Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1677
Revised: May 4, 2024
Accepted: May 20, 2024
Published online: August 15, 2024
Processing time: 117 Days and 11.1 Hours
Diabetic cardiomyopathy (DbCM) is a common but underrecognized compli-cation of patients with diabetes mellitus (DM). Although the pathobiology of other cardiac complications of diabetes such as ischemic heart disease and cardiac autonomic neuropathy are mostly known with reasonable therapeutic options, the mechanisms and management options for DbCM are still not fully understood. In its early stages, DbCM presents with diastolic dysfunction followed by heart failure (HF) with preserved ejection fraction that can progress to systolic dysfunction and HF with reduced ejection fraction in its advanced stages unless appropriately managed. Apart from prompt control of DM with lifestyle changes and antidiabetic medications, disease-modifying therapy for DbCM includes prompt control of hypertension and dyslipidemia inherent to patients with DM as in other forms of heart diseases and the use of treatments with proven efficacy in HF. A basic study by Zhang et al, in a recent issue of the World Journal of Diabetes elaborates the potential pathophysiological alterations and the therapeutic role of teneligliptin in diabetic mouse models with DbCM. Although this preliminary basic study might help to improve our understanding of DbCM and offer a potential new management option for patients with the disease, the positive results from such animal models might not always translate to clinical practice as the pathobiology of DbCM in humans could be different. However, such experimental studies can encourage more scientific efforts to find a better solution to treat patients with this enigmatic disease.
Core Tip: Diabetic cardiomyopathy (DbCM) is a common and underrecognized complication of diabetes mellitus. The pathobiological mechanisms of DbCM are not fully elusive and the therapeutic options are not adequate. Stages of DbCM include diastolic dysfunction followed by heart failure (HF) with preserved ejection fraction, systolic dysfunction and HF with reduced ejection fraction in its order of progression. Zhang et al, in a recent issue of the World Journal of Diabetes, demonstrate the potential pathophysiological changes of DbcM and explore the therapeutic potential of teneligliptin in mouse models of DbCM. Such experimental models might harness better research efforts to find an appropriate therapeutic strategy for DbCM in the near future.
- Citation: Fernandez CJ, Shetty S, Pappachan JM. Diabetic cardiomyopathy: Emerging therapeutic options. World J Diabetes 2024; 15(8): 1677-1682
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1677.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1677
Heart disease is the most common cause of mortality in patients with diabetes mellitus (DM). Diabetic cardiac diseases comprise ischemic heart disease (IHD), cardiac autonomic neuropathy (CAN) and diabetic cardiomyopathy (DbCM)[1]. Although the first 2 forms are well-known, DbCM is not that familiar to most clinicians. The risk of hospitalization for heart failure (HF) is two-fold higher in those with DM than in nondiabetics, and diabetes is identified as an independent risk factor for worse clinical HF outcomes, including a greater decline in left ventricular ejection fraction over time[2]. DbCM, the most important cause of both diastolic and systolic HF in patients with DM, is associated with significantly higher morbidity and mortality risk.
Although there are very good treatment options for diabetics with IHD resulting from coronary artery disease (CAD) to improve the clinical and mortality outcomes, the treatment options for patients with DbCM are mostly symptom control measures. The main reason is the lack of therapeutic avenues targeting the pathobiological mechanisms involved in the development of DbCM. Therapeutics targeting the pathophysiological abnormalities of DbCM are being explored now. The therapies addressing the underlying aetiopathogenesis with the potential to reduce adverse cardiovascular outcomes and mortality are the need of the hour. A basic study by Zhang et al[3] in the recent issue of the World Journal of Diabetes that reveals the beneficial effect of teneligliptin in improving the disease process in mouse models of DbCM, is an example of treatment options targeting the pathobiological processes of DbCM. This editorial briefly discusses the pathobiology and emerging therapeutic options for patients with DbCM.
DbCM is defined as the structural, functional, and metabolic myocardial changes that result in HF in the absence of CAD, valvular heart disease and conventional cardiovascular risk factors such as hypertension and dyslipidaemia[4]. The resultant HF can either be HF with preserved ejection fraction (HFpEF; approximately 2/3rd of DbCM cases) or HF with reduced ejection fraction (HFrEF; approximately 1/3rd of DbCM)[4]. The prevalence of DbCM among community-dwelling adults with diabetes varied between 67.0% when the least restrictive criteria for diagnosis were used to 11.7% when the most restrictive criteria were used[5]. DbCM is associated with a high risk of incident HF with the highest risk when the most restrictive criteria are used [hazard ratio (HR): 2.55; 95% confidence interval (CI): 1.69-3.86] and the lowest risk when the least restrictive criteria are used (HR: 1.99; 95%CI: 1.50-2.65)[5]. These figures clearly explain the higher prevalence of incident HF at any point in time among patients with DM.
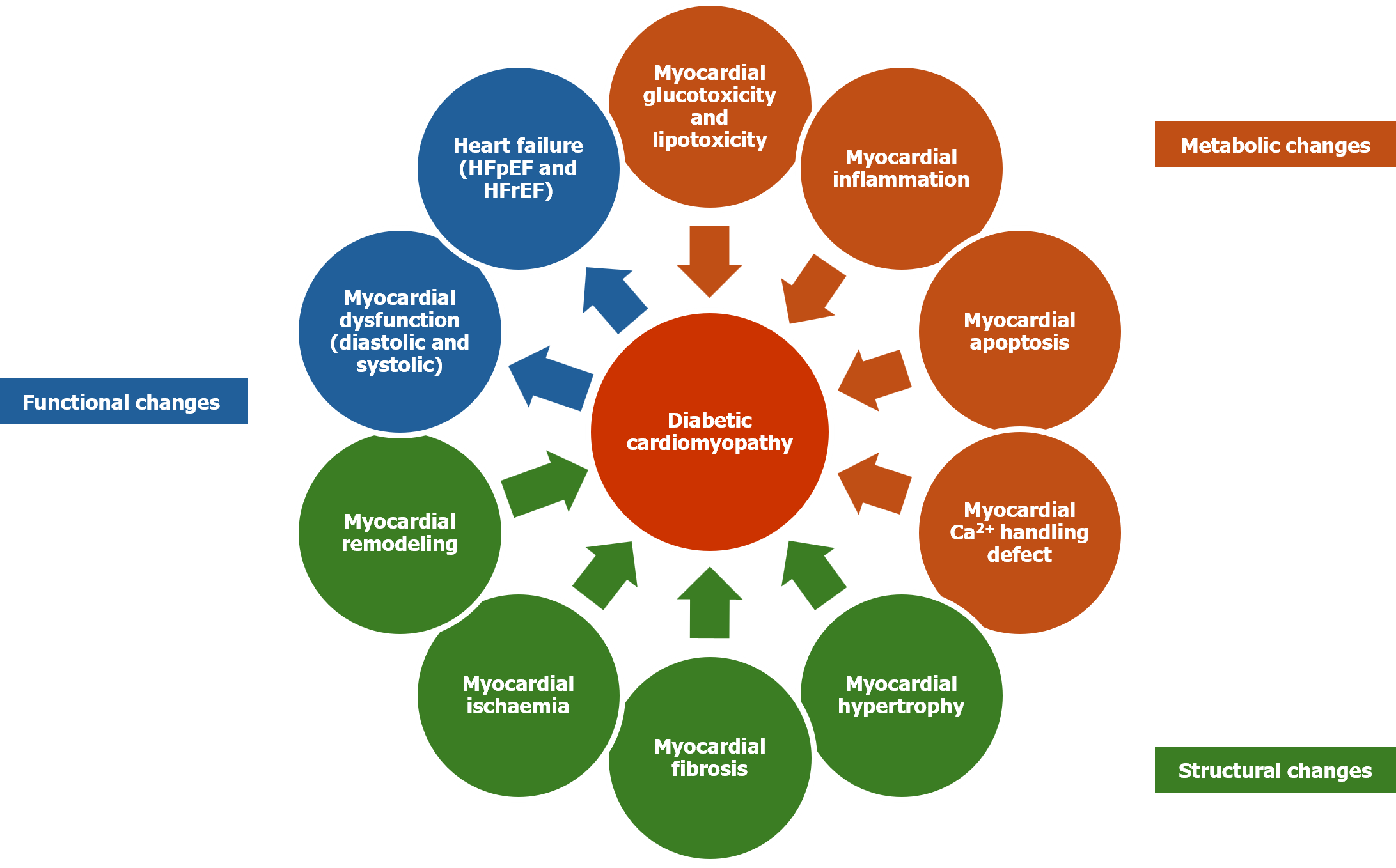
Although not fully elusive, multiple metabolic, structural, and functional alterations have been identified in the etiopathogenesis of DbCM. The pathobiological changes associated with the onset and progression of DbCM include chronic hyperglycemia, insulin resistance, hyperinsulinemia, alterations in the pathways controlling intracellular myocardial energy metabolism, increased myocardial fatty acid oxidation (and the consequent mitochondrial dysfunction); ceramide and diacylglycerol accumulation (with cardiomyocyte lipo-apoptosis), increased reactive oxygen species formation (cardiac oxidative stress), increased generation of advanced glycation end products (AGE) (with nuclear factor-κB mediated inflammation), impaired calcium handling and apoptosis (from mitochondrial dysfunction, and endoplasmic reticulum stress), altered mitochondrial bioenergetics, overactivation of the renin-angiotensin-aldosterone systems (RAAS), impaired nitric oxide and endothelium-derived hyperpolarising factor-mediated vasodilation (leading to microvascular dysfunction), and exosome dysregulation (cardiomyocyte apoptosis)[1,4,6]. Structural changes associated with the pathogenesis of DbCM include myocardial hypertrophy, fibrosis, ischemia, and adverse cardiac remodelling[1,4,6]. Functional changes associated with the pathogenesis of DbCM include the development of diastolic dysfunction, HFpEF, systolic dysfunction, and subsequently HFrEF[1,4,6].
The clinical features, structural changes, functional changes, and biomarkers are not specific enough to accurately diagnose DbCM. Cardiac remodelling is an important feature of DbCM, and various imaging tools used to diagnose cardiac remodelling include echocardiography, cardiac computed tomography (CT), magnetic resonance imaging (MRI), and nuclear imaging techniques[7]. These non-invasive tools can detect changes in myocardial mass, wall thickness and perfusion as indicators of diastolic dysfunction which is the key feature of early stages of DbCM[7].
While 2-D echocardiography can provide details regarding structural changes of DbCM, tissue doppler imaging can provide details regarding functional changes (E/e’ velocity and mitral annular velocity)[7,8]. Dilated left atrium (LA) is an early feature that supports the diagnosis of DbCM. Apart from the LA volume index, 2-D speckle tracking echocardiography provides a measurement of the LA contractile strain rate. Contrast-enhanced cardiac MRI can provide details regarding structural changes (myocardial fibrosis, steatosis, and left ventricular mass), functional changes (diastolic and systolic function), and metabolic changes (MR spectroscopy for myocardial triglyceride content and high-energy phosphate metabolism). In addition to providing a more accurate measurement of LA size over echocardiography, the cardiac MRI can evaluate LA ejection fraction. Similarly, cardiac CT can measure LA total emptying fraction (LATEF) as a measure of global LA function. As CAN is an independent risk factor of DbCM, decreased 123I-MIBG (Iodine-123 metaiodobenzylguanidine) activity can be seen in patients with DbCM[7].
Although the most definitive evidence of diastolic dysfunction is obtainable from cardiac catheterisation studies, it is rarely needed due to the availability of various highly sensitive and specific non-invasive tests. Investigational bio-markers of structural changes are matrix metalloproteinase (MMP) and tissue inhibitor of matrix metalloproteinase (for myocardial fibrosis); and of functional changes include mi-RNA (for contractile function), procollagen 3 N-terminal peptide and troponin (for LV dysfunction), and BNP (brain natriuretic peptide) for LV diastolic and systolic function[1]. Figure 1 shows the metabolic, functional and structural changes in the heart that lead to DbCM.
Initial stages of DbCM are clinically silent and are characterized by structural and functional changes including myocardial hypertrophy, myocardial interstitial fibrosis, and myocardial stiffness[9]. These changes are associated with subclinical diastolic dysfunction, which later evolves into HFpEF and eventually systolic dysfunction accompanied by HFrEF. Early detection and treatment of DbCM are important for preventing the onset and progression of myocardial fibrosis, the irreversible stage of the disease.
Reversing adverse cardiac remodelling of DbCM is challenging and often needs a multi-faceted approach. The best approach to reverse the cardiac remodelling of DbCM is through lifestyle modifications (regular exercise, restricted fat and refined carbohydrate consumption aiming to improve the body weight and metabolic functions), and through drugs that improve systemic and tissue level insulin sensitivity, myocardial glucose uptake and myocardial function[10,11]. For example, metformin acting via the adenosine monophosphate-activated protein kinase (AMPK) pathway and thiazolidinediones acting via peroxisome proliferator-activated receptor gamma(PPAR-γ) modulate insulin sensitivity and myocardial metabolism. In addition, optimising the cholesterol levels and blood pressure is also important[10,11].
Apart from glycaemic control and weight reduction, the glucagon-like peptide 1 receptor agonists exhibit antifibrotic properties by activating the AMPK pathway, reducing the endoplasmic reticulum stress, and inhibiting the expression of type I/III collagen and MMPs, thereby preventing the development of DbCM. Dipeptidyl peptidase-4 (DPP-4) inhibitors could also prevent myocardial hypertrophy and diastolic dysfunction by inhibiting oxidative stress and myocardial fibrosis[10,11].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are also promising drugs in the treatment of DbCM. They enhance sodium excretion and decrease cardiac preload and afterload, thereby improving cardiac output. SGLT2 inhibitors inhibit myocardial fibroblast activation by blocking the transforming growth factor-β (TGF-β)/SMAD signalling pathway[10,11]. SGLT2 inhibitors inhibit the cytosolic sodium-hydrogen (Na+-H+) pump, decrease intracellular calcium and thereby myocardial injury, hypertrophy, and fibrosis. Cardio-protection by SGLT2 inhibitors is also mediated by raised β-hydroxybutyric acid production (increasing the mitochondrial metabolism) and uric acid excretion[10,11].
Drugs with proven efficacy for HFrEF to reverse LV remodelling via inhibition of RAAS such as angiotensin convertase enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists may also be of value in the treatment of HFpEF mediated by DbCM by inhibiting the development of inflammatory and fibrotic responses[10,11]. Similarly, β-blockers reduce myocardial oxygen consumption, improve cardiac metabolism, and reduce myocardial hypertrophy, apoptosis, and fibrosis. Spironolactone could reduce myocardial oxidative stress, inflammatory response, and fibrosis[10,11].
As multiple mechanisms are involved in the pathogenesis of DbCM, the current treatments are not fully effective in the prevention and treatment of DbCM. Many potential therapies are in the preliminary stages of development including sphingosine-1-phosphate modulators, AGE inhibitors, aldose reductase inhibitors, N-acetylcysteine, nicorandil, Nesfatin-1, Schisandrin B, Paeonol, Piceatannol, and flavonoids which are tailored towards different specific pathways[10-12]. On the other hand, gene therapy and stem cell therapy could intervene in the root cause of the disease and might achieve long-term beneficial effects with the potential to reverse myocardial fibrosis[10].
Various types of programmed cell death (apoptosis, pyroptosis, necroptosis, and ferroptosis) are involved in the clearance of cells that lost their function, have an infection, or are affected by neoplasms. Pyroptosis, a highly inflammatory mode of apoptosis, is involved in the pathogenesis of atherosclerosis, ischemia-reperfusion injury, and DbCM. Chronic hyperglycaemia-induced reactive oxygen species (ROS) formation activates NOD-like receptor protein 3 (NLRP3) inflammasome which in turn activates downstream molecules to cause membrane rupture characteristic of pyroptosis and produce pro-inflammatory cytokines[13]. As NLRP3 inflammasome participates in the cardiomyocyte pyroptosis of DbCM, NLRP3 is upregulated in DbCM and the level of NLRP3 correlates with ROS generation. By inhibiting ROS generation, NLRP3 gets inactivated. Thus, NLRP3 is a critical target for treating DbCM. Many diabetic medications including metformin, thiazolidinediones, SGLT2 inhibitors and DPP-4 inhibitors improve DbCM by inhibiting the NLRP3 inflammasome activation[13].
The experimental study by Zhang et al[3] in a recent issue of the World Journal of Diabetes uncovers the potential benefits of the DPP-4 inhibitor teneligliptin in the prevention of DbCM in a diabetic mouse model. The following observations were made by the researchers: In streptozotocin-induced diabetic mice, teneligliptin could ameliorate myocardial hypertrophy and improve heart function. The study also observed that the drug could reduce the activation of NLRP3 inflammasome and the resultant myocardial injury. Finally, the drug increased the AMPK phosphorylation in the hyperglycaemia-induced cardiomyocytes and its beneficial effects on hyperglycaemia-induced cardiomyocytes were abolished by AMPK inhibition, suggesting that the effect of teneligliptin on DbCM is also regulated by AMPK.
This study also sheds light on one of the pathobiological mechanisms of DbCM and the great potential for the prevention and management of this dreaded disease with a readily available therapeutic option used for treating patients with type 2 DM for several years. The study would evoke enthusiasm among researchers in further evaluating the potential role of other DPP-4 inhibitors in the prevention and management of DbCM. However, we should be mindful of the lack of significant benefits of several translational experimental models in our day-to-day clinical practice. Therefore, the small experimental data published by Zhang et al[13] needs further validation in larger laboratory models and human beings to ensure the reproducibility of the positive results to compile clearer scientific evidence.
Moreover, we should also consider the reports on higher hospitalisation risk with some of the DPP-4 inhibitors in patients with advanced HF[14,15]. Real-world cohort studies including retrospective data analysis and randomised controlled trials investigating the use of DPP-4 inhibitors at various stages of DbCM may help us to explore the benefits and risks posed by these drugs shortly.
DbCM is a common, serious and underrecognized complication of DM, the pathobiological mechanisms of which are still not fully elusive. Present evidence suggests that several disease processes are involved in its causation and some available therapeutic avenues for prevention and management of DbCM with the currently available medications. However, there are still great knowledge gaps regarding the pathobiology and treatment options targeting DbCM. More research input is imperative for shedding light on our understanding of the mechanisms and management strategies of this disease. The study by Zhang et al[3], published in the World Journal of Diabetes is one such remarkable attempt. However, more experimental and clinical data with DPP-4 inhibitors in DbCM are necessary to affirm their study findings.
We thank Dr. Marina George Kudiyirickal for providing the voice clip for the audio core tip of this article.
| 1. | Rajbhandari J, Fernandez CJ, Agarwal M, Yeap BXY, Pappachan JM. Diabetic heart disease: A clinical update. World J Diabetes. 2021;12:383-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 2. | Abovich A, Matasic DS, Cardoso R, Ndumele CE, Blumenthal RS, Blankstein R, Gulati M. The AHA/ACC/HFSA 2022 Heart Failure Guidelines: Changing the Focus to Heart Failure Prevention. Am J Prev Cardiol. 2023;15:100527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Zhang GL, Liu Y, Liu YF, Huang XT, Tao Y, Chen ZH, Lai HL. Teneligliptin mitigates diabetic cardiomyopathy by inhibiting activation of the NLRP3 inflammasome. World J Diabetes. 2024;15:724-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (35)] |
| 4. | Prandi FR, Evangelista I, Sergi D, Palazzuoli A, Romeo F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev. 2023;28:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Segar MW, Khan MS, Patel KV, Butler J, Tang WHW, Vaduganathan M, Lam CSP, Verma S, McGuire DK, Pandey A. Prevalence and Prognostic Implications of Diabetes With Cardiomyopathy in Community-Dwelling Adults. J Am Coll Cardiol. 2021;78:1587-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Nakamura K, Miyoshi T, Yoshida M, Akagi S, Saito Y, Ejiri K, Matsuo N, Ichikawa K, Iwasaki K, Naito T, Namba Y, Sugiyama H, Ito H. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 7. | Pan KL, Hsu YC, Chang ST, Chung CM, Lin CL. The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Lee SH, Park JH. The Role of Echocardiography in Evaluating Cardiovascular Diseases in Patients with Diabetes Mellitus. Diabetes Metab J. 2023;47:470-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 589] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 10. | Shou Y, Li X, Fang Q, Xie A, Zhang Y, Fu X, Wang M, Gong W, Zhang X, Yang D. Progress in the treatment of diabetic cardiomyopathy, a systematic review. Pharmacol Res Perspect. 2024;12:e1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 11. | Dhar A, Venkadakrishnan J, Roy U, Vedam S, Lalwani N, Ramos KS, Pandita TK, Bhat A. A comprehensive review of the novel therapeutic targets for the treatment of diabetic cardiomyopathy. Ther Adv Cardiovasc Dis. 2023;17:17539447231210170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Januzzi JL, Del Prato S, Rosenstock J, Butler J, Ezekowitz J, Ibrahim NE, Lam CSP, Marwick T, Wilson Tang WH, Liu Y, Mohebi R, Urbinati A, Zannad F, Perfetti R. Characterizing diabetic cardiomyopathy: baseline results from the ARISE-HF trial. Cardiovasc Diabetol. 2024;23:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 13. | Zhang L, Ai C, Bai M, Niu J, Zhang Z. NLRP3 Inflammasome/Pyroptosis: A Key Driving Force in Diabetic Cardiomyopathy. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 14. | Mannucci E, Nreu B, Montereggi C, Ragghianti B, Gallo M, Giaccari A, Monami M; SID-AMD joint panel for Italian Guidelines on Treatment of Type 2 Diabetes. Cardiovascular events and all-cause mortality in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31:2745-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Cintra RM, Nogueira AC, Bonilha I, Luchiari BM, Coelho-Filho OR, Coelho OR, Schwartzmann P, Muscellie E, Nadruz W, Carvalho LSF, Sposito AC. Glucose-lowering Drugs and Hospitalization for Heart Failure: A Systematic Review and Additive-effects Network Meta-analysis With More Than 500 000 Patient-years. J Clin Endocrinol Metab. 2021;106:3060-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |