
| - | DM was a predictor of shorter survival in patients with sepsis or septic shock | Prospective study | Germany | Schmidt et al[51] |
Nosocomial urinary tract infections (NUTIs) represent a prevalent form of nosocomial infection, with approximately 80% of cases attributed to catheter-associated urinary tract infections (CAUTIs)[2,16]. Between 15% and 25% of hospitalized patients undergo short-term indwelling urinary catheterization[17]. The insertion of urinary catheters, typically via the urethra or pubic bone, facilitates bacterial colonization of catheter surfaces or the formation of biofilms, thereby predisposing to infection[16]. Notably, the risk of CAUTIs increases with the duration of catheterization[2].
Patients with DM are at a heightened risk of developing NUTIs due to the high-sugar environment that favors the proliferation of bacteria[9,17]. The American College of Radiology has explicitly identified DM as a risk factor for acute pyelonephritis[18]. Evidence from a cross-sectional study conducted in Pakistan involving 1074 participants revealed a significantly elevated incidence of NUTIs among diabetic individuals compared to the non-diabetic group[19]. In the study reported by Kang et al[20], DM with chronic complications was an independent risk factor for postoperative NUTIs in patients with colorectal cancer. Furthermore, Girard et al[21] retrospectively analyzed three prospective cohorts of 4669 older patients, including 4045 without catheterization, and reported a lack of significant association between DM and NUTIs, possibly attributed to the inclusion of all diabetic patients irrespective of catheterization status. However, a case-control study involving 7295 hospitalized patients with indwelling urinary catheters ≥ 60 years reported DM as an independent risk factor for CAUTIs[22]. Notably, the heightened risk of NUTIs may predominantly pertain to cases of complex insulin-dependent diabetes[21] and those related to Trichosporon asahii[23].
Crucial strategies for CAUTI prevention include minimizing unnecessary catheterizations and shortening catheterization durations[24]. Emphasis should also be placed on aseptic insertion, proper maintenance of medical devices, and adherence to established practices such as hand hygiene. A notable example is the implementation of comprehensive unit-based safety plans across 603 hospitals in the United States between 2011 and 2013, which led to a reduction in non-ICU catheter-related urinary tract infection rates through coordinated education efforts and resource distribution[25]. Future advancements in catheter materials hold promise for mitigating bacterial growth and CAUTI incidence.
Healthcare-associated pneumonias (HCAPs) represent a significant subset of HAIs with notable lethality. HCAPs are commonly categorized into hospital-acquired pneumonias (HAPs), occurring 48-72 hours post-admission, and ventilator-acquired pneumonias (VAPs), manifesting 48-72 hours post-endotracheal intubation[2]. Patients treated in ICU are at an increased risk of death not only due to their underlying conditions but also attributable to HAIs[1]. Moreover, ICU patients often require endotracheal intubation and invasive mechanical ventilation, with VAPs affecting approximately 9% to 27% of these patients[1]. The primary route of acquisition for non-ventilator HCAPs is bacterial, viral, and fungal aspiration[26]. The etiology of HAPs/VAPs varies geographically, influenced by ICU patient profiles, durations of hospitalization and ICU stays pre-onset, and risk factors for MDR pathogens[27]. In ICUs, gram-negative bacteria (GNB) predominantly cause bacterial pneumonia, often exhibiting high antibiotic resistance, while viral pneumonia is commonly attributed to influenza and respiratory syncytial virus[26,27].
Bacterial pneumococcal pneumonia is relatively common in patients with DM[28]. The adverse effects of DM on morbidity and mortality have been well-documented during influenza and COVID-19 epidemics[28,29]. A prospective longitudinal study in ICUs identified a strong correlation between DM and heightened mortality rates from HAPs[30]. A retrospective observational epidemiological study in Spain demonstrated a higher incidence of pneumonia in patients with type 2 DM (T2DM) compared to those without T2DM[31]. Another recent retrospective analysis of 1093 patients with acute cerebral infarction found that 80% of patients with DM had lung infections caused by GNB, which was significantly higher than those without DM (72.2%)[32]. However, the results are not conclusive. Another study, also from China, reported that DM was not a risk factor for pneumonia in elderly patients with acute ischemic cerebral infarction[33]. Similarly, Vardakas et al[34] reviewed 87 articles between January 1950 to April 2005 and found that DM was not a risk factor for the development of HAP or the increased mortality associated with HAP in hospitals. Moreover, Hu et al[35] conducted a meta-analysis of articles from Jan 1996 to Aug 2022, revealing that MDR bacteria-induced VAPs were not associated with DM. Similarly, another meta-analysis including 11 studies on VAPs after cardiac surgery concluded that the occurrence of VAPs was not associated with DM[36].
Evidence suggests that diabetic immune dysfunction alone may not suffice to precipitate clinically significant respiratory infections in the ICU. Multiple factors, including micro aspirations, illness severity, and mechanical ventilation duration, likely contribute to VAP occurrence[37]. For diabetic patients, prevention of aspiration, disinfection of respiratory apparatus, active hand washing, and reduction of GNB through oral care can reduce the occurrence of VAPs[2]. Factors such as infection control protocols, hospital environment, medication management, and invasive procedure utilization appear to play pivotal roles in HAP development in DM[34,37]. Vaccination emerges as an effective strategy for reducing pneumonia incidence in patients with DM.
Surgical site infections (SSIs) afflict 2%-11% of all surgical interventions[38], with patients’ endogenous pathogens, including skin bacteria or gut bacteria in gastrointestinal surgeries, typically implicated[38]. In hospitalizations surpassing 5-7 days, exogenous and nosocomial flora are dominant, with S. aureus emerging as the most frequently isolated pathogen[38].
DM poses a notable risk factor for SSIs, attributed not only to its strong association with morbid obesity but also the effects of hyperglycemia and glycosylation on cell-mediated immunity[39]. Numerous studies have been published on the effect of diabetes on the increased incidence of SSIs and the potential relationship between hyperglycemia and SSIs. Zhao et al[40] conducted a meta-analysis of 23 studies, which focused on risk factors for SSIs after anterior cruciate ligament reconstruction, with moderate evidence from nine studies affirming the effect of DM on SSI. In a retrospective analysis of 632 patients with head and neck cancer who underwent surgery, Gan et al[41] reported that the risk of SSIs in diabetic patients increased by more than three times compared with those without DM. Similarly, Martin et al[42] summarized the independent association between DM and SSIs across multiple surgical procedures, encompassing 90 published studies between December 1985 and July 2015, and found that DM was an independent risk factor for SSIs for multiple surgical procedure types, with cardiac surgery exhibiting the highest association.
King et al[43] retrospectively analyzed 55408 individuals with DM who underwent various noncardiac surgeries and found that poor glycemic control in the first 24 hours after surgery, during which mean serum glucose concentrations of over 150 mg/dL were associated with an increased incidence of postoperative infectious complications. It is strongly recommended to implement perioperative glycemic control measures, maintaining perioperative plasma glucose levels below 11.1 mmol/L in both diabetic and non-diabetic patients[39]. Moreover, preoperative risk factors can be mitigated through smoking cessation, addressing malnutrition, and judicious antibiotic therapy. Rigorous preoperative skin antiseptic and appropriate surgical preparation are imperative for SSI prevention[2,38].
Nosocomial bloodstream infections (NBSIs) constitute a grave bacterial infection with significant morbidity and mortality, frequently associated with intravascular devices, of which central venous catheters account for 72%[2]. The causative microorganisms of NSSIs are mainly coagulase-negative Staphylococcus, S. aureus, gram-negative Bacilli, and Candida[44,45]. The occurrence of NBSIs correlates with factors such as underlying diseases, catheter type and cleanliness, infused fluid type, and HCWs procedures (e.g., time of catheter placement, frequent catheter manipulation, and catheter location)[45].
Patients with DM experience prolonged abnormal glucose metabolism, fostering a state of negative nitrogen balance characterized by decreased anabolism and increased catabolism, which puts the body in a state of negative nitrogen balance. Concurrently, reduced immunoglobulin synthesis diminishes immunity, augmenting infection susceptibility. Extensive antibiotics used during treatment can disrupt the microbial balance in the body, further increasing the risk of infection. While previous studies have suggested that DM is a risk factor for NBSIs[45-47], two prospective observational studies from Greece have presented conflicting evidence regarding DM’s independent association with NBSIs, yet consistently emphasize a significant association between DM and BSI[37,48]. Similarly, in China, a prospective survey of 2605 ICU patients reported that the incidence of catheter-related BSIs in diabetic patients was 4.32 times higher than that in non-diabetic patients[49]. Furthermore, a recent descriptive cross-sectional study among patients with COVID-19 revealed that patients with DM were at a higher risk of developing NBSIs[50]. A prospective registry study in Germany collected data on the long-term survival of 1975 patients with sepsis, identifying DM as a predictor of shorter survival[51].
Prevention strategies for NBSIs include stringent infection control measures (hand hygiene, environmental dis-infection), meticulous preparation before line insertion (skin preparation), adherence to correct operation protocols, and HCW education (e.g., aseptic operation, removal of unnecessary catheters, insertion site of catheters, immediate replacement of moistened or detached catheter dressings, and timely removal of catheters)[2,52].
Vulnerable populations such as the elderly (> 60 years of age) and infants (< 1 year of age) face heightened susceptibility to HAIs, with underlying diseases, illness severity, and autoimmune status further increasing their susceptibility[2]. A retrospective study from China involving 472 patients with non-Hodgkin lymphoma reported that hospitalization duration, clinical stage, bone marrow infiltrate presence, and peripheral blood neutrophil count were independent risk factors[53]. Another case-control study from China found that different ABO blood types may be susceptible to certain types of HAIs[54]. Notably, in a vicious cycle, HAIs increase the length of hospital stay and prolonged hospitalization increases the risk of exposure to microorganisms in the hospital environment[5]. Microorganisms persist in the environment for a few hours or even months, making the hospital environment a significant contributor to the transmission of hospital pathogens to patients, especially from high-touch infected surfaces such as bedside tables, bed rails, faucets, or doorknobs[55]. In the ICU, the patient's oxygen masks, ventilators, and bed linen are the most contaminated[56]. Additionally, HCWs are susceptible to microbial contamination of their hands and/or gloves, as well as mobile phones used in their daily work, due to their frequent contact with the environmental surfaces of patient’s rooms[57]. Additionally, water reservoirs in aqueous medical devices also provide a favorable environment for the growth of microorganisms such as non-tuberculous mycobacteria[58]. For instance, dental waterlines can be contaminated not only by microorganisms in the aquatic environment but also by bacteria from the patient's oral cavity, posing a risk of infection to both HCWs and patients[59]. Moreover, invasive procedures, nasogastric tubes, urinary and vascular catheters, central venous catheters, antibiotic abuse, and immunosuppressant use are other risk factors for HAIs[2,5,60]. Notably, the most common interventions in the ICU are endotracheal intubation and tracheostomy, as well as mechanical ventilation[61].
Approximately 20%-50% of HAIs occur in the ICU, mainly attributed to the patients’ severe immunocompromised state. The emergence of MDR organisms has reduced the efficacy of antibiotics in treating many common infectious diseases, contributing to increased HAI-related mortality rates[2]. Additionally, the relatively enclosed hospital environment, dense population, and inadequate ventilation pose challenges for infection control[62].
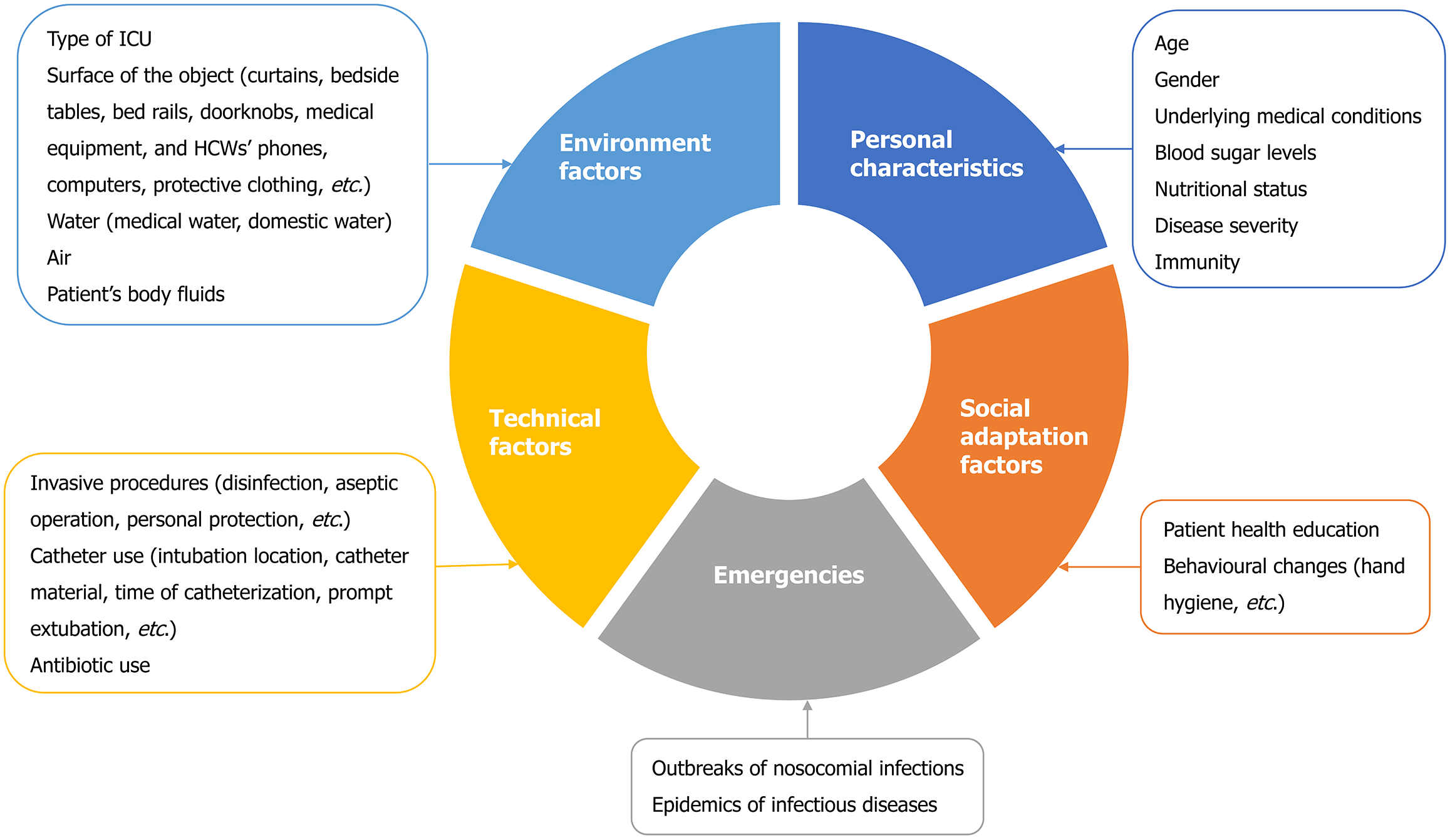
Risk factors for diabetes-associated hospital infections are summarized in Figure 1. These infectious factors can be intervened with strict prevention and control measures. In 2015, the United States Centers for Disease Control and Prevention conducted a point-prevalence survey of 12299 patients from 199 hospitals, wherein 3.2% of hospitalized patients were reported to develop HAIs, which was a decrease from 4% in 2011. This decrease could be attributed to the effective control of surgical site and urinary tract infections[63]. Pathogens can also be transmitted through the hands of HCWs or contaminated objects/aerosols. In a hospital setting, the operating room, ICU environment, and related equipment have strict hygiene requirements, and strict adherence to these requirements can reduce the rate of HAIs[56]. HCWs should pay attention to hand hygiene and the standardized use of medical devices. Additionally, health education for patients can encourage self-management and behavioral changes in the hospital wards. Furthermore, especially in patients with DM, glycemic control and nutritional support during hospitalization can be beneficial for HAI prevention. Nutritional support, such as natural dietary supplements, vitamins, minerals, trace elements, and probiotics in appropriate doses, not only improves blood sugar control but also enhances immune regulation[64].
Hospitalized patients with DM exhibit weakened skin and mucosal barriers, decreasing the body's ability to synthesize immunoglobulins and antibodies, rendering them more susceptible to pathogenic bacterial invasion and infection. Elevated blood glucose concentrations further exacerbate microbial reproduction and provoke inflammatory responses. While the current evidence falls short of conclusively establishing DM’s role in HCAPs, it has unequivocally identified DM as a high-risk factor for NUTIs and SSIs, which may indirectly impact the occurrence of NBSIs. Further studies are required to improve our understanding of the role of DM and glycemic control in HAIs, so as to refine the guidelines for blood glucose control synchronously. Undoubtedly, heightened attention to HAI prevention, especially blood glucose management, is imperative for patients with DM.
We express our gratitude to all colleagues, reviewers, and editors for their valuable contributions toward enhancing the quality of this manuscript.
| 1. | Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections - an overview. Infect Drug Resist. 2018;11:2321-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 674] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 2. | Liu JY, Dickter JK. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest Endosc Clin N Am. 2020;30:637-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Komagamine J, Yabuki T, Kobayashi M, Okabe T. Prevalence of antimicrobial use and active healthcare-associated infections in acute care hospitals: a multicentre prevalence survey in Japan. BMJ Open. 2019;9:e027604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1648] [Cited by in RCA: 1404] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 5. | Biscetti L, Cameriere V, Rossi T, Potente E, Sabbatini D, Bollettini F, Castellani S, Ferrara L, Galeazzi R, Lattanzio F, Di Rosa M, Foresi E, Pelliccioni G. Dementia, stroke, age, use of medical devices and antipsychotic drugs may increase the risk of nosocomial infections among elderly patients hospitalized at Neurology Clinics. Sci Rep. 2023;13:18687. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4143] [Article Influence: 828.6] [Reference Citation Analysis (4)] |
| 7. | Zhao LZ, Li WM, Ma Y. Prevalence and risk factors of diabetes mellitus among elderly patients in the Lugu community. World J Diabetes. 2024;15:638-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Joshi G, Das A, Verma G, Guchhait P. Viral infection and host immune response in diabetes. IUBMB Life. 2024;76:242-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (1)] |
| 9. | Akash MSH, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202:953-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Polk C, Sampson MM, Roshdy D, Davidson LE. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. Infect Dis Clin North Am. 2021;35:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, Li J, Charbonneau C, Baillon-Plot N, Haider S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21 Suppl 2:S27-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Genito CJ, Darwitz BP, Greenwald MA, Wolfgang MC, Thurlow LR. Hyperglycemia potentiates increased Staphylococcus aureus virulence and resistance to growth inhibition by Pseudomonas aeruginosa. Microbiol Spectr. 2023;11:e0229923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Abubakar U, Amir O, Rodríguez-Baño J. Healthcare-associated infections in Africa: a systematic review and meta-analysis of point prevalence studies. J Pharm Policy Pract. 2022;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 14. | Liu X, Long Y, Greenhalgh C, Steeg S, Wilkinson J, Li H, Verma A, Spencer A. A systematic review and meta-analysis of risk factors associated with healthcare-associated infections among hospitalized patients in Chinese general hospitals from 2001 to2022. J Hosp Infect. 2023;135:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. Clin Investig Arterioscler. 2021;33:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 16. | Venkataraman R, Yadav U. Catheter-associated urinary tract infection: an overview. J Basic Clin Physiol Pharmacol. 2023;34:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Finazzi Agrò E. Nosocomial urinary tract infections: A review. Urologia. 2014;81:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Expert Panel on Urological Imaging, Smith AD, Nikolaidis P, Khatri G, Chong ST, De Leon AD, Ganeshan D, Gore JL, Gupta RT, Kwun R, Lyshchik A, Nicola R, Purysko AS, Savage SJ, Taffel MT, Yoo DC, Delaney EW, Lockhart ME. ACR Appropriateness Criteria® Acute Pyelonephritis: 2022 Update. J Am Coll Radiol. 2022;19:S224-S239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 19. | Ramrakhia S, Raja K, Dev K, Kumar A, Kumar V, Kumar B. Comparison of Incidence of Urinary Tract Infection in Diabetic vs Non-Diabetic and Associated Pathogens. Cureus. 2020;12:e10500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Kang CY, Chaudhry OO, Halabi WJ, Nguyen V, Carmichael JC, Mills S, Stamos MJ. Risk factors for postoperative urinary tract infection and urinary retention in patients undergoing surgery for colorectal cancer. Am Surg. 2012;78:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Girard R, Gaujard S, Pergay V, Pornon P, Martin-Gaujard G, Bourguignon L; UTIC Group. Risk factors for urinary tract infections in geriatric hospitals. J Hosp Infect. 2017;97:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Shen L, Fu T, Huang L, Sun H, Wang Y, Sun L, Lu X, Zhang J, Yang Z, Ni C. 7295 elderly hospitalized patients with catheter-associated urinary tract infection: a case-control study. BMC Infect Dis. 2023;23:825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Urs TA, Kadiyala V, Deepak S, Karthik MK. Catheter associated urinary tract infections due to Trichosporon asahii. J Lab Physicians. 2018;10:464-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Vásquez V, Ampuero D, Padilla B. Urinary tract infections in inpatients: that challenge. Rev Esp Quimioter. 2017;30 Suppl 1:39-41. [PubMed] |
| 25. | Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KE, Edson BS, Watson SR, Meyer-Lucas B, Masuga M, Faulkner K, Gould CV, Battles J, Fakih MG. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N Engl J Med. 2016;374:2111-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | Fine LS. Non-ventilator health care-associated pneumonia (NV-HAP): Pathogenesis and microbiology of NV-HAP. Am J Infect Control. 2020;48:A7-A9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Luyt CE, Hékimian G, Koulenti D, Chastre J. Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care. 2018;24:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 29. | Khunti K, Valabhji J, Misra S. Diabetes and the COVID-19 pandemic. Diabetologia. 2023;66:255-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 30. | Yakoub M, Elkhwsky F, El Tayar A, El Sayed I. Hospital-acquired pneumonia pattern in the intensive care units of a governmental hospital: A prospective longitudinal study. Ann Afr Med. 2023;22:94-100. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Lopez-de-Andres A, Albaladejo-Vicente R, de Miguel-Diez J, Hernandez-Barrera V, Ji Z, Zamorano-Leon JJ, Lopez-Herranz M, Jimenez-Garcia R. Incidence and outcomes of hospitalization for community-acquired, ventilator-associated and non-ventilator hospital-acquired pneumonias in patients with type 2 diabetes mellitus in Spain. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Liu YX, Cao QM, Ma BC. Pathogens distribution and drug resistance in patients with acute cerebral infarction complicated with diabetes and nosocomial pulmonary infection. BMC Infect Dis. 2019;19:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Yang NZ, Li X, Yun XH, Chen J, Li M. Risk factors analysis of nosocomial pneumonia in elderly patients with acute cerebral infraction. Medicine (Baltimore). 2019;98:e15045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Vardakas KZ, Siempos II, Falagas ME. Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality. Diabet Med. 2007;24:1168-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Hu JN, Hu SQ, Li ZL, Bao C, Liu Q, Liu C, Xu SY. Risk factors of multidrug-resistant bacteria infection in patients with ventilator-associated pneumonia: A systematic review and meta-analysis. J Infect Chemother. 2023;29:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | He S, Chen B, Li W, Yan J, Chen L, Wang X, Xiao Y. Ventilator-associated pneumonia after cardiac surgery: a meta-analysis and systematic review. J Thorac Cardiovasc Surg. 2014;148:3148-55.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Tsakiridou E, Makris D, Chatzipantazi V, Vlachos O, Xidopoulos G, Charalampidou O, Moraitis G, Zakynthinos E. Diabetes and hemoglobin a1c as risk factors for nosocomial infections in critically ill patients. Crit Care Res Pract. 2013;2013:279479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Kolasiński W. Surgical site infections - review of current knowledge, methods of prevention. Pol Przegl Chir. 2018;91:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Wilson RB, Farooque Y. Risks and Prevention of Surgical Site Infection After Hernia Mesh Repair and the Predictive Utility of ACS-NSQIP. J Gastrointest Surg. 2022;26:950-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 40. | Zhao D, Liang GH, Pan JK, Zeng LF, Luo MH, Huang HT, Han YH, Lin FZ, Xu NJ, Yang WY, Liu J. Risk factors for postoperative surgical site infections after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2023;57:118-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 41. | Gan C, Wang Y, Tang Y, Wang K, Sun B, Wang M, Zhu F. Risk factors for surgical site infection in head and neck cancer. Support Care Cancer. 2022;30:2735-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, Bertran E, Jaber L. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol. 2016;37:88-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 399] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 43. | King JT Jr, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 2011;253:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Sitges-Serra A, Girvent M. Catheter-related bloodstream infections. World J Surg. 1999;23:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Süner A, Karaoğlan I, Mete AO, Namiduru M, Boşnak V, Baydar I. Assessment of bloodstream infections and risk factors in an intensive care unit. Turk J Med Sci. 2015;45:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Ngo JT, Parkins MD, Gregson DB, Pitout JD, Ross T, Church DL, Laupland KB. Population-based assessment of the incidence, risk factors, and outcomes of anaerobic bloodstream infections. Infection. 2013;41:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Trethon A, Prinz G, Varga A, Kocsis I. Characteristics of nosocomial bloodstream infections at a Hungarian cardiac surgery centre. Acta Microbiol Immunol Hung. 2012;59:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Michalia M, Kompoti M, Koutsikou A, Paridou A, Giannopoulou P, Trikka-Graphakos E, Clouva-Molyvdas P. Diabetes mellitus is an independent risk factor for ICU-acquired bloodstream infections. Intensive Care Med. 2009;35:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Jia L, Yu H, Lu J, Zhang Y, Cai Y, Liu Y, Ma X. [Epidemiological characteristics and risk factors for patients with catheter-related bloodstream infections in intensive care unit]. Zhonghua Yi Xue Za Zhi. 2015;95:654-658. [PubMed] |
| 50. | Samantaray S, Karan P, Sharma A, Nag V, Dutt N, Garg MK, Bhatia PK, Misra S. Prevalence, Presentation and Outcome of Secondary Bloodstream Infections among COVID-19 Patients. Infect Disord Drug Targets. 2022;22:e180422203723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Schmidt K, Gensichen J, Fleischmann-Struzek C, Bahr V, Pausch C, Sakr Y, Reinhart K, Vollmar HC, Thiel P, Scherag A, Gantner J, Brunkhorst FM. Long-Term Survival Following Sepsis. Dtsch Arztebl Int. 2020;117:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Buetti N, Timsit JF. Management and Prevention of Central Venous Catheter-Related Infections in the ICU. Semin Respir Crit Care Med. 2019;40:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | DU XH, Zhang XY, Lin XR, Liu QL, Tao G, Lin P. [Clinical Characteristics and Risk Factors of Nosocomial Infection in 472 Patients with Non-Hodgkin Lymphoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Zhong X, Wang DL, Xiao LH, Mo LF, Luo XF. ABO blood groups and nosocomial infection. Epidemiol Infect. 2023;151:e64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Facciolà A, Pellicanò GF, Visalli G, Paolucci IA, Venanzi Rullo E, Ceccarelli M, D'Aleo F, Di Pietro A, Squeri R, Nunnari G, La Fauci V. The role of the hospital environment in the healthcare-associated infections: a general review of the literature. Eur Rev Med Pharmacol Sci. 2019;23:1266-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 56. | Tajeddin E, Rashidan M, Razaghi M, Javadi SS, Sherafat SJ, Alebouyeh M, Sarbazi MR, Mansouri N, Zali MR. The role of the intensive care unit environment and health-care workers in the transmission of bacteria associated with hospital acquired infections. J Infect Public Health. 2016;9:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Dhayhi N, Kameli N, Salawi M, Shajri A, Basode VK, Algaissi A, Alamer E, Darraj M, Shrwani K, Alhazmi AH. Bacterial Contamination of Mobile Phones Used by Healthcare Workers in Critical Care Units: A Cross-Sectional Study from Saudi Arabia. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 58. | Yiek WK, Coenen O, Nillesen M, van Ingen J, Bowles E, Tostmann A. Outbreaks of healthcare-associated infections linked to water-containing hospital equipment: a literature review. Antimicrob Resist Infect Control. 2021;10:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Spagnolo AM, Sartini M, Cristina ML. Microbial Contamination of Dental Unit Waterlines and Potential Risk of Infection: A Narrative Review. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Murni IK, Duke T, Kinney S, Daley AJ, Wirawan MT, Soenarto Y. Risk factors for healthcare-associated infection among children in a low-and middle-income country. BMC Infect Dis. 2022;22:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Wałaszek M, Kosiarska A, Gniadek A, Kołpa M, Wolak Z, Dobroś W, Siadek J. The risk factors for hospital-acquired pneumonia in the Intensive Care Unit. Przegl Epidemiol. 2016;70:15-20, 107. [PubMed] |
| 62. | Du Q, Zhang D, Hu W, Li X, Xia Q, Wen T, Jia H. Nosocomial infection of COVID19: A new challenge for healthcare professionals (Review). Int J Mol Med. 2021;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 63. | Magill SS, O'Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR; Emerging Infections Program Hospital Prevalence Survey Team. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med. 2018;379:1732-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 771] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 64. | Wang MK, Yu XL, Zhou LY, Si HM, Hui JF, Hou DY, Li WP, Yang JS. COVID-19 and liver dysfunction: What nutritionists need to know. World J Gastroenterol. 2022;28:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |