Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1663
Revised: May 22, 2024
Accepted: June 5, 2024
Published online: August 15, 2024
Processing time: 120 Days and 21.7 Hours
Coronavirus disease 2019 (COVID-19) is a highly infectious disease caused by a novel human coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Diabetes is a well-known risk factor for infectious diseases with high prevalence and increased severity. Here, we elucidated the possible factors for the increased vulnerability of diabetic patients to SARS-CoV-2 infection and the more severe COVID-19 illness. The worsened prognosis of patients with both COVID-19 and diabetes may be attributable to host receptor angiotensin-converting enzyme 2-assisted viral uptake. Moreover, insulin resistance is often associated with impaired mucosal and skin barrier integrity, resulting in mic-robiota dysbiosis, which increases susceptibility to viral infections. It may also be associated with higher levels of pro-inflammatory cytokines resulting from an impaired immune system in diabetics, inducing a cytokine storm and excessive inflammation. This review describes diabetes mellitus and its complications, explains the risk factors, such as disease characteristics and patient lifestyle, which may contribute to the high susceptibility of diabetic patients to COVID-19, and discusses preventive and therapeutic strategies for COVID-19-positive diabetic patients.
Core Tip: This paper hypothesizes that the worsening prognosis of diabetic patients with coronavirus disease 2019 (COVID-19) is attributable to host receptor angiotensin-converting enzyme 2-assisted viral uptake. Insulin resistance is often associated with impaired mucosal and skin barrier integrity, resulting in microbiota dysbiosis, which increases susceptibility to viral infections. It may also be associated with higher levels of pro-inflammatory cytokines resulting from an impaired immune system in diabetic patients, which induces a cytokine storm and excessive inflammation. This review discusses the possible factors contributing to the increased susceptibility of diabetic patients to severe acute respiratory syndrome coronavirus 2 infection and the more severe COVID-19 disease and preventive and therapeutic strategies for COVID-19 in diabetic patients.
- Citation: Liu JW, Huang X, Wang MK, Yang JS. Diabetes and susceptibility to COVID-19: Risk factors and preventive and therapeutic strategies. World J Diabetes 2024; 15(8): 1663-1671
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1663.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1663
Coronavirus disease 2019 (COVID-19) is a highly contagious disease caused by a novel human coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of 3 March 2024, 774834254 confirmed COVID-19 cases and 7037007 deaths have been reported globally[1]. According to the specification of the Centers for Disease Control and Prevention, the high-risk groups include people with chronic diseases such as type 2 diabetes, chronic lung, severe heart, and chronic kidney diseases, and obesity, as well as pregnant women[2]. In 2019, the prevalence of diabetes among adults aged 20-79 years worldwide was 9.3% (463 million) and was projected to continue to increase[3]. Diabetes has been a well-known risk factor for infectious diseases, especially increasing the risk of infection and death in severely ill patients[4].
A recent review published in the World Journal of Diabetes by Shukla et al[5] studied and explored the complex link between type 2 diabetes and COVID-19, revealing that reduced ACE2 expression in patients with diabetes mellitus (DM) enhances the activity of renin-angiotensin system, leading to inflammation and fibrosis. Moreover, it was highlighted that people with DM had a higher mortality and higher susceptibility to COVID-19 than those without the disease[5].
Therefore, improving the prognosis of DM and COVID-19 patients requires early diagnosis, prompt treatment, and stringent management. In order to manage patients with comorbid diseases effectively, this review describes the severity of DM, presents an epidemiological analysis of patients with DM comorbid with COVID-19, analyzes the possible factors for the high susceptibility of patients with DM to COVID-19, and examines the preventive and therapeutic strategies of DM patients with COVID-19. These findings could aid in the development of new potential treatments for COVID-19.
Articles that were published in English were retrieved by a computerized search in the PubMed and Google Scholar databases. The search period was from database creation to March 2024. Keywords included “COVID-19”, “coronavirus therapy”, “SARS-CoV-2”, “diabetes”, “angiotensin-converting enzyme”, “insulin resistance”, and “immune system”. Further references were added in the final manuscript based on consensus among all authors by hand-searching in the relevant literature.
DM is a hyperglycemia disease caused by defective insulin secretion and/or action. DM-related chronic hyperglycemia is associated with long-term damage, dysfunction, and failure of various organs, including the eyes, nerves, heart, kidneys, and blood vessels[6]. Nephropathy, cardiovascular disease, retinopathy, and diabetic foot are common complications of DM. Diabetic nephropathy is a serious microvascular complication of type 1 or type 2 DM that damages the kidney filtration system and can lead to life-threatening kidney failure in severe cases. Interstitial fluid glucose accumulation is the basic pathogenesis of blood glucose elevation in DM patients, and its fluctuations in glucose levels with daily hypoglycemia and hyperglycemia are considered important in driving DM-related pathologies[7]. Changes in glucose levels lead to transcriptional changes in renal tubular cells, alterations in the renal extracellular matrix, and metabolic mitochondrial rewiring, which are early indicators of kidney disease that are triggered by hyperglycemia. Furthermore, kidney disease still occurs in DM patients even with strict blood glucose control, suggesting that oxidative stress and other insults, such as lipotoxicity, may also play a key role in its pathogenicity[8].
Cardiovascular disease is a widespread disease of cardiovascular system dysfunction, which is a major complication that contributes to type 2 DM-related deaths. High blood glucose levels in diabetics could induce the production of advanced glycosylation end products (AGEs) which attach to proteins or lipids in a non-enzymatic manner, altering their function and inducing atherosclerosis[9]. Diabetic retinopathy is another common complication of DM, and almost all patients with DM eventually develop this disease. The dysfunction of the retinal cells involved in diabetic retinopathy includes the endothelial cells of the retinal microvessels and pericytes that lie beneath the endothelial cells[10]. One of the earliest features of diabetic retinopathy is pericyte loss. The integrity of the tight junction between endothelial cells is compromised by hyperglycemia, oxidative stress, and AGEs. These factors also cause pericyte detachment and apoptosis.
DM is a known risk factor for infectious diseases with high prevalence and increased severity. According to epidemiologic studies, DM has become one of the most common comorbidities associated with COVID-19 severity and mortality[11]. A report from Wuhan, China suggests that diabetics are overrepresented among deceased patients due to COVID-19[12]. A study from Poland evaluated the impact of the COVID-19 pandemic on DM-related hospitalizations in different age groups and reported an increase of 66.7% and 48.5% in in-hospital mortality in DM patients, respectively[13].
Hospitalization mortality increases with age. Although increasing age is a consistent risk factor for increased COVID-19 severity and mortality, there is growing skepticism that this may not be a simple additive effect of age-related risk in patients with DM. In fact, a recent study has demonstrated that younger individuals with COVID-19 and type 2 DM have significantly higher mortality rates than older individuals[14]. In addition, COVID-19 infection induces acute hyper-glycemic crisis and worsens the prognosis of patients with poorly controlled DM[15]. Therefore, achieving glycemic control is critical to reduce the risk of complications and death after COVID-19 infection.
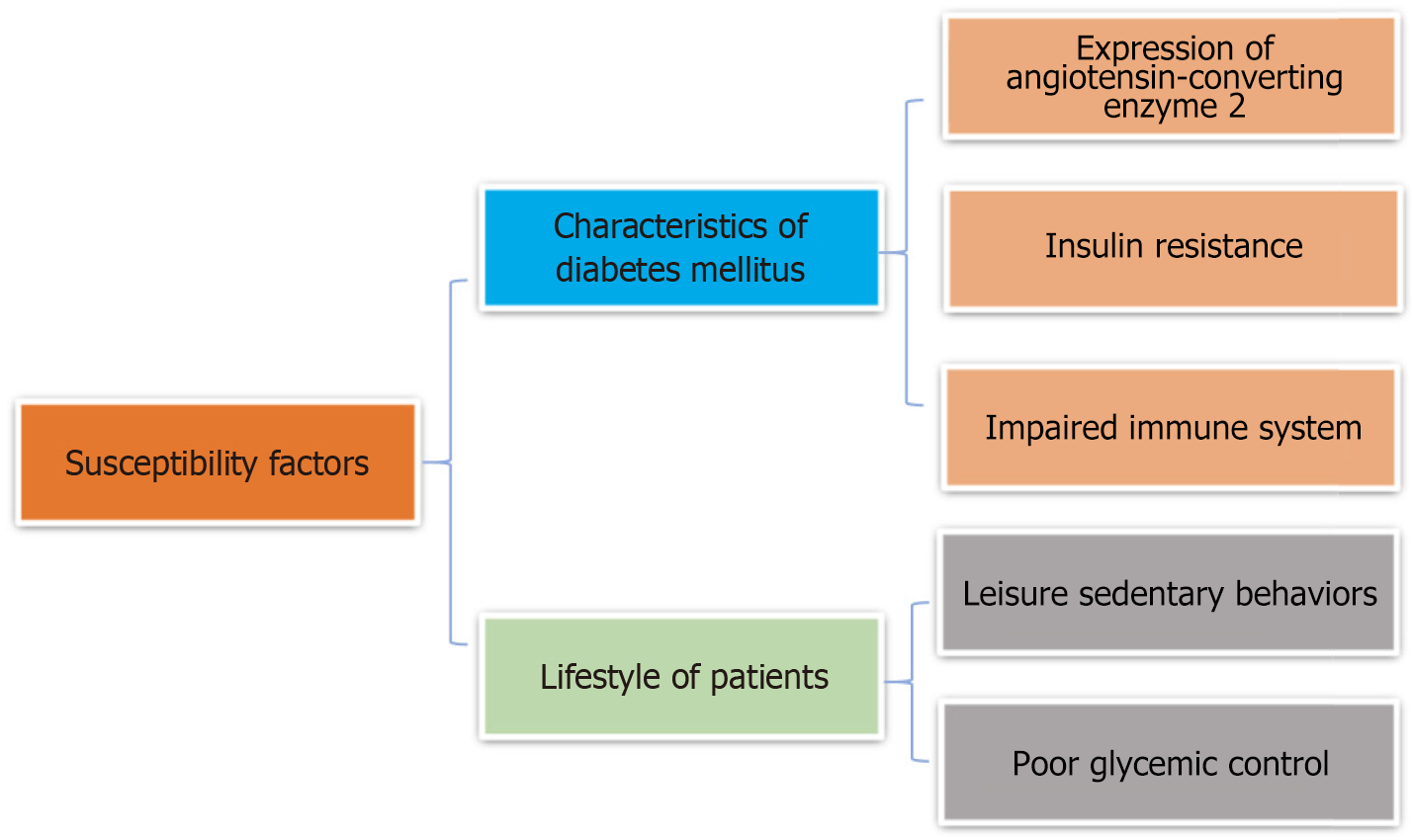
Possible risk factors for high susceptibility to COVID-19 in diabetic patients are summarized in Figure 1.
As an enzymatic functional receptor on the cell surface, angiotensin-converting enzyme 2 (ACE2) plays an important role in the renin-angiotensin-aldosterone system (RAAS). It exerts most of the RAAS physiological and pathophysiological effects, and genetically controls cardiovascular function and injury to several organs, including the lungs, liver, and kidney[16]. SARS-CoV-2 disrupts ACE/ACE2 homeostasis and activates RAAS, eventually leading to COVID-19 progression, especially in patients with comorbidities including hypertension, DM, and cardiovascular disease. In the pancreas, ACE2 antagonizes angiotensin II-mediated oxidative stress and apoptosis in islet cells, exerting islet protective functions. When the body is infected with neocoronaviruses, they reduce ACE2 expression by binding to it or directly damaging ACE2-expressing pancreatic islet β-cells, which ultimately leads to glucose metabolism disorders and acute hyperglycemia[17]. In human kidney-like organs, diabetic disease has recently been revealed to induce ACE2 expression, and single-cell analyses of 436 patients have suggested that increased ACE2 expression in the lungs and kidneys may present an increased risk of SARS-CoV-2 infection[18]. Elevated glucose levels may directly promote SARS-CoV-2 replication and pro-inflammatory cytokine production. Furthermore, SARS-CoV-2 facilitates the shift in monocyte metabolism to aerobic glycolysis, thereby sustaining SARS-CoV-2-induced monocyte responses and their replication.
In addition to the imbalance in ACE2 expression after SARS-CoV-2 infection, cellular metabolism in renal-like organs contributes directly to the increasing viral load. Cells isolated from renal biopsies of DM patients show altered mitochondrial respiration and glycolysis, leading to an increased prevalence of SARS-CoV-2 infection. By contrast, dichloroacetate could reduce SARS-CoV-2 infection in patient cells as an aerobic glycolysis inhibitor[19]. Shukla et al[5] emphasized the potential of ACE2 receptors in the kidney as a treatment and prevention target of diabetic nephropathy, which was a microvascular complication induced by a long-term hyperglycemic environment, manifested by proteinuria and deterioration of the glomerular filtration rate. However, there is concern that ACE2 overexpression leads to a more rapid viral uptake, which may exacerbate lung injury and result in fatal outcomes in COVID-19 patients[5].
In cases of overnutrition, more insulin is secreted under the influence of hyperglycemia, thereby leading to insulin resistance, which may be an aberrant immune response caused by the damaged insulin/IGF signaling pathway in the metabolic organs. This signaling pathway is also impaired by interferon regulator 1 produced through SARS-CoV-2 infection, leading to the development of adverse outcomes, such as disease exacerbation, cell death, and metabolic disorders in COVID-19 patients[20]. Obesity-induced chronic activation of pro-inflammatory signaling pathways (e.g., autocrine/paracrine cytokine signaling and endocrine cytokine signaling) may lead to vascular and metabolic problems and decreased insulin sensitivity, which may be one of the mechanisms of SARS-CoV-2 infection in DM patients[21]. Hyperglycemic states lead to a severe clinical course of COVID-19, which subsequently exhibits deleterious effects on glucose metabolism, and may lead to new-onset DM. In addition, insulin resistance is often associated with impaired mucosal and skin barrier integrity, which may exacerbate systemic inflammation through microbial translocation[22]. One study examined the relationship between the pre-diabetic microbiome and host health by characterizing microbiomes, and showed that insulin-resistant patients exhibited reduced fecal microbiome richness and increased susceptibility in skin microbiome to opportunistic pathogens[23]. Thus, insulin resistance results in microbiota dysbiosis that increases host susceptibility to viral infections.
The key components of innate immunity (e.g., macrophages and neutrophils) are activated by SARS-CoV-2 infection. Subsequently, immune cells in the adaptive immune system (e.g., antibody-producing B and T cells) are also activated following the release of multiple chemokines and cytokines. However, in severe cases, the over-activation of immune cells produces a cytokine storm, and thus causes damage to the organs in these patients, leading to a poor disease prognosis[24]. Elevated blood glucose levels also produce covalent glycoconjugates with a variety of proteins through non-enzymatic glycosylation, which can impair humoral immunity and increase susceptibility to SARS-CoV-2 infection[25]. Elevated levels of adipokines of adipose-tissue origin, interferon, and tumor necrosis factor a in DM patients may impair immune responses to SARS-CoV-2[26]. Moreover, DM patients have an impaired ability to clear SARS-CoV-2 from their circulation[27].
In addition, obesity, as one of the recognized risk factors for type 2 DM, can also be a major trigger of systemic low-level inflammation. Increased responses of the immune system, which are associated with obesity, can lead to metabolic disorders in insulin-sensitive tissues, further aggravating insulin resistance and forming a vicious circle. Subsequently, a compromised immune system and various metabolic imbalances increase patient susceptibility to SARS-CoV-2. DM leads to decreased immune function, increased production of pro-inflammatory cytokines, and a thrombotic state, thus increasing the severity of comorbid COVID-19 in patients with DM. Varga et al[28] found that endothelial cells may be directly infected by SARS-CoV-2, which is followed by necrosis, eventually showing hypercoagulable states[28]. This may be related to the increased incidence of thrombotic events in COVID-19 patients. The presence of increased pro-inflammatory cytokines in DM patients is associated with a “cytokine storm” of excessive inflammation in response to the virus. These responses may produce a fragile environment in the body, leading to exacerbation of COVID-19 symptoms, such as acute respiratory distress syndrome and severe pneumonia, which can increase mortality in COVID-19 patients[29].
Owing to the COVID-19 pandemic, strict measures such as "lockdown" were adopted in many regions. People had to spend less time outdoors and more sedentary time at home. A lack of physical activity may be associated with an increased risk of developing various chronic diseases[30]. A cohort study of adults in the United Kingdom has suggested that unhealthy lifestyle, such as physical inactivity and smoking, increases susceptibility of SARS-CoV-2 infection[31]. Chen et al[32] applied a Mendelian randomization method to assess the potential causal effects of sedentary behavior on COVID-19 susceptibility, hospitalization, and severity characteristics. The results suggest that those with obesity or type 2 DM, especially in older people, comprise a susceptible subgroup to sedentary behavior during a pandemic. Metabolic disorders and smoking behavior may also contribute to COVID-19 susceptibility associated with recreational sedentary behavior.
Maintaining healthy blood glucose levels is beneficial for DM patients to reduce the chances of hospitalization and severity and mortality in COVID-19 DM patients. In a retrospective study of 952 COVID-19 patients comorbid with type 2 DM, a stable blood glycemic control between 3.9 mmol/L and 10.0 mmol/L reduced patient mortality by a factor of 10[33]. A study in Saudi Arabia showed that less than 15% of patients with type 2 DM were able to maintain optimal glycemic control[34]. Regarding the factors associated with adherence to diabetic dietary guidelines, there was no association between educational attainment and dietary adherence. Best dietary adherence was found to exist in patients diagnosed for 3–5 years. In DM patients, poor glycemic control leads to a higher susceptibility to the effects of COVID-19[35]. Overall, fluctuations in blood glucose levels and their complications make it more difficult to treat viral infections in DM patients.
Vaccination is an important measure to prevent the spread of the SARS-CoV-2 virus and the emergence of new variants[36]. The Pfizer (BioNTech) vaccination was reportedly > 95% effective in reducing COVID-19 in people who had never been infected. On the other hand, Moderna’s vaccine was 94.1% effective in preventing the occurrence of symptomatic infections in people who had not been previously infected with COVID-19[37]. In addition to general prophylaxes for COVID-19, including maintaining social distancing, mask-wearing, proper hand hygiene, and vaccinations[38], other prevention strategies are required to protect DM patients with COVID-19. First, they should be advised to adopt healthy lifestyles by reducing sedentary behaviors, promoting appropriate exercise, and maintaining adherence to diets and tight control of blood glucose levels. In addition, increased education on hyperglycemia is an important step in patient management. Various digital solutions can be used to disseminate information, educate persons with disabilities, track individuals, monitor their health, and help them to take care of themselves. In Europe, cooperation between governments and DM associations has enabled the effective implementation and expansion of telemedicine services, thus ensuring continuity of care for people with DM[39]. During the Chinese COVID-19 epidemic, a wide range of online services for blood glycemic control were implemented for patients with DM and the general population. Free educational videos and e-books on DM self-management and COVID-19 prevention were also made available to the public through the WeChat mobile application[40].
Insulin therapy is the preferred treatment for COVID-19 patients with severe hyperglycemia, especially for heavy and critically ill patients. Insulin therapy exerts a powerful anti-inflammatory effect during critical illness and improves blood glucose management in hospitalized patients[41]. Although there is uncertainty about the use or discontinuation of some glucose-lowering medications, controlling blood glucose levels is necessary.
Sodium-glucose cotransporter protein-2 (SGLT 2) inhibitors could not only treat type 2 DM but also exhibit anti-inflammatory effects and reduce cardiovascular and renal complications[42]. However, the use of SGLT 2 inhibitors during COVID-19 infection may cause decreased blood volume due to vomiting and anorexia, as well as diabetic ketoacidosis triggered by the direct cytolytic effect of the virus on β-cells. These factors further lead to reduced endogenous insulin secretion and increased inflammatory response and interleukin-6 levels[43].
Dipeptidyl peptidase-4 inhibitors are transmembrane glycoproteins that play an important role in glucose homeostasis. They may have therapeutic benefits in COVID-19 and may reduce the production of pro-inflammatory cytokines[44]. Glucagon-like peptide-1 receptor agonists have anti-inflammatory effects on mild inflammation and help reduce weight in obese individuals, a condition associated with chronic inflammation and impaired immune response[45]. However, a risk of gastrointestinal side effects, such as nausea, vomiting, and aspiration, have been presented[46]. Although metformin has anti-inflammatory properties and can reduce inflammatory biomarker expression, its immunomodulatory role in the context of COVID-19 remains unclear. Crouse et al[47] found that metformin may improve the prognosis of patients hospitalized with type 2 DM and COVID-19 by reducing mortality. However, it carries a risk of lactic acidosis and is not indicated for critically ill patients. Li et al[48] showed that thiazolidinediones were the peroxisome proliferator-activated receptor-γ agonists and could ameliorate insulin resistance and have anti-inflammatory and anti-atherosclerotic properties[48]. Unfortunately, they are associated with exacerbation of edema and heart failure and are not suitable for critically ill patients[49].
The therapeutic drugs for COVID-19 in DM patients and their properties and risks are shown in Table 1.
| Therapeutic drug | Anti-inflammatory properties | Risks |
| Insulin | Yes | Risk of hypoglycemia |
| SGLT-2 inhibitors | Yes | Risk of euglycemic diabetic ketoacidosis |
| DPP-4 inhibitors | Yes | Relatively safe |
| GLP-1 RA | Yes | Gastrointestinal symptoms |
| Metformin | Yes | Risk of lactic acidosis |
| Pioglitazone | Yes | Risk of fluid retention and heart failure |
To date, there is still no specific remedy for COVID-19 in DM patients; therefore, early diagnosis, timely treatment, and strict management of patients with DM and COVID-19 are important to improve their prognoses. Recent studies have reported that green tea polyphenols possess antiviral activity against SARS-CoV-2, as well as antibacterial, antioxidant, and anti-inflammatory effects[50]. Since drug improvement and vaccine progression require a significant investment of time, alternative strategies also need to be urgently tested. The potential effects of various drugs on the main SARS-CoV-2 protease have been demonstrated through combined computerized and biochemical studies[51]. Seven promising medicines, namely, sapanisertib, ornidazole, napabucasin, lenalidomide, daniquinone, indolimod, and salicylamide were identified through virtual screening against the major proteases of SARS-CoV-2 and are considered essential for the treatment of COVID-19[52]. However, extensive in vivo and in vitro studies are warranted to assess each drug’s activity. In addition, numerous studies have reported that nutritional therapeutic measures, including natural dietary supplements, vitamins, minerals, micronutrients, probiotics, and hydrogen therapy[53] could be promising and effective adjunctive treatments for COVID-19 owing to their antioxidant, antiviral, anti-inflammatory, and positive immunomodulatory properties[54]. For patients with COVID-19 comorbid DM, tailored treatment strategies and optimal glycemic control should be developed based on specific clinical classification, comorbidities, and other influencing factors. All hospitalized patients should control their blood glucose levels to monitor the disease progression and avoid exacerbations. In critically ill patients, early recognition and proper management of adverse drug reactions can prevent the worsening of symptoms. The emphasis on preferred insulin therapy and the development of individualized glycemic management strategies are important. Optimized glycemic management in terms of glucose monitoring, lifestyle, and hyperglycemia education is required to prevent adverse prognoses and improve disease regression. Further epidemiological, clinical, and fundamental studies on the possible factors responsible for the high susceptibility of DM patients to COVID-19 in terms of the characteristics of DM and lifestyle, and preventive and therapeutic strategies for COVID-19 in DM patients, are urgently needed in the future.
We would like to thank Xue-Lu Yu, Xiao-Wen Xing, the reviewers, and the editors for preparing and improving our manuscript.
| 1. | World Health Organization. COVID-19 epidemiological update. Mar 15, 2024. [cited 27 March 2024]. Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update-15-march-2024. |
| 2. | Kumar R, Yeni CM, Utami NA, Masand R, Asrani RK, Patel SK, Kumar A, Yatoo MI, Tiwari R, Natesan S, Vora KS, Nainu F, Bilal M, Dhawan M, Emran TB, Ahmad T, Harapan H, Dhama K. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: Concerns, challenges, management and mitigation strategies-a narrative review. J Infect Public Health. 2021;14:863-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5938] [Article Influence: 989.7] [Reference Citation Analysis (8)] |
| 4. | Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020;11:1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 5. | Shukla AK, Awasthi K, Usman K, Banerjee M. Role of renin-angiotensin system/angiotensin converting enzyme-2 mechanism and enhanced COVID-19 susceptibility in type 2 diabetes mellitus. World J Diabetes. 2024;15:606-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Harreiter J, Roden M. [Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien Klin Wochenschr. 2023;135:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 7. | Vasquez-Muñoz M, Arce-Alvarez A, von Igel M, Veliz C, Ruiz-Esquide G, Ramirez-Campillo R, Alvarez C, Ramirez-Velez R, Crespo FA, Izquierdo M, Del Rio R, Andrade DC. Oscillatory pattern of glycemic control in patients with diabetes mellitus. Sci Rep. 2021;11:5789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, Ytterberg AJ, Olauson H, Axelsson J, Sundelin B, Patrakka J, Scotney P, Nash A, Eriksson U. Reducing VEGF-B Signaling Ameliorates Renal Lipotoxicity and Protects against Diabetic Kidney Disease. Cell Metab. 2017;25:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Stratmann B. Dicarbonyl Stress in Diabetic Vascular Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107:75-83; quiz 84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Papadokostaki E, Tentolouris N, Liberopoulos E. COVID-19 and diabetes: What does the clinician need to know? Prim Care Diabetes. 2020;14:558-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 13. | Sękowski K, Grudziąż-Sękowska J, Goryński P, Pinkas J, Jankowski M. Epidemiological Analysis of Diabetes-Related Hospitalization in Poland before and during the COVID-19 Pandemic, 2014-2020. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4209] [Article Influence: 841.8] [Reference Citation Analysis (0)] |
| 15. | Kazakou P, Lambadiari V, Ikonomidis I, Kountouri A, Panagopoulos G, Athanasopoulos S, Korompoki E, Kalomenidis I, Dimopoulos MA, Mitrakou A. Diabetes and COVID-19; A Bidirectional Interplay. Front Endocrinol (Lausanne). 2022;13:780663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Clarke NE, Turner AJ. Angiotensin-converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, Jiang S, Demeter J, Bevacqua RJ, Chang CA, Whitener RL, Stalder AK, Zhu B, Chen H, Goltsev Y, Tzankov A, Nayak JV, Nolan GP, Matter MS, Andino R, Jackson PK. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33:1565-1576.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 18. | Jiang X, Eales JM, Scannali D, Nazgiewicz A, Prestes P, Maier M, Denniff M, Xu X, Saluja S, Cano-Gamez E, Wystrychowski W, Szulinska M, Antczak A, Byars S, Skrypnik D, Glyda M, Król R, Zywiec J, Zukowska-Szczechowska E, Burrell LM, Woolf AS, Greenstein A, Bogdanski P, Keavney B, Morris AP, Heagerty A, Williams B, Harrap SB, Trynka G, Samani NJ, Guzik TJ, Charchar FJ, Tomaszewski M. Hypertension and renin-angiotensin system blockers are not associated with expression of angiotensin-converting enzyme 2 (ACE2) in the kidney. Eur Heart J. 2020;41:4580-4588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Garreta E, Prado P, Stanifer ML, Monteil V, Marco A, Ullate-Agote A, Moya-Rull D, Vilas-Zornoza A, Tarantino C, Romero JP, Jonsson G, Oria R, Leopoldi A, Hagelkruys A, Gallo M, González F, Domingo-Pedrol P, Gavaldà A, Del Pozo CH, Hasan Ali O, Ventura-Aguiar P, Campistol JM, Prosper F, Mirazimi A, Boulant S, Penninger JM, Montserrat N. A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Cell Metab. 2022;34:857-873.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Shin J, Toyoda S, Nishitani S, Onodera T, Fukuda S, Kita S, Fukuhara A, Shimomura I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism. 2022;133:155236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 809] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 22. | Kawano Y, Edwards M, Huang Y, Bilate AM, Araujo LP, Tanoue T, Atarashi K, Ladinsky MS, Reiner SL, Wang HH, Mucida D, Honda K, Ivanov II. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. 2022;185:3501-3519.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 23. | Zhou X, Shen X, Johnson JS, Spakowicz DJ, Agnello M, Zhou W, Avina M, Honkala A, Chleilat F, Chen SJ, Cha K, Leopold S, Zhu C, Chen L, Lyu L, Hornburg D, Wu S, Zhang X, Jiang C, Jiang L, Jiang L, Jian R, Brooks AW, Wang M, Contrepois K, Gao P, Rose SMS, Tran TDB, Nguyen H, Celli A, Hong BY, Bautista EJ, Dorsett Y, Kavathas PB, Zhou Y, Sodergren E, Weinstock GM, Snyder MP. Longitudinal profiling of the microbiome at four body sites reveals core stability and individualized dynamics during health and disease. Cell Host Microbe. 2024;32:506-526.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 24. | Rabaan AA, Al-Ahmed SH, Garout MA, Al-Qaaneh AM, Sule AA, Tirupathi R, Mutair AA, Alhumaid S, Hasan A, Dhawan M, Tiwari R, Sharun K, Mohapatra RK, Mitra S, Emran TB, Bilal M, Singh R, Alyami SA, Moni MA, Dhama K. Diverse Immunological Factors Influencing Pathogenesis in Patients with COVID-19: A Review on Viral Dissemination, Immunotherapeutic Options to Counter Cytokine Storm and Inflammatory Responses. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 26. | Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 28. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4584] [Article Influence: 916.8] [Reference Citation Analysis (0)] |
| 29. | Johnson BS, Laloraya M. A cytokine super cyclone in COVID-19 patients with risk factors: the therapeutic potential of BCG immunization. Cytokine Growth Factor Rev. 2020;54:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Hossain MJ, Ahmmed F, Rahman SMA, Sanam S, Emran TB, Mitra S. Impact of online education on fear of academic delay and psychological distress among university students following one year of COVID-19 outbreak in Bangladesh. Heliyon. 2021;7:e07388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 32. | Chen X, Hong X, Gao W, Luo S, Cai J, Liu G, Huang Y. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: a Mendelian randomization study. J Transl Med. 2022;20:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 33. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang C, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH, Li H. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1099] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 34. | Alshahri BK, Bamashmoos M, Alnaimi MI, Alsayil S, Basaqer S, Al-Hariri MT, Vallaba Doss CA Sr. Assessment of Self-Management Care and Glycated Hemoglobin Levels Among Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study From the Kingdom of Saudi Arabia. Cureus. 2020;12:e11925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Utli H, Vural Doğru B. The effect of the COVID-19 pandemic on self-management in patients with type 2 diabetics. Prim Care Diabetes. 2021;15:799-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Nainu F, Abidin RS, Bahar MA, Frediansyah A, Emran TB, Rabaan AA, Dhama K, Harapan H. SARS-CoV-2 reinfection and implications for vaccine development. Hum Vaccin Immunother. 2020;16:3061-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Tareq AM, Emran TB, Dhama K, Dhawan M, Tallei TE. Impact of SARS-CoV-2 delta variant (B.1.617.2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccin Immunother. 2021;17:4126-4127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Peng J, Su D, Zhang Z, Wang M. Identification and management of asymptomatic carriers of coronavirus disease 2019 (COVID-19) in China. Influenza Other Respir Viruses. 2020;14:599-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Barone MTU, Ngongo B, Harnik SB, Oliveira LX, Végh D, de Luca PV, Pedrosa HC, Giraudo F, Cardona-Hernandez R, Chaudhury N, Menna-Barreto L. COVID-19 associated with diabetes and other noncommunicable diseases led to a global health crisis. Diabetes Res Clin Pract. 2021;171:108587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 41. | Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 43. | Palermo NE, Sadhu AR, McDonnell ME. Diabetic Ketoacidosis in COVID-19: Unique Concerns and Considerations. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 44. | Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 45. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1107] [Article Influence: 158.1] [Reference Citation Analysis (1)] |
| 46. | Aldhaleei WA, Abegaz TM, Bhagavathula AS. Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort. Pharmaceuticals (Basel). 2024;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 47. | Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin Use Is Associated With Reduced Mortality in a Diverse Population With COVID-19 and Diabetes. Front Endocrinol (Lausanne). 2020;11:600439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 48. | Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 49. | Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O'Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR; IRIS Trial Investigators. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 50. | Tallei TE, Fatimawali, Niode NJ, Idroes R, Zidan BMRM, Mitra S, Celik I, Nainu F, Ağagündüz D, Emran TB, Capasso R. A Comprehensive Review of the Potential Use of Green Tea Polyphenols in the Management of COVID-19. Evid Based Complement Alternat Med. 2021;2021:7170736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Mahmud S, Paul GK, Afroze M, Islam S, Gupt SBR, Razu MH, Biswas S, Zaman S, Uddin MS, Khan M, Cacciola NA, Emran TB, Saleh MA, Capasso R, Simal-Gandara J. Efficacy of Phytochemicals Derived from Avicennia officinalis for the Management of COVID-19: A Combined In Silico and Biochemical Study. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 52. | Chowdhury KH, Chowdhury MR, Mahmud S, Tareq AM, Hanif NB, Banu N, Reza ASMA, Emran TB, Simal-Gandara J. Drug Repurposing Approach against Novel Coronavirus Disease (COVID-19) through Virtual Screening Targeting SARS-CoV-2 Main Protease. Biology (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 53. | Wang M, Peng J, Hui J, Hou D, Li W, Yang J. Hydrogen therapy as an effective and novel adjuvant treatment against COVID-19. QJM. 2021;114:74-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Wang MK, Yu XL, Zhou LY, Si HM, Hui JF, Hou DY, Li WP, Yang JS. COVID-19 and liver dysfunction: What nutritionists need to know. World J Gastroenterol. 2022;28:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |