Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1627
Revised: May 12, 2024
Accepted: June 12, 2024
Published online: July 15, 2024
Processing time: 115 Days and 17.5 Hours
Diabetic foot ulcers (DFUs) are one of the most severe and popular complications of diabetes. The persistent non-healing of DFUs is the leading cause of ampu-tation, which causes significant mental and financial stress to patients and their families. Macrophages are critical cells in wound healing and perform essential roles in all phases of wound healing. However, no studies have been carried out to systematically illustrate this area from a scientometric point of view. Although there have been some bibliometric studies on diabetes, reports focusing on the investigation of macrophages in DFUs are lacking.
To perform a bibliometric analysis to systematically assess the current state of research on macrophage-related DFUs.
The publications of macrophage-related DFUs from January 1, 2004, to December 31, 2023, were retrieved from the Web of Science Core Collection on January 9, 2024. Four different analytical tools: VOSviewer (v1.6.19), CiteSpace (v6.2.R4), HistCite (v12.03.07), and Excel 2021 were used for the scientometric research.
A total of 330 articles on macrophage-related DFUs were retrieved. The most published countries, institutions, journals, and authors in this field were China, Shanghai Jiao Tong University of China, Wound Repair and Regeneration, and Aristidis Veves. Through the analysis of keyword co-occurrence networks, historical direct citation networks, thematic maps, and trend topics maps, we synthesized the prevailing research hotspots and emerging trends in this field.
Our bibliometric analysis provides a comprehensive overview of macrophage-related DFUs research and insights into promising upcoming research.
Core Tip: In This study, we present the first bibliometric analysis of research on macrophage-related diabetic foot ulcers, providing a comprehensive overview of the field. Through keyword and research hotspots analysis, we identified three main research themes: “two main phenotypic transitions in macrophages,” “the complex roles of these phenotypes,” and “the function of exosomes in these transitions,” which are expected to become recent research hotspots in the field. This analysis aims to assist researchers in identifying new study areas and pinpointing emerging hotspots and frontiers.
- Citation: Wen JP, Ou SJ, Liu JB, Zhang W, Qu YD, Li JX, Xia CL, Yang Y, Qi Y, Xu CP. Global trends in publications regarding macrophages-related diabetic foot ulcers in the last two decades. World J Diabetes 2024; 15(7): 1627-1644
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1627.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1627
Diabetic foot ulcers (DFUs) is one of the most severe and common complications of diabetes and the leading cause of lower limb amputations in patients with diabetes[1]. Studies have indicated that 19%–34% of individuals with diabetes develop foot ulcers during their lifetime[2,3]. Healing of DFUs is influenced by multiple factors, such as the production of growth factors[4-6], angiogenic responses[7-9], macrophage function[10-13], and epidermal nerves[14-16]. Macrophages are diverse and plastic cells that are instrumental in maintaining immune homeostasis[17]. These cells can polarize into either the classical M1 or the alternative M2 phenotype in response to different stimuli, including microbes, tissue damage, and lymphocyte activation[18]. Macrophages play a crucial role in resolving inflammation and facilitating wound healing in DFUs[12]. Therefore, quantitative analyses of the current state, key areas, and prospects in the literature regarding macrophages in DFUs are of great significance.
Bibliometrics employs mathematical and statistical methods for qualitative and quantitative analyses of academic literature within a discipline[19]. This approach assesses the trajectory and development of a field of study by evaluating the influence and distribution patterns of published articles[20]. Various information visualization tools such as CiteSpace (6.2. R4)[21], VOSviewer (1.6.19)[22], and online bibliometric analysis platforms (http://bibliometric.com/) are used for co-occurrence and clustering analyses. These tools visualize knowledge structures and application trends in a field, aiding researchers in identifying key resources, pivotal shifts, research hotspots, and prospective research directions[23]. Bibliometrics has been employed in diverse medical fields, including orthopedics[24,25], oncology[26,27], and im-munology[28,29]. Although there have been some bibliometric studies on diabetes[30,31], reports focusing on the investigation of macrophages in DFUs are lacking. Therefore, this study aimed to systematically assess the status, key points, and emerging trends in studies on macrophages in DFUs.
To identify relevant reports, we employed a targeted search strategy using the Web of Science Core Collection (WoSCC). The search terms used were as follows: TS = (diabetic foot OR foot, diabetic OR diabetic feet OR feet, diabetic OR foot ulcer, diabetic OR diabetic foot ulcer OR DFUs) AND TS = (macrophage OR macrophages). We limited our search to articles and reviews published in English between January 1, 2004 and December 31, 2023, and restricted our data source to the Science Citation Index Expanded (SCIE). Literature retrieval and data download were completed on January 9, 2024, to mitigate biases that could arise from frequent database updates. Two investigators independently conducted the initial data retrieval, with a high degree of consistency between their findings.
The initial dataset was extracted from the SCIE, encompassing a wide array of variables, such as the number of articles and citations, publication year, countries/regions, affiliations, authors, journals, references, and keywords. Subsequently, we cleaned the data by manually removing duplicate authors and rectifying misspelled elements. However, some degree of analytical error is unavoidable owing to factors such as multiple versions of cited references, identical abbreviated names for different authors, and variations in journal citation formats. However, we are confident that most of the raw data are reliable. To further refine the dataset, a thesaurus file was used before the analysis, which helped merge duplicate entries, correct misspellings, and eliminate irrelevant terms. Finally, the clean dataset was imported into VOSviewer and CiteSpace for comprehensive bibliometric analyses.
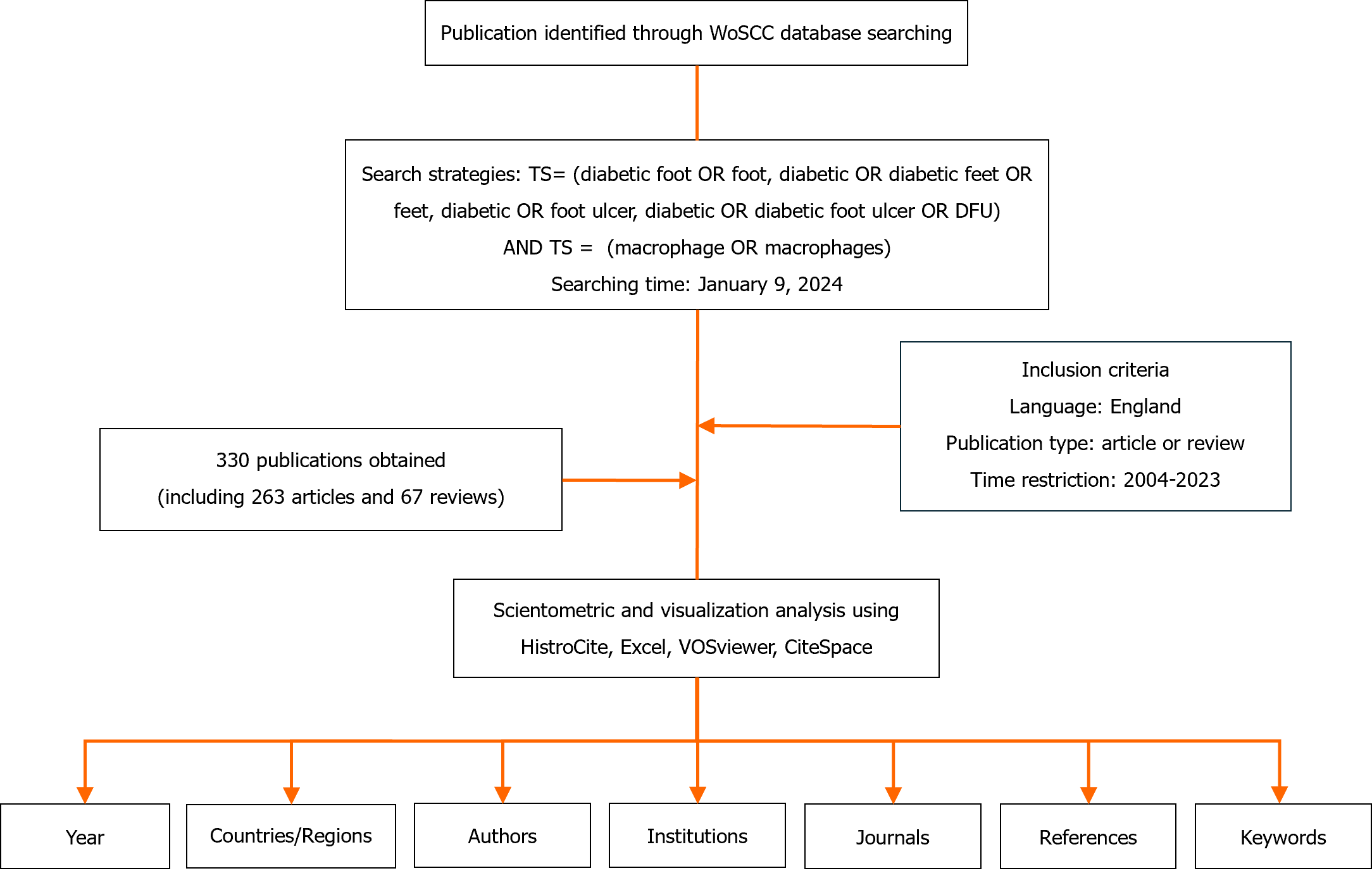
Bibliometric indicators such as the number of articles and citations are commonly used to represent the bibliographic landscape. Herein, we employed HistCite (12.03.07) to examine the annual output, publication language, and publication types. Furthermore, using the most recent edition of Journal Citation Reports, we sourced the impact factor (IF) of the journals in which the articles were published. We used VOSviewer to identify active countries/regions, influential journals, and scholars, as well as the most-cited journals and authors. We used Scimago Graphics (1.0.36.0) to visualize the distribution network of related publications. Within the VOSviewer connection graph, various nodes symbolize countries/regions, institutions, and publications, which represent instance counts or co-occurrence frequencies. The links between the nodes signify co-occurrence associations, and the thickness of these links correlates with the frequency of co-occurrence. We customized the VOSviewer to include a complete counting technique and tailored the threshold values (T) for different elements, such as countries/regions, institutions, journals, authors, and references, based on specific contexts. To explore emerging trends and significant works in specific research areas, we employed CiteSpace for keyword co-occurrence mapping and subsequent clustering. Finally, we managed the data and investigated publication trends using Microsoft Office Excel 2021. A flowchart detailing the methodical approach is presented in Figure 1.
Using our search strategy, we identified 330 relevant studies published between 2004 and 2023, including 263 articles and 67 reviews. The accumulated number of citations for the retrieved articles was 11312, averaging 34.28 citations per article.
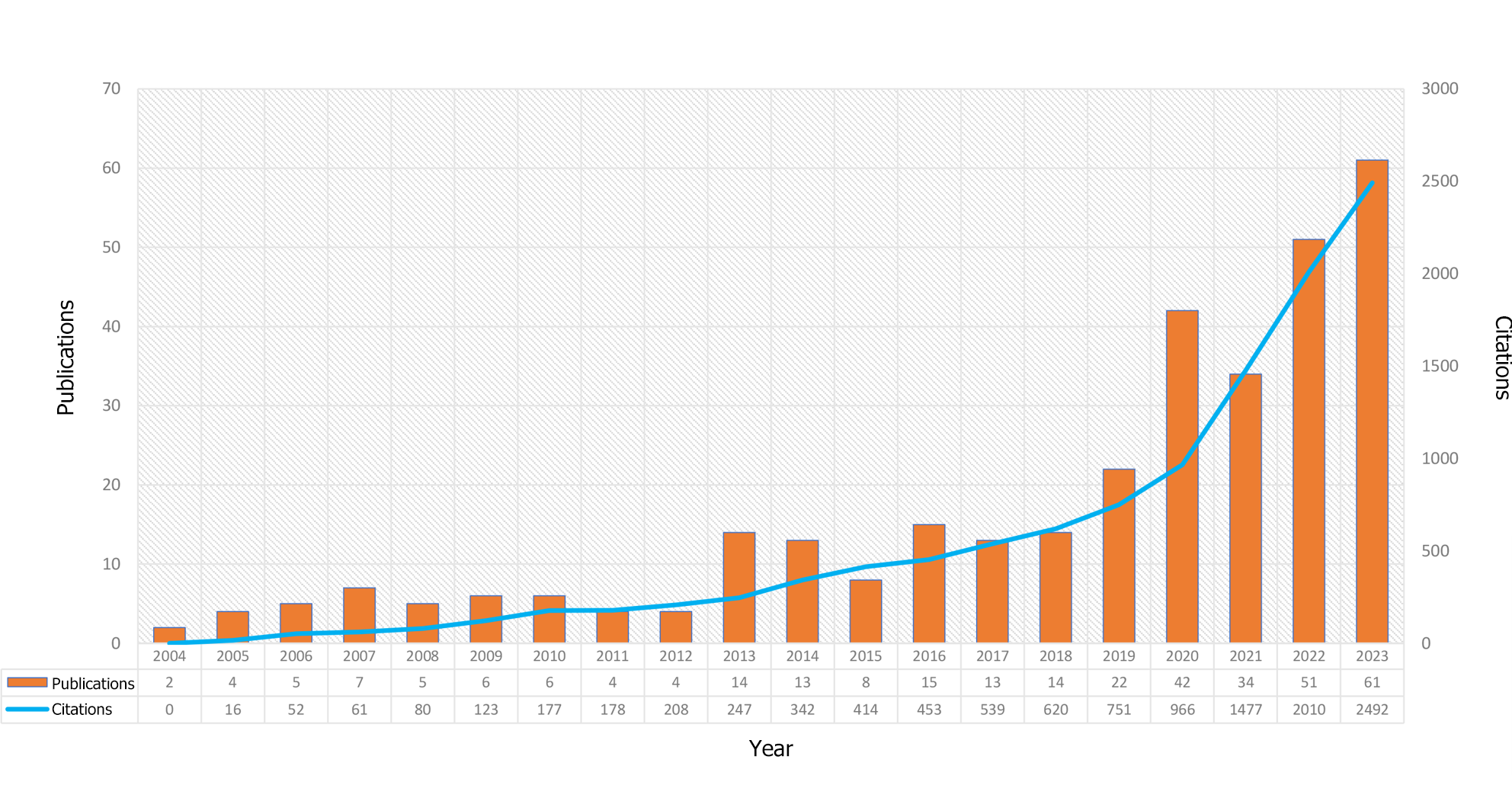
Figure 2 shows the annual number of publications regarding macrophages in DFUs between 2004 and 2023. The dataset revealed a general upward trend in publications over the past two decades, despite some yearly fluctuations. Starting at a modest baseline of only two publications in 2004 (0.61%), the field experienced its most prolific year in 2023, with 61 publications (18.48%). On average, the annual output was approximately 16.5 publications. Furthermore, in the past 5 years, the annual count consistently exceeded 30 publications; 2019 was the only exception to this, with 22 (6.67%) publications.
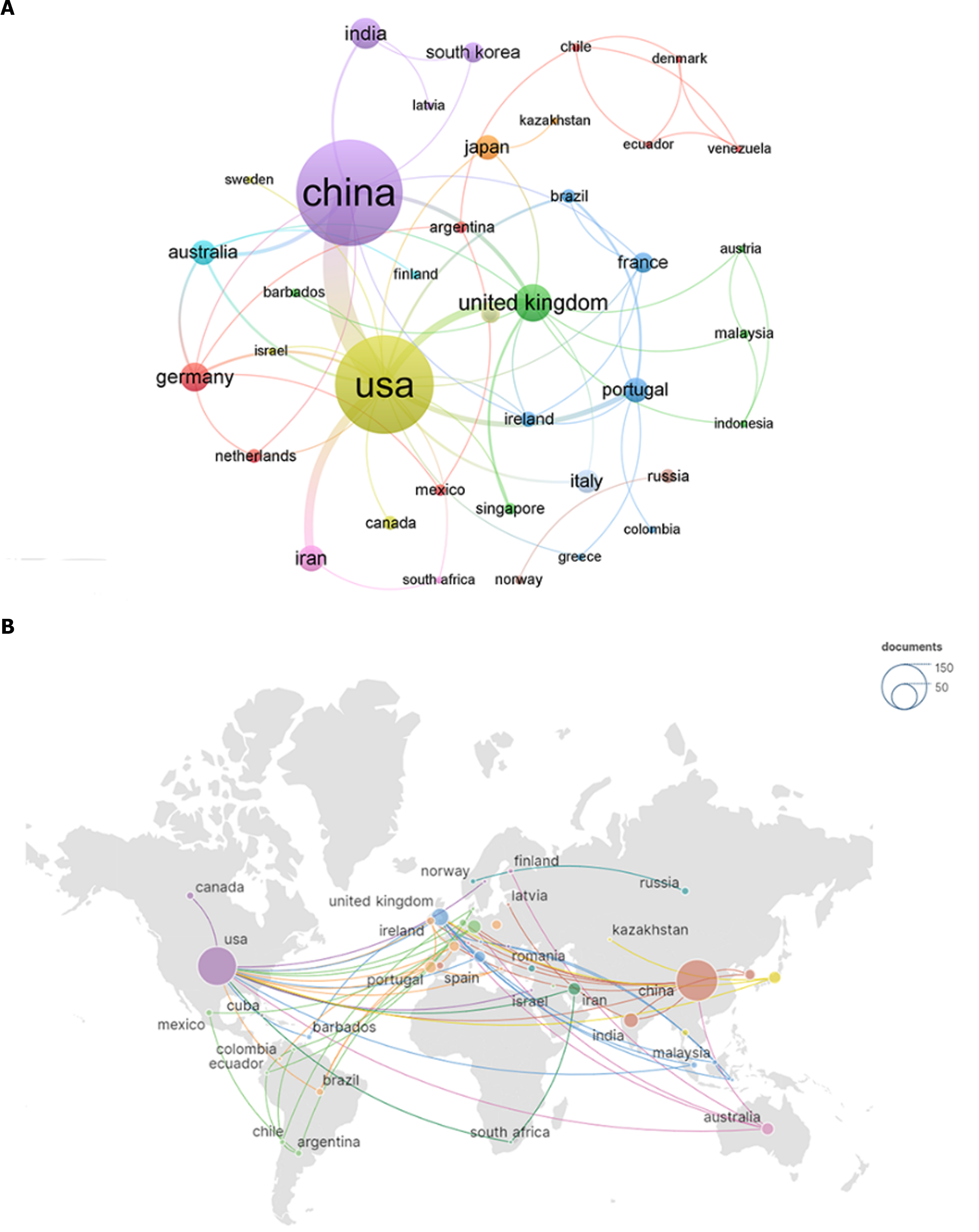
A total of 45 countries/regions contributed to this research field. We ranked the top 10 high-output countries/regions according to the number of publications (Table 1). All these countries/regions produced at least 10 publications. China published the most articles (n = 123, 37.27%), followed by the United States (n = 102, 30.91%), the United Kingdom (n = 21, 6.36%), India (n = 16, 4.85%), and Germany (n = 13, 3.94%). To visualize international collaborations, we used VOSviewer and Scimago Graphics to create a map (Figure 3). Notably, China and the United States had significantly larger nodes, indicating higher numbers of publications. However, regarding citation counts, the United States had 5136 citations (n = 5136, 45.40%), followed by China (n = 2309, 20.41%), and India (n = 1064, 9.41%). Intriguingly, despite having fewer publications than China, the United States had a much higher number of citations. Furthermore, numerous collaborations existed among different countries/regions that warrant attention.
| Rank | Country/region | Documents | Country/region | Citations |
| 1 | China | 123 | United States | 5136 |
| 2 | United States | 102 | China | 2309 |
| 3 | The United Kingdom | 21 | India | 1064 |
| 4 | India | 16 | Germany | 652 |
| 5 | Germany | 13 | Portugal | 631 |
| 6 | Iran | 12 | The United Kingdom | 611 |
| 7 | Japan | 12 | Japan | 544 |
| 8 | Portugal | 11 | Australia | 458 |
| 9 | Australia | 10 | Poland | 354 |
| 10 | Italy | 10 | Ireland | 223 |
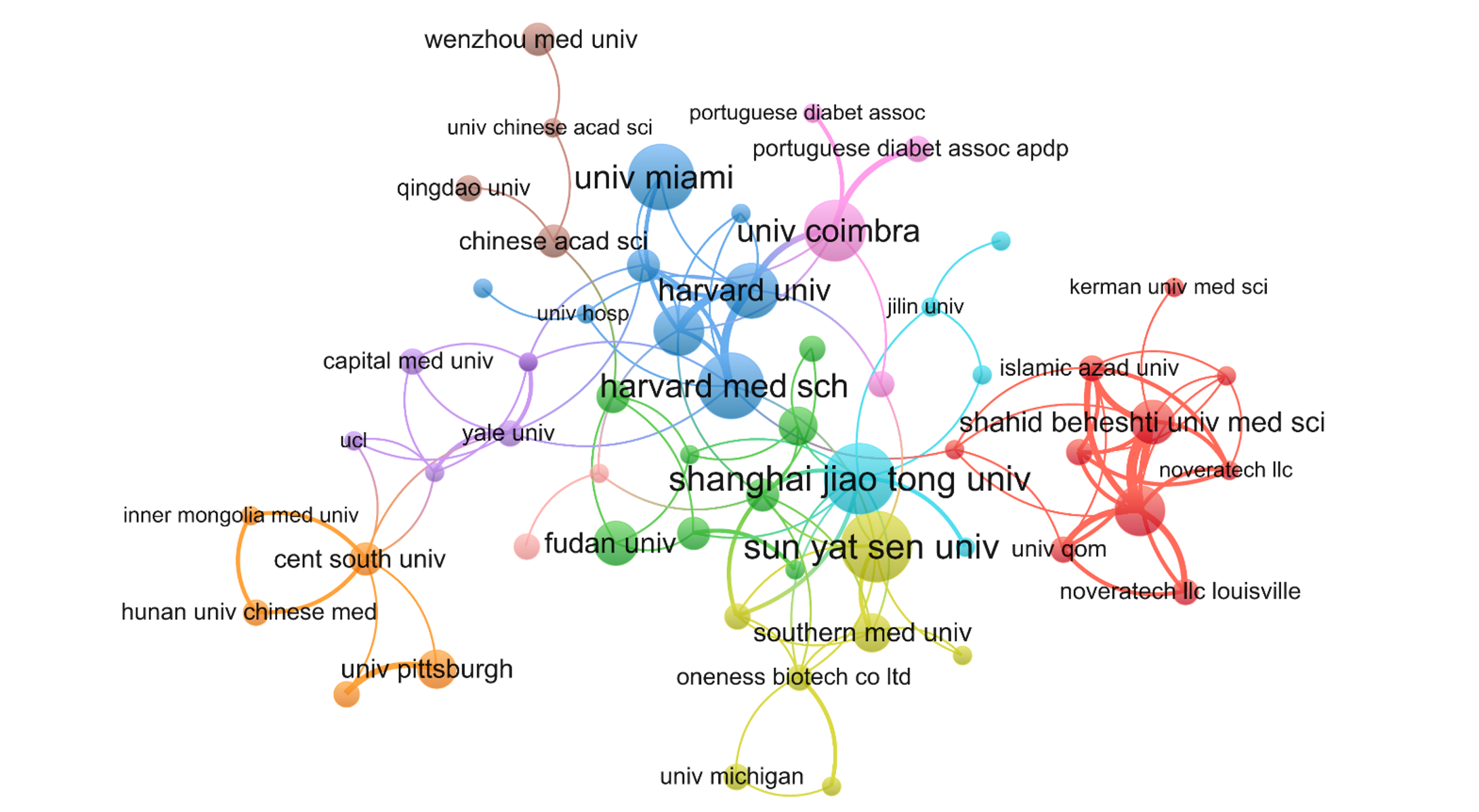
In total, 573 institutions contributed 330 publications regarding macrophage-related DFUs. Table 2 lists the top 10 institutions that contributed to this field, located in three distinct countries/regions: The United States, China, and Portugal. Among these, two Chinese institutions, Shanghai Jiao Tong University and Sun Yat-sen University, each published more than 10 articles (n = 11, 3.33%). Other top institutions included Harvard Medical School (n = 10, 3.03%), the University of Miami (n = 10, 3.03%), and the University of Coimbra (n = 9, 2.96%). To examine institutional collaboration, we built a network of institutions with at least two publications (T = 2) (Figure 4). The figure displays the largest subnetwork, in which nodes representing Shanghai Jiao Tong University, Sun Yat-sen University, Harvard Medical School, the University of Coimbra, and the University of Miami are notably larger, indicating higher publication counts. Additionally, this network revealed active collaborations among these institutions, including close collaborations between Shanghai Jiao Tong University and Sun Yat-sen University, and between the University of Coimbra and Harvard University.
| Rank | Institution | Documents | Institution | Citations |
| 1 | Shanghai Jiao Tong University (China) | 11 | University of Miami (United States) | 967 |
| 2 | Sun Yat-sen University (China) | 11 | University of Coimbra (Portugal) | 554 |
| 3 | Harvard Medical School (United States) | 10 | Harvard University (United States) | 420 |
| 4 | University of Miami (United States) | 10 | University of Mississippi (United States) | 379 |
| 5 | University of Coimbra (Portugal) | 9 | Shanghai Jiao Tong University (China) | 365 |
| 6 | Harvard University (United States) | 8 | University of Pennsylvania (United States) | 353 |
| 7 | Tufts University (United States) | 7 | University of Illinois Urbana-Champaign (United States) | 317 |
| 8 | University of Louisville (United States) | 7 | Ohio State University (United States) | 301 |
| 9 | Zhejiang University (China) | 7 | Tufts University (United States) | 290 |
| 10 | Fudan University (China) | 6 | The Chinese University of Hong Kong (China) | 261 |
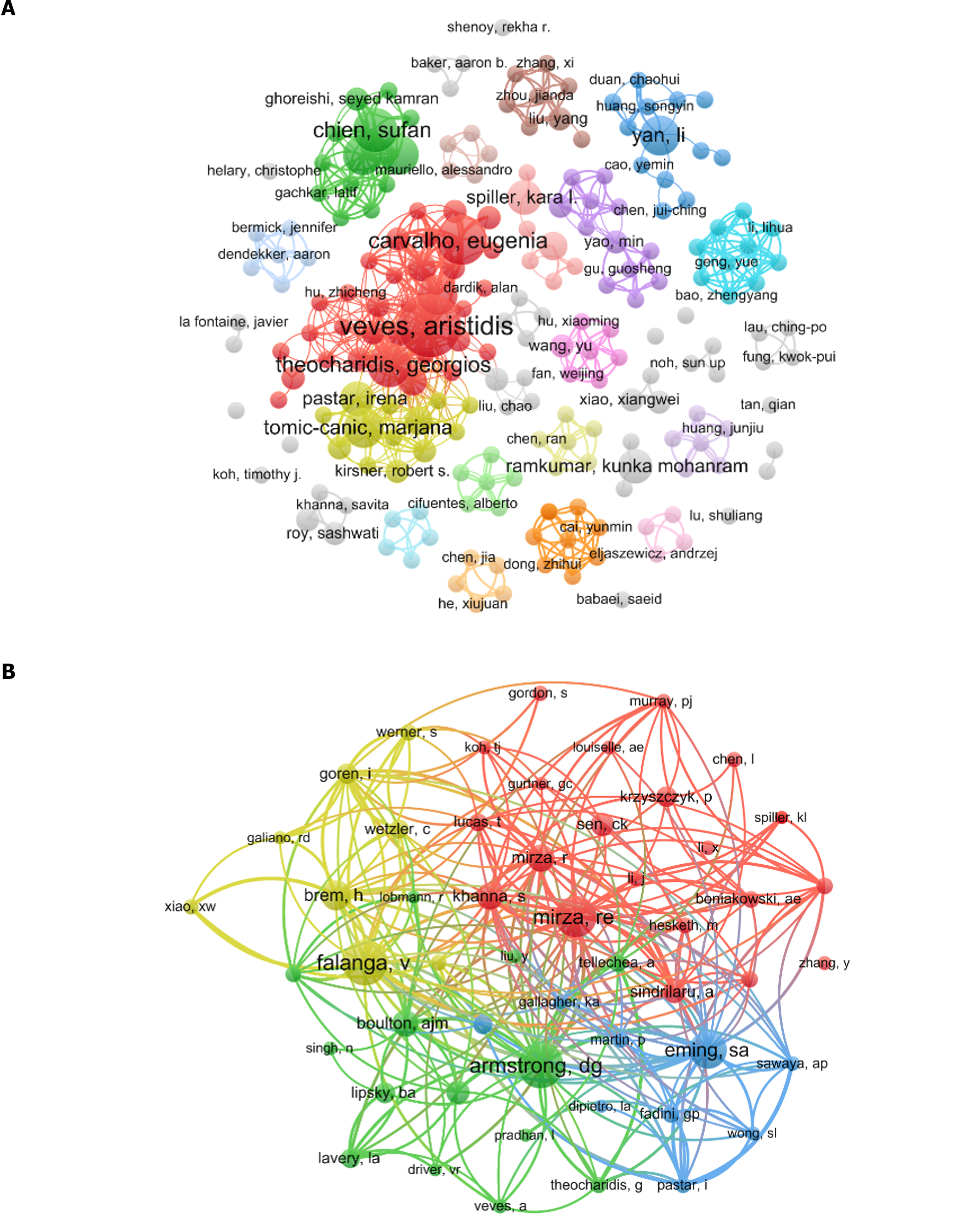
A total of 2145 authors contributed to the research field. The top 10 most productive authors are listed in Table 3. All these authors published more than five papers; however, Aristidis Veves had the highest publication count at 11 articles (3.33%), followed by Eugenia Carvalho (n = 8, 2.42%) and Sufan Chien (n = 7, 2.12%). To elucidate collaborative relationships, a co-authorship graph was constructed using authors with at least two publications (T = 2) (Figure 5A), in which the node representing “Aristidis Veves” was the largest, signifying the highest number of publications among the authors. Noteworthy collaborative ties were visible, particularly among the leading authors.
| Rank | Author | Count | Co-cited author | Co-citation |
| 1 | Aristidis Veves | 11 | David G Armstrong | 71 |
| 2 | Eugenia Carvalho | 8 | Vincent Falanga | 71 |
| 3 | Sufan Chien | 7 | Rita E Mirza | 64 |
| 4 | Georgios Theocharidis | 7 | Sabine A Eming | 58 |
| 5 | Abdollah Amini | 6 | Harold Brem | 46 |
| 6 | Mohamad Bayat | 6 | Andrew J M Boulton | 41 |
| 7 | Ana Tellechea | 6 | Savita Khanna | 41 |
| 8 | Marjana Tomic-Canic | 6 | William J Jeffcoate | 36 |
| 9 | Li Yan | 6 | Chandan K Sen | 36 |
| 10 | Irena Pastar | 5 | Anca Sindrilaru | 35 |
Regarding co-citations, of 13401 co-cited authors, four were co-cited more than 50 times (Table 3): David G Armstrong and Vincent Falanga had 71 co-citations each, followed by Rita E Mirza (n = 64) and Sabine A Eming (n = 58). To further explore this aspect, a co-citation graph was created using authors with at least 20 co-citations (T = 20) (Figure 5B), in which the node representing “David G Armstrong” was the largest, highlighting a high number of co-citations. Moreover, active co-citation relationships were evident among Vincent Falanga, David G Armstrong, and Rita E Mirza, the top three authors regarding co-citations.
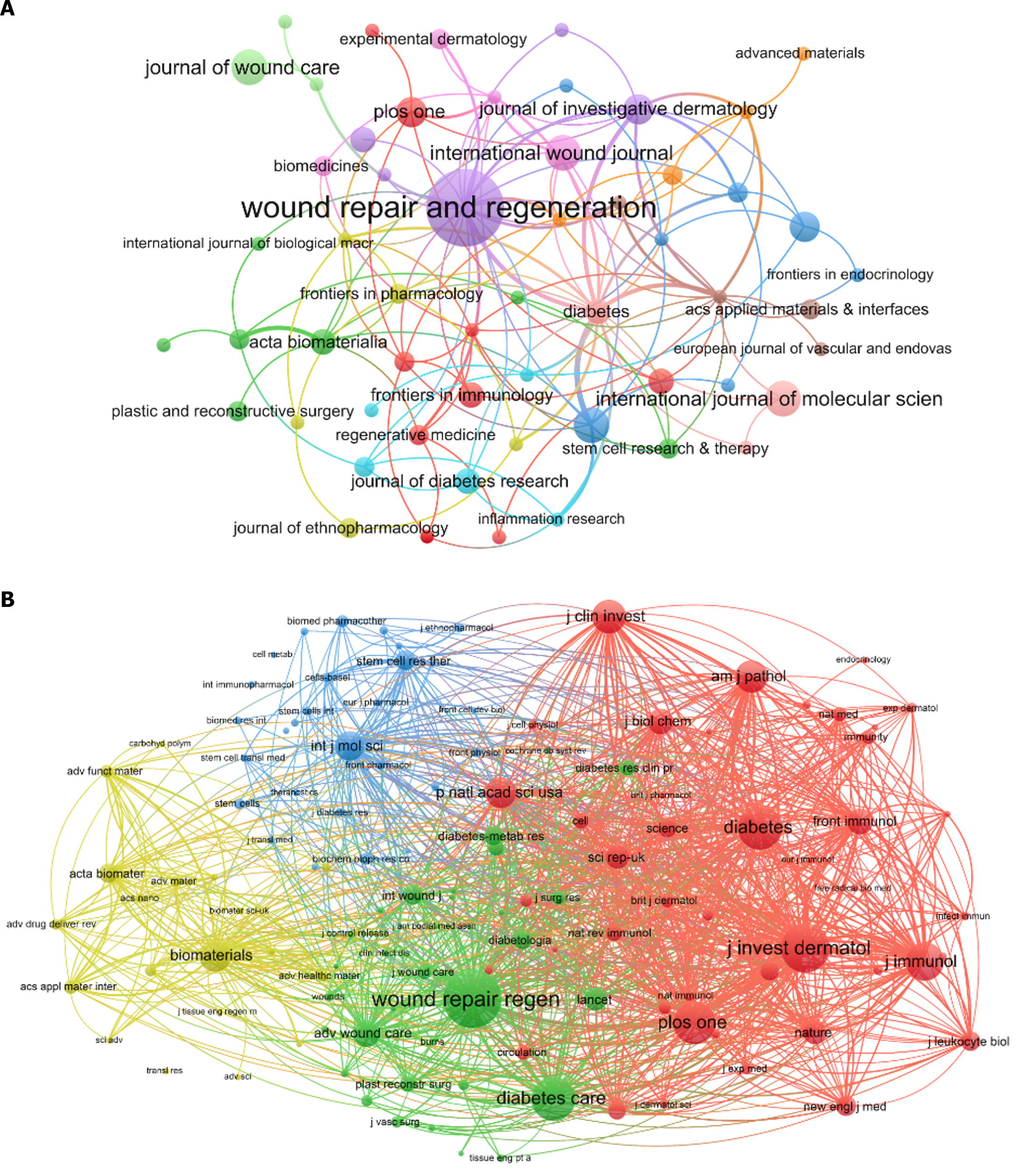
A total of 330 publications regarding this topic were published in 207 academic journals. Of these, five journals published at least six articles on the topic, all of which were based in developed countries (Table 4). Four of these journals were American. “Wound Repair and Regeneration” had the highest publication count at 15 articles (4.55%), followed by “International Journal of Molecular Sciences,” “International Wound Journal,” “Journal of Wound Care” and “Pharmaceutics” (for each journal, n = 6, 1.82%). A journal collaboration network was constructed using journals with at least two publications (T = 2) (Figure 6A), in which the node representing “Wound Repair and Regeneration” was the largest, indicating a higher publication count.
| Rank | Journal | Count | IF 2023 | JCR | Journal | Co-citation | IF 2023 | JCR |
| 1 | Wound Repair and Regeneration (United States) | 15 | 2.9 | Q2 | Wound Repair and Regeneration (United States) | 508 | 2.9 | Q2 |
| 2 | International Journal of Molecular Sciences (United States) | 6 | 5.6 | Q1 | Journal of Investigative Dermatology (United States) | 414 | 6.5 | Q1 |
| 3 | International Wound Journal (England) | 6 | 3.1 | Q2 | Diabetes (United States) | 369 | 7.7 | Q1 |
| 4 | Journal of Wound Care (England) | 6 | 1.9 | Q3 | Diabetes Care (United States) | 364 | 16.2 | Q1 |
| 5 | Pharmaceutics (Switzerland) | 6 | 5.4 | Q1 | Plos One (United States) | 346 | 3.7 | Q2 |
| 6 | Biomedicine & Pharmacotherapy (France) | 5 | 7.5 | Q1 | Journal of Immunology (United States) | 304 | 4.4 | Q2 |
| 7 | Journal of Investigative Dermatology (United States) | 5 | 6.5 | Q1 | Journal of Clinical Investigation (United States) | 266 | 15.9 | Q1 |
| 8 | Plos One (United States) | 5 | 3.7 | Q2 | American Journal of Pathology (United States) | 250 | 6.0 | Q1 |
| 9 | Acta Biomaterialia (England) | 4 | 9.7 | Q1 | Biomaterials (Netherlands) | 249 | 14.0 | Q1 |
| 10 | Advances in Wound Care (United States) | 4 | 4.9 | Q1 | Proceedings of The National Academy of Sciences of United States of America (United States) | 234 | 11.1 | Q1 |
Regarding co-citations journals, among 3203 co-cited academic journals, 10 were cited more than 200 times, including nine journals from the United States and one from the Netherlands. “Wound Repair and Regeneration” had the highest number of co-citations at 508, while “Diabetes Care” had the highest IF of 16.2 among the top 10 journals. A co-citation network was constructed using journals with at least 30 co-citations (T = 30) (Figure 6B), in which nodes representing “Wound Repair and Regeneration,” “Journal of Investigative Dermatology,” and “Diabetes Care” were larger, reflecting their higher co-citation counts. Within this network, close relationships were apparent among various co-cited journals.
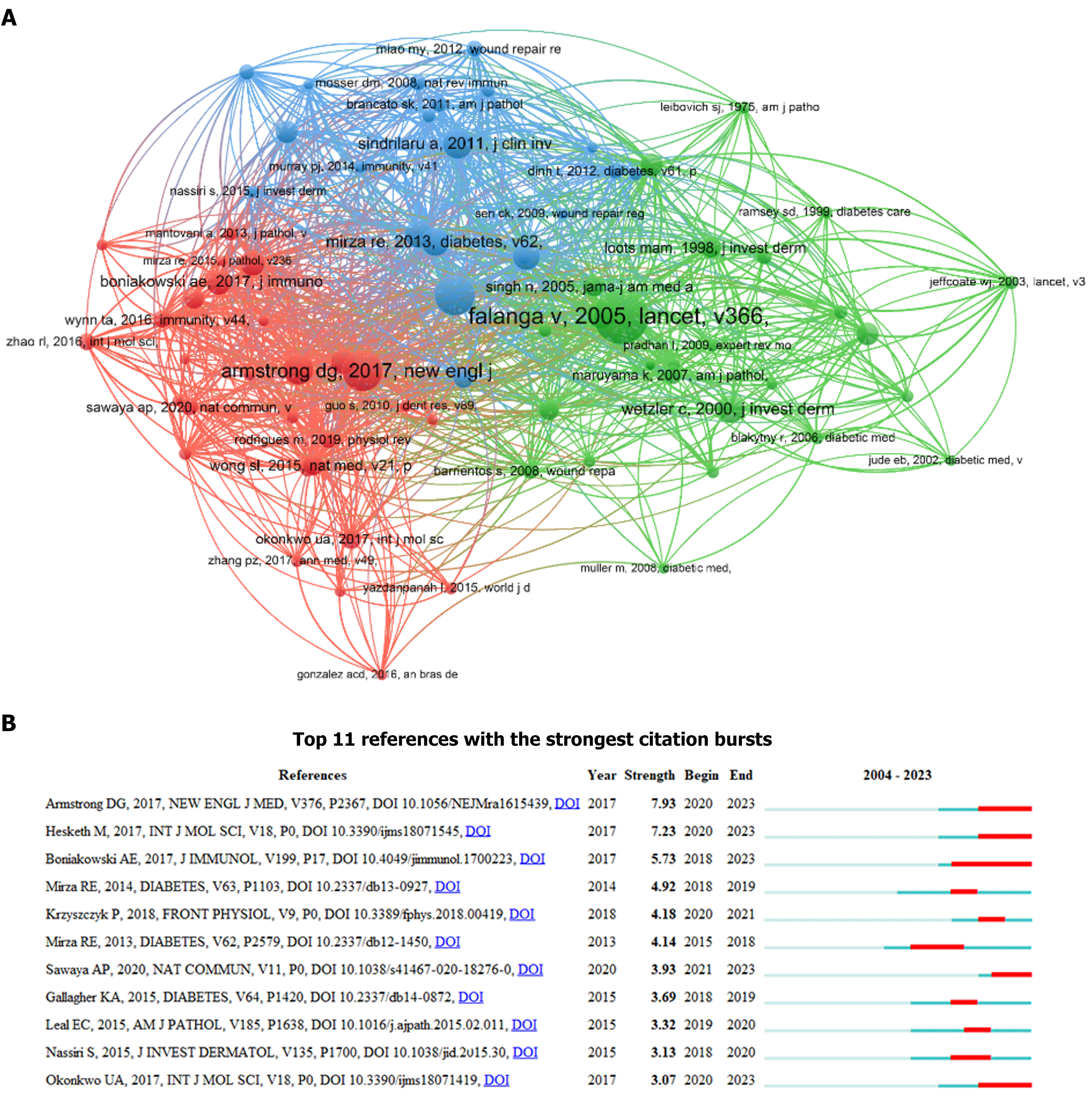
Among the 330 relevant publications, 16596 co-cited references were identified. Table 5 lists the top 10 co-cited references, each with a minimum of 28 co-citations. Of these, three had more than 40 co-citations. The study “Wound healing and its impairment in the diabetic foot“ by Falanga et al[32], published in “The Lancet” in 2005, had the highest number of co-citations at 56, followed by the study “Diabetic Foot Ulcers and Their Recurrence” by Armstrong et al[2], published in the “New England Journal of Medicine” in 2017, with 41 co-citations, and the study “Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice” by Khanna et al[33], published in PLOS ONE in 2010, with 41 co-citations. The co-citation counts of the remaining seven studies on the list ranged from 28 to 31. A co-citation network was constructed using a co-citation threshold of 10 (T = 10) (Figure 7A), in which nodes corresponding to the studies by Vincent Falanga, Savita Khanna, and David G Armstrong were larger, indicating a higher frequency of co-citations. Additionally, close relationships were evident between these key references, suggesting an active pattern of co-citation.
| Rank | Co-cited Ref. | Count | Country |
| 1 | Vincent Falanga, 2005, Lancet, v366, p1736 | 56 | England |
| 2 | David G Armstrong, 2017, New England Journal of Medicine, v376, p2367 | 41 | United States |
| 3 | Savita Khanna, 2010, Plos One, v5 | 41 | United States |
| 4 | Paulina Krzyszczyk, 2018, Frontiers in Physiology, v9 | 31 | United States |
| 5 | Anca Sindrilaru, 2011, Journal of Clinical Investigation, v121, p985 | 30 | United States |
| 6 | Harold Brem, 2007, Journal of Clinical Investigation, v117, p1219 | 29 | United States |
| 7 | Rita E Mirza, 2013, Diabetes, v62, p2579 | 29 | Switzerland |
| 8 | Tina Lucas, 2010, Journal of Immunology, v184, p3964 | 28 | United States |
| 9 | Rita E Mirza, 2011, cytokine, v56, p256 | 28 | England |
| 10 | Wetzler C, 2000, Journal of Investigative Dermatology, v115, p245 | 28 | United States |
Citation burst indicates references that have received increased attention from scholars within a specific timeframe[34]. Using CiteSpace, we identified 11 studies with strong citation bursts (Figure 7B). In this figure, each bar, either red or blue, signifies a 1-year time interval, with red bars indicating the periods of citation bursts. The top 11 references that displayed citation bursts were clustered between 2015 and 2023. Notably, four of these studies experienced bursts in 2020 alone. The most substantial burst was observed for the study “Diabetic Foot Ulcers and Their Recurrence” by Armstrong et al[2] in the burst period spanning from 2020 to 2023, with a burst strength of 7.93. Another article, “Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing” by Hesketh et al[35], published in the “International Journal of Molecular Sciences,” exhibited a citation burst from 2020 to 2023 and ranked second with a burst strength of 7.23. This was followed by the study “Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing” by Boniakowski et al[36] published in the “Journal of Immunology,” which had a burst period from 2018 to 2023, with a burst strength of 5.73.
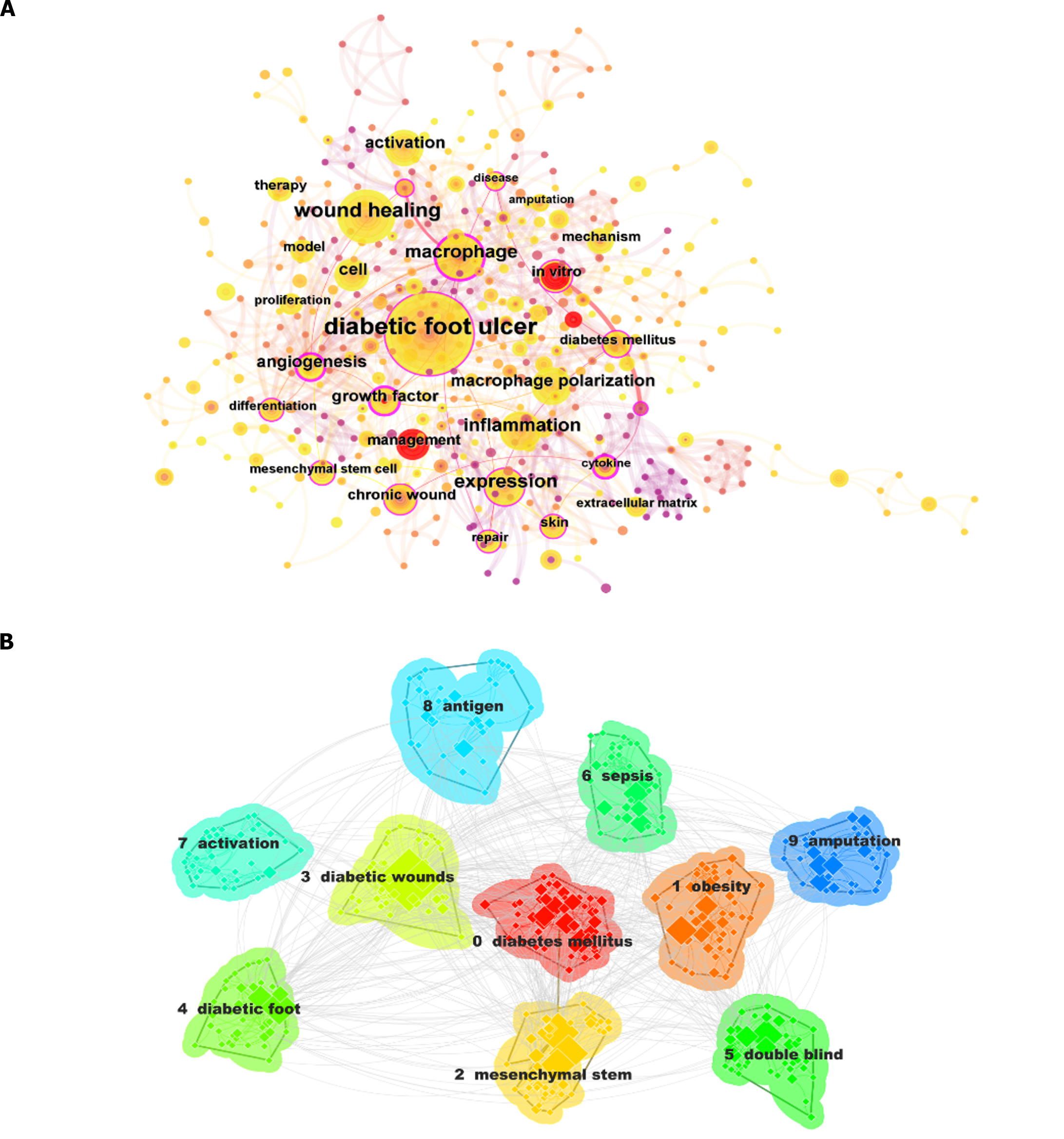
Using CiteSpace software for keyword and cluster analysis, we identified 466 keywords, grouped into 17 distinct clusters. The 10 most frequent keywords, each appearing at least 25 times, were “diabetic foot ulcer,” “wound healing,” “macrophage,“ “expression,“ “inflammation,“ “cell,” “activation,” “macrophage polarization,” “angiogenesis,” and “in vitro” (Table 6). Among these, five keywords were published more than 50 times, with “diabetic foot ulcer” having the highest occurrence at 228, followed by “wound healing” (n = 92), “macrophages” (n = 75), “expression” (n = 66), and “inflammation” (n = 52). The remaining five most frequently used keywords were published 26–50 times. CiteSpace was employed for the co-occurrence analysis of article keywords (Figure 8A). As illustrated in this figure, node labels for keywords that appear more than 10 times are prominently displayed. Specifically, nodes for “diabetic foot ulcers,” “wound healing,” “macrophages” and “expression” were notably larger, signifying their higher frequency.
| Rank | Keywords | Count | Centrality | Year |
| 1 | Diabetic foot ulcer | 228 | 0.37 | 2005 |
| 2 | Wound healing | 92 | 0.1 | 2010 |
| 3 | Macrophage | 75 | 0.15 | 2005 |
| 4 | Expression | 66 | 0.19 | 2004 |
| 5 | Inflammation | 52 | 0.1 | 2010 |
| 6 | Cell | 50 | 0.04 | 2009 |
| 7 | Activation | 43 | 0.05 | 2013 |
| 8 | Macrophage polarization | 43 | 0.04 | 2018 |
| 9 | Angiogenesis | 40 | 0.25 | 2005 |
| 10 | In vitro | 26 | 0.12 | 2007 |
The identified keywords were organized into 17 clusters (Table 7). The top 10 clusters by frequency were “diabetes mellitus,“ “obesity,” “mesenchymal stem,“ “diabetic wounds,“ “diabetic foot,“ “double blind,“ “sepsis,“ “activation,” “antigen,” and “amputation.“ Among these 17 thematic clusters, each of the top 10 clusters contained at least 20 keywords. “Diabetes mellitus” had the highest number of keywords at 64, followed by “obesity” (n = 49) and “mesenchymal stem” (n = 48). The keyword counts of the remaining seven clusters ranged from 27 to 39. A keyword clustering map was constructed based on the 10 most populated clusters (Figure 8B), in which clusters in different colors corresponded to distinct keyword categories, with “diabetes mellitus” featuring the most nodes and the highest occurrence frequency.
| Cluster ID | Size | Silhouette | Mean (year) | Label (LSI) | Label (LLR) | Label (MI) |
| 0 | 64 | 0.63 | 2014 | Wound healing; diabetes mellitus; diabetic foot ulcer; low level laser therapy; tensiometerical properties | chronic wounds; local treatment; immunohistochemical markers; diabetic foot ulcers; venous leg ulcers | Diabetes mellitus (22.75, 1.0E-4); chronic wounds (13.74, 0.001); wound healing (12.48, 0.001); histology (11.05, 0.001); wound closure (7.36, 0.01) | Skin and wound care (0.76); tgf-beta (0.76); metfo |
| 1 | 49 | 0.673 | 2014 | Wound healing; adipose tissue; extracellular vesicles; diabetic foot ulcer; small extracellular vesicles | endothelial progenitor; coronary artery; griffonia simplicifolia; biomimetic hydrogels; tissue engineering | Obesity (9.64, 0.005); expression (9.59, 0.005); coronary artery disease (9.28, 0.005); adipose tissue (9.28, 0.005); endothelial progenitor cells (9.28, 0.005) | Type 2 (0.42); griffonia simplicifolia i b4 (0.42) |
| 2 | 48 | 0.682 | 2015 | Wound healing; diabetic foot ulcer; stromal cells; astragali radix; diabetic wound healing | foot ulcer; mouse model; growth factor; mice; dysfunction | Mesenchymal stem (10.73, 0.005); growth factor (8.23, 0.005); neuropeptides (7.06, 0.01); Chinese medicine (7.06, 0.01); neurotensin (7.06, 0.01) | Animal models (0.27); biologics (0.27); acellular |
| 3 | 39 | 0.738 | 2018 | Wound healing; tensiometric properties; microbial flora; curcumin-loaded superparamagnetic iron oxide nanoparticles; pseudomonas aeruginosa | diabetic foot ulcer; chronic wound; siRNA delivery; lipid nanoparticle; lipidoid nanoparticle | Diabetic wounds (12.93, 0.001); tlr4 (9.12, 0.005); adaptive immunity (5.52, 0.05); siRNA delivery (5.52, 0.05); antibacterial (5.52, 0.05) | Macrophage polarisation (0.44); cell penetrating p |
| 4 | 37 | 0.751 | 2012 | Diabetic foot; wound healing; tumour necrosis factor alpha; foot ulcer; endothelial progenitor | diabetic foot ulcer; foot ulcer; endothelial progenitor; human fibroblasts; tumor necrosis factor-alpha | Diabetic foot (12.14, 0.001); foot (8, 0.005); in vivo (7.47, 0.01); endothelial growth factor (7.05, 0.01); chronic wounds (5.35, 0.05) | Inflammatory arthritis (0.73); surgery (0.73); neu |
| 5 | 37 | 0.871 | 2010 | Extracellular matrix; metal-polyphenol capsule; diabetic wound healing; situ tissue regeneration; split-thickness skin graft | foot ulcers; skin; matrix metalloproteinases; mechanisms; angiogenesis | Double blind (15.79, 1.0e-4); colony stimulating factor (15.79, 1.0e-4); wound healing (11.55, 0.001); in situ tissue regeneration (10.51, 0.005); diabetic neuropathy (10.51, 0.005) | Intraepidermal nerve fibers (0.29); growth factor |
| 6 | 35 | 0.921 | 2006 | Controlled trials; granulocyte colony-stimulating factor; intensive care; acute respiratory distress syndrome; two-hit model | granulocyte-macrophage colony-stimulating factor; diabetic foot infection; colony-stimulating factor; intensive care; two-hit model | Sepsis (17.44, 1.0e-4); acute respiratory distress syndrome (8.68, 0.005); controlled trials (8.68, 0.005); granulocyte colony-stimulating factor (g-csf) (8.68, 0.005); cancer (8.68, 0.005) | Acute respiratory distress syndrome (0.03); control |
| 7 | 30 | 0.732 | 2014 | Wound healing; innate immunity; gene expression; diabetic foot ulcer; diabetic wound | activation; inflammation; diabetic foot disease; neuropeptide substance; skin substitutes | Activation (11.88, 0.001); angiogenesis (5.61, 0.05); nlrp3 inflammasome (5.1, 0.05); inflammation (4.55, 0.05); notch (4.33, 0.05) | Notch (0.51); alveolar macrophages (0.51); behavior |
| 8 | 30 | 0.804 | 2017 | Differentiation; senescence; growth arrest; miR 145; miR expression | presenting cells; intracellular bacteria; staphylococcus aureus; diabetic ulcers; biomimetic hydrogels | Antigen (11.18, 0.001); intracellular bacteria (11.18, 0.001); presenting cells (11.18, 0.001); mesenchymal stem cells (6.83, 0.01); differentiation (6.09, 0.05) | Bm-mscs (0.23); collagen i (0.23); diabetic ulcer |
| 9 | 29 | 0.816 | 2009 | Angiogenesis; skin; tissue; hyperbaric oxygen; osteoradionecrosis | amputation; matrix metalloproteinases; risk factors; binding; potent gelatinase | Amputation (17.25, 1.0e-4); management (11.48, 0.001); angiogenesis (10.17, 0.005); randomized controlled trial (7.78, 0.01); amputations (7.78, 0.01) | Utility (0.21); nanomedicine (0.21); white matter |
| 10 | 27 | 0.676 | 2016 | Wound healing; diabetic foot; no-option critical limb ischemia; cell therapy; peripheral blood mononuclear cells | diabetic foot ulcer; epidermal growth factor; oxidative stress; single cell sequence; angiogenic properties | Migration inhibitory factor (11.07, 0.001); signaling pathway (7.38, 0.01); association (7.38, 0.01); miRNAs-mRNA (5.53, 0.05); synergistic effect (5.53, 0.05) | MiRNAs-mRNA (0.24); synergistic effect (0.24); pat |
| 11 | 23 | 0.865 | 2016 | Chronic wound; nanocomposite hydrogels; rnos eradication; peripheral blood mononuclear cells; wound healing | macrophage polarization; tissue regeneration; diabetic foot; immune system; wound healing | Rnos eradication (12.46, 0.001); immunoregulation (12.46, 0.001); nanocomposite hydrogels (12.46, 0.001); herb-derived (12.46, 0.001); chronic wound repairing (12.46, 0.001) | Immune centric revolution (0.05); revascularization |
| 12 | 11 | 1 | 2004 | Antimicrobial peptides; microbicides; sexually transmitted infections | Microbicides (13.17, 0.001); sexually transmitted infections (13.17, 0.001); antimicrobial peptides (13.17, 0.001); wound healing (0.25, 1.0); inflammation (0.15, 1.0) | Wound healing (0.04); inflammation (0.02); macroph |
| 13 | 6 | 1 | 2008 | Positron emission tomography; fluorodeoxyglucose; infection; inflammation | Fluorodeoxyglucose (12.49, 0.001); positron emission tomography (9.72, 0.005); infection (6.49, 0.05); inflammation (3.09, 0.1); wound healing (0.34, 1.0) | Wound healing (0.03); inflammation (0.02); macroph |
| 14 | 2 | 1 | 2013 | Positron emission tomography; diabetic foot infections; chronic osteomyelitis; 18F-FDG PET/CT; leukocyte scintigraphy; rheumatoid arthritis; joint replacement; bone scintigraphy; nuclear medicine; hybrid pet | Leukocyte scintigraphy (10.49, 0.005); nuclear medicine (10.49, 0.005); bone scintigraphy (10.49, 0.005); chronic osteomyelitis (10.49, 0.005); joint replacement (10.49, 0.005) | Wound healing (0.03); inflammation (0.01); leukocyte |
| 15 | 1 | 0 | 2020 | Diabetic kidney disease; kk-a(y)mouse; sglt2 inhibitor; tofogliflozin | Kk (11.99, 0.001); a (y) mouse (11.99, 0.001); sglt2 inhibitor (11.99, 0.001); tofogliflozin (11.99, 0.001); diabetic kidney disease (9.22, 0.005) | Wound healing (0.03); inflammation (0.02); macroph |
| 16 | 1 | 0 | 2022 | Glomerulonephropathy; laminin alpha 2; mechanosensitive protein; mechanotransduction; podocyte mechanics | Mechan transduction (11.99, 0.001); laminin alpha 2 (11.99, 0.001); mechanosensitive protein (11.99, 0.001); podocyte mechanics (11.99, 0.001); glomerulonephropathy (11.99, 0.001) | Wound healing (0.03); inflammation (0.02); macroph |
Over the past two decades, 573 institutions across 45 countries/regions have published 330 research articles in 207 peer-reviewed journals regarding macrophages in DFUs. At the same time, an increasing trend in the number of publications was observed, indicating that the role of macrophages in DFUs emerged as a significant area of research, particularly between 2017 and 2023. As illustrated in Figure 2, the research landscape before 2019 was characterized by modest growth, with fewer than 20 publications annually. In contrast, the period from 2019 to 2023 experienced a near-exponential increase in publications. Notably, the total number of publications from 2019 to 2023 surpassed the cumulative total from the preceding 16 years, underscoring the recent surge in interest in this field.
Regarding publication volume, the top 10 countries/regions span three major geographical areas - Europe, Asia, and North America - with developed countries comprising 70% of the list. These countries/regions generally maintain high research standards, enabling more advanced studies on the role of macrophages in DFUs. China stands out among Asian nations because of its robust research output, publishing extensively in recent years. While the United States trailed slightly behind China regarding the number of publications, it was significantly ahead in citation counts. This discrepancy suggests a greater depth of research contributions from the United States than that from other countries. Among the top 10 countries/regions by publication volume, only China, India, and Iran were developing nations. This highlights the gap in research capabilities in developing countries/regions and emphasizes the need for them to enhance their scientific research infrastructure. Developing countries and regions should actively assimilate knowledge from their developed counterparts and strive to make meaningful contributions to this burgeoning field.
Among the top 10 most productive institutions that contributed to this research area, five were based in the United States (Table 2), while the remaining five institutes were distributed across two other countries/regions (China and Portugal). The leading institutes were Shanghai Jiao Tong University (China) and Sun Yat-sen University (China) with 11 publications each. This highlights the significance and influence of these institutions within the research field. Moreover, seven of the top 10 most-cited institutions in this field were located in the United States, highlighting its contribution; the remaining three were from Portugal and China, respectively. The University of Miami (United States), the University of Coimbra (Portugal), and Harvard University (United States) ranked among the top three institutions according to citation counts. Collaborating with these institutions could be highly beneficial to researchers in this field.
Regarding journals, “Wound Repair and Regeneration,” which specializes in wound repair, published the highest number of articles (n = 15). Despite the journal’s relatively modest IF of 2.9, it had the highest number of co-citations (n = 508), which can be attributed to its high publication volume. Furthermore, it is noteworthy that nine of the top 10 journals with the highest total co-citation counts were based in the United States, reinforcing the country’s leading role in this scientific domain. Among them, “Diabetes Care” had the highest IF (16.2) and published one article that garnered 364 co-citations. Importantly, journals with higher IFs tended to have more co-cited articles, highlighting their influence in the field. Therefore, researchers should reference articles from frequently co-cited journals in their work and consider these platforms for submission of their research to increase its visibility and impact.
Through a co-occurrence analysis of 466 keywords across 330 articles, we gained valuable insights into prevailing research directions and emerging hotspots in this domain[37]. Figure 8 shows that most studies have focused on the role of macrophages in the wound-healing process in DFUs, specifically the activation, expression activities, and migration of macrophages. The wound healing process is complex and involves four distinct stages, beginning with hemostasis, followed by the inflammatory and proliferative phases, and finally, the remodeling phase[35]. During the inflammatory stage, various immune cells, including macrophages, neutrophils, and monocytes, coordinate with other cells sur-rounding the injured skin to facilitate the repair process[38]. Macrophages play pivotal roles in inflammation, including clearing pathogens and cellular debris and regulating the subsequent proliferative phase, either directly or indirectly. This regulation occurs through the secretion of cytokines and growth factors, which stimulate the proliferation, differentiation, and migration of keratinocytes, fibroblasts, and endothelial cells[39]. However, analysis of this process is complicated because macrophages can adopt different phenotypes, characterized by different functions, during wound healing[40-42]. Proinflammatory macrophages are typically described as M1 macrophages, whereas anti-inflammatory and reparative macrophages are referred to as M2 macrophages. However, it is important to note that this categorization and terminology has certain limitations[43,44].
In the early stages of wound repair, various factors such as cytokines, chemokines, growth factors, and metabolites stimulate the transformation of most inactivated macrophages to M1 macrophages. These M1 macrophages, compared with their undifferentiated counterparts, possess a heightened phagocytic ability and intensify inflammation by releasing various inflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor α (TNF-α), thereby becoming predominant at this stage[39]. However, as the inflammatory process advances, the role of macrophages shifts, with anti-inflammatory and reparative M2 macrophages superseding M1 macrophages. M2 macrophages are instrumental in reducing inflammation and aiding the wound healing process by secreting cytokines such as IL-10, transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF), which marks a critical transition from the inflammatory phase to the remodeling phase of wound healing[36,45]. Consequently, the phenotypic transition of macrophages is not only a feature of the healing process but also a necessary condition for transitioning from the inflammatory to the proliferative phase during wound healing[46].
Typical factors such as IL-4 and IL-13 are known to induce alternative macrophage phenotypes. In vitro wound models using human cell lines have demonstrated that the transition to the M2 phenotype occurs after IL-4/IL-13 stimulation. However, in mouse models of wounds, this transition does not depend solely on these cytokines, suggesting the presence of other yet unclear mechanisms[41]. Research has indicated that M1 macrophages can evolve into a novel M2-like phenotype independent of IL-4/IL-13[47]. This transition involves initial activation through Toll-like receptors (TLR), where adenosine A2A receptor (A2AR) agonists synergize with TLR2, TLR4, TLR7, and TLR9 agonists, leading to the M2d phenotype. Unlike traditional M2a macrophages, M2d macrophages exhibit high levels of VEGF, IL-10, and nitric oxide synthase, with reduced levels of TNF-α and IL-12, and a slight increase in arginase-1. These signaling pathways have been proposed to act as angiogenic switches, bridging innate immunity and wound healing, thus playing a significant role in the wound healing process. Additionally, other factors such as IL-10, glucocorticoids, prostaglandins, and phagocytic processes may also contribute to the induction of the M2-like phenotype. However, within the complex context of wound healing, the precise influence of each stimulus on macrophage phenotype transition and inflammation resolution remains unclear[45]. The phenotypic transition of macrophages is undoubtedly crucial in resolving inflammation and healing wounds in DFUs, with more studies focusing on this area[48-50]. Future studies will likely continue to focus on the transition between the two macrophage phenotypes and their intricate functions in DFUs.
Exosomes, which are cellular vesicles with a lipid bilayer membrane encapsulating functional proteins and nucleic acids, have been extensively studied in diabetic wound healing[51-53]. Macrophages, essential immune cells in diabetic foot pathology, mediate inflammation through polarization. Exosomes can regulate inflammation by influencing macrophage differentiation[54,55]. Specifically, stem cell-derived exosomes transferred to macrophages can induce anti-inflammatory M2 macrophage polarization. This polarization is facilitated by transcriptionally activating β-lactamase-1 and increasing anti-inflammatory factors like IL-10, while not significantly affecting M1-related cytokines. This simultaneously induces an inflammatory response and enhances insulin sensitivity[56]. In myocardial ischemia-reperfusion injury, microRNA (miR)-21-5p from mesenchymal stem cell-derived exosomes stimulates M2 macrophage polarization, reduces inflammation, and aids cardiac cell repair[57]. Additionally, exosomes from adipose-derived stem cells are pivotal in managing obesity-related inflammation and metabolic disorders, promoting anti-inflammatory M2 macrophage polarization through active STAT3 and suppression of macrophage inflammation[56]. In the neurological context, miR-21-5p from exosomes of dorsal root ganglion neurons accelerates rat neuronal injury progression by inducing microglia to adopt a proinflammatory M1 phenotype[58]. Therefore, exosomes are promising targets for studies regarding macrophage-related DFUs.
In addition, keyword co-occurrence analysis revealed a predominance of basic research, necessitating the development of animal models to explore the pathophysiological underpinnings of DFUs more effectively. A highly cited article by Kottaisamy reviewed commonly employed animal models for diabetes research, shedding light on the advantages and limitations of each model[59]. Among these models, streptozotocin is commonly used to induce diabetes in animals by triggering pancreatic β-cell death[60]. Additionally, there are alternative models such as the high-fat diet-induced diabetes model[61], spontaneous diabetes model[62], and genetic diabetes model[63], each of which has its strengths and limitations. Collectively, these animal models offer a rich and diverse platform for an in-depth study of diabetes and DFUs.
Our study has several strengths. First, using a scientific approach for the systematic analysis of publications related to macrophages in DFUs allows clinicians and scholars to gain a clearer understanding of developmental trends in this topic. Second, we utilized four different scientific measurement tools for data visualization analysis - Excel, HistCite, VOSviewer, and CiteSpace - which are the most popular tools in scientometrics, ensuring an objective data analysis process and making the results more convincing. Third, compared with traditional narrative assessments, the visualization offered by scientometric analyses provides a more accurate and easily comprehensible understanding of how research trends and focal points change over time.
However, this study has some limitations. First, the study only considered articles and reviews from the WoSCC without utilizing other databases or languages as supplements. However, it is important to recognize that the WoSCC is a comprehensive database, and it is difficult to simultaneously analyze data from different databases. Second, our data were analyzed using scientometric software rather than being manually compiled as in meta-analyses or systematic reviews. This could introduce inaccuracies due to the inability of the software to discern nuances in publications or differentiate between authors with the same name. Furthermore, our study may have had a time-lag effect, potentially excluding recently published impactful studies. As technology and methodologies for data collection and analysis continue to advance, we look forward to mitigation of these limitations in future research.
Bibliometric analysis revealed rapid developments in research concerning macrophages in DFUs, with a surge in publications since 2019. Leading this trend are Countries such as China and the United States that have made significant contributions to this field. Notably, influential researchers such as Aristidis Veves and Eugenia Carvalho have published multiple widely cited papers in this area. In the foreseeable future, the study of the transition between the two main macrophage phenotypes in DFUs, along with the complex roles these phenotypes play, and the investigation of exosomes in these transitions, are anticipated to remain a research priority. Bibliometric analysis provided a comprehensive overview of the research output in this field from 2004 to 2023, which might serve as a useful reference for scholars investigating similar topics.
| 1. | Yang L, Rong GC, Wu QN. Diabetic foot ulcer: Challenges and future. World J Diabetes. 2022;13:1014-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (6)] |
| 2. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2322] [Article Influence: 290.3] [Reference Citation Analysis (2)] |
| 3. | Boulton AJM, Armstrong DG, Kirsner RS, Attinger CE, Lavery LA, Lipsky BA, Mills JL Sr, Steinberg JS. Diagnosis and Management of Diabetic Foot Complications. Arlington (VA): American Diabetes Association; 2018. [PubMed] |
| 4. | Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015;2015:CD008548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 6. | Zheng SY, Wan XX, Kambey PA, Luo Y, Hu XM, Liu YF, Shan JQ, Chen YW, Xiong K. Therapeutic role of growth factors in treating diabetic wound. World J Diabetes. 2023;14:364-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (7)] |
| 7. | Okonkwo UA, Chen L, Ma D, Haywood VA, Barakat M, Urao N, DiPietro LA. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS One. 2020;15:e0231962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Rai V, Moellmer R, Agrawal DK. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 588] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 10. | Ganesh GV, Ramkumar KM. Macrophage mediation in normal and diabetic wound healing responses. Inflamm Res. 2020;69:347-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Al Sadoun H. Macrophage Phenotypes in Normal and Diabetic Wound Healing and Therapeutic Interventions. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Wu X, He W, Mu X, Liu Y, Deng J, Liu Y, Nie X. Macrophage polarization in diabetic wound healing. Burns Trauma. 2022;10:tkac051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 13. | Wolf SJ, Melvin WJ, Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin Cell Dev Biol. 2021;119:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Nowak NC, Menichella DM, Miller R, Paller AS. Cutaneous innervation in impaired diabetic wound healing. Transl Res. 2021;236:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Galkowska H, Olszewski WL, Wojewodzka U, Rosinski G, Karnafel W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res. 2006;134:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30:3058-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-6440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 3254] [Article Influence: 464.9] [Reference Citation Analysis (0)] |
| 18. | Wang S, Zhou H, Zheng L, Zhu W, Zhu L, Feng D, Wei J, Chen G, Jin X, Yang H, Shi X, Lv X. Global Trends in Research of Macrophages Associated With Acute Lung Injury Over Past 10 Years: A Bibliometric Analysis. Front Immunol. 2021;12:669539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Cheng K, Guo Q, Shen Z, Yang W, Wang Y, Sun Z, Wu H. Bibliometric Analysis of Global Research on Cancer Photodynamic Therapy: Focus on Nano-Related Research. Front Pharmacol. 2022;13:927219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J, Lin T. The Global Research of Artificial Intelligence on Prostate Cancer: A 22-Year Bibliometric Analysis. Front Oncol. 2022;12:843735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. 2006;57:359-377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2057] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 22. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4505] [Cited by in RCA: 5115] [Article Influence: 319.7] [Reference Citation Analysis (0)] |
| 23. | Pan X, Yan E, Cui M, Hua W. Examining the usage, citation, and diffusion patterns of bibliometric mapping software: A comparative study of three tools. J Informetr. 2018;12:481-493. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 24. | Zhang W, Xia CL, Ma JN, Li JX, Chen Q, Ou SJ, Yang Y, Qi Y, Xu CP. Effects of mitochondrial dysfunction on bone metabolism and related diseases: a scientometric study from 2003 to 2022. BMC Musculoskelet Disord. 2022;23:1016. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Hou J, Su H, Kuang X, Qin W, Liu K, Pan K, Zhang B, Yang S, Yang S, Peng X, Nie X, Hua Q. Knowledge Domains and Emerging Trends of Osteoblasts-Osteoclasts in Bone Disease From 2002 to 2021: A Bibliometrics Analysis and Visualization Study. Front Endocrinol (Lausanne). 2022;13:922070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, Qiu F. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J Control Release. 2022;352:211-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 27. | Shi Y, Wei W, Li L, Wei Q, Jiang F, Xia G, Yu H. The global status of research in breast cancer liver metastasis: a bibliometric and visualized analysis. Bioengineered. 2021;12:12246-12262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, Zhang L. Knowledge Mapping of Exosomes in Autoimmune Diseases: A Bibliometric Analysis (2002-2021). Front Immunol. 2022;13:939433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 29. | Miao L, Zhang J, Zhang Z, Wang S, Tang F, Teng M, Li Y. A Bibliometric and Knowledge-Map Analysis of CAR-T Cells From 2009 to 2021. Front Immunol. 2022;13:840956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Zhang W, Zhang S, Dong C, Guo S, Jia W, Jiang Y, Wang C, Zhou M, Gong Y. A bibliometric analysis of RNA methylation in diabetes mellitus and its complications from 2002 to 2022. Front Endocrinol (Lausanne). 2022;13:997034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Zhu Y, Lu J, Wang S, Xu D, Wu M, Xian S, Zhang W, Tong X, Liu Y, Huang J, Jiang L, Guo X, Xie S, Gu M, Jin S, Ma Y, Huang R, Xiao S, Ji S. Mapping intellectual structure and research hotspots in the field of fibroblast-associated DFUs: a bibliometric analysis. Front Endocrinol (Lausanne). 2023;14:1109456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1651] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 33. | Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 462] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Huang X, Fan X, Ying J, Chen S. Emerging trends and research foci in gastrointestinal microbiome. J Transl Med. 2019;17:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 570] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 36. | Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 37. | Zhao J, Li M. Worldwide trends in prediabetes from 1985 to 2022: A bibliometric analysis using bibliometrix R-tool. Front Public Health. 2023;11:1072521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 39. | Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 40. | Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 41. | Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 42. | Koh TJ, Novak ML, Mirza RE. Assessing macrophage phenotype during tissue repair. Methods Mol Biol. 2013;1037:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 1419] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 44. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4521] [Article Influence: 411.0] [Reference Citation Analysis (0)] |
| 45. | Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1113] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 46. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 6845] [Article Influence: 402.6] [Reference Citation Analysis (0)] |
| 47. | Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol. 2003;163:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Huang YY, Lin CW, Cheng NC, Cazzell SM, Chen HH, Huang KF, Tung KY, Huang HL, Lin PY, Perng CK, Shi B, Liu C, Ma Y, Cao Y, Li Y, Xue Y, Yan L, Li Q, Ning G, Chang SC. Effect of a Novel Macrophage-Regulating Drug on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2122607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | Yang Y, Zhang B, Yang Y, Peng B, Ye R. FOXM1 accelerates wound healing in diabetic foot ulcer by inducing M2 macrophage polarization through a mechanism involving SEMA3C/NRP2/Hedgehog signaling. Diabetes Res Clin Pract. 2022;184:109121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | Li Y, Li X, Ju S, Li W, Zhou S, Wang G, Cai Y, Dong Z. Role of M1 macrophages in diabetic foot ulcers and related immune regulatory mechanisms. Front Pharmacol. 2022;13:1098041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 52. | Wang P, Theocharidis G, Vlachos IS, Kounas K, Lobao A, Shu B, Wu B, Xie J, Hu Z, Qi S, Tang B, Zhu J, Veves A. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J Invest Dermatol. 2022;142:2508-2517.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 53. | Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, Xiao Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021;19:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 54. | Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 55. | Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cell Mol Biol Lett. 2022;27:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 56. | Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 486] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 57. | Shen D, He Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann Transl Med. 2021;9:1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 58. | Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, Wang L, Yu J, Li W, Wang Y, Li D, Zhao J, Wang Y, Zuo Y, Li Y, Kong X, Chen F, Lei P. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun. 2020;83:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 59. | Kottaisamy CPD, Raj DS, Prasanth Kumar V, Sankaran U. Experimental animal models for diabetes and its related complications-a review. Lab Anim Res. 2021;37:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 60. | Michaels J 5th, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 61. | Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos MPD, Kawashita NH, França SA. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad Bras Cienc. 2019;91:e20180314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 62. | King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 63. | Kong LL, Wu H, Cui WP, Zhou WH, Luo P, Sun J, Yuan H, Miao LN. Advances in murine models of diabetic nephropathy. J Diabetes Res. 2013;2013:797548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |