Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1518
Revised: April 12, 2024
Accepted: May 14, 2024
Published online: July 15, 2024
Processing time: 139 Days and 1.3 Hours
Heart failure (HF), especially HF with reduced ejection fraction (HFrEF), presents complex challenges, particularly in the aging population where it often coexists with type 2 diabetes mellitus (T2DM).
To analyze the effect of dapagliflozin treatment on cardiac, renal function, and safety in patients with HFrEF combined with T2DM.
Patients with T2DM complicated with HFrEF who underwent treatment in our hospital from February 2018 to March 2023 were retrospectively analyzed as the subjects of this study. The propensity score matching method was used, and a total of 102 eligible samples were scaled. The clinical efficacy of the two groups was evaluated at the end of the treatment, comparing the results of blood glucose, insulin, cardiac function, markers of myocardial injury, renal function indexes, and 6-min walk test (6MWT) before and after the treatment. We compared the occurrence of adverse effects on the treatment process of the two groups of patients. The incidence of adverse outcomes in patients within six months of treatment was counted.
The overall clinical efficacy rate of patients in the study group was significantly higher than that of patients in the control group (P = 0.013). After treatment, the pancreatic beta-cell function index, left ventricular ejection fraction, and glomerular filtration rate of patients in the study group were significantly higher than control group (P < 0.001), while their fasting plasma glucose, 2-h postprandial glucose, glycosylated hemoglobin, insulin resistance index, left ventricular end-systolic diameter, left ventricular end-diastolic diameter, cardiac troponin I, creatine kinase-MB, N-terminal pro b-type natriuretic peptide, serum creatinine, and blood urea nitrogen were significantly lower than those of the control group. After treatment, patients in the study group had a significantly higher 6MWT than those in the control group (P < 0.001). Hypoglycemic reaction (P = 0.647), urinary tract infection (P = 0.558), gastrointestinal adverse effect (P = 0.307), respiratory disturbance (P = 0.558), and angioedema (P = 0.647) were not statistically different. There was no significant difference between the incidence of adverse outcomes between the two groups (P = 0.250).
Dapagliflozin significantly enhances clinical efficacy, cardiac and renal function, and ambulatory capacity in patients with HFrEF and T2DM without an increased risk of adverse effects or outcomes.
Core Tip: This study delves into the complex association between type 2 diabetes mellitus (T2DM) and heart failure (HF) with preserved ejection fraction, focusing on how the two disorders interact through multiple mechanisms, leading to alterations in cardiac structure and function. Special attention is paid to the role of T2DM in promoting myocardial interstitial fibrosis and decreased cardiac function, and how these alterations exacerbate the course of HF, thus providing new perspectives on the treatment of these patients.
- Citation: Yu PL, Yu Y, Li S, Mu BC, Nan MH, Pang M. Dapagliflozin in heart failure and type 2 diabetes: Efficacy, cardiac and renal effects, safety. World J Diabetes 2024; 15(7): 1518-1530
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1518.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1518
Heart failure (HF) is a serious, chronic condition characterized by changes in the structure or function of the heart that results in decreased systolic and/or diastolic function[1]. In particular, patients with HF with reduced ejection fraction (HFrEF), who have a poor long-term prognosis and relatively high mortality, have become the focus of drug and device therapy in recent years[2]. With the aging of the population and the improvement of living standards, type 2 diabetes mellitus (T2DM) has become a common disease in the elderly and often coexists with cardiovascular disease, in which the condition of patients with T2DM combined with cardiac function insufficiency is particularly serious[3].
The pathophysiologic mechanisms that complicate HFrEF in patients with T2DM are extremely complex[4]. In addition to traditional risk factors such as advanced age, race, genetics, hypertension, and smoking, T2DM exacerbates the likelihood of ischemic HF by increasing the risk of coronary artery disease and can directly contribute to cardiac function decline and structural alterations such as increased fibrosis of myocardial interstitial fibrosis and increased left ventricular wall thickness[5]. The mechanisms by which T2DM leads to HFrEF include multiple pathological changes, including abnormalities in calcium signaling, endothelial cell malfunction, disturbances in myocardial energy metabolism, and insulin resistance[6,7]. These changes lead to asymptomatic dysregulation of cardiac diastolic and systolic function, altered microvascular compliance, left ventricular enlargement, and diminished cardiac function, ultimately leading to the onset and progression of symptomatic HFrEF.
As an SGLT2 inhibitor, dapagliflozin was initially developed as a hypoglycemic agent, but its effectiveness in improving cardiovascular function in patients with T2DM has been notable[8]. By binding to the SGLT2 protein target, dapagliflozin reduces glucose reabsorption in renal tubules, increases urinary excretion of glucose and sodium, and reduces glucose and volume load in the body[9]. The 2019 DAPA-HF trial[10] showed that dapagliflozin significantly reduced the rate of primary endpoint events in patients with HFrEF, reducing the risk of progression of HF and the risk of sudden cardiovascular death. Several clinical trials have confirmed[11,12] the cardiovascular protective effects of SGLT2 inhibitors, leading to their recommended use in the treatment of patients with HFrEF. However, the effects of SGLT2 inhibitors on cardiac function, renal function, and safety in patients with HFrEF combined with T2DM are unclear.
The aim of this study was to provide insight into the combined effects of SGLT2 inhibitors, specifically dapagliflozin, in the treatment of patients with HF-and particularly with T2DM-complicated by HFrEF. Considering that the co-morbidity of HF and T2DM has a significant impact on patient prognosis, we are especially interested in how dapagliflozin improves cardiovascular and renal health markers in these patients. We hope to provide a more in-depth and comprehensive therapeutic strategy for the comprehensive management of T2DM complicating HFrEF.
Patients with T2DM complicated with HFrEF who were treated at the Second Hospital of Liaoning University of Tra-ditional Chinese Medicine from February 2018 to March 2023 were retrospectively analyzed as the subjects of this study and were Approval of the Medical Ethics Committee of the Second Affiliated Hospital of Liaoning University of Traditional Chinese Medicine.
Patients were approved for inclusion if they met the diagnostic criteria for HF diagnosis and treatment guidelines[13] of the New York Heart Association (NYHA) class III-IV[14]. Furthermore, they were selected if they had symptoms and/or signs typical of HF, met the diagnostic criteria for T2DM[15], had a history of HF episodes, and their clinical data were complete.
Patients were excluded if they had a combination of severe renal disease, gout attack, and malignant hematological disease, had obvious liver and renal function impairment, had a tumor or other diseases, could not tolerate SGLT2 therapy, or had used initial therapy.
Patients in the control group received the standard HF regimen, including ACEI/ARB/ARNI, beta blockers, and spironolactone, with the addition of a dose of dapagliflozin (10 mg/tablet, Lot No: J20170040; manufactured by AstraZeneca Pharmaceuticals Ltd, United Kingdom) in combination with standard diabetes medications if necessary. Study group patients, on the other hand, received both conventional therapy for HF and T2DM, including glucose-lowering medications such as acarbose, metformin, and insulin. Both groups underwent continuous treatment for six months.
Patient data were collected from electronic medical records and outpatient review notes. Clinical data included sex, age, systolic blood pressure, diastolic blood pressure, heart rate, fasting plasma glucose (FPG), postprandial 2-h blood glucose, glycated hemoglobin, body mass index (BMI), NYHA score, history of coronary heart disease, history of hypertension, history of medications used for treatment of HF, and history of hypoglycemic medications. Laboratory parameters included cardiac troponin I (cTnI), creatine kinase-MB (CK-MB), N-terminal pro b-type natriuretic peptide (NT-proBNP), serum creatinine (Scr), blood urea nitrogen (BUN) test, and glomerular filtration rate (GFR) before and after the treatment, 2-h postprandial glucose (2hPG), FPG, glycosylated hemoglobin (HbAlc), pancreatic beta-cell function index (HOMA-IS), and insulin resistance index (HOMA-IR).
Cardiac function indices included left ventricular ejection fraction (LVEF), left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD) before and after treatment. Patients' post-treatment 6-min walk test (6MWT), post-treatment clinical outcomes, incidence of adverse effects, and incidence of adverse outcomes were also collected (all indicators of the patients were obtained from the medical records and review records, and this study did not directly test the relevant indicators of the patients).
This study used the propensity score matching (PSM) method to reduce selection bias due to non-randomized allocation in observational studies[16]. PSM is a statistical technique that works by estimating the probability that each participant will receive a particular treatment (i.e., propensity scores), and then matching patients in the study group to those in the control group based on these scores. This ensures that the two groups are similar on important baseline variables, thus making comparisons more reliable. In addition, we set a threshold for PSM of 0.02. Two patients were considered matched only if their propensity scores differed within 0.02 of each other.
(1) The study analyzes in depth the pretreatment baseline data of the two groups of patients to ensure comparability at the beginning of the study. This involves comparison of key variables such as age, gender, and medical history; (2) The clinical efficacy of the two groups will be assessed at the end of the treatment, and the criteria for determining the efficacy will be the disappearance of clinical symptoms, such as palpitations, shortness of breath and chest tightness, and the improvement of NYHA cardiac function classification ≥ grade 2. These changes will be considered to be significant. However, the obvious improvement of clinical symptoms, such as palpitations, shortness of breath, and chest tightness, and the improvement of cardiac function ≥ grade 1 will be considered to be ineffective. Finally, the lack of significant improvement or even worsening of clinical symptoms, such as palpitations, shortness of breath, and chest tightness, and the improvement of cardiac function are also considered to be ineffective. The total effective rate of treatment = significant rate + effective rate; (3) We will compare the results of cardiac function, myocardial infarction markers, renal function indexes, and 6MWT before and after the treatment to reveal the specific effects of the treatment on cardiopulmonary health; (4) We will compare the occurrence of any adverse effects during the treatment of the two groups of patients; and (5) We will count the incidence of adverse outcomes in patients within 6 months of treatment.
SPSS 26.0 software was used to process the data. The K-S test was used to analyze the normal distribution of the data. Normally distributed data were expressed as mean ± SD). The t test was used for comparison between the two groups. The independent samples t test was used for comparison between the groups, and the paired t test was used for intragroup comparison. The quartiles were used to express the P50 [P25, P75] for the data that were not normally distributed, and the rank-sum test was used. Count data were expressed as rate (%), and comparisons were made using the χ2 test. GraphPad Prism 9 was used to visualize the data. The nearest-neighbor matching method was used (Caliper = 0.02), and the matching ratio between the two groups was set to 1:1. Statistical differences were indicated when P < 0.05.
To increase the stability of the study results, we performed propensity leveling on the patients' baseline data. One hundred fifty-three patients were deleted by leveling and 51 were removed, resulting in a total of 102 eligible samples from leveling (Table 1).
| Type | Exact matching | Fuzzy matching | Matching variable | P value | Fuzzy match attempt count | Incremental rejection percentage |
| Count | 0 | 51 | PS | 0.02 | 1761 | 97.104 |
We compared the baseline data of patients in both groups of patients after leveling. The results showed that there was no statistically significant difference in gender, age, systolic blood pressure, diastolic blood pressure, heart rate, FPG, 2hPG, glycated hemoglobin, BMI, NYHA, coronary heart disease, hypertension, HF treatment medications, and glucose-lowering drugs between patients of the study group and patients of the control group (P > 0.05; Table 2).
| Clinical characteristics | Control group (n = 51) | Study group (n = 51) | χ2/t | P value |
| Gender | ||||
| Male | 31 | 28 | 0.362 | 0.547 |
| Female | 20 | 23 | ||
| Age (yr) | 70.86 ± 3.99 | 71.04 ± 3.19 | -0.247 | 0.805 |
| Systolic blood pressure (mmHg) | 127.51 ± 12.03 | 126.71 ± 12.41 | 0.332 | 0.74 |
| Diastolic blood pressure (mmHg) | 81.88 ± 6.98 | 81.47 ± 9.93 | 0.242 | 0.809 |
| Heart rate (times/min) | 80.20 ± 10.11 | 80.49 ± 10.76 | -0.142 | 0.887 |
| Fasting plasma glucose (mmol/L) | 8.12 ± 0.75 | 8.03 ± 0.79 | 0.606 | 0.546 |
| 2 h postprandial glucose (mmol/L) | 11.30 ± 0.78 | 11.44 ± 1.04 | -0.775 | 0.44 |
| Glycated hemoglobin (%) | 8.53 ± 0.54 | 8.42 ± 0.63 | 0.948 | 0.346 |
| BMI (kg/m2) | 23.20 ± 1.34 | 23.08 ± 1.81 | 0.393 | 0.696 |
| NYHA score | ||||
| III | 32 | 33 | 0.042 | 0.837 |
| IV | 19 | 18 | ||
| History of coronary heart disease | ||||
| Yes | 44 | 46 | 0.378 | 0.539 |
| No | 7 | 5 | ||
| History of hypertension | ||||
| Yes | 33 | 28 | 1.02 | 0.313 |
| No | 18 | 23 | ||
| Medications for heart failure treatmen | ||||
| Spironolactone | 44 | 48 | 1.774 | 0.183 |
| Other | 7 | 3 | ||
| Glucose-Lowering agents | ||||
| Acarbose | 27 | 25 | 0.157 | 0.692 |
| Other | 24 | 26 |
We have compared the clinical efficacy of the two groups of patients in this study. The data showed that in the control group, 16 (31.37%) were cured, 20 (39.22%) were effective in treatment, while 15 (29.41%) were ineffective in treatment. The overall effective rate of the control group was 70.59%. In contrast, in the study group, 27 (52.94%) were cured, 19 (37.25%) were treated effectively, and only 5 (9.81%) were ineffective, making the total effective rate of the study group 90.19%. Statistical analysis showed that there was a significant difference between the two groups (P = 0.013; Table 3), which indicated that the total clinical effective rate of the patients in the study group was significantly higher than that of the patients in the control group.
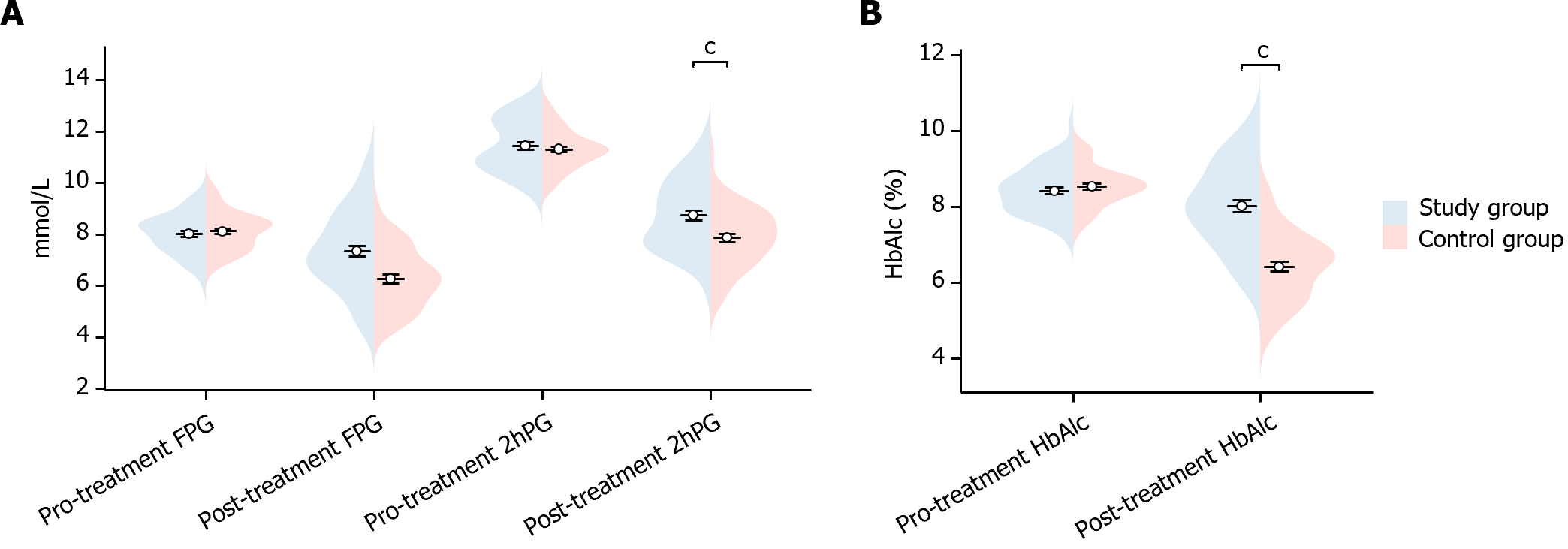
In this study, we compared the glucose indexes of the two groups of patients before and after treatment. Before treatment, there was no statistically significant difference in FPG, 2hPG, and HbAlc indexes between the two groups (FPG P = 0.546, 2hPG P = 0.440, HbAlc P = 0.346). After treatment, study group’s FPG, 2hPG and HbAlc were significantly lower than the control group (FPG P < 0.001, 2hPG P < 0.001, HbAlc P < 0.001), showing statistically significant differences (Figure 1). This indicated that the study group showed significant improvement in glucose after treatment.
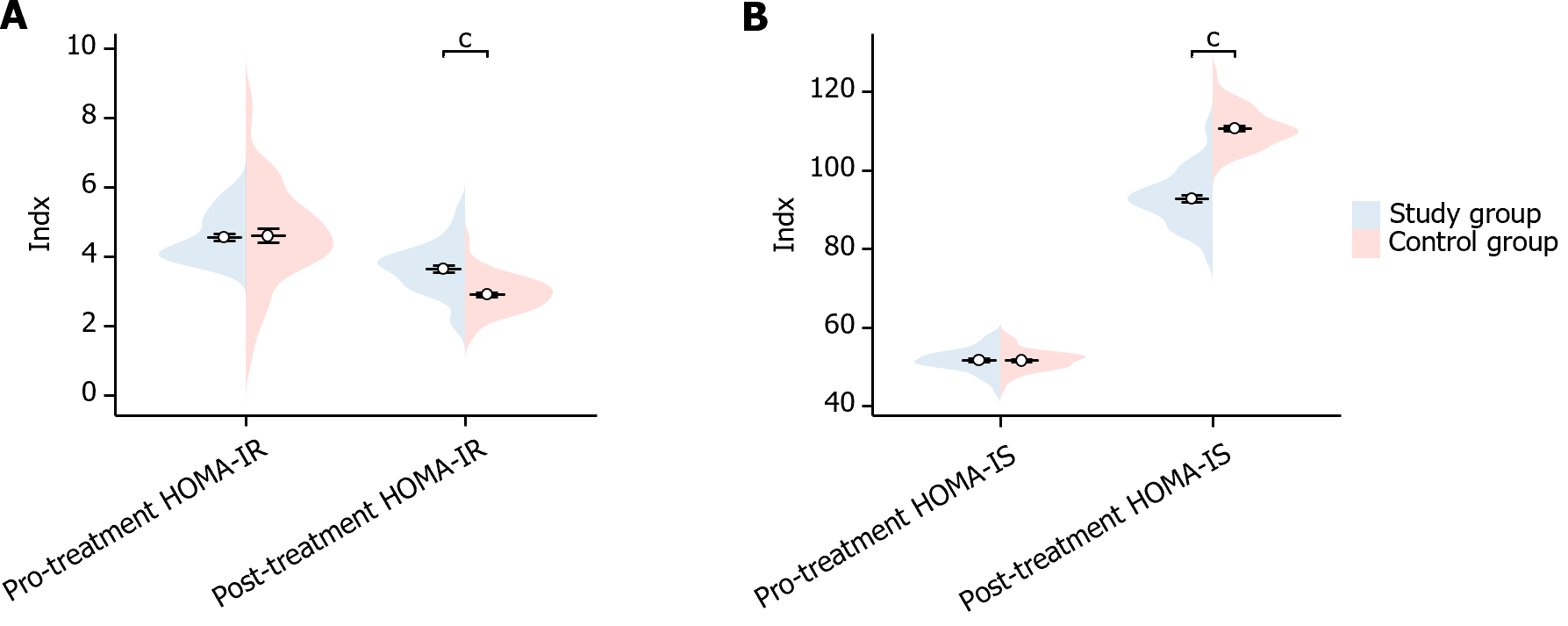
In this study, we compared the insulin indexes of the two groups of patients before and after treatment. Before treatment, there was no statistically significant difference in the HOMA-IR and HOMA-IS indexes between the two groups of patients (HOMA-IR P = 0.700, HOMA-IS P = 0.932). After treatment, the HOMA-IR of patients in the study group was significantly higher than that of the control group (P < 0.001), while their HOMA-IS was significantly lower than that of control group (P < 0.001), showing a statistically meaningful differences (Figure 2). This suggests that the study group showed significant improvement in insulin after treatment.
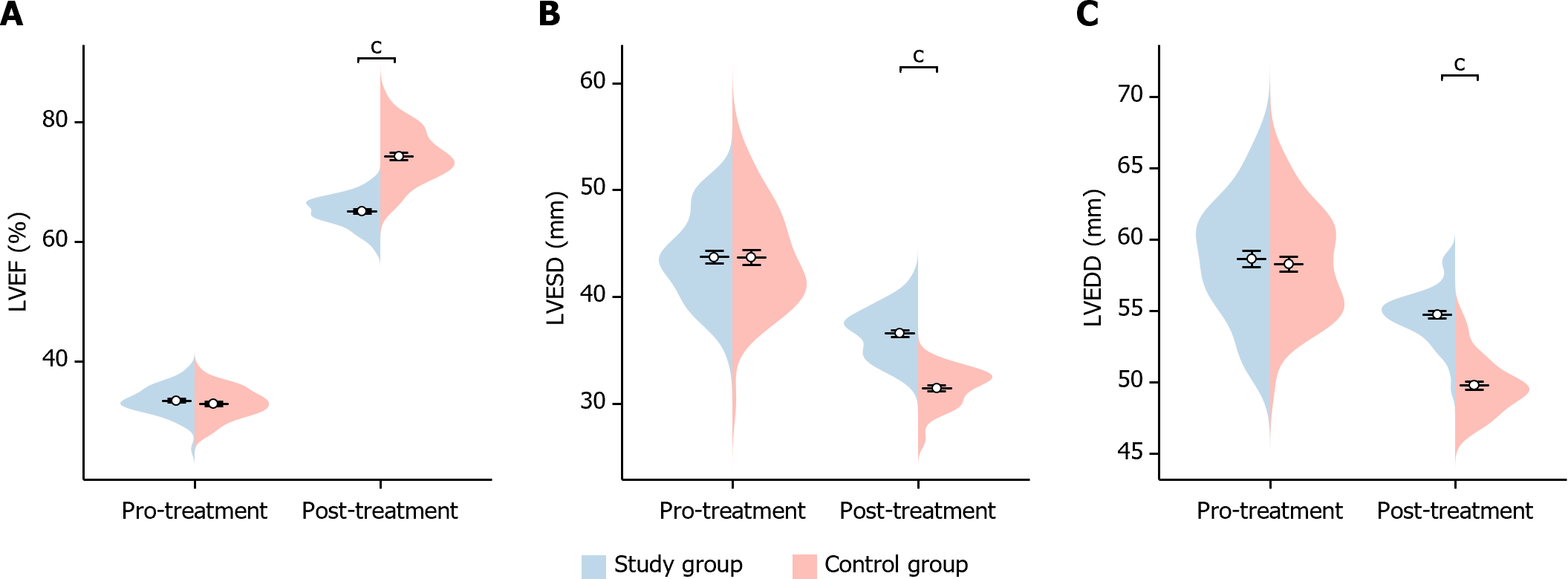
In this study, we compared the cardiac function indexes of the two groups of patients before and after treatment. Before treatment, there was no statistically significant difference in the LVEF, LVEDD and LVESD indexes between the two groups of patients (LVEF P = 0.324, LVEDD P = 0.959, LVESD P = 0.660). After treatment, the LVEF of patients in the study group was significantly higher than that of the control group (P < 0.001), whereas their LVESD and LVEDD were significantly lower than those of the control group (LVESD P < 0.001 and LVEDD P < 0.001), showing statistically significant differences (Figure 3). This indicated that the study group showed significant improvement in cardiac function after treatment.
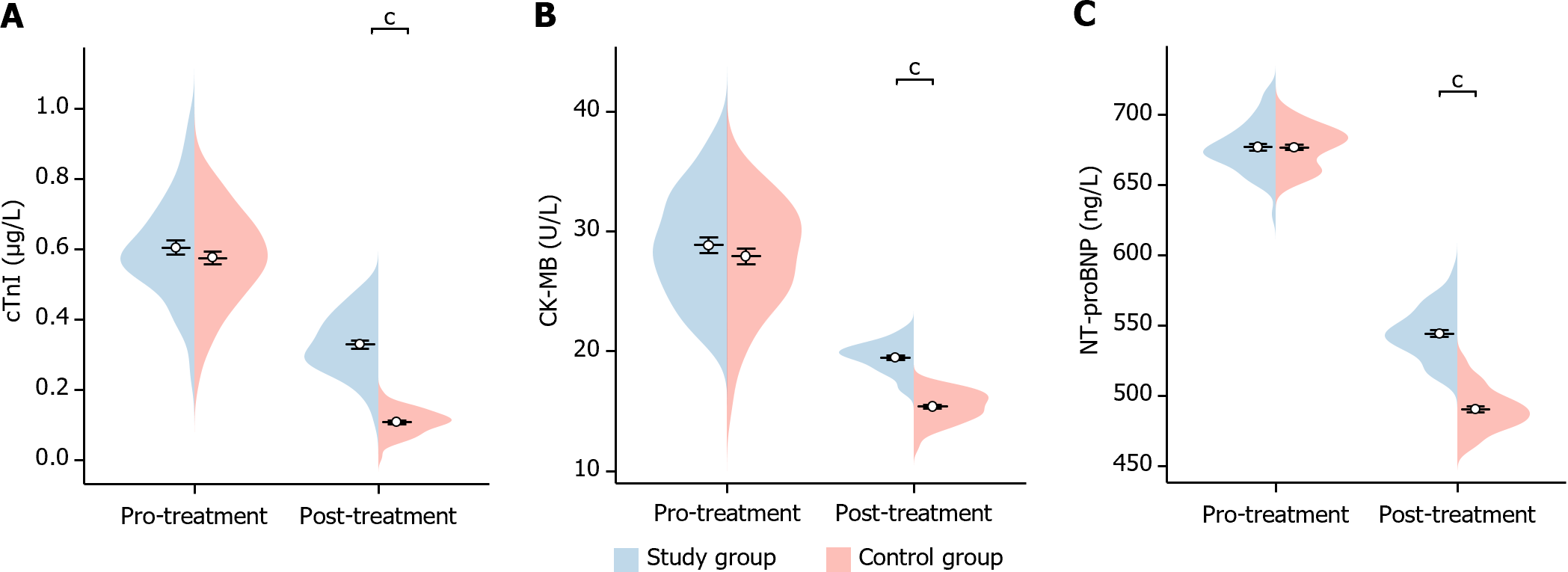
In this study, we compared the changes in markers of myocardial infarction before and after treatment in the two groups of patients. Before treatment, there was no significant difference in the levels of cTnI, CK-MB, and NT-proBNP between the two groups of patients (cTnI P = 0.284, CK-MB P = 0.302, NT-proBNP P = 0.958). After treatment, cTnI, CK-MB, and NT-proBNP were significantly lower in patients in the study group than the control group (cTnI P < 0.001, CK-MB P < 0.001, NT-proBNP P < 0.001), indicating a statistical difference (Figure 4). This suggests that the reduction in markers of myocardial infarction was more significant in the study group after treatment.
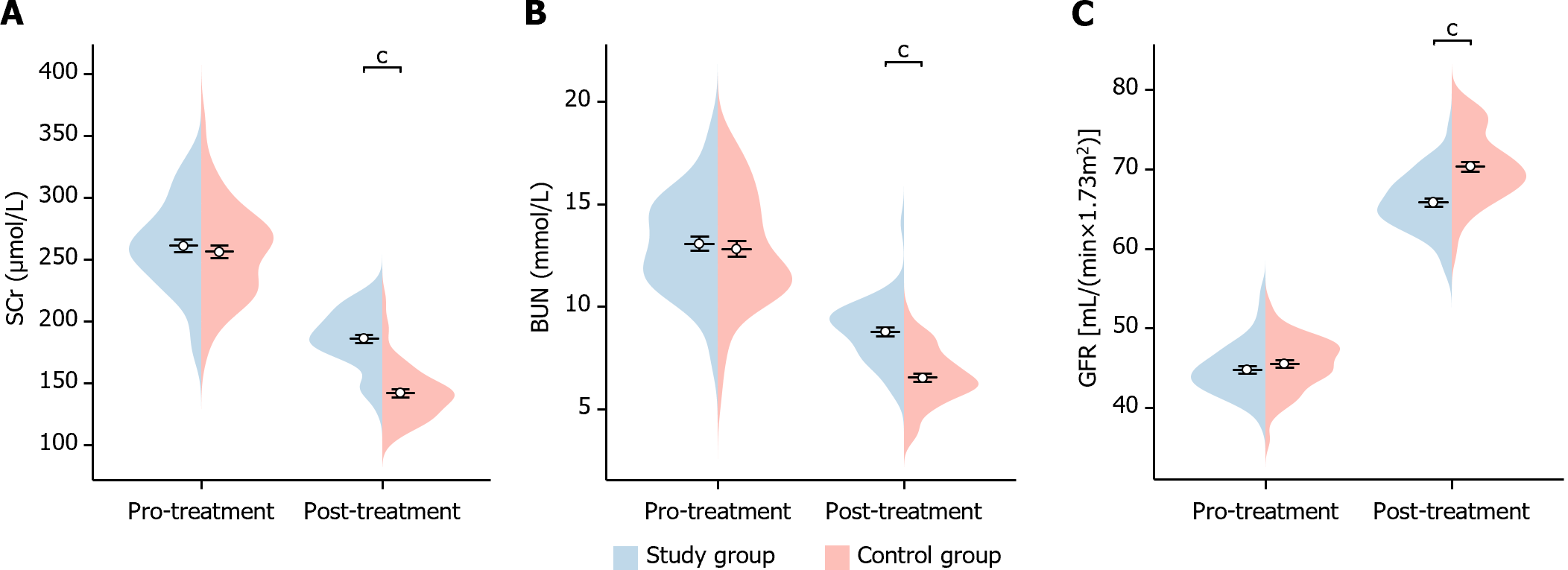
In this study, we analyzed the changes in renal function indexes before and after treatment in the two groups of patients. Before treatment, there was no significant difference in the levels of Scr, BUN, and GFR between the two groups of patients (Scr P = 0.509, BUN P = 0.607, GFR P = 0.275). After treatment, GFR was significantly higher in patients in the study group (GFR P < 0.001), while Scr and BUN were significantly lower than in the control group (Scr P < 0.001, BUN P < 0.001), showing statistical differences (Figure 5). This indicates that the study group showed significant improvement in renal function after treatment.
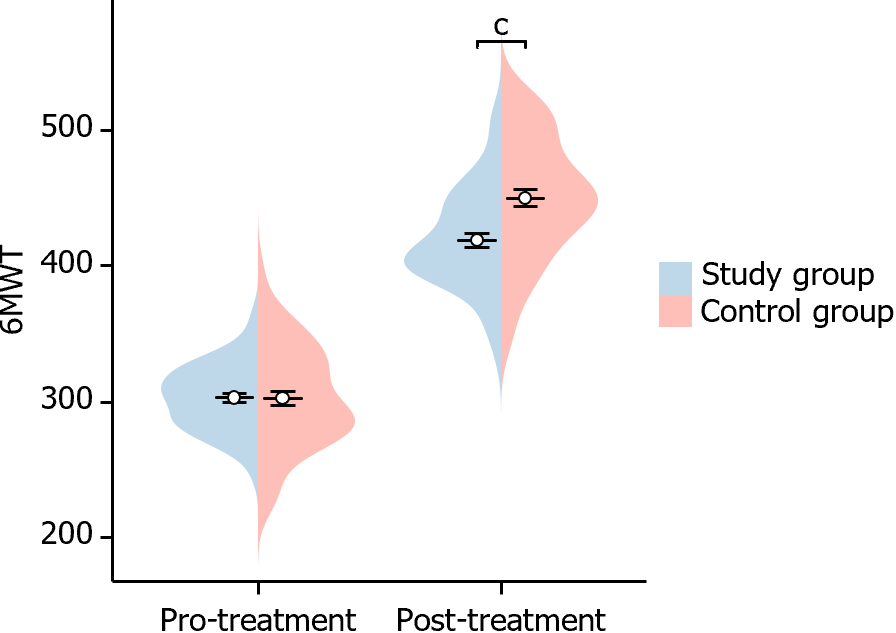
In this study, we evaluated changes in the 6MWT before and after treatment in both groups of patients. Before treatment, there was no significant difference in the 6MWT between the two groups of patients (P = 0.978). However, after treatment, the 6MWT of patients in the study group was significantly higher than that of the control group (P < 0.001), indicating a statistical difference (Figure 6). This suggests that the rise in the 6MWT was more significant in the study group after treatment.
In this study, we have counted the incidence of adverse effects in both groups, and the results showed that hypoglycemic reaction (P = 0.647), urinary tract infection (P = 0.558), gastrointestinal adverse effect (P = 0.307), respiratory distress (P = 0.558), and vascular tract edema (P = 0.647) of the two groups was not statistically different (Table 4).
| Groups | Hypoglycemic reaction | Urinary tract infection | Gastrointestinal adverse effect | Respiratory distress | Vascular edema |
| Control group (n = 51) | 3 (5.88) | 2 (3.92) | 3 (5.88) | 1 (1.96) | 2 (3.92) |
| Study group (n = 51) | 2 (3.92) | 1 (1.96) | 1 (1.96) | 2 (3.92) | 3 (5.88) |
| χ2 | 0.210 | 0.343 | 1.041 | 0.343 | 0.210 |
| P value | 0.647 | 0.558 | 0.307 | 0.558 | 0.647 |
In our present study, we compared the occurrence of adverse outcome events in the two groups of patients. The data showed that in the control group, cardiac death occurred in one patient (1.96%) and HF readmission occurred in eight patients (15.68%) for a total incidence of 17.64%. In contrast, in the study group, there were no cardiac deaths and HF readmissions occurred in five patients (9.80%), with an overall incidence of 9.80%. Statistical analysis showed that there was no significant difference between the incidence of adverse outcomes in the two groups (P = 0.250; Table 5). This indicates that there is no significant difference between the study group and the control group in terms of the incidence of adverse outcomes.
| Groups | Cardiac death | Heart failure readmission | Total incidence |
| Control group (n = 51) | 1 (1.96) | 8 (15.68) | 9 (17.64) |
| Study group (n = 51) | 0 | 5 (9.80) | 5 (9.80) |
| χ2 | 1.010 | 0.793 | 1.325 |
| P value | 0.315 | 0.373 | 0.250 |
In this study, we evaluated the efficacy of dapagliflozin in patients with T2DM complicated with HFrEF and found that it significantly increased the overall clinical efficacy rate, improved cardiac function indices, such as LVEF, LVEDD, and LVESD, and reduced the markers of myocardial infarction, cTnI, CK-MB, and NT-proBNP, as well as elevated the renal function indices, GFR and reduced Scr. In addition, there was a significant increase in the 6MWT performance. The study also found that the safety of dapagliflozin treatment was favorable, with no significant difference in the incidence of adverse effects and adverse outcomes from the control group. These findings suggest that dapagliflozin significantly improves cardiovascular and renal function in patients with T2DM complicated by HFrEF, while maintaining good safety and tolerability.
The significant improvement in therapeutic efficacy is mainly attributed to the combined effects of dapagliflozin on the cardiovascular system and metabolic pathways[17]. As an SGLT2 inhibitor, it reduces glucose levels and volume load in the body by decreasing renal reabsorption of glucose, thereby reducing cardiac burden and improving cardiac function[18]. This was confirmed in a study by Butt et al[19], in which it was observed that dapagliflozin reduced the risk of HF worsening and cardiovascular death. In addition, the effect of dapagliflozin in improving myocardial energy metabolism was a key factor in the enhanced therapeutic effect. In patients with T2DM, cardiomyocytes may not be able to utilize glucose efficiently due to insulin resistance, whereas dapagliflozin helps to enhance the myocardial energy supply by increasing fatty acid oxidation[20]. A study by Martinez et al[21] also showed the potential of dapagliflozin in reducing death and HF worsening. At the same time, the effect of dapagliflozin in reducing myocardial interstitial fibrosis is essential for improving cardiac structure and function. In this way, dapagliflozin may help to maintain or restore normal cardiac function, thereby improving symptoms and elevating the NYHA cardiac function classification[22]. According to one study[23], dapagliflozin is associated with a rapid reduction in the risk of cardiovascular disease, especially in terms of reducing HF worsening and death. Overall, the cardioprotective effects of dapagliflozin and the reduction in the risk of HF worsening provide an effective therapeutic option for patients with T2DM complicated by HFrEF.
In the management of patients with T2DM complicated by HFrEF, glucose control and insulin administration are key components of therapy[24]. Stabilization of glucose levels is essential for the prevention of diabetic complications and also has direct and indirect effects on cardiac function[25]. Insulin resistance is a major feature of T2DM, which not only affects glucose levels but may also affect cardiac function and structure[26]. In the present study, we found significant improvement in blood glucose and modulation of insulin-releasing capacity in patients of the study group after tr-eatment. Dapagliflozin increases urinary excretion of glucose by decreasing reabsorption of glucose in renal tubules. This mechanism leads to lower blood glucose levels as glucose in the body passes through the urine rather than being reabsorbed[27]. Thus, dapagliflozin directly reduces FPG and 2hPG. Also, by reducing the burden on pancreatic β-cells after lowering the patient's glucose, it improves pancreatic β-cell function. This effect of lowering blood glucose levels helps to reduce the stress on pancreatic beta cells from chronic hyperglycemia. A previous multicenter, placebo-controlled, double-blind, randomized trial found[28] that patients treated with 10 mg of dapagliflozin for 12 weeks had significantly lower mean glucose levels of glycated hemoglobin, glycated albumin, and continuous glucose monitoring relative to patients in the placebo group. Another review emphasized[29] that dapagliflozin improved glucose control with a low risk of hypoglycemia, accompanied by the potential for weight loss and possibly lower blood pressure as well. Another study pointed out[30] that significant improvements in glucose control and weight loss were observed after 12 wk of treatment with either 10 or 20 mg of dapagliflozin per day, suggesting that dapagliflozin may be a promising therapeutic option for a wide range of patients with T2DM. Overall, dapagliflozin offers multifaceted health benefits as an effective option for the treatment of T2DM complicated by HFrEF. Not only does it act directly on glucose control, but it may also have a combined effect by improving cardiac and metabolic health.
Improvements in cardiac function and markers of myocardial infarction have been attributed to the multifaceted effects of dapagliflozin on cardiac metabolism and structure. In patients with T2DM, dapagliflozin increased cardiac energy efficiency by improving myocardial energy metabolism, particularly by promoting fatty acid oxidation[31]. This leads to a significant elevation in LVEF and a reduction in LVEDD and LVESD. This effect not only improves the pumping efficiency of the heart but may also contribute to the improvement of overall cardiac function in patients. In a study by Solomon et al[32], dapagliflozin showed the potential to reduce the risk of HF worsening or cardiovascular death. In addition, dapagliflozin reduced myocardial[33]. Structural improvements in the heart help maintain normal cardiac function and morphology and reduce the risk of cardiac overload. This effect may be one of the key reasons for the reduction in markers of myocardial infarction by dapagliflozin. Reductions in markers of myocardial infarction, such as cTnI, CK-MB, and NT-proBNP, further confirm the effectiveness of dapagliflozin in attenuating myocardial stress and injury. A study by Berg et al[34] revealed the effect of dapagliflozin on highly sensitive cTn (hsTnT) levels, demonstrating that it was able to significantly attenuate the increase in hsTnT levels and reduce the risk of HF endpoint events at various hsTnT baseline levels. Overall, dapagliflozin provided a significant cardioprotective effect in patients with T2DM complicated by HFrEF by improving cardiac metabolism and structure, and attenuating myocardial stress and injury, resulting in improved cardiac function and levels of myocardial infarction markers.
The reason for the changes in renal function can be explained by the effects of dapagliflozin on renal physiology and metabolism[35]. Dapagliflozin, as an SGLT2 inhibitor, lowers glucose levels by decreasing tubular reabsorption of glucose while reducing the renal burden, which is particularly important for patients with HF because it helps to reduce renal stress and improve its function[36]. In addition, dapagliflozin elevates renal function by improving renal blood flow and reducing the renal inflammatory response. It improves renal hemodynamics-which may increase GFR-and reduces the inflammatory response, thus helping to protect the kidneys from further damage[37]. Dapagliflozin may also attenuate renal oxidative stress and reduce fibrosis, which are key factors in the decline of renal function. By attenuating these factors, dapagliflozin may help to maintain or improve renal function. A study by Ostrominski et al[38] emphasized that the benefits of dapagliflozin on HF attenuation were independent of baseline renal function, and that in patients with HFrEF, including those without diabetes mellitus, dapagliflozin slowed down the decline in epidermal growth factor receptor (eGFR) rate. Adamson et al[39] also reported that dapagliflozin use was associated with a smaller mean decrease in eGFR and with better clinical outcomes compared with placebo in patients with HF with similarly reduced ejection fraction. Overall, dapagliflozin improves kidney health through multiple actions that are particularly important in patients with HF. These mechanisms not only improve renal function but also play a positive role in overall car-diovascular disease management, providing comprehensive therapeutic benefits for patients with T2DM complicated by HFrEF.
The choice of two treatment regimens does not increase the risk of adverse effects and adverse outcomes in patients, which can be attributed to the favorable safety and tolerability characteristics of dapagliflozin and placebo. In clinical trials, no significant difference in the incidence of adverse events has been observed between patients on dapagliflozin and those on placebo. This suggests that the use of dapagliflozin does not result in an increase in adverse effects. In addition, dapagliflozin has been extensively studied in large-scale clinical trials, where its safety and efficacy have been validated.
In the present study, we confirmed the effect of dapagliflozin treatment in patients with T2DM complicated with HFrEF. However, there are some limitations of this study. First, this was a retrospective study relying on available clinical data and medical records, which may be limited by incomplete information and data quality. Second, the sample size of this study was small, especially after dividing the patients into study group and control group. This may limit the generalizability and statistical confidence of the results. Finally, the time span of the study was only six months, which may not be sufficient to fully assess the long-term effects of the treatment program. Therefore, we hope that future studies will increase the statistical confidence of the study through a larger sample size and extend the time span of the study to assess the long-term effects of the treatment.
This study provides preliminary evidence of the potential benefits of dapagliflozin in the treatment of patients with T2DM complicated by HFrEF, including a significant increase in overall clinical effectiveness, improvement in cardiac function markers, reduction in myocardial infarction markers, elevation of renal function markers, and improvement in ambulatory capacity. Compared with conventional therapy, dapagliflozin treatment was safe and well tolerated, with no increased risk of adverse effects or adverse outcomes.
| 1. | Obesity and Heart Failure. N Engl J Med. 2023;389:e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 2. | Tromp J, Ouwerkerk W, Teng TK, Cleland JGF, Bamadhaj S, Angermann CE, Dahlstrom U, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Collins SP, Filippatos G, Lam CSP. Global disparities in prescription of guideline-recommended drugs for heart failure with reduced ejection fraction. Eur Heart J. 2022;43:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 494] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 4. | Lee MMY, Gillis KA, Brooksbank KJM, Allwood-Spiers S, Hall Barrientos P, Wetherall K, Roditi G, AlHummiany B, Berry C, Campbell RT, Chong V, Coyle L, Docherty KF, Dreisbach JG, Kuehn B, Labinjoh C, Lang NN, Lennie V, Mangion K, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Sharma K, Sourbron S, Speirits IA, Thompson J, Welsh P, Woodward R, Wright A, Radjenovic A, McMurray JJV, Jhund PS, Petrie MC, Sattar N, Mark PB. Effect of Empagliflozin on Kidney Biochemical and Imaging Outcomes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2022;146:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, Green JB, Rosenstock J, Schnaidt S, Brueckmann M, Pocock SJ, Packer M, Anker SD; EMPEROR-Preserved Trial Committees and Investigators. Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes. Circulation. 2022;146:676-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 675] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 7. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1232] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 8. | Heerspink HJL, Kiyosue A, Wheeler DC, Lin M, Wijkmark E, Carlson G, Mercier AK, Åstrand M, Ueckert S, Greasley PJ, Ambery P. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet. 2023;402:2004-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Dia B, Alkhansa S, Njeim R, Al Moussawi S, Farhat T, Haddad A, Riachi ME, Nawfal R, Azar WS, Eid AA. SGLT2 Inhibitor-Dapagliflozin Attenuates Diabetes-Induced Renal Injury by Regulating Inflammation through a CYP4A/20-HETE Signaling Mechanism. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 11. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4394] [Article Influence: 732.3] [Reference Citation Analysis (0)] |
| 12. | Mc Causland FR, Claggett BL, Vaduganathan M, Desai AS, Jhund P, de Boer RA, Docherty K, Fang J, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Saraiva JFK, McGrath MM, Shah SJ, Verma S, Langkilde AM, Petersson M, McMurray JJV, Solomon SD. Dapagliflozin and Kidney Outcomes in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Prespecified Analysis of the DELIVER Randomized Clinical Trial. JAMA Cardiol. 2023;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 13. | Crea F. Heart failure with preserved ejection fraction: innovative diagnostic approaches and therapeutic targets. Eur Heart J. 2023;44:1481-1485. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld J, Januzzi JL, Cohen LS, Ahmad T. Clinical Implications of the New York Heart Association Classification. J Am Heart Assoc. 2019;8:e014240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 15. | Carrillo-Larco RM, Guzman-Vilca WC, Alvizuri-Gómez C, Tamim H, Alqahtani SA, García-Larsen V. Sensitivity and specificity of three diabetes diagnostic criteria in people with non-alcoholic fatty liver disease (NAFLD) and otherwise healthy people: Analysis of NHANES III. Prim Care Diabetes. 2023;17:506-512. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Hong Z, Xu J, Chen Z, Xu H, Huang Z, Weng K, Cai J, Ke S, Chen S, Xie J, Duan H, Kang M. Additional neoadjuvant immunotherapy does not increase the risk of anastomotic leakage after esophagectomy for esophageal squamous cell carcinoma: a multicenter retrospective cohort study. Int J Surg. 2023;109:2168-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Liu J, Chang X, Ding X, He X, Wang J, Wang G. Effect of dapagliflozin on proteomics and metabolomics of serum from patients with type 2 diabetes. Diabetol Metab Syndr. 2023;15:251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Ryaboshapkina M, Ye R, Ye Y, Birnbaum Y. Effects of Dapagliflozin on Myocardial Gene Expression in BTBR Mice with Type 2 Diabetes. Cardiovasc Drugs Ther. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Butt JH, Docherty KF, Petrie MC, Schou M, Kosiborod MN, O'Meara E, Katova T, Ljungman CEA, Diez M, Ogunniyi MO, Langkilde AM, Sjöstrand M, Lindholm D, Bengtsson O, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Jhund PS, McMurray JJV, Køber L. Efficacy and Safety of Dapagliflozin in Men and Women With Heart Failure With Reduced Ejection Fraction: A Prespecified Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial. JAMA Cardiol. 2021;6:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Op den Kamp YJM, de Ligt M, Dautzenberg B, Kornips E, Esterline R, Hesselink MKC, Hoeks J, Schrauwen-Hinderling VB, Havekes B, Oscarsson J, Phielix E, Schrauwen P. Effects of the SGLT2 Inhibitor Dapagliflozin on Energy Metabolism in Patients With Type 2 Diabetes: A Randomized, Double-Blind Crossover Trial. Diabetes Care. 2021;44:1334-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz-Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJV. Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age: Insights From DAPA-HF. Circulation. 2020;141:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 22. | Ostrominski JW, Vaduganathan M, Claggett BL, de Boer RA, Desai AS, Dobreanu D, Hernandez AF, Inzucchi SE, Jhund PS, Kosiborod M, Lam CSP, Langkilde AM, Lindholm D, Martinez FA, O'Meara E, Petersson M, Shah SJ, Thierer J, McMurray JJV, Solomon SD. Dapagliflozin and New York Heart Association functional class in heart failure with mildly reduced or preserved ejection fraction: the DELIVER trial. Eur J Heart Fail. 2022;24:1892-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sjöstrand M, Solomon SD, McMurray JJV, Sabatine MS. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021;6:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 24. | Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis-Tsimikas A, Bao S, Umpierrez G, Davis G, Kruger D, Bhargava A, Young L, McGill JB, Aleppo G, Nguyen QT, Orozco I, Biggs W, Lucas KJ, Polonsky WH, Buse JB, Price D, Bergenstal RM; MOBILE Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA. 2021;325:2262-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 25. | Daly A, Hovorka R. Technology in the management of type 2 diabetes: Present status and future prospects. Diabetes Obes Metab. 2021;23:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Yang W, Jiang W, Guo S. Regulation of Macronutrients in Insulin Resistance and Glucose Homeostasis during Type 2 Diabetes Mellitus. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Lin H, Yang X, Shi J, Sheng X, Wang L, Li T, Quan H, Zhai X, Li W. Effects of dapagliflozin monotherapy and combined aerobic exercise on skeletal muscle mitochondrial quality control and insulin resistance in type 2 diabetes mellitus rats. Biomed Pharmacother. 2023;169:115852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 28. | Lee SH, Min KW, Lee BW, Jeong IK, Yoo SJ, Kwon HS, Choi YH, Yoon KH. Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study. Diabetes Metab J. 2021;45:339-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Paisley AN, Yadav R, Younis N, Rao-Balakrishna P, Soran H. Dapagliflozin: a review on efficacy, clinical effectiveness and safety. Expert Opin Investig Drugs. 2013;22:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab. 2010;12:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, McGrath MM, O'Meara E, Wilderäng U, Lindholm D, Petersson M, Langkilde AM, McMurray JJV, Solomon SD. Dapagliflozin in Patients Recently Hospitalized With Heart Failure and Mildly Reduced or Preserved Ejection Fraction. J Am Coll Cardiol. 2022;80:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 32. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1408] [Article Influence: 469.3] [Reference Citation Analysis (0)] |
| 33. | Myhre PL, Vaduganathan M, Claggett BL, Miao ZM, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Lindholm D, Petersson M, Langkilde AM, McMurray JJV, Solomon SD. Influence of NT-proBNP on Efficacy of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail. 2022;10:902-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Berg DD, Docherty KF, Sattar N, Jarolim P, Welsh P, Jhund PS, Anand IS, Chopra V, de Boer RA, Kosiborod MN, Nicolau JC, O'Meara E, Schou M, Hammarstedt A, Langkilde AM, Lindholm D, Sjöstrand M, McMurray JJV, Sabatine MS, Morrow DA. Serial Assessment of High-Sensitivity Cardiac Troponin and the Effect of Dapagliflozin in Patients With Heart Failure With Reduced Ejection Fraction: An Analysis of the DAPA-HF Trial. Circulation. 2022;145:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Chatur S, Vaduganathan M, Claggett BL, Mc Causland FR, Desai AS, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez FA, Shah SJ, Sabatine MS, Kober L, Ponikowski P, Merkely B, Petersson M, Langkilde AM, McMurray JJV, Solomon SD. Dapagliflozin in Patients With Heart Failure and Deterioration in Renal Function. J Am Coll Cardiol. 2023;82:1854-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O'Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction: Results of DAPA-HF. Circulation. 2021;143:298-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 37. | Nakase M, Ninomiya K, Horiuchi Y, Sekiguchi M, Watanabe Y, Setoguchi N, Asami M, Yahagi K, Yuzawa H, Komiyama K, Tanaka J, Aoki J, Tanabe K. Impact of Dapagliflozin on the Renal Function and Damage in Patients with Heart Failure with a Reduced Ejection Fraction. Intern Med. 2024;63:169-177. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Ostrominski JW, Thierer J, Claggett BL, Miao ZM, Desai AS, Jhund PS, Kosiborod MN, Lam CSP, Inzucchi SE, Martinez FA, de Boer RA, Hernandez AF, Shah SJ, Petersson M, Langkilde AM, McMurray JJV, Solomon SD, Vaduganathan M. Cardio-Renal-Metabolic Overlap, Outcomes, and Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail. 2023;11:1491-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Adamson C, Docherty KF, Heerspink HJL, de Boer RA, Damman K, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Petrie MC, Ponikowski P, Sabatine MS, Schou M, Solomon SD, Verma S, Bengtsson O, Langkilde AM, Sjöstrand M, Vaduganathan M, Jhund PS, McMurray JJV. Initial Decline (Dip) in Estimated Glomerular Filtration Rate After Initiation of Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction: Insights From DAPA-HF. Circulation. 2022;146:438-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |