Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1615
Revised: March 5, 2024
Accepted: April 30, 2024
Published online: July 15, 2024
Processing time: 166 Days and 0.5 Hours
Islets of Langerhans beta cells diminish in autoimmune type 1 diabetes mellitus (T1DM). Teplizumab, a humanized anti-CD3 monoclonal antibody, may help T1DM. Its long-term implications on clinical T1DM development, safety, and efficacy are unknown.
To assess the effectiveness and safety of teplizumab as a therapeutic intervention for individuals with T1DM.
A systematic search was conducted using four electronic databases (PubMed, Embase, Scopus, and Cochrane Library) to select publications published in peer-reviewed journals written in English. The odds ratio (OR) and risk ratio (RR) were calculated, along with their 95%CI. We assessed heterogeneity using Cochrane Q and I2 statistics and the appropriate P value.
There were 8 randomized controlled trials (RCTs) in the current meta-analysis with a total of 1908 T1DM patients from diverse age cohorts, with 1361 patients receiving Teplizumab and 547 patients receiving a placebo. Teplizumab was found to have a substantial link with a decrease in insulin consumption, with an OR of 4.13 (95%CI: 1.72 to 9.90). Teplizumab is associated with an improved C-peptide response (OR 2.49; 95%CI: 1.62 to 3.81) and a significant change in Gly
In type 1 diabetics, teplizumab decreased insulin consumption, improved C-pep
Core Tip: Type 1 diabetes mellitus (T1DM) may benefit from teplizumab. However, there is limited data on its long-term effects on clinical T1DM development, safety, and efficacy. Given its perceived use, this research evaluated the effective
- Citation: Ma XL, Ge D, Hu XJ. Evaluation of teplizumab's efficacy and safety in treatment of type 1 diabetes mellitus: A systematic review and meta-analysis. World J Diabetes 2024; 15(7): 1615-1626
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1615.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1615
Type 1 diabetes mellitus (T1DM) is a pathological condition of an autoimmune type wherein there is a progressive de
The T cells undergo a transient depletion from the peripheral circulation throughout the course of immunotherapy, followed by their reconstitution within a few weeks upon discontinuation of the treatment[10,11]. Based on preclinical and clinical investigations, it has been observed that teplizumab medication has the potential to stimulate regulatory T-cell function, hence indicating an enhancement in immunological tolerance[12-14]. According to recent research, the administration of teplizumab has been found to effectively mitigate beta-cell apoptosis after one year of treatment, modulate CD8+ T lymphocytes as well as enhance C-peptide reactions in clinical trials involving individuals with type 1 diabetes[15,16]. However, additional study is necessary to examine the long-term effects on b-cell activity and survival, as well as to ascertain the safety and efficacy of teplizumab therapy in modifying the progression towards clinical type 1 diabetes. Therefore, in this systematic review and meta-analysis, we assessed the effectiveness and safety of teplizumab as a therapeutic intervention for T1DM via analysis of 8 randomized controlled trials (RCTs)[17-24] selected as per the predetermined inclusion and exclusion criteria.
This meta-analysis and systematic review was conducted with the purpose of determining whether or not teplizumab is an effective and safe treatment intervention for people who have T1DM.
The present meta-analysis was conducted following a comprehensive search across various databases, including PubMed, Embase, Scopus, and Cochrane library. The search covered from the year 2000 to 2023 and utilized specific keywords such as “Type-1 Diabetes mellitus”, “T1DM”, “Teplizumab”, “anti-CD3 monoclonal antibody”, “Insulin”, “Glycated haemoglobin A1c”, “HbA1C”, “C-peptide”, “Adverse events”, “Randomized controlled trials”, “RCT”, “Systematic review” and “meta-analysis”. Based on the PICOs framework[25], the keywords were identified and found to be consistent in both the Medline and Embase databases, as indicated in Table 1. In the context of searching Scopus, the Title (ti)-Abstract (abs)-keyword (key) field was utilized with the aforementioned keywords. The key phrase “Te
| Database | Search strategy |
| Scopus | 11 “Type-1 Diabetes mellitus” OR “T1DM” OR “Teplizumab” OR “anti-CD3 monoclonal antibody” |
| 12 “Insulin” OR “Glycated hemoglobin A1c” OR “HbA1C” OR “C-peptide” OR “Adverse events” OR “Randomized controlled trials” OR “RCT” OR “Systematic review” OR “meta-analysis” | |
| 13 11 AND 12 | |
| PubMed | 11 “Type-1 Diabetes mellitus” OR “T1DM”(MeSH Terms)1 OR “Teplizumab”(all fields) OR “anti-CD3 monoclonal antibody”(all fields) |
| 12 “Insulin”(MeSH Terms) OR “Glycated hemoglobin A1c”(all fields) OR “HbA1C”(all fields) OR “C-peptide”(all fields) OR “Adverse events” OR “Randomized controlled trials”(all fields) OR “RCT”(all fields) OR “systematic review” OR “meta-analysis” | |
| 13 11 AND 12 | |
| Embase | “Type-1 Diabetes mellitus”/ exp2 OR “TIDM”/ exp OR “Teplizumab”/exp OR “anti-CD3 monoclonal antibody”/exp |
| 12 “Insulin”/ exp OR “Glycated hemoglobin A1c” / exp OR “HbA1C”/exp OR “C-peptide”/exp OR “Adverse events”/exp OR “Randomized controlled trials”/exp OR “RCT”/exp OR “systematic review”/ exp OR “meta-analysis” | |
| 13 11 AND 12 | |
| Cochrane library | 11 (Type-1 Diabetes mellitus): Ti, ab, kw3 OR (T1DM): Ti, ab, kw OR (Teplizumab): Ti, ab, kw OR (anti-CD3 monoclonal antibody): Ti, ab, kw OR (Cortisol): Ti, ab, kw (Word variations have been searched) |
| 12 (Insulin): Ti, ab, kw OR (Glycated hemoglobin A1c): Ti, ab, kw OR (HbA1C): Ti, ab, kw or (C-peptide): Ti, ab, kw or (Adverse events): Ti, ab, kw or (Randomized controlled trials): Ti, ab, kw or (systematic review): Ti, ab, kw or (meta-analysis): Ti, ab, kw (Word variations have been searched) | |
| 13 11 AND 12 |
The current analysis comprised studies that showed data on the efficacy and safety of Teplizumab for the treatment of T1DM. Those studies that satisfied the subsequent inclusion criteria were incorporated: (1) Including patients with T1DM; (2) Adolescents and adult patients ranging in age from 7 to 40 years; (3) Evaluating the comparative efficacy and safety of teplizumab for the treatment of T1DM; and (4) Implementation of RCT as the chosen study design. From the year 2000 all the way up until the year 2023, the selection of studies covered the entire time span. We chose papers that were available in full text and offered sufficient information for a table that was two by two. Several clinical outcomes were used as primary measures in this meta-analysis. These outcomes included a decrease in insulin utilization, a change in response to C-peptide, a change in the level of HbA1C, and adverse events that occurred in participants who were treated with teplizumab and those who were in the control group. Additionally, the comparative glycemic control experienced by the teplizumab group in comparison to the control group was also assessed. We did not include references that were either out of date, anecdotal, or based on the opinions of experts. Additionally, we did not include studies that were not cross-sectional, studies that contained experimental data from animal studies or trials, and studies for which we were unable to receive primary data and key information from the authors. Studies that included patients with diabetes in addition to those with HIV, cancer, and other systemic problems were also removed from consideration. Additionally, articles that were not research papers, qualitative studies, and papers published in languages other than English were also removed. Separately, the researchers (Xiao-Lan Ma and Dan Ge) acquired demographic profiles of the patients as well as event data with important components from the studies that were included.
The potential for bias in the papers that were examined was evaluated using a pre-established and standardized qu
For the purpose of evaluating and analyzing the influence of a number of dichotomous and continuous outcomes, the software package Review Manager (RevMan) 5.3[31] was applied. Through the employment of reference management software, the categorization, extraction, and elimination of duplicate references were made easier. The development of forest plots[32] was attempted with the purpose of evaluating the influence of outcome determinants across all of the investigations. A 2 × 2 table[33] that was generated with event data was utilized in order to compute the odds ratio (OR). This was done by employing the DerSimonian Lair method. The examination of dichotomous outcomes included the utilization of OR[34] and risk ratios (RR)[35] in addition to a CI covering 95% of the possible outcomes. The assessment of heterogeneity was examined through the utilization of statistical techniques, such as the χ2 test with a matching P value and the I2 test[36]. A random-effects model was utilized in the event that there was heterogeneity between the studies, which was demonstrated by an I2 value that was greater than fifty percent or a P value that was less than five percent. A fixed-effect model was utilized for the pooled analysis[37], which was otherwise not the case. It was determined that a P value that was lower than 0.05 to be statistically significant[38]. In addition to this, a box and whisker plot[39] was also created in order to assess the glycaemic control[40] in the Teplizumab administration group with the control group.
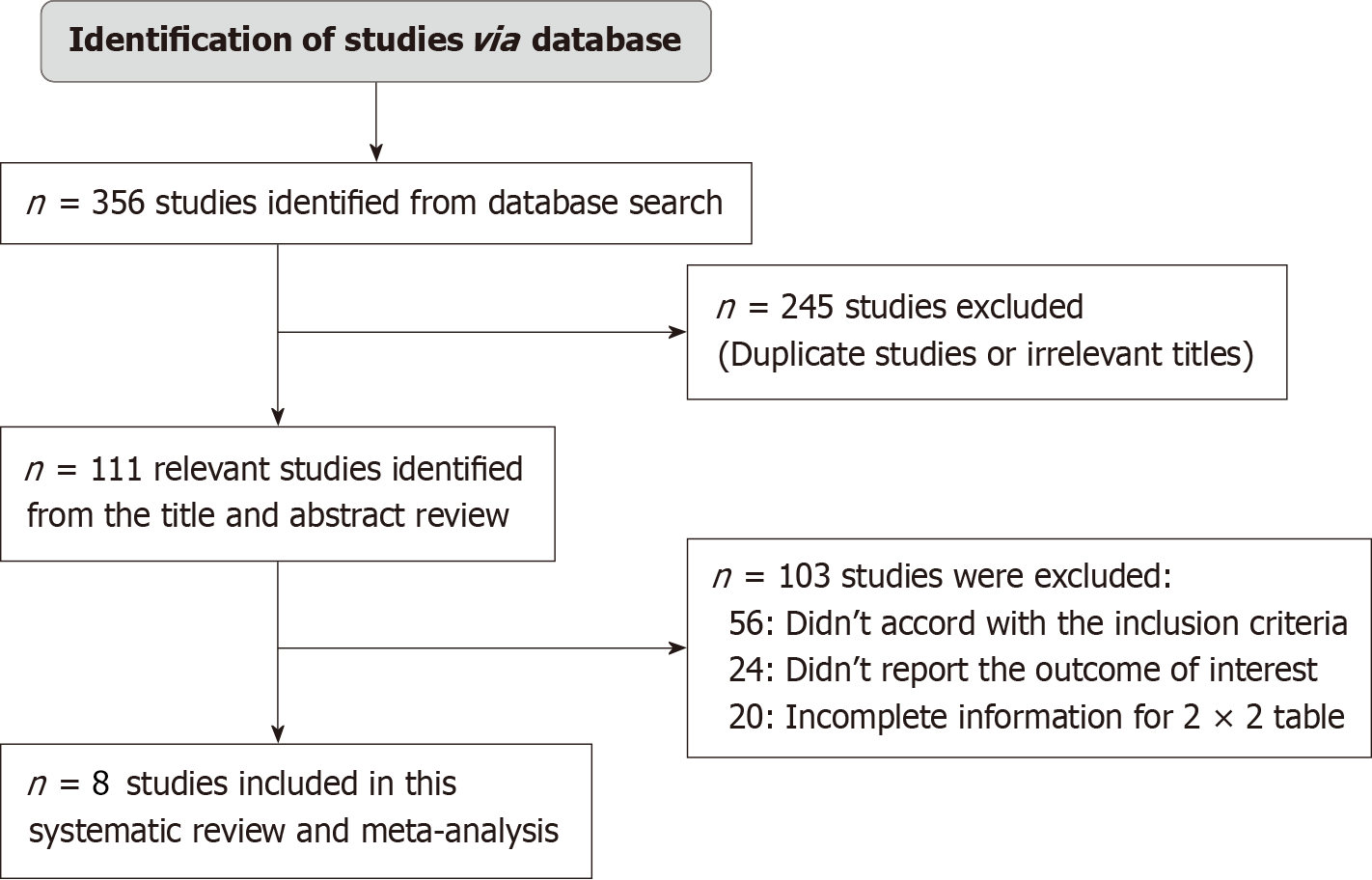
A thorough search of a number of databases was carried out with the use of electronic scanning tools, which led to the discovery of a total of 356 studies that fulfilled the inclusion criteria that were stated by the PICOS framework. We were able to exclude a total of 245 papers after conducting an exhaustive review of their titles and identifying instances of duplication. This left us with 111 records that were taken through additional screening. After applying the inclusion-exclusion criteria, however, a total of 103 studies were determined to be ineligible and were consequently removed from consideration. The absence of inclusion criteria, which included a comparison of the safety and effectiveness of teplizumab with a control drug in patients with T1DM, insufficient data to construct 2 × 2 tables, and the absence of necessary outcome measures, were the primary factors that contributed to the cancellation of studies. As can be seen in Figure 1, this meta-analysis made use of a total of eight RCT that satisfied the inclusion criteria that were stated and that covered the years 2000 to 2023. A total of 1908 type 1 diabetes patients from a variety of age groups were included in the papers that were analyzed for this particular research. The selection of patients for this trial was carried out through the use of a random sampling technique. The group that received Teplizumab consisted of 1361 individuals, whereas the group that served as the control consisted of exactly 547 people. The demographic features of the studies that were utilized in this meta-analysis are presented in Table 2. It is stated in the text that the author identification number, the year of publication, the journal of publication, the country of publication, the study setting, the study design, the total number of participants, the diagnosis, the age of participants, the number of participants in the teplizumab group and the control group, the duration of the study, the gender (male/female ratio), and the primary outcomes that were measured are all included. After that, the data from the events that were described earlier were utilized for the purpose of carrying out the meta-analysis later on.
| Ref. | Year of publication | Journal of publication | Country of study | Study setting | Study design | Total number of participants (n) | Diagnosis | Age of patients | Teplizumab group (n) | Control group (n) | Duration of study | Sex (M/F) | Primary outcomes |
| Herold et al[17] | 2002 | The New England Journal of Medicine | United States | HIC | RCT | 24 | Type-1 diabetes | 7-30 years | 12 | 12 | 1 year | 18/6 | Positive outcomes: Lower insulin uses and decrease in value of HbA1c, Adverse events |
| Hagopian et al[18] | 2013 | Diabetes | Sweden | HIC | RCT | 410 | Type-1 diabetes | 8-35 years | 311 | 99 | 2 years | 295/115 | Positive outcomes: Change in area under the curve for C-peptide, lower insulin uses and decrease in value of HbA1c, Adverse events |
| Herold et al[19] | 2013 | Diabetologia | United States | HIC | RCT | 58 | Type-1 diabetes | 12-15 years | 31 | 27 | 1 year | 30/28 | Positive outcomes: Change in area under the curve for C-peptide, and decrease in value of HbA1c, Adverse events |
| Herold et al[20] | 2019 | New England Journal of medicine | United States | HIC | RCT | 76 | Type-1 diabetes | 12-22 years | 44 | 32 | 2 years | 36/40 | Positive outcomes: Decrease in value of HbA1c, Adverse events |
| Herold et al[21] | 2023 | Diabetes care | United States | HIC | RCT | 609 | Type-1 diabetes | 8-35 years | 375 | 234 | 1 year | 370/239 | Positive outcomes: Change in area under the curve for C-peptide, and decrease in value of HbA1c, Adverse events |
| Perdigoto et al[22] | 2019 | Diabetologia | United States | HIC | RCT | 43 | Type-1 diabetes | 8-30 years | 31 | 12 | 2 years | 26/17 | Positive outcomes: Change in area under the curve for C-peptide, and decrease in value of HbA1c, Adverse events |
| Sherry et al[23] | 2011 | Lancet | United States | HIC | RCT | 763 | Type-1 diabetes | 8-35 years | 513 | 99 | 2 years | 325/438 | Positive outcomes: Lower insulin uses and decrease in value of HbA1c, Adverse events |
| Sims et al[24] | 2021 | Science Translation medicine | United States | HIC | RCT | 76 | Type-1 diabetes | 8-39 years | 44 | 32 | 2 years | 40/36 | Positive outcomes: Change in area under the curve for C-peptide and decrease in value of HbA1c, Adverse events |
Table 3 presents the results of the risk of bias assessment for the studies that were included, which were derived from the questionnaire that was designed beforehand. As can be seen from the graph depicting the risk of bias (Figure 2A) and the summary depicting the risk of bias (Figure 2B), the current meta-analysis appears to have a low probability of being biased. Out of the eight studies that were considered for inclusion in the analysis, six of them had a low risk of bias, while one of them had a substantial risk of bias. It was determined that the bias that occurred as a result of the randomization technique was caused by the moderate risk. In spite of this, there was a specific study that displayed a substantial high risk because of the bias in the selection of the data that were published. The symmetrical form of the funnel plot that is represented in Figure 3 and the statistically insignificant P value of Begg's test (0.249) that is higher than the preset significance level of 0.05 both indicate that there is a minimal risk of publishing bias. In addition, the symmetrical form of the funnel plot is used to illustrate the little risk of publication bias.
| Herold et al[17] | Hagopian et al[18] | Herold et al[19] | Herold et al[20] | Herold et al[21] | Perdigoto et al[22] | Sherry et al[23] | Sims et al[24] | |
| Did the study avoid inappropriate exclusions | Y | Y | Y | Y | Y | Y | Y | Y |
| Did all patients receive the same reference standard | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all patients included in the analysis | N | N | N | N | N | N | N | N |
| Was the sample frame appropriate to address the target population | Y | Y | Y | Y | Y | Y | Y | Y |
| Were study participants sampled in an appropriate way | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the study subjects and the setting described in detail | Y | Y | Y | Y | Y | Y | Y | Y |
| Were valid methods used for the identification of the condition | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the condition measured in a standard, reliable way for all participants | Y | Y | Y | Y | Y | Y | Y | Y |
The current meta-analysis was comprised of a sample of eight RCTs, which included a total of seventeen thousand eight hundred eight individuals with T1DM. Among the whole population, Teplizumab was delivered to 1361 patients, while 547 patients were given a control medication for the treatment of type 1 diabetes. On the basis of the statistical analysis that was carried out on the most important results of the study, the following conclusions were reached:
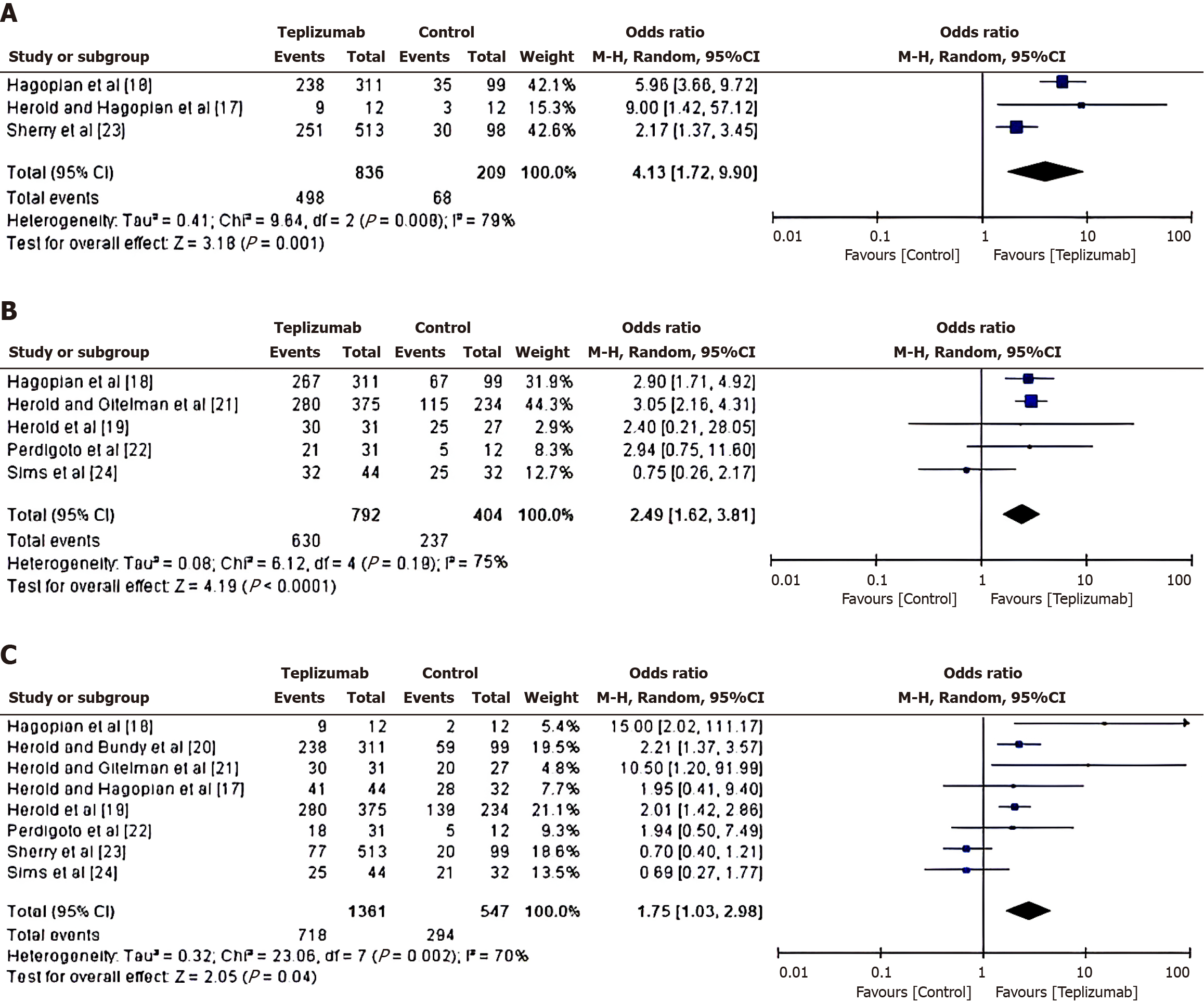
Reduction of insulin use in teplizumab vs control group: To investigate the reduction in insulin-use in patients treated with either Teplizumab or a control drug, an OR was calculated using the event data extracted from the included studies, as depicted in Figure 4A. From the calculated results, it was found that the patients in the teplizumab group have a higher likelihood of reduction in insulin use, with an OR of 4.13 (95%CI: 1.72 to 9.90) and a tau2 value of 0.41, χ2 = 9.64, degree of freedom(df) = 2, Z = 3.18, I2 = 79% and P = 0.001.
Change in C-peptide response in teplizumab vs control group: To examine the change in C-peptide response in patients treated with either Teplizumab or a control drug, an OR was calculated using the event data extracted from the included studies. From the calculated results shown in Figure 4B, it was found that the patients in the teplizumab group have a higher likelihood of change in C-peptide response, with an OR of 2.49 (95%CI: 1.62 to 3.81) and a tau2 value of 0.08, χ2 = 6.12, df = 4, Z = 4.19, I2 = 75% and P < 0.0001.
Change in HbA1C level in teplizumab vs control group: To assess the change in HbA1C level in Teplizumab vs control group, a comparative analysis of glycated haemoglobin value in the patients treated with either teplizumab or control drug was carried out, as depicted in Figure 4C. From the calculations, it was found that the patients in the teplizumab group have a higher likelihood of change in HbA1C level, with an OR of 1.75 (95%CI: 1.03 to 2.98) and a tau2 value of 0.32, χ2 = 23.06, df = 7, Z = 2.05, I2 = 70% and P = 0.04.
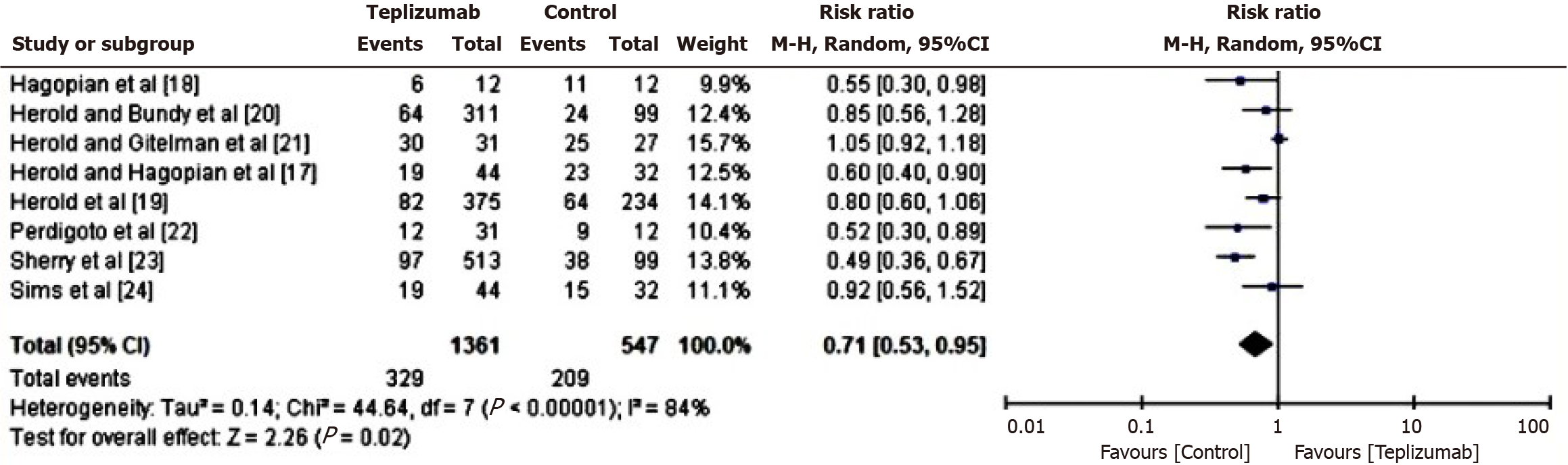
Comparison of adverse events in patients of teplizumab vs control group: In order to assess the comparative risk of adverse events between the Teplizumab and control groups, a RR analysis was conducted. This analysis specifically examined the occurrence of adverse events, such as systemic inflammations and allergic responses, in patients who received either teplizumab or the control medicine. The results of this analysis are presented in Figure 5. Based on the computed data, it was determined that the individuals in the control group exhibit a greater susceptibility to experiencing unfavorable outcomes, as shown by a RR of 0.71 (95%CI: 0.53 to 0.95). Additionally, the tau2 value turned out to be 0.14, the χ2 value was 44.64 with df 7, the Z score was 2.26, the I2 value was 84%, and the P value was 0.02.
Comparison of glycaemic control in patients of teplizumab vs control group: Box and whisker plot was generated using the event data obtained from the studies included in the analysis to evaluate the relative effectiveness of teplizumab vs the control medication in managing glycaemic control in patients. This figure is depicted in Figure 6. The plot exhibited a symmetrical distribution of data points with a median positioned at the centre of the box, and the whiskers extending to almost equal ranges on both sides of the box. The group receiving teplizumab has favorable glycaemic control, characterized by optimal serum glucose concentrations, in comparison to the control group. This effect contributes to the prevention of diabetes complications.
T1DM is an autoimmune disorder resulting from the destruction of pancreatic β-cells responsible for insulin production, either with or without any remaining functional tissue[41]. Type 1 diabetes can be attributed to various factors, such as viral infections, drug-induced effects, and autoimmune mechanisms. These variables contribute to the death of β-cells and result in a complete absence of insulin in the bloodstream, leading to elevated levels of blood glucose[42,43]. Individuals diagnosed with T1DM necessitate the continuous administration of insulin throughout their whole lifespan. The majority of individuals necessitate a minimum of two injections of insulin each day, wherein the dosage is modified in accordance with self-monitoring of blood glucose levels[44]. Teplizumab, also known as teplizumab-mzwv, is a monoclonal antibody of the IgG1 kappa class that has been humanized. It is primarily utilized for the purpose of delaying the onset of type 1 diabetes and as a therapy option for those with T1DM[45]. In November 2022, teplizumab was granted approval as the inaugural medication for the purpose of postponing the initiation of stage 3 type 1 diabetes in individuals aged eight years and older, encompassing both adults and children[46].
Multiple investigations have revealed that Teplizumab demonstrates disease-modifying characteristics through the pr
Our findings align with the aforementioned results, indicating positive correlation between teplizumab and a decrease in insulin usage, with an OR of 4.13 (95%CI: 1.72 to 9.90). Additionally, teplizumab is associated with an improved C-pe
One of the most important aspects of this research is the utilization of all-encompassing search phrases that encompass the investigation of "type-1 diabetes mellitus" and "teplizumab" across a number of different databases.
However, it is necessary to illustrate certain limitations. Firstly, studies done in languages other than English were not included in this analysis. Given that a sizable number of the publications included in our meta-analysis were eliminated, it is also imperative to recognize the possibility of selection bias in our research. Furthermore, it was not possible to determine a correlation between the results and factors like gender, age, or ethnicity, and it is unclear whether these conclusions will apply to people who do not appear to be at risk for type 1 diabetes but do not have first-degree relatives who have the disease. Thirdly, the limited sample size used in the current meta-analysis—just eight studies—showed notable variability and heterogeneity. It is impossible to determine whether repeated doses will prolong therapeutic effects or offer additional advantages in comparison to the drug administered for a single course. Finally, one more limitation of our analysis is that it only includes studies from high-income nations. This emphasizes the importance of doing this kind of research in LMICs, where T1DM is more common in adults and adolescents.
Based on the results of the present meta-analysis, it can be concluded that the utilization of teplizumab is linked to a reduction in insulin use, an enhanced C-peptide response, and a significant alteration in HbA1c levels among individuals diagnosed with type 1 diabetes, while exhibiting minimal adverse effects. The findings indicate that teplizumab exhibits favorable safety and efficacy profiles in promoting improved glycaemic control and managing diabetes mellitus. However, additional study is necessary to investigate the potential synergistic effects of combining immune and metabolic therapy. This research is crucial in order to maintain the immunological responses that are related with the preservation of C-peptide responses and the attainment of good outcomes that have a significant therapeutic impact.
| 1. | Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 799] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 2. | Rodrigues Oliveira SM, Rebocho A, Ahmadpour E, Nissapatorn V, de Lourdes Pereira M. Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices. Micromachines (Basel). 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Galderisi A, Sherr JL. A Technological Revolution: The Integration of New Treatments to Manage Type 1 Diabetes. Pediatr Ann. 2019;48:e311-e318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Burrack AL, Martinov T, Fife BT. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front Endocrinol (Lausanne). 2017;8:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol. 2019;14:50-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 952] [Cited by in RCA: 835] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 6. | Warshauer JT, Bluestone JA, Anderson MS. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 2020;31:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 9. | Song P, Hwang JS, Park HC, Kim KK, Son HJ, Kim YJ, Lee KM. Therapeutic Applications of Type 2 Diabetes Mellitus Drug Metformin in Patients with Osteoarthritis. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Misra S, Shukla AK. Teplizumab: type 1 diabetes mellitus preventable? Eur J Clin Pharmacol. 2023;79:609-616. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Novograd J, Frishman WH. Teplizumab Therapy to Delay the Onset of Type 1 Diabetes. Cardiol Rev. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | Ben-Skowronek I, Sieniawska J, Pach E, Wrobel W, Skowronek A, Tomczyk Z, Rosolowska I. Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Waldron-Lynch F, Henegariu O, Deng S, Preston-Hurlburt P, Tooley J, Flavell R, Herold KC. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med. 2012;4:118ra12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Linsley PS, Greenbaum CJ, Nepom GT. Uncovering Pathways to Personalized Therapies in Type 1 Diabetes. Diabetes. 2021;70:831-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Nagy G, Szekely TE, Somogyi A, Herold M, Herold Z. New therapeutic approaches for type 1 diabetes: Disease-modifying therapies. World J Diabetes. 2022;13:835-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Felton JL, Griffin KJ, Oram RA, Speake C, Long SA, Onengut-Gumuscu S, Rich SS, Monaco GSF, Evans-Molina C, DiMeglio LA, Ismail HM, Steck AK, Dabelea D, Johnson RK, Urazbayeva M, Gitelman S, Wentworth JM, Redondo MJ, Sims EK; ADA/EASD PMDI. Disease-modifying therapies and features linked to treatment response in type 1 diabetes prevention: a systematic review. Commun Med (Lond). 2023;3:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 889] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 18. | Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J; Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901-3908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381:603-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 846] [Cited by in RCA: 713] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 21. | Herold KC, Gitelman SE, Gottlieb PA, Knecht LA, Raymond R, Ramos EL. Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function. Diabetes Care. 2023;46:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 22. | Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 24. | Sims EK, Bundy BN, Stier K, Serti E, Lim N, Long SA, Geyer SM, Moran A, Greenbaum CJ, Evans-Molina C, Herold KC; Type 1 Diabetes TrialNet Study Group. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 25. | Brown D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot Pract. 2020;21:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 26. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13358] [Article Influence: 834.9] [Reference Citation Analysis (0)] |
| 27. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24863] [Article Influence: 1775.9] [Reference Citation Analysis (3)] |
| 28. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2643] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 29. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12186] [Article Influence: 406.2] [Reference Citation Analysis (0)] |
| 30. | Elovic A, Pourmand A. MDCalc Medical Calculator App Review. J Digit Imaging. 2019;32:682-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Schmidt L, Shokraneh F, Steinhausen K, Adams CE. Introducing RAPTOR: RevMan Parsing Tool for Reviewers. Syst Rev. 2019;8:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Dettori JR, Norvell DC, Chapman JR. Seeing the Forest by Looking at the Trees: How to Interpret a Meta-Analysis Forest Plot. Global Spine J. 2021;11:614-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | George BJ, Aban IB. An application of meta-analysis based on DerSimonian and Laird method. J Nucl Cardiol. 2016;23:690-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010;19:227-229. [PubMed] |
| 35. | Viera AJ. Odds ratios and risk ratios: what's the difference and why does it matter? South Med J. 2008;101:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2053] [Cited by in RCA: 2637] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 37. | Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 38. | Andrade C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J Psychol Med. 2019;41:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 39. | Ndako JA, Olisa JA, Ifeanyichukwu IC, Ojo SKS, Okolie CE. Evaluation of diagnostic assay of patients with enteric fever by the box-plot distribution method. New Microbes New Infect. 2020;38:100795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Bin Rakhis SA Sr, AlDuwayhis NM, Aleid N, AlBarrak AN, Aloraini AA. Glycemic Control for Type 2 Diabetes Mellitus Patients: A Systematic Review. Cureus. 2022;14:e26180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 969] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 42. | Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, Donner T, Hamad ARA. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J Diabetes. 2020;11:13-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (9)] |
| 43. | van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 684] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 44. | Janež A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, Tabák AG, Prazny M, Martinka E, Smircic-Duvnjak L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: a Narrative Review. Diabetes Ther. 2020;11:387-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 45. | Masharani UB, Becker J. Teplizumab therapy for type 1 diabetes. Expert Opin Biol Ther. 2010;10:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Keam SJ. Teplizumab: First Approval. Drugs. 2023;83:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 47. | Nourelden AZ, Elshanbary AA, El-Sherif L, Benmelouka AY, Rohim HI, Helmy SK, Sayed MK, Ismail A, Ali AS, Ragab KM, Zaazouee MS. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr Metab Immune Disord Drug Targets. 2021;21:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Ashraf MT, Ahmed Rizvi SH, Kashif MAB, Shakeel Khan MK, Ahmed SH, Asghar MS. Efficacy of anti-CD3 monoclonal antibodies in delaying the progression of recent-onset type 1 diabetes mellitus: A systematic review, meta-analyses and meta-regression. Diabetes Obes Metab. 2023;25:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 49. | Liu Y, Li W, Chen Y, Wang X. Anti-CD3 monoclonal antibodies in treatment of type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2024;83:322-329. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Evans-Molina C, Oram RA. Teplizumab approval for type 1 diabetes in the USA. Lancet Diabetes Endocrinol. 2023;11:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |